Abstract
The hippocampus is a brain region that is particularly susceptible to structural and functional changes in response to chronic stress. Recent literature has focused on changes in gene transcription mediated by post-translational modifications of histones in response to stressful stimuli. Chronic variable stress (CVS) is a rodent model that mimics certain symptoms of depression in humans. Given that stress exhibits distinct effects on the cells of the sub-regions of the hippocampus, we investigated changes in histone acetylation in the CA1, CA3, and dentate gyrus (DG) of the hippocampus in response to CVS. Western blotting revealed a significant decrease in acetylation of histone 4 (H4) at Lys12 in CA3 and DG of CVS animals compared to control animals. Furthermore, phospho-acetyl H3 (Lys9/Ser10) was also decreased in the CA3 and DG regions of the hippocampus of CVS animals. In addition, since histone deacetylases (HDACs) contribute to the acetylation state of histones, we investigated the effects of two HDAC inhibitors, sodium butyrate, a class I and II global HDAC inhibitor, and sirtinol, a class III sirtuin inhibitor, on acetylation of histone 3 (H3) and histone 4 (H4). Application of HDAC inhibitors to hippocampus slices from control and CVS animals revealed increased histone acetylation in CVS animals, suggesting that levels of histone deacetylation by HDACs were higher in the CVS animals compared to control animals. Interestingly, histone acetylation in response to sirtinol was selectively increased in the slices from the CVS animals, with very little effect of sirtuin inhibitors in slices from control animals. In addition, sirtuin activity was increased specifically in CA3 and DG of CVS animals. These results suggest a complex and regionally-specific pattern of changes in histone acetylation within the hippocampus which may contribute to stress-induced pathology.
Keywords: epigenetics, chronic stress, hippocampus, histone deacetylases, depression, sirtuins
Chronic exposure to repeated stress is characterized by hyperactivity of the HPA axis and increased vulnerability to mental health pathology such as depression (Herman et al., 2005, McEwen, 2007). In the hippocampus, chronic stress induces several structural and functional changes including decreased neurogenesis in the dentate gyrus (Heine et al., 2004), suppression of synaptic plasticity in CA1 (Pavlides et al., 2002, Alfarez et al., 2003), atrophy in the apical dendrites of CA3 pyramidal neurons (Watanabe et al., 1992, Conrad et al., 1996, Magarinos et al., 1996), and impairment in hippocampus-dependent learning and memory (Conrad et al., 1996, Kleen et al., 2006, Song et al., 2006, Li et al., 2008a).
Recent literature aimed at uncovering the neurobiological basis underlying stress-induced changes in the hippocampus has focused on epigenetic regulation of chromatin (Weaver et al., 2004, Tsankova et al., 2006, Renthal et al., 2007). Post-translational modifications of amino acid residues on histone tails, one possible mechanism of epigenetic regulation, alter the structural conformation of chromatin, thereby controlling the access to DNA by transcriptional machinery (Herrera et al., 2000). In general, acetylation of lysines and phosphorylation of serines and threonines relaxes the DNA-histone interaction. This modification allows for permissive binding by transcriptional machinery and promotion of gene transcription (Jenuwein and Allis, 2001, Felsenfeld and Groudine, 2003). Post-translational modification of histones in the brain have been demonstrated in several neuropsychological disorders like schizophrenia and bipolar disorder (Tsankova et al., 2007, Jiang et al., 2008), as well as in response to acute and chronic environmental stimuli (Tsankova et al., 2004, Tsankova et al., 2006, Renthal et al., 2007, Jiang et al., 2008). Most importantly, several studies demonstrated that histone modifications underlie processes that occur in the hippocampus including neuronal differentiation, neurodegeneration, synaptic plasticity and memory formation (Reul and Chandramohan, 2007, Abel and Zukin, 2008, Jiang et al., 2008).
Histone acetylation is tightly controlled by the antagonistic actions of two protein families, histone acetyltransferases (HATs) and histone deacetylases (HDACs). HATs catalyze acetylation reactions, whereas HDACs remove acetyl groups from lysine residues thereby controlling gene expression through transcriptional repression (reviewed in Berger, 2007). The HDACs are a super-family of 18 proteins made up of four main classes: Class I (HDAC1-3, 8), Class II (HDAC4-10), Class III (Sirtuin 1-7), and Class IV (HDAC11) (de Ruijter et al., 2003, Denu, 2005). The class III HDACs function via a novel mechanism requiring the co-factor NAD+ (nicotinamide adenine dinucleotide) for enzymatic activity (Denu, 2005). Studies suggest that HDAC inhibitors have antidepressant-like effects in animal models of stress-induced depression (Schroeder et al., 2007, Covington et al., 2009, Gundersen and Blendy, 2009).
The aim of this study was to investigate regulation of post-translational modification of histones in the subregions of the hippocampus of male rats that occur in response to chronic variable stress (CVS). We first confirmed the physiological measures of stress in our CVS rat model. Furthermore, we determined the participation of histone deacetylases (HDACs) and sirtuins in acetylation of lysine 12 (K12) H4 and phospho-acetylation of lysine 9/serine 10 (K9/S10) H3 in the hippocampus of chronically stressed animals. Finally, we investigated Sirtuin 1 (Sirt1) activity in the hippocampus of CVS animals compared to control animals.
Experimental Procedures
Animals
Male Wistar rats (42 days of age upon arrival) were purchased from Harlan Inc. (Indianapolis, IN). On arrival, animals were housed in pairs on a 12:12 light/dark cycle (lights on at 0700 hours) and received food and water ad libitum. Animals were given 14 days to acclimate prior to experimental manipulation. All experimental procedures were conducted during the light cycle and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Tulane University Institutional Animal Care and Use Committee.
Chronic Variable Stress
Animals were randomly assigned to CVS and control groups at 56 days of age. CVS was conducted using a modified method previously reported (Herman et al., 1995). Briefly, the CVS paradigm consisted of twice-daily exposure to stressors applied over 14 consecutive days. Morning stressors were administered between 0830 and 1130, and afternoon stressors were administered between 1330 and 1630. Overnight stressors (social isolation or social crowding) began immediately after cessation of the afternoon stressor and concluded with the start of the next day’s morning stressor. Stressors consisted of warm swim (20 min at 31-33°C), cold swim (10 min at 16-18°C), cold room (1 hr at 4°C, two rats per cage without bedding), rotation (1 hr at 100 rpm), social isolation (overnight, one rat per cage), and social crowding (overnight, 6 rats per cage). With the exception of the overnight stressors, the daily stressors were applied in a semi-randomized manner with each stressor being assigned equally over the 14 days. All animals, control and CVS, were weighed every other day. On the morning of day 15, animals were killed by rapid decapitation, trunk blood collected, and adrenal glands weighed. Animals were killed between 0900 and 1000, when corticosterone levels are lowest, and at least 16 hours after administration after the last stressor.
Sucrose Preference Test
Sucrose preference was assessed based on methodology previously described (Rygula et al., 2005). Briefly, a subset of rats was tested for sucrose preference on day 0 and day 14 of CVS protocol. Rats were given 12 hours (0700 to 1900) to habituate to a free choice between two bottles, both containing tap water. At 1900, one bottle was replaced with 3% sucrose. The other bottle remained as tap water. Animals were given free choice between sucrose and tap water for 12 hours (1900-0700). To prevent possible effects of side preference, the position of the sucrose bottle was randomly distributed among the cages. Consumption was calculated as the percentage of sucrose fluid volume consumed to total fluid volume consumed.
Corticosterone Assay
Trunk blood was allowed to coagulate at room temperature for 90 min. Samples were centrifuged at 2000 × g for 15 min, serum collected and samples stored at −20°C. Samples were sent to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for corticosterone measurements.
Hippocampal Slice Preparation and Pharmacological Manipulations
Animals were killed by rapid decapitation. The brain was rapidly removed and immersed in ice-cold aCSF containing (in mM): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3 2 CaCl2, 1 MgCl2, 25 glucose. Transverse slices (400μm) were prepared with a Vibratome (St. Louis, MO). The hippocampus was isolated from transverse slices and allowed to equilibrate in aCSF for 1hr at 32°C. All solutions were saturated with 95/5% O2/CO2. Slices were treated with the following drugs for 1hr at 32°C: DMSO vehicle, 100 μM Sirtinol (Calbiochem), aCSF vehicle, or 300 μM Sodium Butyrate (Sigma).
Histone Extraction
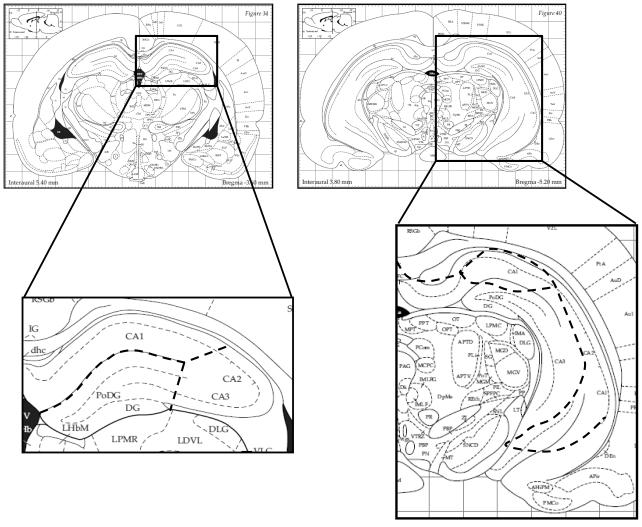
The brain was removed and immersed in ice-cold aCSF. 1mm coronal sections were made using a brain matrix (Braintree Scientific) and the hippocampus was subdivided into CA1, CA3 and DG regions under direct visualization for histone and protein extraction. Figure 1 indicates the regions dissected from anterior and posterior hippocampal slices. Histones were extracted in homogenization buffer containing (in mM): Sucrose 250, Tris pH 7.5 50, KCl 50, PMSF 5, Sodium Butyrate 20, Sodium Orthovanadate. Sample was centrifuged @ 7700 G for 1 min. Pellet was resuspended in 0.4 N H2SO4 and centrifuged at max speed for 10 min at 4°C. Trichloroacetic acid + 4 mg/ml deoxychloric acid was added to the supernatant and centrifuged at max speed for 30 min at 4°C. Histones were extracted with cold acidified acetone and centrifuged at max speed for 5 min at 4°C. The histone pellet was resuspended in 10 mM Tris, ph 8.0 and stored at −80°C. Protein concentration was determined through Lowry protein assay. Samples were normalized to 0.5μg/μl and 4× sample buffer was added (0.3M Tris, 40% Glycerol, 8% SDS, 200 mM DTT, and Bromophenol Blue). Histones were separated on a 15% gel and transferred to Immobilon for Western Blotting.
Figure 1.
Diagram of anterior and posterior hippocampal slice preparations (Paxinos and Watson, 2007). 1mm coronal slices were prepared using a brain matix which allows for uniform slice preparation and accurate tissue blocking. The hippocampus was dissected out under direct visualization. Dashed lines are representative of the subregions of the hippocampus dissected into CA3, CA1, and DG regions.
Western Blotting
Nuclear proteins were extracted using Chemicon’s compartmental protein extraction kit. Protein concentration was determined through Lowry protein assay. Samples were normalized to 0.5μg/μl and 4× sample buffer was added. Nuclear proteins were separated on a 10% SDS-PAGE gel. Blots were probed for anti-acetyl p53 (lysine 379) antibody (1:1000, Cell Signaling Tech), anti-p53 antibody (1:1000, Cell Signaling Tech), anti-sirtuin1 antibody (1:500, Santa Cruz), and anti-HDAC5 antibody (1:1000, Cell Signaling Tech).
Histone fractions were probed for the following antibodies: anti-acetyl lysine 12 (K12) H4 (1:1000, Cell Signaling), anti-histone 3 (1:1000, Cell Signaling), anti-acetyl lysine (K9)-and phospho serine10 (S10) - histone 3 (1:1000, Cell Signaling). Blots for modified histone antibody were stripped and re-probed for total histone 3. The histone 4 antibody was undetectable; therefore all histone modifications (K9/S10 H3 and K12H4) are normalized to total H3 protein expression. Chemiluminescence was detected with either ECL (Amersham, UK) or Super Signal (Pierce, Rockford, IL). Blots were exposed on Kodak XOMAT LS film. Band densitometry was calculated as the mean grey value multiplied by the area (background subtracted) and were analyzed using Image J.
Sirtuin1 Activity Assay
Sirtuin 1 activity was assessed in nuclear extracts from hippocampus preparations via a commercially available kit (Sirtuin 1 Activity Assay Kit; Calbiochem). Briefly, protein concentration was normalized across samples to a final concentration of 1μg/μl. Samples were incubated with 100μM of the sirtuin 1 acetylated (lysine 382) p53 substrate in the presence of 500μM NAD+ for 45min at 37°C. Deacetylation of the Sirt1 substrate produces a fluorescence signal upon the addition of the developer compound. Fluorescence was read in clear, half-volume 96-well plates on SpectraMAX Gemini XS fluorescence plate reader (excitation 355nm, emission 465nm, gain=85). Calculation of net fluorescence was obtained by subtraction of the blank control value from the triplicate mean of each samples fluorescence readings.
Statistical Analysis
Statistical analyses were performed using ANOVA followed by Bonferroni or Tukey’s post-hoc test comparisons when appropriate. The student’s t-test was used for pair-wise comparisons. All analysis was performed using Graph Pad 5.
Results
Physiological and behavioral markers of Chronic Variable Stress
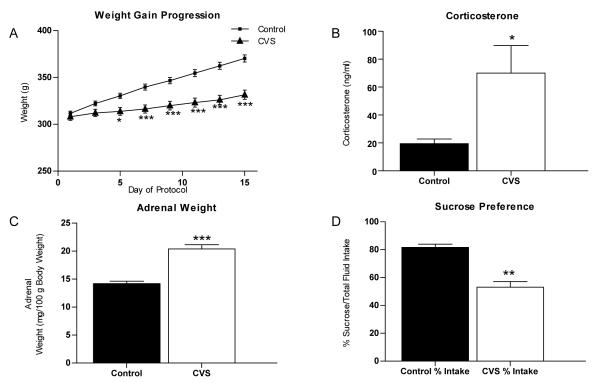
The rodent model of chronic variable stress (CVS) mimics chronic stress in humans and provides a model of neuropsychological conditions like chronic stress-induced depression (Katz et al., 1981, Katz and Sibel, 1982, Sapolsky et al., 1986, Herman et al., 1995). Animals exposed to mild stressors applied over several weeks to several months exhibit many symptoms similar to those observed in depressed patients, including high circulating glucocorticoid levels (Raone et al., 2007), attenuation of weight gain (Marin et al., 2007), adrenal hypertrophy (Ulrich-Lai et al., 2006) and anhedonia (Willner et al., 1987, Willner et al., 1992, Zurita et al., 2000, Rygula et al., 2005) as well as impaired cognitive functions (Bondi et al., 2008). Rats subjected to CVS for 14 days and weighed every other day demonstrated a significant decrease in body weight gain (Fig. 2A) compared to handled controls. Two-way ANOVA revealed a significant effect of stress [F(1,322)=18.84, p<0.0001], time [F(7,322)=98.60, p<0.0001], and stress × time interaction [F(7,322)=493.85, p<0.0001]. Subsequent Bonferroni post-hoc tests confirmed a significant reduction in body weight gain by day 5 (p<0.05), which persisted throughout the remainder of the experiment (day 7, 9, 11, 13, 15 p<0.0001). Additionally, CVS animals showed a significant increase in plasma corticosterone levels [Fig 2B; 70.1 ± 19.7 ng/ml (n=9) compared to control animals 19.5 ± 3.3 ng/ml (n=10), p=0.0281] and adrenal hypertrophy [Fig 2C; 20.4 ± 0.8 mg/100g body weight (n=12) compared to control animals with 14.2 ± 0.4 mg/100g body weight (n=12), p<0.0001].
Figure 2.
Physiological measures of chronic stress. Quantification of physical parameters from animals subjected to either control conditions or 14 days of chronic variable stress (CVS). (A) CVS results in a significant decrease in weight gain (n=12) compared to control (n=12). (B) Serum levels of corticosterone from trunk blood are increased in CVS animals compared to control animals (n=9). (C) A significant increase in adrenal weight relative to body weight was seen in CVS animals compared to control animals (measured as mg/100g body weight; n=12). (D) A reduction in sucrose preference was observed following CVS (measured as % sucrose intake/total fluid intake; n=6). Data are shown as means ± SE; *P<0.05 vs. control; **P<0.01 vs. control; ***P<0.0001 vs. control.
Anhedonia, or the decreased ability to experience pleasure, is one of the core clinical symptoms of depression (American Psychiatric Association., 2000). The sucrose preference test, which measures the rat’s consumption of a palatable rewarding substance over a less appetitive choice, is a behavioral measure of anhedonia in rats that is affected during exposure to chronic stress (Willner et al., 1987, Rygula et al., 2005). Figure 2D shows that control animals demonstrated a clear preference for sucrose (81.6 ± 2.3% total volume consumed, n=6) while CVS animals failed to show sucrose preference (53.1 ± 4.0% total volume consumed, n=6, p=0.004). No difference was observed between control and CVS animals in total fluid intake (p=0.3000; data not shown). This suggests that CVS does not affect fluid consumption but does alter preference for sucrose fluid. Together, these data confirm that our animals showed signs of chronic stress previously described.
Regulation of Histone Acetylation by Chronic Variable Stress
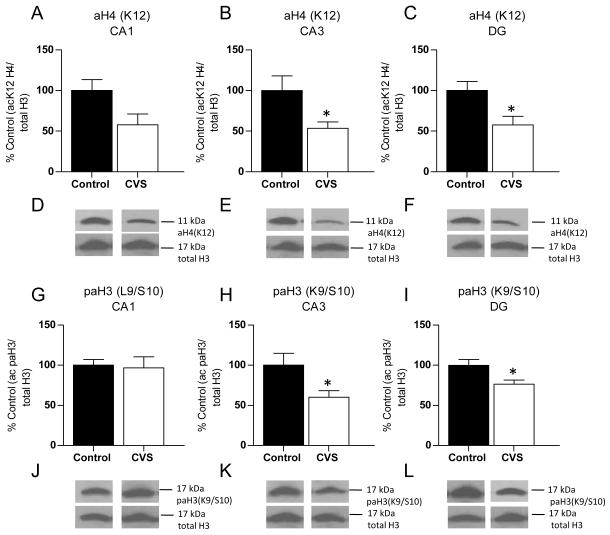
To first assess the effects of chronic stress on histone H3 and histone H4 acetylation in the whole hippocampus, rats were subjected to CVS for 14 days and levels of histone acetylation at several lysine residues were examined. CVS resulted in a significant decrease in band density of the acetylated lysine 12 (K12) H4 antibody (61 ± 12%, n=6 compared to control animals at 100 ± 10%, n=5; p=0.0336) and the phospho-acetylated antibody of lysine 12/serine 10 (K9/S10) H3 (20 ± 5%, n=6 compared to control animals at 100 ± 17%, n=4; p=0.0005; data not shown) in the whole hippocampus. No significant difference in band density between control and CVS animals was observed on lysine 8 (K8) H4, lysine 16 (K16) H4, or lysine 18 (K18) H3 (data not shown). This suggests that acetylation of K12H4 and K9/S10 H3 is decreased in the hippocampus of CVS animals. Given these results, and that chronic stress exhibits distinct effects on cells of the subregions of the hippocampus, we chose to focus on K12H4 and K9/S10 H3 for the detailed localization of the histone acetylation changes in response to chronic variable stress.
No significant change in acetylated K12H4 band density was observed in CA1 although a trend was observed (Fig. 3A, D; n=6, p=0.056). However, a significant decrease in band density of the acetylated K12H4 antibody was observed in CA3 (Fig. 3B, E; 53 ± 8%, n=6) and DG (Fig. 3C, F; 58 ± 10%, n=6) of the hippocampus compared to control animals [CA3 (100 ± 18%, n=5, p=0.033), DG (100 ± 11%, n=5, p=0.022)]. For phospho-acetylated K9/S10 H3, again no significant difference in band density was observed in the CA1 region of the hippocampus (Fig 3G, J). A significant decrease in band density for the phospho-acetylated K9/S10 H3 antibody was observed only in CA3 (Fig. 3H, K; 60 ± 8%, n=6 compared to control animals at 100 ± 15%, n=5, p=0.036) and DG (Fig. 3I, L; 76 ± 5%, n=5 compared to control animals at 100 ± 7%, n=5, p=0.027). These results show a region-specific change in histone acetylation in CA3 and DG of the hippocampus.
Figure 3.
Histone acetylation changes in the hippocampus are subregion-specific. Quantification and representative western blots of histone acetylation extracted from subdissected hippocampus of control and chronic variable stress (CVS) animals. (A) Immunoblotting of acetylated histone 4 lysine 12 [aH4(K12)] antibody showed a trend toward a decrease in the CA1 region of the hippocampus in CVS animals compared to control animals. (B) A significant decrease in acetylation of H4(K12) was observed in CA3. (C) In the dentate gyrus (DG) region of the hippocampus, CVS resulted in a significant decrease in band density of aH4(K12) acetylated antibody compared to control animals. (D, E, F) Representative western blots of aH4(K12) and total H3 for CA3 (D), CA1 (E) and DG (F) regions of the hippocampus. (G) Western blotting of phospho-acetylation of histone 3 at lysine9/serine 10 [paH3(K9/S10)] demonstrated that levels remained unchanged in CA1. (H) A significant decrease in paH3(K9/S10) band density was observed in CA3 of CVS animals compared to control animals. (I) A significant difference in paH3(K9/S10) was observed in the DG between control and CVS animals. (J, K, L) Representative western blots of paH3 (K9/S10) and total H3. Data are shown as means ± SE; *P<0.05 vs. control (n=5-6 animals per group).
The Role of Histone Deacetylases in Chronic Variable Stress
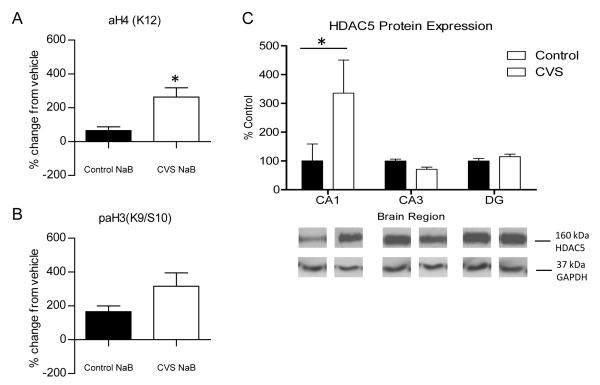
Two possible mechanisms could mediate the decrease in histone acetylation that we observed. One possibility is increased HDAC activity and the other is decreased HAT activity in the CVS animals. HDACs are one of the key enzymes responsible for regulating histone acetylation, and they have shown promise as a potential therapeutic target in several human diseases. Therefore, HDAC activity in the hippocampus of control and CVS animals was investigated. In order to do this, we reasoned that application of HDAC inhibitors to hippocampus slices would cause larger increases in histone acetylation relative to vehicle-treatment in the experimental group where HDACs were most active. Hippocampus slices from control and CVS animals were incubated with either 300 μm sodium butyrate (NaB), a broad inhibitor of class I/II HDACs, or 100 μm sirtinol, an inhibitor of the Class III sirtuins. The changes in histone acetylation of drug-treated slices relative to slices that were treated with vehicle, where no change in activity is expected, were examined. The concentrations of these drugs were previously demonstrated to effectively inhibit HDACs (Levenson et al., 2004) and sirtuins (Renthal et al., 2009) in the slice preparation. A significant increase in the magnitude of change in acetylation in response to HDAC inhibition compared to vehicle-treated slices was observed on K12H4 in the hippocampus slices of CVS animals incubated with NaB [Fig. 4A; 263 ± 54% (n=4) compared to control animals at 65 ± 23% (n=4), p=0.015]. No significant difference was observed in phospho-acetylation of histone H3 between control and CVS slices that were treated with NaB (Fig. 4B). Since NaB caused a significant increase in acetylation of K12H4 and not K9/S10 H3, these results suggest that Class I/II HDAC deacetylation of K12H4 is increased in the hippocampus of CVS animals.
Figure 4.
Deacetylation of histones by Class I/II Histone Deacetylases (HDACs) in control and CVS-treated hippocampus. Western blot analysis of changes in histone 3 and histone 4 acetylation in response to HDAC inhibition in whole hippocampus slices from control and chronic variable stress (CVS) animals treated for one hour with either vehicle or 300 μm sodium butyrate (NaB). (A) Quantification of percentage change from vehicle treated slices in acetylation of lysine 12 on histone 4 [aH4(K12)]. A significant increase from vehicle in K12H4 acetylation was observed in CVS animals compared to control animals. (B) Phospho-acetylation of lysine 9/serine 10 on histone 3 [paH3(K9/S10)] quantification of percentage change mediated by NaB compared to vehicle treated slices. No significant change from vehicle was observed in CVS animals compared to control animals. (C) Quantification and representative western blots from nuclear protein extracted from subdissected hippocampus tissue from control and CVS animals. A significant increase in histone deacetylase 5 (HDAC5) protein expression was observed in the CA1 region of CVS animals compared to control animals with no change in either CA3 or DG. Representative western blot of GAPDH protein expression are shown as a loading control. Data are shown as means ± SE; *P<0.05 vs. control (n=4 animals per group).
Previous research has shown that HDAC5, a class II HDAC, is upregulated in the hippocampus in response to chronic psychological stimuli. This increase in HDAC5 protein expression was attenuated by administration of the antidepressant imipramine (Tsankova et al., 2006). HDAC5 protein expression was examined throughout the subregions of the hippocampus of control and CVS animals (Fig. 4C). Two-way ANOVA of the western blots revealed a significant effect of brain region [F(2,17)=4.966, p<0.05] and brain region × condition interaction [F(2,17)=4.966, p<0.05]. Subsequent Bonferroni post-hoc tests confirmed a significant increase in HDAC5 protein expression in only the CA1 region of the hippocampus (p<0.001). These results suggest that HDAC5 protein expression is increased in the CA1 area of CVS animals, but not CA3 or the DG, where the histone modifications were observed.
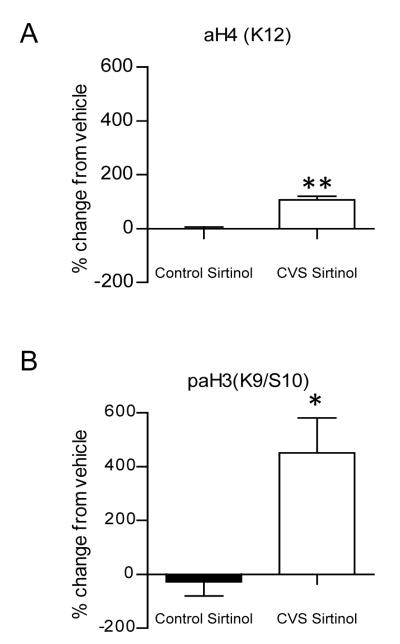
We also investigated the deacetylation of histones by the Class III sirtuin family. In CVS animals, incubation of slices with the sirtuin inhibitor, sirtinol, resulted in a significant increase relative to vehicle-treated slices in acetylation of K12H4 [Fig. 5A; 107 ± 14% (n=4) , p=0.0015]. This effect was not observed in control animals [1 ± 6% (n=4)]. Furthermore, sirtinol produced a significant change from vehicle in phospho-acetylation of K9/S10 H3 in the CVS group [Fig. 5B; 451 ±130% (n=4) compared to control animals at −28 ± 52% (n=4), p=0.0301]. Interestingly, these results suggest that deacetylation of histones by sirtuins is selectively increased in the hippocampus of CVS animals compared to control animals, which demonstrate low levels of histone deacetylation by sirtuins.
Figure 5.
Inhibition of sirtuins increases histone acetylation only in chronic variable stress (CVS) treated hippocampus. Western blot analysis of histone acetylation extracted from whole hippocampus slices from control and chronic variable stress (CVS) animals treated for one hour with either vehicle or 100 μm Sirtinol. (A) Quantification of percentage change from vehicle treated slices in acetylation of K12H4. Pharmacological application of sirtinol resulted in a significant increase from vehicle in CVS animals compared to control animals. (B) Phospho-acetylation of K9/S10 H3 quantification of percentage change from vehicle treated slices. A significant increase from vehicle in phospho-acetylation of H3 was observed in CVS animals compared to control animals. Data are shown as means ± SE; *P<0.05 vs. control; **P<0.01 vs. control (n=4 animals per group).
Chronic Variable Stress increases the activity of Sirtuin 1 in the hippocampus with no effect on protein expression
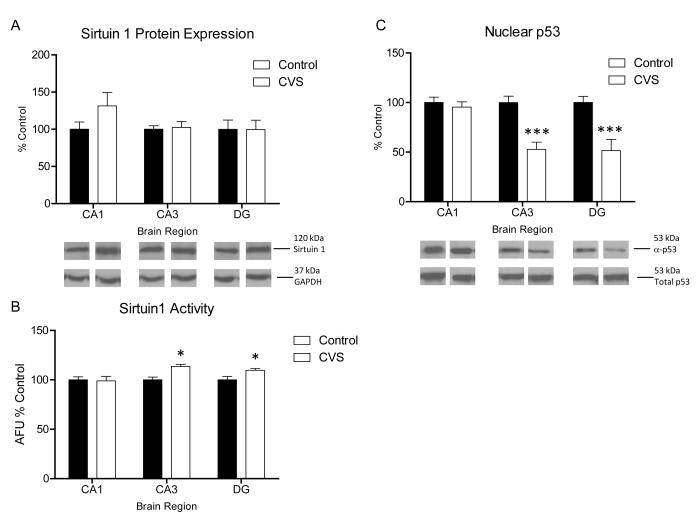
Of the seven mammalian homologues of sirtuins, Sirt1, Sirt2, Sirt3, and Sirt5 have characterized deacetylase functions, however Sirt3 and Sirt5 are functionally restricted to the mitochondria limiting their role in nuclear transcriptional regulation (Whittle et al., 2007). Furthermore Sirt1, Sirt2, and Sirt5 are expressed in the brain (Denu, 2005). Sirt1 demonstrates promiscuous enzymatic activity within the nucleus and the cytoplasm. In addition to its role as an HDAC, has been shown to deacetylate several non-histone targets including hormone receptors (Whittle et al., 2007, Wang et al., 2008), p300 (Bouras et al., 2005), and several transcription factors (Haigis and Guarente, 2006). Sirt1 is highly expressed within the structures of the hippocampus and hypothalamus which have an important role in the HPA axis response to stress (Ramadori et al., 2008). Therefore we investigated whether Sirt1 protein expression was increased in the hippocampus following CVS. Surprisingly, we observed no significant interaction in Sirt1 protein expression in CA1, CA3, or DG regions of the hippocampus following CVS [Fig. 6A; Two-way ANOVA, n=4, p=0.342]. Further examination of the specific activity of Sirt1 (see methods) within the nuclear extract from the subregions of the hippocampus of CVS animals revealed a main effect of condition [Fig. 6B; Two-way ANOVA, F(1,18)=8.377, p<0.01]. Subsequent Bonferroni test revealed a significant increase in Sirt1 activity in the CA3 [n=4, p<0.0001] and DG [n=4, p<0.05]. No significant difference in Sirt1 activity was observed in CA1 between control and CVS animals (Fig. 6B).
Figure 6.
Regulation of Sirtuin 1 activity and protein expression in chronic stress. (A) Quantification and representative western blots of sirtuin 1 expression from nuclear protein extracted from subdissected hippocampus of control and chronic variable stress (CVS) animals. No significant difference from control animals in sirtuin 1 protein expression was seen in CA1, CA3, and DG regions in CVS animals (n=4). Representative blots from GAPDH are shown as protein loading control. (B) Quantification of Sirt 1 activity in nuclear extract from subdissected hippocampus of control and CVS animals. A significant increase in enzymatic activity of Sirt 1 was found in the CA3 and DG regions of the hippocampus of CVS animals compared to control animals. No change from control was seen in CA1 [data is represented as arbitrary florescence units (AFU) relative to control; n=4]. (C) Quantification and representative western blots of p53 acetylation relative to total p53 expression from nuclear protein extracted from subdissected hippocampus of control and CVS animals. A significant decrease in acetylation of p53 was observed in the CA3 and DG hippocampus regions of CVS animals compared to control animals while no difference from control was observed in CA1 (n=6). Data are shown as means ± SE; *P<0.05 vs. control; ***P<0.0001 vs. control.
To confirm these results another substrate for Sirt1, acetylated (lysine 379) p53, was investigated. Immunoblotting for this antibody has been used a marker for Sirt1 activity in vivo (Kim et al., 2007b, Ramadori et al., 2008). Figure 6C shows that a two-way ANOVA of western blot data revealed a significant effect of condition [F(1,30)=31.82, p<0.001], brain region [F(2,30)=5.957, p<0.01], and brain region × condition interaction [F(2,30)=5.957, p<0.01]. Subsequent Bonferroni post-hoc tests confirmed a significant decrease in p53 acetylation in CVS animals in both the CA3 [p<0.001] and DG [p<0.001] regions with no significant difference from control animals in the CA1. Together, these results demonstrate that CVS results in an increase in Sirt1 activity within the CA3 and DG of the hippocampus.
Discussion
The aim of the present study was to examine acetylation of histones in response to CVS. This study is the first to show that CVS, a stress model that exhibits minimal habituation of the HPA axis due to variable stressors, results in decreased histone acetylation at two lysine residues on H3 and H4, specifically in the CA3 and DG regions of the hippocampus. This result is consistent with studies that showed different epigenetic modifications in the hippocampus in response to chronic restraint stress (Hunter et al 2009) and chronic defeat stress (Tsankova et al 2006).
Interestingly, we also found increased levels of acetylation in response to HDAC inhibitors in the hippocampus of CVS animals compared to control animals. NaB resulted in an increase in acetylation of K12H4 in CVS animals, while sirtinol increased acetylation of both K12H4 and phospho-acetylation of K9/S10 H3. Interestingly, sirtuins did not appear to deacetylate histones in control animals as assessed by no change in acetylation in response to application of sirtinol relative to vehicle-treated slices. While we cannot rule out the involvement of other HDACs in these histone acetylation changes, we show novel evidence that stress exposure results in an increase in Sirt1 enzymatic activity in CA3 and DG regions, possibly contributing to the observed hypoacetylation on K9H3 and K12H4.
Until recently, epigenetic changes were considered to be temporally restricted to cellular processes occurring during development. However, it is now widely accepted that epigenetic mechanisms can work in concert with transcription factors and other histone modifying enzymes to confer changes in gene expression in response to environmental stimuli throughout the life span. We found that chronic exposure to variable stress resulted in a subregion specific decrease in H3 and H4 acetylation in the hippocampus. Acetylation of K12H4 and phospho-acetylation of K9/S10 H3 are associated with relaxation of the histone-DNA complex and transcriptional activation (Agalioti et al., 2002, Kurdistani et al., 2004, Berger, 2007). This suggests that the hypoacetylation observed following CVS results in a dynamic restructuring of the chromatin within the cells of CA3 and DG which may confer to measurable changes in transcription on a gene specific level. One possibility is that changes in acetylation of H3 and H4 in the CA3 and DG region of the hippocampus result in transcriptional dysregulation of genes important in hippocampus-dependent processes such as neurogenesis in the DG or maintenance of dendritic morphology in CA3. Both processes are believed to contribute to the pathology observed following chronic stress exposure (Watanabe et al., 1992, Magarinos et al., 1996, Gould et al., 1997, Tanapat et al., 1998, Conrad et al., 1999, Heine et al., 2004). Additionally, while we observed no significant changes in histone acetylation in the CA1 region, we cannot exclude that other post-translational modifications of histones such as methylation may occur in this region resulting in transcriptional changes. Hunter et al. found deceased methylation of K4 and K27 on H3 in CA1 following repeated restraint stress (2009) suggesting that the complexity of post-translational modifications in response to stress deserves further exploration.
HDAC5 expression is decreased in the hippocampus of chronically defeated mice that receive chronic imipramine treatment, and HDAC5 overexpression in the DG blocked the behavioral efficacy of imipramine treatment (Tsankova et al., 2006). Furthermore, a recent study found that decreased mRNA expression of HDAC5 was associated with treatment proficiency in a clinical population recovering from a major depressive episode (Belzeaux et al., 2010), suggesting that HDAC5 may be an important target for novel antidepressants. Although we found a significant upregulation of HDAC5 protein in the CA1 of CVS animals, these results did not correlate with the localized changes in histone acetylation that we observed in CA3 and DG. This implies that while HDAC5 protein expression is regulated in CA1 in response to CVS, these changes in protein expression most likely do not contribute to the post-translational modifications that we observed. However, we cannot exclude changes in HDAC5 activity that may participate in changes in acetylation in CA3 and DG.
Sirt1 is linked to several important processes including energy metabolism, apoptosis, caloric restriction, cancer, and aging (reviewed in Haigis and Sinclair, 2010). While research involving animal models of neurodegenerative diseases indicate that sirtuin activity is neuroprotective in models of Alzheimer’s disease (Marambaud et al., 2005, Qin et al., 2006, Kim et al., 2007a), these results are confounded by research suggesting that inhibition of sirtuin activity can also exhibit neuroprotective properties (Chong et al., 2005, Li et al., 2008b), as well as exert positive benefits on memory and cognition (Green et al., 2008, Morales et al., 2010). In line with this, a recent study showed that Sirt1 inhibition is important in controlling the neuronal fate of adult progenitor cells, an effect directly related to decreased acetylation of K9H3 (Prozorovski et al., 2008). In addition to HDAC activity of sirtuins, deacetylation of other protein substrates may be important for cellular processes involved in stress. Indeed, we showed that acetylated p53 is decreased in the CVS-treated animals. Deacetylation of p53 by sirtuins protects neurons from DNA damage-induced apoptosis (Hasegawa and Yoshikawa, 2008). Furthermore, other protein targets of Sirt1 include transcription factors such as NF-κB (Yeung et al., 2004) and histone acetyltransferases such as p300/CBP (Bouras et al., 2005) that can also participate in regulation of gene transcription. This suggests conflicting roles for mammalian sirtuins that may be mediated by divergent functions of sirtuins.
We observed an increase in Sirt1 activity in the DG and CA3 area of CVS animals, as measured by two different assays, but no concomitant change in Sirt1 protein expression. One possibility for the increase is that in response to environmental stress, Sirt1 activity is upregulated through a positive-feedback mechanism in attempt to compensate for the stress impact. On the other hand, chronic stress exposure may lead to an aberrant increase in Sirt1 activity that contributes to transcriptional dysregulation. The mechanism of the observed activity increase may be mediated by posttranslational modifications of Sirt1. Several proteins interact with Sirt1 and modulate its activity. These include active regulator of Sirt1 (AROS) that activates Sirt1 (Kim et al., 2007b) or the Sirt1 inhibitor, Deleted in Breast Cancer 1 (DBC) (Kim et al., 2008). In addition, posttranslational modifications (Smith et al., 2008), and NAD+ metabolism (Denu, 2007) can also modulate Sirt1 activity. Therefore, each of these mechanisms provides additional potential targets for modulation and will be the subject of further investigation.
Possible Functional Consequences
While the genes that are regulated by the histone modifications reported here are unknown, a possible speculative functional consequence of these modifications is the regulation of hippocampus-dependent memory. In general, chronic stress inhibits hippocampus-dependent memory (Conrad et al., 1996, Kleen et al., 2006, Song et al., 2006, Li et al., 2008a). Both Class I/II HDACs and sirtuins are implicated in modulation of hippocampus-dependent memory. Administration of Class I/II HDAC inhibitors enhances hippocampus-dependent memory (Levenson et al., 2004, Vecsey et al., 2007, Stefanko et al., 2009). Sirt1 over-expressing mice exhibit deficits in the novel object recognition task (Kakefuda et al., 2009), a hippocampus-dependent form of memory that is impaired by chronic stress (Elizalde et al., 2008, Li et al., 2008a). A recent study showed that acetylation of K12 on H4 is also dysregulated in the hippocampus and contributes to aging-related memory impairments (Peleg et al., 2010). Specifically, while K12 and other sites on H4 are acetylated after training for fear conditioning in young animals, K12 specifically is not acetylated after training for fear conditioning in the aged animal. This study further investigated genes that were associated with acetylation of this site, and found regulation of numerous memory-related genes. This suggests that the decrease in H4 acetylation observed here may be partially responsible for the decreased hippocampus-dependent memory in chronically stressed animals.
The CA3 region is hypothesized to participate in pattern completion and spatial memory (Nakazawa et al., 2002, Kirwan et al., 2005, Kesner and Warthen, 2010). The decrease in phospho-acetylation of H3 we observed may also play a role in decreased spatial memory or object recognition in CVS-treated animals (Elizalde et al., 2008, Clarke et al., 2010; unpublished results). Therefore, these studies lay the groundwork for further studies in the epigenetic regulation of hippocampus-dependent memory in the CVS rodent model of depression and implicate sirtuins as a target for therapeutic intervention.
The studies presented above provide a significant contribution into our understanding of the role that chromatin remodeling has in the stress response within the hippocampus. Understanding the molecular mechanisms that contribute to changes in histone acetylation and transcriptional dysregulation as well as the specific genes involved will expand our knowledge into the mechanisms of structural and functional changes observed in the hippocampus following chronic stress, but also lead to a better understanding of how these changes relate to increased vulnerability to pathology such as depression. Furthermore, by investigating the specific enzymes involved in regulation of chromatin open and closed state, we can potentially develop novel therapeutic strategies for the treatment of stress-induced depression.
Acknowledgements
We would like to thank Dr. Carmel McDermott for her research contributions and Dan Liu and Mai Lam for their excellent technical assistance. This work was supported by NIH/COBRE grant P20RR016816: Mentoring Neuroscience in Louisiana and Tulane Research Enhancement funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel T, Zukin RS. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr Opin Pharmacol. 2008;8:57–64. doi: 10.1016/j.coph.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agalioti T, Chen G, Thanos D. Deciphering the transcriptional histone acetylation code for a human gene. Cell. 2002;111:381–392. doi: 10.1016/s0092-8674(02)01077-2. [DOI] [PubMed] [Google Scholar]
- Alfarez DN, Joels M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci. 2003;17:1928–1934. doi: 10.1046/j.1460-9568.2003.02622.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic criteria from DSM-IV-TR. American Psychiatric Association; Washington, D.C.: 2000. [Google Scholar]
- Belzeaux R, Formisano-Treziny C, Loundou A, Boyer L, Gabert J, Samuelian JC, Feron F, Naudin J, Ibrahim EC. Clinical variations modulate patterns of gene expression and define blood biomarkers in major depression. J Psychiatr Res. 2010 doi: 10.1016/j.jpsychires.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Bouras T, Fu M, Sauve AA, Wang F, Quong AA, Perkins ND, Hay RT, Gu W, Pestell RG. SIRT1 deacetylation and repression of p300 involves lysine residues 1020/1024 within the cell cycle regulatory domain 1. J Biol Chem. 2005;280:10264–10276. doi: 10.1074/jbc.M408748200. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Li F, Maiese K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through AKT, BAD, PARP, and mitochondrial associated “anti-apoptotic” pathways. Curr Neurovasc Res. 2005;2:271–285. doi: 10.2174/156720205774322584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JR, Cammarota M, Gruart A, Izquierdo I, Delgado-Garcia JM. Plastic modifications induced by object recognition memory processing. Proc Natl Acad Sci U S A. 2010;107:2652–2657. doi: 10.1073/pnas.0915059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ, 3rd, Wu EY, Ghose S, Krishnan V, Russo SJ, Tamminga C, Haggarty SJ, Nestler EJ. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denu JM. The Sir 2 family of protein deacetylases. Curr Opin Chem Biol. 2005;9:431–440. doi: 10.1016/j.cbpa.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Denu JM. Vitamins and aging: pathways to NAD+ synthesis. Cell. 2007;129:453–454. doi: 10.1016/j.cell.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Elizalde N, Gil-Bea FJ, Ramirez MJ, Aisa B, Lasheras B, Del Rio J, Tordera RM. Long-lasting behavioral effects and recognition memory deficit induced by chronic mild stress in mice: effect of antidepressant treatment. Psychopharmacology (Berl) 2008;199:1–14. doi: 10.1007/s00213-007-1035-1. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G, Groudine M. Controlling the double helix. Nature. 2003;421:448–453. doi: 10.1038/nature01411. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KN, Steffan JS, Martinez-Coria H, Sun X, Schreiber SS, Thompson LM, LaFerla FM. Nicotinamide restores cognition in Alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J Neurosci. 2008;28:11500–11510. doi: 10.1523/JNEUROSCI.3203-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen BB, Blendy JA. Effects of the histone deacetylase inhibitor sodium butyrate in models of depression and anxiety. Neuropharmacology. 2009;57:67–74. doi: 10.1016/j.neuropharm.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Yoshikawa K. Necdin regulates p53 acetylation via Sirtuin1 to modulate DNA damage response in cortical neurons. J Neurosci. 2008;28:8772–8784. doi: 10.1523/JNEUROSCI.3052-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Zareno J, Joels M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci. 2004;19:131–144. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Herrera JE, Schiltz RL, Bustin M. The accessibility of histone H3 tails in chromatin modulates their acetylation by P300/CBP-associated factor. J Biol Chem. 2000;275:12994–12999. doi: 10.1074/jbc.275.17.12994. [DOI] [PubMed] [Google Scholar]
- Hunter RG, McCarthy KJ, Milne TA, Pfaff DW, McEwen BS. Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0911143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Langley B, Lubin FD, Renthal W, Wood MA, Yasui DH, Kumar A, Nestler EJ, Akbarian S, Beckel-Mitchener AC. Epigenetics in the nervous system. J Neurosci. 2008;28:11753–11759. doi: 10.1523/JNEUROSCI.3797-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakefuda K, Fujita Y, Oyagi A, Hyakkoku K, Kojima T, Umemura K, Tsuruma K, Shimazawa M, Ito M, Nozawa Y, Hara H. Sirtuin 1 overexpression mice show a reference memory deficit, but not neuroprotection. Biochem Biophys Res Commun. 2009;387:784–788. doi: 10.1016/j.bbrc.2009.07.119. [DOI] [PubMed] [Google Scholar]
- Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev. 1981;5:247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- Katz RJ, Sibel M. Animal model of depression: tests of three structurally and pharmacologically novel antidepressant compounds. Pharmacol Biochem Behav. 1982;16:973–977. doi: 10.1016/0091-3057(82)90055-7. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Warthen DK. Implications of CA3 NMDA and opiate receptors for spatial pattern completion in rats. Hippocampus. 2010;20:550–557. doi: 10.1002/hipo.20676. [DOI] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007a;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell. 2007b;28:277–290. doi: 10.1016/j.molcel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451:583–586. doi: 10.1038/nature06500. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Gilbert PE, Kesner RP. The role of the hippocampus in the retrieval of a spatial location. Neurobiol Learn Mem. 2005;83:65–71. doi: 10.1016/j.nlm.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kleen JK, Sitomer MT, Killeen PR, Conrad CD. Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore. Behav Neurosci. 2006;120:842–851. doi: 10.1037/0735-7044.120.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdistani SK, Tavazoie S, Grunstein M. Mapping global histone acetylation patterns to gene expression. Cell. 2004;117:721–733. doi: 10.1016/j.cell.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Li S, Wang C, Wang W, Dong H, Hou P, Tang Y. Chronic mild stress impairs cognition in mice: from brain homeostasis to behavior. Life Sci. 2008a;82:934–942. doi: 10.1016/j.lfs.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008b;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- Marin MT, Cruz FC, Planeta CS. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol Behav. 2007;90:29–35. doi: 10.1016/j.physbeh.2006.08.021. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Morales P, Simola N, Bustamante D, Lisboa F, Fiedler J, Gebicke-Haerter PJ, Morelli M, Tasker RA, Herrera-Marschitz M. Nicotinamide prevents the long-term effects of perinatal asphyxia on apoptosis, non-spatial working memory and anxiety in rats. Exp Brain Res. 2010;202:1–14. doi: 10.1007/s00221-009-2103-z. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Nivon LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12:245–257. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press/Elsevier; Amsterdam ; Boston: 2007. [Google Scholar]
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, Salinas-Riester G, Dettenhofer M, Kang H, Farinelli L, Chen W, Fischer A. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schroter F, Ninnemann O, Siegert E, Bendix I, Brustle O, Nitsch R, Zipp F, Aktas O. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol. 2008;10:385–394. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, Puigserver P, Sadoshima J, Deng H, Pedrini S, Gandy S, Sauve AA, Pasinetti GM. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, Anderson J, Elmquist JK, Coppari R. Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci. 2008;28:9989–9996. doi: 10.1523/JNEUROSCI.3257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raone A, Cassanelli A, Scheggi S, Rauggi R, Danielli B, De Montis MG. Hypothalamus-pituitary-adrenal modifications consequent to chronic stress exposure in an experimental model of depression in rats. Neuroscience. 2007;146:1734–1742. doi: 10.1016/j.neuroscience.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE, 3rd, Maze I, Sikder D, Robison AJ, LaPlant Q, Dietz DM, Russo SJ, Vialou V, Chakravarty S, Kodadek TJ, Stack A, Kabbaj M, Nestler EJ. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron. 2009;62:335–348. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Maze I, Krishnan V, Covington HE, 3rd, Xiao G, Kumar A, Russo SJ, Graham A, Tsankova N, Kippin TE, Kerstetter KA, Neve RL, Haggarty SJ, McKinsey TA, Bassel-Duby R, Olson EN, Nestler EJ. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Reul JM, Chandramohan Y. Epigenetic mechanisms in stress-related memory formation. Psychoneuroendocrinology. 2007;32(Suppl 1):S21–25. doi: 10.1016/j.psyneuen.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry. 2007;62:55–64. doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Smith BC, Hallows WC, Denu JM. Mechanisms and molecular probes of sirtuins. Chem Biol. 2008;15:1002–1013. doi: 10.1016/j.chembiol.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Che W, Min-Wei W, Murakami Y, Matsumoto K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacol Biochem Behav. 2006;83:186–193. doi: 10.1016/j.pbb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci U S A. 2009;106:9447–9452. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Galea LA, Gould E. Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. Int J Dev Neurosci. 1998;16:235–239. doi: 10.1016/s0736-5748(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24:5603–5610. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006;291:E965–973. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, Wood MA. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Powell MJ, Popov VM, Pestell RG. Acetylation in nuclear receptor signaling and the role of sirtuins. Mol Endocrinol. 2008;22:539–545. doi: 10.1210/me.2007-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Whittle JR, Powell MJ, Popov VM, Shirley LA, Wang C, Pestell RG. Sirtuins, nuclear hormone receptor acetylation and transcriptional regulation. Trends Endocrinol Metab. 2007;18:356–364. doi: 10.1016/j.tem.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurita A, Martijena I, Cuadra G, Brandao ML, Molina V. Early exposure to chronic variable stress facilitates the occurrence of anhedonia and enhanced emotional reactions to novel stressors: reversal by naltrexone pretreatment. Behav Brain Res. 2000;117:163–171. doi: 10.1016/s0166-4328(00)00302-8. [DOI] [PubMed] [Google Scholar]