Abstract
Very low birth weight preterm (PT) children are at high risk for brain injury. Employing diffusion tensor imaging (DTI), we tested the hypothesis that PT adolescents would demonstrate microstructural white matter disorganization relative to term controls at 16 years of age. Forty-four PT subjects (600 - 1250 grams birth weight) without neonatal brain injury and 41 term controls were evaluated at age 16 years with DTI, the Wechsler Intelligence Scale for Children - III (WISC), the Peabody Picture Vocabulary Test - Revised (PPVT), and the Comprehensive Test of Phonological Processing (CTOPP).
PT subjects scored lower than term subjects on WISC full scale (p = 0.003), verbal (p = 0.043), and performance IQ tests (p = 0.001), as well as CTOPP phonological awareness (p = 0.004), but scored comparably to term subjects on PPVT and CTOPP Rapid Naming tests. PT subjects had lower fractional anisotropy (FA) values in multiple regions including bilateral uncinate fasciculi (left: p = 0.01; right: p = 0.004), bilateral external capsules (left: p < 0.001; right: p < 0.001), the splenium of the corpus callosum (p = 0.008), and white matter serving the inferior frontal gyrus bilaterally (left: p < 0.001; right: p = 0.011). FA values in both the left and right uncinate fasciculi correlated with PPVT scores (a semantic language task) in the PT subjects (left: r = 0.314, p = 0.038; right: r = 0.336, p = 0.026). FA values in the left and right arcuate fasciculi correlated with CTOPP Rapid Naming scores (a phonologic task) in the PT subjects (left: r = 0.424, p = 0.004; right: r = 0.301, p = 0.047).
These data support for the first time that dual pathways underlying language function are present in PT adolescents. The striking bilateral dorsal correlations for the PT group suggest that prematurely born subjects rely more heavily on the right hemisphere than typically developing adults for performance of phonological language tasks. These findings may represent either a delay in maturation or the engagement of alternative neural pathways for language in the developing PT brain.
Keywords: Preterm, adolescence, diffusion tensor imaging, dual language system
Introduction
Premature birth is a pressing public health matter, as nearly 13% of infants in the United States are born preterm and infants weighing under 1500 grams at birth comprise 1.5% of births (Beck et al., 2010; Martin et al., 2008). The brains of infants born prematurely face increased susceptibility to the challenges of ischemia and inflammation. Through the downstream effectors of excitotoxicity and free-radical attack, both glia and axons are disrupted, and the damage is compounded by secondary disturbance of growth patterns (Dyet et al., 2006; Hack et al., 2002; Miller et al., 2005). In addition to feared neurological complications of preterm birth such as cerebral palsy, a range of developmental deficits in cognitive function in preterm children have been observed to persist until early adulthood, indicating long-term disruption of brain function (Hack, 2009; Neubauer et al., 2008). Preterm children with severe brain injury such as periventricular hemorrhagic infarction and periventricular leukomalacia have the greatest risk of neurodevelopmental deficits; however, even those without these forms of brain injury are more likely than control term subjects to have lower IQ scores and require more support in language-based skills (Luu et al., 2009) during childhood and adolescence.
Recent investigations have proposed dual systems of language processing, analogous to dual pathways identified in visual processing (Friederici, 2009; Hickok and Poeppel, 2007; Saur et al., 2008). In this model, a ventral pathway processes comprehension of speech, with mapping of sounds to semantic representations, while a dorsal pathway is involved in matching speech signals to phonological and articulatory representations. The prototypical task calling upon the dorsal pathway is repetition, while the ventral pathway is vital in understanding meaningful speech. The arcuate fasciculus has been identified as the primary component of the dorsal pathway, while the ventral pathways are likely comprised of fibers traveling through both the extreme capsule and the uncinate fasciculus, a ventral pathway connecting the temporal and frontal lobes (Gozzo et al., 2009; Parker et al., 2005). The ventral pathway is thought to be bilateral, while the dorsal pathway tends to be strongly left-dominant (Hickok and Poeppel, 2007; Parker et al., 2005). Furthermore, since investigations of the dual pathway language systems have taken place in adult subjects, the developmental timing of specialization of these pathways is not fully understood. (Cao et al., 2009; Chou et al., 2006; Ment et al., 2006; Peterson et al., 2002).
Diffusion tensor imaging (DTI) provides a sensitive means of assessing the integrity of white matter tracts at a microstructural level. As part of DTI analyses, fractional anisotropy (FA) values provide a quantitative measure of the degree to which water diffusion is restricted along one axis relative to all others. Within cerebral white matter, water preferentially diffuses along axons, with diffusion perpendicular to this axis restricted by structural barriers including cell membranes. Higher FA values are a marker for the coherence of white matter tracts, as the constraints of the tissue organization into axon bundles within well-formed tracts limit the direction of water diffusion. Alterations in FA may result from changes in fiber organization, axonal size (Assaf et al., 2008), or activity-dependent changes in myelination (Als et al., 2004).
Previous studies have shown deficits in FA values in white matter tracts in premature infants at term-equivalent age as compared to term infants (Anjari et al., 2009; Anjari et al., 2007; Berman et al., 2005; Cheong et al., 2009; Dudink et al., 2007; Gimenez et al., 2008; Huppi et al., 1998; Huppi et al., 2001; Miller et al., 2002; Partridge et al., 2004; Rose et al., 2008), and changes in FA values can persist to late childhood and adolescence (Constable et al., 2008; Counsell et al., 2007; Counsell et al., 2008; Gimenez et al., 2006; Kontis et al., 2009; Murakami et al., 2008; Nagy et al., 2003; Reiss et al., 2004; Skranes et al., 2007; Vangberg et al., 2006; Yung et al., 2007). Moreover, FA values have been shown to correlate with multiple measures of neurodevelopmental function (Als et al., 2004; Arzoumanian et al., 2003; Bassi et al., 2008; Berman et al., 2009; Drobyshevsky et al., 2007; Krishnan et al., 2007; Peterson et al., 2000; Rose et al., 2009; Rose et al., 2007; Skranes et al., 2007).
Further, preterm children may develop language pathways differently than normally developing term children. For example, functional connectivity analyses have shown stronger connections between Wernicke’s area and right-sided cortical regions in preterm children than in term children, implying changes in lateralization of language processing (Gozzo et al., 2009). This may represent increased utilization of compensatory alternative pathways in the right hemisphere in preterm children. Thus, for language tasks in particular, preterm children may engage alternative pathways, including increased utilization of the right hemisphere.
We hypothesize that preterm children at age 16 will continue to have deficits in both cognitive testing and microstructural integrity of multiple white matter tracts. By evaluating dorsal and ventral language pathways in preterm children, we will investigate whether preterm children demonstrate departures from the dual language pathway as described in typically developing adults, which may represent delays in maturation or compensations for changes in glio- and/or neurogenesis caused by the injury of preterm birth.
Methods
This study was performed at the Yale University School of Medicine, New Haven, CT and Brown Medical School, Providence, RI. The protocols were reviewed and approved by institutional review boards at each location. Children provided written assent; parent(s) provided written consent for the study. All scans were obtained at Yale University and were analyzed at Yale University and at Stanford University.
Subjects
The preterm cohort consisted of 44 children with no evidence for intraventricular hemorrhage (IVH), periventricular leukomalacia and/or low pressure ventriculomegaly. Subjects had normal findings on conventional MRI, and total ventricular CSF volume within 2 SD of the mean ventricular volume of term control subjects at 12 years of age. They had no contraindications to MRI. All preterm subjects enrolled in the follow-up component of the “Multicenter Randomized Indomethacin IVH Prevention Trial” (Ment et al., 1994a; Ment et al., 1994b) were sequentially recruited for the MRI study when they reached 16 years of age. These children are representative of the cohort of subjects with no evidence of neonatal brain injury with respect to gender, handedness, FSIQ scores, minority status, and maternal education. Forty-one healthy term children, aged 16 years, were recruited from the local community and group-matched with the PT as previously described.
The assessments of neonatal health status and neurologic outcome have been previously described (Peterson et al., 2000). Blinded assessment of intelligence was performed using the Wechsler Intelligence Scale for Children-III (WISC) (Vohr et al., 2003). Children also received the Peabody Picture Vocabulary Test –Revised (PPVT) (Dunn and Dunn, 1981), and the Developmental Test of Visual Motor Integration (VMI) (Beery, 1989), the Comprehensive Test of Phonological Processing (CTOPP) (Wagner et al., 1999), and the Total Word Reading Efficiency test (TOWRE) (Torgesen et al., 1999).
Diffusion Tensor Imaging
Imaging was performed on a Siemens Sonata 1.5 T scanner. DTI data were obtained using a double spin echo EPI sequence with 32 directions, 1 b-value (1000s/mm2) in addition to b=0, and 1 average with TE=87, TR=6200, 128×128 acquisition matrix, Bandwidth 1630, Flip Angle 90, FOV=20×20cm, with 40 slices, 3mm thick, skip 0mm. FA values were calculated from the tensor data using BioImageSuite software (Yale University) and nonlinearly registered to a single subject FA map selected from the control group of children. Both groups of subjects were registered to this single subject template to form composite maps.
An average tensor across subjects was also computed after nonlinear registration of all subjects to a reference FA map, and the control group tensor was used to create a composite tricolor directionality map. This tricolor directionality map from the control group allows fiber bundles to be delineated according to the direction of diffusion along the fibers, and it was used to manually define anatomical regions of interest (ROIs) based on fiber bundle locations as previously described.(Constable et al., 2008) Since all of the subjects are registered in the same composite space, these ROIs were directly applied to each single subject and to group FA maps to generate individual FA values for each ROI for each subject for second level statistical analysis.
Fiber Tracking
ROIs defining the splenium of the corpus callosum and bilateral external capsules were defined using fiber tracking. We used fiber tracking on the tensor data of each subject to extract and define these regions as customized individual ROIs. Preterm subjects are well known to exhibit occipital ventriculomegaly at the time of MR imaging, and we employed this strategy for the splenium because of comparative analyses demonstrating inclusion of splenium fibers in the occipital horns of the lateral ventricles for the preterm group when ROI methods were used. Similarly, we employed fiber tracking for the external capsule so that fibers from the extreme capsule would not be included in the data set.
Volumetric analyses
To complement the DTI analyses, we employed voxel-based morphometry (VBM) to examine volume differences between the preterm and control groups.(Kesler et al., 2008) The optimized VBM process included segmentation and extraction of the brain in native space, normalization of the white matter to a standard space using a custom template created from all study subjects, segmentation and extraction of the normalized brain, modulation of the normalized white matter images to correct for tissue volume differences attributable to the normalization procedure, and smoothing using a 12-mm full width at half-maximum kernel, to reduce the effects of noise.
Statistical Methods
Demographic and cognitive data were analyzed using standard chi-squared statistics for categorical data. Continuous-valued data and ROI-based FA values were analyzed using t-tests to compare groups. General linear models (GLM) including group and gender were employed to compare volume measurements for the two groups, and volumetric data were presented as least square mean (LSM) values ± standard error of the mean (SE); GLM analyses including group, gender and total tissue volume were also used to compare FA values for the groups. Pearson’s correlations were employed to examine the relationships between FA and cognitive outcome measures, while GLM analyses were used to examine the effects of FA, total tissue volume and gender on these measures.
For the purposes of this report, p values < 0.05 were considered significant. All analyses were performed using SAS/STAT® 9.22 (SAS Institute Inc., Cary, NC).
Results
Subject population
Neonatal characteristics of the preterm population are shown in Table 1. The preterm subjects weighed between 600 and 1250 grams at birth, with a mean birth weight of 994 ± 184 grams and mean gestational age of 28.3 ± 1.9 weeks. One quarter of the subjects (26%) developed bronchopulmonary dysplasia.
Table 1.
Preterm Neonatal Data
| N | 44 |
| Male, N (%) | 26 (59) |
| Birth weight, grams, mean ± SD | 994 ± 184 |
| Gestational age, weeks, mean ± SD | 28.3 ± 1.9 |
| Bronchopulmonary dysplasia, N (%) | 11 (26) |
| Intraventricular hemorrhage, N (%) | 0 (0) |
| Periventricular leukomalacia, N (%) | 0 (0) |
| Ventriculomegaly, N (%) | 0 (0) |
Demographic data of the term and preterm cohorts are presented in Table 2. There were no significant differences between preterm and term cohorts in gender, age at scan, number of right-handed subjects, percentage of non-white subjects, subjects who receive special services or subjects whose mothers had less than a high school education.
Table 2.
Demographic Data for the Study Subjects
| Preterm N=44 | Term N=41 | p value | |
|---|---|---|---|
| Male, N (%) | 26 (59%) | 17 (41%) | 0.11 |
| Age at scan, Years ± SD | 16.35 ± 0.31 | 16.26 ± 0.34 | 0.22 |
| Right-handed, N (%) | 38 (86%) | 38 (95%) | 0.27 |
| Special services, N (%) | 8 (18%) | 4 (10%) | 0.35 |
| Non-white, N (%) | 15 (34%) | 14 (34%) | 1.00 |
| Maternal education less than high school, N (%) | 5 (11%) | 2 (5%) | 0.44 |
Results of cognitive testing for the language pathway correlations are presented in Table 3. Preterm subjects scored significantly lower than term subjects on the WISC-III full scale intelligence quotient (IQ) (p=0.003), verbal IQ (p=0.043), and performance IQ (p =0.001). In contrast, there was no significant difference between groups in PPVT scores.
Table 3.
Cognitive Data
| Preterm | Term | p value | |
|---|---|---|---|
| Wechsler Intelligence Scale for Children – III (WISC) | |||
| Full Scale IQ | 94.32 ± 14.25 | 104.56 ± 16.31 | 0.003 |
| Verbal IQ | 96.48 ± 15.29 | 103.38 ± 15.17 | 0.043 |
| Performance IQ | 93.14 ± 15.22 | 105.21 ± 17.44 | 0.001 |
| Peabody Picture Vocabulary Test - Revised (PPVT) | |||
| PPVT | 101.77 ± 20.54 | 104.63 ± 21.39 | 0.53 |
| Comprehensive Test of Phonological Processing (CTOPP) | |||
| Rapid Naming Composite Score | 101.70 ± 18.70 | 97.44 ± 15.32 | 0.26 |
| Phonemic Awareness Composite Score | 82.40 ± 13.64 | 91.40 ± 12.90 | 0.004 |
Preterm subjects performed comparably to term subjects on Non-word Repetition and Phoneme Reversal tasks, as well as Rapid Naming composite scores, which are composed of Rapid Digit Naming and Rapid Letter Naming tasks. The Phonemic Awareness Composite score, made up of tasks involving Blended Non-words and Segmented Non-words, showed significant deficits in preterm subjects compared to term (p=0.004). The subset tasks revealed significantly higher scores in terms than preterms in the Segmented Non-words task but no significant differences in the Blended Non-words task.
Volumetric analyses
VBM data were available for 41 preterm subjects and 41 term control subjects. Least square mean VBM analyses co-varied for gender were performed for total cerebral gray matter, total cerebral white matter and total cerebral tissue for the study groups. As shown in Table 4, preterm subjects had significantly lower values for all three VBM measures than term controls. For gray matter, there was both an effect of group (p < 0.001) and gender (p < 0.001) but no significant group-by-gender interaction (p = 0.16). In addition, for both groups, females had lower values than male subjects. Similarly, there was both an effect of group (p < 0.001) and gender (p < 0.001) but no group-by-gender effect for white matter volumes (p = 0.13) and total tissue volumes (group: p < 0.001; gender: p < 0.001; interaction: p = 0.11). For both white matter and total tissue volume, females had lower volumes than male subjects.
Table 4.
Volumetric data* (cm3)
| Preterm | Term | P value | |
|---|---|---|---|
| Total cerebral gray | 659.3 ± 7.94 | 700.0 ± 8.00 | < 0.001 |
| Total cerebral white | 459.3 ± 6.14 | 494.64 ± 6.18 | < 0.001 |
| Total cerebral tissue | 1118.6 ± 12.79 | 1194.66 | < 0.001 |
Least square means ± SE; data co-varied by gender
Diffusion tensor imaging analysis
Figure 1 shows orthogonal views of the ROIs representing the left arcuate fasciculus (yellow) and the left uncinate fasciculus (white), overlying a FA map of a representative control brain.
Figure 1.
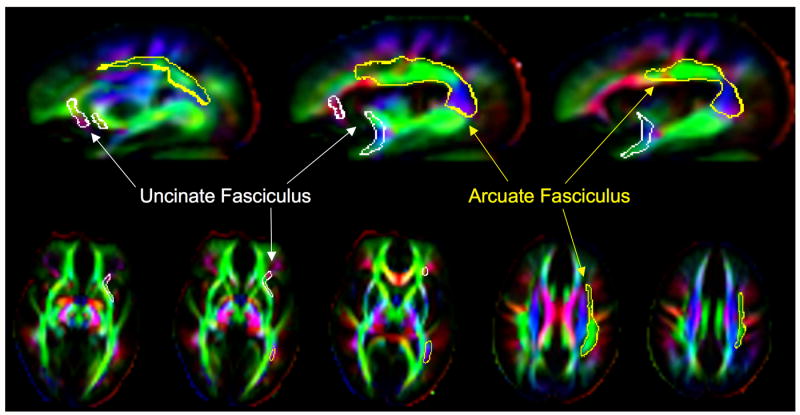
Tri-color fractional anisotropy maps of a control brain are presented in sagittal and axial projections. Colors represent directionality of tracts: blue indicates superior-inferior orientation, green indicates anterior-posterior, and red indicates left-right. Outlines of regions of interest overlie this map: the uncinate fasciculus in white and the arcuate fasciculus in yellow.
Quantitative fractional anisotropy values within a variety of regions of interest within the white matter were calculated for each cohort. Data for the language regions are presented in Table 5. Preterm subjects had significantly lower fractional anisotropy values than term subjects in left and right uncinate fasciculi (p = 0.01 and 0.004, respectively), the left and right external capsule (p < 0.001 for both) and the left and right inferior frontal gyri (left: p < 0.001; right: p = 0.011). Preterms also had significantly lower FA values than term subjects in the genu and splenium of the corpus callosum (p = 0.013 and 0.008, respectively).
Table 5.
Fractional anisotropy values for the language pathways
| Preterm | Term | p value | |
|---|---|---|---|
| Longitudinal fasciculi | |||
| Left Arcuate | 0.359 ± 0.026 | 0.356± 0.022 | 0.46 |
| Right Arcuate | 0.365 ± 0.027 | 0.361 ± 0.025 | 0.48 |
| Left Uncinate | 0.290 ± 0.022 | 0.303 ± 0.021 | 0.01 |
| Right Uncinate | 0.278 ± 0.020 | 0.290 ± 0.020 | 0.004 |
| External Capsule | |||
| Left External Capsule | 0.321 ± 0.017 | 0.347 ± 0.029 | < 0.001 |
| Right External Capsule | 0.327 ± 0.024 | 0.346 ± 0.025 | < 0.001 |
| Corpus Callosum | |||
| Genu | 0.536 ± 0.059 | 0.565 ± 0.045 | 0.013 |
| Body | 0.484 ± 0.060 | 0.500 ± 0.043 | 0.15 |
| Splenium | 0.668 ± 0.055 | 0.694 ± 0.034 | 0.008 |
| Subcortical White Matter Regions | |||
| Left Inferior Frontal Gyrus | 0.247 ± 0.022 | 0.268 ± 0.025 | <0.001 |
| Right Inferior Frontal Gyrus | 0.248 ± 0.025 | 0.262 ± 0.022 | 0.011 |
All FA values are presented as mean value ± SD
In addition, general linear model analyses were performed in which the effect of gender and total tissue volume on FA values were evaluated; results are shown in Supplemental Table 1. While the p value for the contribution of subject group (ie, preterm or term controls) to the genu of the corpus callosum increased from 0.013 (t-test) to 0.08, no other notable changes were noted with this analysis strategy. Furthermore, there were no significant gender effects in these analyses.
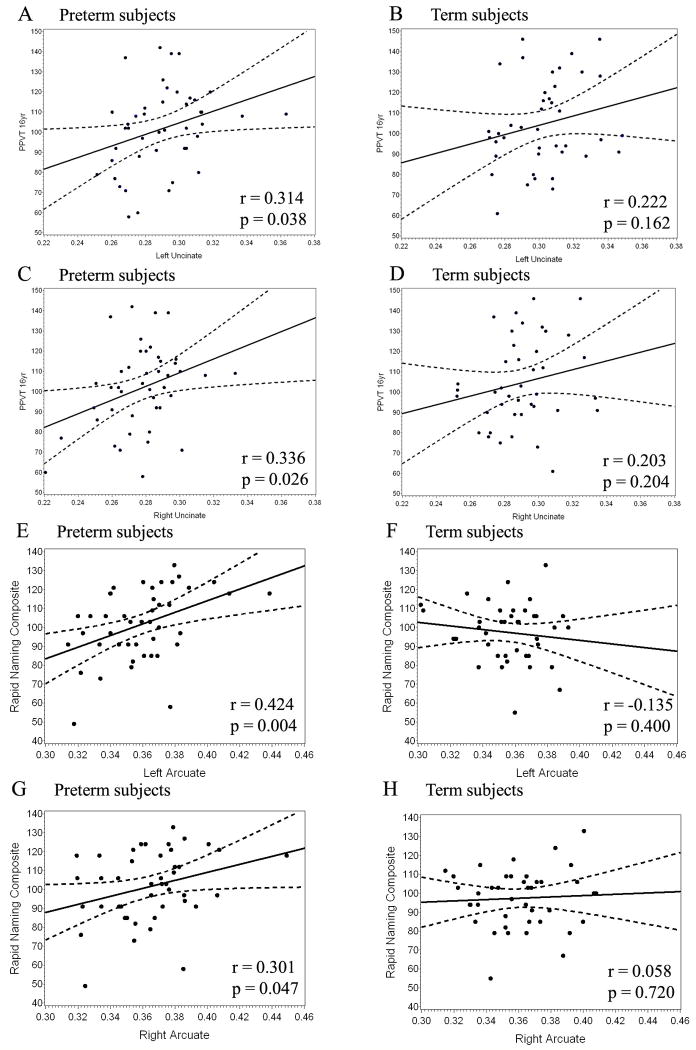
Correlational analyses
Correlations between FA in specific ROIs and selected cognitive tests for the language pathways are presented in Table 6. In analyses including both term and preterm subjects there are significant positive correlations between PPVT scores and bilateral uncinate fasciculi (left: r=0.278, p=0.01; right: r=0.278, p=0.01). There were also trends for positive correlations between Rapid Naming Composite scores and bilateral arcuate fasciculi: on the left, r=0.213; p=0.050, and on the right, r=0.208; p=0.056.
Table 6.
Pearson correlations
| All subjects | Preterm | Term | |||||
|---|---|---|---|---|---|---|---|
| Test | Region | r | p value | r | p value | R | p value |
| PPVT | L uncinate | 0.2782 | 0.01 | 0.3143 | 0.038 | 0.2223 | 0.16 |
| R uncinate | 0.2781 | 0.01 | 0.3358 | 0.026 | 0.2026 | 0.20 | |
| L external capsule | -0.0240 | 0.83 | -0.1901 | 0.22 | 0.0011 | 0.99 | |
| R external capsule | -0.1270 | 0.25 | -0.3013 | 0.047 | -0.0273 | 0.87 | |
| CTOPP Rapid Naming Composite Score | L arcuate | 0.2132 | 0.050 | 0.4238 | 0.004 | -0.1349 | 0.40 |
| R arcuate | 0.2077 | 0.056 | 0.3010 | 0.047 | 0.0578 | 0.72 | |
PPVT: Peabody Picture Vocabulary Test. CTOPP: Comprehensive Test of Phonological Processing. L: left. R: right.
Data from the preterm group alone demonstrated significant positive correlations between FA values in the left uncinate fasciculus and PPVT scores (r=0.314, p=0.038) as well as FA in the right uncinate and PPVT scores (r=0.336, p=0.026). In addition, FA values in bilateral arcuate fasciculi again show significant positive correlations with CTOPP Rapid Naming Composite scores in the preterm group (left: r=0.424, p=0.004; right: r=0.301, p=0.047). In addition, preterm subjects exhibited a negative correlation between right external capsule FA and PPVT scores (r = -0.301, p = 0.047). When analyzed in general linear models taking into account gender, age at scan, bronchopulmonary dysplasia, and birth weight, associations remained significant for preterms in bilateral arcuate fasciculi (left: R-Square=0.337, p=0.005; right: R-Square =0.2796, p=0.028) and right uncinate fasciculus (R-Square =0.171, p=0.027), with trends in the left uncinate fasciculus and the right external capsule. Graphic representations of correlations between FA values in select ROIs and scores on specific cognitive tests are presented in Figure 2.
Figure 2.
Graphs show the relationship between testing scores on the y axis (PPVT in A – D; CTOPP Rapid Naming Composite scores in E – H) and FA values in specific regions on the x axis (left uncinate in A, B; right uncinate in C, D; left arcuate in E, F; right arcuate in G, H). Data are shown for preterm subjects (A, C, E, G) and term subjects (B, D, F, G) separately. The dashed lines represent 95% confidence intervals. Each black dot represents data from a single subject.
There were no significant correlations for the term population. Positive correlations between PPVT scores and FA in left and right uncinate fasciculi did not achieve significance (left: r=0.222, p=0.16; right: r=0.203, p=0.20), while Rapid Naming Composite scores correlated poorly with FA in left and right arcuate fasciculi (left: r=-0.135, p=0.40; right: r=0.058, p=0.72). The smaller number of term subjects and lower degree of variability in FA in term subjects compared to preterm subjects may have contributed to the absence of significant correlations in this population.
There were no significant correlations for white matter volumes and either language measure for both the preterm and term control groups (data not shown; p > 0.25 for all).
Finally, general linear model analyses were performed in which the effect of regional FA, gender and total tissue volume on language outcome measures were evaluated; results are shown in Supplemental Table 2. While the p value for the contribution of the FA of the R arcuate fasciculus to the CTOPP Rapid Naming Composite Score for the preterm group increased slightly from 0.047 (Pearson’s correlation) to 0.057, no other notable changes were noted with this analysis strategy.
Discussion
These data support for the first time that the dual pathways underlying language function are present in prematurely-born subjects at late adolescence. Preterm subjects with no evidence of severe neonatal brain injury exhibit diffuse changes in cerebral white matter microstructure and total cerebral white matter volume compared to term controls at 16 years of age, yet our data show significant positive correlations between language measures and microstructural integrity in both the dorsal and ventral language pathways.
Within the ventral pathway, preterm adolescents showed significantly lower FA than term subjects in bilateral uncinate fasciculi, bilateral external capsules, and bilateral white matter regions subserving the inferior frontal gyrus contiguous with the uncinate fasciculi. Bilateral posterior segments of the inferior fronto-occipital fasciculus, which have also been implicated in semantic processing (Martino et al.), also showed FA deficits in preterm subjects. Increased FA in the uncinate fasciculus positively correlated bilaterally with performance on a semantic language task, the PPVT-R, consistent with previous descriptions of the ventral pathway in normally developing adults.
In contrast, the primary component of the dorsal, phonological language processing pathway, the arcuate fasciculus, showed no significant FA differences between term and preterm groups. FA in bilateral arcuate fasciculi correlated with CTOPP Rapid Naming Composite scores. This correlation between phonological task performance and bilateral dorsal pathway integrity among the preterm subjects, in contrast to the usual left-sided dominance found in populations of both adults and children (Hickok and Poeppel, 2007; Lebel and Beaulieu, 2009), implies that preterm subjects adaptively rely more heavily on the right hemisphere for performance of phonological tasks than typically developing subjects.
Deficits in FA in the preterm group were also noted in regions previously reported to be affected by premature birth, including the genu and splenium of the corpus callosum (Anderson et al., 2006; Caldu et al., 2006; Constable et al., 2008; Narberhaus et al., 2007). Although it is not included within the dual language pathways, corpus callosum disorganization may have implications for reading facility (Carreiras et al., 2009) and memory performance (Caldu et al., 2006).
The lack of correlations of FA values and testing measures for the term control subjects may be attributable to the earlier engrainment of language systems in the preterm group. Because they are at grave risk for neurodevelopmental handicap,(Woodward et al., 2009) premature infants receive special services including speech, language and early intervention from their earliest days(Milgrom et al., 2010; Spittle et al., 2010) and may experience significant improvement in language scores across developmental time periods.(Ment et al., 2003) Of note, preterm subjects at term equivalent age have been found to have higher FA values in corticospinal tracts than term controls,(Gimenez et al., 2008) likely due to the early use of those tracts. Similarly, early intervention has been shown to promote corticogenesis and improve testing scores in preclinical models for neonatal brain injury,(Faverjon et al., 2002) and also to increase FA values in preterm neonates exposed to early intervention therapy.(Als et al., 2004; Milgrom et al., 2010) Finally, our lack of correlation between FA and scores does not indicate that these tracts are not being used by the term controls but rather that the variation in their scores is attributable to other factors. Examining the dual visual streams in the developing brain, Loenneker demonstrated progressive maturation of both intra- and interhemispheric connectivity with increasing age in typically developing subjects.(Loenneker et al., 2010) Similarly, a variety of white matter tracts yet to be pruned may be used in our healthy term population to accomplish language tasks, while the preterms may have specialized earlier.
The development of sophisticated MR imaging strategies has permitted the investigation of those white matter pathways subserving language in the healthy human brain. DTI fiber tracking studies have demonstrated the dual language pathways in adult control subjects and confirm the left-ward dominance of the dorsal phonologic path connecting Broca’s area, L BA44 to the left superior temporal gyrus, also known as Wernicke’s area (Saur et al., 2008). Employing DTI and tractography strategies, Lebel and Beaulieu (Lebel and Beaulieu, 2009) studied the lateralization of the arcuate fasciculus in subjects ranging from ages 5 – 30 years and demonstrated that FA was significantly higher in the arcuate fasciculus in the left hemisphere compared to the right, although 10% of subjects appeared to be right-lateralized. Of note, age and gender did not appear to contribute to lateralization of this dorsal language pathway. Finally, children received cognitive assessments and were divided into three groups; a “left-only” group in which only the left arcuate could be tracked, a left-lateralized group and a right-lateralized group. In this study, scores on the NEPSY Phonological Processing task were significantly better for the left-lateralized group than the right-lateralized children.
In contrast, although task-based functional MRI and functional connectivity studies of language suggest disproportionate engagement of the right hemiphere in preterm subjects compared to term controls at school age, adolescence and young adulthood (Gozzo et al., 2009; Ment et al., 2006), microstructural studies of language are just beginning to emerge. Skranes reported decreased FA values in the external capsules and the superior, middle superior and inferior fasciculi bilaterally in 15 year old preterm subjects compared to term controls (Skranes et al., 2007). Although specific language measures were not addressed, children with low IQ had low FA values in both the external capsule and inferior and middle superior fasciculi. Similarly, our own studies of preterm subjects and term controls at age 12 years demonstrated decreases in the uncinate fasciculi bilaterally in the preterm group; FA values in the uncinate were significantly correlated with performance on a semantic task, the PPVT-R (Constable et al., 2008).
In previous studies, gender has been shown to impact preterm neurocognitive development, with preterm males at higher risk of poor outcome than preterm females (Kesler et al., 2008; Reiss et al., 2004). While our studies revealed marked gender effects at age 12 (Constable et al., 2008), few gender effects were found in our current study at age 16. Further, including gender as a covariate in general linear models associating language testing with FA did not change our results.
Limitations of our study include the sample size and the lack of advanced imaging in the neonatal period. It is probable that some of the preterm subjects had subtle white matter injury undetectable with neonatal cranial ultrasonography. In addition, we have not yet explored the impact of environmental factors, particularly level of maternal education and the impact of receiving special services. Further, the relationship between fractional anisotropy and white matter structure is still being explored; changes seen in preterm brains may be due to changes in axonal size, edema, or myelination patterns. High FA may represent structural organization which develops secondary to high levels of activity and performance within a white matter pathway; conversely, high FA may represent a necessary primary foundation for use of that pathway. Finally, unlike previous reports,(Yung et al., 2007) our data do not support a correlation between total white matter cerebral volumes and language measures, and this finding may be attributable to the age of our subjects, the testing measures examined and/or the lack of neonatal MRI data for our study subjects.
In the future, longitudinal studies including both cognitive testing and neuroimaging correlations will be helpful in confirming the sites of injury in the preterm brain and identifying biomarkers and mechanisms for recovery. The relationship between cognitive development and white matter tracts is still poorly understood and should continue to be actively investigated. Strategies for automatic segmentation of images or development of a neonatal neuroimaging atlas will be useful to engender a common language for disparate studies of closely related neuroanatomic structures. Further, our understanding of the relationship of structure to function will be refined through the use of functional MRI, particularly resting state functional connectivity MRI.
Sixteen years after the insult of preterm birth, preterm subjects continue to show impaired performance on multiple aspects of neuropsychological testing in addition to microstructural abnormalities in diverse white matter tracts. Our data provide the first evidence that dual processing systems underlie language function in adolescents born preterm. Moreover, the bilateral correlations between phonological test performance and FA of the arcuate fasciculus in our preterm population represent a departure from the left-sided lateralization of the dorsal articulatory pathway in typically developing subjects. This suggests increased utilization of the right hemisphere in preterm subjects; future studies will explore the neurobiological basis of these results.
Supplementary Material
Acknowledgments
We thank Drs. Deborah Hirtz and Walter Allan for their scientific expertise; Marjorene Ainley for the follow-up coordination; Jill Maller-Kesselman, Susan Delancy and Victoria Watson for their neurodevelopmental testing; Hedy Sarofin and Terry Hickey for their technical assistance; Mai Manchanda for statistical analysis; and Cleo O’Brien-Udry and Julia Lubsen for their assistance in fiber tracking.
This work was supported by NS 27116 (LRM) and NCRR CTSA-T32 Medical Student Research Fellowship from the National Institutes of Health (KMM).
ABBREVIATIONS
- AIFOF
Anterior inferior fronto-occipital fasciculus
- CTOPP
Comprehensive test of phonologic processing
- Cor Rad
Corona radiata
- DTI
Diffusion tensor imaging
- Ext Cap
External capsule
- FA
Fractional anisotropy
- FSIQ
Full scale IQ
- ILF
Inferior longitudinal fasciculus
- IVH
Intraventricular hemorrhage
- MFG
Middle frontal gyrus
- PCG
Precentral gyrus
- PIFOF
Posterior inferior fronto-occipital fasciculus
- PIQ
Performance IQ
- PT
Preterm
- PPVT
Peabody Picture Vocabulary Test
- ROI
Region of interest
- SFOF
Superior fronto-occipital fasciculus
- SLF
Superior longitudinal fasciculus
- STG
Superior temporal gyrus
- VBM
Voxel based morphometry
- VIQ
Verbal IQ
- VLBW
Very low birth weight
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Als H, Duffy FH, McAnulty GB, Rivkin MJ, Vajapeyam S, Mulkern RV, Warfield SK, Huppi PS, Butler SC, Conneman N, Fischer C, Eichenwald EC. Early experience alters brain function and structure. Pediatrics. 2004;113:846–857. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- Anderson NG, Laurent I, Woodward LJ, Inder TE. Detection of impaired growth of the corpus callosum in premature infants. Pediatrics. 2006;118:951–960. doi: 10.1542/peds.2006-0553. [DOI] [PubMed] [Google Scholar]
- Anjari M, Counsell SJ, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, Edwards AD. The association of lung disease with cerebral white matter abnormalities in preterm infants. Pediatrics. 2009;124:268–276. doi: 10.1542/peds.2008-1294. [DOI] [PubMed] [Google Scholar]
- Anjari M, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, Edwards AD, Counsell SJ. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. Neuroimage. 2007;35:1021–1027. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- Arzoumanian Y, Mirmiran M, Barnes PD, Woolley K, Ariagno RL, Moseley ME, Fleisher BE, Atlas SW. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. AJNR Am J Neuroradiol. 2003;24:1646–1653. [PMC free article] [PubMed] [Google Scholar]
- Assaf Y, Blumenfeld-Katzir T, Yovel Y, Basser PJ. AxCaliber: a method for measuring axon diameter distribution from diffusion MRI. Magn Reson Med. 2008;59:1347–1354. doi: 10.1002/mrm.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi L, Ricci D, Volzone A, Allsop JM, Srinivasan L, Pai A, Ribes C, Ramenghi LA, Mercuri E, Mosca F, Edwards AD, Cowan FM, Rutherford MA, Counsell SJ. Probabilistic diffusion tractography of the optic radiations and visual function in preterm infants at term equivalent age. Brain. 2008;131:573–582. doi: 10.1093/brain/awm327. [DOI] [PubMed] [Google Scholar]
- Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PF. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88:31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery KE. The VMI Developmental Test of Visual Motor Integration. Modern Curriculum Press; Cleveland, OH: 1989. [Google Scholar]
- Berman JI, Glass HC, Miller SP, Mukherjee P, Ferriero DM, Barkovich AJ, Vigneron DB, Henry RG. Quantitative fiber tracking analysis of the optic radiation correlated with visual performance in premature newborns. AJNR Am J Neuroradiol. 2009;30:120–124. doi: 10.3174/ajnr.A1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JI, Mukherjee P, Partridge SC, Miller SP, Ferriero DM, Barkovich AJ, Vigneron DB, Henry RG. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage. 2005;27:862–871. doi: 10.1016/j.neuroimage.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Caldu X, Narberhaus A, Junque C, Gimenez M, Vendrell P, Bargallo N, Segarra D, Botet F. Corpus callosum size and neuropsychologic impairment in adolescents who were born preterm. J Child Neurol. 2006;21:406–410. doi: 10.1177/08830738060210050801. [DOI] [PubMed] [Google Scholar]
- Cao F, Peng D, Liu L, Jin Z, Fan N, Deng Y, Booth JR. Developmental differences of neurocognitive networks for phonological and semantic processing in Chinese word reading. Hum Brain Mapp. 2009;30:797–809. doi: 10.1002/hbm.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreiras M, Seghier ML, Baquero S, Estevez A, Lozano A, Devlin JT, Price CJ. An anatomical signature for literacy. Nature. 2009;461:983–986. doi: 10.1038/nature08461. [DOI] [PubMed] [Google Scholar]
- Cheong JL, Thompson DK, Wang HX, Hunt RW, Anderson PJ, Inder TE, Doyle LW. Abnormal white matter signal on MR imaging is related to abnormal tissue microstructure. AJNR Am J Neuroradiol. 2009;30:623–628. doi: 10.3174/ajnr.A1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TL, Booth JR, Burman DD, Bitan T, Bigio JD, Lu D, Cone NE. Developmental changes in the neural correlates of semantic processing. Neuroimage. 2006;29:1141–1149. doi: 10.1016/j.neuroimage.2005.09.064. [DOI] [PubMed] [Google Scholar]
- Constable RT, Ment LR, Vohr BR, Kesler SR, Fulbright RK, Lacadie C, Delancy S, Katz KH, Schneider KC, Schafer RJ, Makuch RW, Reiss AR. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121:306–316. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- Counsell SJ, Dyet LE, Larkman DJ, Nunes RG, Boardman JP, Allsop JM, Fitzpatrick J, Srinivasan L, Cowan FM, Hajnal JV, Rutherford MA, Edwards AD. Thalamo-cortical connectivity in children born preterm mapped using probabilistic magnetic resonance tractography. Neuroimage. 2007;34:896–904. doi: 10.1016/j.neuroimage.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Counsell SJ, Edwards AD, Chew AT, Anjari M, Dyet LE, Srinivasan L, Boardman JP, Allsop JM, Hajnal JV, Rutherford MA, Cowan FM. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131:3201–3208. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- Drobyshevsky A, Bregman J, Storey P, Meyer J, Prasad PV, Derrick M, MacKendrick W, Tan S. Serial diffusion tensor imaging detects white matter changes that correlate with motor outcome in premature infants. Dev Neurosci. 2007;29:289–301. doi: 10.1159/000105470. [DOI] [PubMed] [Google Scholar]
- Dudink J, Lequin M, van Pul C, Buijs J, Conneman N, van Goudoever J, Govaert P. Fractional anisotropy in white matter tracts of very-low-birth-weight infants. Pediatr Radiol. 2007;37:1216–1223. doi: 10.1007/s00247-007-0626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. PPVT Peabody Picture Vocabulary Test-revised Form. American Guidance Service; Circle Pines, MN: 1981. [Google Scholar]
- Dyet LE, Kennea N, Counsell SJ, Maalouf EF, Ajayi-Obe M, Duggan PJ, Harrison M, Allsop JM, Hajnal J, Herlihy AH, Edwards B, Laroche S, Cowan FM, Rutherford MA, Edwards AD. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118:536–548. doi: 10.1542/peds.2005-1866. [DOI] [PubMed] [Google Scholar]
- Faverjon S, Silveira DC, Fu DD, Cha BH, Akman C, Hu Y, Holmes GL. Beneficial effects of enriched environment following status epilepticus in immature rats. Neurology. 2002;59:1356–1364. doi: 10.1212/01.wnl.0000033588.59005.55. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Pathways to language: fiber tracts in the human brain. Trends Cogn Sci. 2009;13:175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Gimenez M, Junque C, Narberhaus A, Bargallo N, Botet F, Mercader JM. White matter volume and concentration reductions in adolescents with history of very preterm birth: a voxel-based morphometry study. Neuroimage. 2006;32:1485–1498. doi: 10.1016/j.neuroimage.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Gimenez M, Miranda MJ, Born AP, Nagy Z, Rostrup E, Jernigan TL. Accelerated cerebral white matter development in preterm infants: a voxel-based morphometry study with diffusion tensor MR imaging. Neuroimage. 2008;41:728–734. doi: 10.1016/j.neuroimage.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Gozzo Y, Vohr B, Lacadie C, Hampson M, Katz KH, Maller-Kesselman J, Schneider KC, Peterson BS, Rajeevan N, Makuch RW, Constable RT, Ment LR. Alterations in neural connectivity in preterm children at school age. Neuroimage. 2009;48:458–463. doi: 10.1016/j.neuroimage.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack M. Adult outcomes of preterm children. J Dev Behav Pediatr. 2009;30:460–470. doi: 10.1097/DBP.0b013e3181ba0fba. [DOI] [PubMed] [Google Scholar]
- Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, Klein N. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346:149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA, Volpe JJ. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res. 1998;44:584–590. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Murphy B, Maier SE, Zientara GP, Inder TE, Barnes PD, Kikinis R, Jolesz FA, Volpe JJ. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics. 2001;107:455–460. doi: 10.1542/peds.107.3.455. [DOI] [PubMed] [Google Scholar]
- Kesler SR, Reiss AL, Vohr B, Watson C, Schneider KC, Katz KH, Maller-Kesselman J, Silbereis J, Constable RT, Makuch RW, Ment LR. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J Pediatr. 2008;152:513–520. 520–e511. doi: 10.1016/j.jpeds.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontis D, Catani M, Cuddy M, Walshe M, Nosarti C, Jones D, Wyatt J, Rifkin L, Murray R, Allin M. Diffusion tensor MRI of the corpus callosum and cognitive function in adults born preterm. Neuroreport. 2009;20:424–428. doi: 10.1097/WNR.0b013e328325a8f9. [DOI] [PubMed] [Google Scholar]
- Krishnan ML, Dyet LE, Boardman JP, Kapellou O, Allsop JM, Cowan F, Edwards AD, Rutherford MA, Counsell SJ. Relationship between white matter apparent diffusion coefficients in preterm infants at term-equivalent age and developmental outcome at 2 years. Pediatrics. 2007;120:e604–609. doi: 10.1542/peds.2006-3054. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Hum Brain Mapp. 2009;30:3563–3573. doi: 10.1002/hbm.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenneker T, Klaver P, Bucher K, Lichtensteiger J, Imfeld A, Martin E. Microstructural development: Organizational differences of the fiber architecture between children and adults in dorsal and ventral visual streams. Hum Brain Mapp. 2010 doi: 10.1002/hbm.21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu TM, Ment LR, Schneider KC, Katz KH, Allan WC, Vohr BR. Lasting effects of preterm birth and neonatal brain hemorrhage at 12 years of age. Pediatrics. 2009;123:1037–1044. doi: 10.1542/peds.2008-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Kung HC, Mathews TJ, Hoyert DL, Strobino DM, Guyer B, Sutton SR. Annual summary of vital statistics: 2006. Pediatrics. 2008;121:788–801. doi: 10.1542/peds.2007-3753. [DOI] [PubMed] [Google Scholar]
- Martino J, Brogna C, Robles SG, Vergani F, Duffau H. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 46:691–699. doi: 10.1016/j.cortex.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Ment LR, Oh W, Ehrenkranz RA, Philip AG, Vohr B, Allan W, Duncan CC, Scott DT, Taylor KJ, Katz KH, et al. Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics. 1994a;93:543–550. [PubMed] [Google Scholar]
- Ment LR, Oh W, Ehrenkranz RA, Phillip AG, Vohr B, Allan W, Makuch RW, Taylor KJ, Schneider KC, Katz KH, et al. Low-dose indomethacin therapy and extension of intraventricular hemorrhage: a multicenter randomized trial. J Pediatr. 1994b;124:951–955. doi: 10.1016/s0022-3476(05)83191-9. [DOI] [PubMed] [Google Scholar]
- Ment LR, Peterson BS, Vohr B, Allan W, Schneider KC, Lacadie C, Katz KH, Maller-Kesselman J, Pugh K, Duncan CC, Makuch RW, Constable RT. Cortical recruitment patterns in children born prematurely compared with control subjects during a passive listening functional magnetic resonance imaging task. J Pediatr. 2006;149:490–498. doi: 10.1016/j.jpeds.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment LR, Vohr B, Allan W, Katz KH, Schneider KC, Westerveld M, Duncan CC, Makuch RW. Change in cognitive function over time in very low-birth-weight infants. Jama. 2003;289:705–711. doi: 10.1001/jama.289.6.705. [DOI] [PubMed] [Google Scholar]
- Milgrom J, Newnham C, Anderson PJ, Doyle LW, Gemmill AW, Lee K, Hunt RW, Bear M, Inder T. Early Sensitivity Training for Parents of Preterm Infants: Impact on the Developing Brain. Pediatr Res. 2010;67:330–335. doi: 10.1203/PDR.0b013e3181cb8e2f. [DOI] [PubMed] [Google Scholar]
- Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC, Perez M, Mukherjee P, Vigneron DB, Barkovich AJ. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147:609–616. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Miller SP, Vigneron DB, Henry RG, Bohland MA, Ceppi-Cozzio C, Hoffman C, Newton N, Partridge JC, Ferriero DM, Barkovich AJ. Serial quantitative diffusion tensor MRI of the premature brain: development in newborns with and without injury. J Magn Reson Imaging. 2002;16:621–632. doi: 10.1002/jmri.10205. [DOI] [PubMed] [Google Scholar]
- Murakami A, Morimoto M, Yamada K, Kizu O, Nishimura A, Nishimura T, Sugimoto T. Fiber-tracking techniques can predict the degree of neurologic impairment for periventricular leukomalacia. Pediatrics. 2008;122:500–506. doi: 10.1542/peds.2007-2816. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Skare S, Andersson JL, Lilja A, Flodmark O, Fernell E, Holmberg K, Bohm B, Forssberg H, Lagercrantz H, Klingberg T. Preterm children have disturbances of white matter at 11 years of age as shown by diffusion tensor imaging. Pediatr Res. 2003;54:672–679. doi: 10.1203/01.PDR.0000084083.71422.16. [DOI] [PubMed] [Google Scholar]
- Narberhaus A, Segarra D, Caldu X, Gimenez M, Junque C, Pueyo R, Botet F. Gestational age at preterm birth in relation to corpus callosum and general cognitive outcome in adolescents. J Child Neurol. 2007;22:761–765. doi: 10.1177/0883073807304006. [DOI] [PubMed] [Google Scholar]
- Neubauer AP, Voss W, Kattner E. Outcome of extremely low birth weight survivors at school age: the influence of perinatal parameters on neurodevelopment. Eur J Pediatr. 2008;167:87–95. doi: 10.1007/s00431-007-0435-x. [DOI] [PubMed] [Google Scholar]
- Parker GJ, Luzzi S, Alexander DC, Wheeler-Kingshott CA, Ciccarelli O, Lambon Ralph MA. Lateralization of ventral and dorsal auditory-language pathways in the human brain. Neuroimage. 2005;24:656–666. doi: 10.1016/j.neuroimage.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Partridge SC, Mukherjee P, Henry RG, Miller SP, Berman JI, Jin H, Lu Y, Glenn OA, Ferriero DM, Barkovich AJ, Vigneron DB. Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. Neuroimage. 2004;22:1302–1314. doi: 10.1016/j.neuroimage.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Kane MJ, Whalen DH, Schneider KC, Katz KH, Zhang H, Duncan CC, Makuch R, Gore JC, Ment LR. A functional magnetic resonance imaging study of language processing and its cognitive correlates in prematurely born children. Pediatrics. 2002;110:1153–1162. doi: 10.1542/peds.110.6.1153. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. Jama. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Kesler SR, Vohr B, Duncan CC, Katz KH, Pajot S, Schneider KC, Makuch RW, Ment LR. Sex differences in cerebral volumes of 8-year-olds born preterm. J Pediatr. 2004;145:242–249. doi: 10.1016/j.jpeds.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Rose J, Butler EE, Lamont LE, Barnes PD, Atlas SW, Stevenson DK. Neonatal brain structure on MRI and diffusion tensor imaging, sex, and neurodevelopment in very-low-birthweight preterm children. Dev Med Child Neurol. 2009;51:526–535. doi: 10.1111/j.1469-8749.2008.03231.x. [DOI] [PubMed] [Google Scholar]
- Rose J, Mirmiran M, Butler EE, Lin CY, Barnes PD, Kermoian R, Stevenson DK. Neonatal microstructural development of the internal capsule on diffusion tensor imaging correlates with severity of gait and motor deficits. Dev Med Child Neurol. 2007;49:745–750. doi: 10.1111/j.1469-8749.2007.00745.x. [DOI] [PubMed] [Google Scholar]
- Rose SE, Hatzigeorgiou X, Strudwick MW, Durbridge G, Davies PS, Colditz PB. Altered white matter diffusion anisotropy in normal and preterm infants at term-equivalent age. Magn Reson Med. 2008;60:761–767. doi: 10.1002/mrm.21689. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, Umarova R, Musso M, Glauche V, Abel S, Huber W, Rijntjes M, Hennig J, Weiller C. Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A. 2008;105:18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skranes J, Vangberg TR, Kulseng S, Indredavik MS, Evensen KA, Martinussen M, Dale AM, Haraldseth O, Brubakk AM. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130:654–666. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- Spittle AJ, Anderson PJ, Lee KJ, Ferretti C, Eeles A, Orton J, Boyd RN, Inder T, Doyle LW. Preventive care at home for very preterm infants improves infant and caregiver outcomes at 2 years. Pediatrics. 2010;126:e171–178. doi: 10.1542/peds.2009-3137. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of Word Reading Efficiency (TOWRE) Pro-Ed; Austin, TX: 1999. [Google Scholar]
- Vangberg TR, Skranes J, Dale AM, Martinussen M, Brubakk AM, Haraldseth O. Changes in white matter diffusion anisotropy in adolescents born prematurely. Neuroimage. 2006;32:1538–1548. doi: 10.1016/j.neuroimage.2006.04.230. [DOI] [PubMed] [Google Scholar]
- Vohr BR, Allan WC, Westerveld M, Schneider KC, Katz KH, Makuch RW, Ment LR. School-age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 2003;111:e340–346. doi: 10.1542/peds.111.4.e340. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. The Comprehensive Test of Phonological Processing. PRO-ED, Inc.; Austin, TX: 1999. [Google Scholar]
- Woodward LJ, Moor S, Hood KM, Champion PR, Foster-Cohen S, Inder TE, Austin NC. Very preterm children show impairments across multiple neurodevelopmental domains by age 4 years. Arch Dis Child Fetal Neonatal Ed. 2009;94:F339–344. doi: 10.1136/adc.2008.146282. [DOI] [PubMed] [Google Scholar]
- Yung A, Poon G, Qiu DQ, Chu J, Lam B, Leung C, Goh W, Khong PL. White matter volume and anisotropy in preterm children: a pilot study of neurocognitive correlates. Pediatr Res. 2007;61:732–736. doi: 10.1203/pdr.0b013e31805365db. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.