Abstract
Decrease in the level of high density lipoprotein cholesterol (HDLC) has been observed in women who start dieting, but not in men. Patterns of HDLC change during intentional weight loss through 30-months of follow-up, and their association with changes in anthropometric measurements were examined in obese women (N=112) and men (N=100). Missing HDLC values at 6-, 12-, 18-, and 30-month follow-up (N=16, 34, 55, and 50, respectively) due to drop-out were imputed by multiple imputation. Mean ages and body mass indices (BMIs) of subjects at baseline were 47.2 years and 34.8 kg/m2 for women, and 50.4 years and 35.0 kg/m2 for men. On average, participants lost weight steadily for 12 months, followed by slow regain. During the first six months, HDLC decreased significantly in women (−4.1 mg/dl, P=0.0007), but not in men. Significant HDLC increases were observed in both men and women from 6- to 12-month follow-up. HDLC changes in women were positively associated with changes in hip circumference from baseline to 12-month independent of changes in triglycerides, glucose and insulin. Rapid decrease of predominantly subcutaneous fat in the femoral and gluteal area might be associated with HDLC decrease in women during initial weight loss.
Keywords: Obesity, Body Fat Distribution, Women, Lipids, Weight Change
INTRODUCTION
Low level of blood high density lipoprotein cholesterol (HDLC) is a major component of atherogenic dyslipidemia (1). It is an independent predictor of cardiovascular disease (2), and pre- and postmenopausal breast cancer (3, 4). Although it has consistently been associated with obesity in cross-sectional studies, findings remain inconsistent about whether weight loss within individuals leads to a sustained increase in HDLC or not (5). One previous study observed an apparently contradictory decrease of blood HDLC level in obese women after a 10-week weight loss program, which returned to pretreatment levels at 10 months follow-up (6). Another study with longer follow-up (18 months) revealed that women, but not men, who had successfully lost weight and sustained the weight loss experienced continuous increases in HDLC levels even though their weight had stayed the same (7). These observations in women warranted further study with longer follow-up to examine the course of HDLC change beyond 18 months in men and women separately and to explore possible mechanisms underlying sex-specific pattern of HDLC change by incorporating other biomarkers. Associations between changes in anthropometric variables and HDLC should also be examined since each anthropometric variable may have specific association with HDLC (8).
In this study, we followed obese men and women who were attempting weight loss for 30 months. We hypothesized that HDLC would initially decrease after weight loss in women but not in men, and would increase after the initial loss in women. Since independent and opposite associations of waist and hip circumference with HDLC have been observed in a cross-sectional study of men and women (8), we also hypothesized that change in waist circumference would be inversely associated with HDLC change whereas change in hip circumference would be positively associated with HDLC change.
METHODSAND PROCEDURES
LIFE Study participants
The present analyses were done using data collected in a randomized trial (the Lose It For Ever: LIFE Study) that was designed to compare two weight loss strategies. The details of the trial have been described elsewhere (9). Briefly, the LIFE Study recruited obese men and women aged 18 years or older with a body mass index (BMI) 30 to 39 kg/m2 in 2004 and 2005. The upper BMI cut point was used because of concern about the ability of very obese persons to comply with physical activity goals. The primary goal of the trial was to evaluate the effectiveness of maintenance-tailored treatment (MTT) compared to standard behavioral treatment (SBT) in maintaining lost weight long term. Content for the SBT group was modeled after prior work of the investigators (10–12) and closely resembled that used in many recent successful clinical trials, such as those by the Diabetes Prevention Program (13) and the Look AHEAD Research Group (14). MTT received the same number of sessions as SBT but the therapy in the MTT group emphasized variety in both format and content in order to reduce habituation and boredom with the weight loss regimen. Intervention lasted for 18 months for both groups. Subjects were excluded if they met the following criteria: current use of weight-loss medications or participation in another organized weight loss program; history or presence of cancer, cardiovascular disease, diabetes, chronic fatigue, arthritis, or fibromyalgia; inability to walk at least 10 minutes without stopping; current pharmacologic or behavioral treatment for a major psychological disorder; and current use of a thyroid hormone. In addition, women were excluded if they were pregnant, < 6 months postpartum, breastfeeding, or planning to become pregnant in the ensuing 30 months. Participants were also required to participate in either study group. Of 994 individuals screened for the trial, 781 were not included due to their body weight (N=564) and lack of interest or other reasons (N=117), leaving 213 subjects included in the trial. We did not exclude 25 participants who reported cholesterol lowering medication use at baseline since neither HDLC level at baseline nor HCLC changing pattern over time differed by its use. The study protocol was approved by the Institutional Review Board of the University of Minnesota. Although the time pattern of weight change differed significantly between MTT and SBT as previously reported (9), associations between changes in anthropometric variables and HDLC were not significantly different by groups (interaction p>0.10 for all follow-up intervals (baseline to 6-month, 6- to 12-month, 12- to 18-month, and 18- to 30-month). Therefore, the data were treated as an observational study, collapsing across assigned treatment conditions for the present analyses.
Anthropometric and laboratory examination
Participants had BMI, other anthropometric measures and HDLC assessed at baseline, 6-, 12-, 18- and 30-months during the study. BMI (kg/m2) was calculated from weight (kg) and height (cm) measured in light clothing, without shoes (Tanita BWB 800, Tanita corp., Arlington Heights, IL). Waist circumference (cm) was measured at a point equidistant from the iliac crest and the twelfth rib, and hip circumference (cm) was measured at the level of the greater trochartus with a steel measuring tape. Waist-to-hip ratio (WHR) was calculated as a measure of central adiposity.
Fasting blood samples were drawn by a certified phlebotomist at each visit. The serum samples were stored at minus 70 °C until study completion. At that time, high density lipoprotein cholesterol level and triglycerides (TG) (mg/dl) were measured by Fairview Diagnostics Laboratory using the Roche HDL-Cholesterol third generation direct method and Trig/GB (glycerol-blanking) kit (Roche Diagnostics, Indianapolis, IN) on a Roche Modular P Chemistry Analyzer. These methods are standardized against the designated CDC reference method; and calibration of the assay is regularly monitored by the CDC/NHLBI Lipid Standardization Program. The laboratory CV is 2.9 percent for HDLC and 2.0 % (low concentration) and 2.7% (high concentration) for TG. Glucose measurements were also performed on the Roche Modular P Chemistry Analyzer using the Roche Hexokinase Assay. Insulin level was measured by enzyme-linked immunosorbent assays (ELISA) (Mercodia AB, Uppsala, Sweden).
Questionnaire-based assessment
Questionnaires were used at baseline to assess educational level, job and marital status, cigarette smoking, medication use for heart disease, high blood pressure, and high cholesterol, and previous participation in a formal dieting program.
Usual nutritional and alcohol intake of the participants in the previous six month at baseline and at each successive visit were assessed using a 62-item Block Food Frequency Questionnaire (FFQ) (15, 16). We calculated daily total energy intake (kcal), alcohol intake (g), and intakes of total fat, carbohydorates and sweets as percentages of total energy (%E). The Paffenbarger Activity Questionnaire was used to estimate weekly energy expenditure (kcal) (17).
Statistical analyses
Of 213 participants in the trial, one without waist and hip circumference measurements, dietary and physical activity assessment at baseline was excluded, leaving 212 subjects (112 women and 100 men) for the present analysis. Blood HDLC levels were obtained in 212, 196, 178, 157, and 162 individuals at 0, 6, 12, 18, and 30 months, respectively. Missing values for HDLC as well as those for other repeated-measure variables due to drop out, were imputed using Multiple imputation (18), a strategy which replaces each missing value with a set of plausible values that represent the uncertainty about the right value to impute. Imputed variables include anthropometric (BMI, and waist and hip circumferences) and behavioral variables (nutritional and alcohol intake, and physical activity). Since the multiple imputation procedure was carried out under the assumption that missing data were missing at random, we performed completer analysis restricting to those with all HDLC measurements (n=147) as a sensitivity analysis.
Prior to the analyses, variables with skewed distributions (HDLC, TG, insulin, glucose, total energy and alcohol intake, % energy from sweets, and weekly energy expenditure) were logarithmically transformed to approximately normalize the distributions. For these variables, geometric means are presented along with the 95% confidence intervals. Anthropometric variables were divided by the standard deviation at baseline to be examined for the associations with HDLC change.
Pattern and significance of HDLC (or TG) change over time was examined by random coefficient model -- and both linear and quadratic slopes (i.e., month and month-squared), adjusted for baseline levels of age (continuous), race (white, black, others), education (graduate degree, college graduate, others), job (professional, clerical, blue collar/other job, not employed), marital status (married, others), medical history (heart disease, hypertension, high cholesterol), smoking status (never, past, current), previous participation in a formal dieting program (yes, no) treatment assignment (MTT, SBT), and repeated-measured glucose, insulin and TG (or HDLC). Similarly, pattern and significance of changes in anthropometric variables (BMI, waist and hip circumference) were also examined in random growth models allowing random intercept and slopes for month and month-squared.
Associations between change in HDLC and changes in the anthropometric variables were examined separately for each follow-up interval (baseline to 6-month, 6- to 12-month, 12- to 18-month, and 18- to 30-month) by a mixed effect model. In multivariate model 1, we adjusted for baseline levels of age, race, education, job, marital status, medical history, smoking status, previous participation of formal dieting program and treatment assignment as well as longitudinal measurements on total energy and alcohol intake, % energy from fat, carbohydorates, and sweet, and weekly energy expenditure (all continuous). In model 2, we included variables in model 1 plus all the anthropometric variables simultaneously. In model 3, we further included repeated-measure TG, glucose and insulin.
To see whether the pattern of HDLC change is different by baseline HDLC level, we performed an analysis stratified at the median of baseline HDLC in each sex (56 mg/dl in women and 45 mg/dl in men).
All the analyses were conducted separately for men and women using SAS 9.2 for Windows (19), and all reported P values are two-sided. PROC MI was used for Multiple imputation of missing values. Random growth models and mixed models were carried out by PROC MIXED. Summary estimates were derived by the combining the results of analyses carried out on the five imputed datasets with PROC MIANALYZE.
RESULTS
The mean age of subjects was 47.2 years for women and 50.4 years for men (Table 1). More than two-thirds of men and women reported attaining an education level of college graduation or more and most were currently employed. About half the women (52.7 percent) and 81 percent of men in the study were currently married. Approximately, 78.6 percent of women and 31.0 percent of men had been in previous formal dieting programs. Prevalence of current smoking in men and women was 8.9 percent and 2.0 percent, respectively.
Table 1.
Baseline characteristics (Mean, SD and Percentage) of participants according to sex, LIFE Study, 2005 (N=212)
| Women | Men | |
|---|---|---|
| (n=112) | (n=100) | |
| Age (y, mean (SD)) | 47.2 (10.1) | 50.4 (10.7) |
| Race (%) | ||
| White | 55.4 | 81.0 |
| Black | 32.1 | 14.0 |
| Others | 12.5 | 5.0 |
| Education (%) | ||
| Graduate degree | 33.0 | 31.0 |
| College graduate | 39.3 | 40.0 |
| Others | 27.7 | 29.0 |
| Marital status (%) | ||
| Married | 52.7 | 81.0 |
| Others | 47.3 | 19.0 |
| Job (%) | ||
| Professional | 57.1 | 59.0 |
| Clerical | 20.5 | 14.0 |
| Blue collar/other job | 10.7 | 18.0 |
| Not employed | 11.6 | 9.0 |
| Formal dieting program | ||
| Ever (%) | 78.6 | 31.0 |
| Current medical history (%) | ||
| Heart disease | 3.6 | 9.0 |
| Hypertension | 23.2 | 34.0 |
| High cholesterol | 7.1 | 22.0 |
| Smoking status (%) | ||
| Never | 65.2 | 57.0 |
| Past | 25.9 | 41.0 |
| Current | 8.9 | 2.0 |
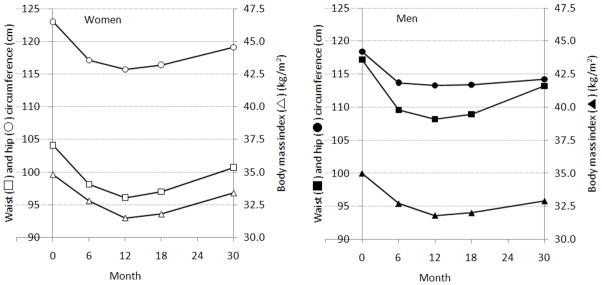
Mean BMIs at baseline were 34.8 kg/m2 in women and 35.0 kg/m2 in men (Table 2, PFigure 1). On average, weight decreased significantly and reached a nadir at 12-months. Weight increased thereafter in both men and women ( for month2 <0.0001). Pattern and significance of change in waist and hip circumference were similar to that of BMI in both men and women. In women, however, WHR at 30-month follow-up had returned to the baseline level, which possibly led to a non-significant linear term for women (P=0.052) but a significant quadratic term (P=0.0007).
Table 2.
Mean (SE) values of anthropometric variablesa during 30 months of follow-up, LIFE Study, 2005–2008 (N=212)
| Baseline | 6 months | 12 months | 18 months | 30 months | P (month)b | P (month2)b | ||
|---|---|---|---|---|---|---|---|---|
| Women | Body mass index (kg/m2) | 34.8 (0.35) | 32.8 (0.37) | 31.5 (0.41) | 31.8 (0.39) | 33.4 (0.41) | <.0001 | <.0001 |
| Waist (cm) | 104.1 (1.02) | 98.1 (1.05) | 96.1 (1.16) | 97.0 (1.08) | 100.7 (1.24) | <.0001 | <.0001 | |
| Hip (cm) | 123.0 (0.85) | 117.1 (0.89) | 115.7 (1.04) | 116.4 (1.00) | 119.1 (1.05) | <.0001 | <.0001 | |
| Waist-to-hip ratio | 0.85 (0.01) | 0.84 (0.01) | 0.83 (0.01) | 0.83 (0.01) | 0.85 (0.01) | 0.052 | 0.0007 | |
| Men | Body mass index (kg/m2) | 35.0 (0.34) | 32.7 (0.34) | 31.8 (0.38) | 32.0 (0.39) | 32.9 (0.38) | <.0001 | <.0001 |
| Waist (cm) | 117.2 (1.04) | 109.6 (1.05) | 108.2 (1.16) | 108.9 (1.13) | 111.3 (1.12) | <.0001 | <.0001 | |
| Hip (cm) | 118.4 (0.72) | 113.7 (0.73) | 113.3 (0.80) | 113.4 (0.77) | 114.2 (0.78) | <.0001 | <.0001 | |
| Waist-to-hip ratio | 0.99 (0.01) | 0.96 (0.01) | 0.95 (0.01) | 0.96 (0.01) | 0.97 (0.01) | <.0001 | <.0001 |
Each estimate is a summary estimate of five repeated analyses for five imputed dataset. Each analysis used a random effect model with random intercept for individual, adjusted for age, race, education, job, marital status, medical history, smoking status, previous participation of formal dieting program and treatment assignment.
P values are derived from a random growth model with random intercept for individual allowing random slope for time and time-squared.
Adjustment factors are same as above. Time in linear scale was used.
Figure 1.
Means of waist and hip circumferences and body mass index during 30-month follow-up adjusted for age, race, education, job, marital status, medical history, smoking status, previous participation of formal dieting program and treatment assignment, LIFE Study, 2004–2005 (n=212)
Open and solid square, circle, and triangle indicate waist circumference, hip circumference and body mass index for women and men, respectively.
Multivariate-adjusted self-reported total energy intake and fat and sweet intake decreased, and carbohydorates intake increased significantly in both men and women from baseline to 6-month follow-up (data not shown in table). Multivariate-adjusted self-reported physical activity significantly increased during the same period of time.
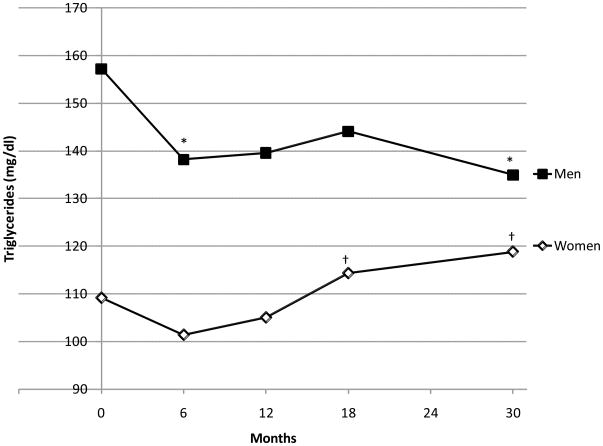
In women, multivariate-adjusted mean HDLC at 6-month follow-up was significantly lower than at baseline (Figure 2, open diamond). At 12-, 18-, and 30-month follow-up, HDLC was significantly higher than at 6-month follow-up. There were no significant differences between 12-, 18- and 30-month HDLC values. Despite the initial decrease in analysis models, only the term for a linear change over time was statistically significant. In men, HDLC did not decrease nor increase during the first six months (Figure 2). HDLCs in men at 12-, 18-, and 30-month follow-up were significantly higher than those at baseline or 6-month follow-up. However, there was a significant decrease in HDLC from 18 to 30 month follow-up in men (P=0.049). Accordingly, both positive linear and quadratic trends were significant in men (both P<0.001). HDLC decreased from 63.7 mg/dl at baseline to 57.2 mg/dl at 6-month in women with higher baseline HDLC (P=0.0006) and from 53.4 mg/dl to 51.9 mg/dl in women with lower baseline HDLC (P=0.11) (P for interaction=0.030). No significant change was observed in men even with higher baseline HDLC (from 53.4 mg/dl to 51.9 mg/dl, P=0.31). TG decreased from baseline to 6-month in both sexes (Figure 3). TG regained thereafter in women.
Figure 2.
Means of high density lipoprotein cholesterol level during 30-month follow-up adjusted for age, race, education, job, marital status, medical history, smoking status, previous participation of formal dieting program treatment assignment, and serum levels of triglycerides, glucose and insulin, LIFE Study, 2004–2005 (n=212)
Open diamond indicates women and solid square, men.
*: Difference from Baseline is statistically significant by Tukey-Kramer adjustment (p<0.05)
†: Difference from 6-month follow-up is statistically significant by Tukey-Kramer adjustment (p<0.05)
‡: Difference from 18-month follow-up is statistically significant by Tukey-Kramer adjustment (p<0.05)
Figure 3.
Means of triglycerides level during 30-month follow-up adjusted for age, race, education, job, marital status, medical history, smoking status, previous participation of formal dieting program, treatment assignment, and serum levels of triglycerides, glucose and insulin, LIFE Study, 2004–2005 (n=212)
Open diamond indicates women and solid square, men.
*: Difference from Baseline is statistically significant by Tukey-Kramer adjustment (p<0.05)
†: Difference from 6-month follow-up is statistically significant by Tukey-Kramer adjustment (p<0.05)
In women, although change in hip circumference from baseline to 6-months was not associated with decrease in HDLC in multivariate model 1, a significant positive association was observed after simultaneous adjustment for BMI and waist circumference (model 2 in Table 3, P=0.041). The significant association remained after further adjustment for TG, glucose, and insulin (P=0.013). From 6- to 12-months increase in HDLC was inversely associated with decrease in BMI and waist circumference. Although HDLC increased overall from 6- to 12-months, decrease in hip circumference during the same period was associated with reduction in HDLC in a model that simultaneously adjusted for changes in BMI and waist circumference (P=0.049 in model 2 and P=0.031 in model 3). On average, change in BMI or waist or hip circumference from 12- to 30-months was not associated with HDLC change independent of TG, glucose, and insulin change during the same period.
Table 3.
Associations of changes in HDL cholesterol with changes in anthropometric variables between each consecutive visit, LIFE Study, 2005–2008 (n=212)
| Sex | Anthropometric variable | From baseline to 6-month |
From 6-month to 12-month |
From 12-month to 18-month |
From 18-month to 30 month |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |||
| Women | Body mass index | Model 1 | −1.39 | 0.20 | −2.30 | 0.036 | −1.98 | 0.062 | −3.81 | 0.001 |
| Model 2 | −1.83 | 0.075 | −2.53 | 0.020 | −1.58 | 0.11 | −1.87 | 0.063 | ||
| Model 3 | −3.41 | 0.007 | −2.31 | 0.026 | 0.42 | 0.68 | −0.27 | 0.79 | ||
| Waist circumference | Model 1 | −1.04 | 0.32 | −1.98 | 0.077 | −2.54 | 0.028 | −3.31 | 0.008 | |
| Model 2 | −1.33 | 0.19 | −2.06 | 0.039 | −3.06 | 0.004 | −2.76 | 0.007 | ||
| Model 3 | −0.72 | 0.48 | −1.36 | 0.20 | −1.65 | 0.13 | −0.72 | 0.49 | ||
| Hip circumference | Model 1 | 0.13 | 0.90 | −0.44 | 0.67 | −0.69 | 0.50 | −3.04 | 0.003 | |
| Model 2 | 2.10 | 0.041 | 2.21 | 0.049 | 2.75 | 0.009 | 1.80 | 0.086 | ||
| Model 3 | 2.56 | 0.013 | 2.34 | 0.031 | 0.10 | 0.92 | −0.28 | 0.78 | ||
| Men | Body mass index | Model 1 | −2.00 | 0.046 | −3.25 | 0.007 | −2.91 | 0.015 | −2.94 | 0.012 |
| Model 2 | −2.08 | 0.037 | −2.79 | 0.006 | −2.07 | 0.051 | −1.84 | 0.089 | ||
| Model 3 | −0.78 | 0.44 | −1.65 | 0.12 | −0.76 | 0.47 | −1.44 | 0.16 | ||
| Waist circumference | Model 1 | −1.27 | 0.21 | −2.43 | 0.041 | −3.72 | 0.001 | −2.86 | 0.014 | |
| Model 2 | 0.23 | 0.82 | −0.10 | 0.92 | −0.11 | 0.91 | 0.59 | 0.56 | ||
| Model 3 | 0.23 | 0.82 | −0.20 | 0.84 | 0.55 | 0.60 | 0.33 | 0.74 | ||
| Hip circumference | Model 1 | −0.63 | 0.53 | −2.27 | 0.035 | −1.38 | 0.19 | −1.30 | 0.22 | |
| Model 2 | 1.04 | 0.30 | 1.32 | 0.19 | 0.84 | 0.41 | 0.33 | 0.75 | ||
| Model 3 | 0.54 | 0.59 | 1.10 | 0.28 | −0.82 | 0.44 | −0.56 | 0.59 | ||
HDL denotes high density lipoprotein.
Standardized coefficient (β) was derived from random effects model using PROC MIXED in SAS with unstructured covariance matrix.
Model 1adjusted for race, education, job, marital status, histories of heart disease, high blood pressure, and high cholesterol, smoking status, previous participation in a formal dieting program, and treatment assignment as well as changes in total energy and alcohol intake, % energy energy intakes from fat, carbohydorates, and sweet, and weekly energy expenditure.
Model 2 includes variable in Model 1 plus all the anthropometric variables simultaneously.
Model 3 includes variable in Model 2 plus triglycerides, glucose and insulin.
Both HDL cholesterol level and anthropometric variables were divided by one standard deviation of its baseline measurement.
HDL cholesterol, triglycerides, glucose, insulin, total energy intake, physical activity, alcohol and sweet intake were logarithmically transformed for their skewed distribution.
Analyses were done separately for men and women, and values in the table are summary estimates of five results using five different imputed datasets, derived from PROC MIANALYZE in SAS.
In men, there was an indication that declines in BMI were associated with increases in HDLC (P=0.046 in model 1 and P=0.037 in model 2) in spite of no significant change in HDLC during the first six months at the agreed significance level. BMI decrease from 6- to 12-months was significantly associated HDLC increase. However, the association of BMI with HDLC was totally mediated by changes in other biomarkers (P=0.44 from baseline to 6-month and P=0.12 from 6- to 12-month in model 3).
Analysis limiting to subjects who had all the HDLC measurement did not materially change the results.
DISCUSSION
The present confirmed previous observations that HDLC decreases during the first six months of weight loss in women prior to an upturn thereafter. The decline was more prominent and statistically significant only in women who had higher HDLC at baseline. The achieved HDLC level remained higher than the 6-month level long-term, despite moderate BMI rebound. In men, we have also confirmed that HDLC did not decrease nor increase during the first six months of weight loss even though weight loss was substantial. Increase in the level of HDLC was observed after six months in men and it remained high, but decreased significantly after 18 months, when significant weight regain was observed. These changes were independent of concomitant changes in TG, glucose and insulin.
From baseline to 12-month follow-up when mean BMI, waist and hip circumference continued to decrease, the changes in hip circumference were positively associated with HDLC in women. This would be in line with a cross-sectional study that found an independent positive association of hip circumference with HDLC (8). Although the finding was accounted for the changes in insulin, glucose and TG, there are several possible mechanisms. First, subcutaneous fat mass in the femoral or gluteal area, which is reflected in hip circumference (20), is probably protective (21, 22) against ectopic fat storage that leads to insulin resistance, and alterations in lipids and glucose metabolisms. Second, decrease in hip circumference may be related to muscle mass loss (20), which is also associated with insufficiency of the insulin effect that would eventually lead to metabolic syndrome. Third, it has been reported that subcutaneous fat loss was more pronounced in women than in men during intentional weight loss (23). Thus, within-individual variation in hip circumference in men might not have been enough to detect significant association with HDLC change. Finally, it would also be possible that the subcutaneous fat actually lost in women was not restricted to femoral or gluteal area directly measured as hip circumference but includes other parts of the body such as trunk and breast, making the impact of subcutaneous fat loss on HDLC level more obvious. Although it is possible that rapid decrease in subcutaneous fat mass in women may be associated with temporal deterioration of lipid metabolism, whether health risks exist or not in association with temporally decreased level of HDLC would need detailed assessment of HDLC function and size (24, 25).
As hypothesized (8, 26, 27), HDLC change was inversely associated with change in waist circumference in women. Waist circumference is a measure of abdominal obesity and is reportedly correlated with both visceral fat and abdominal subcutaneous fat (28, 29). It also has a weak correlation with abdominal lean tissue mass (20, 26). Although it is less clear whether abdominal subcutaneous fat has protective or deteriorative effect on HDLC level in women (26–28, 30), visceral fat has been associated consistently and strongly with low HDLC level especially in women (26–28), a finding in line with ours. We also revealed that waist circumference association with HDLC was totally mediated by TG, glucose and insulin.
On the other hand in men, only BMI change had an independent association with HDLC change which was totally mediated by changes in other biomarkers, suggesting that measurements of waist and hip circumference may not provide significant information above that obtained from BMI in the present sample. However, this does not mean that there were no roles of waist or hip circumference in men since BMI and these anthropometric measures were highly correlated (r=075 for waist and r=0.72 for hip circumference at baseline, both P<0.0001) as well as the fact that analysis excluding BMI yielded significant association between changes in HDLC and waist circumference (significant inverse association from 6- to 12-month and 12- to 18-month follow-up). In light of the previous finding that men in a weight loss program tended to lose more visceral fat and less subcutaneous fat (23), weight (BMI) change could be a useful single measurement in such settings.
There are several limitations in the present study. First, we have only obtained several anthropometric measurements, making it impossible to classify fat from lean tissue or subcutaneous from visceral fat. In order to understand precise physiological mechanisms under the present findings, further studies with such measurements would be meaningful. Second, it is known that HDLC is a class of lipoprotein particles with different sizes and functions (24). Thus, we should be careful in interpreting our finding of significant HDLC decrease in women in the first six months so as not to directly judge it as hazardous until detailed understanding of change in HDL size and function is available. Generalizability of the present finding to nonobse population or extremely obese patients may be limited since subjects were participants of voluntary weight loss program whose BMI ranged between 30 and 39. Furthermore, there were not enough subjects to carry out race-specific analysis. Fourth, we did not obtain information on menopause. But supplemental analysis in women stratified by 50 years of age did not show any remarkable differences by age-groups. Finally, although we have used imputed datasets and did sensitivity analysis on completers, about one-third of participants dropped out, leaving a possibility of potential bias in the observed pattern of HDLC change or its association with anthropometrics. However, it would be less likely that association between anthropometric variables and HDCL differ physiologically by completer and non-completer.
Strengths of the present study include the use of an unusual dataset with both men and women, well represented, carefully standardized and detailed assessment of anthropometric and behavioral variables during the course of long follow-up, and clinically significant weight losses. We have employed multiple imputation techniques for missing values to maintain statistical power which is a novel statistical approach.
Implications of the present finding are potentially different roles of fat depots especially in women, and needs for studies to find behavioral determinants of the respective changes in the anthropometric variables (1). Analyses on the possible mediating biomarkers such as sex hormone-binding globulin (SHBG), insulin, or adipocytokines, are also warranted to understand mechanisms of sex difference in the pattern of HDLC change and the association with anthropometrics (31, 32). Since there may be differences in the associations between pre and postmenopausal women, future studies stratifying by menopausal status are also necessary.
In conclusion, HDLC did decrease during the first six months of weight loss in women, and the decrease was associated with reduction in the hip circumference. In both men and women, HDLC increased after 6-month follow-up, which was associated with waist circumference decrease in women, and BMI decrease in men. Pathophysiological significance of initial decrease in women is not known, but long-term weight loss and the maintenance is important for cardiovascular health and cancer prevention.
Acknowledgments
This research was supported by grant DK064596 from the National Institute of Diabetes and Digestive and Kidney Diseases, grant CA116849 from the National Cancer Institute, and the University of Minnesota Obesity Prevention Center.
This research was supported by grant DK064596 from the National Institute of Diabetes and Digestive and Kidney Diseases, grant CA 116849 from the National Cancer Institute, and the University of Minnesota Obesity Prevention Center. The authors thank the staff and participants of the LIFE study for their important contributions. The author (HY) is also grateful to the Uehara Memorial Foundation which supported his research activities at Division of Epidemiology and Community Health, School of Public Health, University of Minnesota.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
Trial registration: NCT00670462
References
- 1.Mooradian AD, Haas MJ, Wehmeier KR, Wong NC. Obesity-related changes in high-density lipoprotein metabolism. Obesity (Silver Spring) 2008;16:1152–60. doi: 10.1038/oby.2008.202. [DOI] [PubMed] [Google Scholar]
- 2.McNeill AM, Rosamond WD, Girman CJ, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28:385–90. doi: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- 3.Kucharska-Newton AM, Rosamond WD, Mink PJ, Alberg AJ, Shahar E, Folsom AR. HDL-cholesterol and incidence of breast cancer in the ARIC cohort study. Ann Epidemiol. 2008;18:671–7. doi: 10.1016/j.annepidem.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furberg AS, Veierod MB, Wilsgaard T, Bernstein L, Thune I. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J Natl Cancer Inst. 2004;96:1152–60. doi: 10.1093/jnci/djh216. [DOI] [PubMed] [Google Scholar]
- 5.Poobalan A, Aucott L, Smith WC, et al. Effects of weight loss in overweight/obese individuals and long-term lipid outcomes--a systematic review. Obes Rev. 2004;5:43–50. doi: 10.1111/j.1467-789x.2004.00127.x. [DOI] [PubMed] [Google Scholar]
- 6.Thompson PD, Jeffery RW, Wing RR, Wood PD. Unexpected decrease in plasma high density lipoprotein cholesterol with weight loss. Am J Clin Nutr. 1979;32:2016–21. doi: 10.1093/ajcn/32.10.2016. [DOI] [PubMed] [Google Scholar]
- 7.Wing RR, Jeffery RW. Effect of modest weight loss on changes in cardiovascular risk factors: are there differences between men and women or between weight loss and maintenance? Int J Obes Relat Metab Disord. 1995;19:67–73. [PubMed] [Google Scholar]
- 8.Snijder MB, Zimmet PZ, Visser M, Dekker JM, Seidell JC, Shaw JE. Independent and opposite associations of waist and hip circumferences with diabetes, hypertension and dyslipidemia: the AusDiab Study. Int J Obes Relat Metab Disord. 2004;28:402–9. doi: 10.1038/sj.ijo.0802567. [DOI] [PubMed] [Google Scholar]
- 9.Jeffery RW, Levy RL, Langer SL, et al. A comparison of maintenance-tailored therapy (MTT) and standard behavior therapy (SBT) for the treatment of obesity. Prev Med. 2009 doi: 10.1016/j.ypmed.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeffery RW, Bjornson-Benson WM, Rosenthal BS, Lindquist RA, Kurth CL, Johnson SL. Correlates of weight loss and its maintenance over two years of follow-up among middle-aged men. Prev Med. 1984;13:155–68. doi: 10.1016/0091-7435(84)90048-3. [DOI] [PubMed] [Google Scholar]
- 11.Jeffery RW, Wing RR, Thorson C, et al. Strengthening behavioral interventions for weight loss: a randomized trial of food provision and monetary incentives. J Consult Clin Psychol. 1993;61:1038–45. doi: 10.1037//0022-006x.61.6.1038. [DOI] [PubMed] [Google Scholar]
- 12.Jeffery RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? Am J Clin Nutr. 2003;78:684–9. doi: 10.1093/ajcn/78.4.684. [DOI] [PubMed] [Google Scholar]
- 13.Rubin RR, Fujimoto WY, Marrero DG, et al. The Diabetes Prevention Program: recruitment methods and results. Control Clin Trials. 2002;23:157–71. doi: 10.1016/s0197-2456(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 14.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–69. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 16.Wirfalt AK, Jeffery RW, Elmer PJ. Comparison of food frequency questionnaires: the reduced Block and Willett questionnaires differ in ranking on nutrient intakes. Am J Epidemiol. 1998;148:1148–56. doi: 10.1093/oxfordjournals.aje.a009599. [DOI] [PubMed] [Google Scholar]
- 17.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–75. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 18.Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley and Sons, Inc; New York: 1987. [Google Scholar]
- 19.SAS/STAT(R) 9. User’s Guide. 2009 http://support.sas.com/documentation/index.html (2009)
- 20.Snijder MB, Dekker JM, Visser M, et al. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care. 2004;27:372–7. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 21.van Harmelen V, Dicker A, Ryden M, et al. Increased lipolysis and decreased leptin production by human omental as compared with subcutaneous preadipocytes. Diabetes. 2002;51:2029–36. doi: 10.2337/diabetes.51.7.2029. [DOI] [PubMed] [Google Scholar]
- 22.Bos G, Snijder MB, Nijpels G, et al. Opposite contributions of trunk and leg fat mass with plasma lipase activities: The Hoorn Study. Obes Res. 2005;13:1817–23. doi: 10.1038/oby.2005.221. [DOI] [PubMed] [Google Scholar]
- 23.Wirth A, Steinmetz B. Gender differences in changes in subcutaneous and intra-abdominal fat during weight reduction: an ultrasound study. Obes Res. 1998;6:393–9. doi: 10.1002/j.1550-8528.1998.tb00370.x. [DOI] [PubMed] [Google Scholar]
- 24.Santos-Gallego CG, Ibanez B, Badimon JJ. HDL-cholesterol: is it really good? Differences between apoA-I and HDL. Biochem Pharmacol. 2008;76:443–52. doi: 10.1016/j.bcp.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Pascot A, Lemieux I, Bergeron J, et al. HDL particle size: a marker of the gender difference in the metabolic risk profile. Atherosclerosis. 2002;160:399–406. doi: 10.1016/s0021-9150(01)00579-2. [DOI] [PubMed] [Google Scholar]
- 26.Snijder MB, Visser M, Dekker JM, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48:301–8. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- 27.Piche ME, Lapointe A, Weisnagel SJ, et al. Regional body fat distribution and metabolic profile in postmenopausal women. Metabolism. 2008;57:1101–7. doi: 10.1016/j.metabol.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Oka R, Miura K, Sakurai M, et al. Impacts of Visceral Adipose Tissue and Subcutaneous Adipose Tissue on Metabolic Risk Factors in Middle-aged Japanese. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.180. [DOI] [PubMed] [Google Scholar]
- 29.Pou KM, Massaro JM, Hoffmann U, et al. Patterns of abdominal fat distribution: the Framingham Heart Study. Diabetes Care. 2009;32:481–5. doi: 10.2337/dc08-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammed BS, Cohen S, Reeds D, Young VL, Klein S. Long-term effects of large-volume liposuction on metabolic risk factors for coronary heart disease. Obesity (Silver Spring) 2008;16:2648–51. doi: 10.1038/oby.2008.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tchernof A, Toth MJ, Poehlman ET. Sex hormone-binding globulin levels in middle-aged premenopausal women. Associations with visceral obesity and metabolic profile. Diabetes Care. 1999;22:1875–81. doi: 10.2337/diacare.22.11.1875. [DOI] [PubMed] [Google Scholar]
- 32.Westerbacka J, Corner A, Tiikkainen M, et al. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia. 2004;47:1360–9. doi: 10.1007/s00125-004-1460-1. [DOI] [PubMed] [Google Scholar]