Abstract
Objectives
Activation of macrophages may contribute to increased atherosclerosis and coronary artery disease in systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). Neopterin, a pteridine derivative, is a novel marker of monocyte and macrophage activation that is associated with atherosclerosis and cardiovascular risk in the general population. We examined the hypothesis that macrophage activation is associated with accelerated atherosclerosis in SLE and RA.
Methods
We compared serum neopterin concentrations, adjusted for age, race, sex and serum creatinine concentration, in patients with SLE (n=148), RA (n=166) and control subjects (n=177). In patients with SLE or RA, serum neopterin concentrations were then tested for association (adjusted for age, race, sex, serum creatinine and medication use) with measures of disease activity or damage, inflammatory markers and mediators, and coronary artery calcium measured by electron beam computed tomography.
Results
Neopterin concentrations were significantly higher in patients with SLE (median 8.0, IQR [6.5–9.8] nmol/l) and RA (6.7[5.3–8.9] nmol/l) than controls (5.7[4.8–7.1] nmol/l), and were higher in SLE than RA (all p<0.001). In SLE, neopterin was significantly correlated with higher ESR (p=0.001), TNF-α (p<0.001), MCP-1 (p=0.005) and homocysteine concentrations (p=0.01), but in RA only with ESR (p=0.01). Neopterin was not associated with coronary calcium in either SLE (p=0.65) or RA (p=0.21).
Conclusion
Macrophage activation, reflected by increased serum neopterin concentrations, was increased in both SLE and RA. Neopterin was more robustly associated with atherogenic mediators of inflammation and homocysteine in SLE than RA but was not associated with coronary atherosclerosis in either disease.
Keywords: Systemic Lupus Erythematosus, Rheumatoid Arthritis, Neopterin, Homocysteine, Atherosclerosis
Introduction
Morbidity and mortality due to coronary artery disease are increased in both systemic lupus erythematosus (SLE) (1) and rheumatoid arthritis (RA) (2). The mechanisms underlying these increases are unclear, but inflammation is thought to play a role. The involvement of macrophages in the atherosclerosis associated with SLE and RA has not been studied extensively, but is important since monocytes and macrophages play a key role in the early pathogenesis of atherosclerosis by ingesting oxidized LDL, transforming into foam cells, and recruiting additional monocytes and macrophages to the vessel wall (3).
Neopterin, a pteridine derivative, is a marker of macrophage activation. It is formed by GTP cyclohydrolase I that catabolizes GTP into 7,8-dihydroneopterintriphosphate, which is further metabolized to neopterin (4). Interferon-gamma (IFN-γ) is the most important inducer of GTP cyclohydrolase I, and therefore of neopterin formation. Thus, neopterin is considered a marker of TH1 immune activation (4). Atherosclerosis has been described as a TH1 mediated disease (3), and higher concentrations of neopterin are associated with increased cardiovascular risk and atherosclerosis in the general population (5–8).
Several small studies indicate that concentrations of neopterin are elevated, or correlated with disease activity, in patients with SLE (9–13) or RA (14–18), but the association between neopterin and quantitative measures of atherosclerosis such as coronary artery calcification or carotid artery intima-media thickness (IMT) has not been tested in these diseases. Thus, we examined the hypothesis that macrophage activation is associated with atherosclerosis in SLE and RA by assessing whether neopterin concentrations are: 1) higher in patients with SLE or RA compared to controls; 2) associated with disease activity, inflammatory mediators of cardiovascular risk, and coronary calcium in patients with SLE or RA.
Methods
Study Population
We studied patients with SLE (n=148), RA (n=166) and control subjects (n=177). All subjects were 18 years of age or older and patients fulfilled the ACR classification criteria for SLE (19) or RA (20), respectively. The subjects are participants in ongoing studies of cardiovascular risk in cohorts of patients with SLE or RA; details regarding recruitment and methodological procedures have been described (1,2). By design, there were two separate groups of control subjects without inflammatory disease that were frequency-matched for age, race and sex with patients with SLE or RA, respectively (1,2). In the present study we combined these to form a single control group to allow comparison of neopterin concentrations between the three study groups with statistical adjustment for demographic variables. The study was approved by the Vanderbilt University Institutional Review Board and subjects gave written informed consent.
Measurements
Clinical information, laboratory data, current and past medication use and Agatston coronary calcium scores were obtained as described previously (1,2,21,22). In brief, coronary calcium was measured by electron beam computed tomography (EBCT) scanning with an Imatron C-150 scanner (GE/Imatron) except for 37 patients with SLE and 5 controls in whom a 64-row mulitdetector CT (LightSpeed VCT, General Electric, Milwaukee, WI) was used. For logistic reasons scans were not performed in 3 patients. Coronary calcium was quantified as described by Agatston (23) by a single reviewer (PR) blinded to the clinical status of the subjects. Clinical indices of disease activity and damage including the DAS28 (24), SLEDAI (25) and SLICC (26) scores were measured. Medication use was classified according to whether or not patients were currently taking corticosteroids, methotrexate (MTX) or antimalarials (both SLE and RA), or anti-TNF agents (only RA). Erythrocyte sedimentation rate (ESR), plasma homocysteine and serum C-reactive protein (CRP) concentrations were determined in the Vanderbilt University Hospital clinical laboratory. Before 2003, the laboratory did not use a high-sensitivity CRP assay, and low concentrations were reported as <3 mg/l; in 40 patients with SLE and 40 with RA who had CRP concentrations <3 mg/l, CRP concentrations were measured by multiplex ELISA (Lincoplex® Multiplex Immunoassay Kit, Millipore). Serum concentrations of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1) were measured by multiplex ELISA (Millipore). Serum neopterin was measured by ELISA (ALPCO Diagnostics).
Statistical Analysis
Descriptive statistics were calculated as frequencies and proportion, mean with standard deviation (mean ± SD) or median with interquartile range (median [IQR]) according to the distribution of the variables. Demographic and clinical factors were compared by disease group using a Kruskal-Wallis test or Wilcoxon rank-sum test, or a Pearson chi-square test as appropriate.
Serum neopterin concentrations were compared between patients with SLE, RA and control subjects using the Kruskal-Wallis test or Wilcoxon rank-sum test for comparisons. A multivariable linear regression model was used to adjust for age, sex, race, and also serum creatinine as renal function can affect neopterin concentrations (27).
Drugs used for the treatment of SLE and RA affect inflammatory activity, thus we compared neopterin concentrations separately in patients with SLE or RA who were currently receiving corticosteroids, MTX, antimalarials (both SLE and RA) and anti-TNF agents (only RA), and those who were not. Each comparison was performed using multivariable linear regression adjusting for age, race, sex, serum creatinine, and use of the other drugs. For example, in SLE, when neopterin concentrations were compared in patients taking or not taking corticosteroids, the model was adjusted for use of MTX and antimalarials. In RA, the analysis for corticosteroids was adjusted for use of MTX, antimalarials and anti-TNF agents.
Neopterin concentrations were examined for correlation with indices of disease activity and damage (SLE: SLEDAI and SLICC, RA: DAS28), inflammatory mediators (TNF-α, IL-6, MCP-1, ESR and CRP), homocysteine, and coronary calcium score using Spearman’s correlation coefficient in patients with SLE or RA. The relationships were further tested with adjustment for age, sex, race, serum creatinine, current corticosteroid, MTX and antimalarial use in SLE, and additionally for anti-TNF agent use in RA, using either multiple linear regression (inflammatory mediators, DAS28 and homocysteine as the outcome variables) or proportional odds logistic regression (28). The proportional odds model was applied both to skewed continuous variables by using ranks for cut-points and also to skewed ordinal variables including SLEDAI, SLICC, and coronary calcium.
Values for concentrations of inflammatory mediators, neopterin, homocysteine and creatinine were log10-transformed when used in multivariate regression analysis in order to normalize the distribution. Statistical analysis was performed using R 2.9.2 (www.r-project.org). All tests were two-sided and a p value of <0.05 was considered significant.
Results
The clinical characteristics of patients with SLE or RA and control subjects are shown in Table 1. As expected, patients with SLE were younger and more likely to be female than patients with RA. As we have reported previously, concentrations of inflammatory mediators were higher in patients with RA or SLE than control subjects (22,29), and serum IL-6 concentrations were higher in RA than SLE (21). Only 1.7% of controls, 2.7% of SLE and 1.8% of RA patients had a serum creatinine concentration >1.5 mg/dl.
Table 1.
Characteristics of the Study Population
| SLE (N=148) | RA (N=166) | Controls (N=177) | P-value* | |
|---|---|---|---|---|
| Age (Years) | 40[30–48]§† | 54[45–63]† | 47[39–55] | <0.001 |
| Sex (Males %) | 9.5%§† | 31.3% | 25.4% | <0.001 |
| Race (Caucasian %) | 66.9%§† | 88.6%† | 78.5% | <0.001 |
| Hypertension (%) | 44.6%† | 53.0%† | 28.8% | <0.001 |
| Diabetes (%) | 4.7% | 11.4%† | 2.8% | 0.003 |
| Current Smoking (%) | 22.3% | 23.5%† | 13.6% | 0.04 |
| HDL (mg/dl) | 48[36–56] | 43[37–54] | 45[38–46] | 0.35 |
| LDL (mg/dl) | 94[76–126]§† | 111[88–134] | 117[95–138] | <0.001 |
| Triglycerides (mg/dl) | 99.5[73.2–147.0] | 112.0[80.0–158.0]† | 93.0[65.0–127.0] | 0.003 |
| Creatinine (mg/dl) | 0.8[0.7–0.9] | 0.8[0.7–0.9] | 0.8[0.7–0.9] | 0.81 |
| Agatston Score | 0 [0–0.7]¶ | 2.7[0–150.4]¶ | 0 [0–0] | <0.001 |
| SLEDAI | 4 [0–6] | - | - | - |
| SLICC | 1 [0–2] | - | - | - |
| DAS28 | - | 3.89[2.66–4.90] | - | - |
| TNF-α (pg/ml) | 4.8[3.1–7.4]† | 5.6[2.8–11.0]† | 2.7[2.0–3.9] | <0.001 |
| IL-6 (pg/ml) | 5.5[1.9–26.2]§† | 13.9[4.4–43.6]† | 2.5[1.0–10.0] | <0.001 |
| MCP-1 (pg/ml) | 188.2[124.7–294.6]† | 201.8[145.1–289.3]† | 144.1[98.8–206.1] | <0.001 |
| CRP (mg/l) | 3.6[0.8–7.0]§ | 4.0[1.2–10.8] | - | |
| ESR (mm/hr) | 20.0[9.0–34.0] | 16.0[7.0–36.0] | - | |
| Homocysteine (umol/l) | 9.2[7.7–11.2]§† | 10.2[8.1–11.9]† | 8.1[6.9–9.5] | <0.001 |
| Neopterin (nmol/l) | 8.0[6.5–9.8]§† | 6.7[5.3–8.9]† | 5.7[4.8–7.1] | <0.001 |
Data are shown as median [IQR] or percentages(%).
TNF-α, IL-6 and MCP-1 were available in 109 of 148 subjects with SLE, 163 of 166 with RA, and 172 of 177 Controls.
Continuous variables compared with Kruskal-Wallis test and categorical variables with Pearson Chi-Square Test (across all 3 groups)
p<0.05, Wilcoxon rank-sum test comparing SLE and RA.
p<0.05, Wilcoxon rank-sum test comparing SLE and controls, or RA and controls.
P<0.001, proportional odds logistic regression model comparing SLE vs. controls, or RA vs. controls adjusted for age, race and sex.
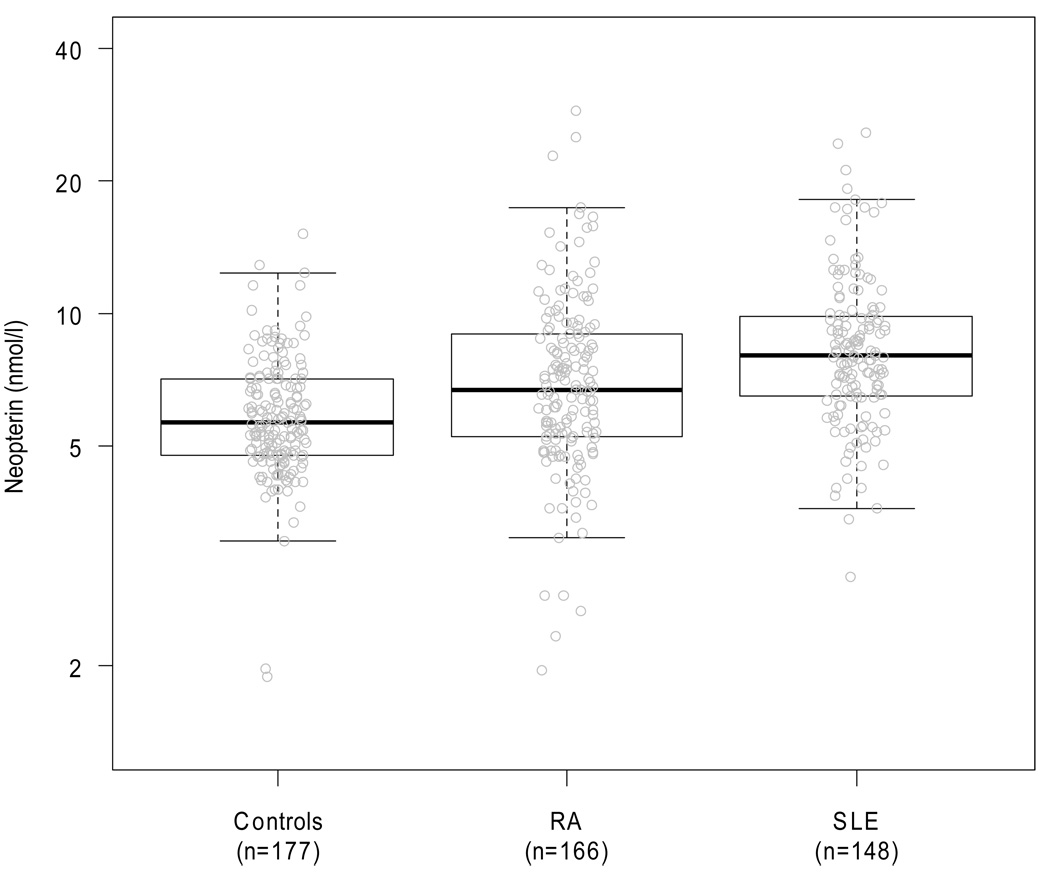
Serum neopterin concentrations were significantly higher in patients with SLE or RA than in controls, and were higher in SLE than in RA (all p values <0.001, Table 1, Figure 1). These differences remained significant after adjusting for age, race, sex and serum creatinine (all p values <0.001). Serum creatinine was significantly associated with higher neopterin values (p <0.001) in this model.
Figure 1. Distribution of Neopterin Concentrations in Control Subjects and Patients with SLE and RA.
In the boxplot the horizontal bold line and the upper and lower hinges of the box represent the median and the interquartile range. The whiskers extends to 1.5 times the upper and lower interquartile range. Neopterin concentrations were significantly higher in patients with SLE or RA compared to controls, and in patients with SLE compared to those with RA (all p<0.001 with or without adjustment).
Medications were associated with neopterin concentration (Table 2). Current antimalarial therapy in SLE and current MTX use in RA were associated with lower neopterin concentrations. Anti-TNF agents did not affect neopterin levels in RA (p=0.18).
Table 2.
Neopterin Concentrations in Patients with SLE and RA and Current Medications
| Current Medication Use | Neopterin (SLE) | Neopterin (RA) | |||||
|---|---|---|---|---|---|---|---|
| n | (nmol/l) | P* | n | (nmol/l) | P* | ||
| Corticosteroids | |||||||
| No | 63 | 7.6 [5.9–9.8] | 0.21 | 76 | 6.6 [5.4–8.6] | 0.94 | |
| Yes | 85 | 8.5 [7.1–9.8] | 90 | 7.0 [4.9–8.9] | |||
| Methotrexate | |||||||
| No | 134 | 8.0 [6.5–9.8] | 0.87 | 48 | 7.6 [6.0–10.7] | 0.01 | |
| Yes | 14 | 8.6 [7.1–11.7] | 118 | 6.4 [4.9–8.3] | |||
| Antimalarials | |||||||
| No | 49 | 8.7 [7.3–12.2] | 0.03 | 124 | 7.1 [5.5–9.3] | 0.12 | |
| Yes | 99 | 7.7 [6.3–9.3] | 42 | 5.8 [4.8–7.5] | |||
| Anti-TNF agents | |||||||
| No | - | - | - | 132 | 6.9 [5.3–9.2] | 0.18 | |
| Yes | - | - | - | 34 | 6.2 [5.0–8.0] | ||
Adjusted P value for age, sex, race, serum creatinine and the current use of other drugs.
The correlations between neopterin and disease indices, inflammatory mediators, homocysteine and coronary calcium score in patients with SLE or RA are shown in Tables 3 and 4, respectively. In SLE neopterin correlated significantly with TNF-α (rho=0.42, adjusted p<0.001), MCP-1 (rho=0.31, adjusted p=0.005), ESR (rho=0.33, adjusted p=0.001) and homocysteine (rho=0.20, adjusted p=0.01), but in RA neopterin correlated only with ESR (rho=0.21, adjusted p=0.01). There were marginal correlations between neopterin and SLICC in SLE (Table 3), and DAS28 in RA (Table 4). The association between SLICC score and neopterin in SLE was attenuated by statistical adjustment that included serum creatinine in the model. Similarly, the univariate correlation between homocysteine and neopterin in RA (rho=0.24, p=0.002), was attenuated by adjusting for serum creatinine (p=0.11). Neopterin was not associated with coronary calcium in either SLE (p=0.65) or RA (p=0.21) in adjusted analyses. When we additionally adjusted the results from Table 3 and 4 for disease activity (SLEDAI and DAS28, respectively) the statistical significance of association between neopterin and coronary calcification did not change (SLE, p=0.70, RA, p=0.22).
Table 3.
Correlations between Neopterin and Disease Indices, Inflammatory Mediators and Coronary Calcium in SLE
| Factor | Rho | p | Adjusted p† |
|---|---|---|---|
| SLEDAI | 0.08 | 0.34 | 0.49* |
| SLICC | 0.19 | 0.02 | 0.08* |
| TNF-α | 0.42 | <0.001 | <0.001 |
| IL-6 | 0.22 | 0.02 | 0.22 |
| MCP-1 | 0.31 | 0.001 | 0.005 |
| CRP | 0.06 | 0.48 | 0.21 |
| ESR | 0.33 | <0.001 | 0.001 |
| Homocysteine | 0.20 | 0.01 | 0.01 |
| Agatston Score | 0.02 | 0.77 | 0.65* |
Rho is the Spearman correlation coefficient
Adjusted for age, sex, race and creatinine and current corticosteroid, MTX and antimalarial use with multivariable linear regression.
Used proportional odds logistic regression.
Table 4.
Correlations between Neopterin and Disease Indices, Inflammatory Mediators and Coronary Calcium in RA
| Factor | Rho | p | Adjusted p† |
|---|---|---|---|
| DAS28 | 0.14 | 0.07 | 0.08 |
| TNF-α | 0.17 | 0.03 | 0.22 |
| IL-6 | 0.04 | 0.63 | 0.75 |
| MCP-1 | 0.12 | 0.13 | 0.24 |
| CRP | 0.13 | 0.10 | 0.07 |
| ESR | 0.21 | 0.01 | 0.01 |
| Homocysteine | 0.24 | 0.002 | 0.11 |
| Agatston Score | 0.20 | 0.01 | 0.21* |
Rho is the Spearman correlation coefficient
Adjusted for age, sex, race and creatinine and current corticosteroid, MTX, antimalarial use and anti-TNF agent use with multivariable linear regression.
Used proportional odds logistic regression.
Discussion
The major finding of this study is that concentrations of neopterin, a marker of macrophage activation and IFN-γ activity, are increased in large cohorts of patients with SLE or RA, and have a more robust association with mediators of inflammation and homocysteine in SLE than in RA.
Previous studies of neopterin in SLE (9–13) and RA (14–17) have been limited largely to defining the relationship between neopterin and disease activity. Generally, they found that neopterin correlated with disease activity in both SLE and RA, and decreased with treatment. Thus, neopterin has been considered as a candidate biomarker for monitoring disease activity. However, these studies most often used the urinary neopterin : creatinine ratio as a biomarker. This ratio adjusts neopterin excretion for variation in urine concentration rather than renal function (30,31). We found that neopterin correlated marginally with DAS28 in RA, but not with SLEDAI or SLICC in SLE. The SLICC score measures damage in SLE and encompasses renal damage. The lack of association between neopterin and the SLICC score after adjustment that included serum creatinine was likely due to the fact that both were positively associated with serum creatinine concentrations. The influence of renal function, as well as medications, on neopterin concentrations suggests that its usefulness as a biomarker for disease activity is limited.
The lower concentrations of neopterin found in patients with SLE taking antimalarials and in those with RA taking MTX suggest that these drugs may inhibit monocyte and macrophage activation. Such associations are interesting, but should be interpreted cautiously given the cross-sectional design of the study.
Higher neopterin concentrations in patients with SLE compared to those with RA have also been observed by others (9). This observation is counterintuitive since current thinking is that SLE is a TH2-mediated disease (32), RA a TH1-mediated (or more recently, TH17) disease (33), and neopterin a marker of TH1 immune activation (4). However, several recent lines of evidence implicate IFN-γ in the pathogenesis of SLE (34), and thus our finding of higher neopterin concentrations in patients with SLE is concordant with those observations.
We have recently reported that TNF-α and IL-6 were independently associated with coronary calcification in SLE and RA (22,29,35). The stronger association of neopterin with TNF-α, IL-6 and MCP-1 (a mediator of monocyte activation) (36) in SLE than in RA, along with the higher neopterin concentrations in SLE than RA, suggests that the activation pathways that drive neopterin production may differ in the two diseases, and that neopterin is associated more closely with atherogenic pathways in SLE than in RA.
Neopterin has been associated with atherosclerosis and coronary risk in the general population. Higher concentrations of neopterin were associated with increased carotid (5) and coronary atherosclerosis (6), and were of prognostic value in patients with acute coronary syndrome (7). These clinical observations suggested a role for monocyte and macrophage activation in the pathogenesis of atherosclerosis and its complications, and were concordant with histological studies in which monocytes and macrophages were prominent in atherosclerotic lesions (37).
Although neopterin concentrations were correlated with some important atherogenic inflammatory mediators in SLE, they were not independently correlated with coronary calcium in either SLE or RA. It is possible that in studies finding an association between neopterin and increased cardiovascular risk (5–7) or carotid artery plaque (8) in the general population, this may have occurred indirectly. For example, if concentrations neopterin and inflammatory mediators were correlated, the observed association between neopterin and atherosclerosis could actually have been due to the relationship between atherosclerosis and inflammatory mediators that were not measured. Alternatively, in chronic inflammatory diseases such as RA and SLE, the increase in neopterin concentration could be driven by generalized inflammation, and this could act to obscure any smaller neopterin signal produced by macrophages associated with atherosclerosis. Finally, some studies suggest that neopterin may identify a patient group with increased cardiovascular risk that is not related to the amount of atherosclerosis present (38). We cannot exclude this possibility in our study.
Homocysteine is a strong predictor of cardiovascular risk (39) and others have also noted an association between homocysteine and neopterin concentrations in a range of populations (17,40,41), including a study of 33 patients with RA (17). The association between neopterin and homocysteine concentrations is thought to occur because immune activation results not only in higher neopterin concentrations but also in oxidative stress, consumption of folate, and impaired metabolism of homocysteine (42). Thus, neopterin and homocysteine levels may both reflect immune activation. Also, because both neopterin (27) and homocysteine (43) are cleared by the kidney, their concentrations would both tend to track with renal function. Our observation that the association between neopterin and homocysteine was attenuated when creatinine was included in the model in RA, but not in SLE, is compatible with the idea that neopterin has a stronger association with inflammation in SLE than in RA.
Our study has several limitations. It was cross-sectional in design, and therefore causal inferences cannot be drawn. Also, we measured coronary calcium, a technique that provides an excellent measure of the atherosclerotic burden, but does not discriminate between stable and unstable lesions. The prognostic association between neopterin concentrations and acute coronary syndrome (7) suggests that additional studies will be required to determine whether the increased concentrations of neopterin in patients with SLE and RA could reflect a greater burden of unstable atheromatous plaque, or an increase in the processes by which stable plaque becomes unstable.
In conclusion, macrophage activation, reflected by increased serum neopterin concentrations, is increased in both SLE and RA compared to control subjects. Neopterin is more robustly associated with atherogenic mediators of inflammation and homocysteine in SLE than in RA, but is not associated with the severity of coronary atherosclerosis in either disease.
Acknowledgements
None.
Sources of Funding: Supported by NIH grants HL65082, HL67964, GM07569, UL1 RR024975 from NCRR/NIH, P60 AR056116 and the Dan May Chair in Medicine.
Footnotes
Disclosures: None of the authors has a conflict of interest related to this work.
References
- 1.Asanuma Y, Oeser A, Shintani AK, Turner E, Olsen N, Fazio S, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349(25):2407–2415. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 2.Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52(10):3045–3053. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 4.Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab. 2002;3(2):175–187. doi: 10.2174/1389200024605082. [DOI] [PubMed] [Google Scholar]
- 5.Weiss G, Willeit J, Kiechl S, Fuchs D, Jarosch E, Oberhollenzer F, et al. Increased concentrations of neopterin in carotid atherosclerosis. Atherosclerosis. 1994;106(2):263–271. doi: 10.1016/0021-9150(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 6.Erren M, Reinecke H, Junker R, Fobker M, Schulte H, Schurek JO, et al. Systemic inflammatory parameters in patients with atherosclerosis of the coronary and peripheral arteries. Arterioscler Thromb Vasc Biol. 1999;19(10):2355–2363. doi: 10.1161/01.atv.19.10.2355. [DOI] [PubMed] [Google Scholar]
- 7.Ray KK, Morrow DA, Sabatine MS, Shui A, Rifai N, Cannon CP, et al. Long-term prognostic value of neopterin: a novel marker of monocyte activation in patients with acute coronary syndrome. Circulation. 2007;115(24):3071–3078. doi: 10.1161/CIRCULATIONAHA.106.666511. [DOI] [PubMed] [Google Scholar]
- 8.Sugioka K, Naruko T, Hozumi T, Nakagawa M, Kitabayashi C, Ikura Y, et al. Elevated levels of neopterin are associated with carotid plaques with complex morphology in patients with stable angina pectoris. Atherosclerosis. 2010;208(2):524–530. doi: 10.1016/j.atherosclerosis.2009.07.054. [DOI] [PubMed] [Google Scholar]
- 9.Hagihara M, Nagatsu T, Ohhashi M, Miura T. Concentrations of neopterin and biopterin in serum from patients with rheumatoid arthritis or systemic lupus erythematosus and in synovial fluid from patients with rheumatoid or osteoarthritis. Clin Chem. 1990;36(4):705–706. [PubMed] [Google Scholar]
- 10.Leohirun L, Thuvasethakul P, Sumethkul V, Pholcharoen T, Boonpucknavig V. Urinary neopterin in patients with systemic lupus erythematosus. Clin Chem. 1991;37(1):47–50. [PubMed] [Google Scholar]
- 11.Lim KL, Jones AC, Brown NS, Powell RJ. Urine neopterin as a parameter of disease activity in patients with systemic lupus erythematosus: comparisons with serum sIL-2R and antibodies to dsDNA, erythrocyte sedimentation rate, and plasma C3, C4, and C3 degradation products. Ann Rheum Dis. 1993;52(6):429–435. doi: 10.1136/ard.52.6.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim KL, Muir K, Powell RJ. Urine neopterin: a new parameter for serial monitoring of disease activity in patients with systemic lupus erythematosus. Ann Rheum Dis. 1994;53(11):743–748. doi: 10.1136/ard.53.11.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wais T, Fierz W, Stoll T, Villiger PM. Subclinical disease activity in systemic lupus erythematosus: immunoinflammatory markers do not normalize in clinical remission. J Rheumatol. 2003;30(10):2133–2139. [PubMed] [Google Scholar]
- 14.Hannonen P, Tikanoja S, Hakola M, Mottonen T, Viinikka L, Oka M. Urinary neopterin index as a measure of rheumatoid activity. Scand J Rheumatol. 1986;15(2):148–152. doi: 10.3109/03009748609102081. [DOI] [PubMed] [Google Scholar]
- 15.Reibnegger G, Egg D, Fuchs D, Gunther R, Hausen A, Werner ER, et al. Urinary neopterin reflects clinical activity in patients with rheumatoid arthritis. Arthritis Rheum. 1986;29(9):1063–1070. doi: 10.1002/art.1780290902. [DOI] [PubMed] [Google Scholar]
- 16.Beckham JC, Caldwell DS, Peterson BL, Pippen AM, Currie MS, Keefe FJ, et al. Disease severity in rheumatoid arthritis: relationships of plasma tumor necrosis factor-alpha, soluble interleukin 2-receptor, soluble CD4/CD8 ratio, neopterin, and fibrin D-dimer to traditional severity and functional measures. J Clin Immunol. 1992;12(5):353–361. doi: 10.1007/BF00920793. [DOI] [PubMed] [Google Scholar]
- 17.Schroecksnadel K, Frick B, Kaser S, Wirleitner B, Ledochowski M, Mur E, et al. Moderate hyperhomocysteinaemia and immune activation in patients with rheumatoid arthritis. Clin Chim Acta. 2003;338(1–2):157–164. doi: 10.1016/j.cccn.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Maerker-Alzer G, Diemer O, Strumper R, Rohe M. Neopterin production in inflamed knee joints: high levels in synovial fluids. Rheumatol Int. 1986;6(4):151–154. doi: 10.1007/BF00541281. [DOI] [PubMed] [Google Scholar]
- 19.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 20.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 21.Chung CP, Oeser A, Solus JF, Gebretsadik T, Shintani A, Avalos I, et al. Inflammation-associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum. 2008;58(7):2105–2112. doi: 10.1002/art.23600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rho YH, Chung CP, Oeser A, Solus J, Asanuma Y, Sokka T, et al. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2009;61(11):1580–1585. doi: 10.1002/art.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 24.Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 25.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 26.Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39(3):363–369. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs D, Hausen A, Reibnegger G, Werner ER, von DP, Wachter H. Neopterin levels in long-term hemodialysis. Clin Nephrol. 1988;30(4):220–224. [PubMed] [Google Scholar]
- 28.Harrel FE. Regression Modeling Strategies - With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer; 2001. Ordinal Logistic Regression; pp. 331–343. [Google Scholar]
- 29.Rho YH, Chung CP, Oeser A, Solus J, Raggi P, Gebretsadik T, et al. Novel cardiovascular risk factors in premature coronary atherosclerosis associated with systemic lupus erythematosus. J Rheumatol. 2008;35(9):1789–1794. [PMC free article] [PubMed] [Google Scholar]
- 30.Toncheva D, Galabov AS, Laich A, Atanassova S, Kamarinchev B, Dimitrov T, et al. Urinary neopterin concentrations in patients with Balkan endemic nephropathy (BEN) Kidney Int. 2003;64(5):1817–1821. doi: 10.1046/j.1523-1755.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs D, Weiss G, Reibnegger G, Wachter H. The role of neopterin as a monitor of cellular immune activation in transplantation, inflammatory, infectious, and malignant diseases. Crit Rev Clin Lab Sci. 1992;29(3–4):307–341. doi: 10.3109/10408369209114604. [DOI] [PubMed] [Google Scholar]
- 32.Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56(7):481–490. doi: 10.1136/jcp.56.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Type 17 T helper cells-origins, features and possible roles in rheumatic disease. Nat Rev Rheumatol. 2009;5(6):325–331. doi: 10.1038/nrrheum.2009.80. [DOI] [PubMed] [Google Scholar]
- 34.Karonitsch T, Feierl E, Steiner CW, Dalwigk K, Korb A, Binder N, et al. Activation of the interferon-gamma signaling pathway in systemic lupus erythematosus peripheral blood mononuclear cells. Arthritis Rheum. 2009;60(5):1463–1471. doi: 10.1002/art.24449. [DOI] [PubMed] [Google Scholar]
- 35.Asanuma Y, Chung CP, Oeser A, Shintani A, Stanley E, Raggi P, et al. Increased concentration of proatherogenic inflammatory cytokines in systemic lupus erythematosus: relationship to cardiovascular risk factors. J Rheumatol. 2006;33(3):539–545. [PubMed] [Google Scholar]
- 36.Coll B, onso-Villaverde C, Joven J. Monocyte chemoattractant protein-1 and atherosclerosis: is there room for an additional biomarker? Clin Chim Acta. 2007;383(1–2):21–29. doi: 10.1016/j.cca.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 37.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 38.Grammer TB, Fuchs D, Boehm BO, Winkelmann BR, Maerz W. Neopterin as a predictor of total and cardiovascular mortality in individuals undergoing angiography in the Ludwigshafen Risk and Cardiovascular Health study. Clin Chem. 2009;55(6):1135–1146. doi: 10.1373/clinchem.2008.118844. [DOI] [PubMed] [Google Scholar]
- 39.Antoniades C, Antonopoulos AS, Tousoulis D, Marinou K, Stefanadis C. Homocysteine and coronary atherosclerosis: from folate fortification to the recent clinical trials. Eur Heart J. 2009;30(1):6–15. doi: 10.1093/eurheartj/ehn515. [DOI] [PubMed] [Google Scholar]
- 40.Turgan N, Habif S, Parildar Z, Ozmen D, Mutaf I, Erdener D, et al. Association between homocysteine and neopterin in healthy subjects measured by a simple HPLC-fluorometric method. Clin Biochem. 2001;34(4):271–275. doi: 10.1016/s0009-9120(01)00226-0. [DOI] [PubMed] [Google Scholar]
- 41.Schroecksnadel K, Frick B, Fiegl M, Winkler C, Denz HA, Fuchs D. Hyperhomocysteinaemia and immune activation in patients with cancer. Clin Chem Lab Med. 2007;45(1):47–53. doi: 10.1515/CCLM.2007.012. [DOI] [PubMed] [Google Scholar]
- 42.Schroecksnadel K, Frick B, Winkler C, Leblhuber F, Wirleitner B, Fuchs D. Hyperhomocysteinemia and immune activation. Clin Chem Lab Med. 2003;41(11):1438–1443. doi: 10.1515/CCLM.2003.221. [DOI] [PubMed] [Google Scholar]
- 43.Castro R, Rivera I, Blom HJ, Jakobs C, Tavares dAI. Homocysteine metabolism, hyperhomocysteinaemia and vascular disease: an overview. J Inherit Metab Dis. 2006;29(1):3–20. doi: 10.1007/s10545-006-0106-5. [DOI] [PubMed] [Google Scholar]