Abstract
Methylphenidate (MPH) is a stimulant drug that amplifies dopamineric and noradrenergic signaling in the brain, which is believed to underlie its cognition enhancing effects. However, the neurobiological effects by which MPH improves cognition are still poorly understood. Here, functional magnetic resonance imaging (fMRI) was used together with working memory (WM) and visual attention (VA) tasks to test the hypothesis that 20 mg oral MPH would increase activation in the dorsal attention network (DAN) and deactivation in the default mode network (DMN) as well as improve performance during cognitive tasks in healthy men. The group of subjects that received MPH (MPH group; N = 16) had higher activation than the group of subjects who received no medication (control group: N = 16) in DAN regions (parietal and prefrontal cortex, regions increasingly activated with increased cognitive load) and had increased deactivation in the insula and posterior cingulate cortex (regions increasingly deactivated with increased cognitive load) and these effects did not differ for the VA and the WM tasks. These findings provide the first evidence that MPH enhances activation of the DAN whereas it alters DMN deactivation. This suggests that MPH (presumably by amplifying dopamine and noradrenergic signaling) modulates cognition in part through its effects on DAN and DMN.
Keywords: BOLD-fMRI, dopamine, cognition, brain function, stimulants, MPH
INTRODUCTION
Dopamine (DA) modulates cognitive performance in part via its regulation of the prefrontal cortex through dopamine D1 and D2 receptors (Goldman-Rakic, 1998). However there is increasing evidence that dopaminergic actions are not limited to the prefrontal cortex and that cognitive operations rely on multiple cortical networks. The role of DA in these functional networks is still largely unknown. Stimulant medications such as amphetamine and methylphenidate (MPH) increase DA signaling in the brain and are used in the treatment of attention deficit hyperactivity disorder (ADHD) and other neuropsychiatric disorders to enhance attention and cognition (Ackerman et al., 1982; Camp-Bruno and Herting, 1994; Clatworthy et al., 2009; Izquierdo et al., 2008). These medications are also abused by healthy individuals as cognitive enhancers (Volkow and Swanson, 2008). In healthy subjects, we have shown that MPH decreased the brain’s glucose demand during arithmetic calculations, an effect that reflected in part enhanced deactivation of default mode network (DMN) regions (Volkow et al., 2008a). However, there is some evidence that MPH effects on brain function may be task dependent (Clatworthy et al., 2009). For instance, MPH has been shown to decrease activation during reversal learning (Dodds et al., 2008) and decision making (Schlösser et al., 2009) tasks, but to increase activation during reaction time tasks (Müller et al., 2005).
Purposeful attention (top-down) is essential for proper cognitive performance and is supported by the activation of a dorsal attention network (DAN) (frontal eye fields, ventral premotor cortex, prefrontal cortex, superior parietal lobule, intraparietal sulcus, motion-sensitive middle temporal areas, and thalamus) (Corbetta and Shulman, 2002) and the deactivation of the DMN, which engages when individuals are not focused on a specific task (Buckner et al., 2008; Raichle et al., 2001). Considering that MPH enhances dopamine (DA) release, which has been postulated to enhance task specific signaling by decreasing background activity of neurons (Kiyatkin and Rebec, 1996), and that MPH induces DA-increases that are associated with enhanced interest on cognitive tasks (Volkow et al., 2004), we hypothesized that for different tasks that cause activation in the same brain regions, MPH would enhance activation in these regions regardless of the cognitive domain (i.e. spatial attention and verbal working memory).
To test this hypothesis we measured brain activation in 32 healthy men using fMRI and two different and well-established cognitive paradigms with parametric increases of cognitive load: n-ball tracking VA (Pylyshyn and Storm, 1988) and n-back WM (Gevins et al., 1987). We selected these tasks because in our previous studies we showed that both tasks cause reliable activation of DAN (Tomasi et al., 2007) and reliable deactivation of DMN (Tomasi et al., 2006). Therefore, the main hypothesis of the study was that the MPH group would have higher fMRI signals in DAN (higher activation in parietal and prefrontal cortex) and DMN (higher deactivation in posterior cingulate cortex and insula) than the control group regardless of the task (VA and WM). Random effects two-way analysis of variance (ANOVA) models were used to test this hypothesis.
MATERIALS AND METHODS
Subjects
Two groups of subjects were tested for this study; one tested with oral MPH (20 mg) and another, which served as control, was tested without pharmacological intervention. All subjects were recruited from the local community using public advertisements. For the MPH group we recruited sixteen healthy, non-smoking, right-handed men (mean ± SD: age 33 ± 3 years, education: 13 ± 1 years). For the control group we used existing data from sixteen healthy, right-handed men (age 36 ± 2 years, education: 14 ± 1 years), who previously participated in an fMRI study using the same tasks and MRI acquisition parameters but without MPH (Tomasi et al., 2009a; Tomasi et al., 2009b; Volkow et al., 2009; Volkow et al., 2008b); a within-subject design was not used to minimize habituation/practice effects on brain activation during VA (Tomasi et al., 2004) and WM (Garavan et al., 2000) tasks. There were no statistically significant differences in age or education between the groups.
Inclusion/Exclusion criteria
All 32 participants provided written informed consent as approved by the Institutional Review Board at Brookhaven National Laboratory. Subjects were screened carefully with a detailed medical history, physical, psychological, and neurological examinations, EKG, breath carbon monoxide, blood tests, and urine toxicology for psychotropic drug to ensure the subjects were healthy at the time of the study. Subjects were included in the study if they were a) 18 to 50 years old, and b) able to understand and give informed consent. Subjects were excluded if they had c) urine positive for psychotropic drugs (cocaine, phencyclidine, benzodiazepines, cannabis, opiates, barbiturates and inhalants); d) present or past history of dependence on alcohol or other drugs of abuse (except for nicotine and caffeine); or e) present or past history of neurological or psychiatric disorder (including ADHD); f) used psychoactive (i.e. opiate analgesics, stimulants, or sedatives), or g) prescription (non-psychiatric; i.e. antihistamines) medications in the past month, or h) medical conditions that may alter cerebral function (i.e cardiovascular, endocrinological and metabolic diseases); i) history of head trauma with loss of consciousness of more than 30 minutes; j) history of sleep disorders; or k) contraindications for MRI (metallic or electronic implants, metallic tattoos in the neck/head, claustrophobia).
MPH administration
Subjects received 20 mg oral MPH and were tested 120 minutes after administration of MPH. Cardiovascular measures were obtained throughout the study to monitor heart rate and blood pressure.
FMRI paradigms
WM task
We used a blocked WM task based on the classical “n-back” verbal WM paradigm (Gevins et al., 1987). During each of the four 30s long “task” epochs, subjects viewed a sequence of single alphabetical letters that were presented in random order at a rate of 1/s. They were instructed to press a response button whenever the current letter was the same as the one displayed one (one-back target) or two (two-back target) steps before in the sequence. There were 27 trials and 5 target events in each task epoch. There were 4 task epochs and 4 resting epochs in each n-back task. XEGDJJITMMODRTQUUYEEBWVCCJK is an example of the sequence of letters for a 1-back epoch that highlights (underline) the five 1-back targets. Similarly, MDUDMWHUMVMKWXPCPJFPFKEZELK is an example of the sequence of letters for a 2-back epoch highlighting the five 2-back targets. Button press events were used to record WM accuracy (the relative difference between hits and false alarms) and reaction times. The remaining 3 seconds were used to display the instructions (“press when one-back” and “press when two-back”). During each of the four 30s long “resting” epochs, subjects passively viewed a sequence of non-alphabetical symbols displayed in a random order at a rate of 1/s, using the same font size and luminescence as for the “task” epochs. During each of the four 30s long “task” epochs of the control (zero-back) task, the letters were presented randomly every 6 seconds and the subjects were instructed to press whenever they saw a letter in the screen; thus, there was the same number of target events during the 0-, 1-, and 2-back epochs. During each of the corresponding 30s long “resting” epochs, symbols were presented at the same rate, font size, and luminescence as for the “task” epochs. Each of these three WM task levels had a total duration of 4:10 minutes (Fig 1).
Fig. 1.
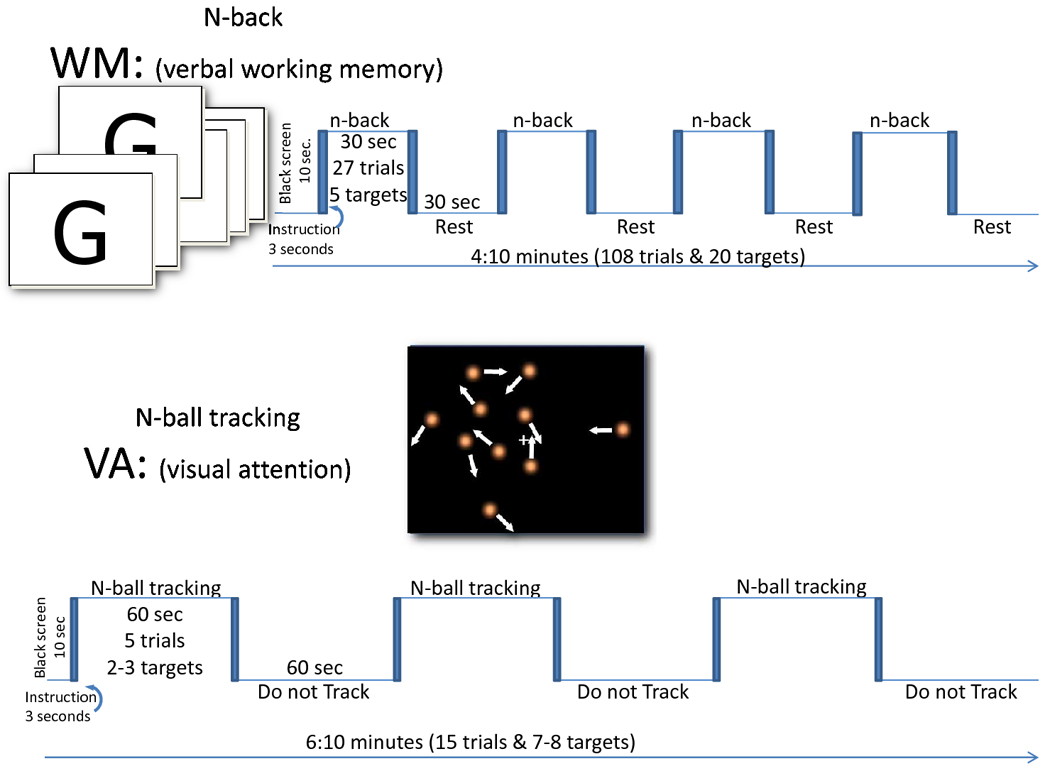
Schematic representations of the fMRI stimulation paradigms.
VA task
We used a blocked visual attention task (Pylyshyn and Storm, 1988) that has been described previously (Tomasi et al., 2007; Tomasi et al., 2004). Briefly, the 60-seconds long “TRACK” epochs are composed of 5 “Ball tracking” trials. In these periods, two, three, or four out of ten target balls are briefly highlighted for 500ms. Then all balls start to move. The subjects’ task was to fixate on the center cross displayed at the center of the visual field and track the target balls as they move randomly across the display (12° of the central visual field) with instantaneous angular speed of 3°/second. During these 11-seconds long tracking trials the 10 balls move in a simulated Brownian motion, and collide with, but do not penetrate each other. At the end of ball-tracking trials, the balls stop moving and a new set of balls is highlighted. The subjects’ were instructed to press a button if the highlighted balls were the target set. Button press events were used to record VA accuracy (the relative difference between hits and false alarms) and reaction times during the fMRI tasks. After a 500 ms delay, the original target balls are re-highlighted to re-focus the subjects’ attention on those balls (the target set). There were a total of 15 trials in each “Ball tracking” task. The 60-seconds long “DO NOT TRACK” baseline epochs are composed of five consecutive “resting” periods. In these periods, all 10 balls move and stop in the same manner as during “TRACK” epochs; however, no balls are highlighted, and subjects were instructed to not track the balls and view them passively. The use of this resting baseline condition allows us to control for the confounding effect of visual input activation.
The tasks were presented to the subjects on MRI-compatible goggles connected to a personal computer. A trigger signal from the scanner was used to synchronize the paradigm with fMRI acquisition.
Functional MRI: data acquisition
Functional MRI data with blood oxygenation level dependent (BOLD) contrast were acquired in a 4-Tesla Varian/Siemens scanner using a T2*-weighted single-shot gradient-echo planar imaging sequence (TE/TR = 20/1600 ms, 4-mm slice thickness, 1-mm gap, 35 coronal slices, 64 × 64 matrix size, 3.1 × 3.1 mm in-plane resolution, 90°-flip angle, time points = 157 for WM or 231 for VA, 200.00 kHz bandwidth) covering the whole brain. Padding was used to minimize motion and subject motion was monitored after each fMRI run using a k-space motion detection algorithm (Caparelli et al., 2003) written in IDL (ITT Visual Information Solutions, Boulder, CO). Earplugs (28 dB attenuation of sound pressure level; Aearo Ear TaperFit 2; Aearo Co.), headphones (30 dB attenuation of sound pressure level; Commander XG MRI Audio System, Resonance Technology inc.), and a “quiet” acquisition approach were used to minimize the interference effect of scanner noise during fMRI (Tomasi et al., 2005). Anatomical images were collected using a T1-weighted 3D-MDEFT sequence (Lee et al., 1995) (TE/TR = 5/15 ms, 0.94 × 0.94 × 1.00 mm3 spatial resolution, axial orientation, 256 readout and 192 × 96 phase-encoding steps, 16 minutes scan time) and a modified T2-weigthed Hyperecho sequence (Hennig and Scheffler, 2001) (TE/TR = 0.042/10 seconds, echo train length = 16, 256 × 256 matrix size, typically 30 coronal slices, 0.86 × 0.86 mm2 in-plane resolution, 5 mm thickness, no gap, 2 min. scan time) and carefully reviewed to rule out gross brain morphological abnormalities.
fMRI analysis
Image reconstruction was performed using a phase correction method that produced minimal ghost artifacts (Caparelli and Tomasi, 2008). The first four imaging time points were discarded to avoid non-equilibrium effects in the fMRI signal. The statistical parametric mapping package SPM2 (Welcome Department of Cognitive Neurology, London UK) was used for standard image realignment, spatial normalization, and spatial smoothing (8-mm). Spatial normalization to the stereotactic space of the Montreal Institute of Neurology (MNI) was carried out using a 12-parameters affine transformation with medium regularization, 16-nonlinear iterations, and voxel size of 3 × 3 × 3 mm3.
The SPM2 general linear model and standard blocked designs with high-pass filtering (cut-off = 1/128Hz for WM or 1/256Hz for VA) were used to calculate the activation maps for each task, cognitive load level, and subject. For the WM task, a blocked design with 4 cycles alternating 30-second long task epochs and 30-second long resting epochs were convolved with the canonical hemodynamic response function. For the VA task, a blocked design with 3 cycles alternating 60-second long task epochs and 60-second long resting epochs were convolved with the canonical hemodynamic response function. Contrast maps reflecting %BOLD signal change from resting baseline to task conditions were computed for each fMRI run and subject.
Group analyses
To assess brain activation differences between groups (task-independent) and between tasks across subjects, the individual BOLD contrast maps were included in a repeated measures ANOVA model1 with six conditions (0-, 1-, and 2-back, and 2-, 3-, and 4-balls) and two groups (MPH and control) in SPM2. To assess brain activation differences between groups, the individual BOLD contrast maps were included in a repeated measures ANOVA model2 with three conditions (for WM: 0-, 1-, and 2-back; for VA: 2-, 3-, and 4-balls) and two groups (MPH and control) in SPM2. Additionally, two independent multiple regression analyses with two regressors (load and RT or load and accuracy) and a constant were used to associate BOLD responses and behavioral variables during fMRI. Activation clusters were displayed using a voxel-level threshold P < 0.005 and a minimum cluster volume of 200 voxels for all analyses except the analysis of non-linear effects of cognitive load on BOLD responses (inverted U-shape analyses); exploratory, a less conservative voxel-level threshold (P < 0.01) was used together with a larger minimum cluster volume (700 voxels) to display activation clusters for inverted U-shape analyses due to the smaller amplitude of these second-order signals. The continuous random field calculation implemented in SPM2 was used to correct for multiple comparisons the statistical significance of brain activation clusters for all analyses. Brain activation and deactivation clusters with at least 60 rescaled voxels (3-mm isotropic, cluster volume 1620 mm3) and p < 0.05, corrected for multiple comparisons with a family-wise error correction based on the random field theory (Worsley et al., 1992), were considered significant. The Montreal Neurological Institute (MNI) coordinates of the cluster maxima were transformed to the Talairach space using a best-fit transform (icbm_spm2tal; http://brainmap.org/icbm2tal/) that minimizes bias associated with reference frame and scaling. Brain regions were labeled according to the Talairach daemon (http://www.talairach.org/) (Lancaster et al., 2000) and a query range of 5 mm.
Functional ROI analyses
Relevant clusters were further evaluated with ROI analyses to identify outliers and to obtain average signal values in a volume comparable to the image smoothness (e.g. resolution elements, or “resels” (Worsley et al., 1992)), which for this study was 13-mm isotropic, rather than single-voxel peak values. Thus, 9-mm isotropic masks containing 27 imaging voxels (0.73 ml) were defined at the centers of relevant activation/deactivation/correlation clusters identified by the SPM analysis (F test from ANOVA model1) to extract the average %BOLD signal from first-level individual BOLD contrast maps (up to 6 contrasts per subject), not from second-level average group contrasts. Note that the variability of the ROI data reflects the between-subjects variability of the BOLD signals in the ROIs. These masks were created and centered at the precise coordinates of the clusters listed in Table 1, which also lists the average statistical significance (t-values) in these ROIs. Note that the coordinates of the ROI masks were kept fixed across subjects, tasks, and conditions and that the Table lists average statistical values only for clusters that were statistically significant (P < 0.05, corrected for multiple comparisons with the random field theory in SPM).
Table 1.
Statistical significance of clusters showing differential activation/deactivation for the MPH and control (CON) groups during working memory (WM) and visual attention (VA) tasks with parametric increases of cognitive load (WM load and VA load, respectively). k: number of 3-mm isotropic voxels in the clusters. MPH > CON lists maximum voxel values from SPM ANOVA model1.
| Region | Talairach [mm] |
MPH > CON (WM & VA) |
Averaged t-values in cubic ROI (volume = 0.73 mm3) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VA | WM | VA > WM | VA-load | WM-load | |||||||||||||
| Name | BA | x | y | z | k | T | Z | CON | MPH | CON | MPH | CON | MPH | CON | MPH | CON | MPH |
| Middle Frontal G** | 6 | −30 | −12 | 48 | 1196 | 8.7 | 7.8 | 4.0 | 11.0 | 3.8 | 10.7 | ns | ns | ns | ns | 2.4 | 5.5 |
| Precentral G | 6 | −54 | 3 | 9 | 7.3 | 6.7 | ns | 2.8 | ns | 3.7 | ns | ns | ns | ns | ns | ns | |
| Inferior Parietal C | 40 | −54 | −30 | 42 | 6.9 | 6.4 | ns | 3.7 | ns | 3.6 | ns | ns | ns | ns | ns | ns | |
| Medial Frontal G** | 6 | 0 | −9 | 48 | 787 | 7.6 | 7.0 | −5.6 | 1.4 | −5.0 | ns | ns | ns | −1.8 | ns | −2.9 | −2.0 |
| Precuneus | 7 | 9 | −48 | 51 | 7.0 | 6.5 | 2.1 | 10.2 | −5.1 | ns | 4.9 | 8.2 | ns | ns | ns | ns | |
| Paracentral Lobule | 5 | 9 | −39 | 54 | 6.2 | 5.8 | −2.3 | 3.4 | −6.4 | −1.9 | 2.5 | 3.8 | −2.2 | ns | −2.0 | −2.2 | |
| Middle Frontal G ** | 10 | 39 | 45 | 12 | 535 | 7.6 | 6.9 | ns | 5.7 | −1.2 | 4.2 | ns | ns | ns | 1.7 | ns | ns |
| Medial Frontal G | 10 | 9 | 57 | 3 | 6.2 | 5.8 | ns | 2.4 | ns | 1.8 | ns | ns | ns | ns | ns | ns | |
| Superior Frontal G | 10 | 24 | 60 | 15 | 6.1 | 5.7 | ns | 5.9 | 2.8 | 4.5 | ns | ns | ns | 1.8 | ns | 2.4 | |
| Inferior Parietal C** | 40 | 33 | −39 | 51 | 229 | 6.0 | 5.7 | 7.6 | 12.9 | 3.1 | 6.8 | 3.7 | 4.8 | ns | 1.8 | 1.9 | 4.0 |
| Precentral G | 6 | 27 | −18 | 57 | 5.1 | 4.9 | −3.3 | ns | −5.1 | −2.8 | ns | 2.8 | −2.4 | ns | ns | ns | |
| Postcentral G | 3 | 45 | −21 | 45 | 4.7 | 4.5 | −2.1 | 4.0 | ns | ns | ns | 2.7 | ns | 1.7 | ns | ns | |
| Superior Parietal C* | 7 | −27 | −60 | 63 | 83 | 5.6 | 5.3 | 5.0 | 8.8 | 3.0 | 5.7 | 1.8 | 2.5 | ns | ns | ns | 2.7 |
| Precuneus | 7 | −9 | −63 | 66 | 3.9 | 3.8 | 4.8 | 6.4 | ns | ns | 4.2 | 3.9 | ns | ns | ns | ns | |
| Superior Parietal C | 7 | −33 | −48 | 48 | 3.6 | 3.6 | 8.9 | 12.2 | 9.0 | 12.4 | ns | ns | 2.9 | ns | 4.0 | 7.4 | |
| Fusiform* | 37 | −33 | −45 | −18 | 111 | 5.4 | 5.2 | ns | 2.8 | ns | 1.9 | ns | ns | ns | ns | 1.8 | 2.7 |
| Cerebellum (tonsil) | −18 | −42 | −33 | 5.4 | 5.2 | 3.4 | 7.4 | ns | 2.4 | 3.0 | 3.7 | ns | ns | ns | 1.7 | ||
| Fusiform G | 20 | −27 | −39 | −21 | 4.9 | 4.7 | ns | 2.4 | ns | ns | ns | 1.8 | ns | ns | ns | 1.7 | |
| Insula** | 13 | 42 | −18 | 0 | 220 | 4.9 | 4.7 | −8.5 | −3.4 | −7.0 | −4.7 | −1.7 | ns | −3.0 | ns | −2.7 | −2.1 |
| Middle Temporal G | 21 | 54 | 3 | −18 | 4.8 | 4.6 | −2.3 | ns | −3.1 | ns | ns | ns | ns | ns | ns | ns | |
| Superior Temporal G | 22 | 54 | −12 | −3 | 4.7 | 4.6 | −3.6 | ns | −4.8 | −2.1 | ns | ns | ns | ns | ns | ns | |
| Cerebellum ** | 27 | −42 | −24 | 178 | 4.9 | 4.7 | 2.0 | 7.6 | ns | 4.1 | ns | 2.7 | ns | ns | ns | ns | |
| Fusiform G | 37 | 39 | −45 | −15 | 4.1 | 4.0 | 1.9 | 3.8 | ns | 2.0 | ns | ns | ns | ns | ns | ns | |
| Parahippocampal G | 35 | 30 | −27 | −18 | 4.1 | 4.0 | ns | 2.2 | −2.9 | ns | ns | 1.9 | ns | ns | ns | ns | |
| Lingual G** | 18 | 12 | −78 | 0 | 366 | −7.4 | −6.8 | 5.6 | −3.3 | 7.6 | 4.6 | ns | −5.6 | 2.2 | ns | 2.2 | ns |
| Cuneus | 18 | −3 | −75 | 6 | −6.4 | −6.0 | ns | −7.3 | 1.7 | −2.2 | −1.8 | −3.8 | ns | −2.8 | ns | −1.7 | |
| Lingual G | 17 | −9 | −87 | −3 | −4.8 | −4.6 | ns | −3.1 | 4.7 | ns | −2.8 | −2.4 | ns | ns | ns | ns | |
| Post Cingulate** | 23 | 9 | −27 | 24 | 362 | −5.8 | −5.5 | 4.3 | Ns | 4.9 | ns | ns | ns | ns | ns | ns | ns |
| Post Cingulate | 31 | −9 | −51 | 21 | −5.8 | −5.4 | −5.6 | −9.9 | −4.3 | −7.9 | ns | −1.8 | ns | ns | −2.5 | −4.2 | |
| Insula** | 13 | −42 | −6 | 0 | 239 | −7.2 | −6.6 | −4.2 | −9.7 | −2.5 | −8.9 | ns | ns | ns | −2.4 | −3.4 | −4.8 |
| Insula | 13 | −42 | 6 | −6 | −6.8 | −6.3 | 1.9 | −3.6 | ns | −4.4 | ns | ns | ns | ns | −1.7 | ns | |
| Temporal Lobe, | 20 | −36 | −12 | −18 | −4.7 | −4.5 | ns | −2.9 | ns | −3.1 | ns | ns | ns | ns | ns | ns | |
| Cerebellum** | 6 | −54 | −36 | 179 | −6.7 | −6.3 | 6.0 | Ns | 6.0 | ns | ns | ns | 2.0 | ns | ns | ns | |
| Cerebellum (tonsil) | 18 | −57 | −36 | −4.3 | −4.2 | 5.5 | 2.1 | 4.6 | 1.6 | ns | ns | ns | ns | ns | ns | ||
| Pretectum** | 0 | −33 | −6 | 187 | −6.0 | −5.7 | 5.8 | 2.5 | 8.6 | 1.9 | ns | ns | 3.1 | ns | 2.2 | ns | |
| Cerebellum (culmen) | −3 | −51 | −6 | −5.2 | −4.9 | ns | −2.8 | 2.2 | −3.3 | ns | ns | ns | ns | ns | ns | ||
| Precuneus* | 7 | −18 | −69 | 36 | 83 | −4.5 | −4.4 | ns | −4.4 | 6.9 | 4.0 | −4.8 | −5.9 | 2.2 | Ns | 4.2 | 2.4 |
| Precuneus | 19 | −27 | −69 | 30 | −4.3 | −4.2 | −4.0 | −7.1 | ns | −3.2 | −3.6 | −2.9 | ns | Ns | ns | −1.7 | |
RESULTS
Cardiovascular effects of MPH
Pulse rate and systolic blood pressure were lower immediately before than 2 hours after oral MPH (e.g. at the time of fMRI acquisition; p = 0.04; Fig 2 top panel). Differences in diastolic blood pressure did not reach statistical significance.
Fig. 2.
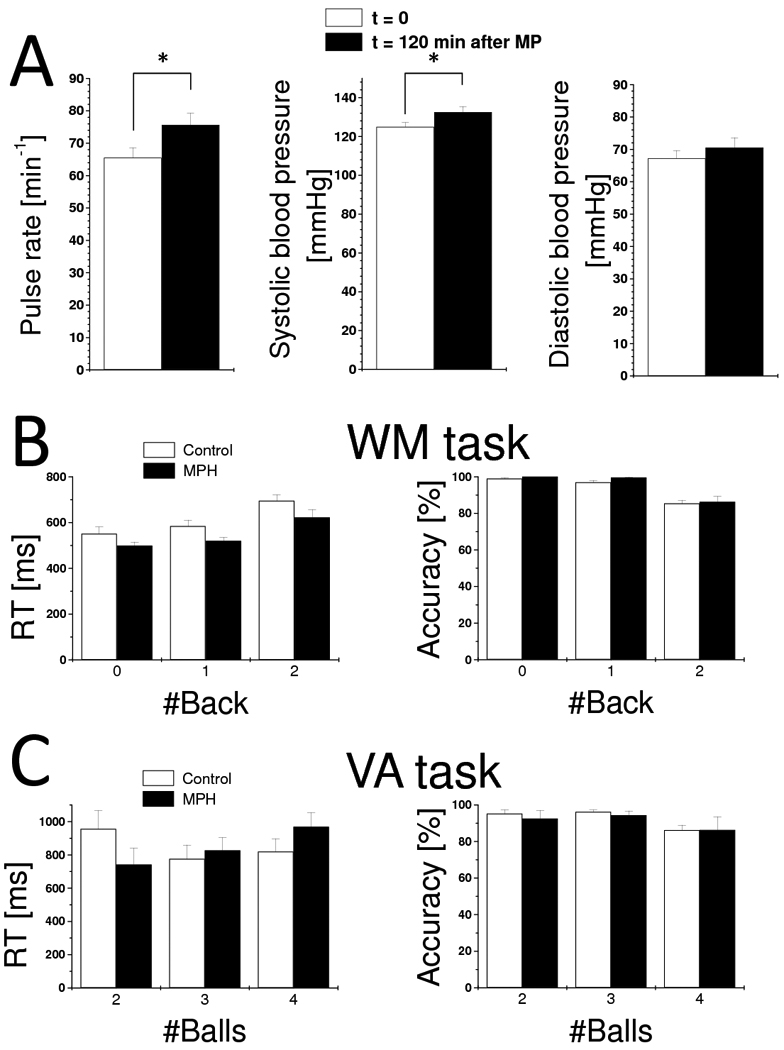
Cardiovascular effects of MPH (A) and behavioral responses during WM (B) and VA (C). Values represent means ± standard deviations. WM sample: 16 MPH and 16 control subjects. VA sample: 15 MPH and 14 control subjects.
Cognitive performance
VA-fMRI data were not acquired for two control subjects and one MPH subject that were unable to achieve acceptable performance accuracy (> 70%; the relative difference between hits and false alarms) on the VA task during training. These subjects completed the WM task with acceptable performance (> 70%) and were included in the analysis. For both tasks, performance accuracy was lower for higher cognitive load conditions than for lower cognitive load conditions (p < 0.001; F > 8.9; separate MPH x load ANOVA for WM and VA) reflecting the cognitive load increases of the tasks (Fig 2 bottom panel). Follow up analyses (paired t-test) revealed that for controls accuracy was lower for the 2-back than for 0- or 1-back (P = 0.0001) and that differences in accuracy between 0-back and 1-back were not statistically significant (P = 0.07); for the MPH group differences in accuracy between the 0-, 1-, and 2-back conditions were not statistically significant (P > 0.1). Similar analyses (paired t-test) for VA task revealed that for controls accuracy was lower for the 4-balls condition than for the 2- or 3-balls conditions (P < 0.01) and that differences in accuracy between 2-balls and 3-balls were not statistically significant (P = 0.67); for the MPH group differences in accuracy between the 2-, 3-, and 4-balls conditions were not statistically significant (P > 0.09).
Reaction time was longer for higher WM-load conditions (p < 0.0001; F(30) = 24.7; MPH x load ANOVA). Follow up analyses (paired t-test) revealed that for controls RT was longer for 2-back than for 0- or 1-back (P < 0.001) and that RT differences between 0-back and 1-back were not statistically significant (P = 0.07); for the MPH group RT was longer for 2-back than for 0- or 1-back (P < 0.01) and RT differences between 0-back and 1-back were not statistically significant (P = 0.07). During the WM task, RT was shorter (p < 0.026; F(30) = 5.5; ANOVA; Fig 2) for the MPH group than for the control group. For the VA task, the effect of increased cognitive load on RT was not significant (p = 0.513; F(27) = 0.675).
WM-load × MPH and VA-load × MPH interaction effects on accuracy and RT were not statistically significant (P > 0.072; F < 2.766). For the WM task, the main effect of MPH on accuracy across all n-back conditions was not significant (P = 0.237; F(30) = 1.458; ANOVA). Similarly for the VA task, there were no significant effects of MPH on RT or accuracy (P > 0.7; F(27) < 0.14). Performance accuracy and RT measures during the WM and VA did not exhibit significant correlations with cardiovascular variables.
Brain activation/deactivation
For both groups (MPH and control), the WM and VA tasks caused strong activation in DAN, occipital cortex, midbrain, and cerebellum, and strong deactivation in DMN, insula and rostral anterior cingulate gyrus (Pcorr < 0.001, corrected for multiple comparisons with the random field theory; Figs. 3A and 3B and Table 1; ANOVA model1). Brain activation in the parietal and right prefrontal cortices (PFC), thalamus and cerebellum and deactivation in insula were higher for VA than for WM (Pcorr < 0.05; Fig. 3D, pink pattern), which is consistent with our previous studies (Tomasi et al., 2006); brain activation in left PFC was higher for WM than for VA (Pcorr < 0.05; Fig. 3D, green pattern).
Fig. 3.
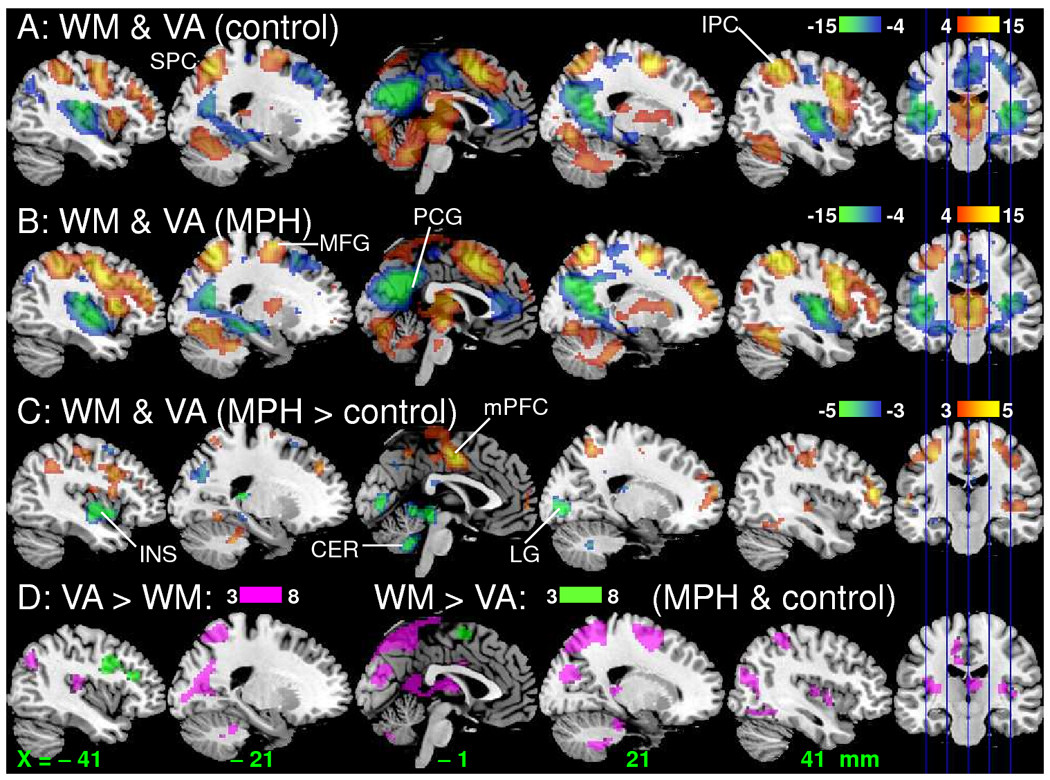
Brain activation patterns rendered to a structural MRI image. Statistical maps (t-value) of BOLD-fMRI responses during the WM and VA tasks (ANOVA model1) for the control (A) and MPH (B) groups as well as for the differential signal between the groups (C) (ANOVA; red-yellow: activation; blue-green deactivation), and between the tasks (D; ANOVA model1). WM sample: 16 MPH and 16 control healthy subjects. VA sample: 15 MPH and 14 control subjects. MFG: middle frontal gyrus; mPFC: medial prefrontal cortex; IPC: inferior parietal cortex; SPC superior parietal cortex; LG: lingual gyrus; PCG: posterior cingulate gyrus; INS: insula; CER: cerebellum.
MPH effects on brain activation patterns
Figure 4 plots BOLD responses (averaged across tasks and conditions) for the MPH group versus controls and shows that in both groups the same brain regions were strongly activated (yellow) or strongly deactivated (green) by the tasks. However, the MPH group had higher activation and higher deactivation than the control group in regions that were less engaged by the tasks in controls. For both tasks, BOLD responses in middle (MFG) and medial (mFG) frontal gyri as well as superior (SPC) and inferior (IPC) parietal were higher for the MPH group than for the control group; BOLD responses in occipital cortices (LG) and cerebellum (CER) were lower for the MPH group than for the control group (Pcorr < 0.05; and Fig 3C; ANOVA model2). Note that for mFG the MPH group showed positive BOLD responses whereas the control group showed deactivation (negative responses) for both tasks (Fig 5B). Deactivation in insula (INS) and precuneus was higher for MPH than for the control group (Pcorr < 0.05; ANOVA model2). Group × task interaction effects on brain activation were not statistically significant (Pcorr > 0.05; ANOVA model2). BOLD responses were not significantly correlated with cardiovascular variables in any of the ROIs.
Fig. 4.
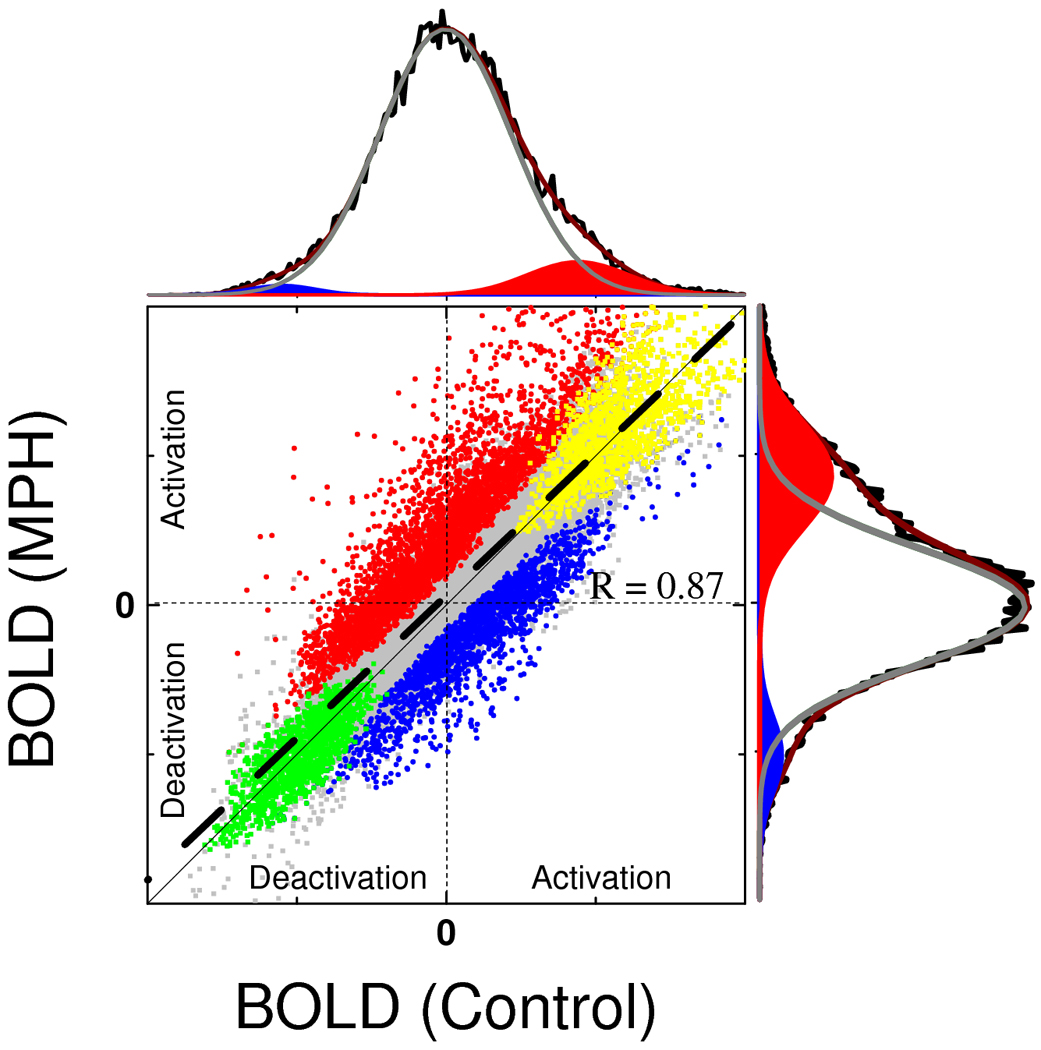
Average BOLD-fMRI responses in the control group vs. those in the MPH group in gray matter voxels. Gray: all gray-matter voxels included; yellow: voxels strongly activated (t-value > 4) by VA and WM; green: voxels strongly deactivated (t-value > 4) by VA and WM; red: voxels with higher activation (t-value > 3) for the MPH group than for control group; blue: voxels with higher deactivation (t-value > 3) in the MPH group. The thick dashed black line reflects a linear fit; R is the Pearson correlation factor. The thin solid black line reflects a 1:1 proportion. The high degree of parallelism of the lines and the high Pearson correlation factor (R) of the linear fit demonstrate the reproducibility of the fMRI results across different groups of subjects. The black histograms are projections of the 2D scatter plot that reflect BOLD signal distribution for controls (top) and MPH (left) groups; the gray curve and the red and blue areas are the Gaussian components that fit (red curve) the histograms. The larger activation (red) and deactivation (blue) gaussian areas for MPH compared to control exemplifies the effect of MPH on hemodynamic responses in the brain during VA and WM tasks.
Fig. 5.
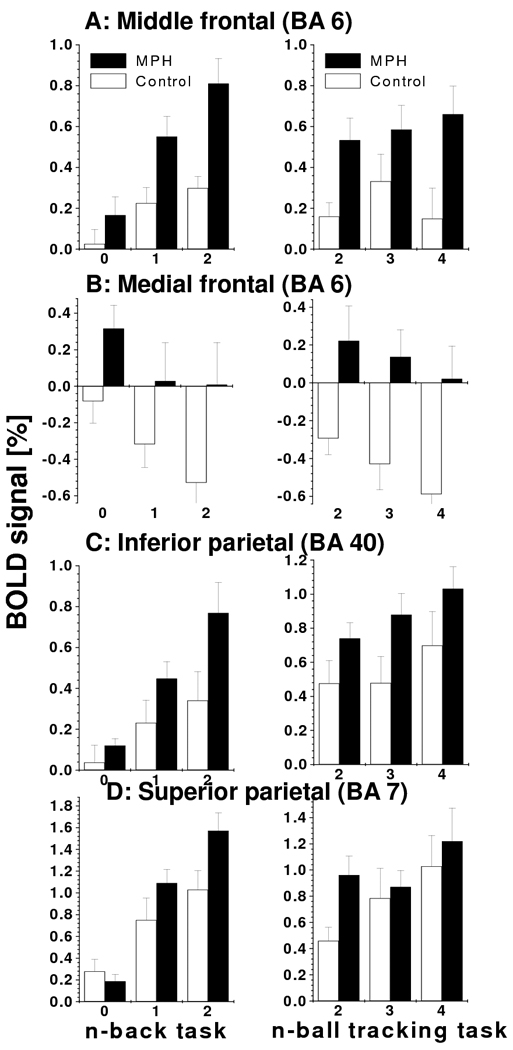
MPH-related activation increases. BOLD-fMRI responses in clusters that show higher activation for MPH (black) than for controls (white) for different VA (left) and WM (right) difficulty levels. Note that for both tasks, increased difficulty (load) caused increased activation (A, C, and D) or deactivation (B). ROI analyses: voxels = 27, volume = 0.73 mm3; see Table 1 for the Talairach coordinates of the ROIs.
MPH effects on VA load and WM load activation patterns
There were both linear and non-linear effects of cognitive load on brain activation during WM and VA. Linear effects: For both tasks, increased cognitive load linearly increased activation in DAN, midbrain and cerebellum and linearly increased deactivation in DMN, insula and rostral ACC (Pcorr < 0.001; ANOVA model2). These WM load activation/deactivation responses in left PFC/insula and cuneus were higher for WM than for VA (Pcorr < 0.05; ANOVA model1), consistently with our previous studies without MPH using the same tasks (Tomasi et al., 2007). WM load activation in left inferior parietal cortex (BA 40) was modestly higher for the MPH group than for the control group (P = 0.011, uncorrected cluster-level; ANOVA model2: MPH × WM-load interaction effect) but the effect did not reach statistical significance after corrections for multiple comparisons. No brain region showed differential VA-load activation/deactivation between the MPH and control groups (Pcorr > 0.75; ANOVA model2: MPH × VA-load interaction effect).
Non-linear effects
For the control group, WM activation exhibited an inverted U-shape as a function of WM load increases that was statistically significant in right MFG (BA 9) and dorsal ACC (BA 32) (Pcorr = 0.05). For the MPH group, the inverted U-shape of WM activation was statistically significant in the lingual gyrus (BA 18) (Pcorr = 0.04). The inverted U-shape of WM activation was higher for the control group than for the MPH group in a bilateral cluster encompassing dorsal ACC (BA 32) and MFG (BA 6) (Pcorr = 0.04). For the control group, the inverted U-shape of VA activation in ventral ACC (BA 32) as a function of cognitive load increases did not reach statistical significance after corrections for multiple comparisons (Puncorr = 0.004). For the MPH group, the inverted U-shape of WM activation was statistically significant in the lingual gyrus (BA 18) (Pcorr = 0.04). The inverted U-shape of VA activation was higher for the control group than for the MPH group in ventral ACC (BA 32) (Pcorr = 0.03). Figure 6 highlights the proximity of the dorsal and ventral ACC regions showing statistically significant inverted U-shape effects on WM and VA.
Fig. 6.
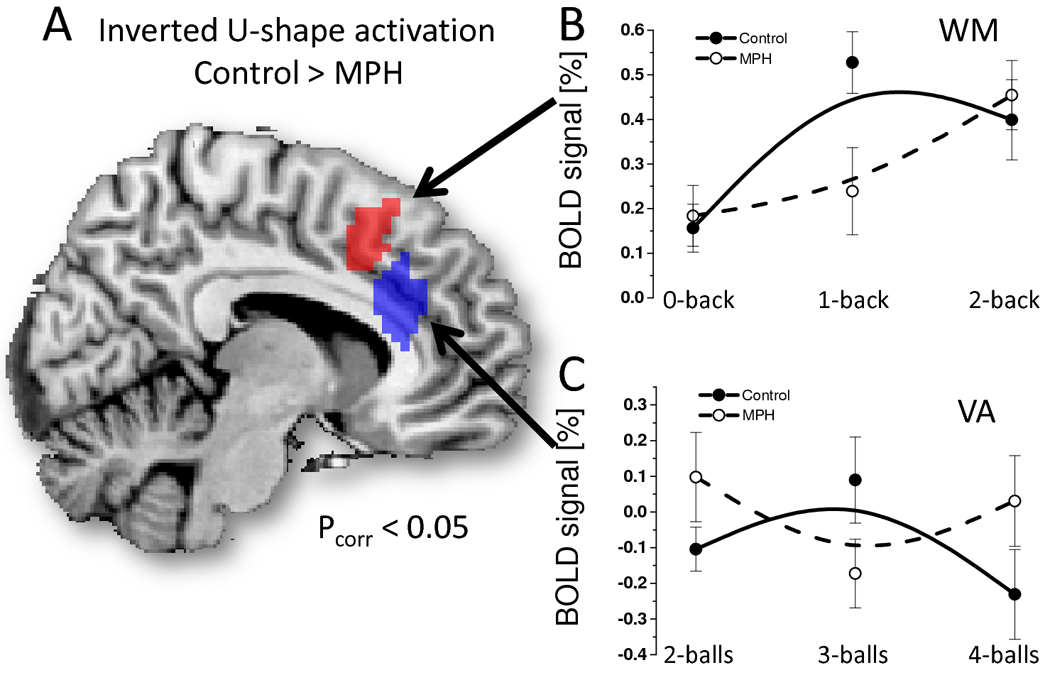
Brain regions exhibiting statistically significant effects of MPH on non-linear BOLD responses (inverted U-shape as a function of cognitive load increases) during WM (red) and VA (blue), superimposed on a sagital view of the human brain (A) and scatter plots showing the %BOLD signal for each cognitive level, group (control: solid circles; MPH: open circles) and task (B: WM; C: VA); ROI analysis. B-spline solid and dashed lines are also shown as guide lines.
BOLD–Performance correlation: neurocognitive effects of MPH
For the WM task, BOLD responses in left SPC (BA 7) and fusiform gyrus (BA 19) showed a negative correlation with RT in the control group (Fig. 7; Pcorr < 0.038) but not in the MPH group (not shown); the higher the activation in these regions the shorter the RT. WM accuracy did not show significant correlation with BOLD responses in any brain region. For the VA task, accuracy and RT did not show significant correlation with BOLD responses in any brain region.
Fig. 7.
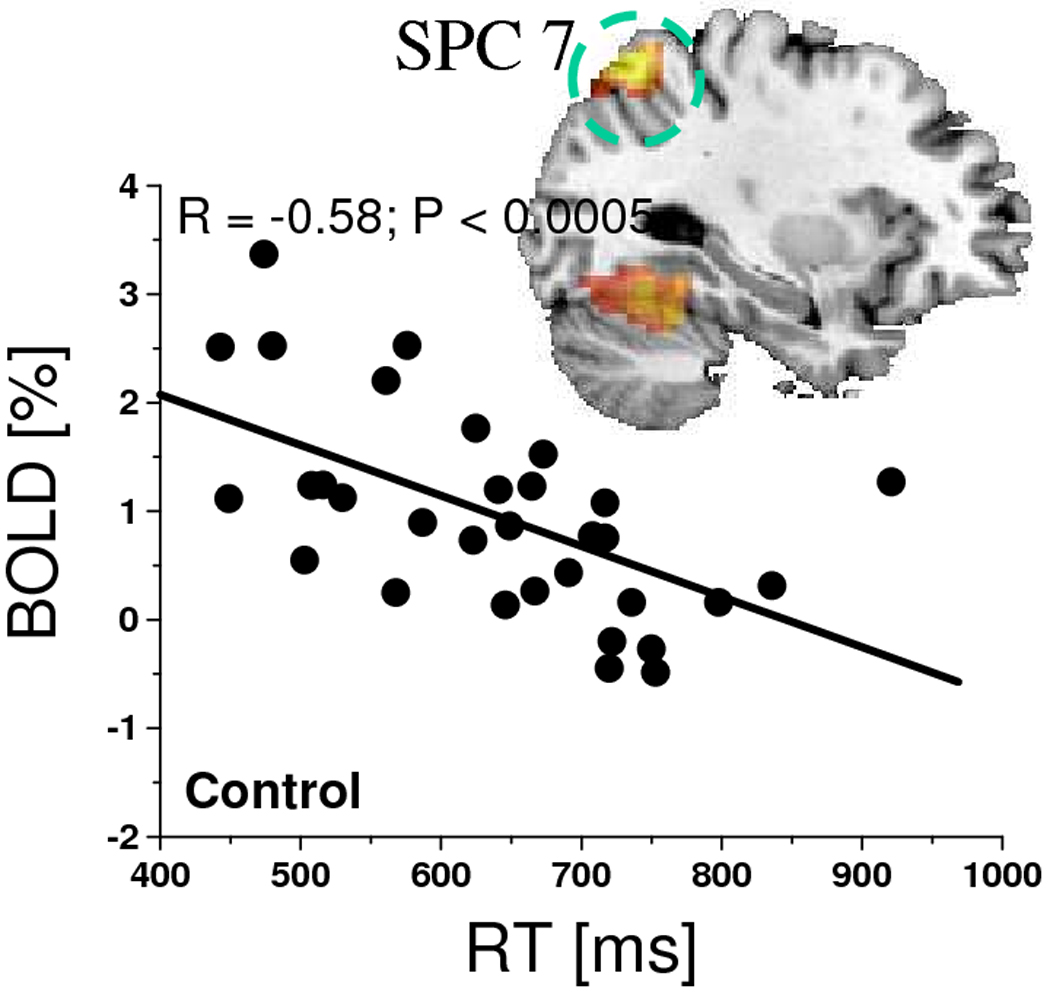
Scatter plot showing the negative correlation between RT and BOLD responses in the left superior parietal cortex (BA 7) during the 1-back and 2-back WM tasks for the control group and the corresponding negative correlation pattern superimposed on a sagital view of the human brain.
DISCUSSION
Here we show that for two different cognitive tasks (WM and VA) MPH increased brain activation in DAN (IPC, SPC, and MFG) and increased deactivation in some DMN regions (insula and posterior cingulate) but decreased it in others (mPFC BA 6; Fig 3). In addition MPH decreased activation in lingual gyrus and cerebellum. Brain activation increases in SPC and fusiform gyrus (BA 19) were associated with shorter RT during the WM task for the control but not for the MPH group (Fig 7). Overall, the MPH group had increased activation and deactivation in brain regions that where moderately activated in controls, suggesting that MPH enhanced BOLD responses simultaneously for the WM and VA tasks (Figs. 4 and 5). However there were no differences in performance accuracy (WM and VA tasks) between the control and the MPH groups (Fig 2).
Effects of MPH on DAN activation
During the WM and VA tasks, the MPH group had higher activation than the control group in IPC, SPC and the posterior PFC, which are part of the DAN network implicated in attention. Indeed, the posterior PFC and parietal cortex have important attentional roles in cognition (Adler et al., 2001; Arrington et al., 2000; Buchel et al., 1998; de Fockert et al., 2001; Fassbender et al., 2004; Lawrence et al., 2003; Le et al., 1998; Leonards et al., 2000; Tomasi et al., 2007) which explains the activation in IPC, SPM, and MFG (Fig. 3D) for different cognitive domains (verbal WM and the spatial VA) observed in this work and reported in previous studies (Tomasi et al., 2007; Tomasi et al., 2006). During WM the control group had longer RT than the MPH group, consistently with previous behavioral studies on WM (Cooper et al., 2005). The longer RT was correlated with decreased activation in SPC and fusiform gyrus in the control group, which implicates these regions in speed of reaction to a working memory task.
Our interpretation that MPH-related activation increases in DAN could reflect enhancement of unique cognitive processes that engage during the WM and VA tasks (sustained attention, spatial orienting, and WM) is partially supported by the shorter RT of the MPH group compared to the control group during the WM task. Furthermore, the BOLD signal in the ACC (BA32) exhibited an inverted U-shape as a function for cognitive load increases, the signature of limitations in cognitive capacity, for the control group in both domains (WM and VA) but not for the MPH group, and this group difference was statistically significant. This also suggests that MPH could enhance cognition in healthy controls under conditions of saturated cognitive capacity (ie. fatigue, difficulty). However, in this study the MPH group had shorter RT only during the WM task and we did not observe differences in accuracy for any of the tasks between control and MPH groups. This could explain why in this group of healthy controls tested under resting conditions, and for low-demanding cognitive tasks, MPH can improve RT but did not improve accuracy.
Effects of MPH on DMN deactivation
Brain deactivation in PCG (BA 23/31), the main functional connectivity hub in the brain (Tomasi and Volkow, 2010), was higher for the MPH group than for the control group, which could facilitate task focusing by filtering irrelevant stimuli. We interpret this as reflecting MPH dopaminergic enhancing effects through its blockade of dopamine transporters. Indeed, we recently showed an inverse correlation between DA transporter levels in the brain and brain deactivation in the posterior DMN during the VA task (Tomasi et al., 2009a); such that lower DA transporter levels (interpreted to predict stronger DA signaling secondary to lower uptake by transporters) were associated with greater deactivation in BA 7. This is also consistent with our findings that MPH reduced the metabolic demand of the brain during numerical calculations, in part by enhancing DMN deactivation (Volkow et al., 2008a). The MPH group also had higher deactivation in left posterior INS than the control group. Our results are also consistent with recent fMRI studies on decision-making, showing increased PCG deactivation after oral (40 mg) MPH (Schlösser et al., 2009), and those on reorienting tasks showing decreased INS activity in ADHD children after 1 year of MPH treatment (Konrad et al., 2007). Note that brain deactivation may reflect changes in the vascular system in response to changes in cerebral blood flow in adjacent regions (“blood stealing”) or active suppression of neural activity to minimize potentially distracting task-irrelevant neural processes (Tomasi et al., 2006).
MPH increases extracelular DA and NE (Berridge et al., 2006), which is a neuromodulator that changes the efficacy of other transmitter signals as a function of ongoing neuronal activity (Kiyatkin and Rebec, 1996). For example DA release decreases the amplitude of spontaneous neuronal activity (up to 90%) in a large fraction (up to 75%) of spontaneously active neostriatal and accumbal neurons and enhances glutamate responses (Kiyatkin and Rebec, 1996), which enhances activation of glutamate-stimulated neurons. Therefore, facilitation of glutamatergic signaling linked with the task and suppression of spontaneous background activity during enhanced dopaminergic and noradrenergic stimulation with MPH could explain the enhanced activation of the DAN and the enhanced deactivation of the DMN in the MPH group compared to the control group. Taken together these findings support the notion that MPH facilitates activation of task relevant circuits (including the DAN) and facilitates the deactivation of the DMN, presumably through enhanced DA neurotransmission (perhaps also noradrenergic). This may be one of the mechanisms that contribute to MPH’s cognition enhancing effects.
Effects of MPH on LG and CER activation
The MPH group had lower activation in the lingual gyrus and cerebellar tonsil than the control group, which could also reflect the dopaminergic enhancing effects of MPH; our findings showing a linear association between striatal DA increases induced during sleep deprivation and decreases in activation (reductions in glucose metabolism) in lingual gyrus and cerebellum (Volkow et al., 2009) support this interpretation. However, this effect is likely to be indirect because neither the cerebellum nor the lingual gyrus receives major DA projections.
Cognitive enhancement
The use of stimulants for cognitive enhancement by normal healthy individuals has increased significantly over the past decade (Maher, 2008; McCabe et al., 2005). Amphetamine and MPH are the most commonly used stimulants and several studies have shown that stimulants can enhance executive function (including impulsivity control and problem solving) in normal healthy individuals under certain conditions (i.e boring repetitive tasks, during sleep deprivation, during fatigue) (Chamberlain et al., 2006; de Wit et al., 2002; Elliott et al., 1997; Farah et al., 2004). These effects are ascribed to stimulant-induced increases in alertness and attention (de Wit et al., 2002). Though others have failed to show improvement in healthy controls, which may reflect the dependency of MPH’s cognitive enhancing effects on the characteristics of the task and on subject’s baseline performance (Mattay et al., 2000). During the WM task the MPH group showed shorter RT than controls but no group differences in accuracy were significant. This indicates that the significant brain activation/deactivation differences were not associated with improved performance (except for shorter RT during WM). Indeed the activation/deactivation responses for neither task were associated with accuracy. This may reflect the fact that during resting conditions the brain can activate reserve neuronal systems in order to maintain accuracy. This capacity may be impaired during fatigue or sleep deprivation, which could then help explain why MPH is particularly beneficial to cognitive performance during those states.
Study limitations
The subjects from the control group participated in a previous study without MPH or placebo. Thus we couldn’t control for the placebo effect. However, the present design minimizes the potential confound of practice on the VA and WM tasks, which has been shown to reduce brain activation in PFC and cerebellum during WM and VA tasks (Garavan et al., 2000; Tomasi et al., 2004), regions where brain activation was significantly affected by MPH. Most subjects had near 100% performance accuracy, especially for low load conditions (0- and 1-back and 2- and 3-ball tracking), which likely limited the sensitivity to MPH-related differences in accuracy.
Summary
We studied the effect of 20 mg oral MPH on brain activation for two different cognitive domains, WM and VA. For both tasks and compared to the control group, the MPH group had higher activation in DAN, higher deactivation in some regions of the DMN (insula and posterior cingulate cortex) but decreased deactivation in others (cingulate gyrus BA 24) and in lingual gyrus. These findings suggest that MPH modulates cognition through its effects on DAN and DMN.
Supplementary Material
Acknowledgements
The National Institute on Alcohol Abuse and Alcoholism (2RO1AA09481) and the National Center for Research Resources (GCRC 5-MO1-RR-10710) supported this work. The authors thank Karen Apelskog-Torres, Barbara Hubbard, Hai-Dee Lee, and Joan Terry for assistance in various aspects of these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors report no biomedical financial interests or potential conflict of interest.
References
- Ackerman P, Dykman R, Holcomb P, McCray D. Methylphenidate effects on cognitive style and reaction time in four groups of children. Psychiatry Res. 1982;7:199–213. doi: 10.1016/0165-1781(92)90093-i. [DOI] [PubMed] [Google Scholar]
- Adler C, Sax K, Holland S, Schmithorst V, Rosenberg L, Strakowski S. Changes in neuronal activation with increasing attention demand in healthy volunteers: an fMRI study. Synapse. 2001;42:266–272. doi: 10.1002/syn.1112. [DOI] [PubMed] [Google Scholar]
- Arrington C, Carr T, Mayer A, Rao S. Neural mechanisms of visual attention: object-based selection of a region in space. J Cogn Neurosci. 2000;12 Suppl 2:106–117. doi: 10.1162/089892900563975. [DOI] [PubMed] [Google Scholar]
- Berridge C, Devilbiss D, Andrzejewski M, Arnsten A, Kelley A, Schmeichel B, Hamilton C, Spencer R. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Buchel C, Josephs O, Rees G, Turner R, Frith CD, Friston KJ. The functional anatomy of attention to visual motion: A functional MRI study. Brain. 1998;121:1281–1294. doi: 10.1093/brain/121.7.1281. [DOI] [PubMed] [Google Scholar]
- Buckner R, Andrews-Hanna J, Schacter D. The Brain’s Default Network: Anatomy, Function, and Relevance to Disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Camp-Bruno J, Herting R. Cognitive effects of milacemide and methylphenidate in healthy young adults. Psychopharmacology (Berl) 1994;115:46–52. doi: 10.1007/BF02244750. [DOI] [PubMed] [Google Scholar]
- Caparelli E, Tomasi D. K-space spatial low-pass filters can increase signal loss artifacts in Echo-Planar Imaging. Biomed Signal Process Control. 2008;3:107–114. doi: 10.1016/j.bspc.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparelli EC, Tomasi D, Arnold S, Chang L, Ernst T. k-Space based summary motion detection for functional magnetic resonance imaging. NeuroImage. 2003;20:1411–1418. doi: 10.1016/S1053-8119(03)00339-2. [DOI] [PubMed] [Google Scholar]
- Chamberlain S, Müller U, Robbins T, Sahakian B. Neuropharmacological modulation of cognition. Curr Opin Neurol. 2006;19:607–612. doi: 10.1097/01.wco.0000247613.28859.77. [DOI] [PubMed] [Google Scholar]
- Clatworthy P, Lewis S, Brichard L, Hong Y, Izquierdo D, Clark L, Cools R, Aigbirhio F, Baron J, Fryer T, Robbins T. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci. 2009;29:4690–4696. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N, Keage H, Hermens D, Williams L, Debrota D, Clark C, Gordon E. The dose-dependent effect of methylphenidate on performance, cognition and psychophysiology. J Integr Neurosci. 2005;4:123–144. doi: 10.1142/s0219635205000744. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman G. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- de Fockert J, Rees G, Frith C, Lavie N. The role of working memory in visual selective attention. Science. 2001;291:1803–1806. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- de Wit H, Enggasser J, Richards J. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Dodds C, Müller U, Clark L, van Loon A, Cools R, Robbins T. Methylphenidate has differential effects on blood oxygenation level-dependent signal related to cognitive subprocesses of reversal learning. J Neurosci. 2008;28:5976–5982. doi: 10.1523/JNEUROSCI.1153-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Sahakian B, Matthews K, Bannerjea A, Rimmer J, Robbins T. Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology (Berl) 1997;131:196–206. doi: 10.1007/s002130050284. [DOI] [PubMed] [Google Scholar]
- Farah M, Illes J, Cook-Deegan R, Gardner H, Kandel E, King P, Parens E, Sahakian B, Wolpe P. Neurocognitive enhancement: what can we do and what should we do. Nat Rev Neurosci. 2004;5:421–425. doi: 10.1038/nrn1390. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Murphy K, Foxe J, Wylie G, Javitt D, Robertson I, Garavan H. A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Brain Res Cogn Brain Res. 2004;20:132–143. doi: 10.1016/j.cogbrainres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Garavan H, Kelley D, Rosen A, Rao SM, Stein EA. Practice-related functional activation changes in a working memory task. Microsc Res Tech. 2000;51:54–63. doi: 10.1002/1097-0029(20001001)51:1<54::AID-JEMT6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Gevins A, Morgan N, Bressler S, Cutillo B, White R, Illes J, Greer D, Doyle J, Zeitlin G. Human neuroelectric patterns predict performance accuracy. Science. 1987;235:580–585. doi: 10.1126/science.3810158. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P. The cortical dopamine system: role in memory and cognition. Adv Pharmacol. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- Hennig J, Scheffler K. Hyperechoes. Magn Reson Med. 2001;46:6–12. doi: 10.1002/mrm.1153. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Bevilaqua L, Rossato J, Lima R, Medina J, Cammarota M. Age-dependent and age-independent human memory persistence is enhanced by delayed posttraining methylphenidate administration. Proc Natl Acad Sci U S A. 2008;105:19504–19507. doi: 10.1073/pnas.0810650105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin E, Rebec G. Dopaminergic modulation of glutamate-induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J Neurophysiol. 1996;75:142–153. doi: 10.1152/jn.1996.75.1.142. [DOI] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Fink G, Herpertz-Dahlmann B. Long-term effects of methylphenidate on neural networks associated with executive attention in children with ADHD: results from a longitudinal functional MRI study. J Am Acad Child Adolesc Psychiatry. 2007;46:1633–1641. doi: 10.1097/chi.0b013e318157cb3b. [DOI] [PubMed] [Google Scholar]
- Lancaster J, Woldorff M, Parsons L, Liotti M, Freitas C, Rainey L, Kochunov P, Nickerson D, Mikiten S, Fox P. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence N, Ross T, Hoffmann R, Garavan H, Stein E. Multiple neuronal networks mediate sustained attention. J Cogn Neurosci. 2003;15:1028–1038. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- Le T, Pardo J, Hu X. 4 T-fMRI study of nonspatial shifting of selective attention: cerebellar and parietal contributions. J Neurophysiol. 1998;79:1535–1548. doi: 10.1152/jn.1998.79.3.1535. [DOI] [PubMed] [Google Scholar]
- Lee JH, Garwood M, Menon R, Adriany G, Andersen P, Truwit CL, Ugurbil K. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med. 1995;34:308–312. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- Leonards U, Sunaert S, Van Hecke P, Orban G. Attention mechanisms in visual search -- an fMRI study. J Cogn Neurosci. 2000;12 Suppl 2:61–75. doi: 10.1162/089892900564073. [DOI] [PubMed] [Google Scholar]
- Maher B. Poll results: look who’s doping. Nature. 2008;452:674–675. doi: 10.1038/452674a. [DOI] [PubMed] [Google Scholar]
- Mattay V, Callicott J, Bertolino A, Heaton I, Frank J, Coppola R, Berman K, Goldberg T, Weinberger D. Effects of dextroamphetamine on cognitive performance and cortical activation. Neuroimage. 2000;12:268–275. doi: 10.1006/nimg.2000.0610. [DOI] [PubMed] [Google Scholar]
- McCabe S, Knight J, Teter C, Wechsler H. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey. Addiction. 2005;100:96–106. doi: 10.1111/j.1360-0443.2005.00944.x. [DOI] [PubMed] [Google Scholar]
- Müller U, Suckling J, Zelaya F, Honey G, Faessel H, Williams S, Routledge C, Brown J, Robbins T, Bullmore E. Plasma level-dependent effects of methylphenidate on task-related functional magnetic resonance imaging signal changes. Psychopharmacology (Berl) 2005;180:624–633. doi: 10.1007/s00213-005-2264-9. [DOI] [PubMed] [Google Scholar]
- Pylyshyn ZW, Storm RW. Tracking multiple independent targets: evidence for a parallel tracking mechanism. Spat Vis. 1988;3:179–197. doi: 10.1163/156856888x00122. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlösser R, Nenadic I, Wagner G, Zysset S, Koch K, Sauer H. Dopaminergic modulation of brain systems subserving decision making under uncertainty: a study with fMRI and methylphenidate challenge. Synapse. 2009;63:429–442. doi: 10.1002/syn.20621. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Caparelli EC, Chang L, Ernst T. fMRI-acoustic noise alters brain activation during working memory tasks. Neuroimage. 2005;27:377–386. doi: 10.1016/j.neuroimage.2005.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Chang L, Caparelli E, Ernst T. Different activation patterns for working memory load and visual attention load. Brain Res. 2007;1132:158–165. doi: 10.1016/j.brainres.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli E, Chang L. Common deactivation patterns during working memory and visual attention tasks: An intra-subject fMRI study at 4 Tesla. Hum Brain Mapp. 2006;27:694–705. doi: 10.1002/hbm.20211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli EC, Chang L. Practice-induced changes of brain function during visual attention: A parametric fMRI study at 4 Tesla. NeuroImage. 2004;23:1414–1421. doi: 10.1016/j.neuroimage.2004.07.065. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow N. Functional Connectivity Density Mapping. Proc Natl Acad Sci U S A. 2010;107:9885–9890. doi: 10.1073/pnas.1001414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow N, Wang R, Telang F, Wang G, Chang L, Ernst T, Fowler J. Dopamine Transporters in Striatum Correlate with Deactivation in the Default Mode Network during Visuospatial Attention. PLoS ONE. 2009a;4:e6102. doi: 10.1371/journal.pone.0006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang R, Telang F, Boronikolas V, Jayne M, Wang G, Fowler J, Volkow N. Impairment of Attentional Networks after 1 Night of Sleep Deprivation. Cereb Cortex. 2009b;19:233–240. doi: 10.1093/cercor/bhn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Fowler J, Wang G, Telang F, Logan J, Wong C, Ma J, Pradhan K, Benveniste H, Swanson J. Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLoS ONE. 2008a;3:e2017. doi: 10.1371/journal.pone.0002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Swanson J. The action of enhancers can lead to addiction. Nature. 2008;451:520. doi: 10.1038/451520a. [DOI] [PubMed] [Google Scholar]
- Volkow N, Tomasi D, Wang G, Telang F, Fowler J, Wang R, Logan J, Wong C, Jayne M, Swanson J. Hyperstimulation of Striatal D2 Receptors with Sleep Deprivation: Implications for Cognitive Impairment. Neuroimage. 2009;45:1232–1240. doi: 10.1016/j.neuroimage.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Telang F, Maynard L, Logan J, Gatley S, Pappas N, Wong C, Vaska P, Zhu W, Swanson J. Evidence that methylphenidate enhances the saliency of a mathematical task by increasing dopamine in the human brain. Am J Psychiatry. 2004;161:1173–1180. doi: 10.1176/appi.ajp.161.7.1173. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Telang F, Fowler J, Logan J, Wong C, Ma J, Pradhan K, Tomasi D, Thanos P, Ferré S, Jayne M. Sleep deprivation decreases binding of [11C]raclopride to dopamine D2/D3 receptors in the human brain. J Neurosci. 2008b;28:8454–8461. doi: 10.1523/JNEUROSCI.1443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley K, Evans A, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


