Abstract
There is increasing recognition that many of the core behavioral impairments that characterize autism potentially emerge from poor neural synchronization across nodes comprising dispersed cortical networks. A likely candidate for the source of this atypical functional connectivity in autism is an alteration in the structural integrity of intra- and inter-hemispheric white matter tracts that form large-scale cortical networks. To test this hypothesis, in a group of adults with high functioning autism (HFA) and matched control participants, we used diffusion tensor tractography to compare the structural integrity of three intra-hemispheric visual-association white matter tracts, the inferior longitudinal fasciculus (ILF), the inferior fronto-occipito fasciculus (IFOF) and the uncinate fasciculus (UF), with the integrity of three sub-portions of the major inter-hemispheric fiber tract, the corpus callosum. Compared with the control group, the HFA group evinced an increase in the volume of the intra-hemispheric fibers, particularly in the left hemisphere, and a reduction in the volume of the forceps minor and body of the corpus callosum. The reduction in the volume of the forceps minor also correlated with an increase in repetitive and stereotypical behavior as measured by the Autism Diagnostic Interview. These findings suggest that the abnormalities in the integrity of key inter-and intra-hemispheric white matter tracts may underlie the atypical information processing observed in these individuals.
Keywords: Autism, White matter, Inferior fronto-occipito fasciculus, Inferior longitudinal fasciculus, Diffusion tensor tractography
1. INTRODUCTION
There is a growing body of literature suggesting that many of the cognitive and social deficits associated with autism might arise from abnormal functional connectivity between and within the distributed cortical networks that mediate complex behavior (Castelli et al., 2002; Just et al., 2004; Kennedy et al., 2006; Villalobos et al., 2005). These alterations in functional connectivity might arise, in turn, from a perturbation in the integrity of white matter tracts that link the spatially distant regions of the network. Indeed, several diffusion tensor imaging (DTI) studies have revealed significant reductions in fractional anisotropy (FA), a measure of microstructural integrity of white matter, and/or other measures of diffusivity, in individuals with autism (Barnea-Goraly et al., 2004; Keller et al., 2007; Lee et al., 2007a). Moreover, there appear to be functional consequences of this white matter perturbation, as reduced microstructural integrity is correlated with lower IQ scores (Alexander et al., 2007), with higher ratings of repetitive behaviour (Thakkar et al., 2008) and with reduced functional connectivity in the Tower of London task (Just et al., 2007). Although these findings suggest an association between alterations in structural and functional connectivity and information processing, they do not pinpoint specific white matter (WM) tracts that are compromised in individuals with autism.
The neurobiological properties of large-scale WM tracts can now be studied in vivo using diffusion tensor tractography (DTT), which essentially uses information about the magnitude of diffusion of water molecules and the direction of maximal diffusion in each voxel to trace the likely trajectory of a white matter tract. Studies that have employed DTT to examine the integrity of specific WM tracts in individuals with autism relative to typically developing individuals have focused primarily on intra-hemispheric tracts. For example, one study reported alterations in the structural integrity of long range fibers in the frontal cortex in children within the autism spectrum (Sundaram et al., 2008), while another reported significant reductions in the micro-structural integrity of the right superior cerebellar peduncle and short intra-cerebellar fibers in adults with Asperger syndrome (Catani et al., 2008a). A more recent study revealed a significant increase in the number of streamlines (i.e. the lines that depict the fibers in a tract) in bilateral inferior longitudinal fasciculus and the cingulum bundle, as well as a reduction in streamlines in the right uncinate fasciculus (Pugliese et al., 2009). Importantly, these tracts are associated with behavioral functions that are known to be impaired in autism. For example, the inferior longitudinal fasciculus and the inferior fronto-occipito fasciculus are critical for higher-level visual and emotion processing (Rudrauf et al., 2008; Thomas et al., 2009), domains shown to be atypical in individuals with autism (Behrmann et al., 2006b; Bertone et al., 2005; Humphreys et al., 2008; Lee et al., 2007b). In summary, these tractography studies reveal perturbations in intra-hemispheric WM tracts in individuals on the autism spectrum, which may account for some of their difficulties in information processing.
Such perturbations do not appear to be specific to intra-hemispheric tracts per se as individuals with autism also show structural abnormalities in the corpus callosum, the largest inter-hemispheric tract, which bridges the two cerebral hemispheres. For example, morphometric studies have found significant reductions in volume along the subdivisions of the callosum in individuals with autism (Frazier et al., 2009). Non-tractography studies that used diffusion tensor imaging (DTI), showed reductions in FA in the corpus callosum in children, adolescents and adults with autism (Alexander et al., 2007; Barnea-Goraly et al., 2004; Keller et al., 2007). Additionally, a reduction in white matter integrity of the corpus callosum has been found to account for the poor performance IQ scores of a subgroup of individuals with autism (Alexander et al., 2007). It should be noted that information processing that requires integrated hemispheric function, such as fine coordination, is affected in individuals with autism (Nyden et al., 2004), suggesting that the perturbation of callosal connectivity may contribute to such impairments.
Taken together, although there is evidence for alterations in intra- and inter-hemispheric WM tracts in autism, a parallel examination of both fiber systems has not been undertaken. Moreover, whether alterations in these two white matter systems are related or whether they account for the core behavioral profile of individuals with autism also remains unknown. Finally, commissural tracts such as the corpus callosum, and intra-hemispheric association tracts such as the ILF, IFOF and UF have distinctly different developmental and maturational trajectories (Keshavan et al., 2002; Rakic et al., 1968) and a parallel investigation of inter- and intra-hemispheric connectivity in autism can potentially help elucidate the neurodevelopmental mechanisms underlying autism.
The goal of the present study, therefore, is to compare the structural integrity of key inter-hemispheric and intra-hemispheric WM tracts in a group of high-functioning adults with autism (HFA) and matched typically developing controls, and to explore the functional relevance of these tracts. To this end, we use diffusion tensor tractography (DTT) to quantify the structural integrity of the largest inter-hemispheric tract, the corpus callosum and its subcomponent tracts, the forceps major (F-Ma) (Dougherty et al., 2005), body and forceps minor (F-Mi), as well as three intra-hemispheric WM tracts: the inferior longitudinal fasciculus, inferior fronto-occipito fasciculus (IFOF), and the uncinate fasciculus (UF) (Catani et al., 2008c). Our hypothesis is that the HFA group will show significant alterations in the structural connectivity profile in both intra- and inter-hemispheric WM tracts.
2. METHODS AND MATERIALS
2.1. Participants
Participants were 30 male adults, 12 diagnosed with high-functioning autism (HFA) and 18 typical individuals. The diagnosis of HFA was based on DSM-IV criteria (2000), the Autism Diagnostic Inventory – Revised (Lord et al., 1994) and the Autism Diagnostic Observational Schedule - Generic (Lord et al., 1999) and was confirmed by expert clinical opinion. Only individuals, who were free of seizures, had no history of brain injury, and no identifiable etiology for the autism profile (e.g. tuberous sclerosis or fragile-X syndrome) were eligible for inclusion. Also, only individuals with full-scale IQ > 80 (Wechsler Adult Intelligence Scale-III) were included (Table 1).
Table 1.
Biographical, IQ, and diagnostic scores of all the individuals in the autism group
| BIOGRAPHICAL | IQ | ADOS | ADI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Participant | Age | Handedness | Verbal | Performance | Full Scale | COM | SOC | SOC | COM | STB |
| 1 | 21 | R | 97 | 119 | 107 | 4 | 8 | 22 | 10 | 3 |
| 2 | 33 | L | 104 | 116 | 110 | 5 | 7 | 21 | 16 | 8 |
| 3 | 26 | R | 116 | 116 | 118 | 5 | 6 | 36 | 17 | 9 |
| 4 | 49 | R | 88 | 103 | 96 | 6 | 11 | 27 | 20 | 8 |
| 5 | 19 | R | 111 | 121 | 118 | 5 | 13 | 20 | 13 | 3 |
| 6 | 28 | R | 118 | 128 | 126 | 4 | 6 | 20 | 11 | 3 |
| 7 | 20 | L | 109 | 86 | 97 | 4 | 7 | 15 | 15 | 3 |
| 8 | 20 | R | 109 | 88 | 99 | 6 | 11 | 27 | 22 | 5 |
| 9 | 24 | R | 97 | 102 | 100 | 4 | 10 | 19 | 13 | 4 |
| 10 | 37 | R | 110 | 96 | 104 | 4 | 10 | 24 | 19 | 5 |
| 11 | 38 | R | 113 | 114 | 115 | 5 | 9 | 15 | 8 | 10 |
| 12 | 22 | R | 89 | 101 | 95 | 5 | 10 | 20 | 16 | 7 |
COM: communication; SOC: Social; STB: stereotypical behaviour.
Each HFA individual was matched to a typical individual (or more than one in some cases) by age, gender, handedness and IQ. All control subjects were in good health with no history of neuropsychiatric disorders, learning disabilities or brain insult. As is evident from table 2, there were no differences across groups in age, verbal IQ, Picture IQ or Full-Scale IQ scores. Written informed consent was obtained from all the participants and all were compensated equally for their participation. This study was approved by the Institutional Review Boards of the University of Pittsburgh Medical Center and of Carnegie Mellon University.
Table 2.
Mean age, full scale IQ (FS-IQ), Verbal IQ (V-IQ) and Performance IQ (P-IQ) and the standard deviation for the HFA and control groups. The p-values from a one way ANOVA do not indicate any significant differences between the two groups across these measures.
| HFA | Control | p - value | |
|---|---|---|---|
| Age |
28.5
9.7 |
22.4
4.1 |
< 0.85 |
| FS-IQ |
106.92
10.47 |
111.6
9.91 |
< 0.30 |
| V-IQ |
105.08
10.09 |
109.8
12.54 |
< 0.34 |
| P-IQ |
107.25
13.9 |
111
10.03 |
< 0.49 |
2.2. Data acquisition
Participants were scanned at the Brain Imaging Research Center in Pittsburgh on a 3T Siemens Allegra scanner equipped with a standard quadrature birdcage head coil. The DTI sequence was based on a single-shot spin-echo, echo-planar imaging (EPI) sequence with diffusion sensitizing gradients applied on either side of the 180° refocusing pulse. Diffusion weighted images of the whole brain were acquired along the horizontal plane along six non-collinear directions: XY, XZ, YZ, −XY, −XZ, and −YZ. In addition, images were acquired with b = 0. Specific DTI parameters: TR = 4900 msec, TE = 82 msec, flip angle = 90°, FOV = 210 × 210 mm2, acquisition matrix size 80 × 128, 34 axial slices, 3 mm thickness (no gap), pixel size = 1.64 × 1.64 mm2, diffusion weighting b = 850 sec/mm2. Twelve repetitions of the complete set were collected and averaged to increase signal-to-noise without introducing motion artifacts. During each scanning session, along with the DTI acquisition (duration 7 minutes), high-resolution anatomical scans and BOLD contrast images were also acquired. The high-resolution anatomical scan (T1-weighted 3-D MPRAGE) was used for co-registration with the b=0 image. Specific T1 scanning parameters: inversion time = 800 msec, TE = 3.04 msec, flip angle = 8 degrees, FOV = 256 × 256 mm2, matrix size = 256 × 256, slice thickness = 1 mm, number of slices = 192.
2.3. DTI methods
Computation of the diffusion tensor and fiber tracking was performed using DTIstudio, operating on a Microsoft Windows platform (Jiang et al., 2006). DTIstudio calculates, using standard computational algorithms (Basser et al., 1996), the diffusion tensors and associated FA maps by solving an over-determined linear equation system using least square fitting. Because long-range fibers can be prematurely terminated (increase in noise as fiber propagation gets longer) (Jones et al., 2005), a tensor smoothing algorithm (Westin et al., 2002) was employed before tractography to reduce residual errors. Tractography was performed by specifying the minimum FA threshold for starting tracking (0.20) and the critical angle threshold (40° or 60°) for stopping tracking in case of sharp turns in the fiber direction. Based on these parameters, DTIstudio generates three-dimensional fiber tracts based on the Fiber Assignment by Continuous Tracking (FACT) algorithm and a brute-force fiber reconstruction approach (Mori et al., 1999; Xue et al., 1999). Note that the white matter fibers of the corpus callosum and the uncinate fasciculus have a highly curvy trajectory and therefore, the critical angle was set to 60 so as to ensure complete extraction of the fibers. However, for the ILF and IFOF the critical angle was set to 40 because of their relatively linear trajectory and also to reduce the likelihood of false positives.
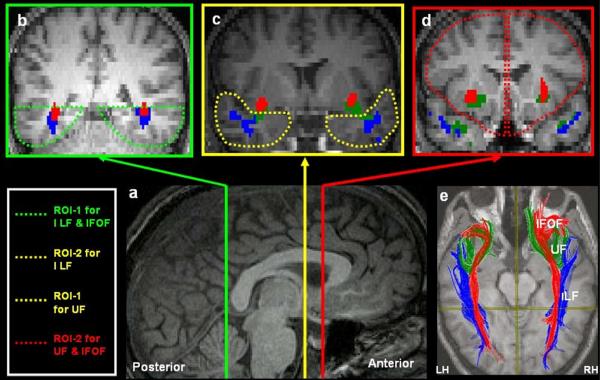
We employed a multiple ROI approach to extract all the tracts of interest, which, in conjunction with the brute-force fiber reconstruction has been found to be less susceptible to noise and partial volume effects, and to ensure robust recovery of the major fiber tracts in the human brain (Huang et al., 2004; Wakana et al., 2004). As illustrated in Figure 1a and 1b, the tracts of interest were extracted and their structural properties were quantified in native space using a previously-defined protocol (Thomas et al., 2009).
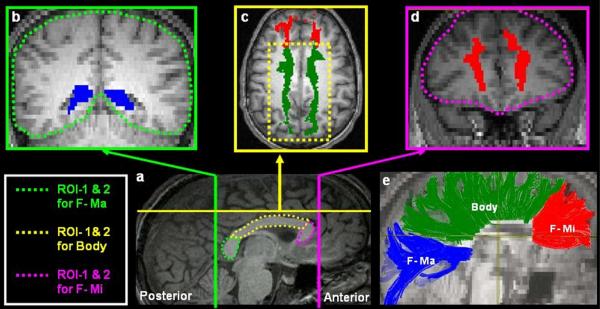
Figure 1a.
Illustration of the protocol used for extracting the three intra-hemispheric visual association tracts. (a) Mid-Sagittal slice shows the relative locations of the coronal slices on which the ROIs for extracting the 3 tracts were defined. All ROIs were defined on each individual's native space. Clockwise from B–D, the coronal slices are color coordinated to indicate their corresponding position on the mid-sagittal plane. To extract the Inferior Longitudinal Fasciculus, the ventral occipito-temporal cortex inferior to the lateral ventricles was defined as ROI-1(b) and the entire temporal cortex anterior to the point where the fornix descends to the mamillary bodies was defined as ROI-2. To extract the inferior fronto occipito fasciculus (IFOF), ROI-1 was the same as the one used for the ILF, but the entire frontal cortex anterior to the rostrum of the callosum was defined as ROI-2 (d). To extract the Uncinate Fasciulus (UF), the anterior temporal cortex region (c) was defined as ROI-1 and the frontal cortex anterior to the rostrum of the callosum was defined as ROI-2. Note that each tract was extracted by including streamlines that project through the 2 ROIs and by excluding tracts that belong to other fiber systems. Thus for extraction of the ILF fibers, the IFOF fibers were removed to avoid inclusion with the ILF fibers. Additionally, for all tracts of interest the ROIs were switched such that ROI-1 was defined as ROI-2 and vice versa and the average number of streamlines and voxels through which the streamlines project were computed from this procedure. (e) The ILF (blue), UF (green) and IFOF (Red) are extracted and displayed on the mid-sagittal plane on a single diffusion weighted slice co-registered to the corresponding T1 high-resolution slice. The data displayed here are from a control subject. All images displayed are in neurological convention.
Figure 1b.
Illustration of the protocol used to extract the three inter-hemispheric callosal tracts. (a) The Splenium, Body and Genu of the corpus callosum were delineated on the mid sagittal slice. For the F-Ma: the Splenium (a) was defined as ROI-1 and the first coronal slice posterior to the splenium of the corpus callosum as ROI-2. For the tracts projecting through the body of the corpus callosum: the body of the callosum was defined as ROI-1. ROI-2 was defined on the axial plane (c) as the white matter caudal to the superior frontal gyrus. For the F-Mi: (a) the genu and rostrum of the callosum were defined as ROI-1 and the first coronal slice anterior to the genu was defined as ROI-2. The same two-ROI technique described in fig 1 a was employed to extract and quantify the 3 intra-hemispheric tracts (e) The approximate trajectory of the three inter-hemispheric tracts. All images displayed are in neurological convention.
2.4. Dependent measures
The macro-structural properties of the tracts were quantified with two measures: the number of streamlines (Pugliese et al., 2009), which depict the fibers within a tract, and the number of voxels through which the streamlines pass. These two measures were used to quantify the volume of the tract and as absolute values they are relevant if there are no significant differences in the overall brain volume between the two groups. Given that increases in brain volume have been reported in individuals with autism (Herbert et al., 2004), we computed the number of streamlines and voxels in the entire brain for each individual. The micro-structural integrity of a tract was quantified by computing the mean FA of all the voxels within the tract of interest. Because one of the parameters required for the extraction of a tract is FA in a voxel, measures like the number of streamlines and volume are likely to be correlated with changes in FA value. However, this is not always the case for all fiber tracts. Therefore, the exact dependent measures one should use are still under debate and so we report number of streamlines, number of voxels, as well as FA. Indeed, if by all 3 measures the pattern in the reduction of the macro- and micro-structural properties of a tract is consistent, this further attests to the reliability of the finding.
2.5 Statistical analysis
Given that previous volumetric studies have reported that individuals with autism exhibit a significant alteration in WM volume at the whole brain level (Herbert et al., 2004; McAlonan et al., 2005; Waiter et al., 2005), at the outset, we explored whether the HFA group differed from the control group with regard to white matter connectivity at the whole brain level. A one-way ANOVA did not indicate any significant differences between the two groups in terms of the number of streamlines (Means: Control: 57323; HFA: 55898.17) (p > .21), or the number of voxels (Means: Control: 81107.80; HFA: 79031.25) through which the streamlines pass (p > .18). The lack of a whole brain difference does not necessarily mean that there are no differences in white matter connectivity between the two groups: for example, an increase in volume in one fiber system (e.g. intra-hemispheric fibers) may be offset by a decrease in a second area (e.g. inter-hemispheric fibers), or vice versa, leading to an equal outcome across the groups. Therefore, the more relevant question for the present study is whether we can uncover differences in intra- and inter-hemispheric white matter fibers between individuals with HFA and their counterparts.
Given that the two groups were comparable in terms of the WM volume at the whole brain level, we focus only on the absolute values for the individual tracts. Before performing parametric tests to examine group differences in the intra-hemispheric (bilateral ILF, IFOF and IFOF) and in the inter-hemispheric callosal tracts (F-Ma, body and F-Mi), the data were subjected to a Kolmogorov-Smirnov (KS) test to verify normality of distribution of the data. The test did not reveal any significant deviations from a normal distribution for any of the dependent measures in any of the tracts (p > .05). In light of this, a repeated measures ANOVA with hemisphere (right/left) and tract (ILF/IFOF/UF) as the within-subjects factor and group (Control/HFA) as the between-subjects factor was performed. For the inter-hemispheric tracts, given that `hemisphere' is not a relevant experimental factor in these homotopic pathways, a separate repeated measure ANOVA with tract (F-Ma/Body/F-Mi) as the only within-subjects factor was performed with group as the between-subject factor. Because the primary objective of the study is to determine the presence of group differences, we focus only on a main effect of group and on interactions with group and the within-subject factors. We report the findings pertaining to the inter-hemispheric tracts first and then go on to explore the group differences in the intra-hemispheric tracts.
3. RESULTS
The forceps major, body and forceps minor of the corpus callosum
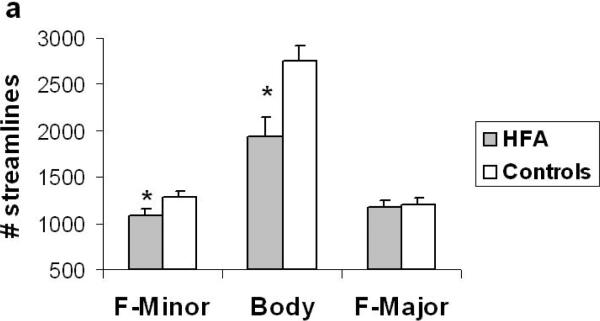
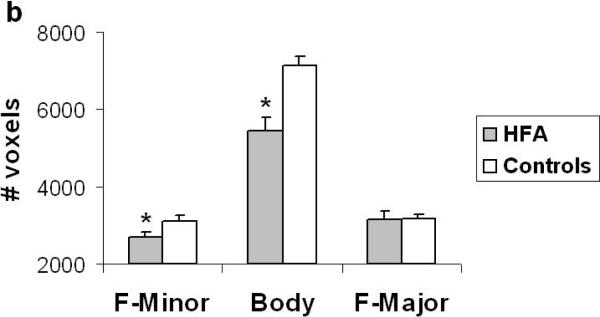
The analysis revealed a significant main effect of group (F (1, 28) = 10.55, p < .003) and a tract by group interaction in terms of the number of streamlines (F (2, 56) = 6.06, p < .004). Similarly, in terms of the number of voxels, the analysis revealed a significant main effect of group (F (1, 28) = 13.41, p < .001) and a tract by group interaction (F (2, 56) = 11.03, p < .001). These findings suggest a significant reduction in the macro-structural integrity of fibers in the corpus callosum in the HFA group relative to controls. Tukey post hoc analysis revealed that the tract by group interaction originated from a significant reduction in the number of streamlines and number of voxels in the F-Mi (p < .05) and in the body of the corpus callosum (p < .05) in the HFA group (Fig. 2a & b). Interestingly, there were no differences in the macro-structural properties of the F-Ma between the two groups. Finally, with regard to the micro-structural integrity of the callosal tracts, the analysis did not reveal any significant differences in mean FA between the two groups.
Figure 2.
(a) Number of streamlines and (b) number of voxels through which the streamlines project in the 3 subcomponents of the corpus callosum in the HFA and control groups. The HFA group showed a significant reduction in streamlines and voxels that project via the F-Minor and Body of the callosum. Error bars indicate standard error of the mean. * p < 0.05
The inferior longitudinal fasciculus, Inferior fronto-occipito fasciculus and Uncinate fasciculus
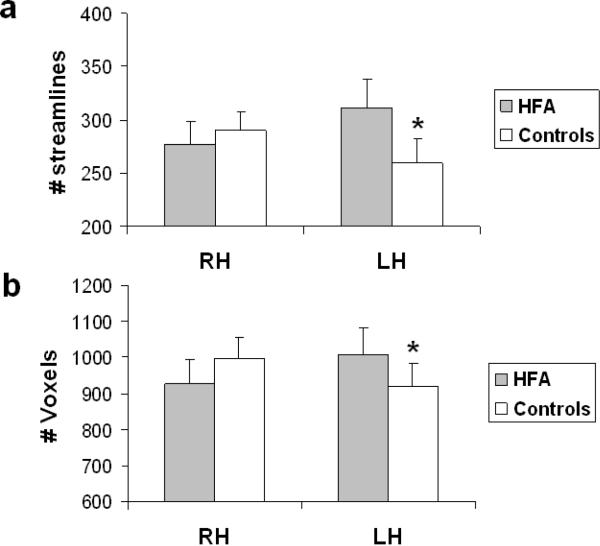
With number of streamlines as the dependent measure, there was a significant two-way interaction of hemisphere × group (F(1, 27) = 8.04, p < .009). Tukey post hoc analysis on this interaction confirmed that, whereas the control group showed no significant difference in the volume of fibers (collapsed across ILF, IFOF and UF) in the two hemispheres, the HFA group exhibited a significantly greater number of streamlines in the left than right hemisphere (p < .05) (see Figure 3a). The post hoc test did not indicate a significant difference between the two groups in the number of streamlines in the right hemisphere suggesting that the increase in left hemisphere was independent of any alteration in the tracts in the right hemisphere. These same findings held with respect to number of voxels as the dependent measure, reinforcing the finding that there are group differences across the hemispheres (F (1, 27) = 5.2, p < .03). As shown in Figure 3b, the analysis revealed significantly greater number of voxels in the left than the right hemisphere (p < .05), relative to the control group, who do not show any differences in the tract measures between the two hemispheres. The absences of neither a main effect of group nor a three-way interaction with group, suggested that the asymmetry observed was equivalent across all three intra-hemispheric tracts of interest.
Figure 3.
(a) Number of streamlines and (b) number of voxels through which the streamlines project collapsed across the 3 intra-hemispheric tracts in the right and left hemisphere for the HFA and control groups. In both cases, compared to the control group, the HFA group shows a significant increase in structural connectivity in the left hemisphere. Error bars indicate standard error of the mean. * p < 0.05.
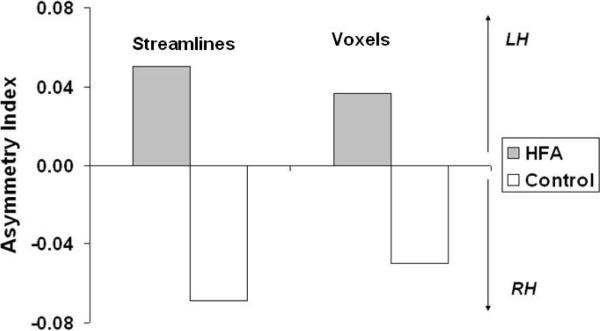
To derive a summary statistic that quantifies the degree of hemispheric asymmetry for each participant, we calculated an asymmetry index defined as (left− right)/(left + right) for number of streamlines and number of voxels. The index ranges from 1 to −1 with positive values indicating an asymmetry towards the left hemisphere. As is evident from Figure 4, a one-way ANOVA for group differences in the asymmetry index further confirmed the significant leftward asymmetry in the distribution of white matter in the autism group in terms of streamlines (F (1, 28) = 8.29, p < .008) as well as number of voxels (F (1, 28)= 4.82, p < .04). With regard to the micro-structural integrity of the three intra-hemispheric visual association tracts, there were neither significant main effects nor an interaction with group.
Figure 4.
Group differences in the asymmetry index for the intra-hemispheric visual association tracts. Positive values indicate a leftward asymmetry. The HFA group showed a significant leftward increase in these intra-hemispheric visual association tracts.
Could the reduction in fibers in the sub-components of the corpus callosum somehow be related to the leftward asymmetric increase in the intra-hemispheric fibers? A Pearson correlation analysis between the intra-hemispheric asymmetry index and the macro-structural measures for the F-Mi and the body of the callosum did not reveal any significant correlations, suggesting that the findings from the intra-hemispheric and inter-hemispheric tracts are independent and likely unrelated.
Finally, to examine the behavioral ramifications of the alteration in white matter integrity in the HFA group, we performed a correlation analysis between the asymmetry index and the macro-structural measures for the forceps minor and the body of the callosum with the components of the ADOS and ADI scores of the HFA group. The analysis revealed a significant negative correlation between an increase in the score for one of the core domains of the ADI-R (restricted, repetitive, and stereotyped behaviors) and reductions in the number of streamlines (r = −60, p < .04) and voxels (r = −64, p < .02) (Uncorrected)1 of the F-Mi.
In summary, our analyses revealed a significant leftward increase in all three visual-association WM tracts in the HFA group, as well as a significant reduction in the volume of the F-Mi and the body of the corpus callosum, the major inter-hemispheric fiber tract in the cerebral cortex, in this same group. Moreover, the reduction in WM volume in the F-Mi correlated with an increase in severity of repetitive and stereotypical behavior in the HFA group. Contrary to previous tractography studies involving autism individuals, a significant group difference in the micro-structural integrity of any of the intra- and inter-hemispheric tracts was not observed. These results raise several provocative questions concerning the potential relationship between structural connectivity, functional connectivity and information processing. These are addressed in turn in the discussion.
4. DISCUSSION
The major finding of the present study is that both inter- and intra-hemispheric connectivity is compromised in individuals with high-functioning autism (HFA). With regard to inter-hemispheric connectivity, our findings reveal that the F-Mi and the fibers projecting through the body of the callosum are significantly reduced in volume (both in number of streamlines and number of voxels through which the streamlines project) in the HFA group, whereas the volume of the F-Ma is well within the range of the control group. Importantly, the reduction in the volume of the F-Mi correlates with an increase in the ADI score for repetitive and stereotypical behavior in the HFA group. The F-Mi comprises predominantly homotopic fibers that project between the bilateral fronto-polar regions, the rostral anterior cingulate cortex (ACC) as well as the ventral and medial prefrontal cortex via the genu and the rostrum of the corpus callosum. Interestingly, a recent DTI study involving a group with autism spectrum disorders found that a reduction in the micro-structural integrity of white matter in the rostral ACC, which extends into the genu of the corpus callosum was associated with hyper-activity in the ACC as well as higher ratings of repetitive behavior (Thakkar et al., 2008). Although, a significant reduction in micro-structural integrity was not observed in our data, the correlation between the reduction in volume of the F-Mi and repetitive behavior further confirms the relationship between abnormalities in the organization of white matter in the F-Mi and increased severity of repetitive behavior in autism.
The HFA group also showed a significant reduction in the volume of the fibers projecting through the body of the callosum, defined here as the homotopic fibers caudal to the prefrontal cortex and rostral to the parieto-occipital cortex. The reductions in the volume that we report here are consistent with the findings from several MRI-based morphometric studies of the callosum involving children (Boger-Megiddo et al., 2006; Vidal et al., 2006), adolescents (Alexander et al., 2007; Piven et al., 1997) and adults (Hardan et al., 2000) within the autism spectrum. Some of these studies also found a reduction in volume in the posterior aspect of the splenium (Alexander et al., 2007; Piven et al., 1997), which corresponds to the region through which the F-Ma projects. However, our data do not reveal any reduction in the volume or micro-structural integrity of the F-Ma as such. One possible reason for this inconsistency could be that all the aforementioned studies focused on children or adolescents within the autism spectrum, whereas the present study focused only on adults with high functioning autism. Thus it may be the case that by the time the HFA individuals reach adulthood, the microstructure of the tracts may be coherent, but the volume and large-scale properties of the tracts may differ from typically developing individuals.
In any case, it is reasonable to conclude that inter-hemispheric connectivity via the corpus callosum is greatly reduced in autism spectrum. The fibers projecting through the body of the callosum link the bilateral primary and secondary motor and somatosensory cortex as well as the posterior parietal cortex (Schmahmann et al., 2009). A reduction in the volume of these fibers would be expected to have concomitant behavioral implications. Our analysis of the data did not reveal any significant correlations between the diagnostic scores and the volume reduction. However, behavioral tests of inter-hemispheric processing in individuals with high functioning autism have revealed deficits in motor and sensory processing tasks that require normal inter-hemispheric information processing (Nyden et al., 2004) and so more fine-grained measures than those we have adopted here might well uncover behavioral changes associated with these inter-hemispheric alterations.
That the HFA group showed no difference in F-Ma volume raises the possibility that the changes in volume anterior to the splenium might offer clues about white matter developmental in autism. Post mortem studies suggest that although the genu grows at a faster rate than the body and the splenium during fetal development, but after birth, the splenium takes the lead in the relative growth of the three parts of the corpus callosum (Rakic et al., 1968). Accordingly, MRI studies have also shown that post-natal myelination in the corpus callosum starts from the splenium much earlier compared to the genu and the body (Barkovich et al., 1988), with a sudden spurt of increase in thickness at 4 to 6 months. Beyond infancy, the most significant growth has been found to be in the splenium rather than in any other subdivision in the corpus callosum (Giedd et al., 1999). This suggests that the reduction in volume in the genu and body revealed in the present study might be the result of some perturbation during prenatal development and offers the possibility of a biological marker for autism.
One would expect the reduction in volume in one of the largest white matter bundles in the cerebral cortex in the HFA group to be evident even at the whole brain WM volume. However, no group differences in whole brain WM volume were observed, suggesting that any reduction in white matter may be compensated by an increase elsewhere. Accordingly, our examination of the three intra-hemispheric visual-association tracts: the ILF, the IFOF and the UF revealed a leftward asymmetric increase in volume in the HFA group. The asymmetry emerged from a significant increase in volume of these tracts in the left hemisphere rather than a reduction in volume of these same tracts in the right hemisphere, relative to controls. There was also no evidence for a relationship between the reduction in WM volume in the components of the corpus callosum and the increase in WM volume of the intra-hemispheric visual-association tracts, which suggests that these differences in WM development might be mediated by independent mechanisms. Hemispheric asymmetries have been known to have a significant genetic component, which influences white matter organization as well (Chiang et al., 2009; Geschwind et al., 2002). One of the white matter pathways that show a striking left lateralization is the arcuate fasciculus (Nucifora et al., 2005), which links Broca's area in the frontal lobe with Wernicke's area in the temporal lobe and is critical for language function (Catani et al., 2008b). Interestingly, the degree of lateralization has functional consequences in that greater left lateralization of the arcuate fasciculus has been found to account for superior phonological processing in typically developing children (Lebel et al., 2009). It is worth noting that the leftward white matter asymmetry in the HFA group that we observe originates from ventral WM tracts that are not known to be lateralized by hemisphere. Bilaterally, the ILF and the IFOF project via the ventral occipito-temporal cortex and project to ventral anterior temporal cortex and inferior frontal cortex respectively, whereas the UF projects from medial and anterior temporal cortex to inferior and orbital frontal cortex. These intra-hemispheric tracts have been found to mediate various critical aspects of visual information processing (Eacott et al., 1992; Thomas et al., 2009). So what might be the functional consequences of the lateralized increase in the volume of these ventral fiber systems in the left hemisphere?
Correlations between the degree of asymmetry and the ADI diagnostic scores did not yield any indications of a brain-behavior relationship, which requires explanation. One well-established finding in the visual perception literature is that the right hemisphere is more sensitive to global information and/or low spatial frequencies, while the left hemisphere is tuned to high spatial frequencies that contain information about local features (Robertson et al., 2000; Yovel et al., 2001). In light of the current findings, one plausible hypothesis would be that the abnormal increase in connectivity within the left hemisphere might potentially be the source or the result of the prominent local visual bias that has been often documented in individuals with HFA (Behrmann et al., 2006a; Happé et al., 2006). Future studies with a combined fine-grained and comprehensive behavioral and tractography approach might potentially test this hypothesis. The left-hemisphere specific increase in WM volume is not entirely consistent with the findings from Pugliese et al (2009) who reported bilateral increase in the volume of the ILF and the cingulum bundle, and a right hemisphere specific reduction in volume in the UF, while the volume of the IFOF was found to be comparable to the control group. Moreover, a significant age-related reduction in micro-structural integrity of the left uncinate fasciculus was also reported. Similar reductions in micro-structural integrity in frontal, temporal and callosal white matter have been reported in studies involving children and adolescents with autism (Alexander et al., 2007; Barnea-Goraly et al., 2004; Keller et al., 2007). This discrepancy may be attributable to a number of possible factors.
First, we acknowledge the relatively small sample size of the present study, although we do note that there is sufficient power in this sample to yield groups differences in intra- and inter-hemispheric connectivity. One likely explanation for this difference could be a developmental change – whereas our study involved adults with high functioning autism, the sample in Pugelise et al. included children and adults with Asperger syndrome. It is also possible, too, that, in addition to the sample size issue, there may be distinct subgroups within the autism population who have different structural connectivity profiles. For example, Alexander et al., (2007) found that in their sample of 43 individuals with autism, only 12 showed a significant reduction in FA relative to the control group. It is, possible then, that the absence of a clear reduction in FA in any of the WM tracts that we focused here might be attributable to particularities of the individuals included in this sample. Finally, in this study, we limited our examination of the structural integrity of WM tracts to the corpus callosum and the three intra-hemispheric visual association tracts. A complete investigation of other fiber systems would help clarify whether there are reductions in other WM tracts that were not examined here.
Our findings shed new light on the nature of the relationship between increased structural connectivity and decreased functional connectivity in autism. Traditionally, a reduction in structural connectivity is assumed to give rise to abnormal function and behavior. Consistent with this expectation, reductions in structural connectivity have been found to account for poor face processing due to aging (Thomas et al., 2008), as well as in patients with white matter disorders, such as multiple sclerosis (Yamasaki et al., 2004). Accordingly, our data suggests that a reduction in connectivity in the F-Mi might contribute to an abnormal increase in repetitive behavior in the autism group. However, the autism group also shows an atypical increase in connectivity in the visual association tracts in the left hemisphere compared to the control group. Although the behavioral correlate of this increase in white matter remains to be established, our findings suggest that both reductions and increases in WM connectivity can result in deficits in information processing. Such alterations in connectivity might give rise to a reduction in functional connectivity by increasing greater cross-talk or noise propagation between spatially distant cortical regions (Dinstein et al., 2010).
Before concluding, a few caveats are warranted. The present study represents a preliminary step toward exploring alterations in the structural connectivity in a select group of inter- and intra-hemispheric tracts in individuals with HFA. Replicating these findings with a larger sample, using sophisticated diffusion imaging techniques will confirm whether the connectivity profile reported here constitutes a part of the neural phenotype of autism. Assuming that this is the case, understanding the origin of the white matter alterations will be critical. The increase in density and volume of the tracts in the HFA group need not necessarily reflect a premorbid alteration in connectivity. Although hemispherical asymmetries are inherited (Geschwind et al., 2002), alterations in white matter structure, particularly with regard to myelination, may be influenced by environmental factors (Barres et al., 1993), too. A final issue concerns the technical limitations of diffusion tensor tractography. DTT does not trace fibers the same way radioactive tracers are used to establish connectivity maps. Thus, the absolute values yielded by DTT, such as streamlines and volume may not directly map on to true anatomical calculations of fiber density and volume of a tract. Indeed, we do not make claims about the meaning of the absolute values of streamlines or voxels and, instead, place the emphasis on the relative group differences.
In conclusion, the present study reveals for the first time, that the major inter-hemispheric and intra-hemispheric visual association tracts are altered in individuals with high functioning autism. With the inter-hemispheric callosal fiber tracts, only the WM fibers that project through the genu and body of the corpus callosum appear to be significantly reduced in volume. However, reduced connectivity specifically through the genu of the callosum may contribute to an increase in repetitive behavior in the HFA individuals. With the intra-hemispheric tracts, a leftward- increase in the volume of the major visual-association WM tracts that project through ventral temporal cortex appears to be evident in HFA individuals. Taken together, the present study provides some provocative pointers that warrant further explorations of the relationship between structural connectivity and behavior in autism.
Table 3.
The mean and standard error of mean for the macrostructural (Number of streamlines and voxels) and microstructural measures (Mean FA) of the three visual association tracts (inferior longitudinal fasciculus (ILF), inferior fronto-occipito fasciculus (IFOF) and uncinate fasciculus (UF)) and the callosal tracts forceps minor (FMi), Body (streamlines projecting through the body of the corpus callosum) and forceps major (FMa). The absolute values for number of streamline and voxels as well as the same values normalized to whole brain WM volume (cells in grey) are presented here.
| Right Hemisphere | Left Hemisphere | Bilateral | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LF | IFOF | UF | Total | ILF | IFOF | UF | Total | F-Mi | Body | F-Ma | |
| HFA |
363.50
55.97 |
286.50
36.62 |
181.46
19.88 |
831.46
75.17 |
519.83
81.63 |
251.75
48.12 |
163.42
15.41 |
935.00
*
111.32 |
1080.50
*
73.62 |
1933.42
*
204.80 |
1164.92
82.71 |
| Controls |
398.89
30.73 |
249.72
34.80 |
219.18
26.37 |
855.61
43.62 |
421.28
33.41 |
158.67
29.19 |
183.76
27.21 |
753.50
46.97 |
1288.39
61.10 |
2746.67
164.16 |
1198.33
74.29 |
| HFA |
1218.50
156.92 |
988.75
89.01 |
573.33
48.58 |
2700.58
220.41 |
1469.50
252.44 |
964.83
103.85 |
591.42
53.07 |
3025.75
*
302.03 |
2715.00
*
129.25 |
5437.58
*
350.09 |
3167.42
190.89 |
| Controls |
1310.43
60.93 |
933.89
104.29 |
752.62
81.99 |
2955.53
150.35 |
1233.06
65.89 |
741.06
97.58 |
746.56
89.77 |
2679.19
141.37 |
3118.50
120.73 |
7119.11
246.72 |
3182.83
108.54 |
| HFA |
0.40
0.01 |
0.47
0.01 |
0.36
0.00 |
0.42
0.01 |
0.46
0.01 |
0.37
0.00 |
0.50
0.01 |
0.50
0.01 |
0.53
0.01 |
||
| Controls |
0.42
0.01 |
0.46
0.01 |
0.37
0.01 |
0.42
0.00 |
0.45
0.01 |
0.38
0.01 |
0.52
0.01 |
0.51
0.01 |
0.54
0.01 |
||
| HFA |
0.65
0.10 |
0.50
0.05 |
0 33
0.04 |
1.48
0.10 |
0.43
0.07 |
0.43
0.07 |
0.30
0.03 |
1.17
*
0.14 |
1.94
0.10 |
3.55
*
0.40 |
2.08
0.09 |
| Controls |
0.68
0.06 |
0.42
0.06 |
0.35
0.05 |
1.45
0.09 |
0.27
0.05 |
0.27
0.05 |
0.30
0.05 |
0.84
0.12 |
2.16
0.08 |
4.63
0.27 |
2.04
0.15 |
| HFA |
1.53
0.17 |
1.24
0.07 |
0.73
0.06 |
3.50
0.17 |
1.79
0.20 |
1.21
0.11 |
0.77
0.08 |
3.77
*
0.21 |
3.45
0.10 |
6.99
*
0.50 |
4.00
0.13 |
| Controls |
1.57
0.08 |
1.11
0.12 |
0.85
0.10 |
3.53
0.18 |
1.47
0.08 |
0.89
0.12 |
0.86
0.12 |
3.22
0.19 |
3.70
0.11 |
8.52
0.33 |
3.80
0.14 |
Indicates significant group differences as per the Tukey post hoc test.
Acknowledgements
This research was funded by a grant from the NICHD/NIDCD PO1/U19 to Marlene Behrmann (PI: Nancy Minshew), which is part of the NICHD/NIDCD Collaborative Programs for Excellence in Autism and by awards from the National Alliance of Autism Research (Autism Speaks) to CT and KH and from the Cure Autism Now foundation to KH. We thank Scott Kurdilla and Debbie Viszlay of the Brain Imaging Research Center for their help in the acquisition of the imaging data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A Bonferroni correction at an alpha of .05 for the full set of comparisons we performed yields a corrected p value of .0014.
REFERENCES
- Alexander A, Lee J, Lazar M, Boudos R, DuBray M, Oakes T, Miller J, Lu J, Jeong E, McMahon W. Diffusion tensor imaging of the corpus callosum in autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Barkovich A, Kjos B. Normal postnatal development of the corpus callosum as demonstrated by mr imaging. American Journal of Neuroradiology. 1988;9:487. [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: Preliminary evidence from diffusion tensor imaging. Biological Psychiatry. 2004;55:323. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor mri. Journal of Magnetic Resonance. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Avidan G, Leonard GL, Kimchi R, Luna B, Humphreys K, Minshew N. Configural processing in autism and its relationship to face processing. Neuropsychologia. 2006a;44:110. doi: 10.1016/j.neuropsychologia.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Thomas C, Humphreys K. Seeing it differently: Visual processing in autism. Trends in cognitive sciences. 2006b;10:258–264. doi: 10.1016/j.tics.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain. 2005;128:2430. doi: 10.1093/brain/awh561. [DOI] [PubMed] [Google Scholar]
- Boger-Megiddo I, Shaw D, Friedman S, Sparks B, Artru A, Giedd J, Dawson G, Dager S. Corpus callosum morphometrics in young children with autism spectrum disorder. Journal of autism and developmental disorders. 2006;36:733–739. doi: 10.1007/s10803-006-0121-2. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–49. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Daly E, Embiricos N, Deeley Q, Pugliese L, Curran S, Robertson D, Murphy DGM. Altered cerebellar feedback projections in asperger syndrome. Neuroimage. 2008a;41:1184. doi: 10.1016/j.neuroimage.2008.03.041. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex. 2008b;44:953–961. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008c;44:1105. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Chiang M, Barysheva M, Shattuck D, Lee A, Madsen S, Avedissian C, Klunder A, Toga A, McMahon K, de Zubicaray G. Genetics of brain fiber architecture and intellectual performance. Journal of Neuroscience. 2009;29:2212. doi: 10.1523/JNEUROSCI.4184-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Thomas C, Humphreys K, Minshew N, Behrmann M, Heeger D. Normal movement selectivity in autism. Neuron. 2010;66:461–469. doi: 10.1016/j.neuron.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty RF, Ben-Shachar M, Bammer R, Brewer AA, Wandell BA. Functional organization of human occipital-callosal fiber tracts. Proceedings of the National Academy of Sciences. 2005;102:7350–7355. doi: 10.1073/pnas.0500003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan D. Inferotemporal-frontal disconnection: The uncinate fascicle and visual associative learning in monkeys. European Journal of Neuroscience. 1992;4:1320–1320. doi: 10.1111/j.1460-9568.1992.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Frazier T, Hardan A. A meta-analysis of the corpus callosum in autism. Biological Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind D, Miller B, Decarli C, Carmelli D. Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3176. doi: 10.1073/pnas.052494999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: A longitudinal mri study. Nature Neuroscience. 1999;2:861–862. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Happé F, Frith U. The weak coherence account: Detail-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2006;36:5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Hardan A, Minshew N, Keshavan M. Corpus callosum size in autism. Neurology. 2000;55:1033. doi: 10.1212/wnl.55.7.1033. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, Sanders HA, Kennedy DN, Caviness VS. Localization of white matter volume increase in autism and developmental language disorder. Annals of Neurology. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang JY, van Zijl PCM, Mori S. Analysis of noise effects on dti-based tractography using the brute-force and multi-roi approach. Magnetic Resonance in Medicine. 2004;52:559–565. doi: 10.1002/mrm.20147. [DOI] [PubMed] [Google Scholar]
- Humphreys K, Hasson U, Avidan G, Minshew NJ, Behrmann M. Cortical patterns of category-selective activation for faces, places & objects in adults with autism. Autism Research. 2008;1:52–63. doi: 10.1002/aur.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HY, van Zijl PCM, Kim J, Pearlson GD, Mori S. Dtistudio: Resource program for diffusion tensor computation and fiber bundle tracking. Computer Methods And Programs In Biomedicine. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Jones DK, Symms MR, Cercignani M, Howard RJ. The effect of filter size on vbm analyses of dt-mri data. Neuroimage. 2005;26:546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: Evidence from an fmri study of an executive function task and corpus callosum morphometry. Cerebral Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18:23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: Resting functional abnormalities in autism. PNAS. 2006;103:8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan M, Diwadkar V, DeBellis M, Dick E, Kotwal R, Rosenberg D, Sweeney J, Minshew N, Pettegrew J. Development of the corpus callosum in childhood, adolescence and early adulthood. Life Sciences. 2002;70:1909–1922. doi: 10.1016/s0024-3205(02)01492-3. [DOI] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Human Brain Mapping. 2009:30. doi: 10.1002/hbm.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK, Johnson M, Morgan J, Miller JN, McMahon WM, Lu J, Jeong E-K, Lainhart JE. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neuroscience Letters. 2007a;424:127. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Lee PS, Foss-Feig J, Henderson JG, Kenworthy LE, Gilotty L, Gaillard WD, Vaidya CJ. Atypical neural substrates of embedded figures task performance in children with autism spectrum disorder. Neuroimage. 2007b;38:184. doi: 10.1016/j.neuroimage.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of autism and developmental disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism diagnostic observation schedule. Western Psychological Services; Los Angeles, CA: 1999. [Google Scholar]
- McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY, Tai KS, Yip L, Murphy DGM, Chua SE. Mapping the brain in autism. A voxel-based mri study of volumetric differences and intercorrelations in autism. Brain. 2005;128:268–276. doi: 10.1093/brain/awh332. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PCM. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Nucifora PGP, Verma R, Melhem ER, Gur RE, Gur RC. Leftward asymmetry in relative fiber density of the arcuate fasciculus. Neuroreport. 2005;16:791–794. doi: 10.1097/00001756-200505310-00002. [DOI] [PubMed] [Google Scholar]
- Nyden A, Carlsson M, Carlsson A, Gillberg C. Interhemispheric transfer in high-functioning children and adolescents with autism spectrum disorders: A controlled pilot study. Developmental Medicine And Child Neurology. 2004;46:448–454. doi: 10.1017/s001216220400074x. [DOI] [PubMed] [Google Scholar]
- Piven J, Bailey J, Ranson B, Arndt S. An mri study of the corpus callosum in autism. American Journal of Psychiatry. 1997;154:1051. doi: 10.1176/ajp.154.8.1051. [DOI] [PubMed] [Google Scholar]
- Pugliese L, Catani M, Ameis S, Dell'Acqua F, de Schotten M, Murphy C, Robertson D, Deeley Q, Daly E, Murphy D. The anatomy of extended limbic pathways in asperger syndrome: A preliminary diffusion tensor imaging tractography study. Neuroimage. 2009;47:427–434. doi: 10.1016/j.neuroimage.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Rakic P, Yakovlev P. Development of the corpus callosum and cavum septi in man. The Journal of Comparative Neurology. 1968;132:45–72. doi: 10.1002/cne.901320103. [DOI] [PubMed] [Google Scholar]
- Robertson LC, Ivry R. Hemispheric asymmetries: Attention to visual and auditory primitives. Current Directions in Psychological Science. 2000;9:59–63. [Google Scholar]
- Rudrauf D, David O, Lachaux J-P, Kovach CK, Martinerie J, Renault B, Damasio A. Rapid interactions between the ventral visual stream and emotion-related structures rely on a two-pathway architecture. J. Neurosci. 2008;28:2793–2803. doi: 10.1523/JNEUROSCI.3476-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J, Pandya D. Fiber pathways of the brain. Oxford Univ Pr: 2009. [Google Scholar]
- Sundaram S, Kumar A, Makki M, Behen M, Chugani H, Chugani D. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cerebral Cortex. 2008 doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar K, Polli F, Joseph R, Tuch D, Hadjikhani N, Barton J, Manoach D. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in asd. Brain. 2008 doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Avidan G, Humphreys K, Jung K, Gao F, Behrmann M. Reduced structural connectivity in ventral visual cortex in congenital prosopagnosia. Nat Neurosci. 2009;12:29–31. doi: 10.1038/nn.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Moya L, Avidan G, Humphreys K, Jung K, Peterson M, Behrmann M. Reduction in white matter connectivity, revealed by diffusion tensor imaging, may account for age-related changes in face perception. Journal of Cognitive Neuroscience. 2008;20:268–284. doi: 10.1162/jocn.2008.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal C, Nicolson R, DeVito T, Hayashi K, Geaga J, Drost D, Williamson P, Rajakumar N, Sui Y, Dutton R. Mapping corpus callosum deficits in autism: An index of aberrant cortical connectivity. Biological Psychiatry. 2006;60:218–225. doi: 10.1016/j.biopsych.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Muller RA. Reduced functional connectivity between v1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage. 2005;25:916–925. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waiter GD, Williams JHG, Murray AD, Gilchrist A, Perrett DI, Whiten A. Structural white matter deficits in high-functioning individuals with autistic spectrum disorder: A voxel-based investigation. Neuroimage. 2005;24:455–461. doi: 10.1016/j.neuroimage.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang HY, Nagae-Poetscher LM, van Zijl P, Mori S. Fiber tract–based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Westin C-F, Maier SE, Mamata H, Nabavi A, Jolesz FA, Kikinis R. Processing and visualization for diffusion tensor mri. Medical Image Analysis. 2002;6:93–108. doi: 10.1016/s1361-8415(02)00053-1. [DOI] [PubMed] [Google Scholar]
- Xue R, van Zijl PCM, Crain BJ, Solaiyappan M, Mori S. In vivo three-dimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magnetic Resonance in Medicine. 1999;42:1123–1127. doi: 10.1002/(sici)1522-2594(199912)42:6<1123::aid-mrm17>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Yamasaki T, Taniwaki T, Tobimatsu S, Arakawa K, Kuba H, Maeda Y, Kuwabara Y, Shida K, Ohyagi Y, Yamada T, Kira JI. Electrophysiological correlates of associative visual agnosia lesioned in the ventral pathway. Journal of the Neurological Sciences. 2004;221:53–60. doi: 10.1016/j.jns.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Yovel G, Yovel I, Levy J. Hemispheric asymmetries for global and local visual perception: Effects of stimulus and task factors. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:1369–1385. [PubMed] [Google Scholar]