Abstract
Hypertension is associated with heightened cardiac sympathetic drive whilst statins reduce angiotensin II (ATII) signalling, superoxide anion production and increase nitric oxide bioavailability, events that can potentially reduce peripheral cardiac sympathetic neurotransmission. We therefore investigated whether pravastatin alters peripheral cardiac sympathetic control in the spontaneously hypertensive rat (SHR). SHRs (16–18 weeks) had significantly (p < 0.05) enhanced atrial 3H-norepinephrine (3H-NE) release to field stimulation compared to normotensive WKYs. 2-week pravastatin supplementation significantly reduced 3H-NE release to levels observed in the WKY. In-vivo, pravastatin lowered resting heart rate (HR) in the SHR despite not affecting arterial blood pressure or serum cholesterol. In SHR atria/right stellate ganglion preparations, the HR response to stellate stimulation (1, 3, and 5 Hz) was also significantly reduced by pravastatin whilst the HR response to exogenous NE (0.025–5 μmol) remained similar. The nitric oxide synthase (NOS) inhibitor l-NAME (1 mmol/l) increased 3H-NE release by similar amounts in atria from supplemented and non-supplemented SHRs, whilst Western blotting showed no difference in protein levels of nNOS, eNOS, guanylyl cyclase, or the NADPH oxidase subunits Gp91 and P40phox. Pravastatin significantly reduced cardiac ATII levels and angiotensin converting enzyme 1 and 2 expressions whilst protein levels of the ATII receptor (ATR1) remained unchanged in the SHR. Immunohistochemistry co-localised ATR1 with tyrosine hydroxylase positive neurons in the stellate ganglion. The ATR1 antagonist Losartan (5 μmol) equalised release of 3H-NE to comparable levels in supplemented and non-supplemented SHRs. These results suggest 2-week pravastatin treatment reduces cardiac ATII, and prevents its facilitatory effect on NE release thus normalising cardiac sympathetic hyper-responsiveness in SHRs.
Keywords: Angiotensin, Autonomic nervous system, Hypertension, Nitric oxide, Statins
Research Highlights
► Pravastatin lowers resting heart rate (HR) in spontaneously hypertensive rats (SHR) ► Cardiac norepinephrine release & tachycardia are reduced by pravastatin in SHRs ► Neuronal NO signalling & NADPH oxidase expression are unchanged by pravastatin ► The effect of pravastatin on sympathetic neurotransmission is reversed by losartan. ► Pravastatin reduces cardiac angiotensin 2 & angiotensin converting enzymes 1 & 2
1. Introduction
HMG-CoA reductase inhibitors have been shown to have many pleiotropic actions in addition to those arising directly from lowering plasma cholesterol. These include inhibition of Rho isoprenylation thereby stabilising eNOS mRNA [1], activation of protein kinase Akt [2] increasing nitric oxide bioavailability, and inhibition of geranylgeranyl dependent modification of Rac1 to reduce agonist-mediated activation of NADPH oxidase/superoxide anion production [3]. They can also downregulate the ATII type 1 receptor (ATR1) expression in a Rho A dependent manner (see [4] and [5] for reviews). These actions can produce overlapping effects to improve vascular endothelial function in a cholesterol independent manner. Moreover, meta-analyses suggest that statins may have antihypertensive effects in patients with elevated arterial blood pressure regardless of plasma cholesterol levels (e.g. [6]).
Hypertension is also associated with dysfunction of the autonomic nervous system in both humans and animal models. Moreover, this neural phenotype has been implicated in the aetiology and progression of the disease [7]. We have previously shown that a significant component of the cardiac sympatho-vagal dysfunction in the spontaneously hypertensive rat (SHR) arises pre-synaptically at the cardiac end organ level secondary to impaired neuronal nitric oxide synthase signalling [8,9]. Adenoviral vector gene transfer of nNOS can restore vagal acetylcholine release and reduce norepinephrine (NE) release to levels seen in control WKY rats [8–10]. It can also decrease post-synaptic excitability to β adrenergic agonists by decreasing the L-type calcium current [11]. Normalisation of NE release can also be achieved by feeding animals with l-arginine to increase peripheral sympathetic NO production [12].
Conversely, oxidative stress is able to augment peripheral sympathetic responsiveness of heart rate by reducing the expression of nNOS [13]. Angiotensin II (ATII) has been shown to facilitate NE release via the ATR1 in the WKY [14]. Reducing oxidative stress and ATII signalling, and increasing NO bioavailability may therefore represent ways by which statin treatment could potentially rectify peripheral cardiac sympathetic hyperactivity associated with hypertension.
We investigated whether 2-week treatment with pravastatin could reduce cardiac sympathetic neurotransmission and heart rate responsiveness to local sympathetic nerve stimulation in the adult SHR with established hypertension via a nitric oxide, oxidative stress and/or ATII dependent mechanisms.
2. Methods
2.1. Animals
Sixteen-week-old male SHRs (n = 73) and Wistar Kyoto (WKY, n = 40) rats (Harlan, UK) were kept under standard laboratory conditions with free access to water and rat chow for 2 weeks. A subgroup of the SHRs (n = 41) and WKYs (n = 20) had their drinking water supplemented with pravastatin (100 mg/l). Experiments conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and the Animals (Scientific Procedures) Act 1986 (UK). Experiments were performed under British Home Office Project License PPL 30/2130 and PPL 30/2630.
2.2. Heart rate and blood pressure measurements
In a subgroup of rats (n = 24 SHRs and n = 16 WKYs), heart rate and arterial blood pressure were measured via a 3F portex catheter in the left carotid artery whilst anaesthetised with 1–3% isoflurane and 100% oxygen prior to in-vitro experimentation as described previously [12].
2.3. Serum cholesterol levels
Rats were euthanised with an intraperitoneal injection of pentobarbitone and blood samples taken via intraventricular puncture before the heart and thorax were removed. Atrial tissue was harvested to characterise sympathetic function in terms of 3H-NE release and heart rate changes whilst ventricular tissue was used for Western blotting and ATII level measurements. Atrial tissue underwent various pharmacological treatments including the addition of ATR1 antagonists, ATII itself and NOS inhibition that would confound measurements of these proteins in this tissue type, which is why it was not used for molecular analysis.
Blood samples from intraventricular puncture were immediately spun at 5000 rpm for 10 min. Red blood cells and serum (in EDTA tubes) were separated and samples frozen in liquid nitrogen before being stored at − 80 °C. Serum lipid analyses were undertaken by the Clinical Biochemistry Department at the John Radcliffe Hospital, Oxford, using the Siemens ADVIA 2400 automated chemistry analyzer (Siemens Healthcare Diagnostics Ltd, Frimley, UK) with a cholesterol oxidase method.
2.4. 3H-Norepinephrine release to field stimulation from right atrial preparations
The spontaneously beating right atrium was isolated and transferred to a preheated (37 ± 0.2 °C), continuously oxygenated, water-jacketed organ bath containing 3 ml Tyrode solution where the atrium was pinned flat on a silver stimulating electrode. The method for determining the local release of 3H-NE to field stimulation 5 Hz (15 V, 1 ms pulse width, for 1 min) was identical to that which we have previously described [12].
2.5. Isolated sinoatrial node/right stellate ganglion preparation
The spontaneously beating atria with intact sympathetic innervation were isolated and transferred to a preheated (37 ± 0.2 °C), continuously oxygenated, water-jacketed organ bath containing 100-ml Tyrode solution. The method for dissecting and measuring responses to sympathetic nerve stimulation (SNS) has been described previously [13]. Before starting each protocol, the mounted atria were allowed to equilibrate for 80 min until beating rate stabilised (± 5 beats per minute, bpm, over 20 min). The Tyrode's solution in the organ bath was replaced approximately every 30 min throughout each protocol. The stellate was stimulated at 1, 3, 5 and 7 Hz, (20 V, 1 ms pulse duration for 30 s). Drugs were applied directly to the organ bath and incubated until a consistent response to sympathetic nerve stimulation (SNS) was obtained.
2.6. Western blotting
Protein was extracted and Western blotting performed as described previously in detail [12]. The following primary antibodies were used: nNOS (Santa Cruz); eNOS (Transduction Laboratory); sGC (Sigma); Gp91 (Santa Cruz); P40phox (Santa Cruz), ACE1 (Abcam), ACE2 (Abcam) and ATR1 (Santa Cruz). The film was digitised and the relative band densities were determined using UN-SCAN-IT, gel 6.1, software according to manufacturer's instructions. Protein band densities were normalised to β-actin (Abcam) band densities that served as a protein loading control.
2.7. Myocardial ATII levels
ATII levels were also measured using an enzyme immunoassay (SPI Bio, #A05880) according to manufacturer's instructions. Results were standardised as pg ATII per mg of extracted protein.
2.8. Immunohistochemistry
Immunohistochemistry was performed as previously described [15] with the following modifications. Right stellate ganglia were dissected free and fixed in 10% formalin at 4 °C. Prior to processing, samples were transferred to a solution containing 30% sucrose and 1% formalin in PBS for 24 hours. Tissue was permeabilised and blocked in 0.3% Triton X100 and 1% bovine serum albumin for one hour. Tyrosine hydroxylase (TH, mouse) primary antibody (Sigma, 1:200) and biotinylated horse anti-mouse (Santa Cruz, 1:200) secondary antibody were used, which were further labeled with streptavidin Texas red. To assess TH/ATR1 co-localisation, the preparation was blocked using a streptavidin kit, and then incubated with ATR1 primary antibody (Santa Cruz, 1:200) and biotinylated secondary antibody and then labeled with streptavidin fluoroscein. Tissue was scanned and digitally photographed at ×20 magnification using a Nikon Eclipse TE2000-U inversion fluorescent light microscope and appropriate filters.
2.9. Solutions and drugs
The Tyrode solution contained (mmol/l) NaCl 120, KCl 4.7, MgSO4 1.2, NaHCO3 25, CaCl2 2, KH2PO4 1.2 and glucose 11, and was aerated with 95% O2/5% CO2 (pH 7.4). Its temperature was continuously monitored (Digitron 1408-K gauge) and kept at 37 ± 0.2 °C.
The effects of SNS were compared to cumulative doses of bath applied NE (0.1–5 μmol, Sigma) in a darkened room to determine if the actions of pravastatin treatment were pre or post-synaptic. Losartan (5 μmol, LKT Laboratories) was used as a specific ATR1 antagonist. The effects of ATII (20 nmol/l, Sigma) on 3H-NE release were also evaluated. l-NAME (1 mmol/l, Sigma) was used as a non-specific inhibitor of both endothelial and neuronal nitric oxide synthase.
2.10. Statistical analysis
Data are presented as mean ± S.E.M. One-way repeated measures ANOVA followed by Tukey's post hoc analysis was used to evaluate the effect of an intervention within a group. For comparison amongst more than 2 groups, between groups ANOVA was used to compare the means with post-hoc Tukey's HSD test to assess individual significance. An unpaired Student's t-test assuming unequal variances and sample sizes was used to evaluate the effect of an intervention between experimental groups of different sizes. Statistical significance was accepted at P < 0.05 and was not affected by analysing the data in terms of absolute beats per minute (bpm) or percentage changes in heart rate or pulse interval.
3. Results
3.1. Animal characteristics
The phenotypical characteristics of both pravastatin supplemented and non-supplemented SHRs and age matched, normotensive WKYs are summarised in Table 1. Two-week pravastatin supplementation did not alter serum cholesterol levels in the SHR. However, the same treatment regime did lower serum cholesterol in WKY rats, although the WKYs had far higher baseline cholesterol levels and a slightly higher voluntary daily intake of pravastatin. As expected, ventricular weight/body weight ratios, an indication of left ventricular hypertrophy, were significantly higher in the SHR compared to the WKY as was mean arterial blood pressure, but neither of these variables were significantly altered by 2-week pravastatin supplementation in either SHR or WKY rats.
Table 1.
Characteristics of pravastatin supplemented and non-supplemented SHRs and WKYs.
| SHR non-supplemented (n = 32) | SHR Pravastatin supplemented (n = 41) | WKY non-supplemented (n = 20) | WKY Pravastatin supplemented (n = 20) | |
|---|---|---|---|---|
| Pravastatin intake (mg/kg) | 0 | 9.42 ± 0.15 (n = 25) | 0 | 10.00 ± 0.20* (n = 12) |
| Serum cholesterol (mmol/l) | 1.0 ± 0.1 (n = 7) | 1.1 ± 0.3 (n = 7) | 2.1 ± 0.1** (n = 7) | 1.6 ± 0.1* (n = 7) |
| Ventricular weight/body weight (×10−3) | 3.23 ± 0.03* (n = 15) | 3.27 ± 0.04* (n = 25) | 2.94 ± 0.03 (n = 12) | 2.88 ± 0.04 (n = 12) |
| Mean ABP (mmHg) | 173 ± 4* (n = 11) | 172 ± 4* (n = 13) | 86 ± 3 (n = 8) | 83 ± 4 (n = 8) |
| Heart rate in-vivo (bpm) | 353 ± 11** (n = 11) | 340 ± 9* (n = 16) | 287 ± 5 (n = 8) | 281 ± 12 (n = 8) |
| Heart rate in-vitro (bpm) | 231 ± 10 (n = 19) | 241 ± 7 (n = 16) | 252 ± 14 (n = 8) | 258 ± 6*** (n = 8) |
*SHR vs. WKY p < 0.05. **SHR vs. WKY and pravastatin supplemented vs. non-supplemented p < 0.05. ***SHR vs. WKY pravastatin supplemented only p < 0.05.
A significant reduction in heart rate measured in-vivo was observed in the SHR group in the resting state following pravastatin treatment although no difference in heart rate was seen after equilibration of isolated atria in the organ bath in-vitro, consistent with the notion that pravastatin altered resting cardiac autonomic tone via a pre-synaptic mechanism. However pravastatin supplementation did not lower resting heart rate in the SHR to the levels observed in the WKYs.
3.2. Pravastatin normalised peripheral cardiac sympathetic hyperactivity by reducing norepinephrine release to levels seen in the WKY
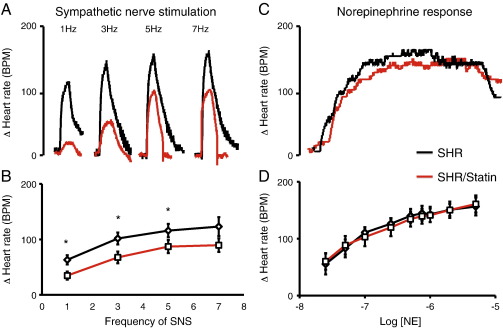
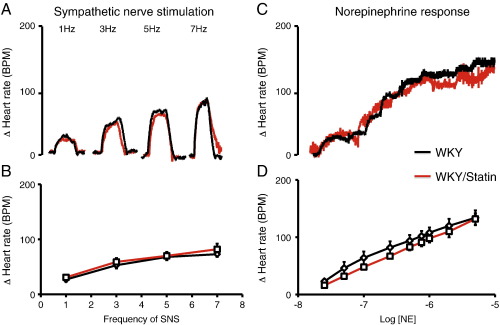
In the SHR the heart rate response to SNS in-vitro was significantly larger than that of the WKY. In addition it was significantly reduced by pravastatin treatment at 1–5 Hz (pravastatin supplemented n = 8 vs. non-supplemented n = 9) as can be seen in Fig. 1, whilst the response to bath applied NE remained unchanged (pravastatin supplemented n = 8 vs. non-supplemented n = 8). The heart rate response to SNS or bath applied NE was not significantly altered by pravastatin treatment in the WKY (pravastatin supplemented n = 8 vs. non-supplemented n = 8) as can be seen in Fig. 2. This suggests that pravastatin reduces peripheral cardiac sympathetic control of heart rate via a pre-synaptic mechanism in the SHR only.
Fig. 1.
(A) Representative raw data traces and (B) group mean data showing significantly reduced heart rate response to sympathetic nerve stimulation (*p < 0.05) in pravastatin treated SHRs (in red, n = 8) compared to non-supplemented SHRs (black, n = 9). (C) Raw data trace and (D) group mean data showing no difference in the heart rate response to cumulative doses of norepinephrine (0.025–5 μmol) between pravastatin supplemented (red, n = 8) and non-supplemented SHRs (black, n = 8).
Fig. 2.
(A) Representative raw data traces and (B) group mean data showing no difference in the heart rate response to sympathetic nerve stimulation in pravastatin treated WKYs (in red, n = 8) compared to non-supplemented WKYs (black, n = 8). (C) Raw data trace and (D) group mean data showing no difference in the heart rate response to cumulative doses of norepinephrine (0.025–5 μmol) between pravastatin supplemented (red, n = 8) and non-supplemented WKYs (black, n = 8).
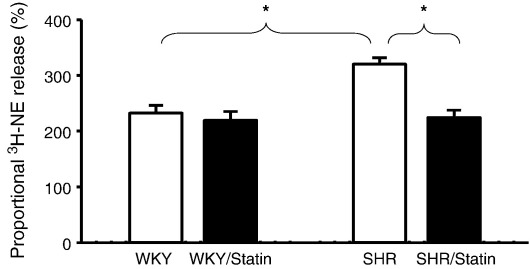
The SHR also had a significantly greater release of 3H-NE to field stimulation compared to the WKY, indicating that a significant component of sympathetic hyper-responsiveness occurs at the end organ level. The release of 3H-NE to field stimulation was significantly reduced by pravastatin treatment in the SHR to levels observed in WKY atria (see Fig. 3, non-supplemented SHRs n = 22 vs. pravastatin supplemented SHRs n = 12). Pravastatin treatment had no effect on 3H-NE release in the normotensive WKY rats however (non-supplemented WKYs n = 12 vs. pravastatin supplemented WKYs n = 12) consistent with the in-vivo and in-vitro recorded heart rate data.
Fig. 3.
Group mean data showing a significantly (*p < 0.05) higher release of 3H-norepinephrine (3H-NE) in response to field stimulation (5 Hz) in non-supplemented SHRs (n = 22) compared to non-supplemented WKYs (n = 12). Whilst pravastatin treatment has no significant effect on 3H-NE release in WKYs (n = 12) it significantly reduces release in the SHR (n = 12) to WKY levels.
3.3. Increased nitric oxide signaling or reduced oxidative stress was not solely responsible for the action of pravastatin on peripheral cardiac sympathetic hyperactivity
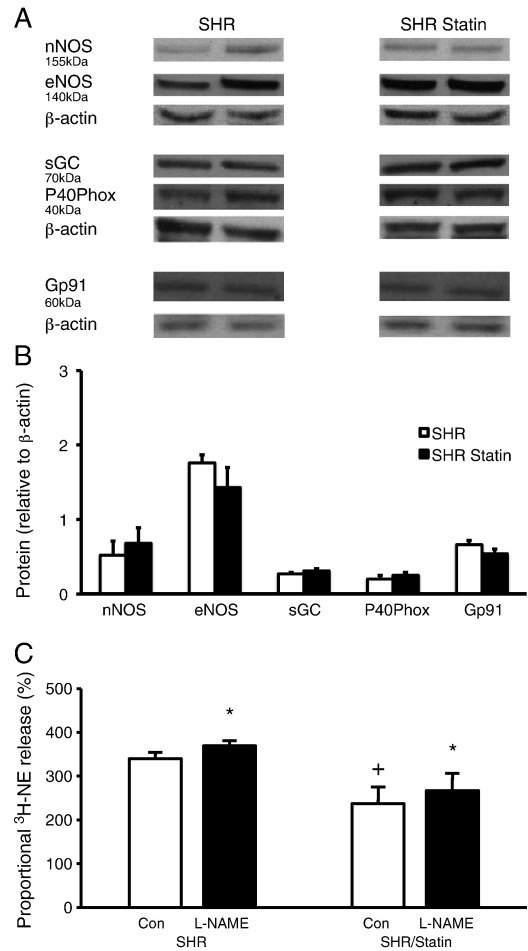
The pleiotropic effects of statins include increasing nitric oxide production and decreasing oxidative stress. We therefore measured the effect of a non-specific inhibitor of nitric oxide synthase (l-NAME) on 3H-NE release during field stimulation in pravastatin supplemented (n = 6) and non-supplemented (n = 6) SHRs. As can be seen in Fig. 4C, l-NAME produced a significant increase in 3H-NE release in both groups although the significant difference between pravastatin supplemented and non-supplemented SHRs remained, suggesting that the nitric oxide pathway is not the predominant route pravastatin acts on to reduce 3H-NE release. Protein levels of nNOS (pravastatin supplemented n = 6 vs. non-supplemented n = 6), eNOS (pravastatin supplemented n = 6 vs. non-supplemented n = 6) and sGC (pravastatin supplemented n = 6 vs. non-supplemented n = 6) normalised to β-actin as a loading control, were also similar between the two groups (Fig. 4A and B). We also measured protein levels of the NADPH oxidase subunits P40phox (pravastatin supplemented n = 6 vs. non-supplemented n = 6), and Gp91 (pravastatin supplemented n = 6 vs. non-supplemented n = 6), which were also similar between the two groups (Fig. 4A and B).
Fig. 4.
Representative Western blots (A) and group mean data (B) showing no difference in the expression of neuronal or endothelial nitric oxide synthase (nNOS, eNOS), soluble guanylyl cyclase (sGC) or the NADPH oxidase subunits P40Phox and Gp91 in myocardial tissue from pravastatin treated (n = 6) and non-supplemented (n = 6) SHRs. Western blot band optical density was normalised to that of β-actin as a loading control. Although pravastatin supplemented SHRs have significantly lower 3H-norepinephrine (3H-NE) release in response to field stimulation (5 Hz) compared to non-supplemented SHRs (+ p < 0.05), the non-specific NOS inhibitor l-NAME (1 mmol/l) significantly (*p < 0.05) increases the release of 3H-NE by similar amounts in both pravastatin treated (n = 6) and non-supplemented (n = 6) groups (C) and does not normalise the difference between the two groups.
3.4. The effect of Pravastatin on ATII mediated control of norepinephrine release
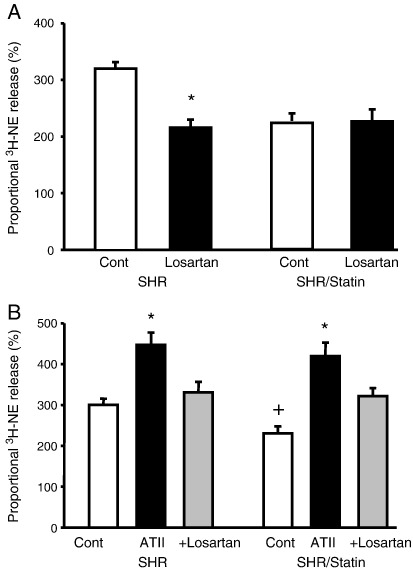
As can be seen in Fig. 5A, the ATR1 antagonist losartan significantly reduced the release of 3H-NE during field stimulation in non-supplemented SHRs (n = 5) to levels observed in pravastatin supplemented SHRs whilst having little effect in the latter group (n = 5). This suggests that pravastatin acted to reduce angiotensin II augmentation of NE release in the SHR. Conversely exogenous ATII was able to significantly increase 3H-NE release during field stimulation in both groups of SHRs (non-supplemented SHRs, n = 6 vs. pravastatin supplemented SHRs, n = 8), an effect abolished by losartan. Losartan was also able to abolish the facilitatory action of ATII on the increase in 3H-NE release during field stimulation in the WKY (n = 6, data not shown), as has been shown by others previously [14].
Fig. 5.
The angiotensin II type 1 (ATII) receptor inhibitor losartan (5 μmol) significantly (*p < 0.05) reduces the release of 3H-norepinephrine (3H-NE) in response to field stimulation (5 Hz) in non-supplemented SHRs (n = 5) to the same levels observed in SHRs treated with pravastatin (n = 5) (A). Exogenous ATII (20 nmol/l) is able to significantly increase 3H-NE release (*p < 0.05) in both non-supplemented (n = 6) and pravastatin treated (n = 8) SHRs, an effect that can be reversed by losartan (B).
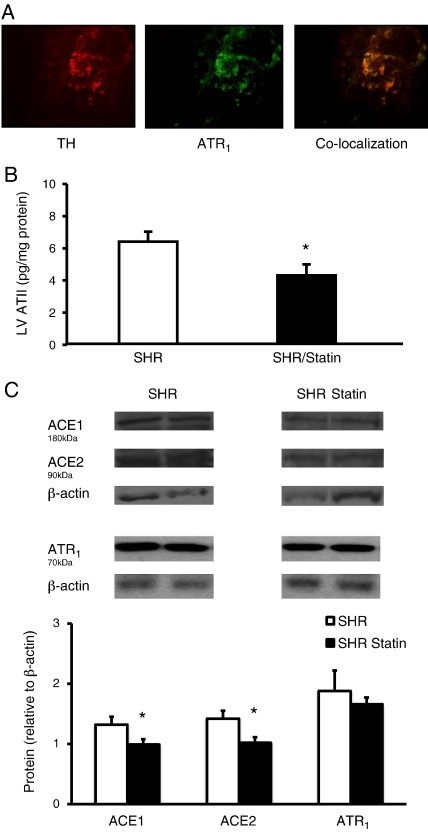
Using immunohistochemistry we demonstrated that the ATR1 is co-localised with tyrosine hydroylase containing sympathetic neurons innervating the heart (see Fig. 6A). Levels of cardiac ATII were significantly reduced by treatment with pravastatin (non-supplemented SHRs, n = 7 vs. pravastatin supplemented SHRs, n = 7, Fig. 6B) although plasma ATII levels were not significantly reduced (non-supplemented SHRs, 52 ± 6 pg/ml, n = 7 vs. pravastatin supplemented SHRs, 45 ± 4 pg/ml, n = 7). No changes were observed in the levels of myocardial ATR1 expression between non-supplemented (n = 6) and pravastatin supplemented (n = 6) SHRs measured by Western blotting. However a significant reduction in ACE 1 and 2 protein levels were observed (non-supplemented n = 6, and pravastatin supplemented SHRs n = 6, in each group) which could account for the lower myocardial ATII levels (Fig. 6C).
Fig. 6.
(A) Immunohistochemistry showing tyrosine hydroxylase (TH) staining with Texas red in a neuron of the right stellate ganglia also staining positive for the angiotensin II type 1 receptor (ATR1) in green (fluroscein) (×20 magnification). Co-localisation is demonstrated by overlap of staining in yellow. (B) Myocardial angiotensin II (ATII) levels are significantly (*p < 0.05) reduced following pravastatin treatment (n = 7) compared to non-supplemented SHRs (n = 7). (C) Representative Western blots and group mean data showing a significant (*p < 0.05) reduction in myocardial angiotensin converting enzyme (ACE) 1 and 2 expression from pravastatin treated (n = 6) and non-supplemented (n = 6) SHRs, with no significant change ATR1 protein expression (n = 6 in each group). Western blot band optical density was normalised to that of β-actin as a loading control.
4. Discussion
The main new findings of this study are as follows.
First, pravastatin supplementation for 2 weeks did not significantly alter mean arterial blood pressure or serum cholesterol in the adult SHR with established hypertension, but significantly reduced resting heart rate in-vivo, although not to the levels observed in the WKY. Exaggerated release of cardiac NE in response to field stimulation in the SHR compared to the WKY in-vitro was normalised (to levels observed in the WKY) by pravastatin treatment. In the SHR this also translated into a reduction in the heart rate response to peripheral cardiac sympathetic nerve stimulation whilst the response to exogenous NE remained unchanged, suggesting the actions of pravastatin were mediated pre-synaptically. Secondly, the reduction in cardiac sympathetic hyperactivity by pravastatin in the SHR cannot be reversed by NOS inhibition. Treatment with pravastatin also did not alter the expression of nNOS, eNOS, sGC or the NADPH oxidase subunits Gp91 or P40phox, suggesting free radical signalling systems are not involved in this neuronal phenotype. Finally, the action of pravastatin in the SHR was completely reversed by the ATR1 antagonist losartan. This was associated with significantly lower levels of cardiac ATII as well as ACE 1 and 2 following treatment with pravastatin, supporting a role for the cardiac angiotensin II system in modulating NE release.
4.1. Pravastatin normalises cardiac sympathetic hyperactivity in the SHR
Sympathetic hyperactivity in the SHR is well documented both centrally [16,17] and at the level of the end organ [8–10]. Others have shown that statins can reduce sympathetic activity in the SHR and have suggested that a centrally mediated mechanism may be at least partly responsible for this. Four-week treatment with high dose atorvastatin (50 mg/kg/day) lowered urinary catecholamines [18] and was associated with increased eNOS expression and reduced oxidative stress in the rostral ventrolateral medulla of the SHR [19], an effect mimicked by eNOS adenoviral vector transfection to this area [20]. Similar sympatholytic actions of statins have been observed in a pacing induced heart failure model in the rabbit. Here simvastatin treatment normalised muscle sympathetic nerve activity, baroreflex function and plasma catecholamines [21,22] and improved left ventricular systolic function [23]. This was associated with downregulation of ATR1, NADPH oxidase subunits and reduced NADPH oxidase activity in the rostral ventrolateral medulla [23,24]. Our study is the first to show normalisation of peripheral cardiac sympathetic hyperactivity in terms of NE release following treatment with pravastatin, establishing that a significant component of the neuronal dysfunction resides at the end organ level.
The fact that there was no change in serum cholesterol levels suggests that the effects on sympathetic hyperactivity we observe are independent from inhibition of HMG Co-A reductase. We observed that the same treatment regime did lower serum cholesterol levels in WKY rats, although the WKY had far higher baseline cholesterol levels than the SHR, which is consistent with other reports (e.g. [25]). Others also observe no change in serum cholesterol in the SHR with both longer durations of treatment and higher doses of pravastatin (e.g. 5 mg/kg for 8 weeks [26], 20 mg/kg/day for 4 weeks [27] or 50 mg/kg/day for 6 weeks [28]). The 9–10 mg/kg/day dose used in this study may appear high when directly compared to the 40–80 mg/day pravastatin dose usually prescribed in humans. However, it is well known that rats metabolise statins to a greater extent than humans, and the dose used in this study is at the lower end of the ranges used in previous studies in the SHR model, which have varied from 5 mg/kg/day [26] to 50 mg/kg/day [28].
We observed a significant reduction in resting heart rate in-vivo following pravastatin treatment, although there was no difference in baseline intrinsic beating rate of the sinoatrial node preparation in-vitro supporting a pre-synaptic reduction in sympathetic neurotransmission. Given the larger differences in the heart rate response to stellate stimulation we observe in-vitro, it is likely that heart rate during daily activity in-vivo may be reduced by an even greater degree by statin treatment although this was not directly measured. We observed no fall in mean arterial blood pressure or reduction in left ventricular hypertrophy (heart weight/body weight ratio) in the SHR. Other studies using longer treatment protocols in younger rats before the hypertensive phenotype clearly manifests have observed antihypertensive effects in both anaesthetised and telemetered SHRs. For example, 4-week treatment with 20 mg/kg/day pravastatin lowers blood pressure, reduces left ventricular hypertrophy and improves vascular function via increased NO bioavailability and reduced oxidative stress in 8-week-old SHRs [27]. Eight-week treatment with 5 mg/kg/day in similarly aged SHRs also reduces blood pressure, left ventricular hypertrophy and susceptibility to arrhythmias [26]. We used a short 2-week treatment protocol in 16–18 week old SHRs with established hypertension. Whether a longer treatment protocol in this age group of SHR would produce a reversal of the hypertensive phentotype and reduce left ventricular hypertrophy remains to be determined.
4.2. Mechanism of action of Pravastatin on cardiac sympathetic hyperactivity
The pleiotropic actions of statins on oxidative stress, NO bioavailability and ATR1 expression have been well characterised (see [4] and [5] for reviews). Here we observe no change in NO dependent modulation of sympathetic neurotransmission following treatment with pravastatin in the SHR, and no change in the expression of either eNOS, nNOS or sGC protein whilst expression of NADPH oxidase subunits Gp91 and P40phox also remains unchanged. We demonstrated that inhibition of ATR1 with losartan equalised the release of 3H-NE between control and pravastatin treated SHRs. We found no significant change in cardiac ATR1 protein following pravastatin treatment, although other groups have found that statins reduced ATR1 expression in the brain [19]. However, we clearly demonstrate that pravastatin lowers cardiac ATII levels as well as expression of ACE 1 and 2 providing a mechanism for our observation. A reduction in overall cardiac ACE activity by statin treatment in conditions associated with left ventricular hypertrophy has been reported previously [29].
Interestingly plasma levels of ATII were not different between pravastatin supplemented and non-supplemented SHRs. If longer treatment protocols in this age group of SHR are able to lower plasma ATII levels then this may very well contribute to an antihypertensive effect in addition to the peripheral cardiac sympatholytic action of the drug. The ATR1 is also known to be up-regulated with hypercholesterolaemia [30], whereas the SHR has low overall cholesterol levels. This may be relevant to human hypertensive patients who also have hypercholesterolaemia where statins may have more of an antihypertensive and sympatholytic effect. Meta-analyses of the small trials using statins in hypertensive patients however suggest that the antihypertensive effects occur regardless of plasma cholesterol levels [6].
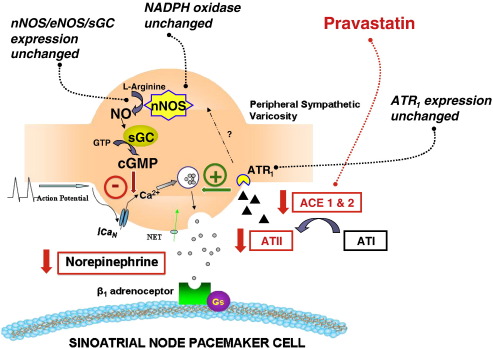
The presence of post-synaptic ATR1 on cardiac myocytes including sinoatrial node cells [31] is well documented. We directly demonstrate the presence of ATR1 on tyrosine hydroxylase positive sympathetic neurons innervating the heart. We also show that exogenous ATII can increase evoked cardiac 3H-NE release and that this can be blocked by the ATR1 antagonist losartan. The effect of ATII on cardiac neurotransmission was first demonstrated in the rabbit [32], but has since been shown to occur via the ATR1 in the WKY rat [14], and in human atria [33]. The mechanisms behind ATII dependent facilitation of NE release is not completely understood but may involve phospholipase C-diacylglycerol-protein kinase C dependent effects on the exocytotic mechanism [34]. It is also possible that the ATR1 indirectly couples to NOS or NOX-superoxide signalling (Fig. 7).
Fig. 7.
A diagram hypothesising the control of norepinephrine (NE) release from a cardiac sympathetic nerve terminal. NE release is inhibited by neuronal nitric oxide synthase (nNOS) via stimulation of soluble guanylyl cyclase (cGC) and inhibition of N type calcium current (ICaN) and subsequent calcium dependent exocytosis. nNOS and NE release are also modulated by oxidative stress. Conversely angiotensin II (ATII) stimulates NE release, although ATII levels are reduced in the SHR with 2-week treatment with pravastatin whilst ATII receptor 1 (ATR1) levels remain unchanged. Two-week statin treatment does not alter neuronal or endothelial NOS or sGC expression or the expression of the NADPH oxidase subunits Gp91 or P40phox.
4.3. Perspectives
Sympathetic hyperactivity is found in both humans and animal models of hypertension and has been implicated in both its aetiology and progression. Statins can produce an antihypertensive action in the SHR when initiated before the hypertensive phenotype manifests itself, and this is associated with a reduction in the activity of the sympathetic nervous system. Here we show that statin treatment reduces peripheral cardiac sympathetic hyperactivity even during established hypertension independent of both serum cholesterol and any antihypertensive effect. We provide evidence that this may occur via a reduction in local cardiac ATII and ACE 1 and 2 levels, a pleiotropic effect of statins not previously described, rather than via a nitric oxide or superoxide dependent mechanism.
As well as being used as antihypertensive agents, statins may also have anti-arrhythmic properties [24] since excessive cardiac sympathetic drive can exacerbate pre-existing cardiac disease and precipitate life-threatening ventricular arrhythmias. NE promotes myocyte calcium influx and increases the inotropic state of the heart, thereby raising myocardial oxygen demand in the context of an elevated heart rate and reduced diastolic coronary perfusion time. Unsurprisingly indices of autonomic function suggest excessive adrenergic drive is a poor prognostic indicator and can predict mortality during many cardiovascular disease states [35,36] as well as in asymptomatic individuals [37]. Targeting excessive cardiac sympathetic drive directly, and combining this with indirect targeting via the use of statins, is likely to be beneficial.
Funding
This work was supported by a British Heart Foundation Centre of Research Excellence Award, Oxford. NH is a Clinical Lecturer in Cardiovascular Medicine at the University of Oxford and Specialist Registrar in Cardiology at the Oxford Radcliffe Hospitals NHS Trust. CWL was a Wellcome Cardiovascular Research Initiative Junior Research Fellow NS and KW hold scholarships at St. Hughes College, Oxford.
Disclosures/conflicts of interest
None.
Contributor Information
Neil Herring, Email: neilherring@doctors.org.uk.
David J. Paterson, Email: david.paterson@dpag.ox.ac.uk.
References
- 1.Laufs U., Liao J.K. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 2.Kureishi Y., Luo Z., Shiojima I., Bialik A., Fulton D., Lefer D.J. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wassmann S., Laufs U., Bäumer A.T., Müller K., Konkol C., Sauer H. Inhibition of geranylgeranylation reduces angiotensin II-mediated free radical production in vascular smooth muscle cells: involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol Pharmacol. 2001;59:646–654. doi: 10.1124/mol.59.3.646. [DOI] [PubMed] [Google Scholar]
- 4.Jasińska M., Owczarek J., Orszulak-Michalak D. Statins: a new insight into their mechanisms of action and consequent pleiotropic effects. Pharmacol Rep. 2007;59(5):483–499. [PubMed] [Google Scholar]
- 5.Wang C.Y., Liu P.Y., Liao J.K. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med. 2008;14(1):37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh K.K., Quon M.J., Waclawiw M.A. Are statins effective for simultaneously treating dyslipidemias and hypertension? Atherosclerosis. 2008;196(1):1–8. doi: 10.1016/j.atherosclerosis.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension. 2009;54(4):690–697. doi: 10.1161/HYPERTENSIONAHA.108.119883. [DOI] [PubMed] [Google Scholar]
- 8.Li D., Wang L., Lee C.W., Dawson T.A., Paterson D.J. Noradrenergic cell specific gene transfer with neuronal nitric oxide synthase reduces cardiac sympathetic neurotransmission in hypertensive rats. Hypertension. 2007;50(1):69–74. doi: 10.1161/HYPERTENSIONAHA.107.088591. [DOI] [PubMed] [Google Scholar]
- 9.Heaton D.A., Li D., Almond S.C., Dawson T.A., Wang L., Channon K.M. Gene transfer of nNOS into intracardiac ganglia reverses vagal impairment in hypertensive rats. Hypertension. 2007;49:380–388. doi: 10.1161/01.HYP.0000255792.97033.f7. [DOI] [PubMed] [Google Scholar]
- 10.Danson E.J., Li D., Wang L., Dawson T.A., Paterson D.J. Targeting cardiac sympatho-vagal imbalance using gene transfer of nitric oxide synthase. J Mol Cell Cardiol. 2009;46(4):482–489. doi: 10.1016/j.yjmcc.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Heaton D.A., Lei M., Li D., Golding S., Dawson T.A., Mohan R.M. Remodelling of cardiac pacemaker I_CaL and beta-adrenergic responsiveness in hypertension following nNOS gene transfer. Hypertension. 2006;48:443–452. doi: 10.1161/01.HYP.0000233383.04280.3c. [DOI] [PubMed] [Google Scholar]
- 12.Lee C.W., Li D., Channon K.M., Paterson D.J. L-Arginine supplementation reduces cardiac noradrenergic neurotransmission in spontaneously hypertensive rat. J Mol Cell Cardiol. 2009;47(1):149–155. doi: 10.1016/j.yjmcc.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohan R.M., Golding S., Paterson D.J. Intermittent hypoxia modulates nNOS expression and heart rate response to sympathetic nerve stimulation. Am J Physiol Heart Circ Physiol. 2001;281(1):H132–138. doi: 10.1152/ajpheart.2001.281.1.H132. [DOI] [PubMed] [Google Scholar]
- 14.Sasaoka T., Egi Y., Tawa M., Yamamoto A., Ohkita M., Takaoka M. Angiotensin II type 2 receptor-mediated inhibition of norepinephrine release in isolated rat hearts. J Cardiovasc Pharmacol. 2008;52(2):176–183. doi: 10.1097/FJC.0b013e31818127f8. [DOI] [PubMed] [Google Scholar]
- 15.Dawson T.A., Li D., Woodward T., Barber Z., Wang L., Paterson D.J. Cardiac cholinergic NO-cGMP signaling following acute myocardial infarction and nNOS gene transfer. Am J Physiol Heart Circ Physiol. 2008;295(3):H990–H998. doi: 10.1152/ajpheart.00492.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Judy W.V., Farrell S.K. Arterial baroreceptor reflex control of sympathetic nerve activity in the spontaneously hypertensive rat. Hypertension. 1979;1:605–614. doi: 10.1161/01.hyp.1.6.605. [DOI] [PubMed] [Google Scholar]
- 17.Kasparov S., Teschemacher A.G. Altered central catecholaminergic transmission and cardio-vascular disease. Exp Physiol. 2008;93:725–740. doi: 10.1113/expphysiol.2007.041814. [DOI] [PubMed] [Google Scholar]
- 18.Kishi T., Hirooka Y., Mukai Y., Shimokawa H., Takeshita A. Atorvastatin causes depressor and sympatho-inhibitory effects with upregulation of nitric oxide synthases in stroke-prone spontaneously hypertensive rats. J Hypertens. 2003;21(2):379–386. doi: 10.1097/00004872-200302000-00030. [DOI] [PubMed] [Google Scholar]
- 19.Kishi T., Hirooka Y., Shimokawa H., Takeshita A., Sunagawa K. Atorvastatin reduces oxidative stress in the rostral ventrolateral medulla of stroke-prone spontaneously hypertensive rats. Clin Exp Hypertens. 2008;30(1):3–11. doi: 10.1080/10641960701813429. [DOI] [PubMed] [Google Scholar]
- 20.Kishi T., Hirooka Y., Ito K., Sakai K., Shimokawa H., Takeshita A. Cardiovascular effects of overexpression of endothelial nitric oxide synthase in the rostral ventrolateral medulla in stroke-prone spontaneously hypertensive rats. Hypertension. 2002;39(2):264–268. doi: 10.1161/hy0202.102701. [DOI] [PubMed] [Google Scholar]
- 21.Pliquett R.U., Cornish K.G., Peuler J.D., Zucker I.H. Simvastatin normalizes autonomic neural control in experimental heart failure. Circulation. 2003;107:2493–2498. doi: 10.1161/01.CIR.0000065606.63163.B9. [DOI] [PubMed] [Google Scholar]
- 22.Pliquett R.U., Cornish K.G., Zucker I.H. Statin therapy restores sympathovagal balance in experimental heart failure. J Appl Physiol. 2003;95(2):700–704. doi: 10.1152/japplphysiol.00265.2003. [DOI] [PubMed] [Google Scholar]
- 23.Gao L., Wang W., Li Y.L., Schultz H.D., Liu D., Cornish K.G. Simvastatin therapy normalizes sympathetic neural control in experimental heart failure: roles of angiotensin II type 1 receptors and NAD(P)H oxidase. Circulation. 2005;112(12):1763–1770. doi: 10.1161/CIRCULATIONAHA.105.552174. [DOI] [PubMed] [Google Scholar]
- 24.Gao L., Wang W., Zucker I.H. Simvastatin inhibits central sympathetic outflow in heart failure by a nitric-oxide synthase mechanism. J Pharmacol Exp Ther. 2008;326(1):278–285. doi: 10.1124/jpet.107.136028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carneado J., Alvarez de Sotomayor M., Perez-Guerrero C., Jimenez L., Herrera M.D., Pamies E. Simvastatin improves endothelial function in spontaneously hypertensive rats through a superoxide dismutase mediated antioxidant effect. J Hypertens. 2002;20(3):429–437. doi: 10.1097/00004872-200203000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Lee T.M., Lin M.S., Chou T.F., Tsai C.H., Chang N.C. Effect of pravastatin on development of left ventricular hypertrophy in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2005;289(1):H220–227. doi: 10.1152/ajpheart.00417.2004. [DOI] [PubMed] [Google Scholar]
- 27.Kassan M., Montero M.J., Sevilla M.A. Chronic treatment with pravastatin prevents early cardiovascular changes in spontaneously hypertensive rats. Br J Pharmacol. 2009;158(2):541–547. doi: 10.1111/j.1476-5381.2009.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bezerra D.G., Mandarim-de-Lacerda C.A. Beneficial effect of simvastatin and pravastatin treatment on adverse cardiac remodelling and glomeruli loss in spontaneously hypertensive rats. Clin Sci (Lond) 2005;108(4):349–355. doi: 10.1042/CS20040292. [DOI] [PubMed] [Google Scholar]
- 29.Luo J.D., Zhang W.W., Zhang G.P., Guan J.X., Chen X. Simvastatin inhibits cardiac hypertrophy and angiotensin-converting enzyme activity in rats with aortic stenosis. Clin Exp Pharmacol Physiol. 1999;26(11):903–908. doi: 10.1046/j.1440-1681.1999.03165.x. [DOI] [PubMed] [Google Scholar]
- 30.Strehlow K., Wassmann S., Bohm M., Nickenig G. Angiotensin AT1 receptor over-expression in hypercholesterolaemia. Ann Med. 2000;32:386–389. doi: 10.3109/07853890008995944. [DOI] [PubMed] [Google Scholar]
- 31.Sechi L.A., Griffin C.A., Grady E.F., Kalinyak J.E., Schambelan M. Characterization of angiotensin II receptor subtypes in rat heart. Circ Res. 1992;71(6):1482–1489. doi: 10.1161/01.res.71.6.1482. [DOI] [PubMed] [Google Scholar]
- 32.Blumberg A.L., Ackerly J.A., Peach M.J. Differentiation of neurogenic and myocardial angiotensin II receptors in isolated rabbit atria. Circ Res. 1975;36(6):719–726. doi: 10.1161/01.res.36.6.719. [DOI] [PubMed] [Google Scholar]
- 33.El Muayed M., Stegbauer J., Oberhauser V., Vonend O., Rump L.C. AT1 and AT2-receptor antagonists inhibit Ang II-mediated facilitation of noradrenaline release in human atria. J Cardiovasc Pharmacol. 2004;43(2):318–324. doi: 10.1097/00005344-200402000-00024. [DOI] [PubMed] [Google Scholar]
- 34.Kubista H., Boehm S. Molecular mechanisms underlying the modulation of exocytotic noradrenaline release via presynaptic receptors. Pharmacol Ther. 2006;112:213–242. doi: 10.1016/j.pharmthera.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Cohn J.N., Levine T.B., Olivari M.T., Garberg V., Lura D., Francis G.S. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 36.La Rovere M.T., Bigger J.T., Jr., Marcus F.I., Mortara A., Schwartz P.J. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 37.Jouven X., Empana J.P., Schwartz P.J., Desnos M., Courbon D., Ducimetière P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352(19):1951–1958. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]