Abstract
Current therapies for treating skeletal pain have significant limitations as available drugs (nonsteroidal anti-inflammatory drugs and opiates) have significant unwanted side effects. Targeting nerve growth factor or it's cognate receptor Tropomysin receptor kinase A (TrkA) has recently become an attractive target for inhibition of adult skeletal pain. Here we explore whether sustained administration of a selective small molecule Trk inhibitor that blocks TrkA, TrkB and TrkC kinase activity with nanomolar affinity reduces skeletal pain while allowing the maintenance of sensory and sympathetic neurons in the adult mouse. Twice-daily administration of a Trk inhibitor was begun 1 day post fracture and within 8 hours of acute administration fracture pain related behaviors were reduced by 50% without significant sedation, weight gain or inhibition of fracture healing. Following administration of the Trk inhibitor for 7 weeks, there was no significant decline in the density of unmyelinated, myelinated sensory or sympathetic nerve fibers, measures of acute thermal pain, acute mechanical pain, or general neuromuscular function. The present results suggest that sustained administration of a peripherally selective TrkA, B & C inhibitor significantly reduces skeletal pain without having any obvious detrimental effects on adult sensory and sympathetic nerve fibers or early fracture healing. As with any potential therapeutic advance, understanding whether the benefits of NGF blockade by ARRY-470 are associated with any risks or unexpected effects will be required to fully appreciate the patient populations that may benefit from this therapy.
INTRODUCTION
Skeletal pain can have a significant impact on the quality of life and functional status of the individual and is a leading cause of age-related morbidity. [1, 2] A major reason skeletal pain remains a significant health problem is the limited repertoire and negative side effects of currently available analgesics. For example, non-steroidal anti-inflammatory drugs (NSAIDs), which are effective in reducing a variety of musculoskeletal pains, have been shown to have significant gastrointestinal (GI) and bone healing side effects. [3, 4] Studies have demonstrated that NSAIDs and selective cyclooxygenase-2 (COX-2) inhibitors hinder callus formation and effective bridging of the fracture site resulting in delayed bone healing, increased incidence of non-union of bone and decreased bone strength. [5, 6] These data, together with reports that show selective prostaglandin agonists of the EP2 receptor accelerate bone healing following fracture, suggest that NSAIDs and COX-2 inhibitors may delay bone healing after fracture.[7, 8]
Opiates are also frequently used to treat moderate to severe skeletal pain. While the effects that opiates have on bone healing remain controversial, opiates as a class cause increased somnolence, agitation, constipation, dizziness, cognitive impairment and respiratory depression. [9, 10] In young individuals with severe fractures, long-term opiate use can result in dependence and a reduced ability to promptly and fully participate in the effective musculoskeletal rehabilitation necessary for early and effective bone healing. [11] In elderly patients, opiate side effects tend to be more pronounced. [12] Following osteoporotic fractures in the elderly minimum bed rest is desired so as to minimize inactivity-induced loss of bone and muscle mass. Use of strong opiates will, in general, reduce the ability of these patients to effectively engage in the exercise and rehabilitation necessary for bone healing.[12] Together, these data highlight the need for the development of novel, mechanism based therapies that can attenuate skeletal pain without negative effects on CNS or bone healing.
Recently, targeting NGF or its cognate receptor TrkA, has become an attractive target for attenuating chronic pain. Four major strategies are currently being pursued in an effort to block the NGF / TrkA axis (Figure 1) and each of these strategies has its potential strengths and limitations. [13, 14] For example, while monoclonal antibodies (mAbs) are extraordinarily target specific, administration of mAbs carries the risk of immune reactions such as acute anaphylaxis, serum sickness and the generation of additional antibodies. In contrast, small molecule inhibitors of kinase activity do not require intravenous or intramuscular injection, are less expensive to make than mAbs and allow greater flexibility in dosing. [13, 14] However, kinase inhibitors are generally less selective than mAbs. Whether the kinases lack the extraordinary specificity of mAbs, provide greater desired efficacy, or greater unwanted side effects, will probably need to be examined with each mAb or kinase(s) that is being targeted.
Figure 1. Major NGF/Trk axis targets to attenuate chronic pain.
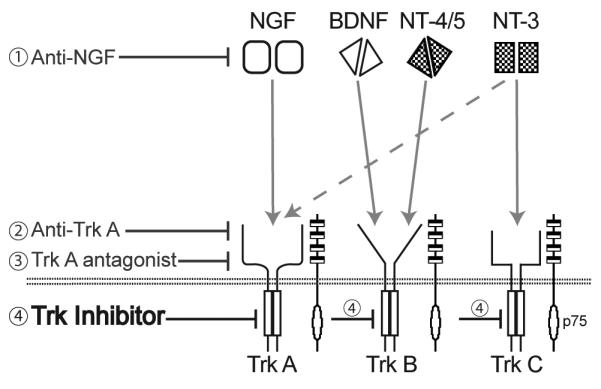
Current strategies for targeting NGF or its cognate receptor TrkA include; monoclonal antibodies that sequester NGF (1), monoclonal antibodies that targe t TrkA and prevent NGF from binding to TrkA (2), small molecule TrkA antagonist therapy (3) and the focus of the current study, a small molecule kinase inhibitor of Trk's (4). The pan-Trk therapy is a small molecule inhibitor demonstrating nanomolar cellular antagonism of TrkA (6.5nM), TrkB (8.1nM), and TrkC (10.6nM) and a high level of selectivity over a panel of kinase and non-kinase receptors (supplemental material Table S1 and S2). Schematic drawing adapted from Pezet and McMahon.
In the present study we explore the effects of a small molecule kinase inhibitor that inhibits TrkA, TrkB and TrkC and determine whether this inhibition reduces skeletal pain, what effect(s) sustained Trk inhibition has on the maintenance of adult sensory and sympathetic nerve fibers, and whether Trk inhibition plays a major role in early aspects of bone fracture healing.
MATERIALS AND METHODS
Experimental animals
Experiments were performed on a total of 163 adult male C3H/HeJ mice (Jackson Laboratories, Bar Harbor, ME) weighing 20-25 g. The mice were housed in accordance with the National Institutes of Health guidelines under specific pathogen free conditions in partial autoclaved cages maintained at 22°C with a 12 hour alternating light and dark cycle and were given food and water ad libitum. All procedures were approved by the Minneapolis VA Medical Center Institutional Animal Care and Use Committee.
Surgical and fracture procedure
To provide stabilization of the mouse femur prior to fracture, a stainless steel pin was surgically implanted into the medullary canal of the left femur. An intraperitoneal injection of 70 mg/kg ketamine and 7 mg/kg xylazine was given to provide a 20 min period of deep anesthesia. The skin of the left hind leg was shaved and swabbed with Betadine prior to surgical incision. An incision of approximately 6 mm was made in the skin dorsal to the knee joint and a 30-gauge needle was used to core through the proximal patellar ligament, between the femoral condyles and into the medullary canal. The femur was immediately radiographed to ensure proper coring. A 29-gauge needle was then used as a pilot to expand the diameter of the cored hole prior to insertion of a stainless steel pin. A pre-cut 0.011 in.-diameter stainless steel pin (Small Parts Inc., Miami Lakes, FL) was then set into the medullary space. Wound clips (MikRon Precision Inc., Gardena, CA) were used to close the skin and bacitracin zinc ointment was applied to the superficial surface of the surgical site. Wound clips were subsequently removed 5 days post-pin placement.
A closed mid-diaphyseal fracture of the left femur was produced 7 days post-pin placement in anesthetized mice (70 mg/kg ketamine and 7 mg/kg xylazine, i.p.) using a 3-point bending method based on the fracture apparatus described by [15]. The left femur of the anesthetized mouse was secured between two lower supports and an upper impactor head. A guillotine-like effect was created by dropping a rod-guided 168 g weight from a height of 10 cm onto the spring-loaded upper impactor head resulting in femoral fracture. [16]
Immediately following fracture, mice were radiographed to ensure localization of fracture to the mid-diaphysis of the femur. Mice with fractures located in the metaphysis (adjacent to the epiphyseal disk), inappropriate pin placement or dislodged pins were excluded from the study. Exclusion criteria were similar to those reported by Gerstenfeld, et al. [17] Following recovery from anesthesia after fracture, mice were allowed unrestricted movement and weight loading on all limbs.
Fracture pain-related behavior analysis
Mice were observed over a 2 minute period before fracture (day 0) and at 1, 2, 4, 7, 10, 14, 17, 21 and 23 days following fracture to assess ongoing (spontaneous) fracture pain-related behaviors, as previously described. [16, 18] Observers scoring the behaviors were blind to the treatment of the animals. Briefly, the number of hindpaw flinches and time spent guarding were recorded as measures of ongoing pain, as these endpoints are similar to observations in patients who protect or suspend their fractured limb. [19]
Behavior analysis of acute nociception and general neuromuscular function
Acute mechanical nociceptive response was used to determine mechanical allodynia of the plantar surface of the contralateral hindpaw following 48 consecutive days of twice-daily ARRY-470 administration (30 mg/kg, p.o.). A series of von Frey monofilaments (Stoelting Co., Wood Dale, IL) were used 1 hour post-dosing to assess the withdrawal threshold by increasing and decreasing the stimulus intensity between 0.4 and 15.1 gram equivalents of force using a Dixon non-parametric test. [20] Two trials of approximately 1-2 minutes each were performed per test period.
Response to acute thermal nociceptive stimulation of the plantar surface of the hindpaw was determined using a PE34 Incremental Hot/Cold Analgesia Meter (IITC, Woodland Hills, CA) following 48 consecutive days of twice-daily ARRY-470 administration. One hour after ARRY-470 or vehicle administration, animals were placed individually on a thermal analgesia meter set to 55°C. The response latency time to exhibition of a nociceptive behavior (in this case, paw licking, hindpaw flick or jump) was recorded.
General neuromuscular function of animals receiving 48 consecutive days of twice-daily ARRY-470 administration was determined using rotorod analysis 1 hour post-dosing. Animals were placed on the rotorod device (Columbus Instruments, Columbus, OH) with control setting of 20 rpm. Forced ambulation was rated by a blinded observer on a scale of 0 to 5: (5) normal use, (4) some limp, but not pronounced, (3) pronounced limp, (2) pronounced limp and prolonged guarding of limb, (1) partial non-use of the limb, and (0) complete lack of use. One trial of approx 1-2 minutes was performed per test.
To monitor the general health of the mice, body weight was recorded at each dose administration time point throughout the experimental period and animals were monitored for side effects (e.g. ataxia, illness, or lethargy) that might be related to the therapy used.
Radiographic analysis
Radiographic analysis of bone bridging and fracture–induced callus was performed to evaluate the effect of drugs on bone healing following fracture. Digital radiographs (Faxitron Specimen Radiography System Model MX-20; Faxitron X-ray Corp., Wheeling, IL) of the fractured femur were acquired immediately after fracture and at days 7, 10, 14, 17, 21, 28, 35, 42, 49 post-fracture (34kV, 12s) and subsequently exported to the Image J software program provided by NIH. The calcified callus area was measured as the difference between digital traces of the femur including the callus and excluding the callus.
Additionally the digital radiographs were used to evaluate bone bridging across the fractured femur at all time points. Scores were assigned based on the appearance of bridging across the left and right peripheries of the callus (1 point each) and the left and right cortices of the fractured femur (1 point each).
Chronic and acute treatment with Trk inhibitor (ARRY-470) therapy
The Trk inhibitor (ARRY-470; Array BioPharma, Boulder, CO) is a potent inhibitor of the tropomyosin kinase family of neurotrophin receptors, demonstrating nanomolar cellular antagonism of TrkA (6.5 nM), TrkB (8.1 nM), and TrkC (10.6 nM) and a high level of selectivity over a panel of kinases run at the ATP Km at 1.0 uM and non-kinase receptors [21](supplemental material Table S1 and S2). At doses of 10-100 mg/kg ARRY-470 reaches high concentrations in plasma and peripheral tissues, while the brain concentrations remain negligible. ARRY-470 at a dose of 30 mg/kg in a mouse xenograft model derived from HEK cells constitutively expressing active human TrkA showed >90% inhibition of phosphorylated TrkA at 1 hour and >70% inhibition over a 12 hour time course. Additionally, both 30 and 100mg/kg of ARRY-470 reduced CFA paw hyperalgesia by 100%, therefore the lower dose was used in the present study. [22]
To evaluate the effect of chronic dosing of a Trk inhibitor on pain-related behaviors, neurochemical changes and bone healing in a mouse model of bone fracture pain, treatment with ARRY-470 was initiated on day 1 post-fracture and was administered chronically twice a day through day 48 post fracture (30 mg/kg, p.o., bid). For behavioral analysis, mice were randomly placed into treatment groups of at least six animals and received either 100% Labrafac (polyglycolyzed glyceride) (pin + vehicle; pin + fracture + vehicle) or ARRY-470 in Labrafac (pin + fracture + ARRY-470, 30 mg/kg, p.o., bid). Analyses were performed within approximately one hour of administration of drug or vehicle.
To evaluate the effect of acute dosing of a Trk inhibitor on pain-related behaviors, ARRY-470 was administered day 4 post-fracture, a time that peak pain behaviors are exhibited. Dosing was one hour prior to initial behavioral analysis and again at three hours post-initial behavioral analysis. For behavioral analysis, mice were randomly placed into treatment groups of 10 animals, receiving either vehicle (pin + vehicle; pin + fracture + vehicle) or ARRY-470 (pin + fracture + ARRY-470, 30 mg/kg, p.o.). Spontaneous behavioral tests (guarding & flinching) were performed 1, 2, 4, 5, 6 and 8 hours post initial acute dose.
Immunohistochemistry and quantification of nerve fiber density
Density of sensory and sympathetic nerve fibers was determined in the plantar surface of the hindpaw from naïve C3H/HeJ mice (n=6 per group; 12 week-old) that had been chronically treated with ARRY-470 or vehicle for 48 days. Mice were sacrificed by carbon dioxide asphyxiation at day 48 post-treatment and perfused intracardially with 20ml of 0.1M phosphate buffered saline (PBS) followed by 20ml of 4% formaldehyde/12.5% picric acid solution in 0.1M PBS. Glabrous skin of the hindpaw was harvested following perfusion, post-fixed for 4 hours in the perfusion fixative, and cryoprotected for 24 hours in 30% sucrose in 0.1M PBS, all at 4°C, and then processed for immunohistochemistry. Serial frozen sections of glabrous skin (40μm) were cut on a cryostat and thaw-mounted on gelatin-coated slides for processing.
The skin sections were then washed in 0.1M PBS three times for 10 minutes each, incubated for 60 minutes at room temperature (RT, 22°C) in a blocking solution of 3% normal donkey serum in PBS with 0.1% Triton X-100 and then incubated overnight at RT with primary antibodies in a solution of 1% normal donkey serum in PBS with 0.3% Triton X-100. Sensory and sympathetic nerve fibers were labeled with a pan neuronal marker PGP 9.5 (1:4000; Ultracone, Cambridge, UK; Catalog number RA95101). Peptide-rich primary afferent sensory neurons were labeled with polyclonal rabbit anti-rat CGRP (1:15,000; Sigma Chemical Co., St. Louis, MO; Catalog Number: C8198). Myelinated primary afferent sensory nerve fibers were labeled with chicken anti-neurofilament 200Kd (NF200, 1:1000; Chemicon, Temecula, CA; Catalog number AB5539). Sympathetic nerve fibers were labeled with polyclonal rabbit anti-rat tyrosine hydroxylase (TH) (1:1,000 dilution; Chemicon, Temecula, CA; Catalog number AB152). Preparations were then washed in PBS and incubated for three hours at RT with secondary antibodies conjugated to fluorescent markers (Cy3 1:600 and Cy2 1:200; Jackson ImmunoResearch, West Grove, PA). Finally, skin sections were washed in PBS and dehydrated through an alcohol gradient (70, 80, 90 and 100%), cleared in xylene and coverslipped with di-nbutylphthalate-polystyrene-xylene (Sigma Chemical Co., St. Louis, MO, USA). The density of PGP9.5+, CGRP+, NF200+ and TH+ nerve fibers in the glabrous skin of the hind paw following chronic treatment with vehicle or ARRY-470 was determined by capturing images of these nerve fibers using an Olympus Fluoview FV1000 laser scanning confocal imaging system (Olympus America Inc, Melville, NY, software v. 5.0). Two digital grayscale images (400X magnification, 323 μm × 285 μm × 40 μm observation field) were acquired per section from a minimum of five sections (spaced 100 μm apart) per animal. Digital confocal grayscale images were compiled from 20 optical sections spaced 2.0μm apart in the z-plane. These confocal images were exported as TIFF files and analyzed using Image Pro Plus (v.6). For CGRP+ and NF200+ nerve fibers, area of evaluation included epidermis, upper dermis and dermis. Upper dermis was defined as the area extending 150 μm below the dermal-epidermal junction. For TH+ post-ganglionic sympathetic nerve fibers, only lower dermis was used as the area of evaluation since under physiological conditions sympathetic nerve fibers are typically absent in the upper dermis. Finally, for PGP+ nerve fibers, area of evaluation included epidermis, upper and lower dermis. The total length of nerve fibers was determined within a defined densitometric threshold applied to all skin sections analyzed and the skin area measurement was determined using computer-assisted tracing. Results were averaged and expressed as total length of nerve fibers (mm) per skin section volume (mm3).
Statistical analysis
Statistical analysis was performed using SPSS v. 11 statistics package (SPSS, Chicago, IL). For behavioral data, group differences in spontaneous guarding and spontaneous flinching were assessed with an analysis of variance for the individual samples collected at each post-fracture time point. Significant ANOVA results were followed by individual two-group comparisons using Fisher's least significant difference (LSD) pairwise multiple comparison test. To determine differences between groups in the expression of PGP 9.5, CGRP, TH and NF200 in sensory nerves of the hind paw glabrous skin, a one-way ANOVA followed by Fisher's PLSD (protected least significant difference) test was performed. For bridging analysis, two-group v2 test of equal proportions was performed. For radiographic data analysis a one-way ANOVA followed by Fisher's PLSD test was performed. Results were considered statistically significant at p<0.05. In all cases, the investigator was blind to the experimental status of each animal.
RESULTS
A Trk inhibitor reduced fracture-induced pain behaviors following femoral fracture
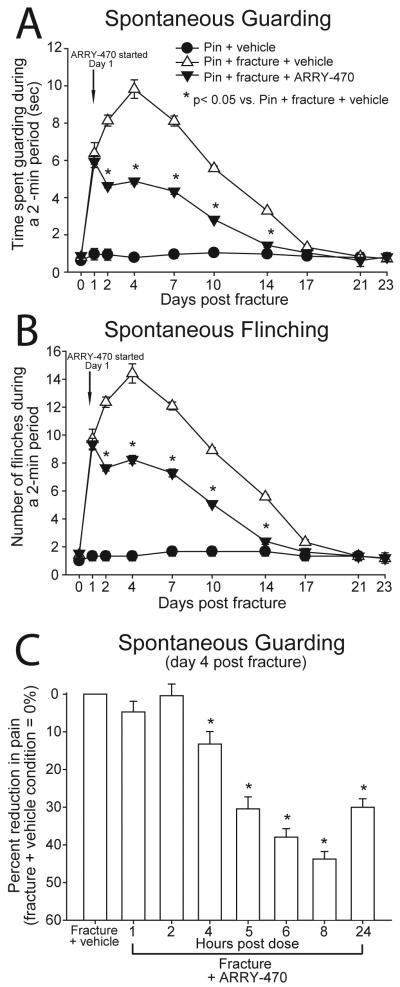
Mice received a Trk inhibitor (ARRY-470; 30 mg/kg, p.o.) twice daily from day 1 through day 48 post-fracture. Ongoing pain (spontaneous guarding and flinching) was assessed in pin and pin + fracture mice. Vehicle-treated mice with pin implantation alone exhibited minimal spontaneous pain behaviors (guarding and flinching) throughout the 23-day behavioral test period and these pain behaviors were not significantly different from baseline values (Figure 2A, B). Animals with femoral fracture receiving vehicle administration exhibited significantly more time guarding and increased number of flinches as compared to pin-alone mice from day 1 through day 14 post-fracture. This increase in pain behavior peaked at day 4 post-fracture and gradually declined such that by 17 days post-fracture pain behaviors in both experimental groups returned to baseline levels. Chronic treatment with ARRY-470 significantly reduced ongoing guarding and flinching behaviors at days 2, 4, 7, 10, and 14 post-fracture as compared with fracture animals treated with vehicle alone (Figure 2A, B).
Figure 2. Chronic administration of a Trk inhibitor significantly reduces bone fracture pain-related behaviors.
The time spent guarding (A) and number of spontaneous flinches (B) of the fractured limb over a 2-minute observation period were used as measures of ongoing pain following mid-diaphyseal fracture of the left femur. The Trk inhibitor ARRY-470 (30 mg/kg, p.o.) was administered twice daily, 1 hour prior to behavioral analysis beginning on day 1 post fracture. Note that ARRY-470 significantly reduced both ongoing fracture pain-related behaviors 2, 4, 7, 10, and 14 days following fracture. ARRY-470 (30 mg/kg, p.o.) was administered once at time 0 and again at 4 hours following the initial dose on day 4 post-fracture. The time spent guarding the fractured limb over a 2-minute observation period was used as a measure of ongoing pain 0, 1, 2, 4, 5, 6, 8 and 24 hours after mid-diaphyseal fracture of the left femur (C). Administration of the Trk inhibitor, ARRY-470, (30 mg/kg, p.o.) significantly reduced ongoing fracture pain 4, 5, 6, 8 and 24 hours following initial dose and achieved approximately 50% pain reduction by 8 hours following initial dose. Results represent the mean ± SEM using an n≥7 for each experimental group.
A separate group of animals received ARRY-470 treatment (acute) only at the time point of peak fracture-induced pain behavior (day 4 post-fracture); one dose at 1 hour prior to behavioral testing and one dose 3 hours later. Acute treatment with ARRY-470 significantly reduced ongoing pain behaviors at 4, 5, 6, 8, and 24 hours post-initial dose and achieved approximately 50% pain reduction 8 hours following the initial dose (Figure 2C).
Chronic Trk inhibitor therapy had minimal effect on bone healing
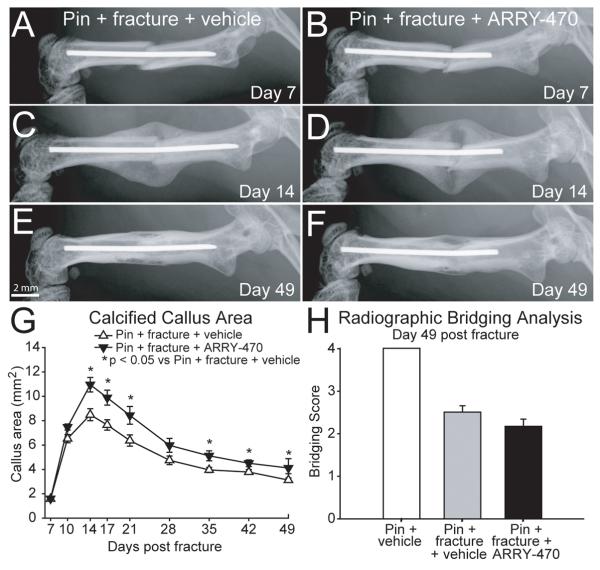
Measures of bone healing were assessed using radiographic analysis of the calcified callus area formed at the site of fracture and bone bridging of the fractured femur. A radiopaque callus was first evident at day 7 post-fracture in pin + fracture + vehicle and pin + fracture + ARRY-470 treated groups and increased progressively until day 14 post-fracture where both groups began a similar gradual callus resorption phase throughout the remaining experimental time period (48 days). Calcified callus area was significantly increased in the pin + fracture + ARRY-470 treated group at days 14, 17, 21, 28, 35, 42, and 49 post-fracture when compared to pin + fracture + vehicle-treated animals (Figure 3A-F, G). No significant difference was observed in the overall fracture site bone bridging score following 48 days of sustained ARRY-470 administration (score of 2.2+/−0.2) compared to vehicle-treated animals (score of 2.5+/−0.2) (Figure 3A-F, H). *p<0.05 vs. pin + fracture + vehicle.
Figure 3. Effects of sustained ARRY-470 treatment on calcified callus and bone bridging measures of bone healing are limited.
Measures of bone healing were assessed using radiographic analysis of the calcified callus area formed at the site of fracture and bone bridging of the fractured femur. Bone bridging scores were assigned based on the appearance of bridging across the left and right peripheries of the callus (1 point each) and the left and right cortices of the fractured femur (1 point each). Although there was a small but statistically significant increase in callus area in the ARRY-470-treated fracture vs. the vehicle-treated fracture group 14, 17, 21, 35, 42, and 49 days post-fracture (A-F, G), there was no significant difference in the overall fracture site bone bridging score following 48 consecutive days of twice-daily ARRY-470 administration (A-F, H). Results represent the mean ± SEM using an n≥7 for each experimental group.
Sensory and sympathetic nerve fibers in the skin remained intact following sustained administration of a Trk inhibitor
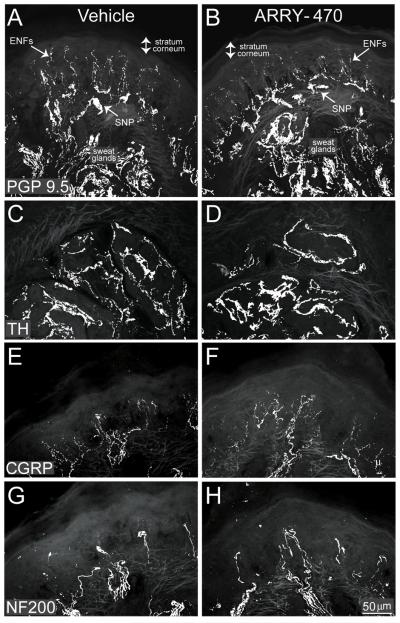
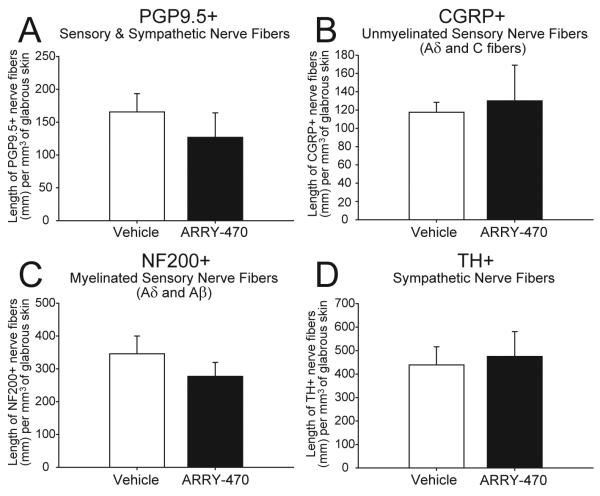
Nerve fibers in hind paw skin sections were immunohistochemically labeled with antibodies against PGP9.5, CGRP, NF200, and TH. PGP 9.5 is known to be a pan-neuronal marker (Figure 4 A, B), CGRP is a neuropeptide found predominantly in unmyelinated (C-fibers) and some thinly myelinated (A-delta) sensory nerve fibers (Figure 4 E, F), whereas NF200 is expressed by myelinated (A-delta and A-beta) primary afferent sensory nerve fibers (Figure 4 G, H) and TH is a marker of sympathetic nerve fibers (Figure 4 C, D). Immunohistochemical analysis revealed localization of CGRP+ nerve fibers in the epidermis and upper dermis, NF200+ nerve fibers were localized in the dermis, while the PGP9.5+ nerve fibers were localized to the epidermis, upper and lower dermis, sweat glands, and SNP, and TH was localized to the sweat gland region. Chronic treatment of non-fractured animals with ARRY-470 over a 48 day period did not reduce the density per mm3 of the hindpaw glabrous skin (Figure 5) of PGP 9.5+ (A), CGRP+ (B), NF200+ (C) or TH+ (D) nerve fibers as compared to vehicle-treated animals where p>0.4 in all cases.
Figure 4. Sensory and sympathetic nerve fiber density in the skin of the hind paw is maintained following 48 days of sustained administration of the Trk inhibitor.
(A-F) Representative confocal images of sensory nerve fibers in hind paw skin sections immunohistochemically labeled with antibodies against PGP 9.5, CGRP or NF200 following 48 consecutive days of ARRY-470 administration (30 mg/kg, p.o., bid). CGRP- labeled nerve fibers were primarily localized in the epidermis and upper dermis, NF200- labeled nerve fibers were localized in the dermis, while PGP 9.5-labeled nerve fibers were localized to the epidermis (epidermal nerve fibers; ENFs), upper and lower dermis, sweat glands, and subepidermal neural plexus (SNP). Note that chronic administration of ARRY-470 in femur fractured mice did not significantly reduce the number of PGP 9.5 (A, B), TH (C, D), CGRP (E, F), or NF200 (G, H) labeled nerve fibers following 48 days of sustained administration as compared to vehicle treated mice. Results represent the mean ± SEM using an n≥6 for each experimental group.
Figure 5. Quantitation of the effects of sustained ARRY-470 treatment on normal hind paw skin nerve fiber density.
(A-D) Hind paw skin sections were stained for PGP9.5, CGRP, NF200 sensory or TH sympathetic nerve fibers following 48 days of sustained ARRY-470 administration (30 mg/kg, p.o., bid) and nerve fiber density was quantified using confocal microscope image analysis. Following confocal image acquisition the total length of nerve fibers labeled with each marker in the glabrous skin was determined within a defined densitometric threshold applied to all sections analyzed. Chronic administration of ARRY-470 in non-fractured mice for 48 days did not significantly reduce the number of PGP9.5 (A), CGRP (B), NF200 (C), or TH (D) labeled nerve fibers, as compared to vehicle-treated mice. Results represent the mean ± SEM using an n≥6 for each experimental group.
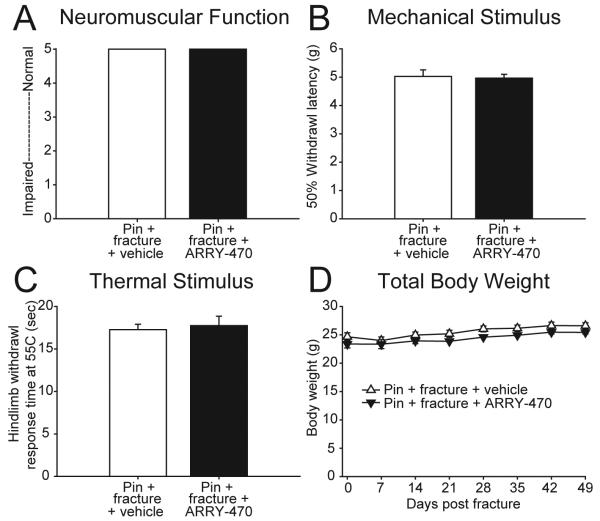
Chronic Trk inhibitor therapy did not reduce behavioral measures of normal thermal nociception and mechanical nociception, general neuromuscular function, or affect body weight
Evaluation of general neuromuscular function using rotorod analysis suggested no significant differences between animals receiving chronic administration of ARRY-470 (score 5.0 +/− 0.0) compared to vehicle-treated animals (score 5.0 +/− 0.0) (Figure 6A). Mechanical stimulation of the hind paw (von Frey monofilaments) was performed on animals following 48 days of either ARRY-470 treatment or vehicle and 50% withdrawal latency was recorded. Results from this assessment of mechanical nociception revealed no significant differences between ARRY-470 (5.0 +/− 0.1 g) and vehicle treated animals (5.0 +/− 0.2 g) (Figure 6B). In addition, assessment of the normal response to a brief 55ºC thermal stimulation of the glabrous skin of the hind paw [23] showed no significant difference in hind paw withdrawal time in animals treated for 48 days with ARRY-470 (17.8 +/− 1.1 s) compared to vehicle treated animals (17.3 +/− 0.6 s) (Figure 6C) where p>0.4 in all cases. Total body weight of the animals was recorded weekly throughout the experimental period and no significant differences were found between ARRY-470 and vehicle treated animals at any time point observed (Figure 6D).
Figure 6. Sustained ARRY-470 treatment does not influence behavioral responses of the skin to noxious thermal or mechanical stimuli, general neuromuscular function, or total body weight.
(A-C) Nociceptive responses were measured using mechanical stimulation (von Frey filament analysis) and thermal stimulation (thermal analgesia analysis). General neuromuscular function was measured using rotorod analysis. Chronic administration of ARRY-470 (30 mg/kg, p.o., bid) in femur-fractured mice for 48 days did not significantly reduce measures of general neuromuscular function (A) the response to mechanical (B) or thermal (C) stimulation, as compared to pin + fracture + vehicle mice. In addition, chronic administration of ARRY-470 in non-fractured mice did not significantly affect total body weight, as compared to pin + fracture + vehicle mice (D). Results represent the mean ± SEM using an n≥3 for each experimental group.
DISCUSSION
Blockade of Trks and skeletal pain
In the present study we show that sustained administration of a Trk inhibitor reduced fracture pain related behaviors by 50% without sedation, changes in total body weight, or inhibition of fracture healing. As the Trk inhibitor has a 50:1 plasma to CSF ratio, the anti-hyperalgesic actions of the inhibitor would appear to occur primarily outside the blood brain barrier. Previous reports have demonstrated that following peripheral inflammation and tissue injury, a variety of inflammatory, immune and stromal cells upregulate the expression of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) whose cognate receptors are TrkA, TrkB and TrkC respectively and a few studies have suggested that peripherally released BDNF and NT-3 may play a role in modulating pain. [24, 25] As the present results show that blockade of all three Trks reduces fracture pain, a key question, which is discussed below, is which neurotrophins and Trks are most likely the major contributors to the generation and maintenance of bone fracture pain.
Previous results have demonstrated that in the adult NGF can directly activate and sensitize sensory neurons involved in the conduction of pain originating from the skin [26, 27], viscera and skeleton. NGF is thought to excite and sensitize sensory neurons by binding to its cognate receptor TrkA that is expressed by a subpopulation of mostly unmyelinated and thinly myelinated sensory neurons. [27] NGF binding to TrkA has been shown to directly depolarize TrkA-expressing nociceptors in vivo and in vitro and binding of NGF to TrkA directly lowers the threshold for depolarization in these neurons. Additionally, NGF has been shown to modulate and/or sensitize a variety of neurotransmitters, receptors, ion channels and structural molecules expressed by nociceptors. [27] It has also been shown that NGF lowers the threshold and enhances the response of nociceptors to mechanical stimuli [28], suggesting it plays a role in activating/sensitizing mechanotransducers expressed by sensory nerve fibers that innervate bone. The NGF produced by target tissues activates TrkA receptors expressed on the terminals of C-fibers [29, 30] presumably including those innervating the skeleton. Whether the Trk inhibitor used here is exerting its effect by interfering with the retrograde signal (the internalized NGF/TrkA complex) that exerts transcriptional control in the neuronal cell or by local modulation at the nociceptor terminal is not clear. However, the fact that significant analgesia is observed 6-8 hours following acute administration of the Trk inhibitor would suggest that local modulation of nerve fibers that innervate bone plays a significant role as transport of the NGF/TrkA from the nerve terminals in the fracture femur to the cell bodies of sensory neurons that innervate the femur (which are located in the L1-L3 ganglia) would be expected to take significantly longer than 8 hours. [31]
While there is strong evidence that TrkB receptors expressed by post-synaptic spinal cord neurons play a significant role in pain transmission, there is significantly less agreement about the role of TrkB expressed by sensory neurons in driving pain. Previous reports have shown that TrkB receptors are expressed by a subpopulation of the DRG, nodose and trigeminal neurons and their terminals in the spinal dorsal horn and trigeminal nucleus [32-34] and that peripheral inflammation in some tissues results in an increase in BDNF levels. [26] Interestingly, in pancreatitis BDNF content was reported to be correlated with pain intensity [35] and exogenous application of BDNF has been shown to excite and sensitize some cutaneous nociceptive terminals [36] (apparently via TrkB). However, the effects of BDNF on TrkB expressing sensory neurons in driving any type of chronic pain are poorly understood. [27] Similarly, there is relatively little evidence to suggest that peripheral TrkC receptors play a significant role in the generation and maintenance of pain in the adult. Thus, while local injection of NT-3 has been reported to induce mild pain at the injection site [37], other reports suggest that NT-3 does not sensitize nociceptive primary afferent fibers [38] and appears to be anti-nociceptive in some models such as CFA-induced skin inflammation. [39]
The above results, together with the present data demonstrating that the analgesic efficacy of the Trk inhibitor in blocking fracture pain is similar to that of anti-NGF sequestering therapy (both reducing fracture pain by approximately 50%), suggests that TrkA plays the prominent role in driving fracture pain. One unique aspect of the sensory innervation of bone, which may partially explain why the blockade of TrkA activation is effective in relieving skeletal pain, is that the majority of C-fibers that innervate the bone are CGRP-expressing fibers, nearly all of which co-express TrkA. [29, 30] Thus, the C-fibers that innervate both human [40] and rodent [41] vertebral discs and bone [42] appear to be CGRP/TrkA expressing fibers. Few unmyelinated non-peptidergic IB4/RET+ nerve fibers are present in these tissues. [42, 43] Thus, since bone appears to lack the redundancy of the C-fiber non-peptidergic IB4/RET+ nerve fibers which are present in skin [43] , blocking TrkA activation may be particularly efficacious in relieving bone pain vs. skin pain.
Trks and their involvement in the maintenance of developing vs. adult sensory and sympathetic neurons
Although Trks clearly play an essential role in the growth and survival of sensory and sympathetic neurons in the developing animal [44, 45], much less is known about the role the Trks play in the maintenance and survival of adult sensory and sympathetic neurons. During development, neurotrophins support the survival of neuronal subpopulations that express appropriate receptors. [45] The low-affinity transmembrane receptor p75NTR binds all neurotrophins, and in addition, each neurotrophin binds with high affinity to one of the Trk family of transmembrane receptors i.e.; NGF to TrkA, BDNF to TrkB, and NT-3 to TrkC. Activation of Trks by their ligands leads to dimerization of the receptor and phosphorylation of different amino acids that in turn promote the activation of a variety of signaling pathways [31] that during early development block apoptosis and promote cell survival and differentiation of sensory and sympathetic neurons.
In the present study using the adult mouse, sustained administration of a Trk inhibitor with nanomolar affinity for all 3 Trks had no observable effect on the density of unmyelinated sensory, myelinated sensory or sympathetic nerve fibers, or on measures of acute thermal pain, acute mechanical pain, or sensory and motor reflexes required for general neuromuscular function. These findings are consistent with previous studies suggesting that in the adult, Trks expressed by sensory and sympathetic neurons play less of a role in the survival and (at least in the case of TrkA) a much greater role regulating neuronal responsiveness and synaptic function particularly for pain-signaling systems. [27]
In the adult, neurotrophins appear to be expressed in most tissues at very low levels, whereas the levels of NGF (and in some tissues BDNF and NT-3) are dramatically up-regulated by inflammation and/or injury. [24, 26] However, it has been shown that chronic NGF deprivation (by exogenous administration of an anti-NGF polyclonal antibody or autoimmunization to NGF) results in a modest hypoalgesia, where animals are less sensitive to some thermal and algogenic stimuli. [46] Whether this hypoalgesic effect observed in rats with polyclonal antibodies or autoimmunization will also be observed in humans treated with monoclonal antibodies or Trk inhibitors is unclear as is how much endogenous NGF, BDNF or NT-3 is required to maintain normal sensory nerve function in the adult. However, the present results do suggest that at least over the time periods examined here, peripheral inhibition of Trk kinase activity significantly reduces skeletal pain without detectable effects on the density or normal neuromuscular and nociceptive functions of sensory nerve fibers.
Trks and bone healing
In the present study the effect that sustained blockade of TrkA, TrkB and TrkC was also examined in terms of callus and/or fracture healing. As assessed radiographically, chronic administration of the Trk inhibitor increased the callus area around the fracture site from days 14 to 49 post-fracture. The mechanism by which Trk inhibition increases callus formation remains unknown, however a similar increase in callus size following fracture was noted in animals that received anti-NGF sequestration therapy [16] and also in animals that received neonatal capsaicin treatment (which ablates CGRP/TrkA expressing sensory fibers). [47] What these three therapies (anti-NGF, Trk inhibition and neonatal capsaicin treatment) share is that during fracture healing, activation of TrkA receptors expressed by sensory nerve fibers would be attenuated. Given these findings and the times required to heal fractures of the femur in the mouse [48] versus human [49] (weeks vs. months), further studies of the role TrkA plays in normal bone remodeling and bone healing are clearly warranted.
Conclusions and limitations of the study
The present study shows that sustained administration of a small molecule Trk inhibitor reduces fracture pain related behaviors without an obvious effect on the density or function of sensory and sympathetic nerve fibers. There are several limitations to the present study. First, since the present study was conducted in normal animals or animals with bone fracture, we did not evaluate the effect the Trk inhibitor may have in animals with direct injury to the peripheral or central nervous system. As some reports have suggested that activation of Trks can be neuroprotective [31], it will be important to gain a better understanding of the effect Trk blockade has following peripheral or central nerve injury. Second, we examined the effect of twice-daily administration of a Trk inhibitor for 48 days post-fracture, but as many chronic skeletal pain conditions may last for months or years, studies examining even longer sustained administration are clearly warranted. Lastly, given that the drug plasma to brain ratio is 50:1 and the lack of predicted CNS side effects if the inhibitor had significant access to the CNS (such as TrkB agonist mediated weight gain [50], these data suggest, but do not prove, that the majority of the Trk inhibitor effects on skeletal pain related behaviors are mediated outside the blood brain barrier.
The current study has not characterized all of the potential effects ARRY-470 may have on early fracture healing, bone quality, and nerve regeneration. As with any potential therapeutic advance, understanding whether the benefits of NGF blockade by ARRY-470 are associated with any risks or unexpected effects will be required to fully appreciate the patient populations that may benefit from this therapy.
Supplementary Material
ACKNOWLEDGEMENTS
Supported by the Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service (O4380-I, A6707-R) and the National Institutes of Health (NS23970, NS048021).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Brooks PM. The burden of musculoskeletal disease-a global perspective. Clin Rheumatol. 2006;25:778–81. doi: 10.1007/s10067-006-0240-3. [DOI] [PubMed] [Google Scholar]
- 2.Woolf AD, Pfleger B. Burden of osteoporosis and fractures in developing countries. Curr Osteoporos Rep. 2005;3:84–91. doi: 10.1007/s11914-005-0015-9. [DOI] [PubMed] [Google Scholar]
- 3.Xian CJ, Zhou XF. Treating skeletal pain: limitations of conventional anti-inflammatory drugs, and anti-neurotrophic factor as a possible alternative. Nat Clin Pract Rheumatol. 2009;5:92–8. doi: 10.1038/ncprheum0982. [DOI] [PubMed] [Google Scholar]
- 4.Balano KB. Anti-inflammatory drugs and myorelaxants. Pharmacology and clinical use in musculoskeletal disease. Prim Care. 1996;23:329–34. doi: 10.1016/s0095-4543(05)70280-3. [DOI] [PubMed] [Google Scholar]
- 5.Simon AM, Manigrasso MB, O'Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res. 2002;17:963–76. doi: 10.1359/jbmr.2002.17.6.963. [DOI] [PubMed] [Google Scholar]
- 6.Abdul-Hadi O, Parvizi J, Austin MS, Viscusi E, Einhorn T. Nonsteroidal anti-inflammatory drugs in orthopaedics. J Bone Joint Surg Am. 2009;91:2020–7. [PubMed] [Google Scholar]
- 7.Li M, Ke HZ, Qi H, Healy DR, Li Y, Crawford DT, Paralkar VM, Owen TA, Cameron KO, Lefker BA, Brown TA, Thompson DD. A novel, non-prostanoid EP2 receptor-selective prostaglandin E2 agonist stimulates local bone formation and enhances fracture healing. J Bone Miner Res. 2003;18:2033–42. doi: 10.1359/jbmr.2003.18.11.2033. [DOI] [PubMed] [Google Scholar]
- 8.Paralkar VM, Borovecki F, Ke HZ, Cameron KO, Lefker B, Grasser WA, Owen TA, Li M, DaSilva-Jardine P, Zhou M, Dunn RL, Dumont F, Korsmeyer R, Krasney P, Brown TA, Plowchalk D, Vukicevic S, Thompson DD. An EP2 receptor-selective prostaglandin E2 agonist induces bone healing. Proc Natl Acad Sci U S A. 2003;100:6736–40. doi: 10.1073/pnas.1037343100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feinberg SD. Prescribing analgesics. How to improve function and avoid toxicity when treating chronic pain. Geriatrics. 2000;55:44. 49-50, 53 passim. [PubMed] [Google Scholar]
- 10.Ivanhoe CB, Hartman ET. Clinical caveats on medical assessment and treatment of pain after TBI. J Head Trauma Rehabil. 2004;19:29–39. doi: 10.1097/00001199-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Massey GM, Dodds HN, Roberts CS, Servoss TJ, Blondell RD. Toxicology screening in orthopedic trauma patients predicting duration of prescription opioid use. J Addict Dis. 2005;24:31–41. doi: 10.1300/j069v24n04_03. [DOI] [PubMed] [Google Scholar]
- 12.Bone LB, Johnson KD, Weigelt J, Scheinberg R. Early versus delayed stabilization of femoral fractures: a prospective randomized study. 1989. Clin Orthop Relat Res. 2004;422:11–16. [PubMed] [Google Scholar]
- 13.Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9:325–38. doi: 10.1038/nrd3003. [DOI] [PubMed] [Google Scholar]
- 14.Opar A. Kinase inhibitors attract attention as oral rheumatoid arthritis drugs. Nat Rev Drug Discov. 2010;9:257–8. doi: 10.1038/nrd3155. [DOI] [PubMed] [Google Scholar]
- 15.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2:97–101. doi: 10.1002/jor.1100020115. [DOI] [PubMed] [Google Scholar]
- 16.Koewler NJ, Freeman KT, Buus RJ, Herrera MB, Jimenez-Andrade JM, Ghilardi JR, Peters CM, Sullivan LJ, Kuskowski MA, Lewis JL, Mantyh PW. Effects of a Monoclonal Antibody Raised Against Nerve Growth Factor on Skeletal Pain and Bone Healing Following Fracture of the C57BL/6J Mouse Femur. J Bone Miner Res. 2007;22:1732–42. doi: 10.1359/jbmr.070711. [DOI] [PubMed] [Google Scholar]
- 17.Gerstenfeld LC, Al-Ghawas M, Alkhiary YM, Cullinane DM, Krall EA, Fitch JL, Webb EG, Thiede MA, Einhorn TA. Selective and nonselective cyclooxygenase-2 inhibitors and experimental fracture-healing. Reversibility of effects after short-term treatment. J Bone Joint Surg Am. 2007;89:114–25. doi: 10.2106/JBJS.F.00495. [DOI] [PubMed] [Google Scholar]
- 18.Jimenez-Andrade JM, Martin CD, Koewler NJ, Freeman KT, Sullivan LJ, Halvorson KG, Barthold CM, Peters CM, Buus RJ, Ghilardi JR, Lewis JL, Kuskowski MA, Mantyh PW. Nerve growth factor sequestering therapy attenuates non-malignant skeletal pain following fracture. Pain. 2007;133:183–96. doi: 10.1016/j.pain.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Santy J, Mackintosh C. A phenomenological study of pain following fractured shaft of femur. J Clin Nurs. 2001;10:521–7. doi: 10.1046/j.1365-2702.2001.00506.x. [DOI] [PubMed] [Google Scholar]
- 20.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 21.Winkler J. 8th IASP Research Symposium. Chicago, IL USA: 2009. Analgesic effects of a potent and selective kinase inhibitor of neurotrophin receptors TrkA, TrkB, and TrkC. [Google Scholar]
- 22.Bouhana KS, Impastato R, Jiang Y, Wallace RD, Hartley DP, Do MG, von Carlowitz I. Keystone Symposium- Neurobiology of Pain. Boulder, CO USA: 2008. Analgesic effects of a potent and selective kinase inhibitor of neurotrophin receptors TrkA, TrkB, and TrkC in a model of inflammatory pain. [Google Scholar]
- 23.Bannon AW, Malmberg AB. Models of nociception: hot-plate, tail-flick, and formalin tests in rodents. Curr Protoc Neurosci. 2007 doi: 10.1002/0471142301.ns0809s41. Chapter 8: Unit 8 9. [DOI] [PubMed] [Google Scholar]
- 24.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMahon SB, Bennett DLH, Bevan S. Inflammatory mediators and modulators of pain. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack's textbook of pain. 5th ed. Elsevier/Churchill Livingstone; Philadelphia: 2006. pp. 49–72. [Google Scholar]
- 26.Paterson S, Schmelz M, McGlone F, Turner G, Rukwied R. Facilitated neurotrophin release in sensitized human skin. Eur J Pain. 2009;13:399–405. doi: 10.1016/j.ejpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–38. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 28.Malik-Hall M, Dina OA, Levine JD. Primary afferent nociceptor mechanisms mediating NGF-induced mechanical hyperalgesia. Eur J Neurosci. 2005;21:3387–94. doi: 10.1111/j.1460-9568.2005.04173.x. [DOI] [PubMed] [Google Scholar]
- 29.Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur. J. Neurosci. 1995;7:1484–1494. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh GS, Krol KM, Kawaja MD. Absence of the p75 neurotrophin receptor alters the pattern of sympathosensory sprouting in the trigeminal ganglia of mice overexpressing nerve growth factor. J. Neurosci. 1999;19:258–73. doi: 10.1523/JNEUROSCI.19-01-00258.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 32.Behnia A, Zhang L, Charles M, Gold MS. Changes in TrkB-like immunoreactivity in rat trigeminal ganglion after tooth injury. J Endod. 2003;29:135–40. doi: 10.1097/00004770-200302000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Kashiba H, Uchida Y, Senba E. Distribution and colocalization of NGF and GDNF family ligand receptor mRNAs in dorsal root and nodose ganglion neurons of adult rats. Brain Res Mol Brain Res. 2003;110:52–62. doi: 10.1016/s0169-328x(02)00584-3. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 35.Zhu ZW, Friess H, Wang L, Bogardus T, Korc M, Kleeff J, Buchler MW. Nerve growth factor exerts differential effects on the growth of human pancreatic cancer cells. Clin Cancer Res. 2001;7:105–12. [PubMed] [Google Scholar]
- 36.Kerr BJ, Bradbury EJ, Bennett DL, Trivedi PM, Dassan P, French J, Shelton DB, McMahon SB, Thompson SW. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J Neurosci. 1999;19:5138–48. doi: 10.1523/JNEUROSCI.19-12-05138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhry V, Giuliani M, Petty BG, Lee D, Seyedsadr M, Hilt D, Cornblath DR. Tolerability of recombinant-methionyl human neurotrophin-3 (r-metHuNT3) in healthy subjects. Muscle Nerve. 2000;23:189–92. doi: 10.1002/(sici)1097-4598(200002)23:2<189::aid-mus7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Shu XQ, Mendell LM. Neurotrophins and hyperalgesia. Proc Natl Acad Sci U S A. 1999;96:7693–6. doi: 10.1073/pnas.96.14.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe M, Endo Y, Kimoto K, Katoh-Semba R, Arakawa Y. Inhibition of adjuvant-induced inflammatory hyperalgesia in rats by local injection of neurotrophin-3. Neurosci Lett. 2000;282:61–4. doi: 10.1016/s0304-3940(00)00842-9. [DOI] [PubMed] [Google Scholar]
- 40.Ozawa T, Ohtori S, Inoue G, Aoki Y, Moriya H, Takahashi K. The degenerated lumbar intervertebral disc is innervated primarily by peptide-containing sensory nerve fibers in humans. Spine. 2006;31:2418–22. doi: 10.1097/01.brs.0000239159.74211.9c. [DOI] [PubMed] [Google Scholar]
- 41.Ozawa T, Aoki Y, Ohtori S, Takahashi K, Chiba T, Ino H, Moriya H. The dorsal portion of the lumbar intervertebral disc is innervated primarily by small peptide-containing dorsal root ganglion neurons in rats. Neurosci Lett. 2003;344:65–7. doi: 10.1016/s0304-3940(03)00416-6. [DOI] [PubMed] [Google Scholar]
- 42.Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, Keyser CP, Clohisy DR, Adams DJ, O'Leary P, Mantyh PW. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113:155–66. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 43.Jimenez-Andrade JM, Mantyh WG, Bloom AP, Xu H, Ferng AS, Dussor G, Vanderah TW, Mantyh PW. A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: Therapeutic opportunity for treating skeletal pain. Bone. 2009 doi: 10.1016/j.bone.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davies AM. Regulation of neuronal survival and death by extracellular signals during development. EMBO J. 2003;22:2537–45. doi: 10.1093/emboj/cdg254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ernsberger U. Role of neurotrophin signalling in the differentiation of neurons from dorsal root ganglia and sympathetic ganglia. Cell Tissue Res. 2009;336:349–84. doi: 10.1007/s00441-009-0784-z. [DOI] [PubMed] [Google Scholar]
- 46.McMahon SB, Bennett DLH, Bevan S. Inflamatory mediators and modulators of pain. In: Wall PD, McMahon SB, Koltzenburg M, editors. Wall and Melzack's Textbook of Pain. 5th ed. Elsevier/Churchill Livingstone; Philadelphia: 2006. pp. 49–72. [Google Scholar]
- 47.Jimenez-Andrade JM, Bloom AP, Mantyh WG, Koewler NJ, Freeman KT, Delong D, Ghilardi JR, Kuskowski MA, Mantyh PW. Capsaicin-sensitive sensory nerve fibers contribute to the generation and maintenance of skeletal fracture pain. Neuroscience. 2009;162:1244–54. doi: 10.1016/j.neuroscience.2009.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gardner MJ, van der Meulen MC, Demetrakopoulos D, Wright TM, Myers ER, Bostrom MP. In vivo cyclic axial compression affects bone healing in the mouse tibia. J Orthop Res. 2006;24:1679–86. doi: 10.1002/jor.20230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Einhorn TA. The science of fracture healing. J Orthop Trauma. 2005;19:S4–6. doi: 10.1097/00005131-200511101-00002. [DOI] [PubMed] [Google Scholar]
- 50.Lin JC, Tsao D, Barras P, Bastarrachea RA, Boyd B, Chou J, Rosete R, Long H, Forgie A, Abdiche Y, Dilley J, Stratton J, Garcia C, Sloane DL, Comuzzie AG, Rosenthal A. Appetite enhancement and weight gain by peripheral administration of TrkB agonists in non-human primates. PLoS One. 2008;3:e1900. doi: 10.1371/journal.pone.0001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.