Abstract
Chronic stress induces dendritic retraction in the hippocampal CA3 subregion, but the mechanisms responsible for this retraction and its impact on neural circuitry are not well understood. To determine the role of NMDA (N-methyl-D-aspartic acid) receptor (NMDAR)-mediated signaling in this process, we compared the effects of chronic immobilization stress (CIS) on hippocampal dendritic morphology, hypothalamic-pituitary-adrenal (HPA) axis activation, and anxiety-related and hippocampus-dependent behaviors, in transgenic male mice in which the NMDAR had been selectively deleted in CA3 pyramidal cells and in non-mutant littermates. We found that CIS exposure for 10 consecutive days in non-mutant mice effectively induces HPA axis activation and dendritic retraction of CA3 short-shaft pyramidal neurons, but not CA3 long-shaft pyramidal neurons, suggesting a differential cellular stress response in this region. Dendritic reorganization of short-shaft neurons occurred throughout the longitudinal axis of the hippocampus and, in particular, in the ventral pole of this structure. We also observed a robust retraction of dendrites in dorsal CA1 pyramidal neurons in the non-mutant C57BL/6 mouse strain. Strikingly, chronic stress-induced dendritic retraction was not evident in any of the neurons in either CA3 or CA1 in the mutant mice that had a functional lack of NMDARs restricted to CA3 pyramidal neurons. Interestingly, the prevention of dendritic retraction in the mutant mice had a minimal effect on HPA axis activation and behavioral alterations that were induced by chronic stress. These data support a role for NMDAR-dependent glutamatergic signaling in CA3 in the cell-type specific induction of dendritic retraction in two hippocampal subregions following chronic stress.
Keywords: HPA axis, CA3, NMDA receptor, chronic stress, dendritic retraction, Golgi staining
Introduction
Chronic stress leads to the development of a number of neural changes that may precipitate the onset of psychiatric disorders. It is therefore important to identify the causal mechanisms that mediate the stress response in order to develop effective clinical intervention strategies. One neural region that may contribute to shaping the brain’s response to chronic stress is the hippocampus, due to the profound structural changes observed in this region and the direct and indirect anatomical connections to the hypothalamic-pituitary axis (HPA). Although not uniquely so, the CA3 subregion of the hippocampus is extremely vulnerable to the effects of long-term stress, as evidenced by a robust stress-induced decrease in complexity and retraction of dendrites (Woolley et al., 1990, Watanabe et al., 1992b).
Regarding the cellular and molecular mechanisms underlying structural remodeling in the hippocampus, the role of adrenal steroids appears to be most crucial (McEwen, 1999). In 1990, McEwen and colleagues made the seminal observation that repeated corticosterone administration induces retraction of apical dendrites in the CA3 subregion of the hippocampus in adult male rats (Woolley et al., 1990). Subsequently, attenuated corticosterone secretion by the steroid synthesis blocker cyanoketone was shown to prevent stress-induced CA3 dendritic retraction (Magariños and McEwen, 1995). Other key neurochemical systems responsible for structural remodeling in the hippocampus may include glutamate release and NMDA (N-methyl-D-aspartic acid) receptors (NMDARs), calcium channels, the serotonin system, GABA receptors, and opioids (McEwen, 1999). For instance, phenytoin, an antiepileptic that interferes with glutamate release and its action, prevents stress-induced CA3 dendritic retraction (Watanabe et al., 1992a, Magariños et al., 1996). These data are consistent with reports showing enhanced glutamate release in response to restraint or immobilization stress (Gilad et al., 1990, Lowy et al., 1993). Increased glutamate appears to act on NMDARs, but not AMPA receptors, to trigger stress-related morphological changes since the competitive NMDAR antagonist CGP43487, but not the AMPA (α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate) receptor antagonist NBQX (2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione), blocks stress-induced dendritic remodeling in CA3c (Magariños and McEwen, 1995). In light of the potential excitotoxic action of NMDARs in response to excess glutamate release (Tapia, 1996), a possible mechanism of CA3 dendritic retraction could be due to glutamatergic excitotoxicity. In particular, McEwen suggests that glutamate release from mossy fiber terminals plays a key role in the remodeling of the CA3 region (McEwen, 2007). Indeed, chronic stress results in the depletion of synaptic vesicles from mossy fiber terminals, suggesting that an increase in glutamate release from mossy fibers (Magariños et al., 1997). However, this idea cannot fully explain the involvement of NMDARs in CA3 dendritic remodeling, because NMDARs are almost absent in the stratum lucidum terminal zone in CA3, the predominant site of mossy fiber projections (Watanabe et al., 1998). Although an earlier study showed that a competitive NMDAR antagonist CGP43487 was effective in preventing CA3 dendritic remodeling (Magariños and McEwen, 1995) the use of systemic injections failed to provide any information on region or synapse specificity in terms of the mechanisms underlying this cellular response to chronic stress.
Based on anatomical evidence showing comparatively high expression levels of postsynaptic NMDARs, two obvious candidate synapses for mediating a glutamatergic influence on stress-induced changes in morphology are the commissural/associational fiber synapse and the stratum lacunosum-moleculare synapse in CA3 pyramidal neurons. To assess the role of NMDARs at these CA3 synapses, we utilized a transgenic line of mice in which the NR1 subunit (Grin1) of this receptor is ablated in CA3 (CA3-NR1-KO mice, hereafter referred to as mutant) after adolescence (Nakazawa et al., 2002). NR1 is a requisite subunit of the operational NMDAR and therefore selective ablation is functionally equivalent to eliminating the receptor itself. One of the distinct advantages of this genetic model is that functional elimination of the NMDAR extends throughout the dorsal-ventral axis of the hippocampus. We assessed hippocampal dendritic morphology following CIS for 10 days, adopting a protocol that had been shown to be sufficient to induce CA3 dendritic retraction in rats and show an NMDA-dependent dendritic atrophy in hippocampal neurons. Using a different cohort of animals, we further conducted tests of hippocampus-dependent behavior and report a dissociation between structural and behavioral modifications following chronic stress.
Experimental Procedures
Animals
Transgenic mice with selective post-adolescent ablation of the NR1 (Grin1) subunit of the NMDAR in CA3 pyramidal neurons were generated as previously described (Fig. 1A-B) (CA3-NR1 knockout mice or simply mutant) (Nakazawa et al., 2002). Male mice between 18 and 24 weeks of age were used for all experiments and their homozygously floxed-NR1 male littermates served as controls (Control). Three separate cohorts of mice were used in the experiments described. In each cohort, mice of each genotype (mutant and non-mutant) were randomly assigned to either a stress or non-stress group. Mice that underwent chronic stress were housed in divided cages (2 mice per cage). Non-stressed mice were group-housed (2–4 mice per cage). All mice were housed in a temperature and humidity-controlled vivarium on a 12 h light-dark cycle and given ad libitum access to food and water. All experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals, and were approved by the NIMH Animal Care and Use Committee.
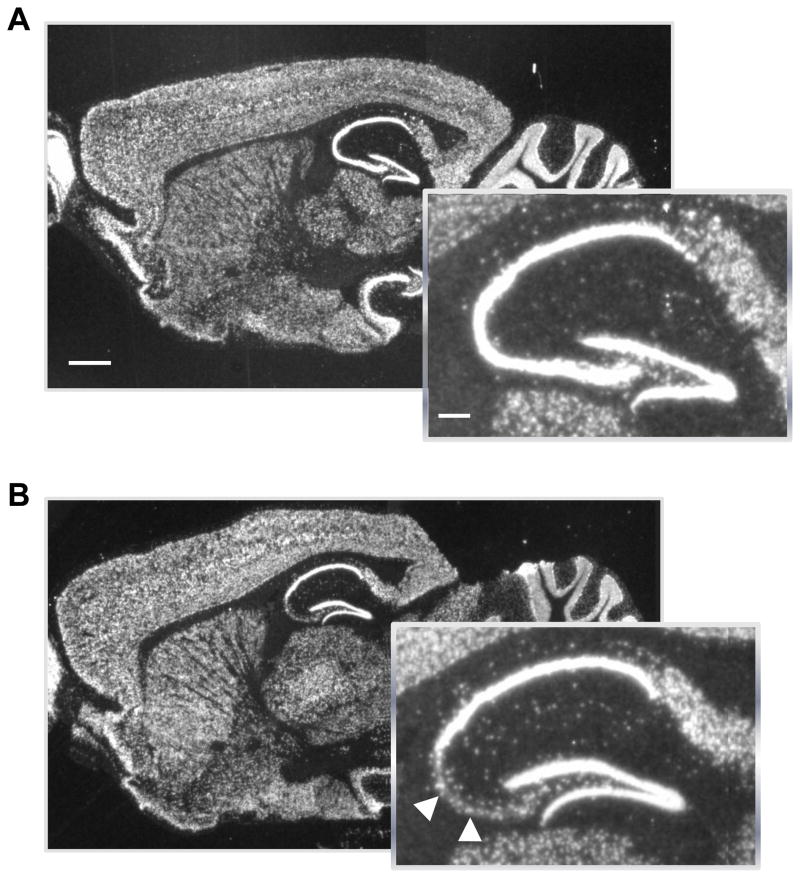
Fig. 1.
In situ hybridization of the NR1 subunit mRNA of the NMDA receptors in 22 week old male mice. Parasagittal brain sections with enlarged inserts of the hippocampus in control (A) and mutant (B) mice. Arrowheads indicate scattered hybridization signals that are likely derived from CA3 interneurons. Scale bar, 100 μm; scale bar in inset, 25 μm.
In situ hybridization
Fresh-frozen parasagittal sections (14 μm-thick) were hybridized with 33P-labeled cRNA probe derived from an AvrII-SphI antisense DNA fragment of rat NR1 cDNA containing from exon 13 to exon 16, as previously described (Nakazawa et al., 2002).
Stress induction
Stressed mice were immobilized for 2 h per day for 10 consecutive days in rodent restraint bags (Vyas et al., 2002) (Braintree Scientific, Inc., Braintree, MA). Non-stressed mice remained in their home cages undisturbed except to be weighed. Mice were weighed on Days 1 and 11 of the experiment. Blood samples were obtained through tail nicks on Days 1 and 10 both before and after the stress session. All samples were collected between 10-2 pm to control for circadian variation in hormonal levels. Samples were centrifuged at 5,000 rpm for 5 min to extract plasma which was then stored at −80 ºC for subsequent analysis with a corticosterone ELISA kit (Assay Designs Inc., Ann Arbor, MI). At the time of perfusion, adrenal glands were bilaterally dissected and the mean wet weight per animal was obtained.
Histology and dendritic analysis
Brains were prepared for analysis by following a modified Golgi-Cox protocol (Glaser and Van der Loos, 1981). Briefly, mice were intracardially perfused with a 0.8 % NaCl/EGTA solution 24 h after the last day of stress exposure (Day 11 for non-stressed controls). Saline-perfused brains were immersed in Golgi solution for 11 days, dehydrated, immersed in 4% then 8% celloidin for 5 days each, embedded in 8% celloidin overnight and cut at 150 μm.
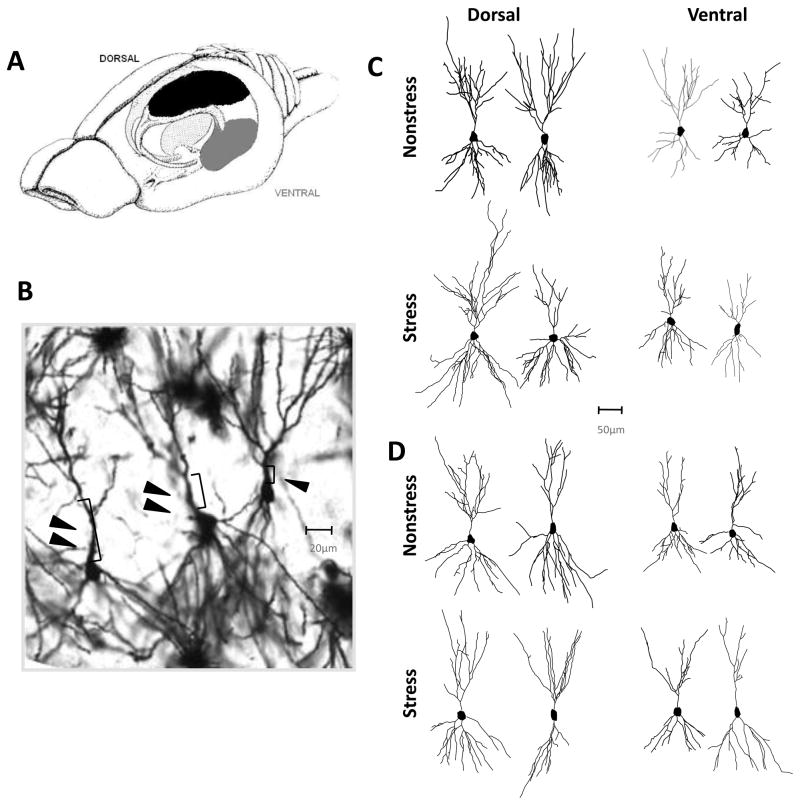
Neurons were selected from three brain regions – dorsal CA1, dorsal CA3, and ventral CA3. For purposes of analyzing differences between poles of the longitudinal axis, neurons in the CA3 region were classified as dorsal or ventral if the neuron was located in the dorsal 40% or ventral 40% of the hippocampus as illustrated in Fig. 3A. In CA3, long-shaft and short-shaft neurons were analyzed separately and identified according to morphological features as previously described (Fitch et al., 1989). Long-shaft neurons have longer primary dendritic shafts, relatively less branching, less extensive radial arborization of dendrites, and a smaller number of thorny excrescences (Figs. 3B-D). In total, neurons were classified into 5 groups: CA1, CA3 Short-shaft Dorsal, CA3 Long-shaft Dorsal, CA3 Short-shaft Ventral, and CA3 Long-shaft Ventral. A minimum of 4 and maximum of 6 pyramidal neurons from each group were selected for analysis from each animal. Data were averaged to derive a single mean value for each neuronal type in each animal. A total of 5 animals were used for each experimental group. Only relatively isolated neurons with completely stained and intact dendritic arbors from the middle third of the section were selected for analysis. Neurons were drawn at 400–600X and morphology was quantified in three dimensions using a computer-based neuron tracing system (Neurolucida, MBF Bioscience, Inc., Williston, VT)..
Fig. 3.
CIS induces atrophy of apical dendrites in short-shaft CA3 pyramidal neurons. (A) Neurons were sampled from the dorsal (black shading) and ventral (gray shading) poles of the hippocampus. (B) Picture of Golgi-Cox stained short-shaft (single arrowhead) and long-shaft (double arrowhead) CA3 pyramidal neurons. Arrowheads indicate relative difference in length of primary dendritic shaft. Image captured at 20x resolution and enlarged to see detail. (C-D) Representative reconstructions of short-shaft (C) and long-shaft (D) from the dorsal and ventral regions in Non-stressed (top row) and Stressed (bottom row) mice. Within each panel, representative tracings from Mutant mice are shown on the left and tracings from Control mice are shown on the right.
For statistical analysis, individual analyses of apical and basal dendritic features were averaged to obtain a mean measurement per animal. This animal mean was used to obtain a group mean for each neuronal type. For CA3, three-way ANOVAs were used to evaluate means across region (Dorsal vs. Ventral), treatment (Stress vs. Non-stress), and genotype (Mutant vs. Control) for dendritic branch length and number. Two-way ANOVAs were used to evaluate CA1 morphological data. When appropriate, significant F tests were further evaluated with Bonferroni post hoc comparisons. An α level of .05 was considered significant. Data are presented as mean ±SEM.
Elevated plus maze
The elevated plus maze (Mikes Machine Co., Attleboro, MA) consisted of two open arms (30 × 5 cm) with 3mm high ledges and two closed arms (30 × 5 cm) with16 cm-high opaque walls and a central square (5 × 5 cm). Mice were allowed to explore the maze for 5 min while movement parameters (e.g. animal location, speed, distance traveled, etc.) were detected through an automated video tracking software system (ANY-maze; Stoelting Co., Wood Dale, IL).
Open field
Mice were placed in a novel open field (54 × 80 cm) for 30 min to measure exploratory behavior and locomotion. Automatic tracking of animals’ behavior was performed with a video-integrated behavioral testing software application (ANY-maze).
Spatial short-term memory in Y maze
Mice were trained in a spatial short-term memory version of the Y-maze task. The maze consists of three equidistant arms (40 cm). During a 10-min habituation trial, mice were placed at the end of a start arm (pseudo-randomly selected and counterbalanced between animals) and allowed to freely explore 2 arms of the maze. The third arm, the “novel” arm, was inaccessible due to an opaque barrier. After a 5-min intertrial interval, mice were returned to the maze and placed in the same start arm as in the previous trial but had free access to all 3 arms. Preference for the novel vs. familiar arm was calculated as a ratio based on time spent in each arm. Data were collected with the aid of video tracking software (ANY-maze).
Fear conditioning
Fear conditioning training and testing were conducted as described previously (Cravens et al., 2006). Briefly, mice were placed in a conditioning chamber consisting of distinct visual cues and a grid floor to deliver scrambled footshocks. Training consisted of 2 footshocks (0.8 mA, 2 s) with an interstimulus interval of 1 min preceded by and coterminating with a 30 s tone (80 dB, 8 kHz). Twenty-four h later, the context-specificity of the conditioned fear memory, as measured by behavioral freezing, was assessed. First, the mice were returned to the chamber used during training for 6 min in the absence of any tone or shock stimuli. Three h later, the animals were placed in a novel context in which the training tone was presented after 3 min. Differences in freezing levels during the first 3 min in the two testing environments reflected the level of contextually modulated fear memory.
Results
NR1 subunit selectively ablated from CA3 pyramidal neurons in mutant mice
We first confirmed that NR1 deletion is selectively targeted to CA3 pyramidal neurons in mutants at 22 weeks of age when all the experiments were conducted using in situ hybridization. (Fig. 1A–B).
Immobilization stress induces changes in plasma corticosterone level, body and adrenal weights
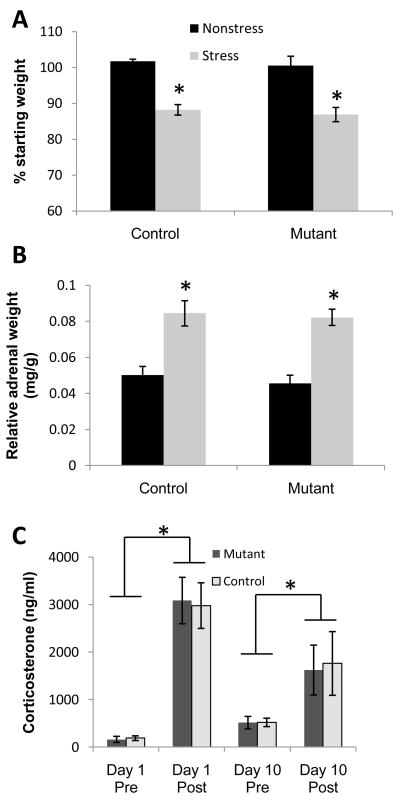
To verify that our protocol elicited a robust stress response in mice at 18–24 weeks old, we measured weight changes of whole body and adrenal gland and plasma corticosterone levels before and after chronic stress. Stressed animals lost weight over the course of the CIS treatment regardless of genotype (treatment: F(1,27) = 57.0; p<.001; genotype: F(1,27) = .64, p>.05) (Fig. 2A) but mean weight of mutant and control mice did not differ in either treatment condition at the end of the ten day experimental period (Nonstress condition: Mutant = 34.6 ± 0.3 g; Control = 31.9 ± 2.3 g; Stress condition: Mutant = 28.0 ± 1.2 g; Control = 27.7 ± 1.0 g). Adrenal weight was significantly increased in the Stress compared to Non-stress groups regardless of genotype (treatment: F(1,27) = 44.61; p<.001; genotype: F(1,27) = .0.47; p>.05) (Fig. 2B). There was a significant increase in blood plasma corticosterone levels following stress induction on Day 1 as revealed by a significant main effect of time of blood collection with respect to stress induction [Pre (before stress) vs. Post (after stress), (F(1,56) = 52.99; p<.001]. Furthermore, on Day 10 both genotypes still showed a substantial post-stress increase in corticosterone levels compared to pre-stress measurements (p=.05 for Mutant, p<.05 for Control, Fig. 2C). These results suggested that our CIS paradigm activates the HPA axis as evidenced by the physiological induction of chronic stress effects. Interestingly, deletion of CA3 NMDARs had no impact on these measures, which is consistent with a report showing HPA activity does not necessarily depend on hippocampal activity (Tuvnes et al., 2003).
Fig. 2. Physiological effects of chronic immobilization stress (CIS).
(A) Significant weight loss over the 10 day treatment period was observed in the Stress groups of both genotypes. (B) Adrenal hypertrophy was also observed in Stress Control and Stress Mutant mice. All stress effects were significant in both CA3-NR1-KO and control mice. No significant differences were observed in any physiological indicator of chronic stress between genotypes. (C) Plasma corticosterone levels increased after CIS on both Day 1 and Day 10. *P<.05; mean ±SEM.
CIS induces NMDAR-dependent retraction of pyramidal cell dendrites in dorsal CA3, ventral CA3 and CA1
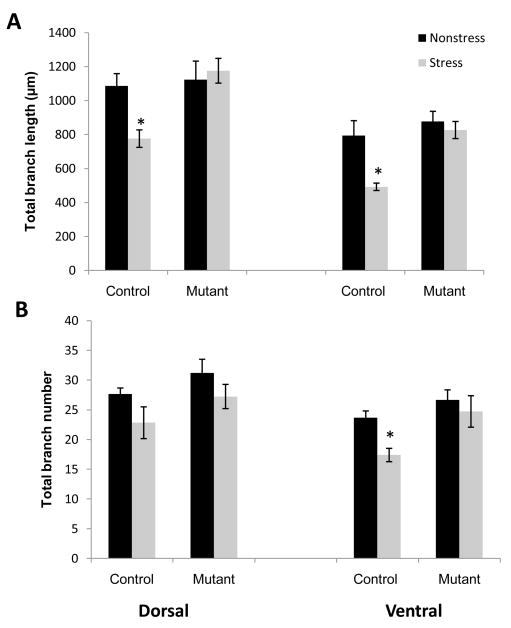
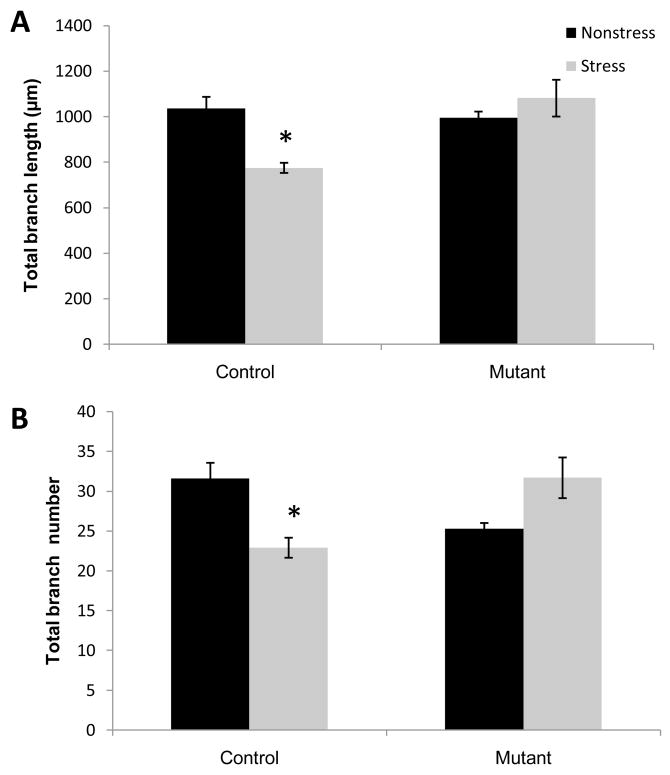
CA3 pyramidal neurons fall into two major categories largely based on the morphology of apical dendrites (Fitch et al., 1989). Short-shaft neurons are characterized by relatively compact apical shafts, a large number of thorny excrescences, and densely branched apical and basilar trees. Long-shaft neurons are characterized by a comparatively elongated primary apical shaft, a small number of thorny excrescences and relatively less dendritic branching in both the apical and basilar trees (Figs. 3B and 3D). To determine whether stress-induced remodeling of dendrites requires functional NMDARs, we first analyzed the morphology of apical and basal dendrites of short-shaft and long-shaft pyramidal neurons from two regions spanning the longitudinal axis of CA3 (Fig. 3A; Table 1). We confirmed in control mice that a significant reduction in dendritic material was observed in the apical dendrites of short-shaft pyramidal neurons in both dorsal and ventral regions of CA3 (Fig. 4). One-way ANOVAs on the data from dorsal CA3 region revealed a significant decrease in branch length (F(1,10) = 12.3; p<.01) (Fig. 4A). In the ventral CA3 region, not only branch length but also branch number of the short-shaft neurons were substantially decreased in the stressed control group (length: F(1,10) = 11.3; p<.01; branch number: F(1,10) = 14.6; p<.01) (Fig. 4B). These results suggest a comparatively enhanced impact of chronic stress on the ventral CA3 region compared to the dorsal CA3 region. In contrast, mutant mice did not exhibit any significant reduction in either apical branch length or number of short-shaft neurons in either dorsal or ventral CA3 (Fig. 4: Dorsal branch length, F(1,10)=.15, p=.7; branch number F(1,10) = .95, p= .35; Ventral: branch length, F(1,10) = .42, p = .52, branch number F(1,10) =1.22, p = .29). These results clearly suggest that NMDAR function is necessary for stress-induced dendritic retraction of short-shaft neurons in area CA3.
Table 1.
Summary data of branch lengths and numbers
|
CA1 | ||||
|---|---|---|---|---|
| Experimental group | Apical dendritic length | Apical branch points | Basal dendritic length | Basal branch points |
| Control nonstress | 1035.57 ± 51.64 | 31.62 ± 1.95 | 770.30 ± 54.61 | 24.55 ± 0.98 |
| Control stress | 775.18 ± 22.26* | 22.90 ± 1.26* | 825.09 ± 105.44 | 27.73 ± 1.97 |
| Mutant nonstress | 995.55 ± 26.89 | 25.30 ± 0.70 | 828.14 ± 105.96 | 26.94 ± 1.67 |
| Mutant stress | 1081.51 ± 80.83 | 31.68 ± 2.55 | 843.59 ± 60.12 | 27.30 ± 1.55 |
|
CA3 Short shaft | ||||
|---|---|---|---|---|
| Experimental group | Apical dendritic length | Apical branch points | Basal dendritic length | Basal branch points |
| Dorsal: | ||||
| Control nonstress | 1086.91 ± 72.08 | 27.64 ± 1.03 | 898.95 ± 132.95 | 25.38 ± 2.59 |
| Control stress | 776.19 ± 51.26* | 22.83 ± 2.67 | 967.02 ± 72.93 | 26.99 ± 1.74 |
| Mutant nonstress | 1123.85 ± 108.68 | 31.20 ± 2.31 | 1068.98 ± 117.79 | 29.01 ± 2.67 |
| Mutant stress | 1175.84 ± 72.90 | 27.24 ± 2.04 | 1145.53 ± 140.16 | 30.89 ± 2.66 |
| Ventral: | ||||
| Control nonstress | 794.71 ± 87.17 | 23.70 ± 1.12 | 800.10 ± 70.06 | 22.61 ± 1.34 |
| Control stress | 492.52 ± 21.76* | 17.40 ± 1.12* | 656.83 ± 94.29 | 21.85 ± 2.00 |
| Mutant nonstress | 877.57 ± 59.50 | 26.64 ± 1.72 | 769.19 ± 61.45 | 23.08 ± 0.97 |
| Mutant stress | 826.78 ± 50.42 | 24.73 ± 2.65 | 867.25 ± 76.67 | 23.13 ± 1.26 |
|
CA3 Long shaft | ||||
|---|---|---|---|---|
| Experimental group | Apical dendritic length | Apical branch points | ||
| Dorsal: | ||||
| Control nonstress | 770.21 ± 90.75 | 18.62 ±1.67 | ||
| Control stress | 634.96 ± 69.11 | 15.88 ±1.33 | ||
| Mutant nonstress | 869.29 ± 41.57 | 23.17 ±0.75 | ||
| Mutant stress | 935.22 ± 83.13 | 22.25 ±2.10 | ||
| Ventral: | ||||
| Control nonstress | 486.33 ± 43.80 | 15.05 ±1.42 | ||
| Control stress | 379.14 ± 58.09 | 11.79 ±1.08 | ||
| Mutant nonstress | 559.74 ± 60.50 | 16.35 ±1.63 | ||
| Mutant stress | 632.62 ± 50.75 | 15.90 ±0.52 | ||
Summary data of branch length and number for each experimental group (n=5) analyzed (control non-stress, control stress, mutant non-stress, mutant stress). Data are further subdivided by anatomical location (CA3 Dorsal, CA3 Ventral, and CA1) and within CA3, by neuronal subtype (Short-shaft, Long-shaft). For control mice, values that denote a significant decrease following CIS are marked with an asterisk (p<.05).
Fig. 4.
Overall dendritic length (A) of neurons in both dorsal and ventral regions of Stress Control mice (n=5) was significantly reduced. Branch number (B) was significantly decreased only in the ventral region of Stress Control mice. Neither parameter was significantly altered in pyramidal neurons of Stress Mutant mice (n=5) in any region of the hippocampus. *P<.05; mean ±SEM.
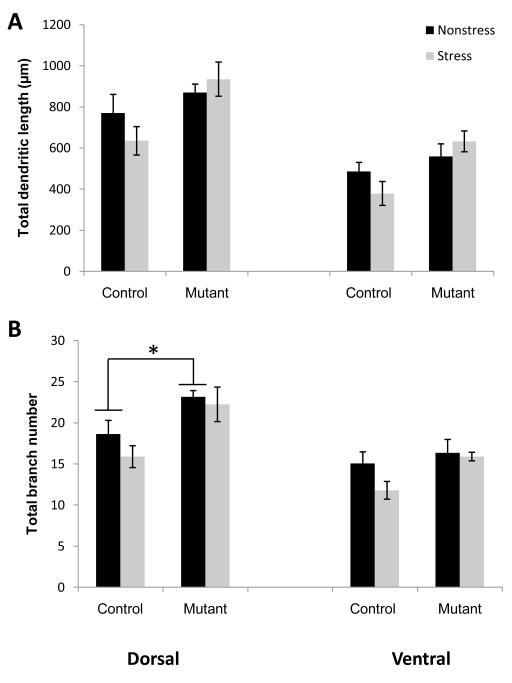
Interestingly, the stress-induced atrophy of CA3 long-shaft neurons was much less pronounced than that of short-shaft neurons. Two-way ANOVAs of apical branch length or branch number in long-shaft neurons did not reveal any significant impact of CIS in either control or mutant mice (Fig. 5). However, we found a significant difference in apical branch number of dorsal neurons, although not length, when we compared mutant and non-mutant mice in the non-stress condition (one way ANOVA; dorsal branch number (F(1,10) = 5.4, p<.05). It is not clear whether this difference reflects a stress-independent compensation in the mutant animals wherein the long-shaft neurons undergo morphological changes in response to the lack of NMDARs or whether the chronic mild stress of laboratory housing induces long-shaft neuron-specific retraction of dendrites which would suggest that these neurons are more, not less, sensitive to stress.
Fig. 5.
Long-shaft apical dendrites in CA3 are resistant to chronic stress. CIS did not induce a significant reduction in either overall dendritic length (A) or branch number (B) in either region of the hippocampus regardless of genotype. Two-way ANOVA revealed a main effect of genotype regardless of treatment but analysis of genotypic differences in the Non-stress groups showed a significant baseline difference between Control (n=5) and Mutant (n=5) only in dorsal branch number (bottom graph). *P<.05; mean ±SEM.
Strikingly, we observed significant alterations in the apical dendrites of CA1 pyramidal neurons in Control mice following CIS (Fig. 6), which was totally absent in the CA3-NR1-KO mutant mice. Two-way ANOVA revealed a significant interaction between genotype and treatment in total length (F(1,20) = 11.5; p<.01) and branch number (F(1,20) = 18.4; p<.001) of apical dendrites. Post-hoc tests identified a significant difference between the stress control group and all other groups (stress mutant, non-stress control and non-stress mutant; p<.05). Lack of CA1 remodeling in the mutant mice indicated that CA1 dendritic retraction is regulated by NMDARs in the upstream CA3 region, suggesting the feedforward effect of CA3 dendritic remodeling on the CA1 subregion of the hippocampus.
Fig. 6.
Genetic ablation of NR1 in CA3 pyramidal neurons modulates stress response in CA1. Both total length (A) and branch number (B) of apical dendrites were significantly decreased in CA1 pyramidal neurons in Control mice (n=5) subjected to CIS. Mutant mice (n=5) showed no significant stress-induced retraction. *P<.05; mean ±SEM.
Stress-induced dendritic remodeling in the hippocampus is independent of stress-induced anxiety and hyperlocomotion
To determine the effect of stress-induced hippocampal dendritic remodeling on behavior, we subjected the mutant and control mice to several tests beginning one day after the cessation of CIS application. Chronically stressed mice, regardless of genotype, showed a decrease in the amount of time spent in the open arms of the elevated plus maze (Fig. 7A), suggesting anxiety-like behavior. Two way ANOVA revealed a significant main effect of treatment (F(1,53)= 13.8; p<.001) but no difference between genotypes or interaction between treatment and genotype. In addition, overall distance traveled during the 5 min test was significantly higher in the stress vs. non-stress groups (main effect of treatment = (F(1,53) = 8.5; p<01), suggesting hyperlocomotion after CIS regardless of the genotypes. Similarly, we observed a stress-induced hyperlocomotion in the open field test (Fig. 7B). Two way ANOVA on the total distance measure revealed a significant main effect of stress treatment (F(1,30)=32.2, p<.001) but no difference between genotypes or interaction between treatment and genotype.
Fig. 7.
CIS-induced hyperlocomotion and anxiety. (A) Chronically stressed mice spent less time in open arms (top row) of the elevated plus maze but were more active overall as reflected in the total distance traveled (bottom row). (B) During 30 min of open field exploration, chronic stress did not affect center stay time in either genotype (top row). Both mutant and control mice subjected to CIS showed higher overall activity levels during the test (bottom row). *P<.05; mean ±SEM.
Chronic stress enhances detection of spatial novelty independently of morphological change to the hippocampus
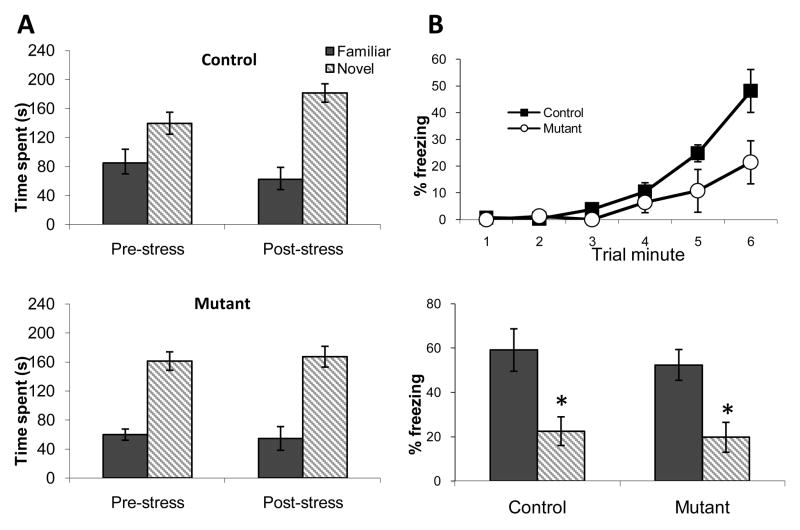
To determine stress-induced changes in short-term spatial memory, we exposed mutant and control mice to a Y maze task before and after CIS induction. A long-standing debate continues as to whether stress-induced CA3 dendritic retraction is detrimental or compensatory in the net effect on hippocampal function (See Discussion). In this study, we attempted to identify the role of CA3 dendritic retraction in the spatial memory. If retraction is compensatory, mutants would show impaired performance in hippocampal dependent behavior following CIS. To test this hypothesis, we used a spatial short term memory task with a short test interval of 5 min because non-mutant mice would be expected to show intact performance and memory formation despite being exposed to CIS (Bellani et al., 2006). Two way ANOVA revealed a statistically significant increase in spatial memory performance for both genotypes as indicated by a main effect of time of testing (pre-stress vs. post-stress; F(1,27) = 4.46, p<.05) (Fig. 8A). These data indicate that spatial short-term memory is enhanced following CIS treatment in the control mice as expected, but the mutants are capable of performing at the same level despite the lack of stress-induced dendritic remodeling. An important caveat is that the mutant mice may be performing close to ceiling level prior to stress induction which could obscure any further stress-related enhancement.
Fig. 8.
Behavioral effects of chronic stress did differ between genotypes. In the Y-maze (A), spatial working memory was enhanced in the non-mutants as shown by a relative increase in exploration of the novel arm during the post-stress test. During contextual fear conditioning (B), stressed Mutant mice showed a significantly decreased level of conditioned freezing during the last minute of the training session (top row), suggesting impaired acquisition but contextual discrimination 24 h later was intact in both Control and Mutant groups (bottom row). *P<.05; mean ±SEM.
Expression of contextual fear discrimination is not affected by CIS
Finally, to evaluate the effect of dendritic remodeling in another hippocampal dependent learning task, we subjected the mice to a contextual discrimination fear conditioning protocol. Performance of this task was normal in the naïve mutants (Cravens et al., 2006). Mutant mice showed a slower rate of acquisition in fear conditioning compared to the CIS-treated controls following CIS, as evidenced by significantly less freezing during the final minute of the training session (Fig. 8B). Repeated measures ANOVA revealed a significant main effect of trial minute (F(1,40) = 21.3; p<.0001) and a significant interaction between genotype and trial minute (F(1,40) = 2.9; p<.05). However, both mutant and control mice subjected to chronic stress prior to fear conditioning were able to distinguish between the training and a novel context when tested for conditioned freezing responses 24 h later (Fig. 8B). Two-way ANOVA showed a significant main effect for context (F(1,20) = 5.6; p<.05) but no significant differences between genotypes or in the interaction between genotype and context. These results suggest that hippocampal dendritic remodeling as a consequence of CA3 NMDAR deletion has little impact on the stress-induced behavioral outcome.
Discussion
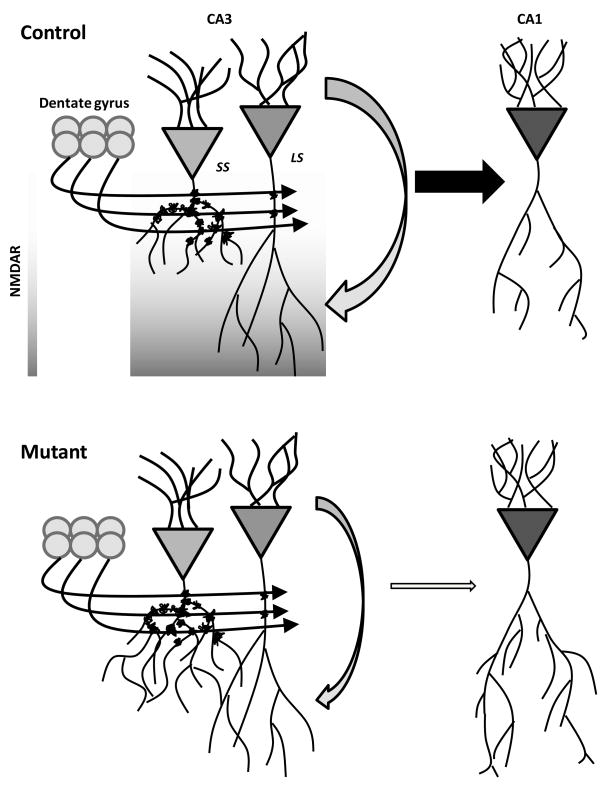
Our results provide the first analysis, to our knowledge, of stress-induced morphological changes throughout the entire longitudinal axis of CA3. We observed that CIS was sufficient to reduce complexity and induce retraction of short-shaft apical dendrites in both dorsal and ventral CA3 regions of non-mutant mice. Many laboratories have reported structural changes in dorsal CA3 as a neural consequence of chronic stress (Watanabe et al., 1992b, Magariños et al., 1996, McEwen and Magariños, 1997, Lambert et al., 1998, Vyas et al., 2002, McLaughlin et al., 2009), but we have also now demonstrated robust dendritic remodeling in the ventral CA3 region. Although dendritic retraction occurred throughout the CA3 region, subtypes of pyramidal neurons were not affected equally. Specifically, we found that apical dendrites of CA3 long-shaft neurons in the non-mutant mice were more resilient to the effects of chronic stress. We predict that this resilience is due to differences in the input to long-shaft and short-shaft neurons with the preponderance of mossy fiber synapses impinging on the proximal dendrites of short-shaft neurons (Fig. 9). However, it is conceivable that non-mutant long-shaft neurons are actually more vulnerable to stress, including the chronic mild stressors arising from laboratory breeding and housing, since there is evidence of less apical branching in non-mutant than mutant mice even without the induction of CIS (Fig. 5B). Although this difference in baseline measures of dendritic branching could be plausibly be explained by morphological consequences of the conditional knockout of the NMDARs.
Fig. 9.
Simplified model depicting how CA3 NMDARs may mediate stress-induced restructuring in both CA3 and CA1 subregions of the hippocampus. Recurrent connections within CA3 are targeted to more distal dendritic regions that are the site of the most dense distribution of NMDARs. A decrease in CA3 activity resulting from NMDAR ablation would have a feedforward effect in reducing the excitatory drive to CA1 pyramidal neurons. Although mossy fiber excitatory input remains the same in the mutant and non-mutant mice (Control), the cumulative excitatory input is the determinant of the cellular susceptibility to stress. A reduction in net excitatory drive from either a decrease in recurrent activity (as in our mutant mice) or from fewer thorny excrescences (as in the long-shaft neurons) results in a protective effect against morphological changes. LS; long-shaft neuron, SS; short-shaft neuron.
Despite this tendency toward more branching and longer dendrites in long-shaft neurons prior to stress, mutant mice were not subject to dendritic retraction in either type of CA3 neuron in which the NMDAR had been functionally ablated. This result demonstrates a cell-autonomous dependence on NMDAR-mediated processes in the induction of atrophy in this stress-sensitive region of the brain. To examine potential non-cell autonomous effects in downstream regions, we analyzed dendritic characteristics in the CA1 region and found a significant reduction in apical branch number and length in stressed, non-mutant mice. Again, mutant mice failed to show CIS-induced dendritic retraction in this region of the hippocampus, suggesting that NMDARs in CA3 are critical for stress-induced morphological effects in CA1. Surprisingly, we observed no demonstrable impact of the mutants’ resistance to dendritic retraction on several behavioral tasks following CIS, suggesting some degree of dissociation between behavioral and cellular consequences of chronic stress.
Potential mechanisms of NMDAR-dependent dendritic retraction in area CA3 and CA1
Putative mechanisms underlying stress-induced CA3 atrophy include glucocorticoid-triggered signaling cascades and activity-dependent glutamate release (Conrad et al., 1996, Smith, 1996, McEwen et al., 2002). With respect to the data presented here, it is unlikely that glucocorticoid receptors are downregulated in NMDAR-deleted CA3 pyramidal cells since activation of NMDARs appears to decrease glucocorticoid mRNA in the hippocampus (Tritos et al., 1999). On the other hand, phenytoin, an anti-epileptic agent that is known to interfere with glutamate release (Skerritt and Johnston, 1983), prevents stress-induced CA3 dendritic retraction (Watanabe et al., 1992a, Magariños et al., 1996), suggesting that chronic stress-induced glutamate release is essential for CA3 dendritic remodeling. Our genetic manipulation, which results in functional elimination of CA3 NMDARs, likely decreases activity in this region. Indeed, in vivo unit recording from the CA3 region of the same mutants used in this study reveals a dramatic reduction in CA3 pyramidal cell firing (Jinde et al., 2009). This reduction in CA3 firing frequency may result in diminished glutamate release within the CA3 recurrent network and may be the critical factor in the prevention of CA3 dendritic retraction in the mutants (Fig. 9). Accordingly, we predict that stress-induced over-stimulation of CA3 NMDA receptors is responsible for eliciting hippocampal dendritic retraction. NMDARs at the commissural/associational fiber synapses may play a preferential role in CA3 dendritic remodeling because these synapses comprise approximately 75% of CA3 synapses and are the basis of the extensive recurrent network (MacVicar and Dudek, 1980). However we cannot exclude the contribution of secondary factors or mechanisms which are also known to be NMDAR-dependent, such as brain-derived neurotrophic factor and serotonin (Vaidya et al., 1999, Joca et al., 2007).
Additional evidence in support of this model is derived from the differential effect on short-shaft and long-shaft neurons in non-mutant mice. This result reflects the cellular and physiological heterogeneity of the CA3 region, which is rarely discussed in terms of the contributions of this subregion to hippocampal processing. Indeed, aside from the initial report that categorized CA3 neurons based on morphology (Fitch et al., 1989), very little is known about the differences between these subtypes in terms of electrophysiological response properties or signaling efficacy. There is a modest positional effect such that short-shaft neurons are predominantly found in the superficial half of the pyramidal layer and conversely, long-shaft neurons are mostly found in the lower half of the layer. But the dominant discriminating feature is the length and arborization pattern of the apical dendrites. Long-shaft neurons have minimally extensive branching in the orthogonal plane. Short-shaft neurons are stouter with dendritic arbors extending in a more concentric manner. However, another readily observable feature of short-shaft neurons is the abundance of thorny excrescences, which are the signature of glutamatergic mossy fiber input from dentate granule neurons. If dendritic remodeling is due to excessive glutamatergic release in affected neurons, then short-shaft neurons would be more susceptible to the excitatory input arriving from mossy fiber afferents. Our model illustrates how invariant input from the dentate gyrus can lead to a cell-selective impact on CA3 pyramidal neurons (Fig. 9).
NMDAR in CA3 pyramidal neurons regulate CA1 dendritic morphology
We found a selective reduction in dendrite length and branch number in CA1 pyramidal neurons of stressed non-mutant mice, which is consistent with previous reports (Lambert et al., 1998, McEwen, 2001). Notably, stress-induced atrophy in the mutant mice was totally absent in CA1. This lack of dendritic retraction in CA1 pyramidal neurons could be explained by the same mechanism we propose for CA3 as described above, i.e. diminished glutamate release in the Schaeffer collateral projections from CA3 pyramidal neurons to CA1 neurons in mutant mice (Fig. 9). Supporting this idea, in vivo unit recording from the CA1 region of the same mutant mice revealed a reduction of feed-forward inhibition from CA3 pyramidal cells to CA1 interneurons. Nonetheless, overall mean firing rates of CA1 pyramidal neurons are normal, suggesting a concomitant decrease in excitatory inputs from CA3 to CA1 pyramidal neurons in the mutants (Jinde et al., 2009). This may explain the lack of CIS-induced dendritic retraction of the mutant CA1 pyramidal neurons. Interestingly, the same mutant mice used in the present study are much more vulnerable to kainic acid-induced excitotoxicity in area CA1 (Jinde et al., 2009). Thus, it appears that although glutamate release is necessary for eliciting dendritic retraction, the retraction may not be the detrimental consequence of an excitotoxic effect. For instance, ibotenic acid-induced excitotoxic damage is enhanced in the CA3 region in rats given 21 days of chronic restraint stress compared with unstressed rats (Conrad et al., 2004), which only demonstrates that stress may increase the vulnerability to neuronal insult. The functional significance of the dendritic remodeling in terms of absolute impact on hippocampal processing has remained elusive. The data of our mutant mice showing a clear dissociation between increased vulnerability to excitotoxicity and dendritic remodeling in CA1 suggest that the dendritic retraction is fundamentally compensatory, rather than detrimental, in terms of maintaining a homeostatic balance in the local hippocampal circuitry.
Morphological impacts of stress on ventral vs dorsal CA3
We hypothesized that there may be heterogeneity in stress-induced changes in the hippocampus because neurons projecting to stress-responsive regions such as the amygdala, prefrontal cortex, and hypothalamus arise almost exclusively from the ventral hippocampus (Swanson, 1981, Cenquizca and Swanson, 2006, Ishikawa and Nakamura, 2006, Ulrich-Lai and Herman, 2009). It is therefore plausible that hippocampal-mediated influences on the HPA axis might preferentially arise from changes in the ventral region. During our analyses of Golgi-impregnated neurons in both the dorsal and ventral hippocampus, we did observe a dramatic region-dependent difference in the cellular morphology of CA3 neurons that was independent of whether the animals had been subjected to chronic stress, a finding that has not been previously reported. Naïve pyramidal neurons in ventral CA3 had reduced dendritic complexity and less extensive processes. While the present study did not address the functional implications of this morphological heterogeneity along the longitudinal axis, it does suggest that this may be a relevant dimension to explore in future investigations of the mechanisms underlying intrahippocampal processing. We did find a relatively more pronounced reduction in overall dendritic material in the ventral region of CA3 indicating that this region shows a similar vulnerability to the effects of chronic stress.
Dissociation between behavioral and cellular consequences of chronic stress
Cognitive deficits associated with chronic stress are well-documented and hypothesized to result, at least in part, from compromised integrity of the hippocampus (McLaughlin et al., 2009). However, the literature is mixed regarding chronic glucocorticoid actions on hippocampal learning and memory. Based on this conflicting evidence, an alternative hypothesis has also been recognized suggesting that the atrophy observed in the dendrites of hippocampal neurons may be a compensatory response to protect the hippocampus from further damage (Ohl and Fuchs, 1999, McEwen, 2001, Bartolomucci et al., 2002, de Quervain et al., 2009). In this view, a reduction in dendritic length may actually preserve function and confer behavioral resistance to stress by minimizing the more severe risk of neurodegeneration. As discussed above, the present morphology study together with our recent study (Jinde et al., 2009) suggested a compensatory role of CA3 dendritic retraction in modulating hippocampal function. If CA3 dendritic remodeling is primarily compensatory, then mutant mice would be predicted to be more severely affected by exposure to chronic stress. Thus, we opted to test this hypothesis using versions of two tasks that have been shown to be sensitive to chronic stress manipulation (Conrad et al., 1996, Kim and Diamond, 2002).
Unexpectedly, our behavioral results suggest that dendritic remodeling of hippocampal neurons has only a marginal effect on stress-induced behavioral phenotypes. Hyperlocomotion, anxiety-like behavior, spatial memory enhancement and contextual discrimination were all indistinguishable between the mutant and non-mutant groups exposed to chronic stress. Taken together with our results from the morphological analysis showing that a CA3-specific NMDAR deletion prevents stress-induced atrophy, we reveal a dissociation between behavioral and cellular consequences of chronic stress. It is puzzling why both mutants and controls displayed similar stress-induced behavioral outcomes. However, we only tested a subset of behaviors that are expected to rely upon the hippocampus or be affected by stress. It is entirely possible that challenging the mice with more complex tasks would reveal selective behavioral deficits in the non-mutant mice. In particular, the extensive training required in incrementally learned tasks, such as the Morris water maze, would allow for a more detailed analysis of acquisition rates over time. In addition, the capacity for behavioral flexibility could be tested, which was not explicitly examined in the one-trial learning protocols used in this study. Although manipulations of the CA3 region can affect performance in rapid acquisition tasks, the differential effects on morphology we observe in CA1 could be predicted to have an impact on more general forms of hippocampal processing involving a broader range of behavioral challenges. Appetitive tasks that rely on motivated behavior or instrumental tasks that require the animal to make a response could also reveal novel deficits in the control mice, although it would be difficult to interpret these data due to nonspecific physiological effects of stress that might obscure an impairment in the hippocampal contribution to learning. It is also possible that extending the exposure time to the stressor would eventually lead to a phenotypic divergence between the mutant and non-mutant animals in learned behavior. Nonetheless, it is clear that there is not a simple correspondence between hippocampal cellular morphology in this exceptionally vulnerable region and stress-related changes in physiological status or behavioral performance in the hippocampal-dependent tasks that we assessed. Given that other corticolimbic structures such as basolateral amygdala and prefrontal cortex show stress-induced changes (e.g., Vyas et al., 2002; Cook & Wellman, 2004) and contribute to many hippocampal-dependent behaviors, perhaps changes in these structures contributed to the stress-induced alterations in the behaviors we assessed. The dissociation we report between cellular structure and systems-level function may raise important questions about the link between this neural hallmark of chronic stress and its regulation of intra- and extra-hippocampal deficits.
Research Highlights.
Chronic stress induces dendritic retraction of CA3 and CA1 pyramidal cells
Stress-induced dendritic remodeling was not evident in CA3 long-shaft pyramidal neurons
Structural changes were absent in stressed CA3-NMDA receptor KO mutant mice
Stimulation of CA3 NMDA receptors may elicit dendritic remodeling in the hippocampus
Acknowledgments
This work was supported by the Intramural Research Programs of the National Institute of Mental Health. We thank Drs. Cameron HA, Chen G, Young WS, and Holmes A for critical reading of the earlier version of the manuscript.
Abbreviations
- ANOVA
analysis of variance
- CIS
chronic immobilization stress
- HPA
hypothalamic-pituitary-adrenal
- NMDA
N-methyl-D-aspartic acid
- NMDAR
NMDA receptor
Footnotes
Financial Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bellani R, Luecken LJ, Conrad CD. Peripubertal anxiety profile can predict predisposition to spatial memory impairments following chronic stress. Behav Brain Res. 2006;166:263–270. doi: 10.1016/j.bbr.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, de Biurrun G, Czeh B, van Kampen M, Fuchs E. Selective enhancement of spatial learning under chronic psychosocial stress. Eur J Neurosci. 2002;15:1863–1866. doi: 10.1046/j.1460-9568.2002.02043.x. [DOI] [PubMed] [Google Scholar]
- Bellani R, Luecken LJ, Conrad CD. Peripubertal anxiety profile can predict predisposition to spatial memory impairments following chronic stress. Behav Brain Res. 2006;166:263–270. doi: 10.1016/j.bbr.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. J Comp Neurol. 2006;497:101–114. doi: 10.1002/cne.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wise LS. Chronic stress enhances ibotenic acid-induced damage selectively within the hippocampal CA3 region of male, but not female rats. Neuroscience. 2004;125:759–767. doi: 10.1016/j.neuroscience.2004.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Cravens CJ, Vargas-Pinto N, Christian KM, Nakazawa K. CA3 NMDA receptors are crucial for rapid and automatic representation of context memory. Eur J Neurosci. 2006;24:1771–1780. doi: 10.1111/j.1460-9568.2006.05044.x. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Front Neuroendocrinol. 2009;30:358–370. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Fitch JM, Juraska JM, Washington LW. The dendritic morphology of pyramidal neurons in the rat hippocampal CA3 area. I. Cell types. Brain Res. 1989;479:105–114. doi: 10.1016/0006-8993(89)91340-1. [DOI] [PubMed] [Google Scholar]
- Gilad GM, Gilad VH, Wyatt RJ, Tizabi Y. Region-selective stress-induced increase of glutamate uptake and release in rat forebrain. Brain Res. 1990;525:335–338. doi: 10.1016/0006-8993(90)90886-g. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Van der Loos H. Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high clarity Golgi-Nissl stain. J Neurosci Methods. 1981;4:117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Ventral hippocampal neurons project axons simultaneously to the medial prefrontal cortex and amygdala in the rat. J Neurophysiol. 2006;96:2134–2138. doi: 10.1152/jn.00069.2006. [DOI] [PubMed] [Google Scholar]
- Jinde S, Belforte JE, Yamamoto J, Wilson MA, Tonegawa S, Nakazawa K. Lack of kainic acid-induced gamma oscillations predicts subsequent CA1 excitotoxic cell death. Eur J Neurosci. 2009;30:1036–1055. doi: 10.1111/j.1460-9568.2009.06896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joca SR, Ferreira FR, Guimaraes FS. Modulation of stress consequences by hippocampal monoaminergic, glutamatergic and nitrergic neurotransmitter systems. Stress. 2007;10:227–249. doi: 10.1080/10253890701223130. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Lambert KG, Buckelew SK, Staffiso-Sandoz G, Gaffga S, Carpenter W, Fisher J, Kinsley CH. Activity-stress induces atrophy of apical dendrites of hippocampal pyramidal neurons in male rats. Physiol Behav. 1998;65:43–49. doi: 10.1016/s0031-9384(98)00114-0. [DOI] [PubMed] [Google Scholar]
- Lowy MT, Gault L, Yamamoto BK. Adrenalectomy attenuates stress-induced elevations in extracellular glutamate concentrations in the hippocampus. J Neurochem. 1993;61:1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- MacVicar BA, Dudek FE. Local synaptic circuits in rat hippocampus: interactions between pyramidal cells. Brain Res. 1980;184:220–223. doi: 10.1016/0006-8993(80)90602-2. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci U S A. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Magariños AM. Stress effects on morphology and function of the hippocampus. Ann N Y Acad Sci. 1997;821:271–284. doi: 10.1111/j.1749-6632.1997.tb48286.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Magariños AM, Reagan LP. Structural plasticity and tianeptine: cellular and molecular targets. Eur Psychiatry. 2002;17(Suppl 3):318–330. doi: 10.1016/s0924-9338(02)00650-8. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Conrad CD. Chronic stress- and sex-specific neuromorphological and functional changes in limbic structures. Mol Neurobiol. 2009;40:166–182. doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl F, Fuchs E. Differential effects of chronic stress on memory processes in the tree shrew. Brain Res Cogn Brain Res. 1999;7:379–387. doi: 10.1016/s0926-6410(98)00042-1. [DOI] [PubMed] [Google Scholar]
- Skerritt JH, Johnston GA. Inhibition of amino acid transmitter release from rat brain slices by phenytoin and related anticonvulsants. Clin Exp Pharmacol Physiol. 1983;10:527–533. doi: 10.1111/j.1440-1681.1983.tb00221.x. [DOI] [PubMed] [Google Scholar]
- Smith MA. Hippocampal vulnerability to stress and aging: possible role of neurotrophic factors. Behav Brain Res. 1996;78:25–36. doi: 10.1016/0166-4328(95)00220-0. [DOI] [PubMed] [Google Scholar]
- Swanson LW. A direct projection from Ammon’s horn to prefrontal cortex in the rat. Brain Res. 1981;217:150–154. doi: 10.1016/0006-8993(81)90192-x. [DOI] [PubMed] [Google Scholar]
- Tapia R. Release and uptake of glutamate as related to excitotoxicity. Rev Bras Biol. 1996;56(Su 1 Pt 1):165–174. [PubMed] [Google Scholar]
- Tritos N, Kitraki E, Philippidis H, Stylianopoulou F. Neurotransmitter modulation of glucocorticoid receptor mRNA levels in the rat hippocampus. Neuroendocrinology. 1999;69:324–330. doi: 10.1159/000054434. [DOI] [PubMed] [Google Scholar]
- Tuvnes FA, Steffenach HA, Murison R, Moser MB, Moser EI. Selective hippocampal lesions do not increase adrenocortical activity. J Neurosci. 2003;23:4345–4354. doi: 10.1523/JNEUROSCI.23-10-04345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya VA, Terwilliger RM, Duman RS. Role of 5-HT2A receptors in the stress-induced down-regulation of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci Lett. 1999;262:1–4. doi: 10.1016/s0304-3940(99)00006-3. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Fukaya M, Sakimura K, Manabe T, Mishina M, Inoue Y. Selective scarcity of NMDA receptor channel subunits in the stratum lucidum (mossy fibre-recipient layer) of the mouse hippocampal CA3 subfield. Eur J Neurosci. 1998;10:478–487. doi: 10.1046/j.1460-9568.1998.00063.x. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992a;2:431–435. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992b;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]