Abstract
T-cell antigen receptor (TCR)-induced thymocyte apoptosis is mediated by calcium-dependent signal transduction pathways leading to the transcriptional activation of members of the Nur77 family. The major calcium- and calcineurin-responsive elements in the Nur77 promoter are binding sites for myocyte enhancer factor-2 (MEF2). It has been shown that nuclear factor of activated T cells (NFAT) interacts with MEF2D and enhances its transcriptional activity, offering a plausible mechanism of activation of MEF2D by calcineurin. We report here that NFATp synergizes with MEF2D to recruit the coactivator p300 for the transcription of Nur77. Surprisingly, the enhancement of transcriptional activity of MEF2D by NFATp does not require its DNA-binding activity, suggesting that NFATp acts as a coactivator for MEF2D. Transient co-expression of p300, MEF2D, NFATp and constitutively active calcineurin is sufficient to recapitulate TCR signaling for the selective induction of the endogenous Nur77 gene. These results implicate NFAT as an important mediator of T-cell apoptosis and suggest that NFAT is capable of integrating the calcineurin signaling pathway and other pathways through direct protein–protein interaction with other transcription factors.
Keywords: apoptosis/calcineurin/MEF2/NFAT/Nur77
Introduction
Signal transduction initiated at the T-cell receptor (TCR) can lead to distinct cellular responses, proliferation or death, depending on the strengths or avidity of the signal and the differentiation states of the cells (Ashton-Rickardt and Tonegawa, 1994; Jameson et al., 1994). During thymocyte development, those thymocytes bearing self-reactive TCRs undergo apoptosis (Nossal, 1994), while those bearing TCR with moderate affinity for self-major histocompatibility complex (MHC)–antigen complex undergo proliferation and differentiation to become single positive T cells (von Boehmer, 1994). For peripheral T cells, the engagement of TCR with foreign antigen presented by self-MHC leads to cytokine production and cell proliferation. Although much is known about TCR signal transduction pathways involved in peripheral T-cell activation (Crabtree and Clipstone, 1994; Rao et al., 1997; DeFranco and Weiss, 1998), little was known about the signaling mechanism involved in thymocyte apoptosis until the discovery of Nur77 as a potential mediator of TCR-induced thymocyte apoptosis.
Nur77 was identified as an immediate early gene responsive to TCR signaling under conditions that led to thymocyte apoptosis (Liu et al., 1994; Woronicz et al., 1994). Much evidence now exists supporting a critical role for Nur77 family members in TCR-mediated thymocyte apoptosis. Transgenic mice overexpressing a dominant-negative form of Nur77 are protected from TCR-induced apoptosis (Calnan et al., 1995; Zhou et al., 1996), while those overexpressing the full-length Nur77 experience a significant increase in thymocyte apoptosis (Calnan et al., 1995; Weih et al., 1996). The transcription activity of Nur77 correlates with the extent of thymocyte apoptosis in vivo (Kuang et al., 1999). Although Nur77 null mice did not have an apoptotic phenotype (Lee et al., 1995), this result appears to be accounted for by the existence of redundant genes such as Nor-1, which exhibits a similar pattern of induction by TCR and possesses pro-apoptotic activity in thymocytes (Cheng et al., 1997).
The mechanism of signal transduction from TCR to the Nur77 promoter has been studied extensively in the past few years. It has been shown that the activation of the Nur77 promoter in response to TCR activation requires the second messenger calcium and appears to involve the calcium-dependent phosphatase calcineurin (Woronicz et al., 1995). Surprisingly, the calcium-responsive elements in the Nur77 promoter were identified as two putative binding sites for the transcription factor myocyte enhancer factor-2 (MEF2). Even though two putative nuclear factor of activated T cells (NFAT)-binding sites are also present within the minimal calcium-responsive Nur77 promoter region, they were not found to be directly responsive to calcium signals upon further promoter mapping (Woronicz et al., 1995), leaving unanswered the question of the relevance of the two putative NFAT-binding sites in TCR-induced Nur77 promoter activation.
The activation of MEF2 by calcineurin signaling has been demonstrated in the expression of several genes in addition to Nur77. The calcineurin signaling pathway has been shown to regulate slow muscle fiber-specific genes via MEF2 (Chin et al., 1998; Wu et al., 2000). The MEF2-mediated activation of the Epstein–Barr virus (EBV) lytic cycle gene BZLF1 in response to surface immunoglobulin cross-linking has been reported to be sensitive to cyclosporin A (CsA), implicating the involvement of calcineurin (Liu et al., 1997). Although how calcineurin activates MEF2 was not known, two alternative modes of regulation have been reported recently. Calcineurin was shown to enhance the DNA-binding activity of MEF2A, thereby increasing its transcriptional activity (Mao and Wiedmann, 1999). Calcineurin has also been reported to activate MEF2 indirectly through NFATp, which forms a ternary complex with MEF2 on the Nur77 promoter, increasing the transcriptional activity of MEF2D (Blaeser et al., 2000). However, how the interaction between NFATp and MEF2D leads to enhanced transcription has remained unknown.
In an effort to unravel the mechanism of Nur77 promoter activation in response to calcium signaling, we recently identified a co-repressor of MEF2 that appears to be responsible for silencing the Nur77 promoter in the absence of TCR or calcium signaling. The co-repressor protein is known as Cabin1 or cain in rat (Lai et al., 1998; Sun et al., 1998). The Cabin1-mediated transcriptional repression is released by calcium signaling through calmodulin, which binds to Cabin1 and displaces it from MEF2, rendering MEF2 competent for transcriptional activation (Youn et al., 1999). More recently, we found that Cabin1 exists as part of a chromatin remodeling complex consisting of mSin3A and histone deacetylases (HDACs) 1 and 2 (Youn and Liu, 2000). In addition, two other co-repressors for MEF2, MITR and HDAC4, have also been reported (Miska et al., 1999; Sparrow et al., 1999). The binding of Cabin1–mSin3–HDACs and other HDAC-containing co-repressors to MEF2 in the absence of calcium signaling necessitates that MEF2 recruits coactivators containing histone acetylases to acetylate the chromatin to activate the Nur77 promoter.
We report here that upon calcineurin activation, NFATp is bound to MEF2D upon nuclear translocation, and the resulting complex synergistically recruits the coactivator p300. Although there were two putative NFAT-binding sites in the Nur77 promoter, they are dispensable for signal integration between calcineurin and MEF2. Co-expression of MEF2, p300, NFATp and constitutively active calcineurin is sufficient to activate the transcription of endogenous Nur77 promoter, suggesting that these signaling proteins may constitute a minimal set of activators required for the expression of the Nur77 gene.
Results
Calcineurin is required for TCR-mediated MEF2 activation
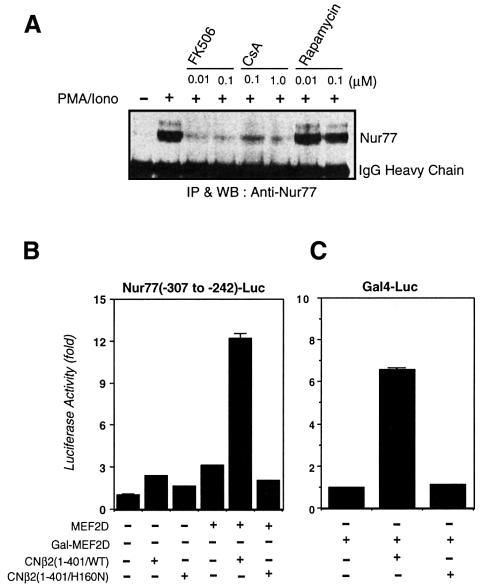
Whether calcineurin is involved in TCR-mediated Nur77 gene expression has been somewhat controversial. While Woronicz et al. (1995) and Blaeser et al. (2000) demonstrated that CsA blocks TCR-mediated Nur77 expression and T-cell apoptosis, Yazdanbakhsh et al. (1995) reported that CsA blocked Nur77-mediated apoptosis at the level of Nur77 DNA binding rather than its expression. To verify the role of calcineurin in Nur77 transcriptional activation, we measured Nur77 production at the protein level in the presence or absence of CsA and FK506. Both drugs inhibited Nur77 expression in response to phorbol 12-myristate 13-acetate (PMA) and ionomycin in DO11.10 T-cell hybridomas (Figure 1A). In contrast, rapamycin, an immunosuppressant that binds to FK506-binding protein (FKBP) but does not inhibit calcineurin (Schreiber, 1992), had no effect on Nur77 expression (Figure 1A). These results provide further support that calcineurin activity is required for TCR-mediated Nur77 expression.
Fig. 1. Calcineurin is necessary for TCR-mediated Nur77 expression and activates an MEF2D-dependent reporter gene. (A) DO11.10 T-hybridoma cells (1 × 107/lane) were pre-treated with the indicated concentrations of FK506, CsA or rapamycin for 30 min prior to a 3 h treatment with PMA (40 nM) and ionomycin (1 µM). Cells were lysed with a lysis buffer (20 mM Tris–HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.5% NP-40 and 1 mM PMSF) and incubated with anti-Nur77 antibody–protein A/G beads for 2 h. Nur77 protein present in the immunoprecipitate was detected with anti-Nur77 antibody. (B) DO11.10 cells were transfected with pSGMEF2D, pGLNur77(–307 to –242) luciferase reporter plasmid along with either pSGCNβ2(1–401/WT) or pSGCNβ2(1–401/H160N), and reporter gene activity was determined as previously described (Sun et al., 1998). (C) DO11.10 cells were transfected with Gal4-MEF2D, pG5-luciferase reporter plasmid along with either pSGCNβ2(1–401/WT) or pSGCNβ2(1–401/H160N). Luciferase activities were normalized to β-galactosidase activity.
As the binding sites for MEF2 have been shown to be the calcium- and calcineurin-responsive elements in the Nur77 promoter, we examined whether a constitutively active form of calcineurin devoid of its C-terminal autoinhibitory domain could activate MEF2 as determined by an MEF2D-driven luciferase reporter gene. MEF2D was chosen among the four different isoforms of MEF2 as it is the most abundant isoform in T cells (Swanson et al., 1998). Indeed, the constitutively active form of calcineurin, CNβ2(1–401), activated MEF2D-dependent transcription (Figure 1B). A catalytically inactive mutant of calcineurin, CNβ2(1–401/H160N), however, did not activate the MEF2 reporter gene, suggesting that the phosphatase activity of calcineurin was required for its activation of MEF2D. To determine whether the calcineurin-dependent MEF2D activation is mediated through its DNA-binding activity, we examined the effect of the constitutively active calcineurin on the activation of a Gal4-luciferase reporter gene driven by a fusion protein between the Gal4 DNA-binding domain and MEF2D. The constitutively active calcineurin activated the Gal4-dependent reporter gene, indicating that stimulation of MEF2D-dependent transcription by calcineurin is not mediated by affecting MEF2 DNA-binding activity (Figure 1C).
NFAT mediates activation of MEF2 by calcineurin
The MEF2 family of transcription factors are known to be regulated by phosphorylation (Black and Olsen, 1998). Furthermore, it has been reported that calcineurin enhances the DNA-binding activity of MEF2A by its dephosphorylation (Mao and Wiedmann, 1999), raising the possibility that calcineurin may regulate MEF2D in the same manner. Using 32Pi to label MEF2D, we found that stimulation of DO11.10 cells by ionomycin did not cause detectable changes in the phosphorylation state of MEF2D (data not shown). It is thus unlikely that calcineurin regulates MEF2D activity through direct dephosphorylation. Together with the requirement for the phosphatase activity of calcineurin for the activation of the MEF2 reporter gene (Figure 1B), these results suggest that another phosphoprotein may be involved in the activation of MEF2D by calcineurin.
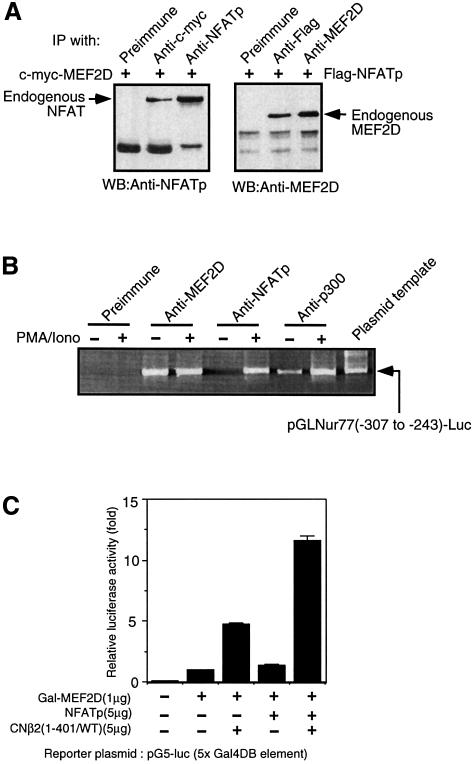
NFAT is a known substrate for calcineurin and translocates from the cytosol into the nucleus upon dephosphorylation by calcineurin (Flanagan et al., 1991; Jain et al., 1993; Rao et al., 1997), making it a likely candidate for a mediator of calcineurin-dependent MEF2D activation. Both MEF2 and NFAT are characterized by their ability to cooperate with other transcription factors to regulate gene expression (Rao et al., 1997; Black and Olsen, 1998). In fact, it was shown recently that co-expressed MEF2D and NFATp do co-immunoprecipitate (Blaeser et al., 2000). To verify further the interaction between MEF2D and NFATp, we ectopically expressed either c-Myc-MEF2D or Flag-NFATp. Endogenous NFAT was found to co-immunoprecipitate with c-Myc-MEF2D (Figure 2A). Similarly, endogenous MEF2D was found to co-immunoprecipitate with Flag-NFATp (Figure 2A). Although we were unsuccessful in detecting the interaction between endogenous MEF2D and endogenous NFAT, this result may be explained by a combination of the amounts of the two proteins in cell lysates being insufficient to be detected after co-immunoprecipitation and the relatively weak affinity of MEF2D for NFAT.
Fig. 2. NFATp interacts with MEF2D and enhances MEF2D-dependent reporter gene activation. (A) NFATp and MEF2D form a complex. Jurkat T cells (4 × 107/lane) were transfected with pSG-flag-NFATp or with pSG-c-myc-MEF2D, respectively. NFAT-overexpressing cell lysates were prepared and immunoprecipitated with anti-Flag antibodies and the bound endogenous MEF2D was detected by western blotting using anti-MEF2D antibodies. A similar procedure was used to detect the presence of endogenous NFAT in c-Myc-MEF2D immuno precipitate. (B) Association of endogenous MEF2D, NFAT and p300 with the Nur77 promoter. Various endogenous proteins were immuno precipitated from nuclear lysates prepared from Jurkat T cells transfected with pGLNur77(–307 to –242)-luc with specific antibodies as indicated, and the co-precipitated Nur77 promoter DNA was amplified using primers specific to the promoter region of Nur77 encoded in pGLNur77(–307 to –242)-luc. (C) NFATp enhances the activation of the MEF2D-dependent Gal4 reporter gene.
We then examined the association of NFATp with the minimal Nur77 promoter containing the two MEF2-binding sites. Using a chromatin immunoprecipitation PCR assay, we found that endogenous MEF2D is bound to the minimal Nur77 promoter in the presence or absence of stimulation with PMA and ionomycin (Figure 2B). In contrast, endogenous NFATp is bound to the same promoter elements only upon treatment of T cells with PMA and ionomycin. This binding is not mediated by the cognate NFAT-binding site present within the same promoter (Figure 4) since mutation of the two NFAT-binding sites does not affect the binding of NFATp while mutation of the two MEF2-binding sites did (H.-D.Youn and J.O.Liu, data not shown). It is thus most likely that NFATp is associated with the Nur77 promoter via its binding to MEF2D. We then determined whether binding of NFATp to MEF2D enhances its transcriptional activity using an MEF2D-dependent reporter gene. Co-expression of NFATp significantly increased the MEF2D-dependent reporter gene activity even when MEF2D is fused to the Gal4 DNA-binding domain (Figure 2C). The reporter gene activation was increased further by expression of the constitutively active form of calcineurin, which dephosphorylates NFATp. These results are consistent with the notion that NFATp associates with MEF2D, enhancing its transcriptional activity.
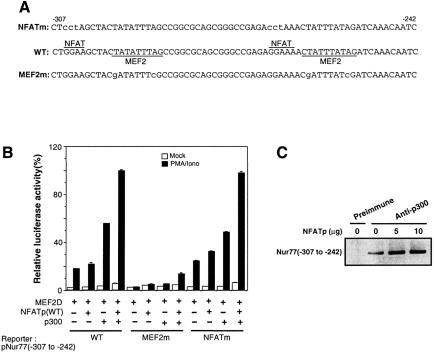
Fig. 4. DNA binding is not required for NFAT-mediated MEF2 transcriptional activation. (A) DNA sequences of the minimal Nur77 promoter. Putative NFAT- and MEF2-binding sites are overlined and underlined, respectively. Nucleotides that were mutated are indicated by lower case letters. (B) NFATp enhancement of the p300-mediated activation of the Nur77-luciferase reporter gene is dependent on the MEF2D-binding site, but independent of the NFAT-binding sites. (C) NFATp enhances p300 binding to the minimal MEF2-responsive element on the Nur77 promoter. DO11.10 cells were transfected with 2.5 µg of MEF2D, 10 µg of p300, 5 µg of pNur77(–347 to –242)-luciferase reporter gene and varying amounts of NFATp (0–10 µg). Transfected cells were stimulated with 40 nM PMA and 1 µM ionomycin for 1.5 h. Upon treatment with 1% formaldehyde, cell lysates were immunoprecipitated with anti-p300 polyclonal antibodies. CHIP assays were carried out as described in Materials and methods.
Determination of the minimal domains from NFATp and MEF2D involved in their interaction
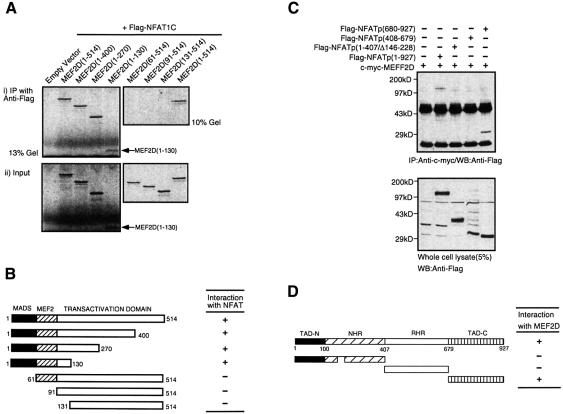
The NFATp-interacting domain in MEF2D was mapped by co-immunoprecipitation. Various truncation mutants of MEF2D were labeled with [35S]methionine by in vitro transcription and translation. Flag-NFATp was expressed in Jurkat T cells, and the corresponding Jurkat lysate was mixed with the [35S]MEF2D mutants followed by immunoprecipitation of NFATp using anti-Flag antibodies. The presence of MEF2D in the NFAT immunoprecipitate was detected by autoradiography (Figure 3A and B). The NFAT-binding domain was mapped to the N-terminal 130 amino acids of MEF2D containing the MADS/MEF2 domain (Figure 3A and B).
Fig. 3. Mapping of NFATp–MEF2D interacting domains. (A and B) NFATp binds to MEF2D through the MADS domain of MEF2D. In vitro transcription/translation products of various [35S]MEF2D deletion mutants were mixed with Jurkat cell lysates containing Flag-tagged NFATp and immunoprecipitated with anti-Flag antibody. Bound [35S]MEF2D mutants were visualized by autoradiography. (C and D) MEF2D associates with the C-terminal fragment of NFATp. Jurkat cells (1.5 × 107) were transfected with 15 µg of pSGMEF2D along with 20 µg of various NFATp deletion mutants. Cell lysates were immunoprecipitated with anti-MEF2D antibody and probed with anti-flag antibody.
The MEF2D-binding domain in NFATp was also determined by co-immunoprecipitation. Both full-length NFATp and a C-terminal fragment NFATp(680–927) were found to bind MEF2D (Figure 3C and D). Neither the N-terminal fragment, NFATp(1–407/Δ146–228), nor the Rel homology region-containing NFATp(408–679) interacted with MEF2D. A short deletion was made in NFATp(1–407/Δ146–228) to allow for visualization of the NFATp mutant in the presence of the signal from the heavy chain of antibodies. Thus, MEF2D binds to NFATp at a site that is different from those required for association with both calcineurin (Rao et al., 1997; Zhu et al., 1998) and p300 (Garcia-Rodriguez and Rao, 1998; Avots et al., 1999), which are located at the N-terminal domain, TAD-N, of NFATp (Figure 3D). This result also suggested that it was possible for NFAT to bind simultaneously to both p300 and MEF2D via its N- and C-termini, respectively.
NFATp and MEF2D synergize to recruit p300
Both MEF2 and NFAT are known to possess weak to moderate transactivation activity on their own. They are also known to interact with the coactivators p300/CBP (Sartorelli et al., 1997; Garcia-Rodriguez and Rao, 1998; Avots et al., 1999), raising the possibility that the MEF2D–NFATp complex can activate transcription by its enhanced ability to recruit p300 in comparison with either protein alone. To determine whether formation of the MEF2D–NFATp complex had a synergistic effect on the activation of the Nur77 promoter, we co-expressed MEF2D, NFATp and p300 along with a luciferase reporter gene under the control of the minimal Nur77 promoter (nucleotides –307 to –242). Although co-expression of NFATp and MEF2D did not give a significant increase in the reporter gene activity over that induced by MEF2D alone, co-expression of p300 and MEF2D did (Figure 4B). In the presence of p300, NFATp significantly enhanced MEF2 reporter gene activity, consistent with the notion that the NFATp–MEF2D complex effectively recruits p300 (Figure 4B).
We also determined the effect of overexpression of NFATp on the recruitment of p300 to the Nur77 promoter by the CHIP assay. Thus, p300 was immunoprecipitated along with the minimal Nur77 reporter plasmid in the presence of increasing amounts of NFATp, and the amount of associated Nur77 reporter plasmid was determined by PCR using primers flanking the MEF2-binding sites in the Nur77 promoter. Overexpression of NFATp led to an increase in the amount of Nur77 promoter bound to p300, presumably via the ternary complex containing MEF2 and NFATp (Figure 4C). This result is consistent with the notion that NFATp synergizes with MEF2D to recruit p300 to the Nur77 promoter and suggests that NFATp and MEF2D signals can be integrated by p300 for activation of Nur77 gene.
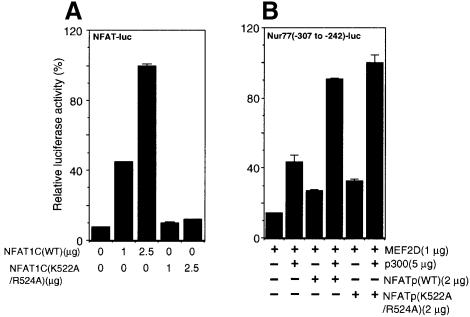
Although the MEF2-binding sites are responsible for Ca2+-dependent transcriptional activation of Nur77 (Woronicz et al., 1995), putative NFAT-binding sequences (GGAAA) are present in the vicinity of the two MEF2-binding sites (Figure 4A), raising the possibility that the two transcription factors cooperate through synergistic binding to the Nur77 promoter similarly to the cases of NFAT–AP1 and MEF2–MyoD cooperation (Rao et al., 1997; Black and Olsen, 1998). To assess the importance of the NFAT-binding sites in the induction of the Nur77 gene, we generated a reporter gene in which the consensus NFAT-binding sequences within the minimal Nur77 promoter are mutated. There was no decrease in reporter gene activation in response to MEF2D, NFATp and p300, indicating that the NFAT-binding sites are dispensable for activation. In contrast, another reporter gene in which the two MEF2-binding sites are mutated did not respond to expression of MEF2D, NFATp and p300 (Figure 4B), in agreement with previous observations (Woronicz et al., 1995). To assess further the importance of DNA binding in the transcriptional activation by NFATp, we mutated Lys522 and Arg524 of NFATp to alanines. These residues have been shown to be critical for DNA binding of NFATp (Sun et al., 1997; Chen et al., 1998). By gel shift assay, NFATp(K522A/R524A) was incapable of binding to the consensus NFAT-binding site (data not shown). The loss of DNA-binding activity of NFATp(K522A/R524A) was confirmed further by its inability to induce an NFAT-luciferase reporter gene in comparison with the wild-type protein (Figure 5A). When NFATp(K522A/R524A) was expressed along with MEF2D and p300, however, it activated the Nur77 reporter gene to the same extent as wild-type NFATp (Figure 5B). Thus, DNA binding is not required for the enhancement of MEF2D transcriptional activity by NFATp, indicating that NFATp is acting as a coactivator for MEF2D without direct DNA binding.
Fig. 5. An NFATp mutant defective in DNA binding remains competent for enhancement of MEF2D-dependent Nur77 reporter gene activity. (A) DNA binding-deficient NFAT mutant NFATp(K522A/R524A) is incapable of activating an NFAT-luciferase reporter gene. (B) NFATp(K522A/R524A) is as active in enhancing MEF2D activity as the wild-type protein. Jurkat T cells were treated with PMA (40 nM) and ionomycin (1 µM) for 12 h and the luciferase activity was normalized to β-gal activity.
Co-expression of MEF2D, p300, NFATp and constitutively active calcineurin is sufficient to activate expression of endogenous Nur77 selectively
To assess further the relevance of the interaction between NFAT and MEF2D in the transcriptional activation of the Nur77 gene, we transiently expressed MEF2D, NFATp, p300 and the constitutively active form of calcineurin, CNβ2(1–401), and determined whether expression of a subset of these proteins was sufficient to induce endogenous Nur77 expression in DO11.10 cells at both the protein and mRNA levels. As shown in Figure 6A, MEF2D, NFATp and p300, when expressed either individually or in combination, did not induce Nur77 expression. However, when CNβ2 (1–401) was co-expressed with MEF2D, NFATp and p300 so that NFATp can be activated, endogenous Nur77 gene expression was clearly detected. Thus, MEF2D and NFATp activated by calcineurin, together with p300, are sufficient to induce endogenous Nur77 expression. In agreement with the western blot analysis, Nur77 mRNA was also induced specifically upon co-expression of MEF2D, NFATp, p300 and the constitutively active form of calcineurin (Figure 6B). Although co-expression of MEF2D, p300 and CNβ2(1–401) also led to moderate production of Nur77 mRNA, the amount is much less than that induced by the co-expression of all four proteins. We also determined endogenous interleukin-2 (IL-2) gene expression using RT–PCR and found that co-expression of constitutively active calcineurin, NFATp, MEF2D and p300 does not lead to IL-2 expression, supporting the notion that the Nur77 expression is induced specifically by the combination of four proteins (Figure 6B). These results strongly suggest that the integration of the calcineurin–NFAT pathway and MEF2D by p300 is likely to be one of the most critical signaling events involved in Nur77 expression in response to calcium signaling.
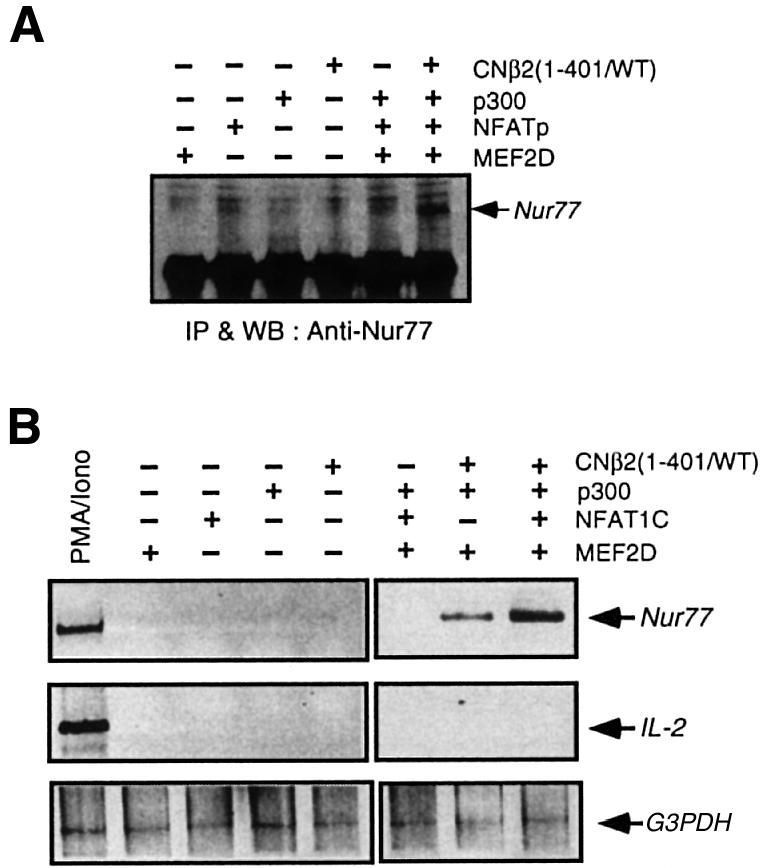
Fig. 6. Reconstitution of signaling pathways leading to endogenous Nur77 expression. (A) Co-expression of MEF2D, NFAT, p300 and constitutively active calcineurin is sufficient to induce expression of the endogenous Nur77. Nur77 protein was detected by a combination of immunoprecipitation and western blotting. (B) Co-expression of MEF2D, NFAT, p300 and constitutively active calcineurin induces the transcription of endogenous Nur77 mRNA, but not that of IL-2. RT–PCR was performed with cDNAs synthesized from total RNAs purified from each of transfected DO11.10. cells.
Discussion
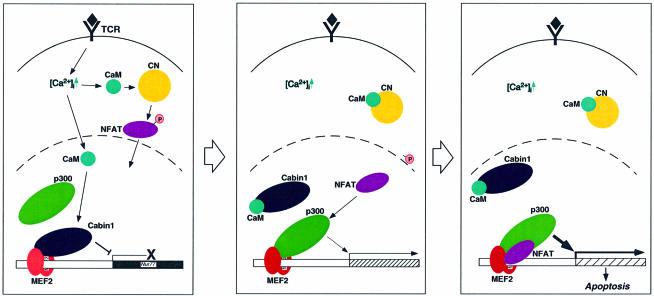
The second messenger Ca2+ is critical for the TCR-mediated signal transduction pathway leading to Nur77 expression and thymocyte apoptosis. Ca2+ signal can be converted into a transcriptional output via different mechanisms including the activation of kinases and phosphatases (Berridge et al., 1998). The most critical Ca2+-responsive elements in the Nur77 promoter were established to be two MEF2-binding sites (Woronicz et al., 1995). Previously, we have shown that MEF2 is bound to a transcriptional co-repressor, Cabin1, and its associated HDACs in unactivated T cells (Youn et al., 1999). One pathway emanating from intracellular Ca2+ leads to direct dissociation of Cabin1 and other functionally redundant repressors including MITR and HDAC4 from MEF2 as a consequence of competitive binding by calmodulin (Youn et al., 1999, 2000). The removal of Cabin1 and HDACs from MEF2 by calmodulin, however, is not sufficient for activation of MEF2 (Youn et al., 1999). A second Ca2+-dependent pathway mediated by calcineurin was also shown to be required for activation of MEF2 and activation of Nur77 transcription (Woronicz et al., 1995; Youn et al., 1999) (Figure 7).
Fig. 7. Two parallel Ca2+-dependent signaling pathways leading to Nur77 expression. Abbreviations: TCR, T-cell receptor; CaM, calmodulin; CN, calcineurin. The black diamond represents the antigen–MHC complex on antigen-presenting cells, and the P contained in the pink circle represents phosphate groups.
In addition to the calcineurin–NFAT signaling pathway, calmodulin-dependent kinases have also been reported to enhance MEF2 transcriptional activity (Blaeser et al., 2000; Lu et al., 2000). In one report, calcium/calmodulin-dependent protein kinase IV (CaMKIV) was shown to synergize with calcineurin to activate MEF2 transcriptional activity possibly through direct phosphorylation of MEF2D for Nur77 expression (Blaeser et al., 2000). It was also demonstrated that CaM kinases activate MEF2 by relieving MEF2 of HDAC repression (Lu et al., 2000), a mechanism distinct from the displacement of Cabin1- and other HDAC-containing repressors from MEF2 by calmodulin (Youn et al., 1999, 2000). Whether these alternative modes of modulation of MEF2 activity by CaM kinases represent cell type-specific or complementary mechanisms of MEF2 regulation remains to be seen. It seems clear that signaling by CaM kinases does represent another signal input into MEF2, making MEF2 a unique integration point of multiple calcium signals to affect transcription.
We and others showed that NFATp is a key mediator for the integration of calcineurin and MEF2 signals; it synergizes with NFATp to recruit p300 for the cooperative transcriptional activation of Nur77, thus completing the signal transduction pathway from calcineurin to MEF2 (Figure 7). It is known that MEF2 binds to the coactivator p300 (Sartorelli et al., 1997). However, this binding does not appear to be sufficient to activate the Nur77 gene fully. MEF2 is known to associate with other transcription factors such as MyoD to induce full activation of transcription of target genes (Black and Olsen, 1998). The demonstration that NFATp binds to MEF2D and that the resulting complex possesses enhanced affinity for p300 provides another mechanism of signal integration and amplification for the Nur77 gene upon calcium signaling. Thus, at least two parallel Ca2+/calmodulin-dependent pathways exist that control the expression of Nur77. This is in contrast to the regulation of IL-2 and other cytokine genes involved in T-cell activation for which the calcium signal is mediated primarily by the calcineurin–NFAT pathway.
NFAT belongs to the Rel superfamily of transcription factors, and a Rel-like DNA-binding domain is conserved among all known isoforms of NFAT (Rao et al., 1997). The apparent affinity of NFAT for DNA can be increased upon its cooperative association with other transcription factors. Transcription factors that cooperate with NFAT, including AP-1, GATA-4 and EGR-1, often have their DNA-binding sites positioned next to that for NFAT (Rao et al., 1997; Decker et al., 1998; Molkentin et al., 1998). Such proximity, in the case of NFAT and AP-1, has been shown unequivocally to increase DNA-binding affinity for the resultant NFAT–AP-1 complex in comparison with either protein alone (Peterson et al., 1996; Sun et al., 1997). NFAT has also been implicated to cooperate with MEF2 in the control of skeletal muscle fiber type (Chin et al., 1998; Wu et al., 2000) and induction of the EBV lytic cycle in response to immunoglobulin cross-linking (Liu et al., 1997). MEF2- and NFAT-binding sites were found to co-exist in the promoter of several genes selectively expressed in slow-oxidative myofibers. Unlike the putative NFAT-binding sites in the Nur77 promoter, that present in slow upstream regulatory elements from slow fiber-selective gene promoters was shown to be important for calcium-dependent promoter activation. In the case of Ig-induced lytic cycle gene induction, no NFAT-binding sites were identified in proximity to the MEF2-binding site (Liu et al., 1997). It is possible that NFAT also acts as a coactivator for MEF2 in response to the calcineurin signal in a manner similar to the Nur77 promoter activation disclosed here. Our observation that NFAT can cooperate with MEF2 to activate gene expression without binding DNA reveals a novel mode of action by this family of transcription factors. It also raises the possibility that the calcineurin–NFAT pathway can be integrated with other pathways via protein–protein interaction between NFAT and other transcription factors. That NFATp is shown to be required for Nur77 expression suggests that NFAT, along with calcineurin, is involved not only in T-cell activation but also in thymocyte apoptosis.
Materials and methods
Cell culture
Jurkat T cells and DO11.10 hybridoma cells were grown in RPMI medium supplemented with 10% serum, 2 mM glutamate and 50 U/ml streptomycin/penicillin.
Transfection of plasmids
T cells were transiently transfected by electroporation (250 V, 950 µF) as described previously (Sun et al., 1998). MEF2D and NFAT deletion mutants, constitutively active and catalytically inactive calcineurin were subcloned into pSG5. The original construct of mouse NFATp(pGLP3-NFATp) was obtained from A.Rao (Harvard Medical School). The mutant reporter gene, Nur77(–307 to –242)/NFATm was generated by PCR and subcloned into pGL2. Wild-type and MEF2D mutant reporter genes were kindly provided by A.Winoto (University of California, Berkeley). More detailed information about plasmids used in this study is available upon request.
Immunoprecipitation
Cells were lysed with a lysis buffer [20 mM Tris–HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.5% NP-40 and 1 mM phenylmethylsulfonyl fluoride (PMSF)] and the resultant lysate was incubated with suitable antibodies–protein A/G beads for 2 h at 4°C, followed by washing three times in lysis buffer. Immunoprecipitates were subjected to SDS–PAGE and probed with appropriate antibodies. Anti-Nur77 antibody was purchased from Santa Cruz, anti-Flag antibody from Sigma, and anti-MEF2D was obtained from R.Prywes (Columbia University).
CHIP assays
CHIP assays were carried out as follows. Jurkat T cells (3 × 107/lane) were transfected with 10 µg of pGLNur77(–307 to –242)-luc reporter gene. After a 24 h incubation, each transfected Jurkat cell pool was treated with PMA (40 nM) and ionomycin (1 µM) for 3 h. Formaldehyde-treated nuclear lysates were subjected to immunoprecipitation with various antibodies. DNA fragments present in the immunoprecipitates were amplified with primers, which specifically recognize a fragment of the Nur77 promoter. As a control, the Nur77 promoter-containing reporter plasmid was precipitated directly from the nuclear lysates and amplified with the same primers used to detect Nur77 promoter.
In vitro transcription/translation
35S-labeled MEF2D deletion mutants were made using an STP3(T7) in vitro transcription/translation kit (Novagen).
RT–PCR
cDNA was prepared from total RNA purified from transfected cells as per the manufacturer’s instructions (cDNA cycle kit, Invitrogen). RT–PCR was carried out using 100 ng of cDNA as template to detect the expression of Nur77, IL-2 and G3PDH. The primers for Nur77 were 5′-primer, 5′-GTTGATGTTCCCGCCTTTGCC-3′, and 3′-primer, 5′-TCAGAAAGACAATGTGTCCAT-3′. The primers for murine IL-2 and G3PDH were purchased from Clontech.
Acknowledgments
Acknowledgements
We thank Drs A.Winoto, S.Burakoff, A.Rao, Ron Prywes and F.McKeon for reagents, and E.Griffith, A.Mondragon and other members of the Liu laboratory for helpful suggestions and comments on the manuscript. This work was supported in part by NIH and the Rita Allen Foundation.
References
- Ashton-Rickardt P.G. and Tonegawa,S. (1994) A differential-avidity model for T-cell selection. Immunol. Today, 15, 362–366. [DOI] [PubMed] [Google Scholar]
- Avots A., Buttmann,M., Chuvpilo,S., Escher,C., Smola,U., Bannister,A.J., Rapp,U.R., Kouzarides,T. and Serfling,E. (1999) CBP/p300 integrates Raf/Rac-signaling pathways in the transcriptional induction of NF-ATc during T cell activation. Immunity, 10, 515–524. [DOI] [PubMed] [Google Scholar]
- Berridge M.J., Bootman,M.D. and Lipp,P. (1998) Calcium—a life and death signal. Nature, 395, 645–648. [DOI] [PubMed] [Google Scholar]
- Black B.L. and Olsen,E.N. (1998) Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu. Rev. Cell Dev. Biol., 14, 167–196. [DOI] [PubMed] [Google Scholar]
- Blaeser F., Ho,N., Prywes,R. and Chatila,T.A. (2000) Ca2+-dependent gene expression mediated by MEF2 transcription factors. J. Biol. Chem., 275, 197–209. [DOI] [PubMed] [Google Scholar]
- Calnan B.J., Szychowski,S., Chan,F.K., Cado,D. and Winoto,A. (1995) A role for the orphan steroid receptor Nur77 in apoptosis accompanying antigen-induced negative selection. Immunity, 3, 273–282. [DOI] [PubMed] [Google Scholar]
- Chen L., Glover,J.N., Hogan,P.G., Rao,A. and Harrison,S.C. (1998) Structure of the DNA-binding domains from NFAT, Fos and Jun bound specifically to DNA. Nature, 392, 42–48. [DOI] [PubMed] [Google Scholar]
- Cheng L.E., Chan,F.K., Cado,D. and Winoto,A. (1997) Functional redundancy of the Nur77 and Nor-1 orphan steroid receptors in T-cell apoptosis. EMBO J., 16, 1865–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin E.R. et al. (1998) A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev., 12, 2499–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree G.R. and Clipstone,N.A. (1994) Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu. Rev. Biochem., 63, 1045–1083. [DOI] [PubMed] [Google Scholar]
- Decker E.L., Skerka,C. and Zipfel,P.F. (1998) The early growth response protein (EGR-1) regulates interleukin-2 transcription by synergistic interaction with the nuclear factor of activated T cells. J. Biol. Chem., 273, 26923–26930. [DOI] [PubMed] [Google Scholar]
- DeFranco A.L. and Weiss,A. (1998) Lymphocyte activation and effector functions. Curr. Opin. Immunol., 10, 243–267. [DOI] [PubMed] [Google Scholar]
- Flanagan W.M., Corthesy,B., Bram,R.J. and Crabtree,G.R. (1991) Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature, 352, 803–807. [DOI] [PubMed] [Google Scholar]
- Garcia-Rodriguez C. and Rao,A. (1998) Nuclear factor of activated T cells (NFAT)-dependent transactivation regulated by the coactivators p300/CREB-binding protein (CBP). J. Exp. Med., 187, 2031–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain J., McCaffrey,P.G., Miner,Z., Kerppola,T.K., Lambert,J.N., Verdine,G.L., Curran,T. and Rao,A. (1993) The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature, 365, 352–355. [DOI] [PubMed] [Google Scholar]
- Jameson S.C., Hogquist,K.A. and Bevan,M.J. (1994) Specificity and flexibility in thymic selection. Annu. Rev. Immunol., 369, 750–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang A.A., Cado,D. and Winoto,A. (1999) Nur77 transcription activity correlates with its apoptotic function in vivo. Eur. J. Immunol., 29, 3722–3728. [DOI] [PubMed] [Google Scholar]
- Lai M.M., Burnett,P.E., Wolosker,H., Blackshaw,S. and Snyder,S.H. (1998) Cain, a novel physiologic protein inhibitor of calcineurin. J. Biol. Chem., 273, 18325–18331. [DOI] [PubMed] [Google Scholar]
- Lee S.L., Wesselschmidt,R.L., Linette,G.P., Kanagawa,O., Russell,J.H. and Milbrandt,J. (1995) Unimpaired thymic and peripheral T cell death in mice lacking the nuclear receptor NGFI-B (Nur77). Science, 269, 532–535. [DOI] [PubMed] [Google Scholar]
- Liu S., Liu,P., Borras,A., Chatila,T. and Speck,S.H. (1997) Cyclosporin A-sensitive induction of the Epstein–Barr virus lytic switch is mediated via a novel pathway involving a MEF2 family member. EMBO J., 16, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.G., Smith,S.W., McLaughlin,K.A., Schwartz,L.M. and Osborne,B.A. (1994) Apoptotic signals delivered through the T-cell receptor of a T-cell hybrid require the immediate-early gene nur77. Nature, 367, 281–284. [DOI] [PubMed] [Google Scholar]
- Lu J., McKinsey,T.A., Nicol,R.L. and Olson,E.N. (2000) Signal-dependent activation of the MEF2 transcription factor by dissociation from histone deacetylases. Proc. Natl Acad. Sci. USA, 97, 4070–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z. and Wiedmann,M. (1999) Calcineurin enhances MEF2 DNA binding activity in calcium-dependent survival of cerebellar granule neurons. J. Biol. Chem., 274, 31102–31107. [DOI] [PubMed] [Google Scholar]
- Miska E.A., Karlsson,C., Langley,E., Nielsen,S.J., Pines,J. and Kouzarides,T. (1999) HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO J., 18, 5099–5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin J.D., Lu,J.R., Antos,C.L., Markham,B., Richardson,J., Robbins,J., Grant,S.R. and Olson,E.N. (1998) A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell, 93, 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G.J.V. (1994) Negative selection of lymphocytes. Cell, 76, 229–239. [DOI] [PubMed] [Google Scholar]
- Peterson B.R., Sun,L.J. and Verdine,G.L. (1996) A critical arginine residue mediates cooperativity in the contact interface between transcription factors NFAT and AP-1. Proc. Natl Acad. Sci. USA, 93, 13671–13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A., Luo,C. and Hogan,P.G. (1997) Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol., 15, 707–747. [DOI] [PubMed] [Google Scholar]
- Sartorelli V., Huang,J., Hamamori,Y. and Kedes,L. (1997) Molecular mechanisms of myogenic coactivation by p300: direct interaction with the activation domain of MyoD and with the MADS box of MEF2C. Mol. Cell. Biol., 17, 1010–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S.L. (1992) Immunophilin-sensitive phosphatase action in cell signaling pathways. Cell, 70, 365–368. [DOI] [PubMed] [Google Scholar]
- Sparrow D.B., Miska,E.A., Langley,E., Reynaud-Deonauth,S., Kotecha,S., Towers,N., Spohr,G., Kouzarides,T. and Mohun,T.J. (1999) MEF-2 function is modified by a novel co-repressor, MITR. EMBO J., 18, 5085–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.J., Peterson,B.R. and Verdine,G.L. (1997) Dual role of the nuclear factor of activated T cells insert region in DNA recognition and cooperative contacts to activator protein 1. Proc. Natl Acad. Sci. USA, 94, 4919–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Youn,H.-D., Loh,C., Stolow,M., He,W. and Liu,J.O. (1998) Cabin 1, a negative regulator for calcineurin signaling in T lymphocytes. Immunity, 8, 703–711. [DOI] [PubMed] [Google Scholar]
- Swanson B.J., Jack,H.M. and Lyons,G.E. (1998) Characterization of myocyte enhancer factor 2 (MEF2) expression in B and T cells: MEF2C is a B cell-restricted transcription factor in lymphocytes. Mol. Immunol., 35, 445–458. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. (1994) Positive selection of lymphocytes. Cell, 76, 219–228. [DOI] [PubMed] [Google Scholar]
- Weih F., Ryseck,R.P., Chen,L. and Bravo,R. (1996) Apoptosis of nur77/N10-transgenic thymocytes involves the Fas/Fas ligand pathway. Proc. Natl Acad. Sci. USA, 93, 5533–5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woronicz J.D., Calnan,B., Ngo,V. and Winoto,A. (1994) Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature, 367, 277–281. [DOI] [PubMed] [Google Scholar]
- Woronicz J.D., Lina,A., Calnan,B.J., Szychowski,S., Cheng,L. and Winoto,A. (1995) Regulation of the Nur77 orphan steroid receptor in activation-induced apoptosis. Mol. Cell. Biol., 15, 6364–6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. et al. (2000) MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J., 19, 1963–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanbakhsh K., Choi,J.W., Li,Y., Lau,L.F. and Choi,Y. (1995) Cyclosporin A blocks apoptosis by inhibiting the DNA binding activity of the transcription factor Nur77. Proc. Natl Acad. Sci. USA, 92, 437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn H.D. and Liu,J.O. (2000) Cabin 1 represses MEF2-dependent Nur77 expression and T cell apoptosis by controlling association of histone deacetylases and acetylases with MEF2. Immunity, in press. [DOI] [PubMed] [Google Scholar]
- Youn H.-D., Sun,L., Prywes,R. and Liu,J.O. (1999) T cell apoptosis regulated by Ca2+-induced release of the transcription factor MEF2. Science, 286, 790–793. [DOI] [PubMed] [Google Scholar]
- Youn H.-D., Grozinger,C.M. and Liu,J.O. (2000) Calcium regulates transcriptional repression of myocyte enhancer factor 2 by histone deacetylase 4. J. Biol. Chem., 275, 22563–22567. [DOI] [PubMed] [Google Scholar]
- Zhou T., Cheng,J., Yang,P., Wang,Z., Liu,C., Su,X., Bluethmann,H. and Mountz,J.D. (1996) Inhibition of Nur77/Nurr1 leads to inefficient clonal deletion of self-reactive T cells. J. Exp. Med., 183, 1879–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Shibasaki,F., Price,R., Guillemot,J.C., Yano,T., Dotsch,V., Wagner,G., Ferrara,P. and McKeon,F. (1998) Intramolecular masking of nuclear import signal on NF-AT4 by casein kinase I and MEKK1. Cell, 93, 851–861. [DOI] [PubMed] [Google Scholar]