Abstract
This review of age-related brain microvascular pathologies focuses on topics studied by this laboratory, including anatomy of the blood supply, tortuous vessels, venous collagenosis, capillary remnants, vascular density, and microembolic brain injury. Our studies feature thick sections, large blocks embedded in celloidin, and vascular staining by alkaline phosphatase (AP). This permits study of the vascular network in three dimensions, and the differentiation of afferent from efferent vessels. Current evidence suggests that there is decreased vascular density in aging, Alzheimer’s disease (AD), and leukoaraiosis (LA), and cerebrovascular dysfunction precedes and accompanies cognitive dysfunction and neurodegeneration. A decline in cerebrovascular angiogenesis may inhibit recovery from hypoxia-induced capillary loss. Cerebral blood flow (CBF) is inhibited by tortuous arterioles and deposition of excessive collagen in veins and venules. Misery perfusion due to capillary loss appears to occur before cell loss in LA, and CBF is also reduced in the normal-appearing white matter. Hypoperfusion occurs early in AD, inducing white matter lesions and correlating with dementia. In vascular dementia, cholinergic reductions are correlated with cognitive impairment, and cholinesterase inhibitors have some benefit. Most lipid microemboli from cardiac surgery pass through the brain in a few days, but some remain for weeks. They can cause what appears to be a type of vascular dementia years after surgery. Donepezil has shown some benefit. Emboli, such as clots, cholesterol crystals, and microspheres can be extruded through the walls of cerebral vessels, but there is no evidence yet that lipid emboli undergo such extravasation.
Keywords: Alzheimer’s disease, Vascular dementia, Leukoaraiosis, Tortuous vessels, Capillary loss, String vessels, Periventricular venous collagenosis, Cerebrovascular lipid emboli
Introduction
Cerebral microvascular pathology precedes and accompanies age-related cognitive dysfunction and neurodegeneration [1–3]. Therefore, knowledge of this pathology is essential to understanding neurodegeneration. This review focuses on several topics studied by this laboratory, including anatomy of the blood supply, tortuous vessels, venous collagenosis, string vessels (capillary remnants), decreased vascular density, and microembolic brain injury. In addition, we will discuss basement membrane (BM) thickening, cerebral perfusion, and extravasation of emboli. These vascular factors are involved in vascular dementia, Alzheimer’s disease (AD), cognitive decline following microembolic injury of the brain, and leukoaraiosis (LA). LA is an age-related white matter degeneration characterized by spongiosis, gliosis, demyelination, and capillary degeneration [4], as well as endothelial dysfunction [5], increased blood-brain barrier (BBB) permeability [6], and cognitive impairment [7–14]. The studies in this laboratory have featured two methods; cutting thick sections from large tissue blocks embedded in celloidin and staining vessels via the endogenous enzyme, alkaline phosphatase (AP) [15]. Large, 100 μm-thick tissue sections provide an overall view of the vascular network in three dimensions, and AP histochemistry stains the afferent vasculature, distinguishing it from the efferent vessels.
Cerebrovascular Anatomy and Pathology
Perilous blood supply
An arterial network covers the surface of the brain and penetrates the brain in the form of end arteries, i.e., they terminate in a capillary bed and do not have shunts to arterioles or veins within the brain [16]. This vascular supply is not uniform, thus some brain regions are more vulnerable than others to chronic hypoperfusion. The deep white matter is particularly vulnerable because its major blood supply is via long medullary arterioles which arise from the border-zone between the middle cerebral artery and the anterior cerebral artery (Figure 1) [17]. Some regions of the deep white matter also receive blood supply from the medial and lateral brain surfaces. An additional blood supply to the deep white matter has also been proposed to originate from the lenticulostriate arteries projecting upward and around the lateral ventricles. In our studies, using AP staining, we have seen no evidence of a lenticulostriate supply to the white matter above the lateral ventricles, although these arteries do appear to project into the white matter lateral to the ventricles. In earlier studies, which used media injected into vessels [18,19], there may have been overfilling of some afferent vessels resulting in unintentional filling of some of the veins that project up from the ventricle into the deep white matter. Those areas supplied by short penetrating vessels, such as the corpus callosum do not exhibit LA, possibly because they are less susceptible to hypoperfusion. Conversely, the deep white mater is subject to both hypoperfusion and LA.
Fig. 1.
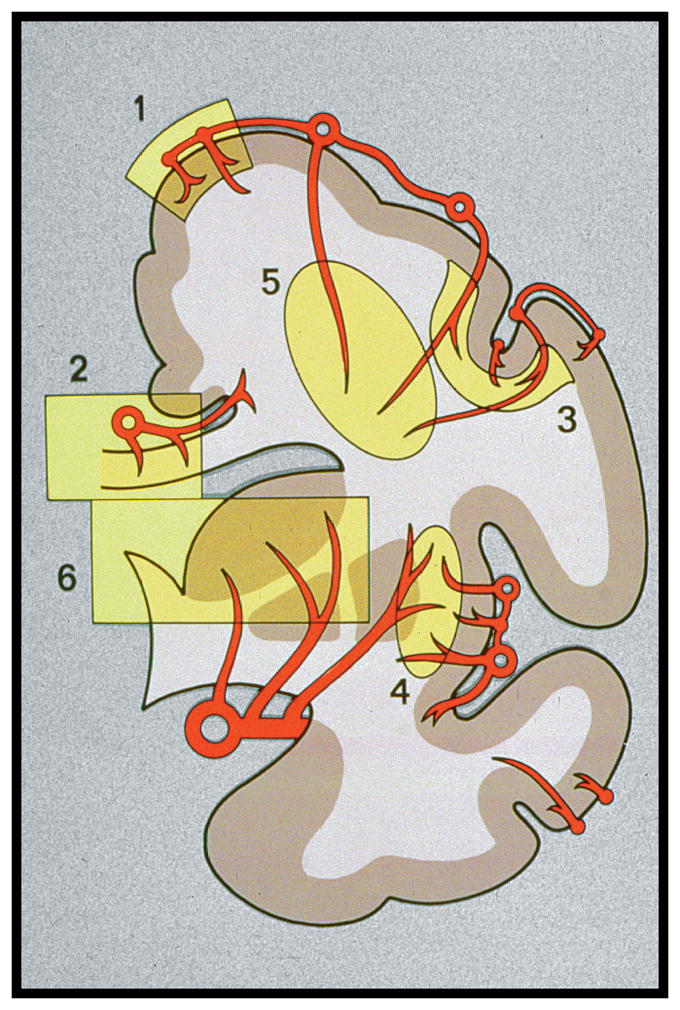
Schematic of the cerebral blood supply. (Reprinted from [17]).
Tortuous vessels
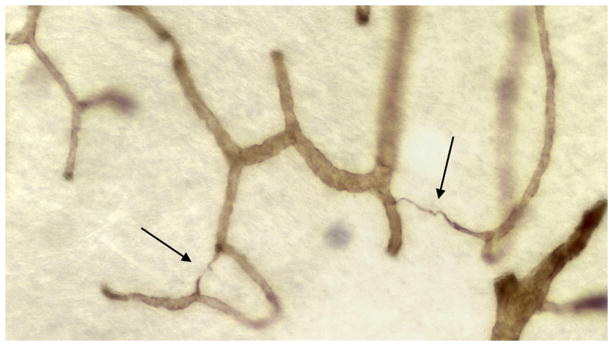
The arterioles supplying the deep white matter have the longest course through the brain, and with aging they often become tortuous [20–29]. Hassler [30] found that they were sparse in subjects under the age of 60, but were common after the age of 70. Akima et al. [24] found them to appear in the 5th decade and to occur in all specimens above 80 years old. Hassler et al. [27] reported that there was no correlation with dementia score. Tortuosity usually begins abruptly as the arteriole passes from the cortex into the white matter (Figure 2), suggesting an intrinsic vulnerability of the white matter. With collagen IV staining in thick celloidin sections, these vessels can be seen to be coiled in a cavity where some brain parenchyma has been lost. We refer to these tortuous vessels and cavities as tortuosity lesions. Tortuosity increases the vessel length, and with each turn and loop there is a loss of kinetic energy such that increased blood pressure is needed to maintain flow in these vessels [22]. We found large amounts of tortuosity after age 50 [25], and there was a trend toward an increase in tortuosity in LA. Thus, tortuous vessels may be a contributing factor to the development of LA in a subset of cases.
Fig. 2.
Tortuous arterioles in the white matter. (A) This thick celloidin section stained with collagen IV shows two tortuous arterioles in cavities. (B) This thick celloidin section stained with AP shows several arterioles with tortuosity beginning as they enter the white matter. (Reprinted from [20]).
Periventricular venous collagenosis
In 1995, we identified a new type of cerebral vascular pathology, periventricular venous collagenosis (PVC), in subjects with LA (Figure 3) [20,31]. An increase in the thickness of the walls of veins and venules in the periventricular white matter was evident with normal aging. In cases with LA, we found a much greater degree of venous wall thickening in the LA lesions, which resulted in narrowed lumina and even occlusion. Using routine stains, these thick-walled veins could be easily mistaken for hyalinized arterioles, but with AP histochemistry it was shown that the affected vessels were exclusively venous. The thickened walls stained for collagens type I and III, which corresponds to excessive collagen deposition as occurs in hyalinization. Staining for collagen IV marked the basal lamina of the endothelium and the glia limitans at the brain parenchyma. These observations have not been repeated by other laboratories, but somewhat similar findings have been reported in multiple sclerosis lesions [32] and in brain capillaries in aging rats and hypertensive rats [33,34].
Fig. 3.
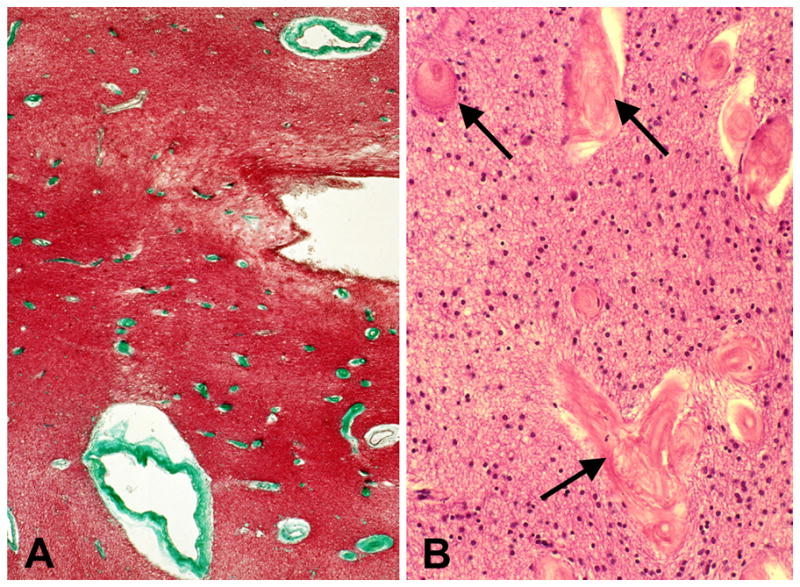
Severe periventricular venous collagenosis in the brain of a subject with leukoaraiosis. (A) This thin paraffin section stained with trichrome shows numerous affected veins (green) near the lateral ventricle. (B) This thin paraffin section stained with &E shows veins with collagenosis (arrows) at higher magnification. (Reprinted from [20]).
We found an association between severe PVC and LA [31]. Although hypertension is often associated with vascular dementia and LA, most of our cases did not have hypertension. Pantoni and Garcia [4] observed that hypertension is not always found in LA cases. In a magnetic resonance imaging (MRI) investigation of PVC, tapering or occlusion of veins at the margin of confluent LA was found, suggesting that LA may involve venous insufficiency [35]. Inhibition of blood flow by the venous stenosis of PVC might induce chronic ischemia and/or edema in the deep white matter, leading to LA.
The thickened venous walls could impair passage into the veins of fluids, solutes, and toxins destined for removal from the brain via the blood stream. In addition, PVC might hinder the function of the perivascular route for drainage through the brain [36,37]. It has been shown that cerebrospinal fluid (CSF) is pumped through the brain via the periarteriolar spaces by the pulsations of the arterioles [37]. Also, arteriolar sclerosis might impede the pulsatile pumping that moves CSF along the arterioles. Among the substances which drain through the perivascular pathway is Aβ [38,39]. The low concentrations of Aβ in the CSF of AD patients is thought to be due to decreased cerebral clearance of Aβ into CSF [40]. Roher et al [41] have speculated that the Aβ deposits in the walls of capillaries could close the lumen and cause degeneration and disappearance of the capillary, leaving a deposit of Aβ that may form a neuritic plaque. Furthermore, Aβ deposition in the perivascular drainage pathway may cause cerebral amyloid angiopathy (CAA) that may block the function of this pathway [39]. In CAA, Aβ deposition occurs in and around the vascular walls (Figure 7), disrupts the BM of small vessels, causes endothelial cell damage, and can reduce the vascular lumen [42]. Perhaps sluggish perivascular flow caused by cerebrovascular pathology, such as venous collagenosis, tortuosity lesions, and thickened arteriolar walls facilitate Aβ deposition in this pathway and further block the clearance of toxins. Clearance of Aβ may be disrupted by microvascular pathology and the reduction of Aβ clearance may contribute to further microvascular pathology.
Fig. 7.
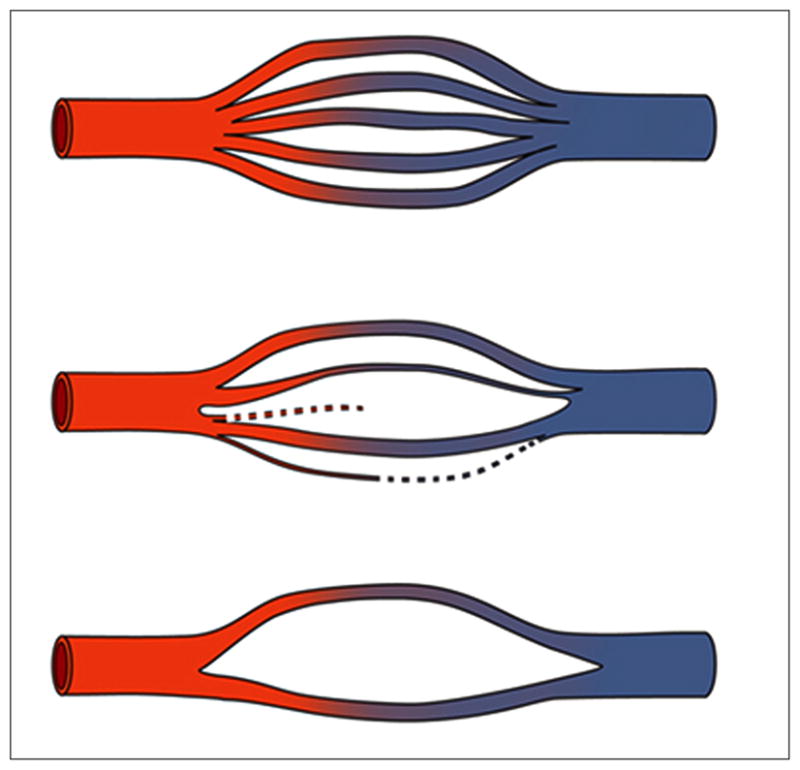
Schematic of capillary loss and string vessel formation. (Reprinted from [44]).
It is unknown why the veins become thickened in the deep white matter and why PVC is associated with LA. We suggest three mechanisms for the development of PVC and ischemia leading to LA: i) a genetic predisposition to excessive collagen deposition in veins; ii) a genetic predisposition to chronic periventricular ischemia that causes overproduction of collagen in the veins; and iii) mechanical damage to small vessels due to abnormally high pulsatile motion in the periventricular white matter, as proposed by Marie-Cecile Henry-Feugeas [43]. These mechanisms need not be mutually exclusive.
String vessels
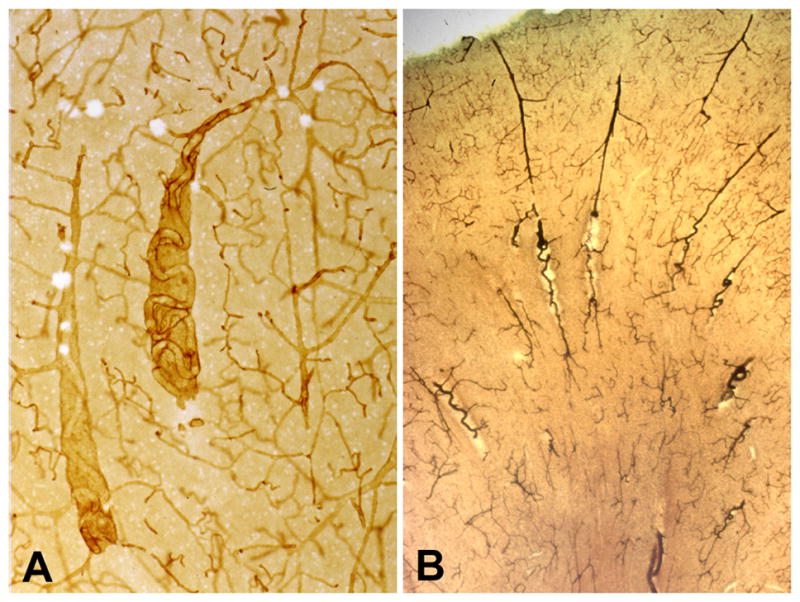
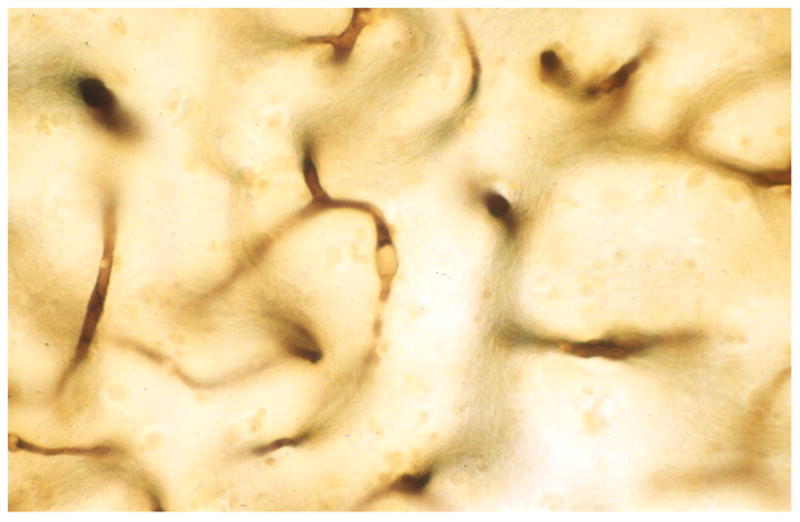
String vessels (Figure 4) are BM remnants of capillaries that have lost their endothelium [44]. They cannot transport blood or plasma [45,46]. In studies of retinal digest preparations, it was found that ischemia caused string vessels to begin to form at 3 to 5 days, with the loss of endothelial cells [47]. By 8 days, some of the acellular capillary remnants were reduced to thin strands, which were still present at 40 days [47]. When capillaries first lose their endothelial cells, the residual BM often shows accordion-like pleating [48]. Shutdown of blood flow for about 1 to 2 days can cause capillary loss [49]. After shutdown, capillary regression occurs by apoptosis, synchronously along capillary segments from one vascular junction to the next, often with macrophage engulfment of apoptotic endothelial cells [50]. During apoptosis, the endothelial cells can bulge into the lumen and detach from the BM [51]. The synchronous apoptosis of endothelial cells may be due to the loss of availability of vascular endothelial growth factor (VEGF), due to the lack of blood flow [50,52]. A second mechanism is the loss of fluid shear stress on the endothelial cell surface, which helps maintain endothelial cell survival [50,53].
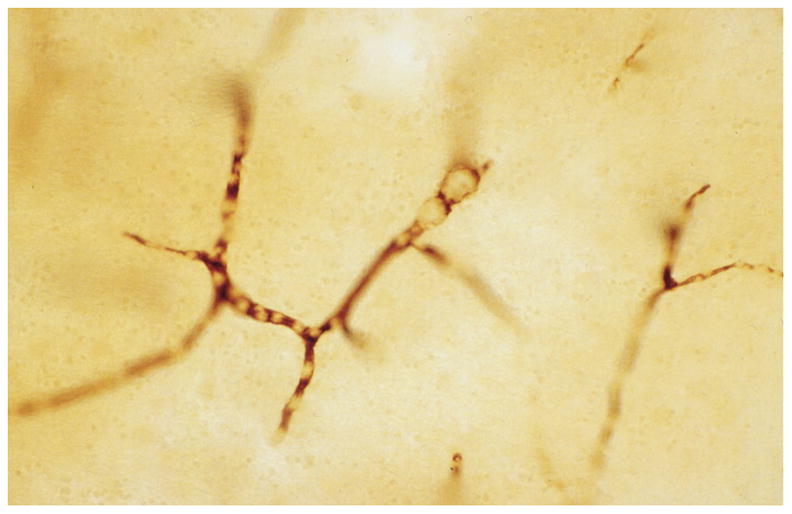
Fig. 4.
String vessels (arrows) in a thick celloidin section stained with antibody to collagen IV.
String vessels occur commonly in the normal brain, spinal cord, and eye of humans and animals [54], and they are increased in ischemia [47], irradiation [55], mesial temporal sclerosis [56], and AD [57–62]. In normal brains, we found string vessels at all ages from preterm babies to the very old [63]. The number of string vessels was highest in pre- and post-natal subjects, when vascular remodeling is prominent. Because string vessels were fewer at older ages, we concluded that they disappear after some months or years. Although string vessels increase in the aged brain and in brain pathology [44], we found the density of string vessels in LA lesions to be decreased (unpublished). The density of capillaries in the deep white matter in LA is also decreased [64,65], but the decrease in string vessels was greater than that of the capillaries. Our data indicate that LA may begin as early as mid-life, with a progressive loss of vessels in the deep white matter. An early loss of capillaries in LA would be marked by an increase in string vessels, but the string vessels gradually disappear as they are resorbed. By the time of death and autopsy, most of the string vessels in LA lesions may have disappeared.
Basement membrane thickening
The BM becomes thicker with age [34,48,66] and in AD [62,67–69] (see review by Kalaria [70]). BMs are highly crosslinked insoluble material made up of approximately 50 proteins, of which about 50% is collagen, especially collagen IV [71]. The molecular composition of the BM is unique for each tissue and the two BM polymer sheets of collagen IV and laminin expose various signaling constituents, such as VEGF, to the endothelial cells [71]. Age-related BM thickening occurs via the deposition of collagen fibrils in and around the BM, and this thickening is correlated with atherosclerosis in large peripheral vessels [34]. BM thickening can also occur when a string vessel is invaded by a newly forming capillary [72]. Empty BM tubes can provide a scaffold and signaling molecules for vessel regrowth [52,71]. Invading capillaries lay down a new BM within the old one, creating a lamellar reduplication. This would likely be rare in aging, AD, and LA.
Capillary Growth and Loss
Hypoxia and angiogenesis in aging
Angiogenesis can occur when VEGF is induced in response to hypoxia via the transcription factor, hypoxia-inducible factor-1 (HIF-1) [73]. The endothelial cells of vascular sprouts have tips that are similar to axonal growth cones, and many of the signaling pathways are shared between the nervous system and the vasculature [74]. For example, VEGFs are growth factors for both endothelial and neural cells, and semaphorins control both axon guidance and vascular patterning [74]. Another factor in capillary stability is pericyte signaling via Tie-2 receptors on endothelial cells [75]. Normally, angiopoietin-1 is released by pericytes and activates the Tie-2 receptors on endothelial cells, helping to maintain vascular integrity [76]. Hypoxia causes the induction of angiopoietin-2 which occupies the Tie-2 receptors, preventing angiopoietin-1 activation and causing the pericytes to move away from the capillaries and destabilize them [76]. In the presence of VEGF, angiogenesis occurs, but in the absence of VEGF, the capillaries undergo apoptotic regression [77]. During angiogenesis, the developing capillaries are more permeable than established vessels. Chronic hypoxia in 2 month-old mice caused an increase in brain vascular density, likely due to angiogenesis [78]. However, there appears to be an age-related decline in the capacity for cerebral angiogenesis [79,80]; the responsiveness of HIF-1 to hypoxia wanes with age, reducing VEGF expression [81–83]. This HIF-1 decline is associated with neuronal loss [84]. Thus, in aging, AD, and LA, there may be a failure of vascular recovery from hypoxia-induced bouts of capillary loss. This failure of vascular recovery and other cerebrovascular changes represent a loss of brain vascular reserve and functional reserve.
Age-related capillary loss
There is considerable evidence of an age-related failure of cerebral vascular recovery in the studies of vessel density in aging, AD, and LA. As described in review by Kalaria [70], in 1996 there were mixed findings. At the time of the review by Riddle et al. [85] in 2003, the preponderance of the 22 studies cited showed declines in vascular density. In the present review of 37 studies, there is strong evidence of decreased vascular density in aging animals, aging humans, AD, LA, and a mouse model of AD. Detailed results are discussed below.
There have been several vascular density studies in aging rats: Jucker et al. [86] reported decreases in capillary number (20%) and length (3%), in the hippocampus, and increased intercapillary distance (24%). In the cortex, they observed reductions in capillary number (25%) and length (7%), and increased intercapillary distance (39%). In brain white matter, Shao et al. [87] found a decrease in capillary length (19%) and volume (24%). Klein and Michel [88] reported a 33% decline in vascular density. Buchweitz-Milton and Weiss [89] found a decrease in capillary length (29%) and volume (16%). Casey and Feldman [90] observed a decreased vascular density (30%) in the brain stem. Hinds and McNelly [91] found a 15% decrease in capillary density. Data from Wilkinson et al. [92] demonstrate a decrease in vessel numbers (11%) and brain blood volume (32%). Sonntag et al. [93] observed a decrease in vascular density (39%) on the cortical surface. Hutchins et al. [94] reported a 39% decrease in arterioles penetrating the cortex. Amenta et al. [95] found a decrease in capillary numbers (43%) and length (20%). Amenta et al. [96] showed decreases in capillary numbers (23%) and length (5%), and increased in intercapillary distance (48%). Data from Burns et al. [97] showed a decrease in capillary density (12%) and an increased intercapillary distance (6%). Knox and Oliveira [98] found a decrease in the capillary numbers (8%). Villena et al. [99] reported decreases in capillary numbers (9%), volume (19%), and length (11%) from 18 to 24 months, but from 24 to 28 months there were increases in capillary numbers (7%), volume (15%), and length (9%). Meier-Ruge and Schulz-Dazzi [100] reported no significant change in capillary density, but their data show decreases in capillary length (7.5%) and number (5%). Black et al. [79] found no change in vessel density. Hughes and Lantos [101] found increases in vessel number, but their old rats were not very old. Bar [102] reported a 3% decrease in vascular volume, but a 16% increase in vessel length. In mice, Sturrock [103] found no change in vessel density. Also, Vaugan and Calvin [104] showed a decrease in hemoglobin in the brains of older mice, which they concluded was due to decreased blood volume.
In studies of human aging, Bell and Ball [105] reported decreases in capillary density in all six brain areas measured (average 16%). They also found a decrease in capillary density (16%) in the calcarine cortex [106,107]. Abernethy et al [108] observed a decrease in vascular density (50%) in the human paraventricular nucleus, but no change in the supraoptic nuclei. Brown et al. [64] found a decline in vessel density between 57 and 90 years in the white matter (Figure 5). Buée et al. [57] reported that the vascular density in two normal elderly subjects (79 years) compared to one young subject (49 years) was decreased by 26%. Mann et al. [109] found a decease in vessel density in frontal cortex, but not in temporal cortex. Hunziker et al. [110,111] found increased vascular density in subjects 64–74 years of age, compared to young subjects, but those 75 and older had a similar vascular density to the young. Meier-Ruge et al. [112] reported that vascular density was increased in the putamen, but unchanged in the cortex. However, their data for cortex showed decreases in capillary length and volume from ages 65–74 to 75–94. Farkas et al. [34] found no age-related change in vascular density in the white matter.
Fig. 5.
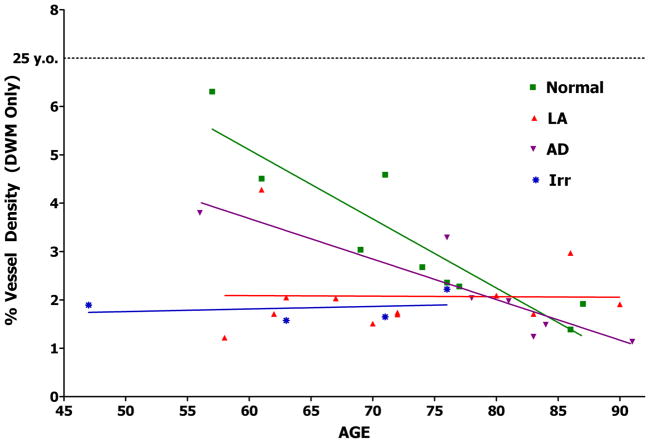
Graph of vascular density in normal, AD, LA, and brain irradiation.
Capillary loss in AD
Bell and Ball [105] reported age-related decreases in capillary density in AD in the six brain areas measured (average 17%). Bell and Ball [106,107] found a decrease in capillary density (18%) in the calcarine cortex in AD. Brown et al. [64] observed an age-related decrease in vessel density in the white matter in seven AD subjects, and the AD vessel densities were lower than those of age-matched controls (Figure 5). Fischer et al. [26] found a decrease in vascular density in AD versus control (30%). Buée et al. [57] reported vascular density in AD to be decreased by 16% compared to age-matched controls, and 38% compared to a 49 year old. Suter et al. [113] commented that small cortical arterioles and capillaries were decreased in three AD cases compared with an age-matched control. Bailey et al. [114] showed a decline in vascular density with increasing Clinical Dementia Rating (CDR) score. In contrast to those studies, Perlmutter et al. [62] reported an anecdotal finding of focal regional increases in capillary density in AD subjects and Desai et al [115] found that vascular density was increased in one of five areas examined. They also found that an endothelial marker of angiogenesis was elevated in AD brains [115]. In a mouse model of AD, Paris et al. [116] found a decrease in vascular density of about 30% in both the hippocampus and cortex. Lee et al. [117] reported a 21% decrease in capillary numbers in the corpus callosum in a mouse model of AD.
Amyloid β (Aβ) accumulations on capillaries may contribute to the reduced brain capillary density in AD via anti-angiogenic activity [116,118]. Although the angiogenic factor VEGF is increased in AD [119], as would be expected in hypoxic conditions, VEGF binds to Aβ and is heavily deposited in plaques. Thus, Aβ may act as a molecular sink for VEGF, reducing its availability in AD [120].
Capillary loss in LA
Our laboratory has published vascular density studies in subjects from 57 to 90 years old comparing vessel density in the deep white mater from non-LA subjects to that in LA lesions, normal-appearing white mater, and cortex [64,65]. Vascular density in LA lesions was decreased (20%). Because there was an age-related decline in vessel density between years 57 and 90 in normal subjects, whereas vessel density in LA lesions did not decrease with age, vessel densities after 80 years were equivalent in the deep white matter of the LA and non-LA subjects (Figure 5). We observed a non-significant decrease in vascular density (11%) in the subcortical white matter in subjects with LA lesions, but in those LA subjects who died before 60 years, the decrease was 47%. Vessel density was also decreased (7%) in the cortex of LA subjects, but in those LA subjects who died before 60 years, the decrease in the cortex was 38% [64,65]. We also studied LA-like white matter lesions in four patients who received whole-brain irradiation for brain tumors. The deep white matter in these patients had low vessel densities, similar to those found in LA (Figure 5). Furthermore, there did not appear to be an age-related decline in vessel density in irradiated patients. After about 80 years of age, vascular density in the white matter tends to drop to levels typical of LA and irradiation. Similarly, it has been shown that the association between Aβ plaques and dementia was strong at 75 years of age, but reduced at 95 years of age [121]. This presents a problem with studies using heavy sampling in subjects over 70 years of age. Younger subjects are more likely to show significant differences in vessel density due to pathologies such as LA and AD. This problem can be partially circumvented by presenting the findings graphically and analytically as age-related changes as in Figure 5.
It is interesting that vascular density in LA lesions does not fall below a level that would cause infarcts and cavitation. Instead, the white matter appears to become hypoxic because of capillary loss, followed by spongeosis and enlargement of the ventricles as cells in the white matter gradually die. In LA we find apoptosis with a loss of oligodendrocytes and perhaps astrocytes [122–124]. Alternatively, a loss of neuropil would result in a decreased need for oxygen and glucose, and lead to a loss of capillaries. However, studies have revealed increased oxygen extraction from the blood in the deep white matter in LA, which would imply that there are too many cells for the existing oxygen supply [125–127]. This suggests that the capillary loss occurs first, causing misery perfusion in the white matter. The capillaries appear to die first, but why do they stop dying? Perhaps a hemodynamic imperative is reached, i.e., the number of capillaries needed to simply transport the arterial blood to the venous system is reached (Figure 6). At that point, capillary loss would cease and no more string vessels would be formed, and as string vessels are resorbed, there would be very few left in the LA lesions at autopsy.
Fig. 6.
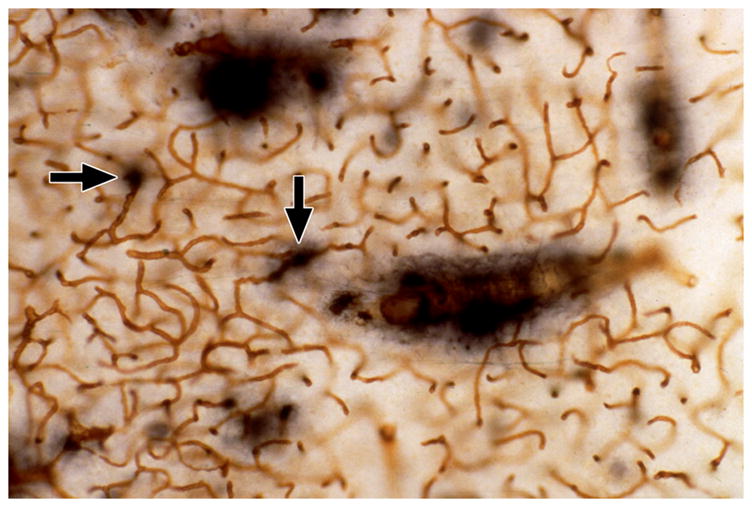
Amyloid β deposited in and around capillaries (arrows) larger blood vessels. Thick celloidin section stained with antibodies to collagen IV and Aβ. (Reprinted from [189]).
Decreases in brain vascular density may be limited by physiological factors. As vessels are lost, hypoxia stimulates angiogenesis, but this may fail in old age. In this case, vessel density may decline, causing neuronal dysfunction and then neuronal and glial cell death. As cell death causes a loss of parenchymal volume, the packing effect reduces the amount of reduction in vascular density. However, some hypoxia can be tolerated by neurons and glia without causing cell death. At that point, there would be some reduction in vascular density.
Cerebral Perfusion
Elevated blood pressure, cerebrovascular pathology, and cognitive dysfunction
Hypertension, elevated blood pressure (BP), and fluctuations in BP are associated with cerebrovascular pathology [128], including the recently described cerebral microbleeds [129,130]. LA lesions were found to be increased with age and linearly increased with increasing BP levels [128]. Hypertension is also associated with decreased cognitive function [131]. In midlife, hypertension appears to injure the vascular system, but with advancing age, the lower boundary of autoregulation in the brain shifts upward, causing vulnerability to hypoperfusion [132]. Such hypoperfusion may cause white matter ischemia and LA [133]. Thus, aggressive treatment of hypertension in old age may cause brain hypoperfusion [133]. On the other hand, hypertension treatment in the elderly has been shown to improve BP levels and restore BP regulation [134], so hypertension treatment may induce some recovery of vascular pathology. Consequently, BP control in the elderly becomes a balancing act between risk and benefit.
Reduced cerebral blood flow and cognitive dysfunction
Reduced vascular density is consistent with findings of reduced cerebral blood flow (CBF) in LA and AD [135,136]. The following are among the factors that influence CBF: perivascular nerves can constrict or dilate arteries and arterioles [137,138], astrocyte end feet can influence arteriolar diameter [139], endothelial cells can release vasodilators such as nitric oxide and vasoconstrictors such as endothelin [140], and pericytes can cause capillary contraction and relaxation [141]. In AD, loss of cholinergic innervation of brain blood vessels [138], even down to the microvessels [142], may contribute to brain hypoperfusion.
LA lesions show increased expression of the hypoxia-inducible factor HIF1α [143], and white matter lesion load correlates with the degree of hypoperfusion [144] and cognitive impairment [7–14]. The cognitive impairment may be affected by the location of the lesions. CBF is also reduced in normal-appearing periventricular white matter in patients with LA [144]. This is consistent with hypoperfusion preceding and inducing LA lesion development. Recent diffusion-weighted imaging studies of normal-appearing white matter in LA patients have shown increased diffusivity in white matter outside of the LA lesions [145,146] and this was more strongly correlated with cognitive decline than was lesion load or brain atrophy [145]. This indication of moderate damage adjacent to the LA lesions parallels our findings of a trend toward decreased vascular density in these areas and in the cortex [64,65]. Regional decreases in white matter integrity (fractional anisotropy) are linked to gray matter hypometabolism in the areas affected in AD and vascular dementia [147].
Hypoperfusion in AD induces white matter lesions and cortical watershed microinfarcts [113]. CBF reductions correlate with dementia and co-localize with cortical atrophy and vascular disease in the white matter [136]. Reduced CBF occurs early in the development of AD, most significantly in areas where tau pathology is associated with AD [148,149]. These perfusion deficits develop in presymptomatic stages before brain atrophy [135,148,149]. Subjects with mild cognitive impairment also exhibit hypoperfusion in the areas most affected in AD [135,149].
Cholinergic stimulation of brain perfusion
The human basal forebrain cholinergic system plays a major role in cognitive function and cholinergic agonists have been shown to enhance cognition in both animals and humans [150]. Baroreceptors monitor BP and induce neural responses to modulate BP [151] and regional blood flow [152]. This function is reduced in the elderly [153]. Cholinergic deficits are observed following chronic hypoperfusion [154] and cholinesterase inhibitors, such as donepezil, can enhance cholinergic transmission [155]. Ischemia of cholinergic nuclei can result in cholinergic neuron loss [156], and this injury may be exacerbated if the damage to the cholinergic nuclei reduces their function. This may occur in vascular dementia [157–159] and AD [160]. Cholinesterase inhibitors have some benefit in treating vascular dementia, although not as much as in AD [161]. In vascular dementia, cholinergic reductions are correlated with cognitive impairment [162]. In AD, excitation of acetylcholine receptors by physostigmine improved the impaired CBF [150]. Furthermore, cholinesterase inhibitors enhanced CBF and cognition in AD patients [163]. Cholinergic fibers are often severed in white matter lesions, resulting in disconnection of the cholinergic projections to the cortex [138]. Possibly, this disconnection contributes to white matter lesion-induced cognitive dysfunction. Indeed, cholinesterase inhibitors can restore CBF after ablation of the nucleus basalis of Meynert [164]. There is reason for optimism that the brain can be rescued from extended periods of hypoperfusion. In Norrie disease, where retinal hypovascularization causes ischemia and vision loss [165], the rods and cones remain intact but unable to transmit signals in their hypoxic milieu. However, their function can be revived with oxygenation.
Cerebrovascular Microemboli and Their Clearance
Microemboli from cardiac surgery
Microemboli released during cardiac surgery assisted by cardiopulmonary bypass (CPB) are a source of microvascular pathology. In brain tissue sections from patients who die within days after cardiac surgery, lipid microemboli can be seen as SCADs (small capillary and arteriolar dilatations) (Figure 8) [166]. The microemboli tend to pump through the vessels, breaking into smaller emboli at branch-points [167]. While most of these emboli pass through the brain in a few hours to a few days, some remain impacted for weeks or longer [168]. This can block blood flow and cause ischemia and capillary loss.
Fig. 8.
Lipid microemboli in brain capillaries in a patient one day after cardiac surgery assisted by CPB. (Reprinted from [190]).
Our findings suggest that SCADs come primarily from fat that drips into the blood in the chest wound during surgery, especially from the fatty marrow of the sternum [167]. When this lipid-laden blood is suctioned into the CPB circuit, the lipid globules can slip through the filters and travel to the brain and other organs. Microvascular occlusion by lipid emboli may be the major contributor to post-CPB encephalopathy. Our studies with experimental dog surgeries have lead to strategies of blood return and blood temperature management to improve surgical outcomes. For example, suctioned blood is cleansed of lipid emboli by passing it through a cell saver, which washes and separates red blood cells by centrifugation [169,170].
Other vascular surgical procedures that can cause lipid microemboli to circulate to the brain include disruption of atheromatous plaque during carotid endarterectomy [171], and left heart catheterization during angioplasty [166,172]. These procedures can also result in cognitive decline [173]. Orthopedic surgery of large bones can also release lipid emboli from the bone marrow into the venous system [174], some of which can pass through the lungs [174,175]. Patients with a patent foramen ovale are especially in danger of having emboli pass into the left side of the heart and travel to the brain. This can cause cognitive dysfunction, stroke, coma, or death [167,176,177]. Another major source of fat embolism is broken bones, as in car accidents, which can cause petechial hemorrhages in the brain, skin, and conjunctiva, and sometimes death [167]. Since fat emboli float in the blood, positioning the patient head down can alter their route [178]. Fat embolism affects the white matter more than it does the cortex [167], perhaps because of its vulnerability to hypoperfusion.
Post-pump encephalopathy appears to be a type of vascular dementia resulting from lipid microemboli [167]. This can cause accelerated cognitive decline some years after surgery [179–183]. With aging, lost capillaries are less likely to be replaced, and this may cause chronic hypoperfusion and exacerbate any bouts of hypoxia. There is an accelerated cognitive decline 5 years after CPB surgery [179–182]. Embolic injury may accelerate age-related cerebrovascular pathology and reduce vascular reserve and brain reserve. It is of interest that the cholinesterase inhibitor, donepezil, has shown some benefit for cognitive decline following CPB surgery [184]. Because cardiac surgery is the most common surgery in the world [185], it may be worth recording as a risk factor in studies of dementia.
Extravasation of emboli
Grutzendler and colleagues have recently shown that certain types of emboli were extruded through the walls of mouse cerebral vessels within 2 to 7 days after injection into the carotid artery [186]. The emboli included fibrin clots and cholesterol crystals filtered through sieves of 8–20 μm pore size, and polystyrene microspheres of 10 or 15 μm diameter. Using new techniques such as a thinned-skull cranial window, high resolution imaging with two-photon laser scanning microscopy, and live video, the process of emboli extravasation was shown in live mice. The emboli that lodged in the microvasculature became covered by endothelial cell membrane projections which completely enveloped them within 24–48 hours. The endothelial projections frequently formed transient adhesions, resembling adherens junctions, with the endothelium of the opposing wall of the vessel. The emboli were then pushed toward the parenchyma as the endothelial cells withdrew from the abluminal side of the embolus, exposing the embolus to the BM. The embolus then passed through the BM into the extravascular space adjacent to the brain parenchyma. Matrix metalloproteinases were detected adjacent to the emboli and extravasation was decreased after treatment with matrix metalloproteinase inhibitors.
There was some vascular degeneration with 15 μm microspheres, but none with 10 μm microspheres [186]. With the numbers of microemboli they administered, they typically found transient hypoxia and transient synaptic pruning rather than cell death. In aged mice, however, extravasation of emboli was delayed and accompanied by persistent hypoxia, dystrophic synapses, and caspase-positive (apoptotic) perivascular cells. Thus the degree of embolic brain injury depends on the number, size, and type of the emboli, as well as the age of the person or animal.
This extravasation of clots, cholesterol crystals, and microspheres appears to differ from what occurs in the case of lipid emboli. We have not seen evidence of lipid embolus extravasation and numerous lipid emboli remain within the cerebral microvasculature for some weeks after cardiac surgery, often in degenerated capillaries (Figures 9 and 10) [167,168]. Antifoam emboli, with fat-like consistency, have also been found up to 8 months after CPB [187]. After fat embolism, excretion occurs via small lipid droplets in the urine and sputum [188]. If lipid emboli are not extravasated, one explanation could be that endothelial cells are unable to extend cell projections over the flexible and motile surface of lipid droplets. Also, the lipid emboli may not trigger an endothelial cell recognition event that induces membrane projections to cover the embolus.
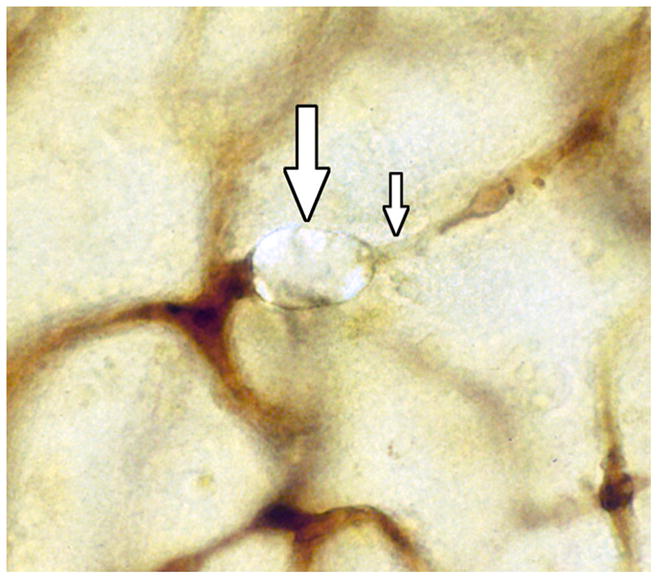
Fig. 9.
A lipid embolus (large arrow) in a brain capillary in a patient 17 days after cardiac surgery assisted by CPB. Note the loss of AP staining and apparent degenerative changes in the capillary down stream from the embolus. (Reprinted from [167]).
Fig. 10.
A lipid embolus (large arrow) showing birefringence in a photograph with a polarizing filter. Note the loss of AP staining and apparent degenerative changes in the capillary down stream from the embolus. (Reprinted from [167]).
Conclusions
Current evidence suggests that there is an age-related decline in microvascular structure. The decline in the capacity for cerebrovascular angiogenesis may result in a failure of vascular recovery from hypoxia-induced bouts of capillary loss in aging, AD, and LA. In concordance with this, there is strong evidence of decreased vascular density in aging, AD, and LA. Reduced capillary density in AD may be due in part to a reduction in angiogenesis caused by VEGF becoming bound to Aβ and sequestered in plaques.
The white matter is vulnerable to suffering hypoperfusion because its major blood supply is via long arterioles which arise from the border-zone. Blood flow can also be inhibited by tortuous arterioles which form in the white matter, coiled in a cavity where brain parenchyma is lost. Another change is the deposition of excessive collagen in the walls of veins and venules in the deep white matter. These deposits can be severe in LA, and they likely contribute to the development of LA. Vascular collagen deposition also occurs in multiple sclerosis lesions. It would be interesting to know its significance and whether the veins are specifically affected. Venous collagenosis in LA likely inhibits blood flow. The thickened walls may inhibit the passage of fluids and toxins into the veins for removal from the brain, and the perivascular drainage may also be impaired, affecting the clearance of Aβ along this channel.
Hypertension appears to injure the vascular system, and with aging, the boundary of autoregulation in the brain shifts upward, causing vulnerability to hypoperfusion. Misery perfusion due to capillary loss appears to occur before cell loss in LA, as there is increased oxygen extraction in the deep white matter, which implies reduced oxygen supply. CBF is also reduced in normal-appearing white matter in LA. This is consistent with hypoperfusion preceding and inducing LA. LA lesions correlate with the degree of hypoperfusion and cognitive impairment. Normal-appearing white matter in LA also has increased diffusivity and this is correlated with cognitive decline. Hypoperfusion in AD induces white matter lesions, and CBF reductions correlate with dementia and vascular disease in the white matter. Reduced CBF occurs early in AD, in areas where tau pathology is associated with AD. These perfusion deficits develop in presymptomatic stages before brain atrophy. Also, in mild cognitive impairment, hypoperfusion occurs in the areas most affected in AD.
In AD, loss of cholinergic innervation of brain blood vessels may contribute to brain hypoperfusion. Neural responses modulate BP and regional blood flow, but this function is reduced in the elderly. Cholinergic deficits occur following chronic hypoperfusion, and ischemia of cholinergic nuclei can cause cholinergic neuron loss. Such injury may be exacerbated if damage to the nuclei reduces cholinergic activity. In vascular dementia, cholinergic reductions are correlated with cognitive impairment, and cholinesterase inhibitors have some benefit, although not as much as in AD. Cholinergic fibers are often severed by lesions in the white matter, disconnecting cholinergic projections to the cortex, which might contribute to cognitive dysfunction. In AD, excitation of acetylcholine receptors improved the impaired CBF, and cholinesterase inhibitors enhanced CBF and cognition.
Lipid microemboli from cardiac surgery pump through the vessels, breaking into smaller emboli at branch-points and occlude some microvessels. Most of the emboli pass through the brain in a few hours to days, but some remain for weeks. They can cause cognitive dysfunction, stroke, coma, or death. Microemboli-induced encephalopathy appears to be a type of vascular dementia which can develop some years after surgery. Donepezil has shown some benefit. It has been recently shown that emboli, such as clots, cholesterol crystals, and microspheres, can be extruded through the walls of cerebral vessels. The emboli became enveloped by endothelial cell membrane projections and then pushed out as the endothelial cells withdrew at the abluminal side of the vessel. Emboli then passed through the BM into the extravascular space. In aged mice, extravasation of emboli was slower. There is no evidence yet that lipid emboli undergo such extravasation.
Acknowledgments
We thank Patricia Wood for histological expertise. This work was supported by NIH grants NS20618, NS 36780, and CA113321.
Reference List
- 1.Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown WR, Moody DM, Thore CR, Anstrom JA, Challa VR. Microvascular changes in the white mater in dementia. J Neurol Sci. 2009;283:28–31. doi: 10.1016/j.jns.2009.02.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke. 2008;39:2712–2719. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- 4.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 5.Hassan A, Hunt BJ, O’Sullivan M, et al. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain. 2003;126:424–432. doi: 10.1093/brain/awg040. [DOI] [PubMed] [Google Scholar]
- 6.Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis. Neurobiol Aging. 2009;30:337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Au R, Massaro JM, Wolf PA, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol. 2006;63:246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- 8.Garde E, Lykke ME, Rostrup E, Paulson OB. Decline in intelligence is associated with progression in white matter hyperintensity volume. J Neurol Neurosurg Psychiatry. 2005;76:1289–1291. doi: 10.1136/jnnp.2004.055905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inzitari D, Simoni M, Pracucci G, et al. Risk of rapid global functional decline in elderly patients with severe cerebral age-related white matter changes: the LADIS study. Arch Intern Med. 2007;167:81–88. doi: 10.1001/archinte.167.1.81. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt R, Petrovic K, Ropele S, Enzinger C, Fazekas F. Progression of leukoaraiosis and cognition. Stroke. 2007;38:2619–2625. doi: 10.1161/STROKEAHA.107.489112. [DOI] [PubMed] [Google Scholar]
- 11.van den Heuvel DM, ten Dam V, De Craen AJ, et al. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. J Neurol Neurosurg Psychiatry. 2006;77:149–153. doi: 10.1136/jnnp.2005.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawamura J, Meyer JS, Terayama Y, Weathers S. Leukoaraiosis correlates with cerebral hypoperfusion in vascular dementia. Stroke. 1991;22:609–614. doi: 10.1161/01.str.22.5.609. [DOI] [PubMed] [Google Scholar]
- 13.Pantoni L, Poggesi A, Basile AM, et al. Leukoaraiosis predicts hidden global functioning impairment in nondisabled older people: the LADIS (Leukoaraiosis and Disability in the Elderly) Study. J Am Geriatr Soc. 2006;54:1095–1101. doi: 10.1111/j.1532-5415.2006.00798.x. [DOI] [PubMed] [Google Scholar]
- 14.Gupta SR, Naheedy MH, Young JC, Ghobrial M, Rubino FA, Hindo W. Periventricular white matter changes and dementia. Clinical, neuropsychological, radiological, and pathological correlation. Arch Neurol. 1988;45:637–641. doi: 10.1001/archneur.1988.00520300057019. [DOI] [PubMed] [Google Scholar]
- 15.Bell MA, Scarrow WG. Staining for microvascular alkaline phosphatase in thick celloidin sections of nervous tissue: morphometric and pathological applications. Microvasc Res. 1984;27:189–203. doi: 10.1016/0026-2862(84)90053-0. [DOI] [PubMed] [Google Scholar]
- 16.Anstrom JA, Brown WR, Moody DM, Thore CR, Challa VR, Block SM. Anatomical analysis of the developing cerebral vasculature in premature neonates: absence of precapillary arteriole-to-venous shunts. Pediatr Res. 2002;52:554–560. doi: 10.1203/00006450-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. AJNR Am J Neuroradiol. 1990;11:431–439. [PMC free article] [PubMed] [Google Scholar]
- 18.De Reuck J. The human periventricular arterial blood supply and the anatomy of cerebral infarctions. Eur Neurol. 1971;5:321–334. doi: 10.1159/000114088. [DOI] [PubMed] [Google Scholar]
- 19.van den Bergh R. Centrifugal elements in the vascular pattern of the deep intracerebral blood supply. Angiology. 1969;20:88–94. doi: 10.1177/000331976902000205. [DOI] [PubMed] [Google Scholar]
- 20.Brown WR, Moody DM, Challa VR, Thore CR, Anstrom JA. Venous collagenosis and arteriolar tortuosity in leukoaraiosis. J Neurol Sci. 2002;203–204:159–163. doi: 10.1016/s0022-510x(02)00283-6. [DOI] [PubMed] [Google Scholar]
- 21.Hassler O. Vascular changes in senile brains. A micro-angiographic study. Acta Neuropathol. 1965;5:40–53. doi: 10.1007/BF00689161. [DOI] [PubMed] [Google Scholar]
- 22.Moody DM, Santamore WP, Bell MA. Does tortuosity in cerebral arterioles impair down-autoregulation in hypertensives and elderly normotensives? A hypothesis and computer model. Clin Neurosurg. 1991;37:372–387. [PubMed] [Google Scholar]
- 23.Ravens JR. Vascular changes in the human senile brain. Adv Neurol. 1978;20:487–501. [PubMed] [Google Scholar]
- 24.Akima M, Nonaka H, Kagesawa M, Tanaka K. A study on the microvasculature of the cerebral cortex. Fundamental architecture and its senile change in the frontal cortex. Lab Invest. 1986;55:482–489. [PubMed] [Google Scholar]
- 25.Thore CR, Anstrom JA, Moody DM, Challa VR, Marion MC, Brown WR. Morphometric analysis of arteriolar tortuosity in human cerebral white matter of preterm, young, and aged subjects. J Neuropathol Exp Neurol. 2007;66:337–345. doi: 10.1097/nen.0b013e3180537147. [DOI] [PubMed] [Google Scholar]
- 26.Fischer VW, Siddiqi A, Yusufaly Y. Altered angioarchitecture in selected areas of brains with Alzheimer’s disease. Acta Neuropathol. 1990;79:672–679. doi: 10.1007/BF00294246. [DOI] [PubMed] [Google Scholar]
- 27.Beskow J, Hassler O, Ottosson JO. Cerebral arterial deformities in relation to senile deterioration. Acta Psychiatr Scand Suppl. 1971;221:111–119. doi: 10.1111/j.1600-0447.1971.tb02143.x. [DOI] [PubMed] [Google Scholar]
- 28.Saunders RL, Bell MA. X-ray microscopy and histochemistry of the human cerebral blood vessels. J Neurosurg. 1971;35:128–140. doi: 10.3171/jns.1971.35.2.0128. [DOI] [PubMed] [Google Scholar]
- 29.Fang HCH, Cervós-Navarro J. Pathology of Cerebral Microcirculation. Berlin: De Guyter; 1974. The studies of cerebral arterioles and capillaries in normal and pathologic status in man; pp. 431–441. [Google Scholar]
- 30.Hassler O. Arterial deformities in senile brains. The occurrence of the deformities in a large autopsy series and some aspects of their functional significance. Acta Neuropathol. 1967;8:219–229. doi: 10.1007/BF00688824. [DOI] [PubMed] [Google Scholar]
- 31.Moody DM, Brown WR, Challa VR, Anderson RL. Periventricular venous collagenosis: association with leukoaraiosis. Radiology. 1995;194:469–476. doi: 10.1148/radiology.194.2.7824728. [DOI] [PubMed] [Google Scholar]
- 32.Mohan H, Krumbholz M, Sharma R, et al. Extracellular matrix in multiple sclerosis lesions: Fibrillar collagens, biglycan and decorin are upregulated and associated with infiltrating immune cells. Brain Pathol. 2010;20:966–975. doi: 10.1111/j.1750-3639.2010.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 34.Farkas E, de Vos RA, Donka G, Jansen Steur EN, Mihaly A, Luiten PG. Age-related microvascular degeneration in the human cerebral periventricular white matter. Acta Neuropathol. 2006;111:150–157. doi: 10.1007/s00401-005-0007-y. [DOI] [PubMed] [Google Scholar]
- 35.Gao FQ, Levy-Copperman N, Scott C, Ramirez J, Bilbao JM, Black SE. White matter hyperintensities a sign of cerebral venous insufficiency. VASCOG Abstracts. 2007:89. [Google Scholar]
- 36.Cserr HF, Ostrach LH. Bulk flow of interstitial fluid after intracranial injection of blue dextran 2000. Exp Neurol. 1974;45:50–60. doi: 10.1016/0014-4886(74)90099-5. [DOI] [PubMed] [Google Scholar]
- 37.Rennels ML, Blaumanis OR, Grady PA. Rapid solute transport throughout the brain via paravascular fluid pathways. Adv Neurol. 1990;52:431–439. [PubMed] [Google Scholar]
- 38.Preston SD, Steart PV, Wilkinson A, Nicoll JA, Weller RO. Capillary and arterial cerebral amyloid angiopathy in Alzheimer’s disease: defining the perivascular route for the elimination of amyloid beta from the human brain. Neuropathol Appl Neurobiol. 2003;29:106–117. doi: 10.1046/j.1365-2990.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 39.Weller RO, Massey A, Newman TA, Hutchings M, Kuo YM, Roher AE. Cerebral amyloid angiopathy: amyloid beta accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. Am J Pathol. 1998;153:725–733. doi: 10.1016/s0002-9440(10)65616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pirttila T, Mehta PD, Soininen H, et al. Cerebrospinal fluid concentrations of soluble amyloid beta-protein and apolipoprotein E in patients with Alzheimer’s disease: correlations with amyloid load in the brain. Arch Neurol. 1996;53:189–193. doi: 10.1001/archneur.1996.00550020105022. [DOI] [PubMed] [Google Scholar]
- 41.Roher AE, Lowenson JD, Clarke S, et al. beta-Amyloid-(1–42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:10836–10840. doi: 10.1073/pnas.90.22.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaguchi H, Yamazaki T, Lemere CA, Frosch MP, Selkoe DJ. Beta amyloid is focally deposited within the outer basement membrane in the amyloid angiopathy of Alzheimer’s disease. An immunoelectron microscopic study. Am J Pathol. 1992;141:249–259. [PMC free article] [PubMed] [Google Scholar]
- 43.Henry-Feugeas MC. Alzheimer’s disease in late-life dementia: a minor toxic consequence of devastating cerebrovascular dysfunction. Med Hypotheses. 2008;70:866–875. doi: 10.1016/j.mehy.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 44.Brown WR. A review of string vessels or collapsed, empty basement membrane tubes. Journal of Alzheimer’s Disease. 2010;21:725–739. doi: 10.3233/JAD-2010-100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Venecia G, Davis M, Engerman R. Clinicopathologic correlations in diabetic retinopathy. I. Histology and fluorescein angiography of microaneurysms. Arch Ophthalmol. 1976;94:1766–1773. doi: 10.1001/archopht.1976.03910040540013. [DOI] [PubMed] [Google Scholar]
- 46.Kohner EM, Henkind P. Correlation of fluorescein angiogram and retinal digest in diabetic retinopathy. Am J Ophthalmol. 1970;69:403–414. doi: 10.1016/0002-9394(70)92273-7. [DOI] [PubMed] [Google Scholar]
- 47.Reinecke RD, Kuwabara T, Cogan DG, Weis DR. Retinal vascular patterns. V. Experimental ischemia of the cat eye. Arch Ophthalmol. 1962;67:470–475. doi: 10.1001/archopht.1962.00960020470015. [DOI] [PubMed] [Google Scholar]
- 48.Cogan DG. Development and senescence of the human retinal vasculature. Trans Ophthalmol Soc U K. 1963;83:465–489. [PubMed] [Google Scholar]
- 49.Clark ER. Studies on the growth of blood vessels in the tail of the frog larva by observation and experiment on the living animal. Am J Anat. 1918;23:37–88. [Google Scholar]
- 50.Lang R, Lustig M, Francois F, Sellinger M, Plesken H. Apoptosis during macrophage-dependent ocular tissue remodelling. Development. 1994;120:3395–3403. doi: 10.1242/dev.120.12.3395. [DOI] [PubMed] [Google Scholar]
- 51.Bartel H, Lametschwandtner A. Regression of blood vessels in the ventral velum of Xenopus laevis Daudin during metamorphosis: light microscopic and transmission electron microscopic study. J Anat. 2000;197 (Pt 2):157–166. doi: 10.1046/j.1469-7580.2000.19720157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inai T, Mancuso M, Hashizume H, et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Resnick N, Einav S, Chen-Konak L, Zilberman M, Yahav H, Shay-Salit A. Hemodynamic forces as a stimulus for arteriogenesis. Endothelium. 2003;10:197–206. doi: 10.1080/10623320390246289. [DOI] [PubMed] [Google Scholar]
- 54.Cammermeyer J. A comparative study of intervascular connective tissue strands in the central nervous system. J Comp Neurol. 1960;114:189–208. doi: 10.1002/cne.901140206. [DOI] [PubMed] [Google Scholar]
- 55.Mao XW, Archambeau JO, Kubinova L, Boyle S, Petersen G, Grove R. Quantification of rat retinal growth and vascular population changes after single and split doses of proton irradiation: translational study using stereology methods. Radiat Res. 2003;160:5–13. doi: 10.1667/rr3007. [DOI] [PubMed] [Google Scholar]
- 56.Mott RT, Thore CR, Moody DM, Glazier SS, Ellis TL, Brown WR. Reduced ratio of afferent to total vascular density in mesial temporal sclerosis. J Neuropathol Exp Neurol. 2009;68:1147–1154. doi: 10.1097/NEN.0b013e3181b9d75f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buee L, Hof PR, Bouras C, et al. Pathological alterations of the cerebral microvasculature in Alzheimer’s disease and related dementing disorders. Acta Neuropathol. 1994;87:469–480. doi: 10.1007/BF00294173. [DOI] [PubMed] [Google Scholar]
- 58.Challa VR, Thore CR, Moody DM, Anstrom JA, Brown WR. Increase of white matter string vessels in Alzheimer’s disease. J Alzheimers Dis. 2004;6:379–383. doi: 10.3233/jad-2004-6404. [DOI] [PubMed] [Google Scholar]
- 59.Kalaria RN, Kroon SN. Expression of leukocyte antigen CD34 by brain capillaries in Alzheimer’s disease and neurologically normal subjects. Acta Neuropathol. 1992;84:606–612. doi: 10.1007/BF00227737. [DOI] [PubMed] [Google Scholar]
- 60.Kalaria RN, Hedera P. Differential degeneration of the cerebral microvasculature in Alzheimer’s disease. Neuroreport. 1995;6:477–480. doi: 10.1097/00001756-199502000-00018. [DOI] [PubMed] [Google Scholar]
- 61.McGeer PL, Zhu SG, Dedhar S. Immunostaining of human brain capillaries by antibodies to very late antigens. J Neuroimmunol. 1990;26:213–218. doi: 10.1016/0165-5728(90)90003-6. [DOI] [PubMed] [Google Scholar]
- 62.Perlmutter LS, Chui HC. Microangiopathy, the vascular basement membrane and Alzheimer’s disease: a review. Brain Res Bull. 1990;24:677–686. doi: 10.1016/0361-9230(90)90007-m. [DOI] [PubMed] [Google Scholar]
- 63.Challa VR, Thore CR, Moody DM, Brown WR, Anstrom JA. A three-dimensional study of brain string vessels using celloidin sections stained with anti-collagen antibodies. J Neurol Sci. 2002;203–204:165–167. doi: 10.1016/s0022-510x(02)00284-8. [DOI] [PubMed] [Google Scholar]
- 64.Brown WR, Moody DM, Thore CR, Challa VR, Anstrom JA. Vascular dementia in leukoaraiosis may be a consequence of capillary loss not only in the lesions, but in normal-appearing white matter and cortex as well. J Neurol Sci. 2007;257:62–66. doi: 10.1016/j.jns.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moody DM, Thore CR, Anstrom JA, Challa VR, Langefeld CD, Brown WR. Quantification of afferent vessels shows reduced brain vascular density in subjects with leukoaraiosis. Radiology. 2004;233:883–890. doi: 10.1148/radiol.2333020981. [DOI] [PubMed] [Google Scholar]
- 66.Alba C, Vidal L, Diaz F, Villena A, de Vargas I. Ultrastructural and quantitative age-related changes in capillaries of the dorsal lateral geniculate nucleus. Brain Res Bull. 2004;64:145–153. doi: 10.1016/j.brainresbull.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 67.Kalaria RN. The blood-brain barrier and cerebral microcirculation in Alzheimer disease. Cerebrovasc Brain Metab Rev. 1992;4:226–260. [PubMed] [Google Scholar]
- 68.Mancardi GL, Perdelli F, Rivano C, Leonardi A, Bugiani O. Thickening of the basement membrane of cortical capillaries in Alzheimer’s disease. Acta Neuropathol. 1980;49:79–83. doi: 10.1007/BF00692225. [DOI] [PubMed] [Google Scholar]
- 69.Kalaria RN, Pax AB. Increased collagen content of cerebral microvessels in Alzheimer’s disease. Brain Res. 1995;705:349–352. doi: 10.1016/0006-8993(95)01250-8. [DOI] [PubMed] [Google Scholar]
- 70.Kalaria RN. Cerebral vessels in ageing and Alzheimer’s disease. Pharmacol Ther. 1996;72:193–214. doi: 10.1016/s0163-7258(96)00116-7. [DOI] [PubMed] [Google Scholar]
- 71.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 72.Mancuso MR, Davis R, Norberg SM, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saint-Geniez M, D’Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol. 2004;48:1045–1058. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- 74.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 75.Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp Cell Res. 2006;312:630–641. doi: 10.1016/j.yexcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 76.Dore-Duffy P, LaManna JC. Physiologic angiodynamics in the brain. Antioxid Redox Signal. 2007;9:1363–1371. doi: 10.1089/ars.2007.1713. [DOI] [PubMed] [Google Scholar]
- 77.LaManna JC, Chavez JC, Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J Exp Biol. 2004;207:3163–3169. doi: 10.1242/jeb.00976. [DOI] [PubMed] [Google Scholar]
- 78.Boero JA, Ascher J, Arregui A, Rovainen C, Woolsey TA. Increased brain capillaries in chronic hypoxia. J Appl Physiol. 1999;86:1211–1219. doi: 10.1152/jappl.1999.86.4.1211. [DOI] [PubMed] [Google Scholar]
- 79.Black JE, Polinsky M, Greenough WT. Progressive failure of cerebral angiogenesis supporting neural plasticity in aging rats. Neurobiol Aging. 1989;10:353–358. doi: 10.1016/0197-4580(89)90048-1. [DOI] [PubMed] [Google Scholar]
- 80.Rivard A, Fabre JE, Silver M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 81.Chavez JC, LaManna JC. Hypoxia-inducible factor-1alpha accumulation in the rat brain in response to hypoxia and ischemia is attenuated during aging. Adv Exp Med Biol. 2003;510:337–341. doi: 10.1007/978-1-4615-0205-0_55. [DOI] [PubMed] [Google Scholar]
- 82.Frenkel-Denkberg G, Gershon D, Levy AP. The function of hypoxia-inducible factor 1 (HIF-1) is impaired in senescent mice. FEBS Lett. 1999;462:341–344. doi: 10.1016/s0014-5793(99)01552-5. [DOI] [PubMed] [Google Scholar]
- 83.Rivard A, Berthou-Soulie L, Principe N, et al. Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J Biol Chem. 2000;275:29643–29647. doi: 10.1074/jbc.M001029200. [DOI] [PubMed] [Google Scholar]
- 84.Rapino C, Bianchi G, Di GC, et al. HIF-1alpha cytoplasmic accumulation is associated with cell death in old rat cerebral cortex exposed to intermittent hypoxia. Aging Cell. 2005;4:177–185. doi: 10.1111/j.1474-9726.2005.00161.x. [DOI] [PubMed] [Google Scholar]
- 85.Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res Rev. 2003;2:149–168. doi: 10.1016/s1568-1637(02)00064-8. [DOI] [PubMed] [Google Scholar]
- 86.Jucker M, Battig K, Meier-Ruge W. Effects of aging and vincamine derivatives on pericapillary microenvironment: stereological characterization of the cerebral capillary network. Neurobiol Aging. 1990;11:39–46. doi: 10.1016/0197-4580(90)90060-d. [DOI] [PubMed] [Google Scholar]
- 87.Shao WH, Li C, Chen L, et al. Stereological investigation of age-related changes of the capillaries in white matter. Anat Rec (Hoboken) 2010;293:1400–1407. doi: 10.1002/ar.21184. [DOI] [PubMed] [Google Scholar]
- 88.Klein AW, Michel ME. A morphometric study of the neocortex of young adult and old maze-differentiated rats. Mech Ageing Dev. 1977;6:441–452. doi: 10.1016/0047-6374(77)90045-8. [DOI] [PubMed] [Google Scholar]
- 89.Buchweitz-Milton E, Weiss HR. Perfused capillary morphometry in the senescent brain. Neurobiol Aging. 1987;8:271–276. doi: 10.1016/0197-4580(87)90012-1. [DOI] [PubMed] [Google Scholar]
- 90.Casey MA, Feldman ML. Aging in the rat medial nucleus of the trapezoid body. III. Alterations in capillaries. Neurobiol Aging. 1985;6:39–46. doi: 10.1016/0197-4580(85)90070-3. [DOI] [PubMed] [Google Scholar]
- 91.Hinds JW, McNelly NA. Capillaries in aging rat olfactory bulb: a quantitative light and electron microscopic analysis. Neurobiol Aging. 1982;3:197–207. doi: 10.1016/0197-4580(82)90040-9. [DOI] [PubMed] [Google Scholar]
- 92.Wilkinson JH, Hopewell JW, Reinhold HS. A quantitative study of age-related changes in the vascular architecture of the rat cerebral cortex. Neuropathol Appl Neurobiol. 1981;7:451–462. doi: 10.1111/j.1365-2990.1981.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 93.Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- 94.Hutchins PM, Lynch CD, Cooney PT, Curseen KA. The microcirculation in experimental hypertension and aging. Cardiovasc Res. 1996;32:772–780. [PubMed] [Google Scholar]
- 95.Amenta F, Cavallotti D, Del VM, et al. Age-related changes in brain microanatomy: sensitivity to treatment with the dihydropyridine calcium channel blocker darodipine (PY 108–068) Brain Res Bull. 1995;36:453–460. doi: 10.1016/0361-9230(94)00210-r. [DOI] [PubMed] [Google Scholar]
- 96.Amenta F, Ferrante F, Mancini M, Sabbatini M, Vega JA, Zaccheo D. Effect of long-term treatment with the dihydropyridine-type calcium channel blocker darodipine (PY 108–068) on the cerebral capillary network in aged rats. Mech Ageing Dev. 1995;78:27–37. doi: 10.1016/0047-6374(94)01513-l. [DOI] [PubMed] [Google Scholar]
- 97.Burns EM, Kruckeberg TW, Gaetano PK. Changes with age in cerebral capillary morphology. Neurobiol Aging. 1981;2:285–291. doi: 10.1016/0197-4580(81)90037-3. [DOI] [PubMed] [Google Scholar]
- 98.Knox CA, Oliveira A. Brain aging in normotensive and hypertensive strains of rats. III. A quantitative study of cerebrovasculature. Acta Neuropathol. 1980;52:17–25. doi: 10.1007/BF00687224. [DOI] [PubMed] [Google Scholar]
- 99.Villena A, Vidal L, Diaz F, Perez DVI. Stereological changes in the capillary network of the aging dorsal lateral geniculate nucleus. Anat Rec A Discov Mol Cell Evol Biol. 2003;274:857–861. doi: 10.1002/ar.a.10100. [DOI] [PubMed] [Google Scholar]
- 100.Meier-Ruge W, Schulz-Dazzi U. Effects of brovincamine on the stereological parameters of corticocerebral capillaries. Life Sci. 1987;40:943–949. doi: 10.1016/0024-3205(87)90313-4. [DOI] [PubMed] [Google Scholar]
- 101.Hughes CC, Lantos PL. A morphometric study of blood vessel, neuron and glial cell distribution in young and old rat brain. J Neurol Sci. 1987;79:101–110. doi: 10.1016/0022-510x(87)90264-4. [DOI] [PubMed] [Google Scholar]
- 102.Bar T. Morphometric evaluation of capillaries in different laminae of rat cerebral cortex by automatic image analysis: changes during development and aging. Adv Neurol. 1978;20:1–9. [PubMed] [Google Scholar]
- 103.Sturrock RR. Quantitative and morphological changes in neurons and neuroglia in the indusium griseum of aging mice. J Gerontol. 1977;32:647–658. doi: 10.1093/geronj/32.6.647. [DOI] [PubMed] [Google Scholar]
- 104.Vaughan WJ, Calvin M. Electrophoretic analysis of brain proteins from young adult and aged mice. Gerontology. 1977;23:110–126. doi: 10.1159/000212179. [DOI] [PubMed] [Google Scholar]
- 105.Bell MA, Ball MJ. Morphometric comparison of hippocampal microvasculature in ageing and demented people: diameters and densities. Acta Neuropathol. 1981;53:299–318. doi: 10.1007/BF00690372. [DOI] [PubMed] [Google Scholar]
- 106.Bell MA, Ball MJ. The correlation of vascular capacity with the parenchymal lesions of Alzheimer’s disease. Can J Neurol Sci. 1986;13:456–461. doi: 10.1017/s0317167100037124. [DOI] [PubMed] [Google Scholar]
- 107.Bell MA, Ball MJ. Neuritic plaques and vessels of visual cortex in aging and Alzheimer’s dementia. Neurobiol Aging. 1990;11:359–370. doi: 10.1016/0197-4580(90)90001-g. [DOI] [PubMed] [Google Scholar]
- 108.Abernethy WB, Bell MA, Morris M, Moody DM. Microvascular density of the human paraventricular nucleus decreases with aging but not hypertension. Exp Neurol. 1993;121:270–274. doi: 10.1006/exnr.1993.1095. [DOI] [PubMed] [Google Scholar]
- 109.Mann DM, Eaves NR, Marcyniuk B, Yates PO. Quantitative changes in cerebral cortical microvasculature in ageing and dementia. Neurobiol Aging. 1986;7:321–330. doi: 10.1016/0197-4580(86)90158-2. [DOI] [PubMed] [Google Scholar]
- 110.Hunziker O, Al SA, Schulz U, Schweizer A. Architecture of cerebral capillaries in aged human subjects with hypertension. Adv Neurol. 1978;20:471–477. [PubMed] [Google Scholar]
- 111.Hunziker O, bdel’Al S, Schulz U. The aging human cerebral cortex: a stereological characterization of changes in the capillary net. J Gerontol. 1979;34:345–350. doi: 10.1093/geronj/34.3.345. [DOI] [PubMed] [Google Scholar]
- 112.Meier-Ruge W, Hunziker O, Schulz U, Tobler HJ, Schweizer A. Stereological changes in the capillary network and nerve cells of the aging human brain. Mech Ageing Dev. 1980;14:233–243. doi: 10.1016/0047-6374(80)90123-2. [DOI] [PubMed] [Google Scholar]
- 113.Suter OC, Sunthorn T, Kraftsik R, et al. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke. 2002;33:1986–1992. doi: 10.1161/01.str.0000024523.82311.77. [DOI] [PubMed] [Google Scholar]
- 114.Bailey TL, Rivara CB, Rocher AB, Hof PR. The nature and effects of cortical microvascular pathology in aging and Alzheimer’s disease. Neurol Res. 2004;26:573–578. doi: 10.1179/016164104225016272. [DOI] [PubMed] [Google Scholar]
- 115.Desai BS, Schneider JA, Li JL, Carvey PM, Hendey B. Evidence of angiogenic vessels in Alzheimer’s disease. J Neural Transm. 2009;116:587–597. doi: 10.1007/s00702-009-0226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paris D, Patel N, DelleDonne A, Quadros A, Smeed R, Mullan M. Impaired angiogenesis in a transgenic mouse model of cerebral amyloidosis. Neurosci Lett. 2004;366:80–85. doi: 10.1016/j.neulet.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 117.Lee GD, Aruna JH, Barrett PM, Lei DL, Ingram DK, Mouton PR. Stereological analysis of microvascular parameters in a double transgenic model of Alzheimer’s disease. Brain Res Bull. 2005;65:317–322. doi: 10.1016/j.brainresbull.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 118.Paris D, Townsend K, Quadros A, et al. Inhibition of angiogenesis by Abeta peptides. Angiogenesis. 2004;7:75–85. doi: 10.1023/B:AGEN.0000037335.17717.bf. [DOI] [PubMed] [Google Scholar]
- 119.Kalaria RN, Cohen DL, Premkumar DR, Nag S, LaManna JC, Lust WD. Vascular endothelial growth factor in Alzheimer’s disease and experimental cerebral ischemia. Brain Res Mol Brain Res. 1998;62:101–105. doi: 10.1016/s0169-328x(98)00190-9. [DOI] [PubMed] [Google Scholar]
- 120.Yang SP, Bae DG, Kang HJ, Gwag BJ, Gho YS, Chae CB. Co-accumulation of vascular endothelial growth factor with beta-amyloid in the brain of patients with Alzheimer’s disease. Neurobiol Aging. 2004;25:283–290. doi: 10.1016/S0197-4580(03)00111-8. [DOI] [PubMed] [Google Scholar]
- 121.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 122.Brown WR, Moody DM, Thore CR, Challa VR. Apoptosis in leukoaraiosis. AJNR Am J Neuroradiol. 2000;21:79–82. [PMC free article] [PubMed] [Google Scholar]
- 123.Brown WR, Moody DM, Challa VR, Thore CR, Anstrom JA. Apoptosis in leukoaraiosis lesions. J Neurol Sci. 2002;203–204:169–171. doi: 10.1016/s0022-510x(02)00285-x. [DOI] [PubMed] [Google Scholar]
- 124.Kobayashi K, Hayashi M, Nakano H, et al. Apoptosis of astrocytes with enhanced lysosomal activity and oligodendrocytes in white matter lesions in Alzheimer’s disease. Neuropathol Appl Neurobiol. 2002;28:238–251. doi: 10.1046/j.1365-2990.2002.00390.x. [DOI] [PubMed] [Google Scholar]
- 125.Meguro K, Hatazawa J, Yamaguchi T, et al. Cerebral circulation and oxygen metabolism associated with subclinical periventricular hyperintensity as shown by magnetic resonance imaging. Ann Neurol. 1990;28:378–383. doi: 10.1002/ana.410280313. [DOI] [PubMed] [Google Scholar]
- 126.Yamauchi H, Fukuyama H, Yamaguchi S, Miyoshi T, Kimura J, Konishi J. High-intensity area in the deep white matter indicating hemodynamic compromise in internal carotid artery occlusive disorders. Arch Neurol. 1991;48:1067–1071. doi: 10.1001/archneur.1991.00530220089024. [DOI] [PubMed] [Google Scholar]
- 127.Yao H, Sadoshima S, Ibayashi S, Kuwabara Y, Ichiya Y, Fujishima M. Leukoaraiosis and dementia in hypertensive patients. Stroke. 1992;23:1673–1677. doi: 10.1161/01.str.23.11.1673. [DOI] [PubMed] [Google Scholar]
- 128.Brickman AM, Reitz C, Luchsinger JA, et al. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010;67:564–569. doi: 10.1001/archneurol.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang LY, Larson EB, Sonnen JA, et al. Blood pressure and brain injury in older adults: findings from a community-based autopsy study. J Am Geriatr Soc. 2009;57:1975–1981. doi: 10.1111/j.1532-5415.2009.02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol. 2007;64:1734–1740. doi: 10.1001/archneur.64.12.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab. 2008;28:1071–1085. doi: 10.1038/jcbfm.2008.13. [DOI] [PubMed] [Google Scholar]
- 133.Birns J, Markus H, Kalra L. Blood pressure reduction for vascular risk: is there a price to be paid? Stroke. 2005;36:1308–1313. doi: 10.1161/01.STR.0000165901.38039.5f. [DOI] [PubMed] [Google Scholar]
- 134.Lipsitz LA, Gagnon M, Vyas M, et al. Antihypertensive therapy increases cerebral blood flow and carotid distensibility in hypertensive elderly subjects. Hypertension. 2005;45:216–221. doi: 10.1161/01.HYP.0000153094.09615.11. [DOI] [PubMed] [Google Scholar]
- 135.Pakrasi S, O’Brien JT. Emission tomography in dementia. Nucl Med Commun. 2005;26:189–196. doi: 10.1097/00006231-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 136.Schuff N, Matsumoto S, Kmiecik J, et al. Cerebral blood flow in ischemic vascular dementia and Alzheimer’s disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement. 2009;5:454–462. doi: 10.1016/j.jalz.2009.04.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Drake CT, Iadecola C. The role of neuronal signaling in controlling cerebral blood flow. Brain Lang. 2007;102:141–152. doi: 10.1016/j.bandl.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 138.Roman GC, Kalaria RN. Vascular determinants of cholinergic deficits in Alzheimer disease and vascular dementia. Neurobiol Aging. 2006;27:1769–1785. doi: 10.1016/j.neurobiolaging.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 139.Takano T, Tian GF, Peng W, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 140.Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 141.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Scheibel AB, Duong TH, Tomiyasu U. Denervation microangiopathy in senile dementia, Alzheimer type. Alzheimer Dis Assoc Disord. 1987;1:19–37. doi: 10.1097/00002093-198701000-00004. [DOI] [PubMed] [Google Scholar]
- 143.Fernando MS, Simpson JE, Matthews F, et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- 144.O’Sullivan M, Lythgoe DJ, Pereira AC, et al. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology. 2002;59:321–326. doi: 10.1212/wnl.59.3.321. [DOI] [PubMed] [Google Scholar]
- 145.Schmidt R, Ropele S, Ferro J, et al. Diffusion-weighted imaging and cognition in the leukoariosis and disability in the elderly study. Stroke. 2010;41:e402–e408. doi: 10.1161/STROKEAHA.109.576629. [DOI] [PubMed] [Google Scholar]
- 146.Vernooij MW, Ikram MA, Vrooman HA, et al. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry. 2009;66:545–553. doi: 10.1001/archgenpsychiatry.2009.5. [DOI] [PubMed] [Google Scholar]
- 147.Kuczynski B, Targan E, Madison C, et al. White matter integrity and cortical metabolic associations in aging and dementia. Alzheimers Dement. 2010;6:54–62. doi: 10.1016/j.jalz.2009.04.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hirao K, Ohnishi T, Hirata Y, et al. The prediction of rapid conversion to Alzheimer’s disease in mild cognitive impairment using regional cerebral blood flow SPECT. Neuroimage. 2005;28:1014–1021. doi: 10.1016/j.neuroimage.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 149.Johnson NA, Jahng GH, Weiner MW, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Geaney DP, Soper N, Shepstone BJ, Cowen PJ. Effect of central cholinergic stimulation on regional cerebral blood flow in Alzheimer disease. Lancet. 1990;335:1484–1487. doi: 10.1016/0140-6736(90)93028-n. [DOI] [PubMed] [Google Scholar]
- 151.Ballard C, Sauter M, Scheltens P, et al. Efficacy, safety and tolerability of rivastigmine capsules in patients with probable vascular dementia: the VantagE study. Curr Med Res Opin. 2008;24:2561–2574. doi: 10.1185/03007990802328142. [DOI] [PubMed] [Google Scholar]
- 152.Hamel E. Cholinergic modulation of the cortical microvascular bed. Prog Brain Res. 2004;145:171–178. doi: 10.1016/S0079-6123(03)45012-7. [DOI] [PubMed] [Google Scholar]
- 153.Uchida S, Suzuki A, Kagitani F, Hotta H. Effects of age on cholinergic vasodilation of cortical cerebral blood vessels in rats. Neurosci Lett. 2000;294:109–112. doi: 10.1016/s0304-3940(00)01556-1. [DOI] [PubMed] [Google Scholar]
- 154.Ogawa N, Asanuma M, Tanaka K, et al. Long-term time course of regional changes in cholinergic indices following transient ischemia in the spontaneously hypertensive rat brain. Brain Res. 1996;712:60–68. doi: 10.1016/0006-8993(95)01446-2. [DOI] [PubMed] [Google Scholar]
- 155.Singer W, Opfer-Gehrking TL, McPhee BR, Hilz MJ, Bharucha AE, Low PA. Acetylcholinesterase inhibition: a novel approach in the treatment of neurogenic orthostatic hypotension. J Neurol Neurosurg Psychiatry. 2003;74:1294–1298. doi: 10.1136/jnnp.74.9.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kavirajan H, Schneider LS. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a meta-analysis of randomised controlled trials. Lancet Neurol. 2007;6:782–792. doi: 10.1016/S1474-4422(07)70195-3. [DOI] [PubMed] [Google Scholar]
- 157.Roman GC. Brain hypoperfusion: a critical factor in vascular dementia. Neurol Res. 2004;26:454–458. doi: 10.1179/016164104225017686. [DOI] [PubMed] [Google Scholar]
- 158.Kasa P, Rakonczay Z, Gulya K. The cholinergic system in Alzheimer’s disease. Prog Neurobiol. 1997;52:511–535. doi: 10.1016/s0301-0082(97)00028-2. [DOI] [PubMed] [Google Scholar]
- 159.Gottfries CG, Blennow K, Karlsson I, Wallin A. The neurochemistry of vascular dementia. Dementia. 1994;5:163–167. doi: 10.1159/000106715. [DOI] [PubMed] [Google Scholar]
- 160.Keverne JS, Court JA, Piggott M, Ince PG, Kalaria RN. Cholinergic neurones and microvascular pathology in dementia. British Neuroscience Association Abstracts. 2003;17:78. [Google Scholar]
- 161.Roman GC, Salloway S, Black SE, et al. Randomized, placebo-controlled, clinical trial of donepezil in vascular dementia: differential effects by hippocampal size. Stroke. 2010;41:1213–1221. doi: 10.1161/STROKEAHA.109.570077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tohgi H, Abe T, Kimura M, Saheki M, Takahashi S. Cerebrospinal fluid acetylcholine and choline in vascular dementia of Binswanger and multiple small infarct types as compared with Alzheimer-type dementia. J Neural Transm. 1996;103:1211–1220. doi: 10.1007/BF01271206. [DOI] [PubMed] [Google Scholar]
- 163.Venneri A, Shanks MF, Staff RT, et al. Cerebral blood flow and cognitive responses to rivastigmine treatment in Alzheimer’s disease. Neuroreport. 2002;13:83–87. doi: 10.1097/00001756-200201210-00020. [DOI] [PubMed] [Google Scholar]
- 164.Peruzzi P, von ED, Lacombe P. Differentiated cerebrovascular effects of physostigmine and tacrine in cortical areas deafferented from the nucleus basalis magnocellularis suggest involvement of basalocortical projections to microvessels. Ann N Y Acad Sci. 2000;903:394–406. doi: 10.1111/j.1749-6632.2000.tb06391.x. [DOI] [PubMed] [Google Scholar]
- 165.Ye X, Wang Y, Cahill H, et al. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Moody DM, Bell MA, Challa VR, Johnston WE, Prough DS. Brain microemboli during cardiac surgery or aortography. Ann Neurol. 1990;28:477–486. doi: 10.1002/ana.410280403. [DOI] [PubMed] [Google Scholar]
- 167.Brown WR, Moody DM, Challa VR. Cerebral fat embolism from cardiopulmonary bypass. J Neuropathol Exp Neurol. 1999;58:109–119. doi: 10.1097/00005072-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 168.Brown WR, Moody DM, Challa VR, Stump DA, Hammon JW. Longer duration of cardiopulmonary bypass is associated with greater numbers of cerebral microemboli. Stroke. 2000;31:707–713. doi: 10.1161/01.str.31.3.707. [DOI] [PubMed] [Google Scholar]
- 169.Brooker RF, Brown WR, Moody DM, et al. Cardiotomy suction: a major source of brain lipid emboli during cardiopulmonary bypass. Ann Thorac Surg. 1998;65:1651–1655. doi: 10.1016/s0003-4975(98)00289-6. [DOI] [PubMed] [Google Scholar]
- 170.Djaiani G, Fedorko L, Borger MA, et al. Continuous-flow cell saver reduces cognitive decline in elderly patients after coronary bypass surgery. Circulation. 2007;116:1888–1895. doi: 10.1161/CIRCULATIONAHA.107.698001. [DOI] [PubMed] [Google Scholar]
- 171.Cogan DG, Kuwabara T, Moser H. Fat emboli in the retina following angiography. Arch Ophthalmol. 1964;71:308–313. doi: 10.1001/archopht.1964.00970010324005. [DOI] [PubMed] [Google Scholar]
- 172.Kennedy JW. Complications associated with cardiac catheterization and angiography. Cathet Cardiovasc Diagn. 1982;8:5–11. doi: 10.1002/ccd.1810080103. [DOI] [PubMed] [Google Scholar]
- 173.Heyer EJ, Sharma R, Rampersad A, et al. A controlled prospective study of neuropsychological dysfunction following carotid endarterectomy. Arch Neurol. 2002;59:217–222. doi: 10.1001/archneur.59.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gauss H. The pathology of fat embolism. Archives of Surgery. 1924;9:593–605. [Google Scholar]
- 175.Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med. 1994;150:1416–1422. doi: 10.1164/ajrccm.150.5.7952570. [DOI] [PubMed] [Google Scholar]
- 176.Rodriguez RA, Tellier A, Grabowski J, et al. Cognitive dysfunction after total knee arthroplasty: effects of intraoperative cerebral embolization and postoperative complications. J Arthroplasty. 2005;20:763–771. doi: 10.1016/j.arth.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 177.Colonna DM, Kilgus D, Brown W, Challa V, Stump DA, Moody DM. Acute brain fat embolization occurring after total hip arthroplasty in the absence of a patent foramen ovale. Anesthesiology. 2002;96:1027–1029. doi: 10.1097/00000542-200204000-00036. [DOI] [PubMed] [Google Scholar]
- 178.Tachakra SS. Distribution of skin petechiae in fat embolism rash. Lancet. 1976;1:284–285. doi: 10.1016/s0140-6736(76)91408-2. [DOI] [PubMed] [Google Scholar]
- 179.Lyketsos CG, Toone L, Tschanz J, et al. A population-based study of the association between coronary artery bypass graft surgery (CABG) and cognitive decline: the Cache County study. Int J Geriatr Psychiatry. 2006;21:509–518. doi: 10.1002/gps.1502. [DOI] [PubMed] [Google Scholar]
- 180.Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 181.Selnes OA, Royall RM, Grega MA, Borowicz LM, Jr, Quaskey S, McKhann GM. Cognitive changes 5 years after coronary artery bypass grafting: is there evidence of late decline? Arch Neurol. 2001;58:598–604. doi: 10.1001/archneur.58.4.598. [DOI] [PubMed] [Google Scholar]
- 182.Sotaniemi KA, Mononen H, Hokkanen TE. Long-term cerebral outcome after open-heart surgery. A five-year neuropsychological follow-up study. Stroke. 1986;17:410–416. doi: 10.1161/01.str.17.3.410. [DOI] [PubMed] [Google Scholar]
- 183.Stygall J, Newman SP, Fitzgerald G, et al. Cognitive change 5 years after coronary artery bypass surgery. Health Psychol. 2003;22:579–586. doi: 10.1037/0278-6133.22.6.579. [DOI] [PubMed] [Google Scholar]
- 184.Doraiswamy PM, Babyak MA, Hennig T, et al. Donepezil for cognitive decline following coronary artery bypass surgery: a pilot randomized controlled trial. Psychopharmacol Bull. 2007;40:54–62. [PubMed] [Google Scholar]
- 185.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 186.Lam CK, Yoo T, Hiner B, Liu Z, Grutzendler J. Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature. 2010;465:478–482. doi: 10.1038/nature09001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Orenstein JM, Sato N, Aaron B, Buchholz B, Bloom S. Microemboli observed in deaths following cardiopulmonary bypass surgery: silicone antifoam agents and polyvinyl chloride tubing as sources of emboli. Hum Pathol. 1982;13:1082–1090. doi: 10.1016/s0046-8177(82)80243-8. [DOI] [PubMed] [Google Scholar]
- 188.Musselman MM, Glas WW, Grekin TD. Fat embolism; a clinical investigation. AMA Arch Surg. 1952;65:551–556. doi: 10.1001/archsurg.1952.01260020567007. [DOI] [PubMed] [Google Scholar]
- 189.Brown WR, Moody DM, Thore CR, Challa VR. Cerebrovascular pathology in Alzheimer’s disease and leukoaraiosis. Ann N Y Acad Sci. 2000;903:39–45. doi: 10.1111/j.1749-6632.2000.tb06348.x. [DOI] [PubMed] [Google Scholar]
- 190.Brown WR, Moody DM, Challa VR, Stump DA. Histologic studies of brain microemboli in humans and dogs after cardiopulmonary bypass. Echocardiography. 1996;13:559–566. doi: 10.1111/j.1540-8175.1996.tb00936.x. [DOI] [PubMed] [Google Scholar]