Abstract
Circulating estrogen levels and hippocampal-dependent cognitive functions decline with aging. Moreover, the responses of hippocampal synaptic structure to estrogens differ between aged and young rats. We recently reported that estrogens increase levels of post-synaptic proteins, including PSD-95, and opioid peptides leu-enkephalin and dynorphin in the hippocampus of young animals. However, the influence of ovarian hormones on synaptic protein and opioid peptide levels in the aging hippocampus is understudied. Here, young (3–5 mo old), middle-aged (9–12 mo old), and aged (about 22 mo old) female rats were ovariectomized for 4 weeks and then subcutaneously implanted with a silastic capsule containing vehicle or 17β-estradiol. After 48 hours, rats were subcutaneously injected with progesterone or vehicle and sacrificed one day later. Coronal sections through the dorsal hippocampus were processed for quantitative peroxidase immunohistochemistry of leu-enkephalin, dynorphin, synaptophysin, and PSD-95. With age, females showed opposing changes in leu-enkephalin and dynorphin levels in the mossy fiber pathway, particularly within the hilus, and regionally specific changes in synaptic protein levels. 17β-estradiol, with or without progesterone, altered leu-enkephalin levels in the dentate gyrus and synaptophysin levels in the CA1 of young but not middle-aged or aged females. Additionally, 17β-estradiol decreased synaptophysin levels in the CA3 of middle-aged females. Our results support and extend previous findings indicating 17β-estradiol modulation of hippocampal opioid peptides and synaptic proteins while demonstrating regional and age-specific effects. Moreover, they lend credence to the “window of opportunity” hypothesis during which hormone replacement can modulate hippocampal structure and circuitry to improve cognitive outcomes.
Keywords: aging, estrogen, hippocampus, opioids, synaptic protein
1. Introduction
Ovarian hormones, particularly estrogens, have been shown to play beneficial roles in the maintenance of cognitive ability in preclinical and clinical studies (Rapp et al., 2003; Sandstrom & Williams, 2004; Wallace et al., 2006; Sherwin, 2009). Specifically, research studies using rodents demonstrate that in the hippocampal formation (HF), a region of the brain noted for its involvement in learning and memory processes, estrogens can modulate neuronal firing and synaptic plasticity in ways that would facilitate performance on cognitive tasks (McEwen & Woolley, 1994; Daniel, 2006).
In particular, ovarian steroid hormones have been shown to influence levels of the endogenous hippocampal opioid peptides, enkephalin and dynorphin, which directly modulate hippocampal excitability. Both enkephalins and dynorphins are abundant in dentate granule cell mossy fibers (reviewed by Drake et al., 2007). Pharmacological and immunohistochemical reports show that ovarian steroids can modulate leu-enkephalin and dynorphin peptide levels within the mossy fiber pathway (Roman et al., 2006; Torres-Reveron et al., 2008; Torres-Reveron et al., 2009). Specifically, leu-enkephalin levels are increased in sub-regions of the dentate gyrus (DG) and CA3 of young adult females when estrogen levels are relatively high (Torres-Reveron et al., 2008). Similarly, dynorphin levels are increased in the DG and select CA3 lamina 24 hours following estrogen exposure and 2 weeks following chronic medroxyprogesterone exposure (Torres-Reveron et al., 2009). Although both opioid peptides are released by high frequency stimulation (Wagner et al., 1990; Wagner et al., 1991; Caudle et al., 1991; Xie & Lewis, 1991), enkephalins enhance while dynorphins suppress long term potentiation at glutamatergic mossy fiber-CA3 pyramidal cell synapses (Weisskopf et al., 1993; Wagner et al., 1993; Derrick & Martinez, Jr., 1994; Simmons & Chavkin, 1996; Jin & Chavkin, 1999; Chavkin, 2000). Thus, estrogen modulation of opioid peptide levels would directly impact CA3 pyramidal cell firing.
Similarly, ovarian steroid hormones also influence hippocampal function by modulating synapse structure and synaptic proteins involved in signal transduction. In particular, estrogens increase while progestins decrease CA1 pyramidal cell dendritic spine number and axospinous synapse density (Woolley et al., 1990; Gould et al., 1990; Woolley & McEwen, 1992; Woolley & McEwen, 1993; McEwen & Woolley, 1994). Estrogen receptor (ER) α LIM-kinase (LIMK) is increased in CA1 synapses in response to estrogens in young rats (Adams et al., 2002). Moreover, estrogens (either in young ovariectomized (OVX) female rodents replaced with 17β-estradiol or in late proestrus, the high estrogen phase of the estrous cycle) increase the levels of pre- and post-synaptic proteins synaptophysin, PSD-95, and spinophilin (Lee et al., 2004; Spencer et al., 2008; Waters et al., 2009). However, studies demonstrate that the aged hippocampal synapse structure and response to estrogens are fundamentally different from the young synapse. Age-related decreases in axospinous synapse density are observed in DG dendritic spines of female rats (Miranda et al., 1999) and aged rats demonstrate a 50% reduction in extranuclear ERα labeled synapses and decreased phosphorylated-LIMK immunoreactivity (-ir) in the CA1 compared to young rats (Adams et al., 2002). Furthermore, while neurotrophin ligands and receptors are largely insensitive to estrogens in older, reproductively senescent rats (Jezierski and Sohrabji, 2001), estrogen modulation of ERβ and phosphorylation of signaling molecule Akt has been observed in both young and aged females (Waters et al.,; Yildirim et al., submitted this issue). Whether estrogen modulation of pre- and post-synaptic markers, synaptophysin and PSD-95 respectively, is similarly observed in both young and aged female rats has not been investigated.
As estrogen’s effect on synaptic communication in the hippocampus is particularly significant in the context of aging, when both circulating estrogen levels change and hippocampal-dependent functions decline (Woolley, 1998; Sherwin, 2000), the present study examined the effect of age and steroid hormone replacement on endogenous opioid peptide and synaptic protein levels in the dorsal HF. To reflect the most common steroid hormone replacement regimens prescribed to women, we administered 17β-estradiol alone and in combination with progesterone, as women with a uterus that are taking estrogens need a progestin for prevention of endometrial hyperplasia and carcinoma (Smith et al., 1975; Thom & Studd, 1980; Van & Neven, 2002; Hersh et al., 2004). Furthermore, as some preclinical studies have demonstrated that estrogen’s effects on cognition are abolished (Chesler & Juraska, 2000; Bimonte-Nelson et al., 2006) or enhanced (Gibbs, 2000) by co-administration of progesterone, the impact of progesterone on estrogen modulation of hippocampal opioid peptides and synaptic proteins merited examination. For this, we used quantitative light microscopic densitometry to measure levels of leu-enkephalin and dynorphin peptide as well as pre-synaptic protein synaptophysin and post-synaptic protein PSD-95 in the dorsal hippocampus of young, middle aged and aged rats with and without hormone replacement.
2. Results
2.1 Age and hormone replacement differentially regulate levels of endogenous opioid peptides and synaptic proteins in the dorsal hippocampus
Endogenous opioid peptide and synaptic protein levels were examined in the dorsal hippocampus of OVX young, middle-aged, and aged female rats after administration of estradiol alone, estradiol in combination with progesterone, or vehicle. 17β-estradiol or vehicle was administered continuously via a subcutaneously implanted silastic capsule. Progesterone or vehicle was administered via subcutaneous injection 48 hrs later and tissue was collected 24 hrs after progesterone administration. Hence, nine groups of animals were used in the current study: (1) Young Control, (2) Young Estradiol, (3) Young Estradiol/Progesterone (Est/Prog), (4) Middle-aged Control, (5) Middle-aged Estradiol, (6) Middle-aged Est/Prog, (7) Aged Control, (8) Aged Estradiol, and (9) Aged Est/Prog. Using quantitative densitometry, levels of dynorphin (Fig. 1A) and leu-enkephalin (Fig. 1B) were measured in the mossy fiber pathway in the hilus of the DG and stratum lucidum (SLu) of CA3. As previously reported effects of ovarian steroids on opioid peptides within the mossy fiber pathway were not homogeneous but varied likely due to anatomical and neurochemical distinctions between different portions of the hilus and CA3 (Claiborne et al., 1986; Pierce et al., 1999; Torres-Reveron et al., 2008; Torres-Reveron et al., 2009), the DG and CA3 were each divided into three sub-regions for analysis. Similarly, levels of the pre-synaptic protein synaptophysin (Fig. 1G) and the post-synaptic protein PSD-95 (Fig. 1H) were measured in the six sub-regions of the mossy fiber pathway as well as in the stratum radiatum (SR) and stratum oriens (SO) of CA1 as previously described (Waters et al., 2009). Interestingly, females showed opposing changes in leu-enkephalin and dynorphin peptide levels with age in the mossy fiber pathway, particularly within the hilus. Regionally specific changes in synaptic protein levels also were observed with age. Most importantly, this study demonstrated that hormone replacement was only able to alter opioid peptide or synaptic protein levels in the dorsal HF of young and middle-aged females.
Fig. 1. Examination of opioid peptide and synaptic protein immunoreactivity (-ir) in the hippocampal formation.
A: Representative light photomicrograph showing dynorphin (DYN)-ir in the hilus of the dentate gyrus (DG) and stratum lucidum (SLu) of CA3. Three different sub-regions of the hilus (corresponding to levels between 3.80 and 4.30 caudal to Bregma, level 32) (Swanson, 2000) were analyzed as demonstrated by the dotted lines: (1) the tip, (2) the body or central region, and (3) the dorsal blade. SLu of the CA3 region was also divided into three different sub-regions demonstrated by the dotted lines: (1) CA3a, (2) CA3b, and (3) CA3c based on the classical divisions of (Lorente de No, 1934). B: Representative light photomicrograph showing leu-enkephalin (LE)-ir in the hilus of the DG and SLu of the CA3 region. C: Both large and small processes with DYN labeling were visible in SLu of CA3b. D: Both diffuse and punctuate DYN-immunoreactive processes were present in the body of the hilus. E: Many large processes with LE labeling were visible in SLu of CA3a. F: Both diffuse and punctuate LE-immunoreactive processes were observed in the body of the hilus. G: Representative light photomicrograph showing synaptophysin (SYP)-ir in the hilus, SLu of CA3, and stratum oriens (SO) and stratum radiatum (SR) of the CA1 region. H: Representative light photomicrograph showing PSD-95-ir in the hilus, SLu of CA3, and SO and SR of CA1. Dotted lines represent regions examined by densitometry in the CA1. I: Many large processes with SYP labeling were visible in SLu of CA3a. J: SYP-ir was diffusely distributed within SO and SR of CA1. K: Diffuse PSD-95 labeling was observed in SO of CA1 and distinct PSD95-immunoreactive processes were observed in SR of CA1. L: Representative light photomicrograph of a tissue section processed with omission of primary antisera showing lack of SYP-ir or PSD-95-ir in SO and SR of the CA1 region. GCL: Granule cell layer. SP: Stratum pyramidale. Scale bars = 300 μm (A, B, G, H); 100 μm (C – F, I – L). All photomicrographs were taken from young females.
2.2 Age and hormone replacement influence dynorphin-ir in the mossy fiber pathway
In agreement with previous studies (Pierce et al., 1999; Torres-Reveron et al., 2009), dynorphin-ir (DYN-ir) was diffuse as well as in punctuate varicosities within the mossy fiber pathway of all animal groups (Fig. 1A). Whereas large and small processes with DYN labeling were visible in SLu of CA3 (Fig. 1C), both diffuse and punctuate DYN-immunoreactive processes were present in the body of the hilus (Fig. 1D). The level of DYN-ir in three sub-regions of the DG hilus and three sub-regions of the SLu of CA3 was compared between animal groups to determine age or hormone associated differences.
An interaction between the variables age and hormone treatment was observed between the groups examined for DYN-ir in the dorsal blade of the DG (F(4,53)=2.92, p<0.05). Post-hoc analysis revealed that, in the dorsal blade of the DG hilus, middle-aged and aged females that did not receive hormone replacement had significantly less DYN-ir than young females that did not receive hormone replacement (p<0.01, all comparisons; Fig. 2A). When the three sub-regions of the DG were averaged together (Fig. 2B), we again found a significant effect of age on DYN-ir levels (DG-whole: F(2,54)=12.67, p<0.001). Post hoc analysis revealed that middle-aged and aged females displayed significantly less DYN-ir than young females in the DG (p<0.01, all comparisons). A trend for reduced DYN-ir in the DG of aged females in comparison to middle-aged females was also observed (p=0.08). Hormone replacement did not influence DYN-ir levels in the DG, either by sub-region (tip: F(2,53)=0.323, p>0.05; body: F(2,53)=1.35, p>0.05; dorsal blade: F(2,53)=2.27, p>0.05) or when the sub-regions were averaged together (DG-whole: F(2,54)=1.54, p>0.05; Fig. 2B).
Fig. 2. Age and hormone replacement influence DYN-ir in the mossy fiber pathway.
As a statistical interaction between the variables age and hormone treatment was observed for DYN-ir in the dorsal blade of the DG hilus and SLu of CA3a, graphs present data from all age and treatment groups separately (9 groups). A: In the dorsal blade of the DG hilus, middle-aged and aged females that did not receive hormone replacement had significantly less DYN-ir than young females that did not receive hormone replacement (**p<0.01, ***p < 0.001, compared to young). B: In all hilar subregions (DG-whole), middle-aged and aged females displayed significantly less DYN-ir than young females (***p < 0.001, compared to young). C: In SLu of CA3a, middle-aged and aged females that did not receive hormone replacement had significantly less DYN-ir than young females that did not receive hormone replacement (*p<0.05, ***p < 0.001, compared to young). In addition, in young females only, hormone replacement decreased DYN-ir (^p < 0.05, compared to control). D: In all CA3 sub-regions (CA3-whole), middle-aged and aged females displayed significantly less DYN-ir than young females (*p < 0.05, ***p < 0.001, compared to young). The numbers at the bottom of each bar represent the number of animals per group and error bars represent SEM.
An interaction between the variables age and hormone treatment was also observed between the groups examined for DYN-ir in SLu of the CA3a (F(4,53)=2.64, p<0.05). Post-hoc analysis revealed that middle-aged and aged females that did not receive hormone replacement had significantly less DYN-ir than young females that did not receive hormone replacement (p<0.05, all comparisons; Fig. 2C). In addition, in young females only, hormone replacement (either 17β-estradiol alone or in combination with progesterone) decreased DYN-ir in SLu of the CA3a (p<0.05, all comparisons; Fig. 2C). When the three sub-regions of the CA3 were averaged together (Fig. 2D), we again found a significant effect of age on DYN-ir levels (CA3-whole: F(2,54)=8.22, p<0.001). Post hoc analysis revealed that middle-aged and aged females displayed significantly less DYN-ir than young females in the CA3 (p<0.05, all comparisons). No significant effects of hormone replacement on DYN-ir were observed in CA3b or CA3c (CA3b: F(2,53)=0.83, p>0.05; CA3c: F(2,53)=0.65, p>0.05) nor when the sub-regions were averaged together (CA3-whole: F(2,54)=1.54, p>0.05; Fig. 2D).
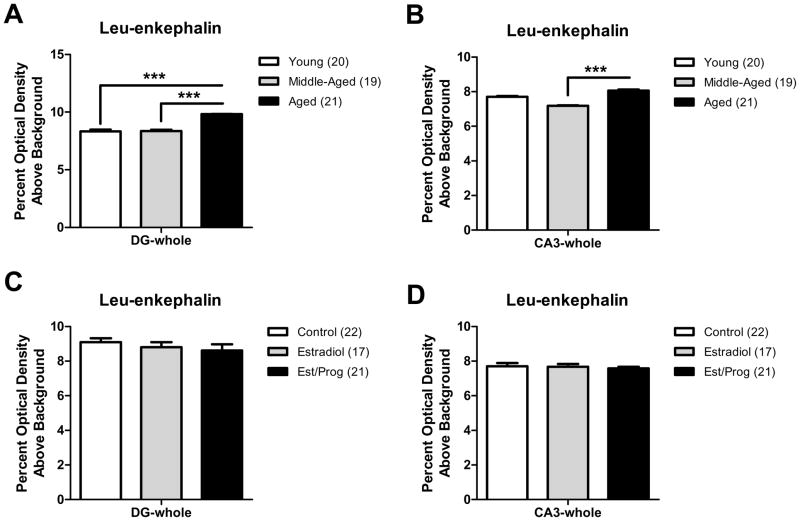
2.3 Leu-enkephalin immunoreactivity increases with age in the mossy fiber pathway, but does not vary with hormone replacement
Consistent with prior studies (Commons & Milner, 1996; Drake et al., 2002; Torres-Reveron et al., 2008), leu-enkephalin-ir (LE-ir) was abundant in the DG hilus and observed in both small and large punctuate processes of all animal groups (Fig. 1B, F). Within SLu of CA3, which contains large mossy fiber terminals, LE-ir was observed in large punctuate varicosities of all animal groups (Fig. 1E). The level of LE-ir in three sub-regions of the DG hilus and three sub-regions of the SLu of CA3 was compared between animal groups to determine age or hormone associated differences. As age and hormone treatment effects were identical for each sub-region, data is only presented for all sub-regions averaged together (i.e., DG-whole and CA3-whole). Moreover, no interaction between the variables age and hormone treatment was observed between the groups examined for LE-ir in the DG or CA3 (DG-whole: F(4,51)=0.49, p>0.05; CA3-whole: F(4,51)=0.59, p>0.05).
A significant effect of age on LE-ir levels was found in the hilus of the DG (DG-whole: F(2,51)=11.54, p<0.001). Post hoc analysis revealed that young and middle-aged females displayed significantly less LE-ir than aged females in the DG (p<0.001, all comparisons; Fig. 3A). LE-ir also increased significantly as a function of age in the CA3 (CA3-whole: F(2,51)=9.38, p<0.001). Post hoc analysis revealed that middle-aged females displayed significantly less LE-ir than aged females in the CA3 (p<0.001; Fig. 3B). A trend for reduced LE-ir in the CA3 of middle-aged females in comparison to young females was also observed (p=0.06). In contrast, hormone replacement did not influence LE-ir levels in the DG (DG-whole: F(2,51)=1.06, p>0.05; Fig. 3C) or CA3 (CA3-whole: F(2,51)=0.37, p>0.05; Fig. 3D).
Fig. 3. LE-ir increases with age but not hormone replacement in the mossy fiber pathway.
As no statistical interaction between the variables age and hormone treatment was observed for LE-ir in any region, graphs demonstrating age effects pool data from all treatment groups and graphs demonstrating treatment effects pool data from all age groups. A: In all hilar sub-regions (DG-whole), young and middle-aged females displayed significantly less LE-ir than aged females (post-hoc test ***p < 0.001, compared to aged). B: In all CA3 sub-regions (CA3-whole), middle-aged females displayed significantly less LE-ir than aged females (***p < 0.001). C: LE-ir did not vary with steroid hormone replacement in the DG. D: LE-ir did not vary with steroid hormone replacement in the CA3. For this and all subsequent figures error bars represent SEM and numbers in parentheses represent number of animals per group.
2.4 Synaptophysin-ir varies with age but not hormone replacement in the mossy fiber pathway and CA1
Synaptophysin (SYP) is a membrane component of neuronal synaptic vesicles and is expressed in many terminals within the mossy fiber pathway and CA1 lamina (Fykse et al., 1993; Waters et al., 2009). SYP-ir was diffusely distributed within the hilus of the DG, in SLu of the CA3 where mossy fiber terminals are abundant, and in SR and SO in the CA1 (Fig. 1G, I, J). The level of SYP-ir in (1) three sub-regions of the DG hilus, (2) three sub-regions of SLu of CA3, and (3) SR and SO of CA1 was compared between animal groups to determine age or hormone associated differences. As age and hormone treatment effects were identical for each sub-region, data is only presented for all subregions averaged together (i.e., DG-whole and CA3-whole). Moreover, no interaction between the variables age and hormone treatment was observed between the groups examined in the DG, CA3, or CA1 lamina (DG-whole: F(4,46)=1.60, p>0.05; CA3-whole: F(4,46)=1.12, p>0.05; SR: F(4,52)=1.52, p>0.05; SO: F(4,52)=1.27, p>0.05).
SYP-ir decreased significantly as a function of age in the DG (DG-whole: F(2,46)=6.36, p<0.01; Fig. 4A). Post hoc analysis revealed that aged females displayed significantly less SYP-ir than young females in the DG (p<0.01). In contrast, SYP-ir increased significantly as a function of age in the CA3 (CA3-whole: F(2,46)=13.56, p<0.001; Fig. 4B). Post hoc analysis revealed that young and middle-aged females displayed significantly less SYP-ir than aged females in the CA3 (p<0.01, all comparisons). In the CA1 (Fig. 4C), we observed a significant effect of age on SYP-ir in SO (F(2,52)=7.80, p<0.01) but not SR (F(2,52)=1.25, p>0.05). Post-hoc analysis demonstrated that middle-aged and aged females showed significantly less SYP-ir than young females in SO of CA1 (p<0.05, all comparisons). In contrast, hormone replacement did not influence SYP-ir levels in the DG (DG-whole: F(2,46)=1.52, p>0.05) or CA3 (CA3-whole: F(2,46)=1.42, p>0.05). No effect of hormone replacement was observed on SYP-ir levels in SR or SO of CA1 (SR: F(2,52)=1.63, p>0.05; SO: F(2,52)=1.93, p>0.05).
Fig. 4. SYP-ir varies with age in the mossy fiber pathway and CA1.
As no statistical interaction between the variables age and hormone treatment was observed for SYP-ir in any region, graphs demonstrating age effects pool data from all treatment groups. A: In all hilar sub-regions, aged females displayed significantly less SYP-ir than young females (**p < 0.01). B: In all CA3 sub-regions, young and middle-aged females displayed significantly less SYP-ir than aged females (**p < 0.01, ***p < 0.001, compared to aged). C: In SO of CA1, middle-aged and aged females displayed significantly less SYP-ir than young females (*p < 0.05, **p < 0.01, compared to young).
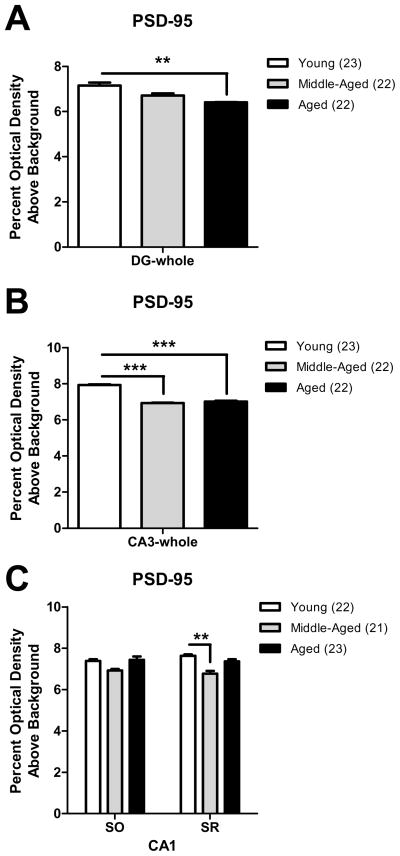
2.5 PSD-95-ir decreases with age but not hormone replacement in the mossy fiber pathway and CA1
PSD-95, a scaffolding protein involved in the organization of constituents of the postsynaptic density, was found diffusely distributed in the hilus of the DG, SLu of the CA3, and SR and SO of the CA1 (Fig. 1H, K). The level of PSD-95-ir in (1) three subregions of the DG hilus, (2) three sub-regions of SLu of CA3, and (3) SR and SO of CA1 was compared between animal groups to determine age or hormone associated differences. As age and hormone treatment effects were identical for each sub-region, data is only presented for all sub-regions averaged together (i.e., DG-whole and CA3-whole). Moreover, no interaction between the variables age and hormone treatment was observed between the groups examined in the DG, CA3, or CA1 lamina (DG-whole: F(4,58)=0.81, p>0.05; CA3-whole: F(4,58)=0.29, p>0.05; SR: F(4,57)=1.51, p>0.05; SO: F(4,57)=1.10, p>0.05).
PSD-95-ir decreased significantly as a function of age in the DG (DG-whole: F(2,58)=6.50, p<0.01). Post hoc analysis revealed that aged females displayed significantly less PSD-95-ir than young females in the DG (p<0.01; Fig. 5A). Similarly, PSD-95-ir decreased significantly as a function of age in the CA3 (CA3-whole: F(2,58)=20.80, p<0.001). Post hoc analysis revealed that middle-aged and aged females displayed significantly less PSD-95-ir than young females in the CA3 (p<0.001, all comparisons; Fig. 5B). In the CA1 (Fig. 5C), we observed a significant effect of age on PSD-95-ir in SR (F(2,57)=7.14, p<0.01) but not SO (F(2,57)=2.29, p>0.05). Post-hoc analysis demonstrated that middle-aged females showed significantly less PSD-95-ir than young females in SR of CA1 (p<0.01). A trend for reduced PSD-95-ir in SR of middle-aged females in comparison to aged females was also observed (p=0.07). In contrast, hormone replacement did not alter PSD-95-ir levels in the DG (DG-whole: F(2,58)=1.96, p>0.05) or CA3 (CA3-whole: F(2,58)=0.26, p>0.05). Similarly, no effect of hormone replacement was observed on PSD-95-ir levels in SR or SO of CA1 (SR: F(2,57)=0.70, p>0.05; SO: F(2,57)=0.99, p>0.05).
Fig. 5. PSD-95-ir decreases with age in the mossy fiber pathway and CA1.
As no statistical interaction between the variables age and hormone treatment was observed for PSD-95-ir in any region, graphs demonstrating age effects pool data from all treatment groups. A: In all hilar sub-regions, aged females displayed significantly less PSD-95-ir than young females (**p < 0.01). B: In all CA3 sub-regions, middle-aged and aged females displayed significantly less PSD-95-ir than young females (***p < 0.001, compared to young). C: In SR of CA1, middle-aged females displayed significantly less PSD-95-ir than young females (**p < 0.01).
2.6 Hormone replacement selectively alters LE-ir and SYP-ir in young and middle-aged females
As prior studies indicated that estrogens modulate endogenous opioid peptide and synaptic protein levels in young adult females (Torres-Reveron et al., 2008; Spencer et al., 2008; Waters et al., 2009; Torres-Reveron et al., 2009), we specifically analyzed the effect of hormone replacement in the dorsal hippocampus of young females. Hormone replacement significantly altered LE-ir and DYN-ir levels in the DG (LE: F(2,19)=7.13, p<0.01; DYN: F(2,19)=3.69, p<0.05). Post-hoc analysis demonstrated that young females receiving 17β–estradiol with and without progesterone showed significantly less LE-ir in the hilus of the DG than young control females (p<0.05, all comparisons; Fig. 6A). While there was a slight decrease in DYN-ir in the DG of young females receiving 17β–estradiol and progesterone in comparison to young control females, no significant difference was observed (p>0.05, all comparisons). In the CA1, hormone replacement significantly influenced SYP-ir levels in the SR and SO of young females (SR: F(2,19)=13.16, p<0.001; SO: F(2,19)=9.27, p<0.01). Post-hoc analysis demonstrated that young females receiving 17β–estradiol with and without progesterone showed significantly higher SYP-ir in the SR (Fig. 6B) and SO (data not shown) than young control females (p<0.05, all comparisons).
Fig. 6. Hormone replacement selectively alters LE-ir and SYP-ir in young and middle-aged females.
A: In the DG-whole of young females, hormone replacement significantly decreased LE-ir (*p < 0.05, compared to control). B: In the CA1 SR of young females, hormone replacement significantly increased SYP-ir (*p < 0.05, **p < 0.01, compared to control). C: In the CA3-whole of middle-aged females, hormone replacement with 17β-estradiol alone significantly decreased SYP-ir (*p < 0.05, compared to control). The numbers at the bottom of each bar represent the number of animals per group.
We performed a similar analysis of the effects of hormone replacement on opioid peptide and synaptic protein levels in the dorsal hippocampus of middle-aged and old females. We found a significant effect of hormone replacement on SYP-ir levels in the CA3 of middle-aged females only (CA3-whole: F(2,18)=4.68, p<0.05). Post-hoc analysis showed decreased SYP-ir in the CA3 of middle-aged females receiving 17β–estradiol when compared to middle-aged control females (p<0.05; Fig. 6C). In summary, hormone replacement was only effective in modulating opioid peptide levels in young females and synaptic protein levels in young or middle-aged females.
3. Discussion
The current study addressed whether levels of endogenous opioid and synaptic proteins are significantly altered by steroid hormone replacement in the dorsal hippocampus of OVX young, middle-aged, and aged female rats. While opioid peptide and synaptic protein levels significantly varied by chronological age in all hippocampal regions examined (Fig. 7A), steroid hormone modulation of opioid peptide and synaptic protein levels were only apparent in young or middle-aged females (Fig. 7B).
Fig. 7. Summary of age and treatment effects on opioid peptide and synaptic protein levels in the dorsal hippocampal formation.
A: With age, females showed opposing changes in leu-enkephalin and dynorphin levels in the mossy fiber pathway, particularly within the hilus, and regionally specific changes in levels of pre-synaptic protein synaptophysin and post-synaptic protein PSD-95. For each region, significant changes with age, if evident, were found in comparison to the young (Y) or middle-aged (M) group noted by the thick black outline. B: Hormone replacement (E or P) significantly altered leu-enkephalin and dynorphin peptide levels within sub-regions of the mossy fiber pathway and synaptophysin levels in the CA1 of young but not middle-aged or aged female rats. Additionally, 17β-estradiol (E) decreased synaptophysin levels in the CA3 of middle-aged females. For each region, significant changes with hormone treatment, if evident, were found in comparison to the control (C) group noted by the thick black outline.
3.1. Methodological considerations
The current study used an established ovariectomy model (Adams et al., 2001; Gore et al., 2002; Maffucci et al., 2009) to elucidate the specific role of 17β-estradiol in opioid peptide and synaptic protein modulation. In this model, females receive bilateral ovariectomies and after a 4-week interval are implanted with a 17β-estradiol-containing silastic capsule followed by an injection of progesterone 24 hours prior to sacrifice. Studies using this steroid hormone replacement paradigm have demonstrated estrogen-induced changes in N-methyl-D-aspartate (NMDA) receptor mRNA levels in the HF and NMDA receptor subunit mRNA levels in the hypothalamus of female rats (Adams et al., 2001; Gore et al., 2002; Maffucci et al., 2009). Indeed, the present study was performed on the same female animal cohort that demonstrated that the subunit composition of NMDA receptors in the anteroventral periventricular nucleus of the hypothalamus undergo both age- and hormone-regulation (Maffucci et al., 2009). Thus, our findings extend observations of age- and hormone-regulation to opioid peptides and synaptic proteins within the dorsal HF. However, it is worth noting that prior studies in our laboratory exploring the effects of steroid hormone replacement on hippocampal opioid peptide and synaptic protein levels in young animals employed different ovariectomy and hormone replacement paradigms generally characterized by a post-ovariectomy interval of less than 2 weeks and estrogen replacement via subcutaneous injection (Torres-Reveron et al., 2008; Torres-Reveron et al., 2009; Waters et al., 2009). As the effect of estrogens depend strongly on steroid dose, time examined after steroid administration, interval following ovariectomy, and whether progestins are given concurrently (Adams et al., 2001; Tanapat et al., 2005; Torres-Reveron et al., 2008; Conrad & Bimonte-Nelson, 2010), current study findings were not expected to exactly reproduce those of prior studies but rather to demonstrate areas of estrogen sensitivity within the HF that could be compared between age groups. Still, to enable a high level of comparison of our findings with the established literature, we chose to assess opioid peptide and synaptic protein levels using quantitative densitometric approaches. Not only do light microscopic optical density measurements correlate linearly with ultrastructural observations, particularly for opioid peptides like dynorphin, and allow for sub-region specific analysis to avoid masking effects, but they have also been used previously to investigate age or hormone effects on each of the proteins of interest in the current study (Pierce et al., 1999; Croll et al., 1999; Nyffeler et al., 2007; Wang et al., 2007; Torres-Reveron et al., 2008; Torres-Reveron et al., 2009; Waters et al., 2009).
Furthermore, the current study was designed to investigate the effect of hormone replacement on opioid peptides and synaptic proteins in a model of reproductive senescence, using females of different ages and potentially different sensitivities to estrogen, an inherent concept in the critical window hypothesis of hormone replacement as aged females may be less sensitive to the beneficial cognitive effects of estrogen than young and middle-aged females. Reproductive senescence, or menopause, in humans is characterized by loss of ovarian-derived circulating hormones, including estrogens and progesterone, due to age-related alterations of the hypothalamus, pituitary, and ovary leading to follicular depletion (Timaras et al., 1995). Female rats also undergo some of the same processes of reproductive aging as women, including cessation of reproductive cycles and loss of fertility. However, in contrast to humans, estradiol levels in rats during middle-age do not drop precipitously but often remain elevated during a state of persistent estrus or estropause. Rodents subsequently enter a pseudopregnant or persistent diestrus state characterized by very low levels of estradiol and high progesterone levels (Meites & Lu, 1994; Maffucci & Gore, 2006). Thus, the ultimate hormone profile of the older reproductively senescent female rat and woman differ, limiting the use of the ovary-intact female rat as an optimal model of human menopause. For this reason, ovariectomy to induce the cessation of ovarian function in rats during the chronological equivalent of middle-age is a commonly used model for menopause, particularly surgical menopause, as it reproduces the drop in ovarian steroid levels and the advanced age that characterize human menopause (Maffucci & Gore, 2006; Morrison et al., 2006; Daniel & Bohacek, 2010). In the current study, female rats of all ages were (1) ovariectomized, both to model human menopause and evaluate activational effects of estradiol on a zero-level circulating hormone background, and (2) administered hormone replacement paradigms of estradiol alone or estradiol in combination with progesterone. Thus, the chosen experimental model of ovariectomy plus estradiol/progesterone, while important for clearly distinguishing the effects of select steroid hormones in neuroendocrine physiology, does not perfectly reflect age-related changes in ovary-intact animals. The use of ovary-intact females would therefore help in distinguishing the effects of aging from the effects of ovariectomy. Although the tissues from the current cohort of aging rats were exhausted for the present analysis, we intend to incorporate intact animals in future studies specifically addressing the influence of age on opioid peptide and synaptic protein modulation. Given that ovary-intact female rats are an imperfect model of human reproductive senescence, as previously discussed, we are particularly interested in also utilizing rodent models of transitional hormone loss that chemically induce follicular depletion by selectively destroying primordial and primary follicles via acceleration of the natural atresia process and, therefore, produce hormonal profiles more comparable to those found in naturally menopausal women (Timaras et al., 1995; Springer et al., 1996; Conrad & Bimonte-Nelson, 2010).
3.2. Age-associated changes in endogenous opioid peptides and synaptic proteins in OVX females
Studies in humans suggest that changes in brain structure and function occur with increasing age, particularly in the prefrontal cortex and hippocampus (Esiri, 2007). Enkephalin-binding studies performed in humans report decreased leu-enkephalin receptor binding in the hippocampus of Alzheimer’s disease (AD) patients but no change in leu-enkephalin receptor binding or content in the hippocampus with age in general (Rinne et al., 1993a; Rinne et al., 1993b). However, preclinical studies using a transgenic mouse model of AD demonstrate that elevations in enkephalin, in this case met-enkephalin, observed in AD mice correlate with AD pathology and memory deficits when compared to controls (Meilandt et al., 2008). Similarly, preclinical studies in males suggest that age-associated memory impairment correlates with dynorphin levels, but the direction of this correlation is unclear as studies note memory impairment with elevations in dynorphin in Long Evans rats (Jiang et al., 1989) or with reductions in dynorphin in Sprague-Dawley (SD) rats (Croll et al., 1999). The present study, for the first time, demonstrated significant age-related changes in dynorphin and leu-enkephalin peptide levels in OVX female SD rats. Specifically, dynorphin levels decreased with age while leu-enkephalin levels increased with age in the hilus of the DG and SLu of the CA3 (Fig. 7A). Thus, further behavioral studies are warranted in middle-aged and aged female SD rats to determine if, like SD males or transgenic AD mice, decreased dynorphin levels and increased leu-enkephalin levels correlate with memory impairment.
In addition, synaptophysin levels have been shown to decrease with age in the hippocampus and various cortical regions (Masliah et al., 1993; Eastwood et al., 1994). Some studies in male rodents have found similar age-related reductions in hippocampal synaptophysin (Smith et al., 2000; Davies et al., 2003; Wang et al., 2007; Adams et al., 2008; Canas et al., 2009) whereas other studies report increases or no age-related changes in this protein (Calhoun et al., 1998; Nicolle et al., 1999). The few studies performed in females, using C57BL/6 mice, suggest for the most part that synaptophysin increases with age in the hippocampus (Frick et al., 2002; Benice et al., 2006), although one study did show a trend for decreased synaptophysin with age (Frick & Fernandez, 2003). Our findings using densitometry reveal that OVX middle-aged and aged SD females show reduced SYP-ir in the DG and CA1 in comparison to OVX young SD females (Fig. 7A), a result consistent with previously reported findings in the DG and CA1 of males (Davies et al., 2003; Wang et al., 2007). Such changes with age pose important functional consequences as synaptophysin content correlates with synaptic vesicle content (Leal-Galicia et al., 2008). Thus, the finding of reduced synaptophysin immunolabeling with age could indicate that there are fewer vesicles per synaptic bouton in OVX aged rats, leading to reduced vesicle fusion with the pre-synaptic membrane following stimulation (Davies et al., 2003). Indeed, aged SD male rats showing decreased synaptophysin in the CA1 also exhibited fewer synapses and number of synaptic vesicles as well as impaired performance on memory tasks (Wang et al., 2007).
In contrast, we observed increased SYP-ir in the CA3 of OVX aged females. In line with similar observations in the aforementioned female mice, increases in synaptophysin with age may constitute a mechanism whereby the aged brain tries to compensate for cognitive decline as behavioral testing revealed that robust increases in synaptophysin, particularly within the CA3, correlated with greater cognitive decline in reproductively senescent females (Frick et al., 2002; Benice et al., 2006). Further behavioral studies are warranted in aged female SD rats to determine whether the observed regional changes in synaptophysin level in the dorsal HF correlate with memory impairment.
The current study also demonstrated changes in the post-synaptic protein PSD-95 with age. Specifically, OVX middle-aged females display reduced PSD-95-ir in all three regions of the dorsal HF examined – DG, CA3, and CA1 – while OVX aged females show reduced PSD-95-ir in the mossy fiber pathway (Fig. 7A). Unlike synaptophysin, age-related changes in PSD-95 levels have been understudied. Still, one study in male Wistar rats using semi-quantitative immunohistochemistry found increased PSD-95 expression with age in stratum moleculare of the DG, SLu of CA3, and SR of CA1 (Nyffeler et al., 2007). As increases in PSD-95 protein levels in the CA3, but not DG or CA1, correlated with impaired spatial learning performance in males, it is possible that the observed increase in PSD-95 is part of a larger compensatory increase in post-synaptic density protein expression aimed at maintaining synaptic area contact levels in learning-impaired aged rats (Nyffeler et al., 2007). The difference between the observation of increased PSD-95-ir with age in male Wistar rats versus the observation of decreased PSD-95-ir with age in OVX female SD rats could be attributable to synaptic changes occurring during the 4-week post-ovariectomy period experienced by females in the current study, as ovariectomy itself can lead to reductions in post-synaptic spine density that are associated with impaired performance on memory tasks (Wallace et al., 2006). Thus, further study is warranted to explore the relationship between altered PSD-95 levels and cognitive function in both OVX and intact aging females.
3.3. Steroid hormones influence opioid peptides and synaptic proteins in young and middle-aged females
In the current study, we found that 17β-estradiol, with or without progesterone, significantly decreased dynorphin and leu-enkephalin levels in the mossy fiber pathway of young but not middle-aged or aged female rats. It is of note that the direction of the observed hormonal modulation, specifically a decrease in opioid peptide level with steroid hormone replacement, contrasts with previously reported findings in our laboratory (Torres-Reveron et al., 2008; Torres-Reveron et al., 2009). In our prior study, young females received bilateral ovariectomies 2 weeks prior to 17β-estradiol replacement via subcutaneous injection and we observed an increase in leu-enkephalin and dynorphin levels. We attribute the difference between study findings more to the form of 17β-estradiol administration (cyclic versus tonic) rather than post-ovariectomy interval. Indeed, our prior studies clearly demonstrate that the ability of 17β-estradiol to modulate opioid peptide levels is largely time-dependent. In particular, LE-ir increased in OVX females 24 hrs but not 6 or 72hrs following 17β-estradiol injection (Torres-Reveron et al., 2008). Still, both the prior and current studies demonstrate that leu-enkephalin and dynorphin levels change in the same direction in response to 17β-estradiol. As opioid peptides have differential effects on neuronal plasticity in the HF, in which enkephalins enhance and dynorphins suppress neurotransmission, the relative balance between leu-enkephalin and dynorphin effects in the mossy fiber pathway is preserved in young circuitry and sensitive to modulation by estrogens. Taking into account the observed opposing changes in opioid peptide levels with age (increased leu-enkephalin and decreased dynorphin), we posit that a greater leu-enkephalin (excitatory) effect exists in aged circuitry that is unresponsive to hormonal modulation.
We also found that 17β-estradiol, with or without progesterone, significantly increased synaptophysin levels in the CA1 of young but not middle-aged or aged female rats. Interestingly, our prior study found increased levels of PSD-95-ir but not SYP-ir within the SR of CA1 following 17β-estradiol administration (Waters et al., 2009). We ascribe the differences between the findings of the current and prior study to differences in steroid replacement paradigm. In the prior study, OVX females, three days post-ovariectomy, received two subcutaneous injections of 17β-estradiol 24 hrs apart and were perfused 48 hrs after the last injection (Waters et al., 2009). As that model has consistently been used to reproduce the modifications of dendritic spines in CA1 normally produced during the rodent estrous cycle (Woolley & McEwen, 1993), one might expect a particular sensitivity to hormone-induced changes in post-synaptic proteins that play a role in this process, like PSD-95. The paradigm employed by the current study, in contrast, exhibits an ability to detect hormone-induced changes at the pre-synaptic site – specifically, changes in leu-enkephalin and dynorphin found in mossy fiber axons and axon terminals. Thus, one might expect an increased sensitivity to detection of changes in pre-synaptic proteins, like synaptophysin. Moreover, the observed increase in synaptophysin in the SR and SO of CA1 following 17β-estradiol administration could suggest a hormone sensitive increase in synaptic vesicle representation or synaptic connectivity at both the basal and apical dendritic arbor of CA1 pyramidal cells. Indeed, studies in mice indicate that estrogens can significantly increase hippocampal synaptophysin expression and estrogen-induced increases in synaptophysin content correlate with improved spatial memory (Frick et al., 2002; Spencer et al., 2008).
Additionally, our findings demonstrated that middle-aged females receiving 17β-estradiol exhibit decreased synaptophysin levels in SLu of the CA3 when compared to middle-aged control females. As the CA3 was the only region to display changes in synaptic protein levels in response to 17β-estradiol in middle-aged females, it suggests that of the three hippocampal areas examined – DG, CA3, and CA1 – the CA3, or more specifically inputs to the CA3, are either the most resilient following absence of estrogen exposure or are the last to show inherently reduced responsivity. Still, it is important to note that the direction of estrogen-induced effect in middle aged females opposes that observed in young females. The observed decrease in synaptophysin in the SLu of CA3 following 17β-estradiol administration could suggest a hormone sensitive decrease in synaptic vesicle representation or synaptic connectivity at the apical dendritic arbor of CA3 pyramidal cells, a site critical for age-related spatial learning (Nyffeler et al., 2007). Thus, administration of estrogens at this time point in middle-aged females may negatively impact cognition.
Despite evidence that progesterone co-administration with 17β-estradiol can in some cases alter the ability of 17β-estradiol to influence animal performance on cognitive tasks (Chesler & Juraska, 2000; Gibbs, 2000; Bimonte-Nelson et al., 2006), the effects of 17β-estradiol alone or in combination with progesterone were for the most part indistinguishable on any of the parameters measured in the current study. Thus, future studies addressing the functional outcomes of the observed changes in opioid peptide or synaptic protein level with steroid hormone replacement can focus on replacement with 17β-estradiol alone. Given the principal motivation for combined estrogen/progestin hormone replacement therapy in the clinical setting is often preventing the development of endometrial hyperplasia associated with unopposed estrogen treatment (Smith et al., 1975) and the combination has been associated with increased breast density and pathology in women (McTiernan et al., 2005; Martin & Manson, 2008), the findings of the current study - that acute administration of progesterone does not influence estrogen’s actions on key mediators of hippocampal function - would support restricting the use of progesterone for primarily preventative purposes in women with an intact uterus.
3.4. Clinical Implications
The susceptibility of aged neuronal synapses to estrogen-induced changes is particularly relevant in light of recent hormone replacement trial results in humans. Randomized control, cross-sectional, and longitudinal studies have all been undertaken to investigate whether administration of estrogens to postmenopausal women can delay or protect against memory deterioration in aging individuals. Although several of these trials produced seemingly conflicting results largely due to variations in study design, for the most part estrogen replacement therapy is beneficial if (1) estrogens are administered intramuscularly or transdermally as 17β-estradiol and not orally as conjugated equine estrogen, (2) verbal or working memory performance is assessed as an outcome measure using specific neuropsychological tests, and (3) hormone replacement is initiated near the time of transition to menopause and continued for a few years (reviewed by Sherwin, 2006; Sherwin, 2009). This last finding coupled with the aforementioned animal studies have led to the critical period hypothesis of hormone replacement: estrogens may protect neurons involved in memory processes only when given soon after menopause and not when significant time has elapsed since cessation of ovarian function. Furthermore, estrogen therapy has no effect, or might even cause harm, when treatment is initiated too long after the menopausal transition (Sherwin, 2007; Sherwin, 2009).
The current study sought to experimentally evaluate the effects of estrogens, with and without progestins, in an aging animal model to better understand and define how estrogens regulate hippocampal opioid peptides and synaptic proteins in an older cohort. Using a rodent 4 week - ovariectomy model, we report that steroid hormone replacement can successfully alter synaptophysin levels in young and middle-aged but not aged OVX females. Prior to ovariectomy, young and middle-aged females experienced exposure to either fluctuating or persistently high estrogen levels as they transitioned to estropause. In contrast, aged females experienced little to no estrogen exposure as ovarian function had effectively ceased. Consequently, young and middle-aged females experienced 4 weeks of absence of estrogen exposure, the duration of the post-ovariectomy interval, while aged females experienced a longer period of absence. Thus, the observation of a lack of estrogen effect in aged females supports a key tenet of the critical period hypothesis - estrogen therapy has no effect when significant time has elapsed since cessation of ovarian function. Importantly, the direction and location of estrogen-induced changes in synaptophysin level differs in young and middle-aged females and may influence functional outcomes as changes in synaptophysin content correlate with spatial memory (Frick et al., 2002; Spencer et al., 2008). Future studies designed to assess cognitive outcomes of the observed estrogen-induced changes in synaptophysin content in young and middle-aged OVX female SD rats will help understand and optimize hormone therapies given to women. In sum, the findings of the present study lend credence to the “window of opportunity” hypothesis during which hormone replacement can modulate hippocampal structure and circuitry to improve cognitive outcomes.
4. Experimental procedure
4.1. Animals
A total of 75 adult female Sprague-Dawley rats were used in the present study. Rats were obtained from the University of Texas at Austin Animal Resource Facility rat colony (Austin, TX), which contains animals received and bred from Harlan Sprague-Dawley, Inc. (Houston, TX). Animals were young (3–5 months), middle-aged (9–12 months) or aged (approximately 22 months). Vaginal smears taken daily showed all young animals to be regularly cycling; middle-aged animals were irregularly cycling or acyclic (persistent estrus); and aged animals were acyclic (persistent estrus or diestrus). Animals were housed two to three per cage in a temperature controlled (21–22°C) room with ad libitum access to food and water and under a 12:12 light/dark cycle. Cages received 8cm long PVC pipes for enrichment. All animal protocols were approved by the University of Texas at Austin Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health guidelines.
4.2. Ovariectomy and hormone replacement
Animals were OVX and hormone replaced with estradiol and progesterone as previously described (Maffucci et al., 2009). Briefly, all rats were bilaterally OVX under isoflurane anesthesia between 9am and 12pm. Four weeks later, at 10am, rats were subcutaneously implanted with a Silastic capsule (inner diameter 1.96 mm; outer diameter 3.18 mm) containing either 17β-estradiol/95% cholesterol or 100% cholesterol (both from Sigma-Aldrich, St. Louis, MO) under isoflurane anesthesia. Young, middle-aged, and aged animals received an implant 1.0cm, 1.5cm, or 2.0cm in length, respectively. Different capsule implant sizes were assigned according to body weight to provide the same implant length per gram of body weight across different ages (Lauber et al., 1990; Funabashi et al., 1998; Adams et al., 2001). These capsule lengths and steroid hormone concentration have previously been shown to restore circulating estradiol levels in all three age groups to a level comparable with that of a young proestrus female rat (Nass et al., 1984; Adams et al., 2001; Gore et al., 2002). Two days later, at 10am, one third of the animals received a subcutaneous injection of progesterone (Sigma) dissolved in ethanol and diluted in sesame oil (0.59 mg). The remaining animals received a vehicle control injection (sesame oil). Animals were sacrificed 24 hrs after the progesterone injection. Hence, nine groups of animals were used in the current study: (1) Young Control, (2) Young Estradiol (Estrogen), (3) Young Estradiol/Progesterone (Est/Prog), (4) Middle-aged Control, (5) Middle-aged Estrogen, (6) Middle-aged Est/Prog, (7) Aged Control, (8) Aged Estrogen, and (9) Aged Est/Prog. Previously reported serum progesterone levels, serum luteinizing hormone levels, pituitary weights, and uterine diameters of these animals (Maffucci et al., 2009) confirmed that the estradiol alone and estradiol with progesterone treated groups had appropriate physiological responses that were lacking in vehicle treated animals.
4.3. Section preparation
Rats were deeply anesthetized with 0.4 ml of ketamine (100 mg/ml) and 0.4 ml of xylazine (20 mg/ml). Blood was collected via cardiac puncture and animals were then perfused at a rate of 50 ml/min with 1% paraformaldehyde, followed by 4% paraformaldehyde and 0.125% glutaraldehyde in phosphate-buffered saline (PBS). Brains were removed from the skull and tissues post-fixed in 4% paraformaldehyde and 0.125% glutaraldehyde in PBS overnight at 4°C. Brains were stored in PBS with 0.1% sodium azide for shipment. At Weill Cornell Medical College, coronal sections of 40 μm thickness were cut through the hippocampal formation on a Leica Vibratome (VT1000S, Leica, Wein, Austria) in chilled 0.1 M phosphate buffer (PB) and stored in cryoprotectant (30% sucrose and 30% ethylene glycol in PB) at −20°C until immunohistochemical processing.
4.4. Antisera
The antibody to leu-enkephalin (LE, mouse monoclonal; 1:15,000) was purchased from Sera Labs (Crawley Down, UK) (Drake et al., 2002) and has been previously characterized for specificity (Milner et al., 1989; Commons & Milner, 1995). The antibody recognizing dynorphin B 1–13 (DYN, rabbit polyclonal; 1:30,000) was a generous gift from Stanley Watson (Molecular and Behavioral Neuroscience Institute, Univ. of Michigan, Ann Arbor, MI) and has been previously characterized for specificity using both self-blocking and cross-blocking adsorption controls (Neal, Jr. & Newman, 1989). The antibody to PSD-95 (mouse monoclonal, 1:3,000) was purchased from Sigma and recognized a single band of 95 kDa by western blot (manufacturer’s technical information). The antibody to synaptophysin (SYP, mouse monoclonal, 1:75,000 or 1:800,000) was purchased from Sigma. It was generated using clone SVP-38 (Wiedenmann & Franke, 1985) and detects a single band of 38 kDa by western blot (Brake et al., 2001). Each antibody has been previously used by our laboratory in immunohistochemical studies (Pierce et al., 1999; Torres-Reveron et al., 2008; Waters et al., 2008; Torres-Reveron et al., 2009).
4.5. Immunohistochemistry
For quantitative light microscopic localization of opioid peptides and synaptic proteins, serial dilutions for each antibody were established and a linear function of antibody concentration against labeling intensity was obtained using densitometry, as previously described (Chang et al., 2000; Torres-Reveron et al., 2008). The dilution producing slightly less than half-maximal labeling was chosen to allow for variations in labeling intensity in either direction that might be produced by the groups examined (Chang et al., 2000). To ensure identical labeling conditions during immunohistochemistry (Pierce at al., 1999), sections of each treatment group were rinsed in PB, coded with hole-punches in the cortex, and pooled into single containers. Sections were treated with 1% sodium borohydride in PB for 30 minutes to neutralize free aldehydes. The tissue was processed according to the avidin-biotin complex (ABC) method (Hsu et al., 1981). Briefly, sections were rinsed in PB followed by Tris-buffered saline (TS; pH 7.6) and incubated in: (1) 0.5% bovine serum albumin (BSA) in TS to block nonspecific antibody binding, 30 min; (2) the primary antisera in 0.1% BSA/TS with 0.1% Triton (SYP) or 0.25% Triton (LE, DYN, PSD-95) for 1 day at room temperature followed by 1–2 days at 4°C; (3) 1:400 dilution of anti-mouse or anti-rabbit biotinylated-IgG (Vector Labs, Burlingame, CA), 30 min; (4) 1:100 dilution of peroxidase-avidin complex (Vectastain Elite Kit), 30 min; and (5) 3,3′-diaminobenzidine (DAB; Sigma) and H2O2 in TS for 4–8 minutes. All incubations were separated by washes in TS. Sections were mounted on gelatin coated slides, dehydrated in ascending concentrations of alcohols, and cover-slipped with D.P.X. neutral mounting medium (Sigma).
For synaptophysin only, as young animals were processed using a different antibody dilution than middle-aged and aged animals, additional hippocampal sections of two animals from each of the nine groups were processed for synaptophysin labeling at the same 1:800,000 dilution to establish a correction factor for age comparisons.
4.6. Analysis
For quantitative densitometry, images of regions of interest (R.O.I) were captured using a Dage MTI CCD-72 camera and NIH Image 1.50 software on a Nikon Eclipse 80i microscope (Pierce et al., 1999). The mean gray value (of 256 gray levels) for each selected R.O.I was determined as previously described (Pierce et al., 1999; Torres-Reveron et al., 2008; Torres-Reveron et al., 2009; Waters et al., 2009). To compensate for background staining and control for variations in illumination level between images, the average pixel density for three regions lacking labeling was subtracted. Tissue from control and experimental animals was processed together in the same crucibles. Sections from each animal were selected from the dorsal midseptotemporal level of the hippocampal formation (AP −3.80 to −4.30 caudal to Bregma; Swanson approximate level 32) (Swanson, 2000) and matched sections were selected for processing with each antibody. A single hippocampus from each animal with the best morphology and consistent immunoperoxidase labeling was included in the analysis. Optical density values were measured using Image J. Net optical density values obtained after subtracting background values were converted to a percentage scale of 256 preset gray values ranging from 0 to 100%.
For statistical analysis, two-way ANOVA was used to determine group differences by age or hormone treatment using SPSS, Version 11 (SPSS Inc., Chicago IL) for Windows (variables: age and steroid hormone treatment). When indicated, Bonferroni post-hoc analyses were performed. To verify previously observed differences in opioid peptide and synaptic protein levels by hormone treatment in young animals (Torres-Reveron et al., 2008; Waters et al., 2009; Torres-Reveron et al., 2009), this group also was assessed using one-way ANOVA followed by Bonferroni post-hoc analyses.
Graphs were prepared with Graph Pad Prism 5.2 (Graph Pad Software, Inc., San Diego CA). For construction of Fig. 1, immunoperoxidase labeled sections were photographed with a Nikon Eclipse 80i microscope equipped with bright-field and DIC optics and a Micropublisher digital camera (Q-imaging, Barnaby, British Columbia). Levels, brightness, and contrast were adjusted for each image in Adobe Photoshop CS and final figures assembled in Microsoft Office PowerPoint 2007.
Acknowledgments
This work was supported by National Institutes of Health grants DA08259, HL18974, HL096571, DK07313, NS07080, AG016765 and AG028051, NIH-MSTP grant GM07739, the American Psychological Association Diversity Program in Neuroscience, and the UNCF-Merck Science Initiative. Thanks to Nicole Lou, Sarah Dickerson, Sonya Hughes, Kristen Reynolds, and Deena Walker for technical support.
GRANT SUPPORT: NIH grants DA08259 (TAM), HL18974 (TAM), HL096571 (TAM), DK07313 (EMW), NS007080 (BSM), AG016765 (BSM; ACG), and AG028051 (ACG), and NIH MSTP grant GM07739 (TJW)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, et al. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Oung T, Morrison JH, Gore AC. Length of postovariectomy interval and age, but not estrogen replacement, regulate N-methyl-D-aspartate receptor mRNA levels in the hippocampus of female rats. Exp Neurol. 2001;170:345–356. doi: 10.1006/exnr.2001.7716. [DOI] [PubMed] [Google Scholar]
- Adams MM, Shi L, Linville MC, Forbes ME, Long AB, Bennett C, et al. Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability. Exp Neurol. 2008;211:141–149. doi: 10.1016/j.expneurol.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137:413–423. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24:229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Brake WG, Alves SE, Dunlop JC, Lee SJ, Bulloch K, Allen PB, et al. Novel target sites for estrogen action in the dorsal hippocampus: an examination of synaptic proteins. Endocrinology. 2001;142:1284–1289. doi: 10.1210/endo.142.3.8036. [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Kurth D, Phinney AL, Long JM, Hengemihle J, Mouton PR, et al. Hippocampal neuron and synaptophysin-positive bouton number in aging C57BL/6 mice. Neurobiol Aging. 1998;19:599–606. doi: 10.1016/s0197-4580(98)00098-0. [DOI] [PubMed] [Google Scholar]
- Canas PM, Duarte JM, Rodrigues RJ, Kofalvi A, Cunha RA. Modification upon aging of the density of presynaptic modulation systems in the hippocampus. Neurobiol Aging. 2009;30:1877–1884. doi: 10.1016/j.neurobiolaging.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Caudle RM, Wagner JJ, Chavkin C. Endogenous opioids released from perforant path modulate norepinephrine actions and inhibitory postsynaptic potentials in guinea pig CA3 pyramidal cells. J Pharmacol Exp Ther. 1991;258:18–26. [PubMed] [Google Scholar]
- Chang PC, Aicher SA, Drake CT. Kappa opioid receptors in rat spinal cord vary across the estrous cycle. Brain Res. 2000;861:168–172. doi: 10.1016/s0006-8993(99)02461-0. [DOI] [PubMed] [Google Scholar]
- Chavkin C. Dynorphins are endogenous opioid peptides released from granule cells to act neurohumorly and inhibit excitatory neurotransmission in the hippocampus. Prog Brain Res. 2000;125:363–367. doi: 10.1016/S0079-6123(00)25025-5. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Juraska JM. Acute administration of estrogen and progesterone impairs the acquisition of the spatial morris water maze in ovariectomized rats. Horm Behav. 2000;38:234–242. doi: 10.1006/hbeh.2000.1626. [DOI] [PubMed] [Google Scholar]
- Claiborne BJ, Amaral DG, Cowan WM. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J Comp Neurol. 1986;246:435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- Commons KG, Milner TA. Ultrastructural heterogeneity of enkephalin-containing terminals in the rat hippocampal formation. J Comp Neurol. 1995;358:324–342. doi: 10.1002/cne.903580303. [DOI] [PubMed] [Google Scholar]
- Commons KG, Milner TA. Ultrastructural relationships between leu-enkephalin- and GABA-containing neurons differ within the hippocampal formation. Brain Res. 1996;724:1–15. doi: 10.1016/0006-8993(96)00236-3. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Bimonte-Nelson HA. Impact of the hypothalamic-pituitary-adrenal/gonadal axes on trajectory of age-related cognitive decline. Prog Brain Res. 2010;182:31–76. doi: 10.1016/S0079-6123(10)82002-3. [DOI] [PubMed] [Google Scholar]
- Croll SD, Chesnutt CR, Greene NA, Lindsay RM, Wiegand SJ. Peptide immunoreactivity in aged rat cortex and hippocampus as a function of memory and BDNF infusion. Pharmacol Biochem Behav. 1999;64:625–635. doi: 10.1016/s0091-3057(99)00122-7. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18:787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Bohacek J. The critical period hypothesis of estrogen effects on cognition: Insights from basic research. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbagen.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Davies HA, Kelly A, Dhanrajan TM, Lynch MA, Rodriguez JJ, Stewart MG. Synaptophysin immunogold labelling of synapses decreases in dentate gyrus of the hippocampus of aged rats. Brain Res. 2003;986:191–195. doi: 10.1016/s0006-8993(03)03251-7. [DOI] [PubMed] [Google Scholar]
- Derrick BE, Martinez JL., Jr Frequency-dependent associative long-term potentiation at the hippocampal mossy fiber-CA3 synapse. Proc Natl Acad Sci U S A. 1994;91:10290–10294. doi: 10.1073/pnas.91.22.10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Chang PC, Harris JA, Milner TA. Neurons with mu opioid receptors interact indirectly with enkephalin-containing neurons in the rat dentate gyrus. Exp Neurol. 2002;176:254–261. doi: 10.1006/exnr.2002.7948. [DOI] [PubMed] [Google Scholar]
- Drake CT, Chavkin C, Milner TA. Opioid systems in the dentate gyrus. Prog Brain Res. 2007;163:245–263. doi: 10.1016/S0079-6123(07)63015-5. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Burnet PW, McDonald B, Clinton J, Harrison PJ. Synaptophysin gene expression in human brain: a quantitative in situ hybridization and immunocytochemical study. Neuroscience. 1994;59:881–892. doi: 10.1016/0306-4522(94)90292-5. [DOI] [PubMed] [Google Scholar]
- Esiri MM. Ageing and the brain. J Pathol. 2007;211:181–187. doi: 10.1002/path.2089. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol Aging. 2003;24:615–626. doi: 10.1016/s0197-4580(02)00138-0. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience. 2002;115:547–558. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Funabashi T, Kleopoulos SP, Kimura F, Mobbs CV. Changes in neurotensin mRNA by estrogen in the female rat preoptic area during aging: an in situ hybridization histochemistry study. Gen Comp Endocrinol. 1998;112:364–371. doi: 10.1006/gcen.1998.7139. [DOI] [PubMed] [Google Scholar]
- Fykse EM, Takei K, Walch-Solimena C, Geppert M, Jahn R, De CP, et al. Relative properties and localizations of synaptic vesicle protein isoforms: the case of the synaptophysins. J Neurosci. 1993;13:4997–5007. doi: 10.1523/JNEUROSCI.13-11-04997.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gore AC, Oung T, Woller MJ. Age-related changes in hypothalamic gonadotropin-releasing hormone and N-methyl-D-aspartate receptor gene expression, and their regulation by oestrogen, in the female rat. J Neuroendocrinol. 2002;14:300–309. doi: 10.1046/j.1365-2826.2002.00777.x. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- Jiang HK, Owyang VV, Hong JS, Gallagher M. Elevated dynorphin in the hippocampal formation of aged rats: relation to cognitive impairment on a spatial learning task. Proc Natl Acad Sci U S A. 1989;86:2948–2951. doi: 10.1073/pnas.86.8.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Chavkin C. Mu opioids enhance mossy fiber synaptic transmission indirectly by reducing GABAB receptor activation. Brain Res. 1999;821:286–293. doi: 10.1016/s0006-8993(99)01089-6. [DOI] [PubMed] [Google Scholar]
- Lauber AH, Romano GJ, Mobbs CV, Howells RD, Pfaff DW. Estradiol induction of proenkephalin messenger RNA in hypothalamus: dose-response and relation to reproductive behavior in the female rat. Brain Res Mol Brain Res. 1990;8:47–54. doi: 10.1016/0169-328x(90)90008-2. [DOI] [PubMed] [Google Scholar]
- Leal-Galicia P, Castaneda-Bueno M, Quiroz-Baez R, Arias C. Long-term exposure to environmental enrichment since youth prevents recognition memory decline and increases synaptic plasticity markers in aging. Neurobiol Learn Mem. 2008;90:511–518. doi: 10.1016/j.nlm.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Romeo RD, Svenningsson P, Campomanes CR, Allen PB, Greengard P, et al. Estradiol affects spinophilin protein differently in gonadectomized males and females. Neuroscience. 2004;127:983–988. doi: 10.1016/j.neuroscience.2004.05.049. [DOI] [PubMed] [Google Scholar]
- Lorente de No R. Studies on the structure of the cerebral cortex-II. Continuation of the study of the ammonic system. J Psychol Neurol. 1934;46:113–117. [Google Scholar]
- Maffucci JA, Gore AC. Age-related changes in hormones and their receptors in animal models of female reproductive senescence. In: Conn PM, editor. Handbook of Models for Human Aging. New York: Elsevier; 2006. pp. 533–552. [Google Scholar]
- Maffucci JA, Noel ML, Gillette R, Wu D, Gore AC. Age- and hormone-regulation of NMDA receptor subunit NR2b in the anteroventral periventricular nucleus (AVPV) of the female rat: Implications for reproductive senescence. J Neuroendocrinol. 2009 doi: 10.1111/j.1365-2826.2009.01860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KA, Manson JE. Approach to the patient with menopausal symptoms. J Clin Endocrinol Metab. 2008;93:4567–4575. doi: 10.1210/jc.2008-1272. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Hansen L, DeTeresa R, Terry RD. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology. 1993;43:192–197. doi: 10.1212/wnl.43.1_part_1.192. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Woolley CS. Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Exp Gerontol. 1994;29:431–436. doi: 10.1016/0531-5565(94)90022-1. [DOI] [PubMed] [Google Scholar]
- McTiernan A, Martin CF, Peck JD, Aragaki AK, Chlebowski RT, Pisano ED, et al. Estrogen-plus-progestin use and mammographic density in postmenopausal women: Women’s Health Initiative randomized trial. J Natl Cancer Inst. 2005;97:1366–1376. doi: 10.1093/jnci/dji279. [DOI] [PubMed] [Google Scholar]
- Meilandt WJ, Yu GQ, Chin J, Roberson ED, Palop JJ, Wu T, et al. Enkephalin elevations contribute to neuronal and behavioral impairments in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2008;28:5007–5017. doi: 10.1523/JNEUROSCI.0590-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meites J, Lu JKH. Reproductive aging and neuroendocrine function. In: Charlton HM, editor. Oxford review of reproductive biology. New York: Oxford Press; 1994. [Google Scholar]
- Milner TA, Pickel VM, Reis DJ. Ultrastructural basis for interactions between central opioids and catecholamines: I. Rostral ventrolateral medulla. J Neurosci. 1989;9:2114–2130. doi: 10.1523/JNEUROSCI.09-06-02114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda P, Williams CL, Einstein G. Granule cells in aging rats are sexually dimorphic in their response to estradiol. J Neurosci. 1999;19:3316–3325. doi: 10.1523/JNEUROSCI.19-09-03316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci. 2006;26:10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass TE, LaPolt PS, Judd HL, Lu JK. Alterations in ovarian steroid and gonadotrophin secretion preceding the cessation of regular oestrous cycles in ageing female rats. J Endocrinol. 1984;100:43–50. doi: 10.1677/joe.0.1000043. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr, Newman SW. Prodynorphin peptide distribution in the forebrain of the Syrian hamster and rat: a comparative study with antisera against dynorphin A, dynorphin B, and the C-terminus of the prodynorphin precursor molecule. J Comp Neurol. 1989;288:353–386. doi: 10.1002/cne.902880302. [DOI] [PubMed] [Google Scholar]
- Nicolle MM, Gallagher M, McKinney M. No loss of synaptic proteins in the hippocampus of aged, behaviorally impaired rats. Neurobiol Aging. 1999;20:343–348. doi: 10.1016/s0197-4580(99)00054-8. [DOI] [PubMed] [Google Scholar]
- Nyffeler M, Zhang WN, Feldon J, Knuesel I. Differential expression of PSD proteins in age-related spatial learning impairments. Neurobiol Aging. 2007;28:143–155. doi: 10.1016/j.neurobiolaging.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Kurucz OS, Milner TA. Morphometry of a peptidergic transmitter system: dynorphin B-like immunoreactivity in the rat hippocampal mossy fiber pathway before and after seizures. Hippocampus. 1999;9:255–276. doi: 10.1002/(SICI)1098-1063(1999)9:3<255::AID-HIPO6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne JO, Lonnberg P, Marjamaki P. Human brain methionine- and leucine-enkephalins and their receptors during ageing. Brain Res. 1993a;624:131–136. doi: 10.1016/0006-8993(93)90070-4. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Lonnberg P, Marjamaki P, Molsa P, Sako E, Paljarvi L. Brain methionine- and leucine-enkephalin receptors in patients with dementia. Neurosci Lett. 1993b;161:77–80. doi: 10.1016/0304-3940(93)90144-a. [DOI] [PubMed] [Google Scholar]
- Roman E, Ploj K, Gustafsson L, Meyerson BJ, Nylander I. Variations in opioid peptide levels during the estrous cycle in Sprague-Dawley rats. Neuropeptides. 2006;40:195–206. doi: 10.1016/j.npep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav. 2004;45:128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138:1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? J Neuroendocrinol. 2007;19:77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen therapy: is time of initiation critical for neuroprotection? Nat Rev Endocrinol. 2009;5:620–627. doi: 10.1038/nrendo.2009.193. [DOI] [PubMed] [Google Scholar]
- Simmons ML, Chavkin C. Endogenous opioid regulation of hippocampal function. Int Rev Neurobiol. 1996;39:145–196. doi: 10.1016/s0074-7742(08)60666-2. [DOI] [PubMed] [Google Scholar]
- Smith DC, Prentice R, Thompson DJ, Herrmann WL. Association of exogenous estrogen and endometrial carcinoma. N Engl J Med. 1975;293:1164–1167. doi: 10.1056/NEJM197512042932302. [DOI] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, McEwen BS. Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience. 2008;155:1106–1119. doi: 10.1016/j.neuroscience.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmacol. 1996;139:394–401. doi: 10.1006/taap.1996.0180. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. 2. San Diego, CA: Academic Press; 2000. [Google Scholar]
- Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol. 2005;481:252–265. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- Thom MH, Studd JW. Oestrogens and endometrial hyperplasia. Br J Hosp Med. 1980;23:506–508. 3. [PubMed] [Google Scholar]
- Timaras P, Quay W, Vernadakis A. Hormones and aging. Boca Raton: CRC Press; 1995. [Google Scholar]
- Torres-Reveron A, Khalid S, Williams TJ, Waters EM, Drake CT, McEwen BS, et al. Ovarian steroids modulate leu-enkephalin levels and target leu-enkephalinergic profiles in the female hippocampal mossy fiber pathway. Brain Res. 2008;1232:70–84. doi: 10.1016/j.brainres.2008.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Khalid S, Williams TJ, Waters EM, Jacome L, Luine VN, et al. Hippocampal dynorphin immunoreactivity increases in response to gonadal steroids and is positioned for direct modulation by ovarian steroid receptors. Neuroscience. 2009;159:204–216. doi: 10.1016/j.neuroscience.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van GT, Neven P. Endometrial safety of hormone replacement therapy: review of literature. Maturitas. 2002;42:93–104. doi: 10.1016/s0378-5122(02)00031-2. [DOI] [PubMed] [Google Scholar]
- Wagner JJ, Caudle RM, Neumaier JF, Chavkin C. Stimulation of endogenous opioid release displaces mu receptor binding in rat hippocampus. Neuroscience. 1990;37:45–53. doi: 10.1016/0306-4522(90)90190-f. [DOI] [PubMed] [Google Scholar]
- Wagner JJ, Evans CJ, Chavkin C. Focal stimulation of the mossy fibers releases endogenous dynorphins that bind kappa 1-opioid receptors in guinea pig hippocampus. J Neurochem. 1991;57:333–343. doi: 10.1111/j.1471-4159.1991.tb02132.x. [DOI] [PubMed] [Google Scholar]
- Wagner JJ, Terman GW, Chavkin C. Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature. 1993;363:451–454. doi: 10.1038/363451a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006;1126:176–182. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- Wang R, Tang Y, Feng B, Ye C, Fang L, Zhang L, et al. Changes in hippocampal synapses and learning-memory abilities in age-increasing rats and effects of tetrahydroxystilbene glucoside in aged rats. Neuroscience. 2007;149:739–746. doi: 10.1016/j.neuroscience.2007.07.065. [DOI] [PubMed] [Google Scholar]
- Waters EM, Mitterling K, Spencer JL, Mazid S, McEwen BS, Milner TA. Estrogen receptor alpha and beta specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res. 2009;1290:1–11. doi: 10.1016/j.brainres.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Torres-Reveron A, McEwen BS, Milner TA. Ultrastructural localization of extranuclear progestin receptors in the rat hippocampal formation. J Comp Neurol. 2008;511:34–46. doi: 10.1002/cne.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Zalutsky RA, Nicoll RA. The opioid peptide dynorphin mediates heterosynaptic depression of hippocampal mossy fibre synapses and modulates long-term potentiation. Nature. 1993;365:188. doi: 10.1038/365188a0. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Xie CW, Lewis DV. Opioid-mediated facilitation of long-term potentiation at the lateral perforant path-dentate granule cell synapse. J Pharmacol Exp Ther. 1991;256:289–296. [PubMed] [Google Scholar]