Abstract
Chronic restraint stress produces morphological changes in medial prefrontal cortex and disrupts a prefrontally mediated behavior, retrieval of extinction. To assess potential physiological correlates of these alterations, we compared neural activity in infralimbic and prelimbic cortex of unstressed versus stressed rats during fear conditioning and extinction. After implantation of microwire bundles into infralimbic or prelimbic cortex, rats were either unstressed or stressed via placement in a plastic restrainer (3 h/day for 1 week). Rats then underwent fear conditioning and extinction while activity of neurons in infralimbic or prelimbic cortex was recorded. Percent freezing and neural activity were assessed during all phases of training. Chronic stress enhanced freezing during acquisition of conditioned fear, and altered both prelimbic and infralimbic activity during this phase. Stress did not alter initial extinction or conditioned stimulus (CS)-related activity during this phase. However, stress impaired retrieval of extinction assessed 24 h later, and this was accompanied by alterations in neuronal activity in both prelimbic and infralimbic cortex. In prelimbic cortex, unstressed rats showed decreased activity in response to CS presentation, whereas stressed rats showed no change. In infralimbic cortex, neurons in unstressed rats exhibited increased firing in response to the CS, whereas stressed rats showed no increase in infralimbic firing during the tone. Finally, CS-related firing in infralimbic but not prelimbic cortex was correlated with extinction retrieval. Thus, the stress-induced alteration of neuronal activity in infralimbic cortex may be responsible for the stress-induced deficit in retrieval of extinction.
Keywords: Stress, Medial prefrontal cortex, Infralimbic cortex, Prelimbic cortex, Extracellular Recording, Extinction Retrieval
Introduction
Stress can precipitate or exacerbate many psychological disorders, most notably depression, schizophrenia, and posttraumatic stress disorder (e.g., Brown and Harris, 1989;Ventura et al., 1989), and can also disrupt cognitive and emotional behavior (Holmes and Wellman, 2009). Prefrontal cortex has been implicated in many stress-related disorders (Baxter et al., 1989;Drevets et al., 1992;Carter et al., 2001;Takahashi et al., 2004), is involved in many of the cognitive processes that are influenced by chronic stress (e.g., Dias et al., 1996), and is a target for the hormones involved in the stress response (Meaney and Aitken, 1985).
Chronic stress produces profound changes in the morphology of neurons in both the infralimbic and prelimbic regions of medial prefrontal cortex (mPFC) of male rats (Cook and Wellman, 2004;Izquierdo et al., 2006;Radley et al., 2006). Interestingly, dendritic morphology of mPFC appears to be exquisitely sensitive to stress: Just one week of brief daily restraint reduces mPFC apical dendritic branch number and length (Brown et al., 2005).
Exposure to chronic stress also produces deficits in retrieval of extinction of cued fear conditioning (Miracle et al., 2006;Garcia et al., 2008;Baran et al., 2009;Farrell et al., 2010), a behavior mediated by the infralimbic region of mPFC (Quirk et al., 2000). This suggests that chronic stress may compromise the function of mPFC. Indeed, acute stress produces sustained activation of infralimbic neurons (Jackson and Moghaddam, 2006) and impairs the induction of long term potentiation (LTP) in prelimbic cortex (Maroun and Richter-Levin, 2003); and chronic stress attenuates 5-HT-induced excitatory post-synaptic potentials (EPSP) in prelimbic neurons (Liu and Aghajanian, 2008) and impairs induction of LTP in infralimbic cortex (Goldwater et al., 2009). However, no studies to date have assessed the effects of chronic stress on neuronal physiology in mPFC in behaving animals. Likewise, the relationship between stress-induced changes in neuronal firing in mPFC and stress-induced changes in fear conditioning and extinction is unknown. Therefore, we assessed the effects of chronic stress on activity of mPFC neurons during fear conditioning and extinction.
Previous studies suggest different roles for prelimbic (PL) and infralimbic cortex (IL) in expression of cued fear conditioning and acquisition and retrieval of extinction. For instance, lesions of dorsal mPFC facilitate reactivity to the conditioned stimulus (Morgan and LeDoux, 1995), neurons in PL respond to the CS during fear conditioning (Baeg et al., 2001), temporary inactivation of PL disrupts the expression of conditioned fear (Corcoran and Quirk, 2007), stimulation of PL increases expression of conditioned fear and retards its extinction (Vidal-Gonzalez et al., 2006), and activity in PL is associated with increased freezing during extinction (Burgos-Robles et al., 2009). Thus, PL may contribute to expression of conditioned fear. On the other hand, IL appears to play a critical role in retrieval of extinction: Although there is some debate (Garcia et al., 2006), several laboratories have now shown that lesions of IL impair retrieval of extinction (Quirk et al., 2000;Baran et al., 2010;Farrell et al., 2010). Further, neuronal firing in this region increases in response to the CS during retrieval of extinction (Milad and Quirk, 2002), and stimulation of IL facilitates retrieval of extinction (Milad et al., 2004). Therefore, we assessed potential stress-induced changes in neuronal activity in each region.
Experimental Procedures
Animals
Male Sprague–Dawley rats (175–200 g, approximately 50 days old on arrival; Harlan, Indianapolis, IN), were individually housed in a vivarium with a 12:12 h light/dark cycle (lights on at 6:30 AM) and ambient temperature of 23–25 °C. To motivate rats for bar pressing, weights were gradually reduced to 85% of free-feeding weight. As in previous studies (Quirk et al., 2000;Miracle et al., 2006;Farrell et al., 2010), rats were then maintained at this weight, with weekly increases allowing for normally occurring weight gain, throughout the duration of the experiment. All experimental procedures occurred between 7:00 AM and 5:00 PM. All experimental procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Bloomington Institutional Animal Care and Use Committee.
Bar Press Training
When rats had reached their target weight behavioral training and testing commenced. To obtain a baseline level of activity against which to measure freezing, rats were trained to bar press for food reinforcement. (Quirk et al., 2000;Miracle et al., 2006;Farrell et al., 2010). Each rat was placed in an operant chamber within a sound-attenuating cabinet (Med Associates, St. Albans, VT). The chamber contained one operant lever on the left side of a food receptacle, an illuminated cue light over the lever, and a floor consisting of metal rods. Rats were shaped to press the lever for a food pellet reinforcer (BioServ pellets, Holton Industries, Frenchtown, NJ); shaping lasted 1–2 sessions, after which the reinforcement schedule was gradually reduced over four days from FR-1 to VI-60. As in previous studies (Quirk et al., 2000;Miracle et al., 2006;Farrell et al., 2010), during all subsequent phases of training and testing, rats were allowed to bar press for pellets on a VI-60 schedule. Computer-based operant software (MedPCIV; Med Associates, St. Albans, VT) controlled pellet delivery.
Surgery
After bar press training was complete (at approximately 60 days of age), electrode bundles were implanted unilaterally into either PL or IL. Unilateral implantation was used to minimize electrode track damage to the dorsal mPFC, as such damage may potentiate conditioned freezing (Milad and Quirk, 2002;Milad et al., 2004). Each electrode bundle consisted of one external stainless steel groundwire plus either one, two, or four pairs of 25µm formvar insulated, stainless steel microwires (California Fine Wire, Grover Beach, CA) twisted together to form stereotrodes. All wires were friction fitted to gold pins and attached to a plastic connector (Omnetics Connector; Minneapolis, MN) with epoxy adhesive. Rats were anesthetized with ketamine (74 mg/kg), xylazine (3.7 mg/kg), and acepromazine (0.74 mg/kg) and placed in a stereotaxic instrument (Kopf) with the incisor bar set so that bregma and lambda were in the same horizontal plane. The scalp was incised and retracted, a hole was drilled at 2.7 mm anterior and 0.5 mm lateral to bregma, and the underlying dura carefully retracted. Three additional holes were drilled for stainless steel anchoring screws, and the ground wire was secured to two of the anchoring screws. Electrode bundles were lowered to either 3.5 mm (PL) or 4.3 mm (IL) ventral to the skull, and cemented to the skull and anchoring screws with dental acrylic.
Restraint Stress
Approximately 7 days after surgery, rats were randomly assigned to either unstressed (n=19) or stressed conditions (n=17). Stressed rats were placed in a small plastic semi-cylindrical restrainer (6.35 cm dia × 15.24 cm l, modified so the tail piece locks into place; Braintree Scientific, Braintree, MA) for 3 h per day for 1 week, a manipulation that produces significant increases in plasma corticosterone levels (Cook & Wellman, 2004) and has been shown to impair retrieval of extinction (Miracle et al., 2006;Farrell et al., 2010).
Fear Conditioning and Extinction
Within 24 h of the final day of restraint, rats were placed in the operant chamber for a final session of bar press training (VI-60 schedule) and the headstage connector was plugged into a tether for acquisition of electrophysiological data while allowing the rat to move freely. During all subsequent phases of training and testing, rats were allowed to bar press for pellets on a VI-60 schedule. Fear conditioning and extinction took place over the following 2 days using a procedure similar to that of Quirk et al. (2000). On day 1, rats were placed in the operant chambers and underwent fear conditioning. After a 3-min acclimation period, rats received five habituation trials consisting of a 30-s tone (4.5-kHz, 80 db). Habituation trials were included to mitigate ceiling effects (Baran et al., 2009), and because previous studies describing the role of neural activity in IL (Milad and Quirk, 2002;Milad et al., 2004;Burgos-Robles et al., 2007) and PL (Burgos-Robles et al., 2009) in fear conditioning and extinction, as well as stress effects on extinction retrieval (Miracle et al., 2006;Baran et al., 2009;Farrell et al., 2010) have utilized habituation trials. Rats then underwent fear conditioning, consisting of seven pairings of the tone conditioned stimulus (CS) with a footshock unconditioned stimulus (US; 500-ms, 0.5mA) coterminating with the tone CS. Rats were then returned to their home cages for 1 h, after which they were returned to the chambers and given extinction trials consisting of tone alone. As previously described (Miracle et al., 2006), to ensure comparable levels of extinction learning across both groups, on day 1 extinction trials continued until the rat exhibited less than 10% (3 s) freezing on four consecutive trials (i.e., criterion). The following day, rats were given another 15 CS-alone trials to assess retrieval of extinction. For all phases of conditioning and extinction, variable intertrial intervals averaged 4 min. Computer-based operant software (Spike2; CED, London, UK) controlled the delivery of tones and shocks. For all trials, the duration of freezing (defined as the absence of any visible movement except that due to breathing) during the tone was measured with a digital stopwatch by an observer blind to experimental condition, and expressed as percent freezing (seconds spent freezing/30 s) during habituation, fear conditioning, extinction on day 1 (initial extinction), and extinction on day 2 (retrieval of extinction).
Single-Unit Recording
Neural activity was acquired and stored with a Power 1401 625 kHz data acquisition system and computer running Spike2 software (CED, London, UK) connected to a Lynx 8 amplifier (Neuralynx, Bozeman, MT). Data were amplified (20 khz), bandpass filtered (0.3 – 9 khz), and continuously sampled (20 kHz) throughout fear conditioning and extinction for subsequent analysis. All spike sorting occurred offline using Spike2. After a voltage threshold was established, individual units with similar waveforms and amplitudes were discriminated using principle components analysis. Time stamps from electrophysiological data were imported into NeuroExplorer (Nex Technologies, Littleton, MA) and power spectral densities, autocorrelograms, and interspike interval histograms were used to ensure that units were free of noise and represented individual neurons. All units used in subsequent analyses were clearly identified, with signal-to-noise ratios of approximately 2 to 1. Because the primary focus of this experiment was to assess potential stress-induced differences in the responding of populations of neurons in mPFC, rather than to analyze differences in unit responses within animals across days, individual units were not tracked across recording sessions. Cortical neurons can be classified into fast-spiking (>10 spikes/s with narrow afterhypolarizations, AHP) and regular-spiking (<10 spikes/s with wide AHP) units (McCormick et al., 1985;Connors and Gutnick, 1990;Jung et al., 1998;Baeg et al., 2001;Homayoun et al., 2005;Homayoun and Moghaddam, 2007), which are thought to correspond to interneurons and pyramidal cells, respectively (McCormick et al., 1985;Connors and Gutnick, 1990;Homayoun and Moghaddam, 2007). Similar to previous reports, putative interneurons constituted <10% of our total neuronal sample (Jung et al., 1998;Baeg et al., 2001;Homayoun and Moghaddam, 2007) and were excluded from subsequent analyses. Data collected from putative pyramidal neurons were then analyzed in NeuroExplorer.
Statistical Analyses
Percent freezing during habituation, fear conditioning, initial extinction, and retrieval of extinction were compared between unstressed and stressed groups. Data were analyzed using 2-way repeated measures ANOVAs (stress × trial); when appropriate, follow-up planned comparisons were conducted consisting of two-group F-tests done within the context of the overall ANOVA (Hays, 1994;Maxwell and Delaney, 2003). Finally, trials to criterion for initial extinction were calculated and compared between unstressed and stressed groups using a t-test (unpaired).
For the single-unit data, we first examined baseline firing rates by constructing histograms for a 10 s pre-tone period for all trials during habituation, conditioning, and extinction sessions. Baseline firing rates were compared across stress group, brain region, and training phase using a three-way ANOVA. Significant effects were followed by appropriate planned comparisons.
To evaluate neuronal responses to the CS, peri-stimulus time histograms (PSTH) were constructed for individual neurons for the 1 s preceding and 2 s following CS onset. Spikes were summed into 50-ms bins and expressed as percent change from baseline, defined as average firing rate during the 1 s preceding the onset of the CS (Milad and Quirk, 2002). For statistical comparisons, PSTHs for individual neurons were averaged in unstressed versus stressed rats for all habituation trials, conditioning trials 2 to 7, initial extinction trials 2 to criterion, and all retrieval of extinction trials. The first trial was excluded for conditioning and initial extinction because learning about the CS-US association (conditioning) or lack thereof (extinction) could not occur prior to the second trial of each phase. CS-induced activity for stressed and unstressed rats was compared for the 2-s post-CS period using 2-way repeated measures ANOVAs (stress × time bin).
Significant group differences observed on these PSTHs were followed up by analyzing CS responses of individual neurons. We calculated 95% confidence intervals (Abeles, 1982) for raw baseline firing rate for each IL and PL neuron recorded during conditioning or retrieval of extinction. Each neuron was then categorized as either excited by the CS (2 or more post-CS bins > upper 95% confidence interval), inhibited by the CS (2 or more post-CS bins < lower 95% confidence interval), or not responsive to the CS (neither excited nor inhibited as defined above). Differences in proportions of neurons meeting these criteria in unstressed and stressed groups were then assessed using Chi-square analyses.
In addition, to more closely examine potential group differences during particular phases of training that might be obscured by collapsing across trials, we constructed single-trial PSTHs for CS-induced activity in unstressed and stressed rats during the final conditioning, initial extinction, final extinction, and initial retrieval of extinction trial for PL and IL. Single trial CS-induced activity was calculated for IL for the first 200 ms of the post-CS period and for PL for the first 2 s of the post-CS period. These intervals were selected based on previous data on the duration of CS-induced activity in each region (Milad and Quirk, 2002;Burgos-Robles et al., 2009). Percent change was calculated as described above and was compared across unstressed and stressed rats using 2-way repeated measures ANOVAs (stress × training phase). When appropriate, follow-up planned comparisons were conducted consisting of two-group F-tests for across group comparisons and one-group t-tests to measure CS-induced excitation or inhibition within a group (Hays, 1994;Maxwell and Delaney, 2003).
Unpaired Controls
To assess the specificity of differences in unit activity, a separate group of underwent bar press training followed by implantation of either one or two 4-wire electrode bundles as described above. Rats were either unstressed (n=9) or stressed (n=10) as described above. Neural activity was then recorded during fear conditioning and extinction. However, following habituation trials, the CS and US were presented in an explicitly unpaired fashion, with tones and footshocks occurring 1–3 min apart and inter-trial intervals averaging 2 min. Thus, total time in the conditioning phase was the same as for paired conditioning. Statistical analyses were performed as described for the rats undergoing paired conditioning.
Histology
After fear conditioning and extinction, all rats were deeply anesthetized with urethane and marking lesions were made by applying a current across each wire. Rats were transcardially perfused with saline followed by 10% formalin. Brains were removed and cryoprotected and frozen sections through mPFC were cut coronally at 40 µm on a sliding microtome. Sections were stained with cresyl echt violet; marking lesions were visualized using a Prussian blue reaction. Electrode placement was verified based on standard cytoarchitectural criteria (Zilles and Wree, 1995).
Results
Paired Conditioning
To rule out potential confounds due to differences in activity level between stressed and unstressed groups, average bar presses per min on the day immediately preceding fear conditioning and extinction were compared across groups using a t-test. Stress did not significantly alter bar pressing (t = 1.46, p = 0.15).
Chronic Stress Facilitates Freezing During Fear Conditioning and Impairs Retrieval of Extinction
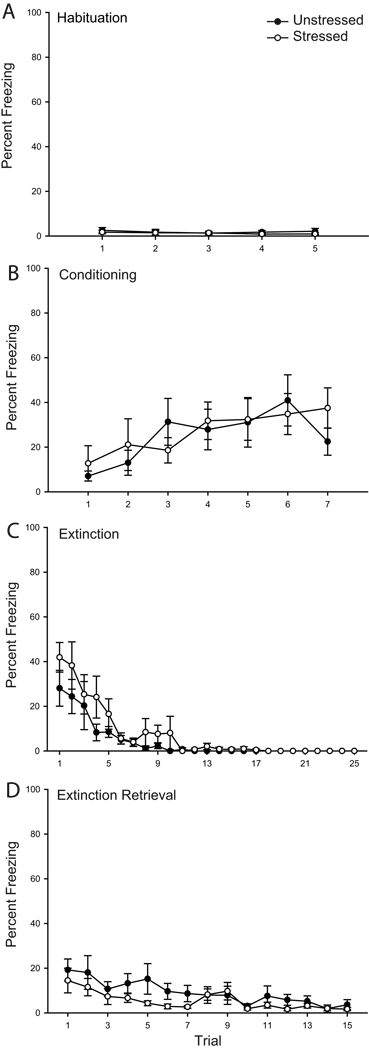
Stress did not appear to alter unconditioned responding to the tone (Fig. 1 A). Although repeated-measures ANOVA suggested a significant effect of stress during habituation (main effect of stress, F(1,34) = 6.28, p ≤ 0.05; stress by trial interaction, F(4,136) = 0.69, p = 0.69), planned comparisons revealed that unstressed and stressed rats did not differ on any individual trial (Fs(1,34) < 3.71, ns), nor when comparisons were collapsed across trials (F(1,34) = 2.40, ns).
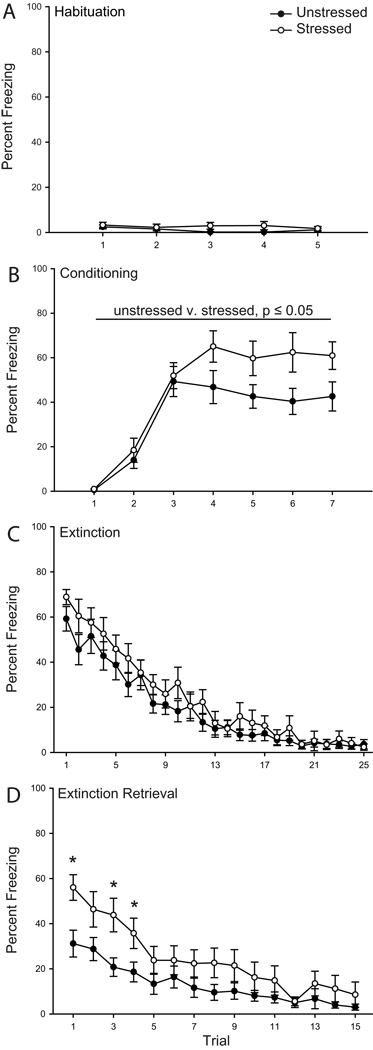
Figure 1.
Chronic stress increases freezing during fear conditioning and retrieval of extinction. Mean percent freezing to tone in rats that were unstressed (filled circles) versus stressed (open circles) across habituation (A), conditioning (B), initial extinction (C), and extinction retrieval (D) trials. Vertical bars represent SEMs. Asterisks (*) indicate significant difference relative to unstressed.
Freezing during acquisition of the conditioned fear response was facilitated by stress (Fig. 1 B). Both groups acquired the conditioned fear response, with a significant increase in freezing across trials (F(6,204) = 46.78, p ≤ 0.05). However, freezing significantly differed between unstressed and stressed rats (F(1,34) = 5.74, p ≤ 0.05), and this effect did not vary across trials (stress by trial interaction, F(6,204) = 1.98, p = 0.07). The facilitated freezing during conditioning did not significantly alter freezing to the tone 1 h later during the first trial of extinction (F(1,34) = 1.98, p = 0.17).
During initial extinction, freezing diminished across trials (Fig. 1 C; main effect of trial, F(24,816) = 57.12, p ≤ 0.05), and this did not vary with stress (main effect of stress, F(1,34) = 1.18, p = 0.29; stress by trial interaction, F(24,816) = 1.04, p = 0.41). Furthermore, trials to criterion did not differ significantly between unstressed and stressed rats (t(34) = 0.38, p = 0.70; unstressed, 17.37 ± 1.44; stressed, 16.59 ± 1.42). Therefore, chronic restraint stress did not significantly alter initial extinction learning.
However, stress significantly altered extinction retrieval assessed on day 2 (Fig. 1 D). Whereas both groups showed a decrease in freezing across trials (main effect of trial, F(14,476) = 25.18, p ≤ 0.05), stressed animals showed impaired extinction retrieval (main effect of stress, F(1,34) = 4.08, p ≤ 0.05; stress by trial interaction, F(14,476) = 2.07, p ≤ 0.05). Planned comparisons indicated that stressed animals had significantly increased freezing during trials 1, 3 and 4 (Fs(1,34) > 4.73, p ≤ 0.05). However, consistent with performance on initial extinction, freezing was not significantly different between unstressed and stressed rats on any other trial (all Fs(1,34) < 1.89, ns).
Neuron Characteristics
Unstressed rats had 10 IL and 12 PL recording sites (3 rats had dual electrode placements: 1 IL and 1 PL 2-wire or 4-wire bundles) and stressed rats had 10 IL and 9 PL recording sites (2 rats had dual electrode placements: 1 IL and 1 PL 4-wire bundles; Fig. 2A). We recorded from a total of 513 putative pyramidal neurons (unstressed IL: 32 cells during habituation and conditioning, 34 cells during extinction, and 49 cells during extinction retrieval; stressed IL: 35 cells for habituation and conditioning, 40 cells for extinction, and 43 cells for extinction retrieval; unstressed PL: 50 cells for habituation and conditioning, 49 cells for extinction, and 57 cells for extinction retrieval; stressed PL: 38 cells for habituation and conditioning, 40 cells for extinction, and 46 cells for extinction retrieval).
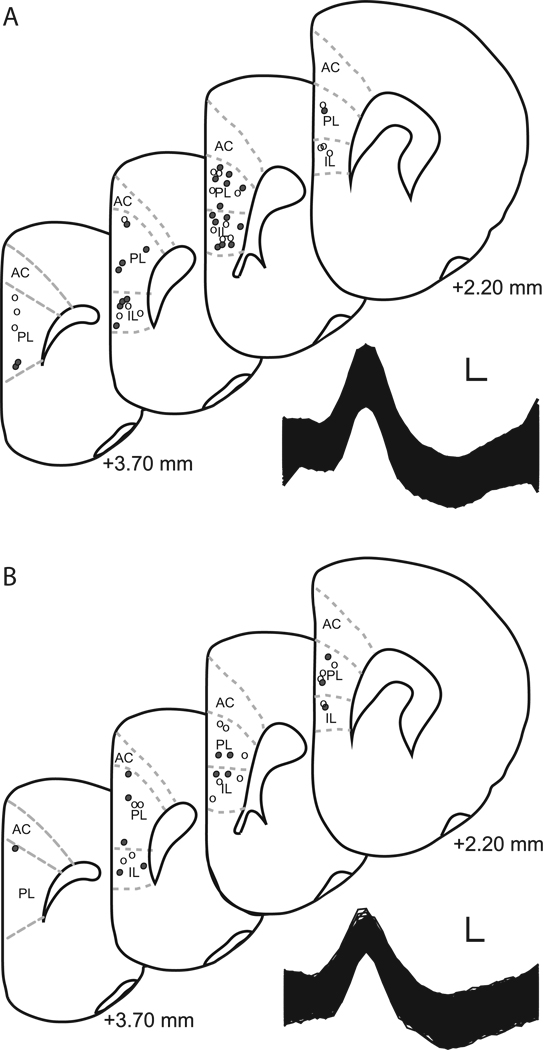
Figure 2.
A. Above. Recording sites in infralimbic (IL) and prelimbic (PL) cortex for unstressed (filled circles; IL n = 10, PL n= 12; 3 rats had dual IL and PL placed electrode) and stressed rats (open circles; IL n = 10, PL n=9; 2 rats had dual IL and PL placed electrode) that underwent paired fear conditioning. Numbers indicate position relative to bregma. Below. Raw waveform of a representative neuron recorded from PL of an unstressed rat. Scale bar 25 µV by 0.1 ms. B. Above. Recording sites in infralimbic (IL) and prelimbic (PL) cortex for unstressed (filled circles; IL n = 5, PL n= 9) and stressed rats (open circles; IL n = 6, PL n=8) receiving explicitly unpaired presentations of tone and shock. Numbers indicate position relative to bregma. Below. Raw waveform of a representative neuron recorded from IL of an unstressed rat. Scale bar 25 µV by 0.1 ms.
Chronic stress Alters Baseline Firing Rate in mPFC During Conditioning
To assess potential stress effects on baseline activity, we examined baseline firing rates averaged across all trials for each phase of training (Table 1). Overall, there was a significant main effect of stress (F(1,658) = 5.77, p ≤ 0.05), brain region (F(1,658) = 23.50, p ≤ 0.05), and training phase (F(3,658) = 13.40, p ≤ 0.05) on baseline firing rate. In addition, there were significant stress × training phase (F(3,658) = 4.44, p ≤ 0.05) and brain region × training phase (F(3,658) = 3.37, p ≤ 0.05) interactions, but not stress × brain region (F(1,658) = 0.10, p = 0.75) or stress × brain region × training phase interactions (F(3,658) = 1.02, p = 0.38). Thus, for each phase of training, baseline firing rate within each brain region was compared between unstressed and stressed rats using F-tests. In addition, one-way ANOVAs were used to compare firing rates across training phases within PL and IL for unstressed and stressed rats.
Table 1.
Means and SEMs for baseline firing rates in infralimbic and prelimbic cortex of unstressed and stressed rats across phases of training.
| Infralimbic Cortex | ||
| Training Phase | Unstressed | Stressed |
| Habituation | 4.45 (0.56) | 4.25 (0.67) |
| Conditioning | 2.33 (0.40)† | 3.11 (0.66) |
| Extinction | 6.55 (1.26) | 4.10 (0.48) |
| Extinction Retrieval | 3.30 (0.30)‡ | 3.00 (0.37) |
| Prelimbic Cortex | ||
| Training Phase | Unstressed | Stressed |
| Habituation | 3.56 (0.39) | 3.18 (0.44) |
| Conditioning | 1.88 (0.31)† | 1.22 (0.21)† |
| Extinction | 3.87 (0.45) | 2.07 (0.21)*§ |
| Extinction Retrieval | 2.85 (0.34) | 2.96 (0.46) |
Asterisk (*), significant difference relative to unstressed within a training phase and brain region;
dagger (†), significant difference relative to habituation, initial extinction, and extinction retrieval within a treatment group and brain region;
double dagger (‡), significant difference relative to habituation and initial extinction within a treatment group and brain region;
section sign (§), significant difference relative to habituation within a treatment group and brain region
In IL, there was no effect of stress on baseline firing rate during habituation (F(1,65) = 0.05, p = 0.82), conditioning (F(1,65) = 0.05, p = 0.82), extinction (F(1,72) = 3.70, p = 0.06), or retrieval of extinction (F(1,90) = 2.11, p = 0.06). Baseline firing rate varied significantly across training phases for unstressed (F(3,143) = 6.53, p ≤ 0.05) but not stressed (F(3,149) = 6.53, p = 0.22) rats. Planned comparisons indicated that baseline firing rate was reduced during conditioning relative to habituation, initial extinction, and extinction retrieval for unstressed rats (Fs(1,62–79) > 3.87, p ≤ 0.05), and baseline firing rate was decreased during extinction retrieval relative to habituation and initial extinction (Fs(1, 79&81) > 3.92, p ≤ 0.05).
In PL, baseline firing rates did not differ between unstressed and stressed rats for habituation (F(1,86) = 0.43, p = 0.51), conditioning (F(1,86) = 2.71, p = 0.10), or retrieval of extinction (F(1,101) = 0.04, p = 0.84), but were significantly decreased in stressed relative to unstressed rats during initial extinction (F(1,87) = 11.25, p ≤ 0.05). In PL, baseline firing rate varied significantly across training phase for unstressed (F(3,202) = 5.41, p ≤ 0.05) and stressed rats (F(3,158) = 5.88, p ≤ 0.05). Planned comparisons indicated that baseline firing rate was reduced during conditioning relative to habituation, initial extinction, and extinction retrieval for both unstressed and stressed rats (Fs(1,74–105) > 4.36, p ≤ 0.05), and baseline firing rate was also reduced during initial extinction relative to habituation for stressed rats (F(1,76) = 5.26, p ≤ 0.05). Thus, in unstressed rats, baseline firing rate in both IL and PL was reduced during conditioning, but stress eliminated this reduction in IL.
Chronic Stress Alters CS-Related Neural Activity in mPFC during Conditioning and Extinction Retrieval
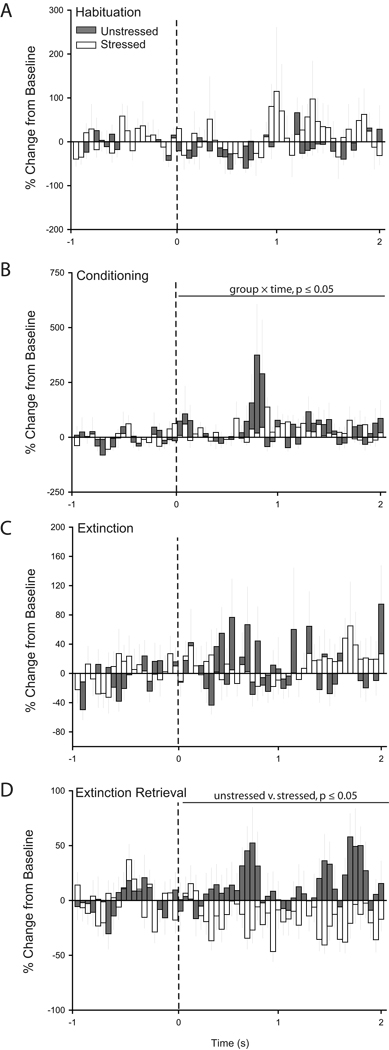
Next, we examined CS-related activity for each phase of training (Figs. 3 and 4). Visual inspection of PSTHs suggested that IL neurons were not responsive to the tone during habituation, slightly increased firing during conditioning for unstressed but not stressed rats, decreased firing during initial extinction for both unstressed and stressed rats, and increased firing in response to the tone during extinction retrieval for unstressed but not stressed rats (Fig. 3). Two-way repeated measures ANOVAs confirmed that stress did not significantly alter CS-related activity during habituation (main effect of stress, F(1,65) = 0.72, p = 0.40; stress × time interaction, F(39, 2535) = 0.83, p = 0.76) and initial extinction (main effect of stress, F(1,72) = 0.21, p = 0.65; stress × time interaction, F(39, 2808) = 0.97, p = 0.53). However, during conditioning, units in unstressed rats showed significantly more activity in response to the CS compared to neurons in stressed rats (main effect of stress, F(1,65) = 9.43, p ≤ 0.05; stress × time interaction, F(39,2535) = 1.29, p = 0.10). A more profound difference between unstressed and stressed rats was apparent during retrieval of extinction: units in stressed rats showed significantly less activity in response to the CS compared to neurons in unstressed rats (Fig. 3, main effect of stress, F(1,65) = 13.00, p ≤ 0.05; stress × time interaction, F(39,3510) = 0.59, p = 0.98). Thus, during extinction retrieval the CS-related increase in IL activity in unstressed rats was similar to previous reports (Milad and Quirk, 2002), but this normal pattern of activity was absent in stressed rats.
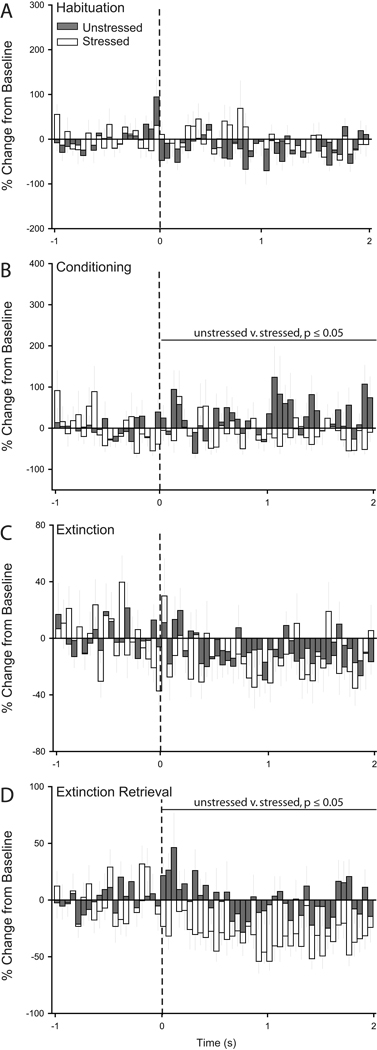
Figure 3.
Chronic stress decreases CS-evoked firing in infralimbic cortex during retrieval of extinction. Peri-stimulus time histograms averaged across trials for habituation (A), conditioning (B), initial extinction (C), and extinction retrieval (D) showing percent change from baseline (50 ms bin size) for the 2 s following tone onset (dashed line) for units recorded from infralimbic cortex of unstressed (32 cells for Habituation/Conditioning, 34 cells for Extinction, and 49 cells for Extinction Retrieval) and stressed rats (35 cells for Habituation/Conditioning, 40 cells for Extinction, and 43 cells for Extinction Retrieval) that underwent paired fear conditioning.
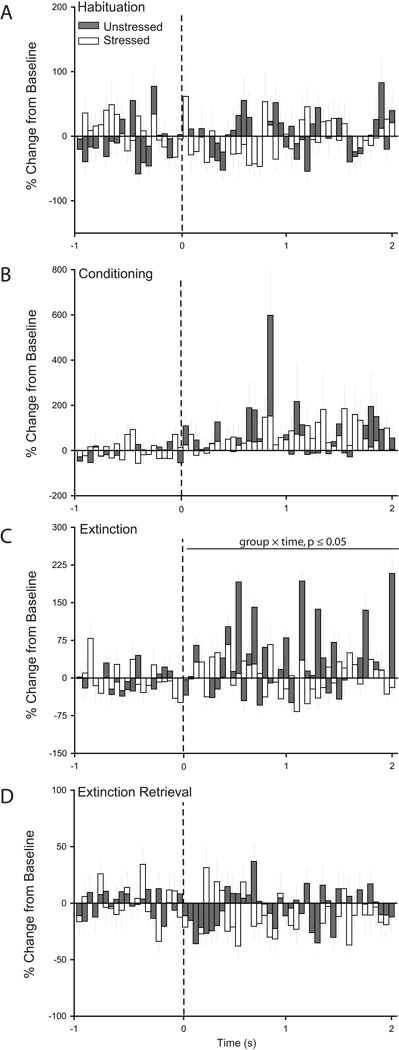
Figure 4.
Chronic stress increases CS-evoked firing in prelimbic cortex during retrieval of extinction. Peri-stimulus time histograms averaged across trials for habituation (A), conditioning (B), initial extinction (C), and extinction retrieval (D) showing percent change from baseline (50 ms bin size) for the 2 s following tone onset (dashed line) for units recorded from prelimbic cortex of unstressed (50 cells for Habituation/Conditioning, 49 cells for Extinction, and 57 cells for Extinction Retrieval) and stressed rats (38 cells for Habituation/Conditioning, 40 cells for Extinction, and 46 cells for Extinction Retrieval) that underwent paired fear conditioning.
Visual inspection of PSTHs suggested that in unstressed rats, PL neurons were not responsive to the tone during habituation, increased CS-related firing during conditioning, and were inhibited during both initial extinction and extinction retrieval. On the other hand, in stressed rats, PL neurons appeared to be relatively unresponsive throughout all phases of training (Fig. 4). Two-way repeated-measures ANOVAs indicated that stress did not significantly alter CS-evoked firing of PL neurons during habituation (main effect of stress, F(1,86) = 0.12, ns; stress × time interaction, F(39, 3354) = 1.15, p = 0.25), initial extinction (main effect of stress, F(1,87) = 1.64, p = 0.20; stress × time interaction, F(39,3939) < 1.33, p = 0.08), and retrieval of extinction (main effect of stress, F(1,101) = 2.70, p = 0.10; stress × time interaction, F(39,3939) = 0.94, p = 0.58). However, during conditioning, stress significantly decreased the tone response (F(1,86) = 7.93, p ≤ 0.05), and this effect did not vary with time (F(39,3354) = 0.91, p = 0.63).
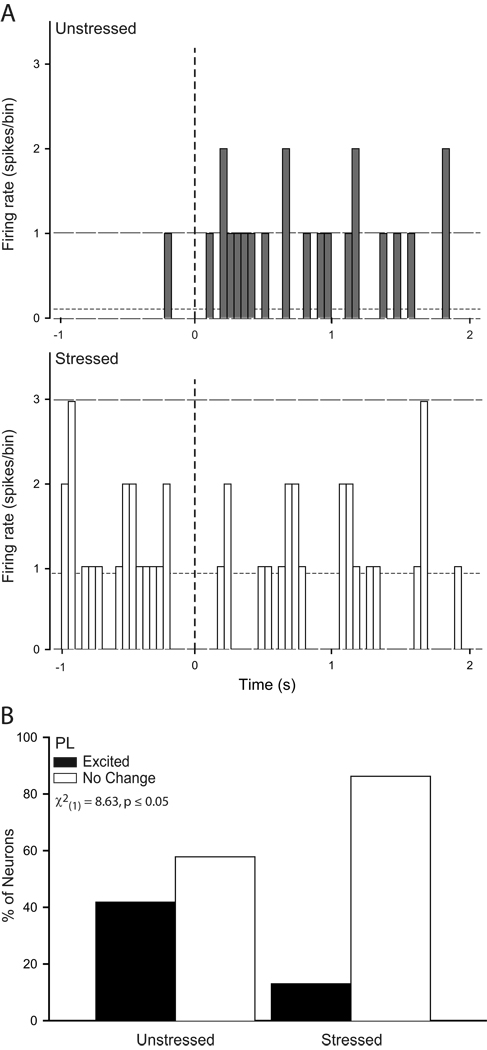
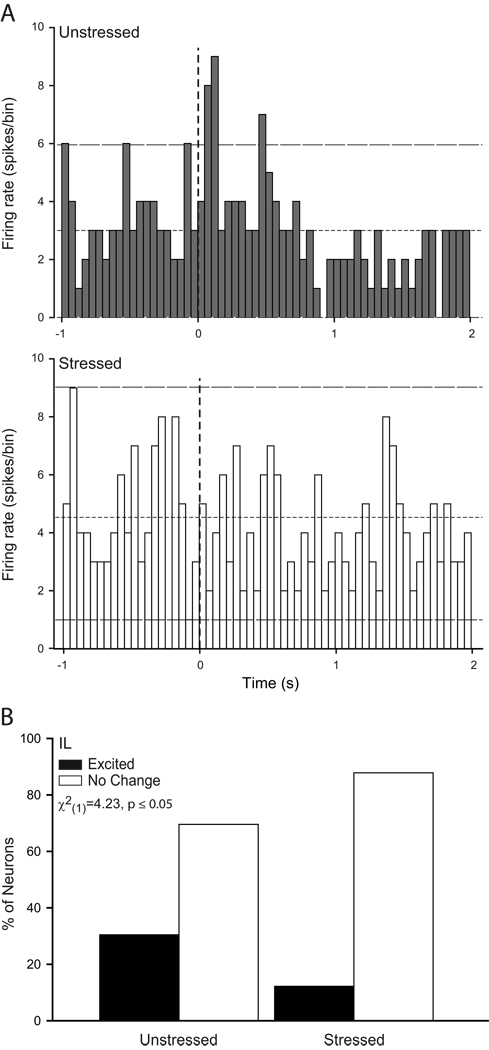
Because the overall PSTHs revealed significant stress effects on neural activity during conditioning and extinction retrieval, individual-neuron analyses focused on these training phases. The activity of individual neurons in these phases paralleled that seen in the group PSTHs (see Figs. 5 and 6). Because the number of neurons meeting the criterion for inhibition was quite low (in IL, less than 6% during either phase examined; in PL, less than 4% in either phase examined), inhibited neurons were excluded from further analysis. Thus, we compared the proportion of excited versus unresponsive neurons using chi-square analyses. During conditioning, the proportion of excited neurons did not significantly differ in IL of unstressed versus stressed rats (unstressed, 31%; stressed, 17%; X2(1) = 1.83, p = 0.18; data not shown), whereas three times as many PL neurons were excited by the tone in unstressed compared to stressed rats (Fig. 5; X2(1) = 8.63, p ≤ 0.05). Thus, the proportion of CS-excited neurons in PL of unstressed rats was consistent with previous reports (Burgos-Robles et al., 2009), but this normal pattern of excitation was absent in stressed rats during conditioning. On the other hand, during extinction retrieval, twice as many IL neurons were excited by the tone in unstressed compared to stressed rats (Fig. 6; X2(1) = 4.23, p ≤ 0.05), while the proportion of excited PL neurons did not vary with stress (15% of unstressed and 17% of stressed; X2(1) = 0.07, p = 0.79; data not shown).
Figure 5.
Chronic stress decreases the proportion of neurons in prelimbic cortex that show CS-induced excitation during paired conditioning. (A) Representative raw firing rate (50 ms bin size) for the 1 s preceding and 2 s following CS onset (vertical dashed line) for a neuron in prelimbic cortex of an unstressed (above) and a stressed (below) rat. Horizontal lines indicate mean baseline firing rate (short dash) and 95% confidence interval for increased and decreased (long dash) firing relative to baseline. (B) Percentage of CS-responsive cells that were classified as excited (≥ 2 bins that crossed the upper 95% confidence limit) or no change (neither excited nor inhibited in response to the CS) recorded in prelimbic cortex of unstressed and stressed rats during extinction retrieval. There was a significantly higher proportion of excited PL neurons in unstressed rats.
Figure 6.
Chronic stress decreases the proportion of neurons in infralimbic cortex that show CS-induced excitation during extinction retrieval following paired conditioning. (A) Representative raw firing rate (50 ms bin size) for the 1 s preceding and 2 s following CS onset (vertical dashed line) for a neuron in infralimbic cortex of an unstressed (above) and a stressed (below) rat. Horizontal lines indicate mean baseline firing rate (short dash) and 95% confidence interval for increased and decreased (long dash) firing relative to baseline. (B) Percentage of CS-responsive cells that were classified as excited (≥ 2 bins that crossed the upper 95% confidence limit) or no change (neither excited nor inhibited in response to the CS) recorded in infralimbic cortex of unstressed and stressed rats during extinction retrieval. There was a significantly higher proportion of excited IL neurons in unstressed rats.
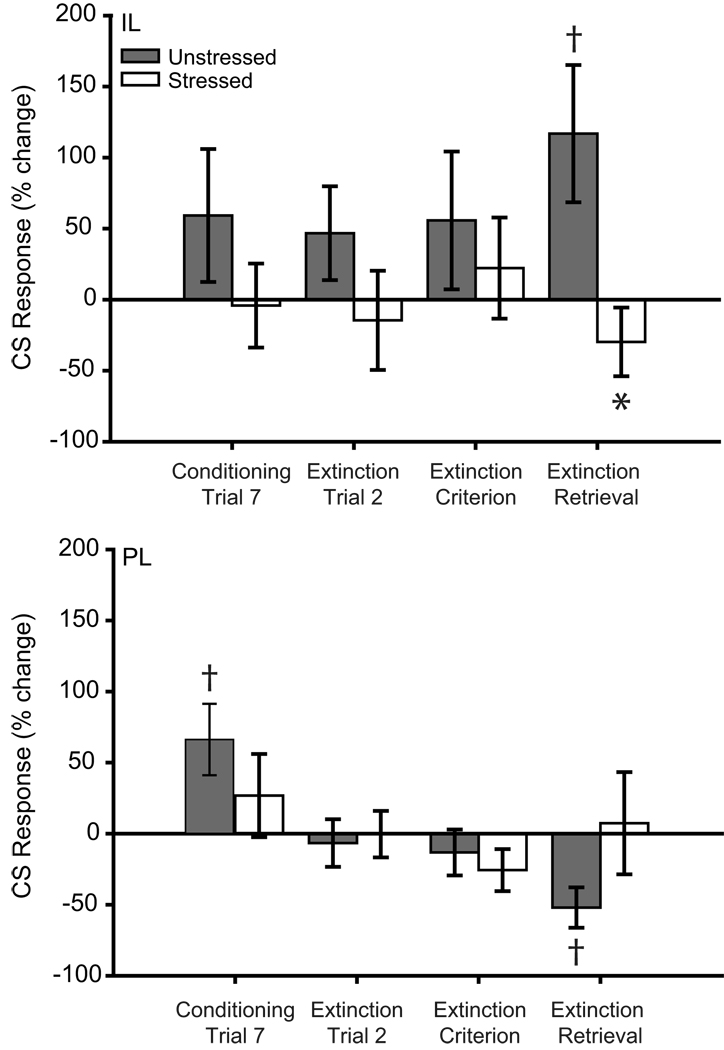
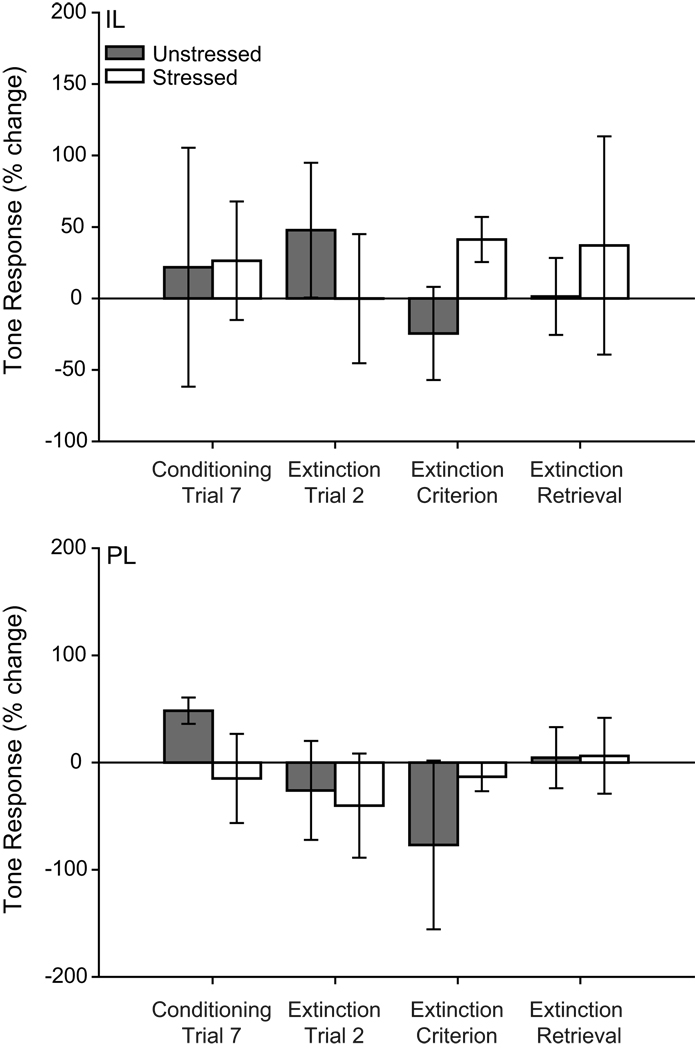
Comparisons of activity on individual trials indicated that in IL, stress significantly altered CS-induced responding (Fig. 7; main effect of stress, F(1,17) = 5.50, p ≤ 0.05; stress × training phase interaction, F(3,51) = 0.91, p = 0.44; main effect of training phase, F(3,51) = 0.21, p = 89). Consistent with the PSTH analyses, for the first trial of extinction retrieval, unstressed rats showed a significant CS-induced increase in firing rate (t(9) = 2.71, p ≤ 0.05), whereas stressed rats (t(9) = −1.23, p = 0.25) did not. Moreover, unstressed animals had significantly more CS-induced excitation than stressed rats (t(18) = 2.42, p ≤ 0.05). However, no significant differences were present for either the final trial of conditioning or the first extinction trial (all ts(9) < 1.84, all ps ≥ 0.10). Thus, IL activity was significantly increased in response to the tone during extinction retrieval in unstressed rats, consistent with previous reports (Milad and Quirk, 2002), but this normal pattern of activity was absent in stressed rats.
Figure 7.
Neurons in infralimbic and prelimbic cortex of chronically stressed rats that underwent paired fear conditioning are unresponsive to CS during conditioning and retrieval of extinction. Mean CS-related activity (expressed as percent change from baseline) of infralimbic (above) and prelimbic (below) units in the first 200ms (infralimbic) or 2 s (prelimbic) after CS onset for the initial or final trial for conditioning, extinction and extinction retrieval. Asterisk (*) indicates significant difference relative to unstressed; daggers (†) indicate significant differences relative to baseline.
For PL, CS-induced responding varied across phases of training (main effect of training phase, F(3,54) = 4.56, p ≤ 0.05; main effect of stress, F(1,18) = 0.03, p = 0.98; stress × training phase interaction, F(3,54) = 2.10, p = 0.11). For the last trial of conditioning there was significant CS-induced excitation from baseline in unstressed rats (t(10) = 2.61, p ≤ 0.05) but not stressed rats (t(8) = 0.92, p = 0.38). For the first trial of extinction retrieval, unstressed rats had significant CS-induced PL inhibition (t(11) = −3.66, p ≤ 0.05), whereas stressed rats did not have a significant CS-response (t(8) = 0.20, p = 0.85). All other comparisons were nonsignificant (all ts(19) ≤ 1.70, ps ≥ 0.11; all ts(11) ≤ −0.82, ps ≥ 0.43; all ts(8) ≤ −1.74, ps ≥ 0.12). Thus, CS responses of PL neurons in unstressed rats were consistent with previous data (Burgos-Robles et al., 2009), while PL neurons in stressed rats did not respond to the CS.
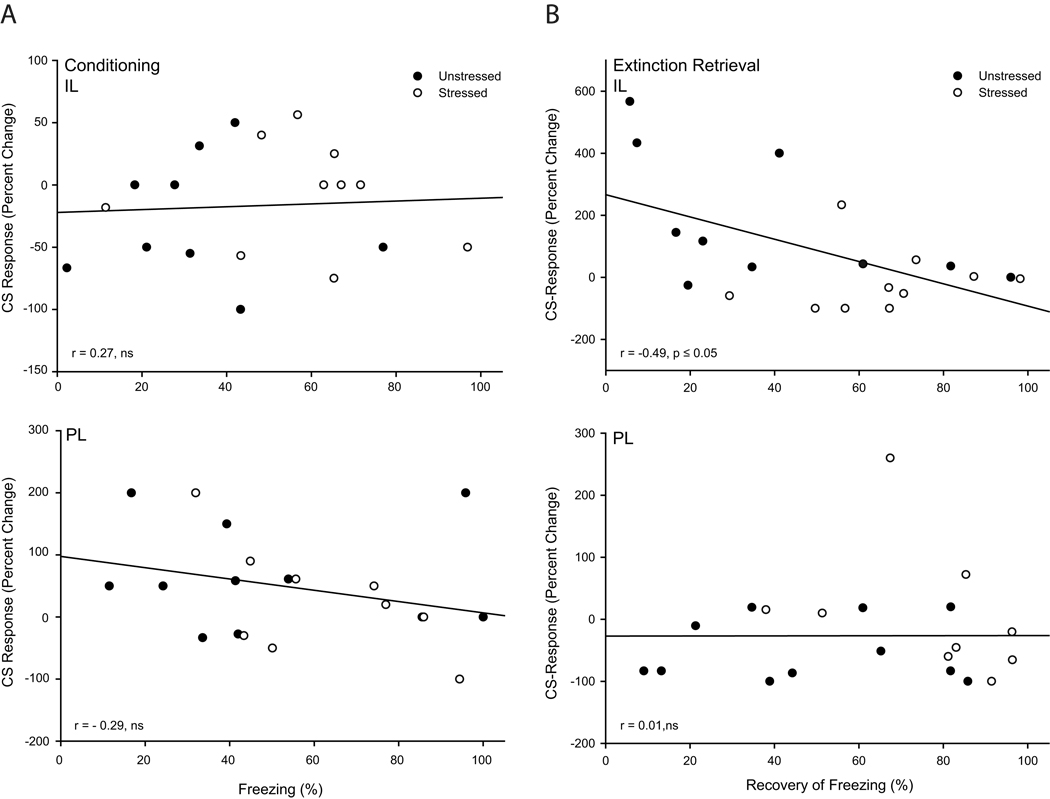
Activity in IL but not PL Is Correlated with Retrieval of Extinction
The relationship between activity in mPFC and performance during the two phases of training during which stress significantly altered behavior were examined. Correlations between unit activity (expressed as percent change from baseline on trial one of extinction retrieval; calculated as described in Methods above) in either IL or PL, and percent freezing during the last trial of conditioning and spontaneous recovery of freezing on day 2 (defined as percent freezing on trial 1 of extinction retrieval/maximum freezing during initial extinction) were computed. Unit activity during the last trial of fear acquisition in either PL or IL and average freezing on this trial were not significantly correlated (Fig. 8; for PL, r=−0.29, p = 0.22; for IL, r=0.27, p = 0.61). For IL, CS-evoked unit activity was significantly and negatively correlated with spontaneous recovery, with greater CS-evoked inhibition associated with more freezing during extinction retrieval (Fig. 8; r = −0.49, p ≤ 0.05). On the other hand, PL unit activity was not significantly correlated with freezing during extinction retrieval (Fig. 8; r = 0.01, p = 0.98). Thus, stress-induced alterations in IL but not PL unit activity were associated with deficits in retrieval of extinction.
Figure 8.
Stress-induced inhibition of infralimbic activity is associated with poor extinction retrieval following paired conditioning. (A) Linear regression for average unit activity for each rat (percent change from baseline during the first 2 s of CS on trial 1 of extinction retrieval) versus freezing on the last trial of conditioning for neurons in infralimbic (above) and prelimbic cortex (below). (B) Linear regression for average unit activity for each rat (percent change from baseline during the first 200ms of CS on trial 1 of extinction retrieval) versus recovery of freezing on day 2 (percent freezing on trial 1 of extinction retrieval/maximum freezing during initial extinction) for neurons in infralimbic (above) and prelimbic cortex (below).
Unpaired Controls
Behavior
As in rats undergoing paired conditioning, stress did not alter unconditioned responding to the tone during habituation trials (Fig. 9 A; main effect of stress, F(1,16) =0.51, p=.48; stress by trial interaction, F(4,64) = 0.22, p = 0.93). Likewise, although both groups’ freezing to tone increased significantly across unpaired conditioning trials (Fig. 9 B; F(6,96) = 3.93, p ≤ 0.05), this did not significantly differ between unstressed and stressed rats (F(1,16) = 0.06, p = 0.80), and this was consistent across trials (stress by trial interaction, F(6,96) = 0.92, p = .49). During initial extinction, freezing diminished across trials (Fig. 9 C; main effect of trial, F(24,384) = 15.84, p ≤ 0.05), and this did not vary with stress (main effect of stress, F(1,16) = 1.90, p = 0.19; stress by trial interaction, F(24,384) = 0.99, p = 0.48). Furthermore, trials to criterion did not differ significantly between unstressed and stressed rats (t(16) = 1.31, p = 0.21; unstressed, 6.89 ± 0.89; stressed, 9.00 ± 1.34). Finally, stress did not significantly alter extinction retrieval assessed on day 2 (Fig. 9 D). Stressed and unstressed rats showed comparable decreases in freezing across trials (main effect of trial, F(14,224) = 5.13, p ≤ 0.05; main effect of stress, F(1,16) = 1.54, p = 0.23; stress × trial interaction, F(14,224) = 0.72, p = 0.76). Thus, the stress-induced deficit in extinction retrieval in the paired rats reflects learning-specific alterations.
Figure 9.
Chronic stress does not alter freezing during unpaired fear conditioning, extinction, or retrieval of extinction. Mean percent freezing to tone in unstressed (filled circles) versus stressed (open circles) rats across habituation (A), unpaired conditioning (B), initial extinction (C), and extinction retrieval (D) trials. Vertical bars represent SEMs.
Unit Activity
Unstressed rats had 5 IL and 8 PL recording sites and stressed rats had 6 IL and 8 PL recording sites (Fig. 2B). Five unstressed and six stressed rats had dual electrode placements (1 IL and 1 PL 4-wire bundle). For unstressed IL, we recorded from an additional 967 putative pyramidal neurons (for unstressed PL, 41 cells during habituation, 31 cells during conditioning, 47 cells during extinction, and 45 cells during extinction retrieval; for stressed IL, 77 cells for habituation, 68 for conditioning, and 70 each for extinction and extinction retrieval; for unstressed PL, 58 cells for habituation, 46 for conditioning, 69 for extinction, and 74 for extinction retrieval; for stressed PL, 70 cells for habituation, 50 for conditioning, 76 for extinction, and 75 for extinction retrieval).
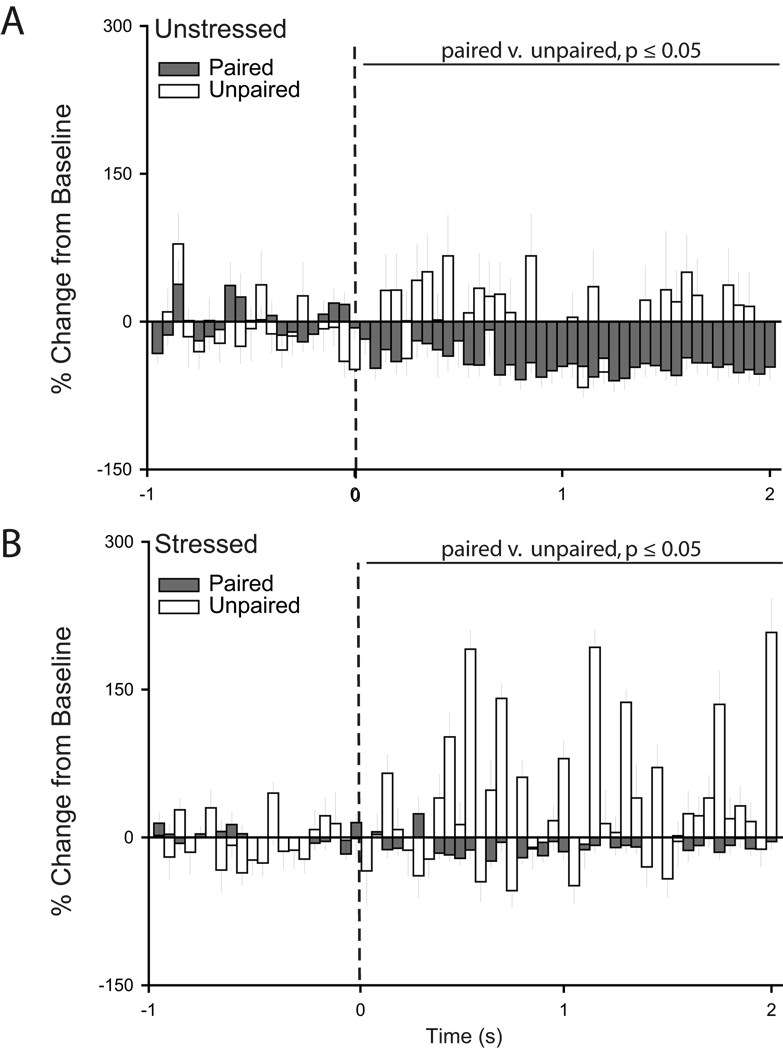
Two-way repeated measures ANOVAs showed that for IL units, stress did not significantly alter tone-related activity during habituation (Fig. 10A; main effect of stress, F(1,116) = 1.93, p = 0.17; stress × time interaction, F(39, 4524) = 1.15, p = 0.24) and initial extinction (Fig. 10C; main effect of stress, F(1,115) = 0.00, p = 0.99; stress × time interaction, F(39, 4485) = 0.63, p = 0.97). However, during conditioning, whereas there was no overall difference in unit activity (main effect of stress, F(1,97) = 1.78, p = 0.19), the pattern of tone-related activity was significantly different in unstressed versus stressed rats (Fig. 10B; stress × time interaction, F(39,3783) = 1.76, p ≤ 0.05). Further, during extinction retrieval, units in stressed rats showed significantly less activity in response to the tone compared to neurons in unstressed rats (Fig. 10D, main effect of stress, F(1,120) = 10.82, p ≤ 0.05; stress × time interaction, F(39,4680) = 1.58, p ≤ 0.05).
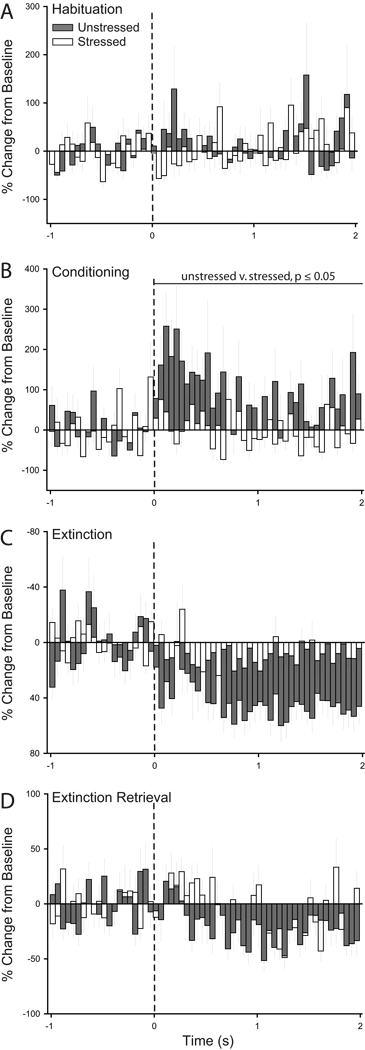
Figure 10.
Peri-stimulus time histograms averaged across trials for habituation (A), conditioning (B), initial extinction (C), and extinction retrieval (D) showing percent change from baseline (50 ms bin size) for the 2 s following tone onset (dashed line) for units recorded from infralimbic cortex of unstressed and stressed rats that underwent unpaired fear conditioning.
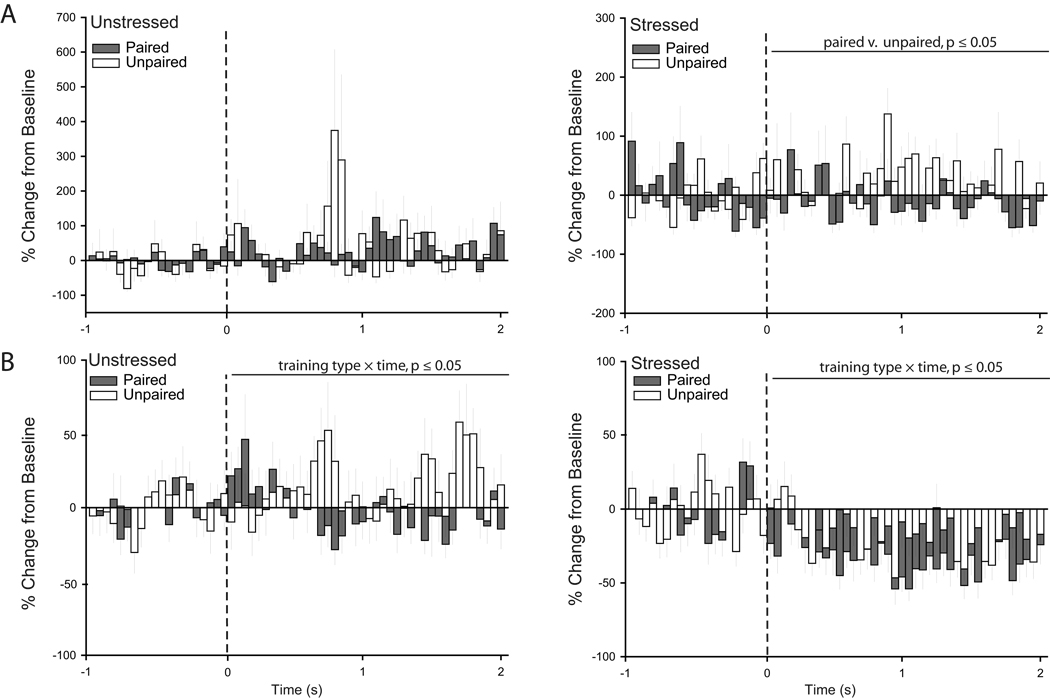
This difference in activity in unstressed versus stressed rats was present despite the fact that visual inspection of PSTHs suggested that the patterns of responding in IL neurons were different in unstressed rats that underwent paired versus unpaired conditioning. Two-way repeated measures ANOVAs confirmed that although tone-related IL activity was similar in unstressed unpaired and paired rats during conditioning (Fig. 11A; main effect of training type, F(1,61) = 1.40, p = 0.24; training type × time interaction, F(39,2379) = 1.20, p = 0.18), the pattern of tone-related activity during extinction retrieval was different (Fig. 11B; training type × time interaction, F(39,3588) = 1.47, p ≤ 0.05; main effect of training type, F(1,92) = 2.30, p = 0.13). Similarly, patterns of firing were significantly different in stressed paired versus unpaired rats during both conditioning (Fig. 11A; main effect of training type, F(1,101) = 6.86, p ≤ 0.05; training type × time interaction, F(39,3939) = 0.97, p = 0.52) and extinction retrieval (Fig. 11B; training type × time interaction, F(39,4602) = 1.46, p ≤ 0.05; main effect of training type, F(1,118) = 0.55, p = 0.82).
Figure 11.
Differential patterns of IL unit activity result from paired versus unpaired conditioning. While the tone-induced inhibition apparent in stressed animals appears during unpaired and paired extinction retrieval, it is more pronounced during paired training relative to unpaired training. A. Peri-stimulus time histograms averaged across conditioning trials showing percent change from baseline (50 ms bin size) for the 2 s following tone onset (dashed line) for units recorded from infralimbic cortex of unstressed (left) and stressed rats (right) that underwent either paired or unpaired fear conditioning. B. Peri-stimulus time histograms averaged across extinction retrieval trials showing percent change from baseline (50 ms bin size) for the 2 s following tone onset (dashed line) for units recorded from infralimbic cortex of unstressed (left) and stressed rats (right) that underwent either paired or unpaired fear conditioning.
For PL units, stress did not significantly alter tone-related activity during habituation (Fig. 12A; main effect of stress, F(1,126) = 0.15, p = 0.70; stress × time interaction, F(39, 4914) = 1.24, p = 0.15), conditioning (Fig. 12B; main effect of stress, F(1,94) = 0.11, p = 0.74; stress × time interaction, F(39, 3666) = 1.05, p = 0.38), or extinction retrieval (Fig. 12D; main effect of stress, F(1,147) = 1.30, p = 0.26; stress × time interaction, F(39, 5733) = 1.32, p = 0.09). However, during initial extinction, whereas there was no overall difference in unit activity (main effect of stress, F(1,143) = 1.33, p = 0.25), the pattern of tone-related activity was significantly different in unstressed versus stressed rats (Fig. 12C; stress × time interaction, F(39,5577) = 1.44, p ≤ 0.05). This difference in activity in unstressed versus stressed rats was again present despite the fact that the patterns of responding in PL neurons in unstressed rats undergoing paired versus unpaired conditioning were strikingly different (Fig. 13; main effect of training type, F(1,116) = 8.04, ≤ 0.05; training type × time interaction, F(39,4524) = 0.74, p = 0.88). Similar differences were found in stressed paired versus unpaired rats (Fig. 13; main effect of training type, F(1,114) = 7.82, ≤ 0.05; training type × time interaction, F(39,4446) = 0.82, p = 0.78).
Figure 12.
Peri-stimulus time histograms averaged across trials for habituation (A), conditioning (B), initial extinction (C), and extinction retrieval (D) showing percent change from baseline (50 ms bin size) for the 2 s following tone onset (dashed line) for units recorded from prelimbic cortex of unstressed and stressed rats that underwent unpaired fear conditioning.
Figure 13.
Differential patterns of PL unit activity occur during paired versus unpaired conditioning. Peri-stimulus time histograms averaged across initial extinction trials showing percent change from baseline (50 ms bin size) for the 2 s following tone onset (dashed line) for units recorded from infralimbic cortex of unstressed (above) and stressed rats (below) that underwent either paired or unpaired fear conditioning.
Individual neuron analyses focused on conditioning and extinction retrieval, the training phases in which stress effects were found for paired conditioning. Chi-square analyses indicated that the proportion of excited neurons did not significantly differ in IL of unstressed versus stressed rats during conditioning (unstressed, 29%; stressed, 16%; X2(1) = 2.18, p = 0.14; data not shown) or extinction retrieval (unstressed, 22%; stressed, 14%; X2(1) = 1.26, p =.26; data not shown), nor did they significantly differ in PL (conditioning: unstressed, 24%; stressed, 20%; X2(1) = 0.21, p = 0.65; extinction retrieval: unstressed, 15%; stressed, 15%; X2(1) = 0.001, p =0.97). Likewise, comparisons of activity on individual trials indicated that there were no significant differences in tone-induced responding during any training phase (Fig. 14; IL: main effect of stress, F(1,9) = 0.09, p = 0.77; stress × training phase interaction, F(3,27) = 0.60, p = 0.62; PL: main effect of stress, F(1,14) = 0.01, p = 0.92; stress × training phase interaction, F(3,42) = 0.70, p = 0.56).
Figure 14.
Tone-related activity for specific trials does not significantly differ for unstressed and stressed rats that underwent unpaired conditioning. Mean tone-related activity (expressed as percent change from baseline) of infralimbic (above) and prelimbic (below) units in the first 200ms (infralimbic) or 2 s (prelimbic) after CS onset for the initial or final trial for conditioning, extinction and extinction retrieval.
Discussion
We found that stress enhanced freezing during acquisition of conditioned fear, and altered activity of both PL and IL neurons during this phase of training. While unstressed rats showed reduced baseline firing of both PL and IL neurons during conditioning, stressed rats did not show this reduction in IL. Further, CS-evoked activity in stressed rats was reduced relative to that in unstressed rats in both PL and IL. In contrast, while stressed rats showed decreased baseline firing rates in PL during initial extinction, stress did not significantly alter initial extinction or CS-related mPFC activity during this phase. However, stress impaired retrieval of extinction assessed 24 h later, and this was accompanied by alterations in neuronal activity in PL and IL. In PL, stress prevented the CS-evoked inhibition of activity seen in unstressed rats. In IL, neurons in unstressed rats exhibited increased firing rate in response to the CS, whereas stressed rats showed inhibition of IL firing during the tone. Finally, CS-related firing in IL but not PL was correlated with extinction retrieval.
Importantly, stress did not significantly alter freezing during any training phase in rats that underwent explicitly unpaired presentations of the tone and shock during conditioning, suggesting that the behavioral changes seen with paired training reflect learning-related alterations. Further, unstressed rats undergoing unpaired training did not show the significant alterations in tone-related firing in IL or PL seen in unstressed rats receiving paired conditioning, indicating that the changes in unit activity in the unstressed rats are specific to learning. Similarly, stressed rats undergoing unpaired training had significantly different tone-related firing in IL and PL compared to stressed rats that underwent paired training, indicating that the changes in unit activity in the stressed rats were also specific to paired presentations. However, alterations in tone-related activity in stressed versus unstressed rats were present with either unpaired or paired training, suggesting that stress produced mPFC dysfunction regardless of what is being learned. This is consistent with the hypothesis that chronic stress produces pervasive changes not just in the morphology of mPFC (e.g., Cook & Wellman, 2004) but also its physiology (e.g., Goldwater et al., 2009). Indeed, visual inspection of our data suggest a relatively generalized suppression of responding in mPFC throughout the training sessions in the stressed rats, which becomes more pronounced during the final session. Thus, while the pattern of activity in unstressed mPFC is learning-specific, the dysfunction in stressed mPFC is not, but sets the stage for a disruption of extinction learning.
Chronic Stress Alters Fear Conditioning and Extinction Retrieval
One week of daily restraint stress increased freezing during acquisition of conditioned fear. Although stressed rats showed increased freezing during the final trials of conditioning, they did not show more freezing during the initial extinction trials, nor did they require more trials to reach extinction criterion. This dissociation may reflect a selective effect on expression of conditioned fear during acquisition. This is in contrast to our previous study (Miracle et al., 2006), in which one week of restraint stress produced significant changes in retrieval of extinction but not acquisition of conditioned fear. Differences in the stressors involved in the two studies could explain this difference. Longer-term chronic restraint stress can both facilitate fear conditioning (Conrad et al., 1999) and increase dendritic extent and spine density in basolateral amygdala (Vyas et al., 2002;Vyas et al., 2006). It is interesting to speculate that the combination of a prior stressor—e.g, the surgery and/or food restriction—and the short-term, moderately intense restraint stress in the present study resulted in a facilitation of fear conditioning, perhaps mediated by changes in basolateral amygdala. Alternatively, it is possible that nonsignificant trends present in the original study simply reached significance here.
Consistent with previous reports, stress also impaired retrieval of extinction (Miracle et al., 2006;Garcia et al., 2008;Baran et al., 2009;Farrell et al., 2010). The impairment in extinction retrieval was not likely due to facilitation of fear conditioning, as there were no differences in freezing on the initial extinction trials, and the number of trials required to reach criterion did not vary between stressed and unstressed rats. Further, others have shown that chronic restraint stress in male rats does not increase reinstatement of conditioned fear via unsignaled footshock, or expression of the conditioned fear memory in the absence of prior extinction trials (Baran et al., 2009). Nonetheless, it is possible that the effect seen during extinction retrieval might be due to strengthening of the CS-US association and therefore increased spontaneous recovery on day 2 rather than an actual decrement in extinction retrieval. Therefore, we cannot rule out the possibility that the stress-induced facilitation of freezing during fear conditioning contributed to the changes seen during extinction retrieval. A future study could address this possibility by stressing animals after fear conditioning rather than before.
In the present study, we demonstrated that unstressed and stressed rats did not differ in freezing response to the tone during any phase of testing when the tone and shock were explicitly unpaired. Others have shown that unstressed and stressed rats show comparable behavioral responses (jumping, flinching) to unsignaled footshock (Baran et al., 2009). Thus, nonassociative processes such as heightened emotionality or sensitization are unlikely to explain the increased freezing during extinction retrieval. Finally, Baran and colleagues (2009) demonstrated stress-induced deficits in retrieval of extinction but no difference between stressed and unstressed rats in freezing to context during this phase. Thus, while we trained and tested rats in the same context, the increased freezing seen during extinction retrieval likely reflects responses to tone rather than context. Taken together, these results suggest that prior exposure to chronic stress has distinct and dissociable effects on different aspects of fear conditioning and extinction, likely due to differential effects on the neural regions underlying these components.
Chronic Stress-Induced Alterations in Neuronal Activity in mPFC
The impairment in retrieval of extinction was accompanied by alterations in neural activity in PL and IL. Furthermore, analysis of PSTHs demonstrated that activity of PL neurons was inhibited by the CS in unstressed but not stressed rats. Additionally, unstressed rats exhibited an increase in neuronal firing in IL in response to the CS during retrieval of extinction, whereas stressed rats failed to increase firing in IL during the CS. Finally, CS-related activity in IL but not PL was correlated with extinction retrieval. Thus, while we found stress-induced alterations in activity of both PL and IL, only functional alterations in IL were associated with stress-induced impairment of extinction retrieval.
Baseline firing in mPFC was suppressed in unstressed rats during fear conditioning. Basolateral amygdala provides projections to mPFC (McDonald, 1991;Verwer et al., 1996), and activation of amygdalar inputs inhibits output neurons in mPFC (Garcia et al., 1999). Thus, the suppression of baseline activity that we observed during conditioning could reflect inhibition by the amygdala.
Furthermore, in unstressed rats, PL neurons increased firing in response to the CS during conditioning, and decreased firing in response to the CS during extinction retrieval. This pattern is consistent with recent evidence for PL contributions to expression of conditioned fear (Corcoran and Quirk, 2007;Burgos-Robles et al., 2009), and an inverse relationship between PL activity and successful retrieval of extinction (Burgos-Robles et al., 2009). Stress prevented CS-elicited changes in PL activity during conditioning and extinction retrieval, suggesting that stress-induced changes in PL neurons render them less responsive to inputs—either inhibitory on excitatory—during fear conditioning and extinction. Interestingly, this decreased responsiveness did not correlate with expression of conditioned fear either late in acquisition or during extinction retrieval, again suggesting that changes in other corticolimbic structures, for instance the amygdala, likely also contribute to the stress-induced behavioral changes.
IL plays a critical role in retrieval of extinction: Lesions of IL impair retrieval of extinction (Quirk et al., 2000), neuronal firing in this region increases in response to the CS during retrieval of extinction (Milad and Quirk, 2002), and stimulation of IL facilitates retrieval of extinction (Milad et al., 2004). Consistent with this role, we found increased IL activity during extinction retrieval in unstressed rats. However, in stressed rats, IL neurons failed to increase their firing rates in response to the CS. Furthermore, consistent with previous reports in unstressed rats (Milad and Quirk, 2002), firing in IL was correlated with extinction retrieval. Thus, stress-induced changes in the activity of IL may be responsible for the stress-induced changes in extinction retrieval. In addition, although stress-induced alterations in IL activity were present during conditioning as well, activity in IL was not correlated with expression of conditioned fear in late acquisition—despite the significant stress-induced facilitation of freezing during acquisition. Thus, as expected given evidence for a discrete role for IL in extinction retrieval but not acquisition of fear conditioning (Quirk et al., 2000), the contribution of stress-induced dysfunction of IL to stress-induced impairment of retrieval of extinction can be dissociated from stress-induced changes mediating facilitation of conditioned fear.
As with PL, the altered IL activity in stressed rats is consistent with the hypothesis that stress-induced changes in IL neurons render them less responsive to inputs. This is consistent with morphological alterations in both PL and IL seen after chronic stress (e.g., Cook and Wellman, 2004;Izquierdo et al., 2006). Alterations in neuronal excitability are associated with changes in dendritic morphology. For instance, repeated high-frequency stimulation of callosal fibers both increases dendritic length and potentiates excitability of cortical pyramidal cells (Monfils et al., 2004), while repeated low-frequency stimulation produces dendritic retraction and concomitant decreases in the excitability (Monfils and Teskey, 2004). Furthermore, stimulation of apical tufts of cortical pyramidal neurons disproportionately excites these cells (Rhodes and Llinás, 2001). Thus, stress-induced retraction of apical dendrites of IL neurons could decrease their excitability, impairing retrieval of extinction of conditioned fear.
Likewise, chronic administration of the stress hormone corticosterone downregulates expression of the NR2B subunit of the NMDA receptor in mPFC (Gourley et al., 2008). Given that activation of NMDA receptors in IL is necessary for consolidation and retrieval of extinction (Burgos-Robles et al., 2007), chronic stress-induced alterations in NMDA receptor expression in IL could also contribute to the stress-induced alteration in IL activity during, and deficit in, retrieval of extinction.
Nonetheless, IL is part of a circuit whose coordinated activation is critical for retrieval of extinction, and multiple structures in the circuit—for instance, hippocampus and basolateral amygdala—are influenced by chronic stress (e.g., Magariños and McEwen, 1995;Vyas et al., 2002). Potentiation of hippocampal synapses in mPFC is associated with extinction (Farinelli et al., 2006), and disruption of this potentiation, via either low-frequency stimulation or prior exposure to chronic stress, impairs retrieval of extinction (Garcia et al., 2008). Likewise, activation of basolateral amygdala inputs to IL may suppress IL activity through activation of inhibitory interneurons (Cunningham et al., 2008). Thus, the inhibition of activity in IL in stressed rats, and its association with impaired extinction retrieval, could reflect stress-induced alterations in structures such as the hippocampus and amygdala that provide input to mPFC. Indeed, even if the stress effects we have shown are intrinsic to IL, potential morphological contributions to them nonetheless may reside at the circuit level, as recent data suggest that in IL, some neurons–for instance, those that project directly to basolateral amygdala–may not show the stress-induced changes in dendritic morphology that are apparent in other IL pyramidal neurons (Shansky et al., 2009). However, Shansky and colleagues’ (2009) data are not inconsistent with the hypothesis that morphological changes in IL neurons are directly responsible for stress-induced deficits in extinction retrieval: The major amygdalar targets of IL are the intercalated neurons of the amygdala and the central nucleus of the amygdala (Vertes, 2004). IL likely participates in extinction via activation of inhibitory interneurons in these structures (Quirk et al., 2006).
In summary, the present study demonstrates that chronic stress alters activity of neurons in mPFC, with concomitant impairment of retrieval of extinction. Therefore, stress-induced alterations in activity of neurons in mPFC during retrieval of extinction may be responsible for this deficit. Given the strong link between stress and psychological disorders (Garcia, 2002), understanding the mechanisms whereby stress alters prefrontal function should help to elucidate causal pathways in mental illness.
Acknowledgements
This research was supported by the Indiana METACyt Initiative of Indiana University, funded in part through a major grant from the Lilly Endowment, Inc., and by MHR03087794 to C.L.W.
Abbreviations
- CS
conditioned stimulus
- US
unconditioned stimulus
- mPFC
medial prefrontal cortex
- PL
prelimbic
- IL
infralimbic cortex
- PSTH
peri-stimulus time histograms
- LTP
long term potentiation
- EPSP
excitatory post-synaptic potentials
- AHP
afterhypolarizations
- NMDA
N-methyl-d-aspartic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abeles M. Quantification, smoothing, and confidence limits for single-units' histograms. J Neurosci Methods. 1982;5:317–325. doi: 10.1016/0165-0270(82)90002-4. [DOI] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb Cortex. 2001;11:441–451. doi: 10.1093/cercor/11.5.441. [DOI] [PubMed] [Google Scholar]
- Baran SE, Armstrong CE, Niren DC, Conrad CD. Prefrontal cortex lesions and sex differences in fear extinction and perseveration. Learn Mem. 2010;17:267–278. doi: 10.1101/lm.1778010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiology of Learning and Memory. 2009;91:323–332. doi: 10.1016/j.nlm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter LR, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, Gerner RH, Sumida RM. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiat. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Depression. In: Brown GW, Harris TO, editors. Life Events and Illness. New York: Guilford; 1989. pp. 49–93. [Google Scholar]
- Brown SM, Henning S, Wellman CL. Short-term, mild stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Carter CS, MacDonald AW, III, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatr. 2001;158:1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. TINS. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;24:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Increasing Interaction of amygdalar afferents with GABAergic interneurons between birth and adulthood. Cereb Cortex. 2008;18:1529–1535. doi: 10.1093/cercor/bhm183. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinelli M, Deschaux O, Hugues S, Thevenet A, Garcia R. Hippocampal train stimulation modulates recall of fear extinction independently of prefrontal cortex synaptic plasticity and lesions. Learn Mem. 2006;13:329–334. doi: 10.1101/lm.204806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MR, Sayed JA, Underwood AR, Wellman CL. Lesion of infralimbic cortex occludes stress effects on retrieval of extinction but not fear conditioning. Neurobiology of Learning and Memory. 2010;94:240–246. doi: 10.1016/j.nlm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Garcia R. Stress, synaptic plasticity, and psychopathology. Rev Neurosci. 2002;13:195–208. doi: 10.1515/revneuro.2002.13.3.195. [DOI] [PubMed] [Google Scholar]
- Garcia R, Chang C, Maren S. Electrolytic lesions of the medial prefrontal cortex do not interfere with long-term memory of extinction of conditioned fear. Learn Mem. 2006;13:14–17. doi: 10.1101/lm.60406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R, Spennato G, Nilsson-Todd L, Moreau J-L, Deschaux O. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiol Learning Memory. 2008;89:560–566. doi: 10.1016/j.nlm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Garcia R, Voloumba RM, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402:294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, Morrison JH. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neurosci. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2008;34:707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays WL. Statistics. Fort Worth, TX: Harcourt Brace; 1994. [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neuroscience and Biobehavioral Reviews. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Jackson ME, Moghaddam B. Activation of metabotropic glutamate 2/3 receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. Journal of Neurophysiology. 2005;93:1989–2001. doi: 10.1152/jn.00875.2004. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Rapid dendritic retraction in medial prefrontal neurons and impaired fear extinction following exposure to uncontrollable stress. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ME, Moghaddam B. Distinct patterns of plasticity in prefrontal cortex neurons that encode slow and fast responses to stress. European Journal of Neuroscience. 2006;24:1702–1710. doi: 10.1111/j.1460-9568.2006.05054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, Qin Y, McNaughton BL, Barnes CA. Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cereb Cortex. 1998;8:437–450. doi: 10.1093/cercor/8.5.437. [DOI] [PubMed] [Google Scholar]
- Liu R-J, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: Role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci USA. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neurosci. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J Neurosci. 2003;23:4406–4409. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell SE, Delaney HD. Designing experiments and analyzing data: A model comparison perspective. Mahwah, New Jersey: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neurosci. 1991;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH. [3H]Dexamethasone binding in rat frontal cortex. Brain Res. 1985;328:176–180. doi: 10.1016/0006-8993(85)91340-x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learning Memory. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Teskey GC. Induction of long-term depression is associated with decreased dendritic length and spine density in layers II and V of sensorimotor neocortex. Synapse. 2004;53:114–121. doi: 10.1002/syn.20039. [DOI] [PubMed] [Google Scholar]
- Monfils MH, VandenBerg PM, Kleim JA. Long-term potentiation induces expanded movement representation and dendritic hypertrophy in layer V of rat sensorimotor neocortex. Cereb Cortex. 2004;14:586–593. doi: 10.1093/cercor/bhh020. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatr. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WGM, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Rhodes PA, Llinás RR. Apical tuft input efficacy in layer 5 pyramidal cells from rat visual cortex. J Physiol. 2001;5361:167–187. doi: 10.1111/j.1469-7793.2001.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex. 2009;19:2479–2484. doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Koeda M, Oda K, Matsuda T, Matsushima E, Matsuura M, Asai K, Okubo Y. An fMRI study of differential neural response to affective pictures in schizophrenia. NeuroImage. 2004;22:1247–1254. doi: 10.1016/j.neuroimage.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Ventura J, Neuchterlein KH, Lukoff D, Hardesty JD. A prospective study of stressful life events and schizophrenic relapse. J Abnormal Psychol. 1989;98:407–411. doi: 10.1037//0021-843x.98.4.407. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Verwer RW, Van Vulpen EH, Van Uum JF. Postnatal development of amygdaloid projections to the prefrontal cortex in the rat studied with retrograde and anterograde tracers. J Comp Neurol. 1996;376:75–96. doi: 10.1002/(SICI)1096-9861(19961202)376:1<75::AID-CNE5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neurosci. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Wree A. Cortex: areal and laminar structure. In: Paxinos G, editor. The rat nervous system. San Diego: Academic Press; 1995. pp. 649–685. [Google Scholar]