Abstract
Metabolic syndrome (MetS) is a complex entity consisting of multiple interrelated factors including insulin resistance, central adiposity, dyslipidemia, endothelial dysfunction and atherosclerotic disease, low-grade inflammation, and in males, low testosterone levels. MetS has been linked to a number of urologic diseases including nephrolithiasis, benign prostatic hyperplasia and lower urinary tract symptoms, erectile dysfunction, male infertility, female incontinence, and prostate cancer. This article reviews the relationships between MetS and these entities. Urologists need to be cognizant of the impact that MetS has on urologic diseases as well as on overall patient health.
Key words: Metabolic syndrome, Insulin resistance, Obesity, Cardiovascular disease, Hypogonadism, Nephrolithiasis, Endothelial dysfunction, Benign prostatic hyperplasia, Lower urinary tract symptoms
Known in the past as syndrome X, deadly quartet, and insulin resistance syndrome,1 metabolic syndrome (MetS) generally refers to a constellation of interrelated cardiac risk factors consisting of insulin resistance (IR) (impaired insulin action), visceral obesity, atherogenic dyslipidemia, endothelial dysfunction, and systemic inflammation.2 Throughout the years, numerous definitions of MetS have been proposed by various organizations, including the World Health Organization (WHO), European Group for the Study of Insulin Resistance (EGIR), International Diabetes Foundation (IDF), the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III), and the American Association of Clinical Endocrinologists (AACE) (Table 1). Each of the definitions shares many similarities, including the presence of criteria relating to obesity, hyperglycemia, dyslipidemia, and hypertension (HTN). However, several differences among the classifications are noted. The laboratory values that define these aforementioned criteria and the number of positive criteria necessary to be classified as having MetS vary among the organizations. Additionally, the WHO, AACE, EGIR, and IDF require the presence of certain criteria (eg, evidence of insulin resistance, hyperinsulinemia, or central obesity) to fulfill their classification of MetS, whereas the NCEP ATP III lacks this constraint. The NCEP ATP III definition is the one most used today because it incorporates the key concepts of MetS, relies on commonly used laboratory studies available to most physicians, and is less restrictive than the other classifications.2
Table 1.
Metabolic Syndrome Definitions
| WHO (1998) | EGIR (1999) | AACE (2003) | IDF (2005) | NCEP ATP III (2005 Revision) | |
| Required component | IR (IGT, IFG, T2DM, or additional evidence of IR) | Hyperinsulinemiaa (plasma insulin > 75th percentile) | IR (IGT or IFG) | CO (WC)b | None |
| Criteria | Required component and ≥ 2/5 below | Required component and ≥ 2/4 below | Required component and any below based on clinical judgment | Required component and ≥ 2/4 below | ≥ 3/5 below |
| Obesity | WHR > 0.9 (M), > 0.85 (F) or BMI > 30 kg/m2 | WC ≥ 94 cm (M), ≥ 80 cm (F) | BMI ≥ 25 kg/m2 | — | WC > 102 cm (M) > 88 cm (F) |
| Hyperglycemia (mg/dL) | + | + | + | Fasting glucose ≥ 100 | Fasting glucose ≥ 100 or Rx |
| Dyslipidemia (mg/dL) | TG ≥ 150 or HDL-C < 35 (M), < 39 (F) | TG ≥ 150 or HDL-C < 39 | TG ≥ 150 and HDL-C < 40 (M), < 50 (F) | TG ≥ 150 or Rx | TG ≥ 150 or Rx |
| HDL < 40 (M), < 50 (F), or Rx | HDL < 40 (M), 50 (F), or Rx | ||||
| Hypertension (mm Hg) | > 140/90 | > 140/90 or Rx | > 130/85 | > (S), > 85 (D) or Rx | > 130 (S), > 85 (D) or Rx |
| Other criteria | Microalbuminuriac | Other features of IRd |
ACCE, American Association of Clinical Endocrinologists; BMI, body mass index; CO, central obesity; D, diastolic; Dx, diagnosis; EGIR, European Group for the Study of Insulin Resistance; HDL, high-density lipoprotein; IDF, International Diabetes Foundation; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; IR, insulin resistance; NCEP ATP III, National Cholesterol Education Program Adult Treatment Panel III; Rx, pharmacologic intervention for that criterion; S, systolic; T2DM: type 2 diabetes mellitus; TG, triglycerides; WC, waist circumference; WHO, World Health Organization; WHR, waist-hip ratio.
+ Criteria fulfilled with required component.
In patients without T2DM.
Values population dependent.
Urinary albumin excretion of 20 μg/min or albumin-to-creatinine ratio of ≥ 30 mg/g.
This includes family history of T2DM, polycystic ovary syndrome, sedentary lifestyle, advancing age, and ethnic groups susceptible to T2DM.
Epidemiology of MetS
MetS has become quite prevalent within our society. Approximately 35% to 39% of the adult population in the United States has MetS, with similar rates in men and women.3 This is not surprising because the percentage of overweight and obese individuals in the United States is growing, with the greatest escalation seen in the so-called superobese (body mass index [BMI] ≥ 50).4,5 Currently, approximately 32% of our population is obese, with higher rates seen in African Americans and Hispanics.3 The prevalence6,7 and incidence7,8 of type 2 diabetes mellitus (T2DM) are increasing as well. The United States does not appear to be unique in this trend as obesity and MetS have generally become prevalent throughout the world.9,10
Sadly, the growing epidemic of obesity and T2DM and their respective consequences are not limited to the adult population. In the past 30 years, the number of overweight children has more than doubled in the United States.11 As in adults, obesity in children is associated with an increase in MetS features such as IR, HTN, dyslipidemia, and T2DM.11,12 T2DM itself is also increasing in children and adolescents in the United States, with T2DM now accounting for approximately 20% to 25% of childhood diabetes.12
MetS and Cardiovascular Disease
It is well established that MetS imposes numerous cardiovascular risks. A diagnosis of MetS (or even 1 to 2 of its components) increases the risk for both cardiovascular disease (CVD) and T2DM.13,14 One study demonstrated that men with 4 or 5 features of MetS, compared with those with none, had a 3.7-fold increase in coronary heart disease (CHD) and an impressive 24.5-fold increase in T2DM.15 The risks of all-cause mortality as well as death from CHD and CVD were also increased.14 Wilson and colleagues noted that more than any 3 of the following including low highdensity lipoprotein (HDL) cholesterol, elevated BMI, elevated systolic blood pressure (SBP), elevated triglycerides (TG), or elevated serum glucose were associated with more than double the risk for coronary artery disease (CAD).16
MetS is not only associated with the aforementioned cardiovascular and systemic diseases but it also impacts a number of urologic disorders. Prior to elaborating on this relationship, we review pertinent concepts regarding MetS. Subsequently, the influence of MetS on these various urologic maladies is examined.
MetS and Inflammation
MetS has been associated with elevated levels of C-reactive protein (CRP), a nonspecific marker of inflammation, as well as the proinflammatory cytokines IL-1β, IL-6, and tumor necrosis factor-α (TNF-α).17–19 One etiology may be inflamed adipose tissue. Obesity induces adipose cell enlargement and chemokine release, leading to macrophage infiltration of adipose tissue.20 Macrophage infiltration further perpetuates the proinflammatory state and may account for the adipose secretion of adipokines such as IL-1β, IL-6, IL-8, CRP, and TNF-α.20–23 Macrophage infiltration of adipose tissue has also been shown to contribute to obesity-related IR.20,24 CRP is associated with numerous MetS features including abdominal obesity, IR and T2DM, HTN, low HDL, as well as elevated TG, IL-6, and TNF-α.18,25 CRP has also been shown to stimulate synthesis of IL-8 in human aortic endothelial cells,26 suggesting a role for this cytokine in MetS. A recent study determined that IL-8 is significantly elevated in heart failure patients with MetS as compared with those without MetS.26 Higher IL-8 levels have also been noted in patients with T2DM and it has been further directly correlated with hemoglobin A1c (HbA1c) levels.27 Additionally, IL-6, IL-1β, and CRP are all known to impair insulin signaling so it is not surprising that they too are associated with increased risk of developing diabetes.18,21,22 Therefore, a proinflammatory state may contribute to MetS and its features.
MetS and Endothelial Dysfunction
Features of MetS such as IR, hyperglycemia and resulting advanced glycation end-products (AGEs), free fatty acids (FFAs), and chronic inflammation may lead to endothelial damage and atherosclerosis.2,28–30 With endothelial dysfunction, there is a decrease in vascular nitric oxide (NO) levels.2 NO has crucial functions in maintaining vascular health. It defends against the initiation of atherosclerosis by inhibiting adhesion of platelets and leukocytes to the vascular wall as well as decreasing proliferation of vascular smooth muscle.30 A decrease in NO levels may therefore abolish these protective functions. Increased CRP levels have been shown to decrease NO synthesis in endothelial cells.31 Another component of MetS-hyperglycemia-also promotes increased production of free radicals such as superoxide anion resulting in NO inactivation.30 Hyperinsulinemia and endothelial dysfunction in T2DM may contribute to increased levels of the vasoconstrictor endothelin-1 (ET-1).30,32 Diminished insulin regulation of NO and ET-1 secondary to IR may decrease NO production while ET-1 production is maintained, thus promoting vasoconstriction.29,33 Elevated FFAs, associated with dyslipidemia in MetS, can cause endothelial dysfunction through increased free radical production and inhibition of NO synthesis via activation of protein kinase C, a pathway that ultimately leads to decreased NO synthase (NOS) activation.30 Components of MetS can also lead to increased arterial stiffness.29 AGEs formed on the vascular wall secondary to hyperglycemia lead to collagen cross-linking and loss of vascular elasticity.29 Hyperinsulinemia and hyperglycemia may locally activate the renin-aldosteroneangiotensin system, leading to elevated angiotension-1 receptor expression and subsequent vascular fibrosis and hypertrophy.29 These aforementioned findings demonstrate the strong relationship between MetS and endothelial dysfunction as well as the importance of NO in this physiology.
MetS and Male Hypogonadism
There is growing evidence that testosterone in men plays a major role not only in aspects of sexual health, but also in muscle and bone mass maintenance, erythropoiesis, and even glucose and lipid metabolism.34 Low testosterone levels are significantly associated with prevalence of MetS.35–37 Men with prostate cancer (PCa) who undergo long-term androgen deprivation therapy (ADT) also have significantly higher rates of MetS than those who do not.38 Numerous studies have demonstrated that MetS features such as HTN,39,40 obesity,36,38,41 hyperinsulinemia,41–44 T2DM,45,46 hyperglycemia44,47 (including elevated HbA1c48), hypertriglyceridemia,36,41,49 elevated CRP,47,50 as well as low HDL38,41,47 are associated with diminished serum testosterone levels. A reduction in sex hormone-binding globulin (SHBG) has also been reported.35,37,40,46,48 Weight loss may reverse these trends. Niskanen and colleagues51 demonstrated that maintained weight loss in obese men with MetS at 12 months increased free testosterone levels and reduced hypogonadism risk. At the same endpoint, they also noted a beneficial impact on insulin sensitivity, fasting plasma glucose, TG, and serum HDL levels.51 Moreover, treatment with testosterone appears to alleviate features of MetS. Boyanov and colleagues52 randomized 48 males with T2DM to either oral testosterone undecanoate (TU) therapy for 3 months or placebo, and demonstrated that the treatment arm had significant improvements in weight, waist-hip ratio (WHR) (a measure of abdominal obesity), and percentage of body fat. Additionally, fasting blood glucose (FBG) and HbA1c in the TU group significantly improved in both compared with baseline TU and control groups.52
A link between hypogonadism and MetS may be leptin, a 16-kDa protein synthesized and secreted mostly by adipocytes.53,54 Leptin is believed to regulate energy intake and utilization, principally via the hypothalamus.50 Serum leptin is directly associated with BMI50,54 and studies have demonstrated that leptin is inversely associated with testosterone levels. 55,56 Isidori and associates55 reported that elevated levels of leptin in obese men may lead to decreased luteinizing hormone (LH)-stimulated testosterone synthesis. Additionally, Luukkaa and colleagues56 noted that, in elderly men without diabetes, testosterone enanthate injections significantly lowered leptin levels while increasing testosterone levels, and that 3 months after treatment was stopped, levels of both hormones returned to baseline. Similarly, in men with T2DM and hypogonadism, testosterone replacement also resulted in significantly decreased serum leptin levels.50
Hypogonadism associated with MetS in males may be related to the low-grade inflammatory state known to occur in the latter. It has been reported that in Leydig cells, TNF-α inhibits steroidogenesis and IL-1 inhibits cholesterol side chain cleavage by cytochrome P450, thereby leading to diminished testosterone synthesis.17
Increased visceral adiposity, a feature of MetS, may contribute to hypogonadism through aromatase activity. Aromatase is an adipose enzyme that is involved in the irreversible conversion of testosterone to estrogen. 57 Logically, because persons with MetS tend to have visceral adiposity, higher aromatase activity should lower testosterone and increase estradiol levels. Indeed, Vermeulen and coauthors58 demonstrated that obese men have significantly higher plasma estradiol levels than controls. The resultant decrease in testosterone and increase in estrogen can cause excessive visceral adipose deposition, leading to further elevated aromatase activity, creating a self-perpetuating loop coined the hypogonadal-obesity cycle.57 The impact of aromatase has been indirectly demonstrated by experiments in which its action has been blocked. Zumoff and colleagues59 treated 6 obese men with oral testolactone, an aromatase inhibitor, and after 6 weeks, noted significantly higher levels of testosterone and LH as well as decreased levels of estrogen compared with baseline. Elevated estrogen levels may, in turn, cause pituitary suppression leading to hypogonadotropic hypogonadism (HHG).59 There have been inconsistent data regarding plasma levels of gonadotropins such as gonadotropin-releasinghormone(GRH), LH, and follicle-stimulating hormone in obese individuals. Vermeulen and colleagues58 studied pulsatile LH secretion in 8 obese men and normal controls and found a lower mean diurnal LH level, LH pulse amplitude, and sum of LH pulse amplitudes in obese individuals. Additionally, in the obese group, the sum of LH pulse amplitudes was directly associated with testosterone levels.58 The increase in serum LH that was associated with a decrease in serum estrogen in the study by Zumoff and colleagues59 may also be a manifestation of reduced estrogen inhibition on the normal pituitary response.
Therefore, there does appear to be a link between MetS and hypogonadism, and furthermore, MetS features such as low-grade inflammation, elevated leptin and estrogen levels, and higher aromatase activity might contribute.
MetS and Urologic Diseases
Nephrolithiasis
Much like MetS and obesity, the prevalence of nephrolithiasis in the United States and other countries is increasing.60,61 There is evidence that these parallel changes might be linked. Studies have shown that MetS and its components (obesity/increased waist circumference, HTN, etc) are associated with increased rates of nephrolithiasis. A recent cross-sectional study from Italy demonstrated that individuals with MetS are twice as likely to have ultrasonographic evidence of nephrolithiasis.62 Using the NHANES III (Third National Health and Nutrition Examination Survey) data, West and associates63 similarly showed that the odds of self-reported stone disease are approximately twice as likely in individuals with MetS than those without.63 They also demonstrated that increasing numbers of MetS components are associated with greater stone reporting: 3% with no MetS components, 7.5% with 3 components, and 9.8% with 5 components.63 Waist circumference (WC), elevated BMI, diabetes, and HTN have also been correlated to nephrolithiasis risk.62,64,65
MetS may be contributing to the changing gender ratio of stone formers. Although nephrolithiasis remains more common in men than in women, there is evidence that this gender gap is decreasing. Scales and colleagues66 reported a decrease in the manwoman ratio of inpatient discharges for kidney stones from 1.7:1 in 1997 to 1.3:1 in 2002. They hypothesized that the disproportionate increase of overweight and obese women compared with men might explain this phenomenon. Furthermore, with respect to BMI, women have a higher percentage of body fat than men, which could result in metabolic differences influencing stone risk.64,66,67 In children, the epidemiology of nephrolithiasis is different from adults as boys are more prone to stones in the first decade of life whereas girls prevail in the second.68
There are a number of possible reasons for the association between MetS and nephrolithiasis. It has been demonstrated that features of MetS are associated with decreased urine pH. One study noted that increasing IR (measured by comparing glucose disposal rates via euglycemic clamp with 24-hour urine studies) is associated with more acidic urine.69 Maalouf and associates70 showed the same relationship, but used the homeostasis model assessment of IR. Several investigators have determined that urine pH decreases with increasing BMI.71–74 Another study demonstrated that as the number of MetS components increased (0, 1, 2, 3, 4, 5, respectively) urine pH decreased (6.15, 6.10, 5.99, 5.85, and 5.69, respectively).70 They also found that the components of MetS significantly associated with urinary pH are BMI, SBP, serum glucose, and serum HDL.70
Low urine pH is a well-described feature of uric acid urolithiasis, so it is not surprising that people with features of MetS tend to preferentially have uric acid stones or risk factors for their development. A higher prevalence of T2DM, glucose intolerance, and hypertriglyceridemia in pure uric acid stone formers has been reported.75 Others have demonstrated that patients with T2DM have a significantly higher prevalence of uric acid stones.76 Li and coauthors71 reported that a higher percentage of uric acid stone formers were obese (53.1%), as compared with those with calcium phosphate (34.3%), mixed calcium stones (38.7%), and calcium oxalate stones (42.7%). Studies have shown that BMI is also associated directly with uric acid excretion,74,77 serum uric acid level,74 and supersaturation of uric acid.77 Additionally, hypertensive stone formers more commonly have uric acid stones than normotensive stone formers.78
MetS may impact stone risk factors that promote the formation of alternate stone types such as calcium oxalate, calcium phosphate, or mixed stones (uric acid/calcium oxalate or calcium oxalate/calcium phosphate). Lower urine pH results in more uric acid crystals, which may contribute to heterogeneous nucleation and epitaxial crystal growth.79 Lemann and associates80 found a positive correlation between oxalate and lean body mass estimated by urinary creatinine excretion. In a retrospective study of 1021 patients with a history of nephrolithiasis, Ekeruo and colleagues72 demonstrated that obese individuals had a significantly higher prevalence of gouty diathesis (54%), hypocitraturia (54%), hyperuricosuria (43%), and hyperoxaluria (31%) compared with their nonobese counterparts. Additionally, calcium, oxalate, and uric acid excretion were higher in the obese cohort and these differences were more profound as the extent of obesity increased.72 Siener and colleagues74 also reported a positive correlation between BMI and uric acid excretion in a group of idiopathic calcium oxalate stone formers. They noted some gender differences, reporting that BMI was positively correlated with calcium excretion in men and oxalate excretion in women.74 Duffey and associates81 analyzed 24-hour urine samples in morbidly obese patients (mean BMI, 49.5) prior to bariatric surgery and found that greater than 97% had at least 1 risk factor for stone formation, with 80% having at least 3, the most common being low urine volume (71%). In both sexes, BMI was inversely related with urine magnesium excretion, whereas in females, BMI was also inversely associated with urinary citrate excretion.81 Similar to Ekeruo and colleagues, they also found that elevated BMI (> 45) was strongly associated with hypercalciuria (odds ratio [OR], 18).81 Taylor and Curhan73 analyzed individuals with and without nephrolithiasis in the Health Professionals Follow-Up Study (HPFS) (men), Nurses’ Health Study I (older women), and Nurses’ Health Study II (younger women). They found that BMI was directly related to oxalate excretion in women and also detected a direct correlation between BMI and urinary calcium excretion in both the male and younger female cohorts.73 However, individuals with a lower BMI consumed less sodium and animal protein, and when controlling for these dietary factors, the relationship between BMI and calcium excretion was not significant. Increased BMI was also indirectly associated with urinary citrate excretion, although this was seen only in men.73 One explanation of this relationship is that BMI is inversely related to urine pH, and low urine pH results in increased citrate resorption.82
Bidirectional associations between HTN and nephrolithiasis have been reported. Hypertensive patients are more at risk to develop stones,83,84 and those with stones are more likely to develop HTN.85–87 HTN has been shown to impact urinary stone risk factors. Positive correlations between HTN and calcium excretion in both those with a history of nephrolithiasis88 and those without84,89,90 have been reported. Taylor and colleagues also found this relationship in the HPFS.77 Borghi and colleagues84 reported that hypertensive men and women had significantly increased oxalate excretion and also found that men had increased uric acid excretion. Losito and associates78 demonstrated that the presence of HTN was associated with lower urinary pH and citrate excretion and higher titratable acid excretion. The authors also noted an inverse association between HTN severity and urinary pH and a direct relationship with titratable acid.78
Studies comparing increased weight in children and nephrolithiasis are limited. Several articles, however, have found associations between being overweight and lithogenic alterations in urine composition. One study showed that, in children with a history of kidney stones, increased BMI was associated with a decrease in urine oxalate excretion and an increase in calcium phosphate supersaturation. 91 Another group compared overweight and normal children, and found significant hypocitraturia, hyperoxaluria, and hypercalciuria in the former group.92
Several etiologies have been proposed for the association between MetS and risk factors for uric acid stones. As described before, low urine pH is an important and the most prevalent component of uric acid stone development. Other known risk factors include hyperuricosuria and low urinary volume.93 Causes of low urinary pH include excessive net acid excretion (NAE) and impaired ammonium (NH4+) excretion resulting in poor urine buffering.70,79 It has been demonstrated that NAE is significantly elevated in persons with MetS compared with those without the condition.70 Causes of amplified NAE include increased dietary acid intake (via correlation between urine sulfate and NAE), decreased dietary alkali intake (via urinary potassium and NAE), or excessive endogenous acid production.70 Ammonium is a key urinary buffer.94 Maalouf and colleagues70 noted that as the number of MetS features increased, ammonium excretion was impaired. One explanation may be IR, which has been linked to decreased ammonium excretion. Insulin has been shown to stimulate renal ammoniagenesis in rat,95 canine,96 and opossum kidney (OK) cells.97 Additionally, Klisic and colleagues98 demonstrated that insulin stimulates the Na+/H+ exchanger 3 (NHE3) in OKP cells (OK cells with proximal tubule characteristics). This allows tubular ammonia to be converted to ammonium, thereby trapping the molecule, which under low pH conditions within the tubule, may be the prevailing mechanism of ammonia excretion.99 When considering these effects of insulin on the kidney, it is conceivable that IR could also impair both ammoniagenesis and ammonium excretion.93
Lipotoxicity refers to the concept of excessive lipid accumulation in nonadipose tissues (eg, skeletal and cardiac myocytes, hepatocytes, pancreatic β cells) resulting in cell damage and impaired functioning.100,101 Lipotoxicity may exist in those with MetS100 and result in altered renal structure and function.102 Bobulescu and colleagues103 noted that Zucker diabetic fatty (ZDF) rats (a model of MetS) have increased TG steatosis, predominantly around the tubular portions of the kidney, and that this is associated with low urinary pH and NH4+ in addition to high titratable acidity. These urinary abnormalities are similar to those described in humans with MetS features. Additionally, they noted that the observed decrease in NH4+ excretion might be attributed to a lipotoxicity-induced reduction in NHE3 activity.103 These investigators also incubated OK clone P cells with FFAs and found that ammonium secretion decreased as the concentration of FFA in the medium increased.103 They also demonstrated that fat accumulation within these cells increased with higher concentrations of FFA in the medium.103 In a follow-up study, Bobulescu and colleagues demonstrated that reduction of renal steatosis in ZDF rats improved urinary NH4+, increased pH, reduced titratable acidity, and additionally increased citrate and brush border NHE3 levels and activity.94
Evidence linking features of MetS and hypercalciuria are limited. Schwille and colleagues104 compared males with idiopathic hypercalciuria (n = 30) with normal controls (n = 8) and noted that the former group demonstrated postprandial hyperinsulinemia and IR without hyperglycemia. Worcester and colleagues105 demonstrated that patients with idiopathic hypercalciuria postprandially excreted more calcium (compared with normal controls) secondary to reduced tubular calcium resorption, not an increased filtered load. Perhaps IR is the cause of this decreased resorption, resulting in hypercalciuria.61 Because IR is a component of MetS, it is possible that this might explain hypercalciuria seen in those with HTN or increased BMI.
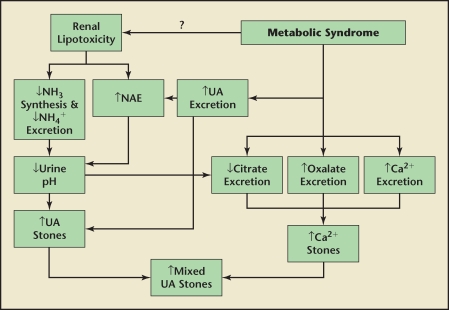
The cause for the positive correlation with body weight and oxalate excretion is unknown. This could be due to an increase in the endogenous synthesis of oxalate. IR may hypothetically increase the glucose pool and it is possible that the metabolism of this sugar is linked to endogenous oxalate synthesis. Figure 1 displays a diagram of the proposed associations between MetS and nephrolithiasis.
Figure 1.
Possible mechanisms of nephrolithiasis in metabolic syndrome (MetS). Several lithogenic factors are noted in MetS including increased uric acid (UA), oxalate, and calcium (Ca2+) excretion as well as decreased citrate excretion. Renal lipotoxicity may be a cause of elevated net acid excretion (NAE) in addition to decreased ammonia (NH3) synthesis and ammonium (NH4+) excretion, leading to lower urinary pH. These abnormalities respectively contribute to elevated UA, Ca2+, and mixed stones.
Benign Prostatic Hyperplasia and Lower Urinary Tract Symptoms
Components of MetS such as HTN, T2DM, hyperinsulinemia, abdominal obesity, and dyslipidemia have all been associated with an increased benign prostatic hyperplasia (BPH) risk.106–110 Hammarsten and colleagues108 demonstrated that men with T2DM, treated HTN, and dyslipidemia had higher median average BPH growth rates. The authors further noted that annual BPH growth rate was directly associated with diastolic blood pressure (DBP) and obesity and inversely associated with HDL levels. Multivariate analysis revealed that serum insulin levels were directly correlated with total prostatic volume.108 Ozden and colleagues106 studied 78 men with BPH and lower urinary tract symptoms (LUTS) and determined that presence versus absence of MetS was associated with significantly higher total prostate (TP) growth rate (1.0 vs 0.64 mL/y, respectively) and median annual transition zone (TZ) growth rate (1.25 vs 0.93 mL/y, respectively). Additionally, MetS patients were noted to have significantly higher serum prostate-specific antigen (PSA) levels,106 which may be an index for prostatic volume106,111 and LUTS.112
Investigators have reported conflicting associations between MetS and LUTS. Using data from the Boston Area Community Health Survey, Kupelian and colleagues113 demonstrated that the presence of MetS was significantly associated with an elevated American Urological Association (AUA)-Symptom Index Score (score ≥ 2; multivariate OR, 1.68). When adjusting for age, men younger than 60 years with MetS were more likely to report intermittency, incomplete emptying, and nocturia.113 Demir and associates114 reported in a univariate analysis that MetS and its features such as HTN or treatment of HTN, elevated FBG or treatment of diabetes, and increased WC were all associated with greater BPH-related LUTS severity. Michel and colleagues115 noted in a retrospective, multivariate analysis that HTN was directly associated with BPH-related LUTS as indexed by International Prostate Symptom Score (IPSS) and inversely with maximal flow rate (Qmax). Similarly, diabetes was also associated with a higher IPSS and lower baseline Qmax.116 In a Japanese cross-sectional study, multivariate analysis revealed that men undergoing treatment of HTN and T2DM were more likely to report LUTS.117 Conversely, Temml and colleagues118 found no significant relationship between LUTS and MetS.
There is evidence of a relationship between MetS features and BPH/LUTS, and because the two are intertwined, both need to be considered when discussing pathophysiologic associations with MetS. One explanation involves hyperinsulinemia and autonomic hyperactivity. Hyperinsulinemia is associated with increased sympathetic activity via enhanced glucose metabolism in ventromedial hypothalamic neurons119 leading to increased activation of α-adrenergic receptors on smooth muscle in the prostatic capsule and bladder neck.106 This concept has been studied in both animal models and human subjects. In the rat model, elevated autonomic activity can induce BPH.120 McVary and colleagues121 assessed men with LUTS secondary to BPH for autonomic nervous system hyperactivity after tilt-table testing. They demonstrated that markers of autonomic hyperactivity (BP elevation, heart rate elevation, and elevated serum or urine catecholamines) were positively associated to subjective markers of LUTS (AUA Symptom Score, Quality of Life Score, and BPH Impact Index).121 Additional multivariate analysis showed a significant positive correlation between plasma norepinephrine levels after tilt testing and transition zone (TZ) volume.121 Mc-Vary hypothesized that increased autonomic activity can impact α-receptors, and therefore smooth muscle contraction, throughout male genitourinary tract structures including the prostate, bladder neck, and urethra, thereby contributing to LUTS.120 BPH/LUTS may also occur secondary to decreased NOS and NO activity in the prostate. Decreased nitrogenic (NO-related) innervation of the prostate coupled with lower NOS and NO activity in the TZ of BPH prostates has been reported.120,122 This diminished prostatic NOS and NO activity may lead to increased smooth muscle proliferation, prostatic enlargement, and subsequent LUTS.120 In addition, the distribution of eNOS and nNOS suggests that NO might serve to regulate regional prostatic vasculature.122 The aforementioned findings illustrate the potential importance of NO and NOS in BPH/LUTS and thereby further link MetS with these entities as endothelial dysfunction may be present in both. Additionally, NO and NOS may also play a role in another possible etiology of BPH/LUTS and MetS-pelvic atherosclerosis. Numerous animal studies have linked atherosclerosis and chronic ischemia to bladder and prostatic changes.120 Rabbits with induced pelvic atherosclerosis developed bladder fibrosis, smooth muscle atrophy, and decreased bladder compliance123 as well as chronic prostatic ischemia with resultant stromal and capsular fibrosis, glandular cystic atrophy, impaired smooth muscle relaxation, and increased prostatic weight.124,125 Moreover, it appears that in ischemic prostate models, diminished smooth muscle relaxation is regulated via an NO-dependent mechanism, 125 much like the cavernosal vascular bed.
The RHO-kinase (ROK) system may further contribute to the MetS and BPH/LUTS relationship. The ROK pathway appears to be important in maintenance of tonic contraction or high basal tone126 and may possibly contribute to prostate contractility. ROK causes smooth muscle contraction not by altering intracellular calcium levels but through modifying calcium sensitivity of the contractile machinery.120,127 The impact of ROK activity on smooth muscle is thought to be via inhibition of myosin-light chain phosphatase (MLCP), thereby promoting myosin-light chain phosphorylation and contraction through actin-myosin interaction.120,128,129 NO counteracts this by indirectly favoring the active form of MLCP.129 On the contrary, α-adrenergic activity stimulates the ROK pathway,120 providing a link between ROK and autonomic hyperactivity. IL-8 has also been shown to stimulate BPH cell proliferation via the ROK pathway.130 ET-1 stimulates this pathway as well,120,127 and we have previously noted that ET-1 production may be increased in patients with diabetes. Takahashi and colleagues127 demonstrated in cultured human prostatic stromal cells that ET-1-induced contraction was increased by ρ-A (a ROK activator) overexpression and decreased by ROK inhibition. Further experiments with human127 and rat126 prostate tissues revealed that ROK inhibition caused decreased NE-induced contraction. Rees and colleagues126 demonstrated that human and rat prostate smooth muscle cells showed decreased proliferation in the presence of Y-27632, a ROK inhibitor. Therefore, autonomic hyperactivity, higher ET-1 and IL-8 levels in addition to lower NO levels found in MetS may result in increased ROK activity and subsequent BPH/LUTS.
We have noted the ability of IL-8 to stimulate prostatic growth, suggesting that inflammation may also play a role in BPH/LUTS. Macrophage and T-lymphocyte infiltrates are commonly found in prostate tissue removed during open prostatectomy and transurethral resection of prostate (TURP).131 Additionally, the level of inflammation has been directly correlated with prostatic volume and IPSS.131 Several proinflammatory cytokines are upregulated in BPH, suggesting an immunologic etiology to the inflammation.130 In patients with BPH, seminal IL-8 levels positively correlate with LUTS via IPSS and PSA.132 The cytokines IL-6 and IL-8 are elevated in MetS, and may contribute to inflammation in BPH/LUTS as both can be secreted by stromal cells with cytokine stimulation, and both result in proliferation of prostatic tissues.133 Yet some question the importance of inflammation in these entities. Rohrmann and colleagues134 failed to show a definitive relationship between elevated CRP levels and BPH-related LUTS, but did note a stronger, albeit nonsignificant, relationship in those with MetS.
The sex hormonal milieu may further contribute to this linkage of BPH/LUTS and MetS. Similar to MetS, men with LUTS/BPH may have lower androgen and higher estrogen levels. Analyzing 260 men older than 60 years from the NHANES III Trial, Rohrmann and associates135 noted that elevated estrogen levels and molar estradiol/testosterone ratios as well as lower androstanediol glucuronide (a metabolite of dihydroxytestosterone [DHT]) levels were associated with greater LUTS risk. Schatzl and colleagues136 analyzed 312 men (mean age, 62.8 years) with LUTS secondary to untreated BPH and found a direct correlation between elevated estradiol levels and prostatic volume determined with transrectal ultrasound. Multivariate analysis from the Physician’s Health Study also showed that increasing estradiol levels were significantly associated with BPH surgery in men with low testosterone and/or DHT levels.137 However, others have not found evidence of such definitive relationships.138,139 Urodynamic parameters can also be impacted by these hormonal abnormalities. Koritsiadis and colleagues140 analyzed 25 men with confirmed bladder outlet obstruction who had undergone either TURP or suprapubic prostatectomy with hormonal measurements and urodynamics. They demonstrated a significant inverse correlation between testosterone values and detrusor pressure at the end of urinary flow and detrusor pressure at Qmax.140 Additionally, they noted that low testosterone levels were associated with detrusor overactivity, defined as biphasic detrusor contraction during the filling phase.140 Although there have been no definitive data linking low testosterone levels to BPH/LUTS, testosterone replacement appears to improve LUTS. Haider and colleagues141 studied hypogonadal men (mean age, 59.5 years) and demonstrated significant decreases in IPSS score and residual bladder volume after 9 months of TU treatment. Kalinchenko and colleagues142 assessed 30 men (mean age, 51 years) with hypogonadism, and subsequently divided them into 2 treatment groups (10 with testosterone gel and 20 with TU). At the 26-week endpoint, the authors noted significant improvements in IPSS scores of both groups.142
A discussion of the mechanistic role that estrogens play in BPH/LUTS is germane given the aforementioned relationships. Recent experiments have shown interesting mechanisms of estrogen impact on BPH. Classically, it is known that estrogens passively cross the cellular membrane, bind an intracellular receptor (estrogen receptor [ER] α or β), and after transfer to the nucleus, function as a transcriptional regulator.143,144 There is some evidence that high estradiol concentrations can result in upregulation of ERs in prostatic stromal cells.145 In cultured BPH specimens, estradiol stimulates proliferation of the stromal but not epithelial component of the prostate, via intracellular ERs.143 Additionally, Ho and associates143 demonstrated that aromatase activity was limited to the prostatic stroma, and that this might serve to regulate local estradiol levels and subsequent proliferation. In the dog model, estrogen activation may cause formation of radical species, resulting in prostate damage and an increased susceptibility to DHTinduced growth.146 Estrogens have also been demonstrated to induce an inflammatory response in lateral prostates of castrated rats and increasing transcript levels of IL-1β and IL-6.133 Interestingly, a recent study compared stromal prostate cells from normal and BPH specimens and found that both responded to lower levels of estradiol than previously believed. 144 The authors demonstrated that estradiol activates a different, nongenomic pathway in normal prostate cells, resulting in estradioldependent signaling via a mitogenactivated protein (MAP) kinase cascade, Erk1/2.144 However, stromal cells derived from BPH prostate specimens responded to estradiol via the classic genomic pathway, and additionally, were noted to have consistent levels of Erk1/2 activity regardless of estradiol levels.144 The authors hypothesized that an increased estradiol/androgen ratio, which normally occurs with aging, may evoke changes such as constitutive Erk1/2 activation, resulting in stromal proliferation and differentiation, and perhaps, BPH.144 If MetS results in a similar alteration in the hormonal milieu, this can further explain how MetS might contribute to BPH/LUTS. Overall, the regulation and impact of estrogens on prostatic growth and BPH/LUTS is quite complex and requires further scientific inquiry.
Erectile Dysfunction
Studies have demonstrated that erectile dysfunction (ED) is up to 3 times more prevalent in individuals with MetS.147–152 Direct relationships between ED and various components of MetS including obesity,148,149,153–155 HTN,148,153,155 T2DM,156 and hypertriglyceridemia157 have also been shown. Esposito and colleagues150 noted that in age- and BMI-matched controls, higher ED prevalence was seen when 3 or more MetS components were present. Similarly, Bal and associates149 demonstrated that the OR of ED increased with the number of MetS components including the following: increased WC only, 1.94; increased WC plus abnormal HDL or TG level, 2.97; increased WC plus pathologic HDL and TG, 3.38. Using data from the Massachusetts Male Aging Study, Kupelian and colleagues158 showed that presence of MetS in those with a BMI < 25 increased ED risk approximately 2-fold. They postulated that ED could even be used to predict the development of MetS in the nonobese. Direct relationships between ED severity and MetS149,151 as well as number of MetS features148 have also been established. In a cross-sectional study, Heidler and colleagues noted that presence of MetS, T2DM, elevated WHR (a marker of central obesity), and HTN correlated with ED severity151 whereas Aslan and associates reported that only T2DM is a significant risk factor for severe ED.156
A brief review of erectile physiology is presented to facilitate an understanding of the mechanisms by which MetS can contribute to the development of ED. An erection involves dilatation of the penile arterial bed, relaxation of the trabecular smooth muscle, and decreased venous outflow, all of which increase cavernosal pressure.159 These events are mediated by the NO-cyclic guanosine monophosphate (cGMP) pathway. Nitrous oxide is synthesized in the endothelium of penile arteries and cavernosal sinuses and in nonadrenergic, noncholinergic nerves. This synthesis is catalyzed by NO synthases: endothelial (e)NOS, more crucial in maintaining erection; neuronal (n)NOS, more crucial in initiating erection. Nitrous oxide synthase converts L-arginine to NO, which subsequently diffuses into smooth muscle cells where it activates guanylate cyclase that converts guanosine-5-triphosphate (GTP) to cGMP. cGMP decreases intracellular calcium levels leading to vascular smooth muscle relaxation and corporal engorgement. The cGMP is degraded by phosphodiesterase-5 in the cavernosa, thereby promoting detumescence.160–165
Several interrelated reasons may explain the observed relationship between MetS and ED. Hypogonadism has been associated with MetS and its association with sexual dysfunction and ED is well known. Corona and colleagues36 demonstrated that low testosterone levels are associated with severe ED, hypoactive sexual desire, decreased nocturnal erections, and reduced intercourse frequency. The strongest association between low testosterone levels and decreased intercourse frequency and severe ED was seen in men over 62 years, suggesting that hypogonadism has a greater impact on older individuals.36 Additionally, in men over 62 years, low testosterone levels were significantly associated with decreased penile peak systolic velocity.36 Because testosterone has been demonstrated to increase cavernosal nNOS mRNA levels in rats,166 hypogonadism may result in diminished NO synthesis and subsequent ED.
Another attractive etiology is atherosclerotic disease, as the same mechanisms causing CAD in those afflicted with MetS could similarly impact the penile vascular bed. Shamloul and colleagues167 showed that, in men age > 40 years, presence of arterial vasculogenic ED was significantly associated with ischemic heart disease diagnosed with cardiac stress testing. Blumentals and colleagues168 analyzed information on 12,825 men in the Integrated Healthcare Information Services National Managed Care Benchmark Database and showed in a multivariate analysis that men with ED were almost twice as likely to have sustained a myocardial infarction. Other atherosclerotic risk factors such as prior or current smoking are associated with ED as well.152–155 Several studies have shown that physical activity reduces the probability of ED147,154 and this is also known to reduce the risks of CVD and MetS.169–171 The link between cardiac ischemic disease and ED is not surprising. Given the smaller size of the penile vasculature in relation to the coronary vessels, similar amounts of atherosclerotic burden may cause significant occlusion in the former prior to the latter, and as such, symptomatic ED might present prior to CAD.172 In fact, many have suggested that ED can serve as a marker of early vascular disease in men.158,173,174 In addition to diminished blood flow, atherosclerotic disease may actually lead to structural damage within the penis. Animal models of pelvic ischemia have shown similar structural alterations in bladder and prostate as well as penile tissues.120 In rabbit cavernosa, for example, ischemia and hypercholesterolemia have been shown to induce smooth muscle atrophy and fibrosis,175 possibly increasing ED predisposition.
Atherosclerotic disease, endothelial dysfunction, and other MetS factors may further impact penile NOS activity, and subsequently, ED. Yono and colleagues176 demonstrated that spontaneously hypertensive rats (SHR) had diminished circulation to the penis and that this was associated with lower mRNA expression of penile nNOS and eNOS than control rats. Decreased corporal expression of these constitutive NOS subtypes was also noted in a T2DM rat model and was associated with ED.177 Similarly, in rabbits with induced chronic penile ischemia, impaired cavernosal relaxation was again associated with decreased expression of cavernosal nNOS and eNOS and a concomitant increase in inducible (i)NOS expression.178 This may be partially explained by the ability of iNOS to decrease eNOS synthesis both in vitro and in vivo.179 Saenz de Tejada and colleagues180 analyzed penile corporal tissue from both patients with diabetes and nondiabetics with ED and found that the former group had decreased neural and endothelial-dependent smooth muscle relaxation. They concluded that a poor relaxation response was caused not by insensitivity to, but reduced synthesis or release of endothelium-derived NO.180 Esposito and colleagues150 compared 100 men with MetS with normal ageand BMI-matched controls and assessed ED and endothelial function (via L-arginine test). The investigators demonstrated that ED was directly associated with decreased NO availability. Chronic low-grade inflammation seen in MetS may further contribute to this phenomenon. We have previously noted that serum CRP levels are inversely related with NO synthesis. In addition, multivariate analysis has shown that markers of inflammation known to be elevated in MetS have also been demonstrated to be higher in those with ED, including (high sensitivity) CRP150,181,182 as well as IL-1β, IL-6, and TNF-α.181 CRP levels have been further directly correlated to extent of penile ischemia, assessed with penile Doppler studies.182 Increased iNOS levels have been previously related to ED in animal models, and its expression is increased by IL-1β, IL-6, and TNF-α.178
It is known that α-adrenergic activity results in corporal smooth muscle relaxation, a concept that is exploited during treatment of priapism with α-agonists.183,184 We have previously noted that hyperglycemia is associated with autonomic hyperactivity, and in the rat model, elevated autonomic activity was shown to induce ED.120 This was further corroborated by a recent study demonstrating that patients with nonorganic ED had significantly higher cardiac sympathetic activity than controls.185 In patients with BPH/LUTS, several studies have noted that nonselective α1-antagonists such as doxazosin186,187 and prostate-selective subtypes such as alfuzosin188,189 lead to improvements in sexual dysfunction, including ED. In SHR, treatment with doxazosin (α1-receptor antagonist) results in increased penile blood flow as well as increased penile expression of eNOS and nNOS compared with untreated or calcium channel antagonist (nifedipine)-treated SHR.190 Thus, excessive corporal adrenergic activity can further contribute to ED.
Features of MetS have been previously noted to stimulate the ROK pathway, which may further increase ED by inhibiting cavernosal relaxation. In T2DM rat models, ED is associated with increased cavernosal levels of a ROK receptor, ROK1, and a G-protein activator of ROK, RhoA.177 To a lesser extent, increased cavernosal type A endothelin receptor, was also noted in this study, and these receptors are involved in regulation of vasoconstriction.177 This finding is not surprising because ET-1 is known to stimulate the ROK pathway and elevated ET-1 levels have been associated with both T2DM30,32 and ED.191 The importance of ROK activity in erectile function was further demonstrated in an experiment where ROK inhibition resulted in diminished contraction of human and rat cavernosal tissue to α-adrenoreceptor stimulation with phenylephrine.192 In rats, NO can also inhibit ROK activity, and this is believed to contribute to NO-induced erection.193 Thus, disturbances found in MetS may result in increased ROK activation, further leading to ED.
LUTS/BPH, ED, and MetS: Common Etiological Links?
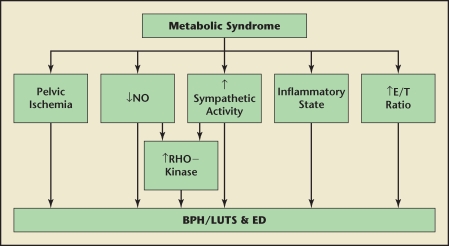
The association with ED and LUTS/BPH warrants further discussion as MetS is encountered in those afflicted with both entities. Demir and colleagues showed that ED was the only variable found to be an independent predictor of severe LUTS.114 Rosen and colleagues194 found that LUTS severity was significantly associated with increased frequency of ED, ejaculatory dysfunction, and decreased sexual activity. Multiple aspects of sexual dysfunction (including libido, erectile function, ejaculation, bother due to sexual problems, and overall sexual satisfaction) have also been positively correlated with LUTS.120,195 Several reasons for the link between ED and BPH/LUTS have been proposed, including pelvic atherosclerosis, decreased NO/NOS activity as well as increased autonomic and ROK activation.120 The relationship of these concepts with MetS has been examined. Pelvic atherosclerosis, or more specifically ischemia, can be linked to MetS as endothelial dysfunction, atherosclerosis, and increased CVD are all features seen in MetS. Multiple aspects of MetS including IR, hyperglycemia, AGEs, FFAs, endothelial dysfunction, and chronic inflammation all contribute to decreased NO bioavailability. Likewise, hyperinsulinemia has been shown to contribute to autonomic hyperactivity. Finally, excessive ROK activation can result in greater tonic muscle contraction contributing to both BPH/LUTS and ED. Moreover, this pathway may be augmented by ET-1, autonomic hyperactivity, and IL-8 activity. Low testosterone levels are linked definitively with ED, and some evidence suggests that deficient androgens and/or excessive estrogens may contribute to BPH/LUTS. Therefore, these processes, working independently or in concert with one another, may explain the association with both BPH/LUTS and ED. These relationships are summarized in Figure 2.
Figure 2.
Possible mechanisms of benign prostatic hyperplasia, lower urinary tract symptoms (LUTS), and erectile dysfunction (ED) in metabolic syndrome (MetS). Features that may be seen in MetS, including pelvic atherosclerosis and ischemia, decreased nitric oxide (NO) availability, elevated sympathetic activity, low-grade inflammatory state, and elevated estrogen/testosterone ratio can contribute to benign prostatic hyperplasia (BPH), LUTS, and ED. Lower NO availability and elevated sympathetic activity can stimulate RHO-kinase activity, further exacerbating the process.
Female Incontinence
There is evidence that features of MetS may be contributing to urinary incontinence (UI) in women. A cross-sectional study from Taiwan assessed 371 women with the Incontinence-Quality of Life (I-QOL) questionnaire, and noted that stress urinary incontinence (SUI) was the most frequent subtype reported (28.6%), followed by mixed (24.5%) and urge (16.2%) incontinence. 196 Univariate analysis showed a significant association between urinary incontinence and HTN and obesity (BMI > 27) in this study, and the latter condition significantly correlated with obesity on multivariate analysis (OR, 3.38).196 In the Look AHEAD (Action for Health in Diabetes) trial, the prevalence of incontinence in overweight and obese women with T2DM was 27%; higher than retinopathy (7.5%), microalbuminuria (2.2%), and neuropathy (1.5%).197 In that trial, incontinence was also associated with increasing BMI (BMI = 35–39, OR, 1.65; and BMI ≥ 40, OR, 1.84).197
No studies have shown direct links between MetS and SUI in women, but associations between features of MetS such as elevated BMI, abdominal obesity, diabetes, and HTN have been demonstrated. In a study of approximately 4000 women, multivariate analysis revealed that SUI was most prevalent in individuals with both obesity and diabetes (OR, 3.67). Presence of either obesity or diabetes was also associated with increased SUI prevalence (OR, 2.62 and 1.81, respectively). 198 In the Look AHEAD trial, SUI was associated with increasing BMI (BMI = 35-39, OR, 1.56; and BMI ≥ 40, OR, 1.64).197 Multivariate analysis from HERS (Heart & Estrogen/Progestin Replacement Study) showed that SUI was 10% more likely with elevated BMI and 20% more likely with increased WHR.199 Cross-sectional studies from China have further demonstrated that SUI is independently associated with being overweight (OR, 1.31) or obese (OR, 1.44), as well as having high WC (> 80 cm; OR, 1.38) or HTN (OR, 1.2).200,201
Overactive bladder (OAB) and urinary urge incontinence (UUI) are associated with MetS components as well. Lawrence and colleagues198 noted that rates of OAB are almost 3 times higher in obese women, regardless of diabetes status. In multivariate analysis from HERS data, diabetes was significantly associated with UUI (OR, 1.5).199 Similarly, in data from the first and second Nurses’ Health Studies (NHSI and NHSII), T2DM was significantly associated with UUI in a multivariate analysis.202 In the Look AHEAD trial, UUI had only a modest correlation with WC.197
Interestingly, weight loss appears to alleviate UI in women. The recent PRIDE trial (Program to Reduce Incontinence by Diet and Exercise) randomized overweight or obese women with > 10 episodes of incontinence per week to either an intensive 6-month behavioral weight loss program or a structured 4-session educational program.203 Those in the intensive program lost more weight and their SUI improved more than the other group. However, this relationship was not seen with UUI. Bump and colleagues204 assessed 13 morbidly obese women with urodynamic studies before and after gastric bypass surgery (prebypass mean BMI, 49.4; postbypass mean BMI, 33.1), and reported significant resolution of both SUI and UUI.
No associations between features of MetS and incontinence in males have been demonstrated.205–208 However, a group of investigators did find a positive correlation between ED and OAB in men (OR, 1.5), which was previously noted to be more frequent in MetS.209
Obesity appears to be the major MetS feature associated with SUI in women. SUI occurs when intravesical pressure exceeds bladder outlet pressure either secondary to urethral hypermobility and/or an intrinsic sphincter deficiency, resulting in involuntary urine loss.210,211 Associations between central obesity and bladder pressures have been noted. Sugerman and colleagues212 measured intravesical pressures in anesthetized male (n = 17) and female (n = 67) patients and noted significant positive associations with obesity and central obesity. Furthermore, weight loss in morbidly obese women improved numerous SUI parameters including lower elevations in bladder pressure with maximal effort coughs, better cough pressure transmission from bladder to urethra, and lower urethral axial mobility with coughing.204 Another etiology has been proposed by Kim and colleagues,210 who noted that SUI is associated with increased size of retropubic space, and stated that this area might be larger in overweight women.
Components of MetS that contribute to ED and BPH/LUTS can also impact UUI and OAB. The pathophysiology of UUI is secondary to inappropriate bladder contractions during the storage phase, thereby leading to the sensation of urgency, whereas reductions in functional capacity manifest as frequency.128 At the cellular level, detrusor contractions are stimulated by parasympathetic activity via acetylcholine (ACh) binding to muscarinic M3 receptors.128 Increased ROK activity is associated with MetS features, and this pathway may also contribute to increased detrusor activity not only via ACh receptors, but also purinoceptors, as well as neurokinin and bradykinin receptors.128 Elevated ROK or RhoA activity has been associated in animal models of bladder hypertrophy as well as diabetes- and HTN-associated bladder dysfunction.128 Bladder hypertrophy often leads to components of OAB such as urgency and frequency,128 and we have previously noted that chronic pelvic ischemia in the rabbit also leads to increased bladder fibrosis and smooth muscle atrophy, resulting in decreased compliance. HTN-associated bladder dysfunction has been studied. Persson and colleagues213 demonstrated that SHR bladders exhibited reduced micturition volume, bladder capacity, and increased amplitude of nonvoiding bladder contractions, compared with control Wistar-Kyoto rats. Intrathecal doxazosin (a nonspecific α-antagonist) resulted in higher bladder capacity and diminished amplitude of nonvoiding bladder contractions, and in tissue studies, increasing norepinephrine concentrations resulted in paradoxical increases in bladder contractions.213
It is known that SHRs have increased bladder sympathetic innervation as well as efferent and afferent bladder neuronal hypertrophy, and that altered noradrenergic control of the micturition reflex might account for the distorted SHR bladder function. 213 Given these data as well as the positive association between α1 receptors and ROK activity, sympathetic hyperactivity may be an etiology of UUI in humans. The link between diabetes and UUI has been attributed to axonal atrophy of autonomic neurons to the bladder.214 In streptazosin-induced diabetic rats (SIDR), findings consistent with detrusor overactivity (eg, larger gap junctions between detrusor myocytes) were seen in conjunction with degenerated nerve fibers.214 SIDR have further demonstrated detrusor hyperactivity that is associated with increased muscarinic receptor expression and sensitivity to muscarinic stimulation.215 Nevertheless, alternate etiologies such as increased diuresis secondary to diabetes leading to urinary frequency202 must also be considered when discussing relationships between MetS and incontinence. These explanations between MetS and female incontinence are depicted in Figure 3.
Figure 3.
Possible mechanisms of female incontinence in metabolic syndrome (MetS). Features of MetS including diabetes and obesity may contribute to stress urinary incontinence (SUI). In addition, diabetes (DM) and other possible MetS components such as elevated sympathetic activity and pelvic ischemia may further lead to overactive bladder (OAB) and urinary urge incontinence (UUI) via the denoted pathways. M receptor, muscarinic receptor.
Male Infertility
Features of MetS have been linked to male infertility. A retrospective study revealed that overweight (BMI 25–29) and obese (BMI ≥ 30) men were significantly more likely to experience infertility (OR, 1.19 and 1.36, respectively) compared with lean men (BMI 20–22.4).216 This association with higher BMI and greater infertility was maintained even after numerous adjustments, including age, partner’s BMI, parity, and coital frequency.216 A retrospective Japanese study similarly showed that men with higher BMI were less likely to experience the birth of a new child compared with lower BMI counterparts, even after adjustment for age, SBP, DBP, LDL, TG, and HbA1c.217 In a cross-sectional study from Qatar, male infertility was significantly related with T2DM (OR, 1.958) and HTN (OR, 2.558).218 Multivariate analysis further revealed that obese (BMI > 30) T2DM patients were more than 3 times as likely to be infertile when compared with their thin (BMI < 25) T2DM counterparts.218
Numerous factors may contribute to male infertility in those with MetS features. Obesity, for example, has been demonstrated to negatively impact semen quality in several studies. 219–221 Increasing BMI is associated with low sperm motility221,222 and ejaculate volume.220 BMI has also been positively associated with high spermatic DNA damage as assessed by a measure of chromatin integrity, the DNA fragmentation index.220,222 Abdominal obesity has also been inversely correlated with sperm volume, sperm count, and sperm motility. 223 Diminished sperm motility and semen volume have been noted in T2DM patients as well.224 Hypogonadism, a condition linked to MetS, may impact fertility as testosterone initiates and qualitatively maintains spermatogenesis in humans. The ability to deliver sperm may also be influenced by MetS. Individuals with T2DM are more apt to have ED and ejaculatory dysfunction (EjD) including retrograde ejaculation and premature ejaculation.225-228
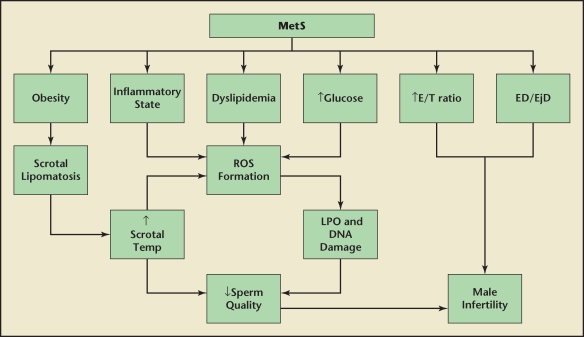
Several explanations may account for the seminal abnormalities noted in those with MetS features. Kasturi and colleagues229 proposed that dyslipidemia and a proinflammatory state may result in increased reactive oxygen species (ROS) formation and subsequent lipid peroxidation of the spermatic membranes. Given that hyperglycemia is associated with increased free radical production, it may further contribute to this process in MetS. The end result may be increased DNA damage and decreased spermatic motility.229 Obesity may increase scrotal temperature, which is thought to induce oxidative damage and apoptosis of sperm.230 This is probably not directly related to varicocele, a condition thought to increase scrotal temperature, as varicocele is less prevalent in obese subjects.231,232 Nevertheless, scrotal adiposity may be an underlying cause. Shafik and Olfat233 performed scrotal dissections on men with idiopathic infertility and noted that extra and intratunicary fat distributions, which they coined scrotal lipomatosis, were more prevalent in obese subjects. They proposed that scrotal lipomatosis may lead to elevated scrotal temperatures via increased adipose insulation, venous stasis, and higher testicular position within the scrotum.233 These investigators performed scrotal lipectomy in infertile subjects, the majority of whom were obese, resulting in increased sperm number and motility, and improved morphology.234 The associations between MetS and male infertility are summarized in Figure 4.
Figure 4.
Possible mechanisms of male infertility in metabolic syndrome (MetS). Several features of MetS may contribute to male infertility. Obesity might lead to scrotal lipomatosis and elevated scrotal temperatures, depressing sperm quality. This may be further augmented by dyslipidemia, hyperglycemia, and a proinflammatory state contributing to reactive oxygen species (ROS) formation and sperm damage. Other contributors may include elevated estrogen/testosterone ratio (E/T), erectile dysfunction (ED) and ejaculatory dysfunction (EjD). LPO, lipid peroxidation. Data from Kasturi SS et al.229
Prostate Cancer
Some studies have demonstrated positive correlations between MetS, its features, and PCa. A prospective trial from Finland in which 1880 patients were followed demonstrated in a multivariate analysis that, in those with MetS, the PCa risk doubled.235 Furthermore, those with MetS and a BMI ≥ 27 had a 3 times greater risk of developing PCa.235 In univariate analysis, positive associations with PCa prevalence and hyperglycemia (OR, 7.31), low HDL-cholesterol (OR, 9.93), as well as elevated WHR, SBP, and DBP have also been noted.236,237 Another study demonstrated that each 12-mm increase in DBP was independently associated with an 8% increase in PCa incidence.238 Data from the Helsinki Heart Study showed that patients with an elevated BMI (> 28) and SBP (> 150 mm Hg) were greater than 2 times more likely to have PCa, and greater than 3 times more likely with the addition of low HDL (≤ 1.05 mmol/L).239 In a large prospective cohort study of 950,000 Norwegian men, stratification by age revealed that obese men age 50 to 59 years had a > 50% increased PCa incidence compared with those with a normal BMI.240 Additionally, a significant positive correlation with BMI and PCa incidence was shown.240 A meta-analysis of 56 trials with 68,753 total cases demonstrated an overall 5% increased PCa risk per 5 kg/m2 (BMI) increment.241
Some studies suggest that components of MetS may also result in more aggressive PCa. In the previously mentioned meta-analysis,241 each 5-point BMI increment increased the risk of advanced PCa stage significantly (relative risk [RR], 1.12). A recent retrospective study showed that white men with a BMI ≥ 35 were approximately 2 to 3 times more likely to have worse pathologic findings during radical prostatectomy (including Gleason sum ≥ 7, positive surgical margins, extraprostatic extension, and seminal vesicle invasion) than lean counterparts (BMI < 25).242 Biochemical PCa recurrences were also higher in overweight or obese Caucasian and African-American men.242 Hammarsten and Högstedt243 demonstrated that both PCa stage and grade are directly associated with BMI, waist measurement, TG, and fasting plasma insulin, and indirectly with HDL. A prospective study further demonstrated that significantly higher plasma insulin levels were noted in those who succumbed versus those who survived PCa, and moreover, PCa mortality was significantly associated with number of MetS features present.244 Investigators have also reported positive associations among WHR, DBP, and serum PSA in those with PCa, as well as an inverse relationship between HDL and serum PSA.236,245 Low testosterone, a condition associated with MetS, has also been linked to more severe PCa. In retrospective analysis, men with low total plasma testosterone (< 3 ng/mL) had an elevated risk of high-grade PCa (Gleason sum ≥ 7; OR, 2.59).246 Low testosterone levels have also been significantly related to more advanced PCa stages247 and positive margins in radical retropubic prostatectomy. 248 Yet other investigators have failed to find relationships between testosterone levels and risk of PCa or more aggressive PCa.249
There is also evidence that MetS and some of its components may actually limit the risk of PCa. Tande and colleagues followed 6429 men in the Atherosclerosis Risk in Communities (ARIC) Study and determined that MetS, either with or without diabetes, was significantly associated with a reduced risk of PCa (RR, 0.71 and 0.77, respectively).250 Kasper and colleagues251 demonstrated in multivariate analysis that diabetes was associated with a 17% reduced risk for total PCa, 28% reduced risk for localized PCa, 31% reduced risk for high-grade PCa, and 24% reduced risk for low-grade PCa. Further analysis demonstrated that overweight patients with diabetes (BMI ≥ 25) had a 19% lower PCa risk than overweight individuals without diabetes.251 Further prospective multivariate analysis of 72,670 men from the Cancer Prevention Study II Nutritional Cohort (CPSII NC) revealed that after 4 years from diagnosis of diabetes, there was a 37% decrease in PCa incidence.252 Similarly, data from the CPSII NC showed that diabetes decreased the incidence of both nonaggressive (stages I and II with Gleason < 8) and aggressive (stages III and IV and those with Gleason ≥ 8) PCa (RR, 0.71 and 0.51, respectively).252 Other MetS features have also been shown to decrease PCa risk. In the ARIC Study, increased MetS features showed a trend toward decreasing PCa, with diabetes excluded. 250 An inverse correlation between TG and incidence of PCa has also been reported.253 On the contrary, other investigators have found no relationship between MetS features and PCa.238,254–256
A number of mechanisms may play a role in how the aforementioned factors either positively or negatively influence PCa risk. Low testosterone levels may disrupt hormonal balance resulting in tumor cells that proliferate in an androgen-independent manner and a more aggressive phenotype.247,257 Angiogenesis is crucial for tumor survival, and it has been demonstrated that androgens regulate the steady-state expression of vascular endothelial growth factor (VEG-F) in PCa models. 258 Indeed, Schatzl and colleagues259 demonstrated that microvessel density (MVD) is inversely related to serum testosterone levels in men with newly diagnosed PCa. Low testosterone levels are also closely related to hyperinsulinemia, yet the etiology behind the relationship has not been fully elucidated. 260 Nevertheless, insulin is known to have promitotic and antiapoptotic actions, and elevated insulin levels have been associated with increased growth in a PCa cell line (LNCaP).261 5 α-reductase (AR) activity has been found to be elevated in patients with T2DM and obesity262 and may raise cancer risk through greater prostatic stimulation. 263,264 There is ample evidence that IGF-1 is linked to MetS and that it may influence the development of PCa. An increasing number of MetS components have been inversely correlated with serum IGF-1 levels as well as IGF-1/insulin growth factor-binding protein-3 (IGFBP-3) ratio, a marker of IGF-1 bioavailability. 265–267 Additionally, WC, SBP, TG, and FPG are inversely correlated with IGF-1/IGFBP-3 ratio, and HDL is directly correlated.266 IGF-1 levels are inversely correlated with risk of T2DM development,266 levels of HbA1c268 and CRP,265 as well as HTN.269 Significant direct relationships between IGF-1 levels and increased total PCa risk as well as that for low- and high-grade PCa have also been reported.270–273 In PCa cell lines, IGF-1 has been shown to cause proliferation and inhibit apoptosis. 251 Prostate epithelial cells have further demonstrated the ability to synthesize low levels of VEG-F in response to IGF-1 stimulation, providing a mechanism for increased angiogenesis.274 The relationship of T2DM and PCa initially appears paradoxical. T2DM is associated with hyperinsulinemia, which itself is related to worse PCa disease. Nevertheless, T2DM has been shown to be protective for high-grade disease. This phenomenon may be due to timing. Although T2DM is associated with IR and initial hyperinsulinemia, β-islet cell desensitization and insulin depletion (termed β-cell exhaustion) may develop over time, promoting a low insulin state.275 This concept is further corroborated by the previously mentioned findings of Rodriguez and coauthors252 who demonstrated a decreased PCa incidence at 4 years post-diabetes diagnosis.
The chronic inflammatory state associated with MetS may contribute to increased PCa risk. We have previously mentioned that IL-1β, IL-6, TNF-α, CRP, and perhaps IL-8 are increased in MetS. TNF-α, IL-6, and IL-8 have been associated with increased PCa risk and PCa stage, as well as metastatic disease.276–278 IL-1β has also been associated with metastatic disease.279 IL-8 has chemotactic and angiogenic activity and can stimulate androgen-independent growth of LNCaP cells.276,280 Both IL-1β and TNF-α have been shown to induce IL-8 expression in androgen-dependent (LNCaP) and androgen-independent (DU-145 and PC-3) PCa cell lines.276 These cell lines have also demonstrated the ability to secrete IL-6, which might function as a paracrine signal in LNCaP cells and an autocrine signal in DU-145 and PC-3 cell lines.281 In vivo, PCa cells have shown the capacity to secrete TNF-α, and the ability of TNF-α to decrease AR expression and DHT sensitivity in LNCaP cells suggests that it may contribute to the development of androgen insensitivity in PCa.282 Additionally, IL-1β, IL-6, IL-8, and TNF-α are known to stimulate the nuclear factor-κB (NF-κB) pathway, and this has been hypothesized as a possible link between elevated inflammatory cytokine levels and PCa development.283 Increased NF-κB activity has been linked to PCa. In both PC-3 and DU- 145 cell lines, NF-κB was shown to be constitutively activated.284 Inverse correlations between NF-κB activity and AR sensitivity in PCa models suggests a role for NF-κB in androgenindependent PCa progression.285 Indeed, inhibition of NF-κB in metastatic PC-3M PCa cell lines resulted in decreased VEGF and IL-8 expression, and this correlated with decreased revascularization and lymph node metastasis in prostates of nude mice.284 Furthermore, Lessard and colleagues286 demonstrated that, in radical prostatectomy specimens, nuclear NF-κB localization is directly related to extent of lymph node invasion. IGF-1 appears to modulate proinflammatory cytokines as it has been shown to stimulate IL-8 expression in DU-145 cells independently or synergistically with IL-1β.276 IGF-1 can also cause IL-6, IL-8, and TNF-α expression in human immune cells.276 Interestingly, IL-6 and TNF-α have themselves been demonstrated to decrease serum IGF-1 levels while increasing hepatic CRP synthesis,265 possibly explaining the aforementioned inverse relationship between CRP and IGF-1. Thus, lower IGF-1 levels seen in MetS may be associated with decreased levels of these proinflammatory cytokines, thereby reducing PCa risk. However, it is important to note that some investigators have shown no correlations between these adipokines and PCa risk.267,287 These interactions are summarized in Figure 5.
Figure 5.
Possible mechanisms of prostate cancer (PCa) in metabolic syndrome (MetS). Features of MetS such as elevated BMI, hypertension (↑BP), and low HDL levels may increase risk of PCa. Hyperinsulinemia (↑insulin) and low testosterone levels are interrelated and may further contribute. Increases in proinflammatory cytokines might lead to prostatic malignancy via increased NF-κB (↑NF-κB) activity. However, lower IGF-1 activity seen in MetS may decrease risk of PCa. Additionally, lower PCa levels in late T2DM may be secondary to a hypoinsulinemic state.
Conclusions
MetS is a complex disorder consisting of numerous interrelated pathophysiologic entities including obesity, HTN, dyslipidemia, hyperglycemia/IR, and endothelial dysfunction. These problems and a number of systemic responses reviewed in this article may promote the development of several multifactorial urologic disorders including nephrolithiasis, ED, male infertility, BPH/LUTS, female incontinence, and PCa. Furthermore, this group of patients is subject to a number of systemic diseases that can influence quality of life and longevity. Therefore, it is important that urologists be cognizant of this disorder and the aforementioned relationships with urologic diseases.
Main Points.
Approximately 35% to 39% of the adult population in the United States has metabolic syndrome (MetS), with similar rates in males and females. The percentage of overweight and obese individuals in the United States continues to grow, with the greatest escalation seen in the so-called “superobese”.
MetS is a complex disorder consisting of numerous interrelated pathophysiologic entities including obesity, hypertension (HTN), dyslipidemia, hyperglycemia/insulin resistance (IR), and endothelial dysfunction. These problems may promote the development of several multifactorial urologic disorders including nephrolithiasis, erectile dysfunction (ED), male infertility, benign prostatic hyperplasia/lower urinary tract symptoms, female incontinence, and prostate cancer (PCa).
Studies have shown the MetS and its components (obesity/increased waist circumference, HTN, etc) are associated with increased rates of nephrolithiasis. A recent cross-sectional study from Italy demonstrated that individuals with MetS are twice as likely to have ultrasonographic evidence of nephrolithiasis.
ED is up to 3 times more prevalent in individuals with MetS. Direct relationships between ED and various components of MetS including obesity, HTN, diabetes, and hypertriglyceridemia have also been shown. Atherosclerotic disease, endothelial dysfunction, and other MetS factors may further impact penile NOS activity, and subsequently, ED.
Obesity appears to be the major MetS feature associated with stress urinary incontinence (SUI) in women. Weight loss in morbidly obese women improved numerous SUI parameters including lower elevations in bladder pressure with maximal effort coughs, better cough pressure transmission from bladder to urethra, and lower urethral axial mobility with coughing.
Features of MetS have been linked to male infertility. A retrospective study revealed that overweight (body mass index [BMI] 25–29) and obese (BMI ≥ 30) men were significantly more likely to experience infertility (odds ratio, 1.19 and 1.36, respectively) compared with lean men (BMI 20–22.4).
Studies have demonstrated conflicting information with regard to MetS and PCa risk. One factor may be alterations within insulin state in early versus late type 2 diabetes mellitus. Further research into this and other MetS components would be prudent and may help in better elucidating these complex relationships.
References
- 1.Alberti G. Introduction to the metabolic syndrome. Eur Heart J. 2005;7(suppl D):D3–D5. [Google Scholar]
- 2.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golden SH, Robinson KA, Saldanha I, et al. Clinical review: prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94:1853–1878. doi: 10.1210/jc.2008-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 5.Sturm R. Increases in clinically severe obesity in the United States, 1986–2000. Arch Intern Med. 2003;163:2146–2148. doi: 10.1001/archinte.163.18.2146. [DOI] [PubMed] [Google Scholar]
- 6.Mokdad AH, Ford ES, Bowman BA, et al. Diabetes trends in the U.S.: 1990–1998. Diabetes Care. 2000;23:1278–1283. doi: 10.2337/diacare.23.9.1278. [DOI] [PubMed] [Google Scholar]
- 7.Leibson CL, O’Brien PC, Atkinson E, et al. Relative contributions of incidence and survival to increasing prevalence of adult-onset diabetes mellitus: a population-based study. Am J Epidemiol. 1997;146:12–22. doi: 10.1093/oxfordjournals.aje.a009187. [DOI] [PubMed] [Google Scholar]
- 8.Geiss LS, Pan L, Cadwell B, et al. Changes in incidence of diabetes in U.S. adults, 1997–2003. Am J Prev Med. 2006;30:371–377. doi: 10.1016/j.amepre.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Low S, Chin MC, Deurenberg-Yap M. Review on epidemic of obesity. Ann Acad Med Singapore. 2009;38:57–59. [PubMed] [Google Scholar]
- 10.Termizy HM, Mafauzy M. Metabolic syndrome and its characteristics among obese patients attending an obesity clinic. Singapore Med J. 2009;50:390–394. [PubMed] [Google Scholar]
- 11.Deckelbaum RJ, Williams CL. Childhood obesity: the health issue. Obes Res. 2001;9(suppl 4):239S–243S. doi: 10.1038/oby.2001.125. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman FR, Shaw J. Type 2 diabetes in youth: rates, antecedents, treatment, problems and prevention. Pediatr Diabetes. 2007;8(suppl 9):4–6. doi: 10.1111/j.1399-5448.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 14.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 15.Sattar N, Gaw A, Scherbakova O, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414–419. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 16.Wilson PW, Kannel WB, Silbershatz H, D’Agostino RB. Clustering of metabolic factors and coronary heart disease. Arch Intern Med. 1999;159:1104–1109. doi: 10.1001/archinte.159.10.1104. [DOI] [PubMed] [Google Scholar]
- 17.Kalyani RR, Dobs AS. Androgen deficiency, diabetes, and the metabolic syndrome in men. Curr Opin Endocrinol Diabetes Obes. 2007;14:226–234. doi: 10.1097/MED.0b013e32814db856. [DOI] [PubMed] [Google Scholar]
- 18.Devaraj S, Singh U, Jialal I. Human C-reactive protein and the metabolic syndrome. Curr Opin Lipidol. 2009;20:182–189. doi: 10.1097/MOL.0b013e32832ac03e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Cordero L, Garcia JJ, Giraldo E, et al. Influence of exercise on the circulating levels and macrophage production of IL-1beta and IFNgamma affected by metabolic syndrome: an obese Zucker rat experimental animal model. Eur J Appl Physiol. 2009;107:535–543. doi: 10.1007/s00421-009-1140-4. [DOI] [PubMed] [Google Scholar]
- 20.Gustafson B, Hammarstedt A, Andersson CX, Smith U. Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2276–2283. doi: 10.1161/ATVBAHA.107.147835. [DOI] [PubMed] [Google Scholar]
- 21.Murdolo G, Smith U. The dysregulated adipose tissue: a connecting link between insulin resistance, type 2 diabetes mellitus and atherosclerosis. Nutr Metab Cardiovasc Dis. 2006;16(suppl 1):S35–S38. doi: 10.1016/j.numecd.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Alexandraki K, Piperi C, Kalofoutis C, et al. Inflammatory process in type 2 diabetes: the role of cytokines. Ann N Y Acad Sci. 2006;1084:89–117. doi: 10.1196/annals.1372.039. [DOI] [PubMed] [Google Scholar]
- 23.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto Y, Folco EJ, Minami M, et al. Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ Res. 2008;102:218–225. doi: 10.1161/CIRCRESAHA.107.164988. [DOI] [PubMed] [Google Scholar]
- 25.Fagerberg B, Behre CJ, Wikstrand J, et al. C-reactive protein and tumor necrosis factor-alpha in relation to insulin-mediated glucose uptake, smoking and atherosclerosis. Scand J Clin Lab Invest. 2008;68:534–541. doi: 10.1080/00365510701870898. [DOI] [PubMed] [Google Scholar]
- 26.Devaraj S, Kumaresan PR, Jialal I. Effect of C-reactive protein on chemokine expression in human aortic endothelial cells. J Mol Cell Cardiol. 2004;36:405–410. doi: 10.1016/j.yjmcc.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Zozuliñska D, Majchrzak A, Sobieska M, et al. Serum interleukin-8 level is increased in diabetic patients. Diabetologia. 1999;42:117–118. doi: 10.1007/s001250051124. [DOI] [PubMed] [Google Scholar]
- 28.Baron AD. Insulin resistance and vascular function. J Diabetes Complications. 2002;16:92–102. doi: 10.1016/s1056-8727(01)00209-4. [DOI] [PubMed] [Google Scholar]
- 29.Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51:527–539. doi: 10.1007/s00125-007-0918-3. [DOI] [PubMed] [Google Scholar]
- 30.Creager MA, Löscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation. 2003;108:1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 31.Verma S, Wang CH, Li SH, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–919. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 32.Khan ZA, Chakrabarti S. Endothelins in chronic diabetic complications. Can J Physiol Pharmacol. 2003;81:622–634. doi: 10.1139/y03-053. [DOI] [PubMed] [Google Scholar]
- 33.Corona G, Manucci E, Forti G, Maggi M. Hypogonadism, ED, metabolic syndrome and obesity: a pathological link supporting cardiovascular diseases. Int J Androl. 2009;32:587–598. doi: 10.1111/j.1365-2605.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- 34.Yassin AA, Saad F, Gooren LJ. Metabolic syndrome, testosterone deficiency and erectile dysfunction never come alone. Andrologia. 2008;40:259–264. doi: 10.1111/j.1439-0272.2008.00851.x. [DOI] [PubMed] [Google Scholar]
- 35.Kupelian V, Hayes FJ, Link CL, et al. Inverse association of testosterone and the metabolic syndrome in men is consistent across race and ethnic groups. J Clin Endocrinol Metab. 2008;93:3403–3410. doi: 10.1210/jc.2008-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corona G, Mannucci E, Ricca V, et al. The agerelated decline of testosterone is associated with different specific symptoms and signs in patients with sexual dysfunction. Int J Androl. 2009;32:720–728. doi: 10.1111/j.1365-2605.2009.00952.x. [DOI] [PubMed] [Google Scholar]
- 37.Goncharov NP, Katsya GV, Chagina NA, Gooren LJ. Three definitions of metabolic syndrome applied to a sample of young obese men and their relation with plasma testosterone. Aging Male. 2008;11:118–122. doi: 10.1080/13685530802204629. [DOI] [PubMed] [Google Scholar]
- 38.Braga-Basaria M, Dobs AS, Muller DC, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24:3979–3983. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- 39.Khaw KT, Barrett-Connor E. Blood pressure and endogenous testosterone in men: an inverse relationship. J Hypertens. 1988;6:329–332. [PubMed] [Google Scholar]
- 40.Svartberg J, von Möhlen D, Schirmer H, et al. Association of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromsø Study. Eur J Endocrinol. 2004;150:65–71. doi: 10.1530/eje.0.1500065. [DOI] [PubMed] [Google Scholar]
- 41.Phillips GB, Jing T, Heymsfield SB. Relationships in men of sex hormones, insulin, adiposity, and risk factors for myocardial infarction. Metabolism. 2003;52:784–790. doi: 10.1016/s0026-0495(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 42.Simon D, Preziosi P, Barrett-Connor E, et al. Interrelation between plasma testosterone and plasma insulin in healthy adult men: the Telecom Study. Diabetologia. 1992;35:173–177. doi: 10.1007/BF00402551. [DOI] [PubMed] [Google Scholar]
- 43.Haffner SM, Karhapää , Mykkänen L, Laakso M. Insulin resistance, body fat distribution, and sex hormones in men. Diabetes. 1994;43:212–219. doi: 10.2337/diab.43.2.212. [DOI] [PubMed] [Google Scholar]
- 44.Haffner SM, Valdez RA, Mykkänen MP, et al. Decreased testosterone and dehydroepiandrosterone sulfate concentrations are associated with increased insulin and glucose concentrations in nondiabetic men. Metabolism. 1994;43:599–603. doi: 10.1016/0026-0495(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 45.Selvin E, Feinleib M, Zhang L, et al. Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III) Diabetes Care. 2007;30:234–238. doi: 10.2337/dc06-1579. [DOI] [PubMed] [Google Scholar]
- 46.Stellato RK, Feldman HA, Hamdy O, et al. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490–494. doi: 10.2337/diacare.23.4.490. [DOI] [PubMed] [Google Scholar]
- 47.Laaksonen DE, Niskanen L, Punnonen K, et al. Sex hormones, inflammation and the metabolic syndrome: a population-based study. Eur J Endocrinol. 2003;149:601–608. doi: 10.1530/eje.0.1490601. [DOI] [PubMed] [Google Scholar]
- 48.Svartberg J, Jenssen T, Sundsfjord J, Jorde R. The associations of endogenous testosterone and sex hormone-binding globulin with glycosylated hemoglobin levels, in community dwelling men. The Tromsø Study. Diabetes Metab. 2004;30:29–34. doi: 10.1016/s1262-3636(07)70086-1. [DOI] [PubMed] [Google Scholar]
- 49.Atlantis E, Martin SA, Haren MT, et al. Demographic, physical and lifestyle factors associated with androgen status: the Florey Adelaide Male Ageing Study (FAMAS) Clin Endocrinol (Oxf) 2009;71:261–272. doi: 10.1111/j.1365-2265.2008.03463.x. [DOI] [PubMed] [Google Scholar]
- 50.Kapoor D, Clarke S, Stanworth R, et al. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007;156:595–602. doi: 10.1530/EJE-06-0737. [DOI] [PubMed] [Google Scholar]
- 51.Niskanen L, Laaksonen DE, Punnonen K, et al. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab. 2004;6:208–215. doi: 10.1111/j.1462-8902.2004.00335.x. [DOI] [PubMed] [Google Scholar]
- 52.Boyanov MA, Boneva Z, Christov VG. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male. 2003;6:1–7. [PubMed] [Google Scholar]
- 53.Lee MJ, Fried SK. Integration of hormonal and nutrient signals that regulate leptin synthesis and secretion. Am J Physiol Endocrinol Metab. 2009;296:E1230–E1238. doi: 10.1152/ajpendo.90927.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McConway MG, Johnson D, Kelly A, et al. Differences in circulating concentrations of total, free and bound leptin relate to gender and body composition in adult humans. Ann Clin Biochem. 2000;37:717–723. doi: 10.1258/0004563001899771. [DOI] [PubMed] [Google Scholar]
- 55.Isidori AM, Caprio M, Strollo F, et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab. 1999;84:3673–3680. doi: 10.1210/jcem.84.10.6082. [DOI] [PubMed] [Google Scholar]
- 56.Luukkaa V, Pesonen U, Huhtaniemi I, et al. Inverse correlation between serum testosterone and leptin in men. J Clin Endocrinol Metab. 1998;83:3243–3246. doi: 10.1210/jcem.83.9.5134. [DOI] [PubMed] [Google Scholar]
- 57.Cohen PG. Obesity in men: the hypogonadalestrogen receptor relationship and its effect on glucose homeostasis. Med Hypotheses. 2008;70:358–360. doi: 10.1016/j.mehy.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 58.Vermeulen A, Kaufman JM, Deslypere JP, Thomas G. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab. 1993;76:1140–1146. doi: 10.1210/jcem.76.5.8496304. [DOI] [PubMed] [Google Scholar]
- 59.Zumoff B, Miller LK, Strain GW. Reversal of the hypogonadotropic hypogonadism of obese men by administration of the aromatase inhibitor testolactone. Metabolism. 2003;52:1126–1128. doi: 10.1016/s0026-0495(03)00186-0. [DOI] [PubMed] [Google Scholar]
- 60.Stamatelou KK, Francis ME, Jones CA, et al. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 61.Sakhaee K. Nephrolithiasis as a systemic disorder. Curr Opin Nephrol Hypertens. 2008;17:304–309. doi: 10.1097/MNH.0b013e3282f8b34d. [DOI] [PubMed] [Google Scholar]
- 62.Rendina D, Mossetti G, De Filippo G, et al. Association between metabolic syndrome and nephrolithiasis in an inpatient population in southern Italy: role of gender, hypertension and abdominal obesity. Nephrol Dial Transplant. 2009;24:900–906. doi: 10.1093/ndt/gfn548. [DOI] [PubMed] [Google Scholar]
- 63.West B, Luke A, Durazo-Arvizu RA, et al. Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988–1994. Am J Kidney Dis. 2008;51:741–747. doi: 10.1053/j.ajkd.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 64.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–462. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 65.Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68:1230–1235. doi: 10.1111/j.1523-1755.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 66.Scales CD , Jr, Curtis LH, Norris RD, et al. Changing gender prevalence of stone disease. J Urol. 2007;177:979–982. doi: 10.1016/j.juro.2006.10.069. [DOI] [PubMed] [Google Scholar]
- 67.Blaak E. Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care. 2001;4:499–502. doi: 10.1097/00075197-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 68.Novak TE, Lakshmanan Y, Trock BJ, et al. Sex prevalence of pediatric kidney stone disease in the United States: an epidemiologic investigation. Urology. 2009;74:104–107. doi: 10.1016/j.urology.2008.12.079. [DOI] [PubMed] [Google Scholar]
- 69.Abate N, Chandalia M, Cabo-Chan AV, Jr, et al. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65:386–392. doi: 10.1111/j.1523-1755.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 70.Maalouf NM, Cameron MA, Moe OW, et al. Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2:883–888. doi: 10.2215/CJN.00670207. [DOI] [PubMed] [Google Scholar]
- 71.Li WM, Chou YH, Li CC, et al. Association of body mass index and urine pH in patients with urolithiasis. Urol Res. 2009;37:193–196. doi: 10.1007/s00240-009-0194-4. [DOI] [PubMed] [Google Scholar]
- 72.Ekeruo WO, Tan YH, Young MD, et al. Metabolic risk factors and the impact of medical therapy on the management of nephrolithiasis in obese patients. J Urol. 2004;172:159–163. doi: 10.1097/01.ju.0000128574.50588.97. [DOI] [PubMed] [Google Scholar]
- 73.Taylor EN, Curhan GC. Body size and 24-hour urine composition. Am J Kidney Dis. 2006;48:905–915. doi: 10.1053/j.ajkd.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 74.Siener R, Glatz S, Nicolay C, Hesse A. The role of overweight and obesity in calcium oxalate stone formation. Obes Res. 2004;12:106–113. doi: 10.1038/oby.2004.14. [DOI] [PubMed] [Google Scholar]
- 75.Sakhaee K, Adams-Huet B, Moe OW, Pak CY. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62:971–979. doi: 10.1046/j.1523-1755.2002.00508.x. [DOI] [PubMed] [Google Scholar]
- 76.Pak CY, Sakhaee K, Moe O, et al. Biochemical profile of stone-forming patients with diabetes mellitus. Urology. 2003;61:523–527. doi: 10.1016/s0090-4295(02)02421-4. [DOI] [PubMed] [Google Scholar]
- 77.Taylor EN, Mount DB, Forman JP, Curhan GC. Association of prevalent hypertension with 24-hour urinary excretion of calcium, citrate, and other factors. Am J Kidney Dis. 2006;47:780–789. doi: 10.1053/j.ajkd.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 78.Losito A, Nunzi EG, Covarelli E, et al. Increased acid excretion in kidney stone formers with essential hypertension. Nephrol Dial Transplant. 2009;24:137–141. doi: 10.1093/ndt/gfn468. [DOI] [PubMed] [Google Scholar]
- 79.Sakhaee K, Maalouf NM. Metabolic syndrome and uric acid nephrolithiasis. Semin Nephrol. 2008;28:174–180. doi: 10.1016/j.semnephrol.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 80.Lemann J , Jr, Pleuss JA, Worcester EM, et al. Urinary oxalate excretion increases with body size and decreases with increasing dietary calcium intake among healthy adults. Kidney Int. 1996;49:200–208. doi: 10.1038/ki.1996.27. [DOI] [PubMed] [Google Scholar]
- 81.Duffey BG, Pedro RN, Kiredberg C, et al. Lithogenic risk factors in the morbidly obese population. J Urol. 2008;179:1401–1406. doi: 10.1016/j.juro.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 82.Hamm LL, Hering-Smith KS. Pathophysiology of hypocitraturic nephrolithiasis. Endocrinol Metab Clin North Am. 2002;31:885–893. doi: 10.1016/s0889-8529(02)00031-2. [DOI] [PubMed] [Google Scholar]
- 83.Cappuccio FP, Siani A, Barba G, et al. A prospective study of hypertension and the incidence of kidney stones in men. J Hypertens. 1999;17:1017–1022. doi: 10.1097/00004872-199917070-00019. [DOI] [PubMed] [Google Scholar]
- 84.Borghi L, Meschi T, Guerra A, et al. Essential arterial hypertension and stone disease. Kidney Int. 1999;55:2397–2406. doi: 10.1046/j.1523-1755.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- 85.Strazzullo P, Barba G, Vuotto P, et al. Past history of nephrolithiasis and incidence of hypertension in men: a reappraisal based on the results of the Olivetti Prospective Heart Study. Nephrol Dial Transplant. 2001;16:2232–2235. doi: 10.1093/ndt/16.11.2232. [DOI] [PubMed] [Google Scholar]
- 86.Madore F, Stampfer MJ, Rimm EB, Curhan GC. Nephrolithiasis and risk of hypertension. Am J Hypertens. 1998;11:46–53. doi: 10.1016/s0895-7061(97)00371-3. [DOI] [PubMed] [Google Scholar]
- 87.Madore F, Stampfer MJ, Willett WC, et al. Nephrolithiasis and risk of hypertension in women. Am J Kidney Dis. 1998;32:802–807. doi: 10.1016/s0272-6386(98)70136-2. [DOI] [PubMed] [Google Scholar]
- 88.Timio F, Kerry SM, Anson KM, et al. Calcium urolithiasis, blood pressure and salt intake. Blood Press. 2003;12:122–127. doi: 10.1080/08037050310001084. [DOI] [PubMed] [Google Scholar]
- 89.Young EW, Morris CD, McCarron DA. Urinary calcium excretion in essential hypertension. J Lab Clin Med. 1992;120:624–632. [PubMed] [Google Scholar]
- 90.Strazzullo P, Nunziata V, Cirillo M, et al. Abnormalities of calcium metabolism in essential hypertension. Clin Sci (Lond) 1983;65:137–141. doi: 10.1042/cs0650137. [DOI] [PubMed] [Google Scholar]
- 91.Eisner BH, Eisenberg ML, Stoller ML. Influence of body mass index on quantitative 24-hour urine chemistry studies in children with nephrolithiasis. J Urol. 2009;182:1142–1145. doi: 10.1016/j.juro.2009.05.052. [DOI] [PubMed] [Google Scholar]
- 92.Sarica K, Eryildirim B, Yencilek F, Kuyumcuoglu U. Role of overweight status on stone-forming risk factors in children: a prospective study. Urology. 2009;73:1003–1007. doi: 10.1016/j.urology.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 93.Maalouf NM, Cameron MA, Moe OW, Sakhaee K. Novel insights into the pathogenesis of uric acid nephrolithiasis. Curr Opin Nephrol Hypertens. 2004;13:181–189. doi: 10.1097/00041552-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 94.Bobulescu IA, Dubree M, Zhang J, et al. Reduction of renal triglyceride accumulation: effects on proximal tubule Na+/H+ exchange and urinary acidification. Am J Physiol Renal Physiol. 2009;297:F1419–F1426. doi: 10.1152/ajprenal.00177.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krivosiková Z, Spustová V, Dzúrik R. Participation of P-dependent and P-independent glutaminases in rat kidney ammoniagenesis and their modulation by metabolic acidosis, hippurate and insulin. Physiol Res. 1998;47:177–183. [PubMed] [Google Scholar]
- 96.Chobanian MC, Hammerman MR. Insulin stimulates ammoniagenesis in canine renal proximal tubular segments. Am J Physiol. 1987;253:F1171–F1177. doi: 10.1152/ajprenal.1987.253.6.F1171. [DOI] [PubMed] [Google Scholar]
- 97.Nissim I, States B, Nissim I, et al. Hormonal regulation of glutamine metabolism by OK cells. Kidney Int. 1995;47:96–105. doi: 10.1038/ki.1995.11. [DOI] [PubMed] [Google Scholar]
- 98.Klisic J, Hu MC, Nief V, et al. Insulin activates Na(+)/H(+) exchanger 3: biphasic response and glucocorticoid dependence. Am J Physiol Renal Physiol. 2002;283:F532–F539. doi: 10.1152/ajprenal.00365.2001. [DOI] [PubMed] [Google Scholar]
- 99.Nagami GT. Luminal secretion of ammonia in the mouse proximal tubule perfused in vitro. J Clin Invest. 1988;81:159–164. doi: 10.1172/JCI113287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weinberg JM. Lipotoxicity. Kidney Int. 2006;70:1560–1566. doi: 10.1038/sj.ki.5001834. [DOI] [PubMed] [Google Scholar]
- 101.Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 102.Wahba IM, Mak RH. Obesity and obesityinitiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:550–562. doi: 10.2215/CJN.04071206. [DOI] [PubMed] [Google Scholar]
- 103.Bobulescu IA, Dubree M, Zhang J, et al. Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and ammonium secretion. Am J Physiol Renal Physiol. 2008;294:F1315–F1322. doi: 10.1152/ajprenal.00550.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schwille PO, Schmiedl A, Herrmann U, Wipplinger J. Postprandial hyperinsulinaemia, insulin resistance and inappropriately high phosphaturia are features of younger males with idiopathic calcium urolithiasis: attenuation by ascorbic acid supplementation of a test meal. Urol Res. 1997;25:49–58. doi: 10.1007/BF00941906. [DOI] [PubMed] [Google Scholar]
- 105.Worcester EM, Gillen DL, Evan AP, et al. Evidence that postprandial reduction of renal calcium reabsorption mediates hypercalciuria of patients with calcium nephrolithiasis. Am J Physiol Renal Physiol. 2007;292:F66–F75. doi: 10.1152/ajprenal.00115.2006. [DOI] [PubMed] [Google Scholar]
- 106.Ozden C, Ozdal OL, Urgancioglu G, et al. The correlation between metabolic syndrome and prostatic growth in patients with benign prostatic hyperplasia. Eur Urol. 2007;51:199–203. doi: 10.1016/j.eururo.2006.05.040. discussion 204–206. [DOI] [PubMed] [Google Scholar]
- 107.Ziada A, Rosenblum M, Crawford ED. Benign prostatic hyperplasia: an overview. Urology. 1999;53(suppl 3a):1–6. doi: 10.1016/s0090-4295(98)00532-9. [DOI] [PubMed] [Google Scholar]
- 108.Hammarsten J, Högstedt B, Holthuis N, Mellström D. Components of the metabolic syndrome-risk factors for the development of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 1998;1:157–162. doi: 10.1038/sj.pcan.4500221. [DOI] [PubMed] [Google Scholar]
- 109.Gupta A, Gupta S, Pavuk M, Roehrborn CG. Anthropometric and metabolic factors and risk of benign prostatic hyperplasia: a prospective cohort study of Air Force veterans. Urology. 2006;68:1198–1205. doi: 10.1016/j.urology.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 110.Kristal AR, Arnold KB, Schenk JM, et al. Race/ethnicity, obesity, health related behaviors and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. J Urol. 2007;177:1395–1400. doi: 10.1016/j.juro.2006.11.065. [DOI] [PubMed] [Google Scholar]
- 111.Oreste M, Giuseppe PG, Gianna P, et al. Betweensubject variations of transition zone epithelial volume and serum PSA levels in men with benign prostatic hyperplasia. World J Urol. 2010;28:379–383. doi: 10.1007/s00345-009-0465-2. [DOI] [PubMed] [Google Scholar]
- 112.Collin SM, Metcalfe C, Donovan J, et al. Associations of lower urinary tract symptoms with prostate-specific antigen levels, and screendetected localized and advanced prostate cancer: a case-control study nested within the UK population-based ProtecT (Prostate testing for cancer and Treatment) study. BJU Int. 2008;102:1400–1406. doi: 10.1111/j.1464-410X.2008.07817.x. [DOI] [PubMed] [Google Scholar]
- 113.Kupelian V, McVary KT, Kaplan SA, et al. Association of lower urinary tract symptoms and the metabolic syndrome: results from the Boston Area Community Health Survey. J Urol. 2009;182:616–624. doi: 10.1016/j.juro.2009.04.025. discussion 624–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Demir O, Akgul K, Akar Z, et al. Association between severity of lower urinary tract symptoms, erectile dysfunction and metabolic syndrome. Aging Male. 2009;12:29–34. doi: 10.1080/13685530902777425. [DOI] [PubMed] [Google Scholar]
- 115.Michel MC, Heemann U, Schumacher H, et al. Association of hypertension with symptoms of benign prostatic hyperplasia. J Urol. 2004;172:1390–1393. doi: 10.1097/01.ju.0000139995.85780.d8. [DOI] [PubMed] [Google Scholar]
- 116.Michel MC, Mehlburger L, Schumacher H, et al. Effect of diabetes on lower urinary tract symptoms in patients with benign prostatic hyperplasia. J Urol. 2000;163:1725–1729. [PubMed] [Google Scholar]
- 117.Tomita K, Mizoue T, Matsumoto T. Lower urinary tract symptoms in relation to lifestyle and medical conditions in Japanese workers. Int J Urol. 2009;16:493–498. doi: 10.1111/j.1442-2042.2009.02276.x. discussion 498. [DOI] [PubMed] [Google Scholar]
- 118.Temml C, Obermayr R, Marszalek M, et al. Are lower urinary tract symptoms influenced by metabolic syndrome? Urology. 2009;73:544–548. doi: 10.1016/j.urology.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 119.Landsberg L. Diet, obesity and hypertension: an hypothesis involving insulin, the sympathetic nervous system, and adaptive thermogenesis. Q J Med. 1986;61:1081–1090. [PubMed] [Google Scholar]
- 120.McVary K. Lower urinary tract symptoms and sexual dysfunction: epidemiology and pathophysiology. BJU Int. 2006;97(suppl 2):23–28. doi: 10.1111/j.1464-410X.2006.06102.x. discussion 44–45. [DOI] [PubMed] [Google Scholar]
- 121.McVary KT, Rademaker A, Lloyd GL, Gann P. Autonomic nervous system overactivity in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2005;174(4 Pt 1):1327–1433. doi: 10.1097/01.ju.0000173072.73702.64. [DOI] [PubMed] [Google Scholar]
- 122.Bloch W, Klotz T, Loch C, et al. Distribution of nitric oxide synthase implies a regulation of circulation, smooth muscle tone, and secretory function in the human prostate by nitric oxide. Prostate. 1997;33:1–8. doi: 10.1002/(sici)1097-0045(19970915)33:1<1::aid-pros1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 123.Azadzoi KM, Tarcan T, Siroky MB, Krane RJ. Atherosclerosis-induced chronic ischemia causes bladder fibrosis and non-compliance in the rabbit. J Urol. 1999;161:1626–1635. [PubMed] [Google Scholar]
- 124.Kozlowski R, Kershen RT, Siroky MB, et al. Chronic ischemia alters prostate structure and reactivity in rabbits. J Urol. 2001;165:1019–1026. [PubMed] [Google Scholar]
- 125.Azadzoi KM, Babayan RK, Kozlowski R, Siroky MB. Chronic ischemia increases prostatic smooth muscle contraction in the rabbit. J Urol. 2003;170(2 Pt 1):659–663. doi: 10.1097/01.ju.0000064923.29954.7e. [DOI] [PubMed] [Google Scholar]
- 126.Rees RW, Foxwell NA, Ralph DJ, et al. Y-27632, a Rho-kinase inhibitor, inhibits proliferation and adrenergic contraction of prostatic smooth muscle cells. J Urol. 2003;170(6 Pt 1):2517–2522. doi: 10.1097/01.ju.0000085024.47406.6c. [DOI] [PubMed] [Google Scholar]
- 127.Takahashi R, Nishimura J, Seki N, et al. RhoA/Rho kinase-mediated Ca2+ sensitization in the contraction of human prostate. Neurourol Urodyn. 2007;26:547–551. doi: 10.1002/nau.20365. [DOI] [PubMed] [Google Scholar]
- 128.Peters SL, Schmidt M, Michel MC. Rho kinase: a target for treating urinary bladder dysfunction? Trends Pharmacol Sci. 2006;27:492–497. doi: 10.1016/j.tips.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 129.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, et al. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- 130.Penna G, Fibbi B, Amuchastegui S, et al. The vitamin D receptor agonist elocalcitol inhibits IL-8-dependent benign prostatic hyperplasia stromal cell proliferation and inflammatory response by targeting the RhoA/Rho kinase and NF-kappaB pathways. Prostate. 2009;69:480–493. doi: 10.1002/pros.20896. [DOI] [PubMed] [Google Scholar]
- 131.Robert G, Descazeaud A, Nicolaïew N, et al. Inflammation in benign prostatic hyperplasia: a 282 patients’ immunohistochemical analysis. Prostate. 2009;69:1774–1780. doi: 10.1002/pros.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Penna G, Mondaini N, Amuchastegui S, et al. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol. 2007;51:524–533. doi: 10.1016/j.eururo.2006.07.016. discussion 533. [DOI] [PubMed] [Google Scholar]
- 133.Fibbi B, Penna G, Morelli A, et al. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int J Androl. 2010;33:475–488. doi: 10.1111/j.1365-2605.2009.00972.x. [DOI] [PubMed] [Google Scholar]
- 134.Rohrmann S, De Marzo AM, Smit E, et al. Serum C-reactive protein concentration and lower urinary tract symptoms in older men in the Third National Health and Nutrition Examination Survey (NHANES III) Prostate. 2005;62:27–33. doi: 10.1002/pros.20110. [DOI] [PubMed] [Google Scholar]
- 135.Rohrmann S, Nelson WG, Rifai N, et al. Serum sex steroid hormones and lower urinary tract symptoms in Third National Health and Nutrition Examination Survey (NHANES III) Urology. 2007;69:708–713. doi: 10.1016/j.urology.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 136.Schatzl G, Brössner C, Schmid S, et al. Endocrine status in elderly men with lower urinary tract symptoms: correlation of age, hormonal status, and lower urinary tract function. The Prostate Study Group of the Austrian Society of Urology. Urology. 2000;55:397–402. doi: 10.1016/s0090-4295(99)00473-2. [DOI] [PubMed] [Google Scholar]
- 137.Gann PH, Hennekens CH, Longcope C, et al. A prospective study of plasma hormone levels, nonhormonal factors, and development of benign prostatic hyperplasia. Prostate. 1995;26:40–49. doi: 10.1002/pros.2990260109. [DOI] [PubMed] [Google Scholar]
- 138.Ansari MA, Begum D, Islam F. Serum sex steroids, gonadotrophins and sex hormonebinding globulin in prostatic hyperplasia. Ann Saudi Med. 2008;28:174–178. doi: 10.5144/0256-4947.2008.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Roberts RO, Jacobson DJ, Rhodes T, et al. Serum sex hormones and measures of benign prostatic hyperplasia. Prostate. 2004;61:124–131. doi: 10.1002/pros.20080. [DOI] [PubMed] [Google Scholar]
- 140.Koritsiadis G, Stravodimos K, Mitropoulos D, et al. Androgens and bladder outlet obstruction: a correlation with pressure-flow variables in a preliminary study. BJU Int. 2008;101:1542–1546. doi: 10.1111/j.1464-410X.2008.07521.x. [DOI] [PubMed] [Google Scholar]
- 141.Haider A, Gooren LJ, Padungtod P, Saad F. Concurrent improvement of the metabolic syndrome and lower urinary tract symptoms upon normalisation of plasma testosterone levels in hypogonadal elderly men. Andrologia. 2009;41:7–13. doi: 10.1111/j.1439-0272.2008.00880.x. [DOI] [PubMed] [Google Scholar]
- 142.Kalinchenko S, Vishnevsky EL, Koval AN, et al. Beneficial effects of testosterone administration on symptoms of the lower urinary tract in men with late-onset hypogonadism: a pilot study. Aging Male. 2008;11:57–61. doi: 10.1080/13685530801953994. [DOI] [PubMed] [Google Scholar]
- 143.Ho CK, Nanda J, Chapman KE, Habib FK. Oestrogen and benign prostatic hyperplasia: effects on stromal cell proliferation and local formation from androgen. J Endocrinol. 2008;197:483–491. doi: 10.1677/JOE-07-0470. [DOI] [PubMed] [Google Scholar]
- 144.Park II, Zhang Q, Liu V, et al. 17Beta-estradiol at low concentrations acts through distinct pathways in normal versus BPH-derived prostate stromal cells. Endocrinology. 2009;150:4594–4605. doi: 10.1210/en.2008-1591. [DOI] [PubMed] [Google Scholar]
- 145.Smith P, Rhodes NP, Ke Y, Foster CS. Upregulation of estrogen and androgen receptors modulate expression of FGF-2 and FGF-7 in human, cultured, prostatic stromal cells exposed to high concentrations of estradiol. Prostate Cancer Prostatic Dis. 2002;5:105–110. doi: 10.1038/sj.pcan.4500571. [DOI] [PubMed] [Google Scholar]
- 146.Winter ML, Liehr JG. Possible mechanism of induction of benign prostatic hyperplasia by estradiol and dihydrotestosterone in dogs. Toxicol Appl Pharmacol. 1996;136:211–219. doi: 10.1006/taap.1996.0027. [DOI] [PubMed] [Google Scholar]
- 147.Rosen RC, Wing RR, Schneider S, et al. Erectile dysfunction in type 2 diabetic men: relationship to exercise fitness and cardiovascular risk factors in the Look AHEAD trial. J Sex Med. 2009;6:1414–1422. doi: 10.1111/j.1743-6109.2008.01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Demir T, Demir O, Kefi A, et al. Prevalence of erectile dysfunction in patients with metabolic syndrome. Int J Urol. 2006;13:385–388. doi: 10.1111/j.1442-2042.2006.01310.x. [DOI] [PubMed] [Google Scholar]
- 149.Bal K, Oder M, Sahin AS, et al. Prevalence of metabolic syndrome and its association with erectile dysfunction among urologic patients: metabolic backgrounds of erectile dysfunction. Urology. 2007;69:356–360. doi: 10.1016/j.urology.2006.09.057. [DOI] [PubMed] [Google Scholar]
- 150.Esposito K, Giugliano F, Martedì E, et al. High proportions of erectile dysfunction in men with the metabolic syndrome. Diabetes Care. 2005;28:1201–1203. doi: 10.2337/diacare.28.5.1201. [DOI] [PubMed] [Google Scholar]
- 151.Heidler S, Temml C, Broessner C, et al. Is the metabolic syndrome an independent risk factor for erectile dysfunction? J Urol. 2007;177:651–654. doi: 10.1016/j.juro.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 152.Grover SA, Lowensteyn I, Kaouache M, et al. The prevalence of erectile dysfunction in the primary care setting: importance of risk factors for diabetes and vascular disease. Arch Intern Med. 2006;166:213–219. doi: 10.1001/archinte.166.2.213. [DOI] [PubMed] [Google Scholar]
- 153.Al-Hunayan A, Al-Mutar M, Kehinde EO, et al. The prevalence and predictors of erectile dysfunction in men with newly diagnosed type 2 diabetes mellitus. BJU Int. 2007;99:130–134. doi: 10.1111/j.1464-410X.2006.06550.x. [DOI] [PubMed] [Google Scholar]
- 154.Bacon CG, Mittleman MA, Kawachi I, et al. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Ann Intern Med. 2003;139:161–168. doi: 10.7326/0003-4819-139-3-200308050-00005. [DOI] [PubMed] [Google Scholar]
- 155.Saigal CS, Wessells H, Pace J, et al. Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med. 2006;166:207–212. doi: 10.1001/archinte.166.2.207. [DOI] [PubMed] [Google Scholar]
- 156.Aslan Y, Sezgin T, Tuncel A, et al. Is type 2 diabetes mellitus a cause of severe erectile dysfunction in patients with metabolic syndrome? Urology. 2009;74:561–564. doi: 10.1016/j.urology.2009.02.073. [DOI] [PubMed] [Google Scholar]
- 157.Eaton CB, Liu YL, Mittleman MA, et al. A retrospective study of the relationship between biomarkers of atherosclerosis and erectile dysfunction in 988 men. Int J Impot Res. 2007;19:218–225. doi: 10.1038/sj.ijir.3901519. [DOI] [PubMed] [Google Scholar]
- 158.Kupelian V, Shabsigh R, Araujo AB, et al. Erectile dysfunction as a predictor of the metabolic syndrome in aging men: results from the Massachusetts Male Aging Study. J Urol. 2006;176:222–226. doi: 10.1016/S0022-5347(06)00503-9. [DOI] [PubMed] [Google Scholar]
- 159.Andersson KE, Wagner G. Physiology of penile erection. Physiol Rev. 1995;75:191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- 160.Rajfer J, Aronson WJ, Bush PA, et al. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med. 1992;326:90–94. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- 161.Ganz P. Erectile dysfunction: pathophysiologic mechanisms pointing to underlying cardiovascular disease. Am J Cardiol. 2005;96(12B):8M–12M. doi: 10.1016/j.amjcard.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 162.Barrett KE, Barman SM, Boitano S, Brooks H. The gonads: development and function of the reproductive system. In: Barrett KE, Barman SM, Boitano S, Brooks H, editors. Ganong’s Review of Medical Physiology. 23 ed. New York: McGraw-Hill Medical; 2009. pp. 391–428. [Google Scholar]
- 163.Champion HC, Bivalacqua TJ, Hyman AL, et al. Gene transfer of endothelial nitric oxide synthase to the penis augments erectile responses in the aged rat. Proc Natl Acad Sci U S A. 1999;96:11648–11652. doi: 10.1073/pnas.96.20.11648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Giuliano F. New horizons in erectile and endothelial dysfunction research and therapies. Int J Impot Res. 2008;20(suppl 2):S2–S8. doi: 10.1038/ijir.2008.46. [DOI] [PubMed] [Google Scholar]
- 165.Andersson KE. Erectile physiological and pathophysiological pathways involved in erectile dysfunction. J Urol. 2003;170(2 Pt 2):S6–S13. doi: 10.1097/01.ju.0000075362.08363.a4. discussion S13–S14. [DOI] [PubMed] [Google Scholar]
- 166.Reilly CM, Zamorano P, Stopper VS, Mills TM. Androgenic regulation of NO availability in rat penile erection. J Androl. 1997;18:110–115. [PubMed] [Google Scholar]
- 167.Shamloul R, Ghanem HM, Salem A, et al. Correlation between penile duplex findings and stress electrocardiography in men with erectile dysfunction. Int J Impot Res. 2004;16:235–237. doi: 10.1038/sj.ijir.3901148. [DOI] [PubMed] [Google Scholar]
- 168.Blumentals WA, Gomez-Caminero A, Joos S, Vannappagari V. Should erectile dysfunction be considered as a marker for acute myocardial infarction? Results from a retrospective cohort study. Int J Impot Res. 2004;16:350–353. doi: 10.1038/sj.ijir.3901174. [DOI] [PubMed] [Google Scholar]
- 169.Hamer M, Stamatakis E. Physical activity and mortality in men and women with diagnosed cardiovascular disease. Eur J Cardiovasc Prev Rehabil. 2009;16:156–160. doi: 10.1097/HJR.0b013e32831f1b77. [DOI] [PubMed] [Google Scholar]
- 170.Esteghamati A, Khalilzadeh O, Rashidi A, et al. Association between physical activity and metabolic syndrome in Iranian adults: national surveillance of risk factors of noncommunicable diseases (SuRFNCD-2007) Metabolism. 2009;58:1347–1355. doi: 10.1016/j.metabol.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 171.Gelaye B, Revilla L, Lopez T, et al. Prevalence of metabolic syndrome and its relationship with leisure time physical activity among Peruvian adults. Eur J Clin Invest. 2009;39:891–898. doi: 10.1111/j.1365-2362.2009.02191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Montorsi P, Montorsi F, Schulman CC. Is erectile dysfunction the “tip of the iceberg” of a systemic vascular disorder? Eur Urol. 2003;44:352–354. doi: 10.1016/s0302-2838(03)00307-5. [DOI] [PubMed] [Google Scholar]
- 173.Guay AT. ED2: erectile dysfunction = endothelial dysfunction. Endocrinol Metab Clin North Am. 2007;36:453–463. doi: 10.1016/j.ecl.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 174.Kaiser DR, Billups K, Mason C, et al. Impaired brachial artery endothelium-dependent and -independent vasodilation in men with erectile dysfunction and no other clinical cardiovascular disease. J Am Coll Cardiol. 2004;43:179–184. doi: 10.1016/j.jacc.2003.07.042. [DOI] [PubMed] [Google Scholar]
- 175.Nehra A, Azadzoi KM, Moreland RB, et al. Cavernosal expandability is an erectile tissue mechanical property which predicts trabecular histology in an animal model of vasculogenic erectile dysfunction. J Urol. 1998;159:2229–2236. doi: 10.1016/S0022-5347(01)63311-1. [DOI] [PubMed] [Google Scholar]
- 176.Yono M, Yamamoto Y, Imanishi A, et al. Differential effects of prazosin and naftopidil on pelvic blood flow and nitric oxide synthase levels in spontaneously hypertensive rats. J Recept Signal Transduct Res. 2008;28:403–412. doi: 10.1080/10799890802176626. [DOI] [PubMed] [Google Scholar]
- 177.Chiou WF, Liu HK, Juan CW. Abnormal protein expression in the corpus cavernosum impairs erectile function in type 2 diabetes. BJU Int. 2010;105:674–680. doi: 10.1111/j.1464-410X.2009.08852.x. [DOI] [PubMed] [Google Scholar]
- 178.Azadzoi KM, Master TA, Siroky MB. Effect of chronic ischemia on constitutive and inducible nitric oxide synthase expression in erectile tissue. J Androl. 2004;25:382–388. doi: 10.1002/j.1939-4640.2004.tb02804.x. [DOI] [PubMed] [Google Scholar]
- 179.Wessells H, Teal TH, Luttrell IP, Sullivan CJ. Effect of endothelial cell-based iNOS gene transfer on cavernosal eNOS expression and mouse erectile responses. Int J Impot Res. 2006;18:438–445. doi: 10.1038/sj.ijir.3901464. [DOI] [PubMed] [Google Scholar]
- 180.Saenz de Tejada I, Tejada I, Goldstein I, et al. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989;320:1025–1030. doi: 10.1056/NEJM198904203201601. [DOI] [PubMed] [Google Scholar]
- 181.Vlachopoulos C, Aznaouridis K, Ioakeimidis N, et al. Unfavourable endothelial and inflammatory state in erectile dysfunction patients with or without coronary artery disease. Eur Heart J. 2006;27:2640–2648. doi: 10.1093/eurheartj/ehl341. [DOI] [PubMed] [Google Scholar]
- 182.Billups KL, Kaiser DR, Kelly AS, et al. Relation of Creactive protein and other cardiovascular risk factors to penile vascular disease in men with erectile dysfunction. Int J Impot Res. 2003;15:231–236. doi: 10.1038/sj.ijir.3901012. [DOI] [PubMed] [Google Scholar]
- 183.Harrison BC, Bell ML, Allen DL, et al. Skeletal muscle adaptations in response to voluntary wheel running in myosin heavy chain null mice. J Appl Physiol. 2002;92:313–322. doi: 10.1152/japplphysiol.00832.2001. [DOI] [PubMed] [Google Scholar]
- 184.Muneer A, Minhas S, Freeman A, et al. Investigating the effects of high-dose phenylephrine in the management of prolonged ischaemic priapism. J Sex Med. 2008;5:2152–2159. doi: 10.1111/j.1743-6109.2008.00862.x. [DOI] [PubMed] [Google Scholar]
- 185.Chen CJ, Kuo TB, Tseng YJ, Yanc CC. Combined cardiac sympathetic excitation and vagal impairment in patients with non-organic erectile dysfunction. Clin Neurophysiol. 2009;120:348–352. doi: 10.1016/j.clinph.2008.10.155. [DOI] [PubMed] [Google Scholar]
- 186.Kirby RS, O’Leary MP, Carson C. Efficacy of extended-release doxazosin and doxazosin standard in patients with concomitant benign prostatic hyperplasia and sexual dysfunction. BJU Int. 2005;95:103–109. doi: 10.1111/j.1464-410X.2004.05252.x. discussion 109. [DOI] [PubMed] [Google Scholar]
- 187.De Rose AF, Carmignani G, Corbu C, et al. Observational multicentric trial performed with doxazosin: evaluation of sexual effects on patients with diagnosed benign prostatic hyperplasia. Urol Int. 2002;68:95–98. doi: 10.1159/000048426. [DOI] [PubMed] [Google Scholar]
- 188.Liguori G, Tombetta C, De Giorgi G, et al. Efficacy and safety of combined oral therapy with tadalafil and alfuzosin: an integrated approach to the management of patients with lower urinary tract symptoms and erectile dysfunction. Preliminary report. J Sex Med. 2009;6:544–552. doi: 10.1111/j.1743-6109.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 189.Kaplan SA, Gonzalez RR, Te AE. Combination of alfuzosin and sildenafil is superior to monotherapy in treating lower urinary tract symptoms and erectile dysfunction. Eur Urol. 2007;51:1717–1723. doi: 10.1016/j.eururo.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 190.Yono M, Yamamoto Y, Yoshida M, et al. Effects of doxazosin on blood flow and mRNA expression of nitric oxide synthase in the spontaneously hypertensive rat genitourinary tract. Life Sci. 2007;81:218–222. doi: 10.1016/j.lfs.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bocchio M, Desideri G, Scarpelli P, et al. Endothelial cell activation in men with erectile dysfunction without cardiovascular risk factors and overt vascular damage. J Urol. 2004;171:1601–1604. doi: 10.1097/01.ju.0000116325.06572.85. [DOI] [PubMed] [Google Scholar]
- 192.Rees RW, Ralph DJ, Royle M, et al. Y-27632, an inhibitor of Rho-kinase, antagonizes noradrenergic contractions in the rabbit and human penile corpus cavernosum. Br J Pharmacol. 2001;133:455–458. doi: 10.1038/sj.bjp.0704124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mills TM, Chitaley K, Lewis RW, Webb RC. Nitric oxide inhibits RhoA/Rho-kinase signaling to cause penile erection. Eur J Pharmacol. 2002;439:173–174. doi: 10.1016/s0014-2999(02)01408-5. [DOI] [PubMed] [Google Scholar]
- 194.Rosen R, Altwein J, Boyle P, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7) Eur Urol. 2003;44:637–649. doi: 10.1016/j.eururo.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 195.McVary KT, Bautista OM, Kusek J. The association of lower urinary tract symptoms (LUTS) with sexual function in men participating in a clinical trial of medical therapy for benign prostatic hyperplasia. J Urol. 2003;169(suppl):322, A1252. [Google Scholar]
- 196.Tsai YC, Liu CH. Urinary incontinence among Taiwanese women: an outpatient study of prevalence, comorbidity, risk factors, and quality of life. Int Urol Nephrol. 2009;41:795–803. doi: 10.1007/s11255-009-9523-3. [DOI] [PubMed] [Google Scholar]
- 197.Phelan S, Kanaya AM, Subak LL, et al. Prevalence and risk factors for urinary incontinence in overweight and obese diabetic women: action for health in diabetes (look ahead) study. Diabetes Care. 2009;32:1391–1397. doi: 10.2337/dc09-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lawrence JM, Lukacz ES, Liu IL, et al. Pelvic floor disorders, diabetes, and obesity in women: findings from the Kaiser Permanente Continence Associated Risk Epidemiology Study. Diabetes Care. 2007;30:2536–2541. doi: 10.2337/dc07-0262. [DOI] [PubMed] [Google Scholar]
- 199.Brown JS, Grady D, Ouslander JG, et al. Prevalence of urinary incontinence and associated risk factors in postmenopausal women. Heart & Estrogen/Progestin Replacement Study (HERS) Research Group. Obstet Gynecol. 1999;94:66–70. doi: 10.1016/s0029-7844(99)00263-x. [DOI] [PubMed] [Google Scholar]
- 200.Zhu L, Lang J, Wang H, et al. The prevalence of and potential risk factors for female urinary incontinence in Beijing, China. Menopause. 2008;15:566–569. doi: 10.1097/gme.0b013e31816054ac. [DOI] [PubMed] [Google Scholar]
- 201.Zhu L, Lang J, Liu C, et al. The epidemiological study of women with urinary incontinence and risk factors for stress urinary incontinence in China. Menopause. 2009;16:831–836. doi: 10.1097/gme.0b013e3181967b5d. [DOI] [PubMed] [Google Scholar]
- 202.Danforth KN, Townsend MK, Curhan GC, et al. Type 2 diabetes mellitus and risk of stress, urge and mixed urinary incontinence. J Urol. 2009;181:193–197. doi: 10.1016/j.juro.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Subak LL, Wing R, West DS, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. 2009;360:481–490. doi: 10.1056/NEJMoa0806375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bump RC, Sugerman HJ, Fantl JA, McClish DK. Obesity and lower urinary tract function in women: effect of surgically induced weight loss. Am J Obstet Gynecol. 1992;167:392–397. doi: 10.1016/s0002-9378(11)91418-5. discussion 397–399. [DOI] [PubMed] [Google Scholar]
- 205.Correia S, Dinis P, Rolo F, Lunet N. Prevalence, treatment and known risk factors of urinary incontinence and overactive bladder in the noninstitutionalized Portuguese population. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:1481–1489. doi: 10.1007/s00192-009-0975-x. [DOI] [PubMed] [Google Scholar]
- 206.Tennstedt SL, Link CL, Steers WD, McKinlay JB. Prevalence of and risk factors for urine leakage in a racially and ethnically diverse population of adults: the Boston Area Community Health (BACH) Survey. Am J Epidemiol. 2008;167:390–399. doi: 10.1093/aje/kwm356. [DOI] [PubMed] [Google Scholar]
- 207.Larrieu S, Pérès K, Letenneur L, et al. Relationship between body mass index and different domains of disability in older persons: the 3C study. Int J Obes Relat Metab Disord. 2004;28:1555–1560. doi: 10.1038/sj.ijo.0802755. [DOI] [PubMed] [Google Scholar]
- 208.Janssen I. Morbidity and mortality risk associated with an overweight BMI in older men and women. Obesity (Silver Spring) 2007;15:1827–1840. doi: 10.1038/oby.2007.217. [DOI] [PubMed] [Google Scholar]
- 209.Irwin DE, Milsom I, Reilly K, et al. Overactive bladder is associated with erectile dysfunction and reduced sexual quality of life in men. J Sex Med. 2008;5:2904–2910. doi: 10.1111/j.1743-6109.2008.01000.x. [DOI] [PubMed] [Google Scholar]
- 210.Kim JK, Kim YJ, Choo MS, Cho KS. The urethra and its supporting structures in women with stress urinary incontinence: MR imaging using an endovaginal coil. AJR Am J Roentgenol. 2003;180:1037–1044. doi: 10.2214/ajr.180.4.1801037. [DOI] [PubMed] [Google Scholar]
- 211.Macura KJ, Genadry RR. Female urinary incontinence: pathophysiology, methods of evaluation and role of MR imaging. Abdom Imaging. 2008;33:371–380. doi: 10.1007/s00261-007-9257-6. [DOI] [PubMed] [Google Scholar]
- 212.Sugerman H, Windsor A, Bessos M, Wolfe L. Intra-abdominal pressure, sagittal abdominal diameter and obesity comorbidity. J Intern Med. 1997;241:71–79. doi: 10.1046/j.1365-2796.1997.89104000.x. [DOI] [PubMed] [Google Scholar]
- 213.Persson K, Pandita RK, Spitsbergen JM, et al. Spinal and peripheral mechanisms contributing to hyperactive voiding in spontaneously hypertensive rats. Am J Physiol. 1998;275:R1366–R1373. doi: 10.1152/ajpregu.1998.275.4.R1366. [DOI] [PubMed] [Google Scholar]
- 214.Rizk DE, Padmanabhan RK, Tariq S, et al. Ultrastructural morphological abnormalities of the urinary bladder in streptozotocin-induced diabetic female rats. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:143–154. doi: 10.1007/s00192-005-1359-5. [DOI] [PubMed] [Google Scholar]
- 215.Stevens LA, Sellers DJ, McKay NG, et al. Muscarinic receptor function, density and G-protein coupling in the overactive diabetic rat bladder. Auton Autacoid Pharmacol. 2006;26:303–309. doi: 10.1111/j.1474-8673.2006.00371.x. [DOI] [PubMed] [Google Scholar]
- 216.Nguyen RH, Wilcox AJ, Skaerven R, Baird DD. Men’s body mass index and infertility. Hum Reprod. 2007;22:2488–2493. doi: 10.1093/humrep/dem139. [DOI] [PubMed] [Google Scholar]
- 217.Ohwaki K, Endo F, Yano E. Relationship between body mass index and infertility in healthy male Japanese workers: a pilot study. Andrologia. 2009;41:100–104. doi: 10.1111/j.1439-0272.2008.00896.x. [DOI] [PubMed] [Google Scholar]
- 218.Bener A, Al-Ansari AA, Zirie M, Al-Hamaq AO. Is male fertility associated with type 2 diabetes mellitus? Int Urol Nephrol. 2009;41:777–784. doi: 10.1007/s11255-009-9565-6. [DOI] [PubMed] [Google Scholar]
- 219.Stewart TM, Liu DY, Garrett C, et al. Associations between andrological measures, hormones and semen quality in fertile Australian men: inverse relationship between obesity and sperm output. Hum Reprod. 2009;24:1561–1568. doi: 10.1093/humrep/dep075. [DOI] [PubMed] [Google Scholar]
- 220.Chavarro JE, Toth TL, Wright DL, et al. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril. 2010;93:2222–2231. doi: 10.1016/j.fertnstert.2009.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hammoud AO, Wilde N, Gibson M, et al. Male obesity and alteration in sperm parameters. Fertil Steril. 2008;90:2222–2225. doi: 10.1016/j.fertnstert.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 222.Kort HI, Massey JB, Elsner CW, et al. Impact of body mass index values on sperm quantity and quality. J Androl. 2006;27:450–452. doi: 10.2164/jandrol.05124. [DOI] [PubMed] [Google Scholar]
- 223.Fejes I, Koloszár S, Szöllosi J, et al. Is semen quality affected by male body fat distribution? Andrologia. 2005;37:155–159. doi: 10.1111/j.1439-0272.2005.00671.x. [DOI] [PubMed] [Google Scholar]
- 224.Ali ST, Shaikh RN, Siddiqi AN, Siddiqi PQ. Semen analysis in insulin-dependent/noninsulin-dependent diabetic men with/without neuropathy. Arch Androl. 1993;30:47–54. doi: 10.3109/01485019308988368. [DOI] [PubMed] [Google Scholar]
- 225.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 226.Gilja I, Parazajder J, Radej M, et al. Retrograde ejaculation and loss of emission: possibilities of conservative treatment. Eur Urol. 1994;25:226–228. doi: 10.1159/000475288. [DOI] [PubMed] [Google Scholar]
- 227.el-Rufaie OE, Bener A, Abuzeid MS, Ali TA. Sexual dysfunction among type II diabetic men: a controlled study. J Psychosom Res. 1997;43:605–612. doi: 10.1016/s0022-3999(97)00183-9. [DOI] [PubMed] [Google Scholar]
- 228.El-Sakka AI. Premature ejaculation in noninsulin- dependent diabetic patients. Int J Androl. 2003;26:329–334. doi: 10.1111/j.1365-2605.2003.00433.x. [DOI] [PubMed] [Google Scholar]
- 229.Kasturi SS, Tannir J, Brannigan RE. The metabolic syndrome and male infertility. J Androl. 2008;29:251–259. doi: 10.2164/jandrol.107.003731. [DOI] [PubMed] [Google Scholar]
- 230.Shiraishi K, Takihara H, Matsuyama H. Elevated scrotal temperature, but not varicocele grade, reflects testicular oxidative stress-mediated apoptosis. World J Urol. 2010;28:359–364. doi: 10.1007/s00345-009-0462-5. [DOI] [PubMed] [Google Scholar]
- 231.Tsao CW, Hsu CY, Chou YC, et al. The relationship between varicoceles and obesity in a young adult population. Int J Androl. 2009;32:385–390. doi: 10.1111/j.1365-2605.2008.00926.x. [DOI] [PubMed] [Google Scholar]
- 232.Handel LN, Shetty R, Sigman M. The relationship between varicoceles and obesity. J Urol. 2006;176:2138–2140. doi: 10.1016/j.juro.2006.07.023. discussion 2140. [DOI] [PubMed] [Google Scholar]
- 233.Shafik A, Olfat S. Scrotal lipomatosis. Br J Urol. 1981;53:50–54. doi: 10.1111/j.1464-410x.1981.tb03128.x. [DOI] [PubMed] [Google Scholar]
- 234.Shafik A, Olfat S. Lipectomy in the treatment of scrotal lipomatosis. Br J Urol. 1981;53:55–61. doi: 10.1111/j.1464-410x.1981.tb03129.x. [DOI] [PubMed] [Google Scholar]
- 235.Laukkanen JA, Laukkanen JA, Niskanen L, et al. Metabolic syndrome and the risk of prostate cancer in Finnish men: a population-based study. Cancer Epidemiol Biomarkers Prev. 2004;13:1646–1650. [PubMed] [Google Scholar]
- 236.de Santana IA, Moura GS, Vieira NF, Cipolotti R. Metabolic syndrome in patients with prostate cancer. Sao Paulo Med J. 2008;126:274–278. doi: 10.1590/S1516-31802008000500006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Coman I, Cri-an N, Marian A, et al. Prevalence of the metabolic syndrome in patients with prostate cancer. J Gastrointestin Liver Dis. 2008;17:237–238. [PubMed] [Google Scholar]
- 238.Martin RM, Vatten L, Gunnell D, et al. Components of the metabolic syndrome and risk of prostate cancer: the HUNT 2 cohort, Norway. Cancer Causes Control. 2009;20:1181–1192. doi: 10.1007/s10552-009-9319-x. [DOI] [PubMed] [Google Scholar]
- 239.Tuohimaa P, Tenkanen L, Syvälä H, et al. Interaction of factors related to the metabolic syndrome and vitamin D on risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:302–307. doi: 10.1158/1055-9965.EPI-06-0777. [DOI] [PubMed] [Google Scholar]
- 240.Engeland A, Tretli S, Bjørge T. Height, body mass index, and prostate cancer: a follow-up of 950000 Norwegian men. Br J Cancer. 2003;89:1237–1242. doi: 10.1038/sj.bjc.6601206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006;17:989–1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 242.Jayachandran J, Bañez LL, Aronson WJ, et al. Obesity as a predictor of adverse outcome across black and white race: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer. 2009;115:5263–5271. doi: 10.1002/cncr.24571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hammarsten J, Högstedt B. Clinical, haemodynamic, anthropometric, metabolic and insulin profile of men with high-stage and high-grade clinical prostate cancer. Blood Press. 2004;13:47–55. doi: 10.1080/08037050310025735. [DOI] [PubMed] [Google Scholar]
- 244.Hammarsten J, Högstedt B. Hyperinsulinaemia: a prospective risk factor for lethal clinical prostate cancer. Eur J Cancer. 2005;41:2887–2895. doi: 10.1016/j.ejca.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 245.Han JH, Choi NY, Bang SH, et al. Relationship between serum prostate-specific antigen levels and components of metabolic syndrome in healthy men. Urology. 2008;72:749–754. doi: 10.1016/j.urology.2008.01.084. discussion 754–755. [DOI] [PubMed] [Google Scholar]
- 246.Platz EA, Leitzmann MF, Rifai N, et al. Sex steroid hormones and the androgen receptor gene CAG repeat and subsequent risk of prostate cancer in the prostate-specific antigen era. Cancer Epidemiol Biomarkers Prev. 2005;14:1262–1269. doi: 10.1158/1055-9965.EPI-04-0371. [DOI] [PubMed] [Google Scholar]
- 247.Isom-Batz G, Bianco FJ , Jr, Kattan MW, et al. Testosterone as a predictor of pathological stage in clinically localized prostate cancer. J Urol. 2005;173:1935–1937. doi: 10.1097/01.ju.0000158040.33531.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Teloken C, Da Ros CT, Caraver F, et al. Low serum testosterone levels are associated with positive surgical margins in radical retropubic prostatectomy: hypogonadism represents bad prognosis in prostate cancer. J Urol. 2005;174:2178–2180. doi: 10.1097/01.ju.0000181818.51977.29. [DOI] [PubMed] [Google Scholar]
- 249.Morote J, Ramirez C, Gómez E, et al. The relationship between total and free serum testosterone and the risk of prostate cancer and tumour aggressiveness. BJU Int. 2009;104:486–489. doi: 10.1111/j.1464-410X.2009.08378.x. [DOI] [PubMed] [Google Scholar]
- 250.Tande AJ, Platz EA, Folsom AR. The metabolic syndrome is associated with reduced risk of prostate cancer. Am J Epidemiol. 2006;164:1094–1102. doi: 10.1093/aje/kwj320. [DOI] [PubMed] [Google Scholar]
- 251.Kasper JS, Liu Y, Giovannucci E. Diabetes mellitus and risk of prostate cancer in the health professionals follow-up study. Int J Cancer. 2009;124:1398–1403. doi: 10.1002/ijc.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Rodriguez C, Patel AV, Mondul AM, et al. Diabetes and risk of prostate cancer in a prospective cohort of US men. Am J Epidemiol. 2005;161:147–152. doi: 10.1093/aje/kwh334. [DOI] [PubMed] [Google Scholar]
- 253.Ulmer H, Borena W, Rapp K, et al. Serum triglyceride concentrations and cancer risk in a large cohort study in Austria. Br J Cancer. 2009;101:1202–1206. doi: 10.1038/sj.bjc.6605264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kurahashi N, Iwasaki M, Sasazuki S, et al. Association of body mass index and height with risk of prostate cancer among middle-aged Japanese men. Br J Cancer. 2006;94:740–742. doi: 10.1038/sj.bjc.6602983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tavani A, Gallus S, Bertuzzi M, et al. Diabetes mellitus and the risk of prostate cancer in Italy. Eur Urol. 2005;47:313–317. doi: 10.1016/j.eururo.2004.10.027. discussion 317. [DOI] [PubMed] [Google Scholar]
- 256.Hsieh CC, Thanos A, Mitropoulos D, et al. Risk factors for prostate cancer: a case-control study in Greece. Int J Cancer. 1999;80:699–703. doi: 10.1002/(sici)1097-0215(19990301)80:5<699::aid-ijc12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 257.Hsing AW, Sakoda LC, Chua S., Jr Obesity, metabolic syndrome, and prostate cancer. Am J Clin Nutr. 2007;86:s843–s857. doi: 10.1093/ajcn/86.3.843S. [DOI] [PubMed] [Google Scholar]
- 258.Stewart RJ, Panigraphy D, Flynn E, Folkman J. Vascular endothelial growth factor expression and tumor angiogenesis are regulated by androgens in hormone responsive human prostate carcinoma: evidence for androgen dependent destabilization of vascular endothelial growth factor transcripts. J Urol. 2001;165:688–693. doi: 10.1097/00005392-200102000-00095. [DOI] [PubMed] [Google Scholar]
- 259.Schatzl G, Madersbacher S, Haitel A, et al. Associations of serum testosterone with microvessel density, androgen receptor density and androgen receptor gene polymorphism in prostate cancer. J Urol. 2003;169:1312–1315. doi: 10.1097/01.ju.0000056900.26628.16. [DOI] [PubMed] [Google Scholar]
- 260.Traish AM, Saad F, Guay A. The dark side of testosterone deficiency: II. Type 2 diabetes and insulin resistance. J Androl. 2009;30:23–32. doi: 10.2164/jandrol.108.005751. [DOI] [PubMed] [Google Scholar]
- 261.Albanes D, Weinstein SJ, Wright ME, et al. Serum insulin, glucose, indices of insulin resistance, and risk of prostate cancer. J Natl Cancer Inst. 2009;101:1272–1279. doi: 10.1093/jnci/djp260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Tomlinson JW, Finney J, Hughes BA, et al. Reduced glucocorticoid production rate, decreased 5alpha-reductase activity, and adipose tissue insulin sensitization after weight loss. Diabetes. 2008;57:1536–1543. doi: 10.2337/db08-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Pflug BR, Pecher SM, Brink AW, et al. Increased fatty acid synthase expression and activity during progression of prostate cancer in the TRAMP model. Prostate. 2003;57:245–254. doi: 10.1002/pros.10297. [DOI] [PubMed] [Google Scholar]
- 264.Vale S. Increased activity of the oncogenic fatty acid synthase and the impaired glucose uptake in the metabolic syndrome. Cancer Epidemiol Biomarkers Prev. 2009;18:2151. doi: 10.1158/1055-9965.EPI-09-0418. [DOI] [PubMed] [Google Scholar]
- 265.Efstratiadis G, Tsiaousis G, Athyros VG, et al. Total serum insulin-like growth factor-1 and C-reactive protein in metabolic syndrome with or without diabetes. Angiology. 2006;57:303–311. doi: 10.1177/000331970605700306. [DOI] [PubMed] [Google Scholar]
- 266.Sierra-Johnson J, Romero-Corral A, Somers VK, et al. IGF-I/IGFBP-3 ratio: a mechanistic insight into the metabolic syndrome. Clin Sci (Lond) 2009;116:507–512. doi: 10.1042/CS20080382. [DOI] [PubMed] [Google Scholar]
- 267.Moore SC, Leitzmann MF, Albanes D, et al. Adipokine genes and prostate cancer risk. Int J Cancer. 2009;124:869–876. doi: 10.1002/ijc.24043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Garay-Sevilla ME, Nava LE, Malacara JM, et al. Advanced glycosylation end products (AGEs), insulin- like growth factor-1 (IGF-1) and IGF-binding protein-3 (IGFBP-3) in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2000;16:106–113. doi: 10.3904/kjim.2007.22.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Colao A, Di Somma C, Cascella T, et al. Relationships between serum IGF1 levels, blood pressure, and glucose tolerance: an observational, exploratory study in 404 subjects. Eur J Endocrinol. 2008;159:389–397. doi: 10.1530/EJE-08-0201. [DOI] [PubMed] [Google Scholar]
- 270.Roddam AW, Allen NE, Appleby P, et al. Insulin-like growth factors, their binding proteins, and prostate cancer risk: analysis of individual patient data from 12 prospective studies. Ann Intern Med. 2008;149:461–471. doi: 10.7326/0003-4819-149-7-200810070-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Renehan AG, Zwahlen M, Minder C, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 272.Chan JM, Stampfer MJ, Ma J, et al. Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer. J Natl Cancer Inst. 2002;94:1099–1106. doi: 10.1093/jnci/94.14.1099. [DOI] [PubMed] [Google Scholar]
- 273.Chan JM, Stampfer MJ, Giovannucci E, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 274.Stearns M, Tran J, Francis MK, et al. Activated Ras enhances insulin-like growth factor I induction of vascular endothelial growth factor in prostate epithelial cells. Cancer Res. 2005;65:2085–2088. doi: 10.1158/0008-5472.CAN-04-4100. [DOI] [PubMed] [Google Scholar]
- 275.Rustenbeck I. Desensitization of insulin secretion. Biochem Pharmacol. 2002;63:1921–1935. doi: 10.1016/s0006-2952(02)00996-6. [DOI] [PubMed] [Google Scholar]
- 276.Kooijman R, Himpe E, Potikanond S, Coppens A. Regulation of interleukin-8 expression in human prostate cancer cells by insulin-like growth factor-I and inflammatory cytokines. Growth Horm IGF Res. 2007;17:383–391. doi: 10.1016/j.ghir.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 277.Michalaki V, Syrigos K, Charles P, Waxman J. Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer. 2004;90:2312–2316. doi: 10.1038/sj.bjc.6601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lehrer S, Diamond EJ, Mamkine B, et al. Serum interleukin-8 is elevated in men with prostate cancer and bone metastases. Technol Cancer Res Treat. 2004;3:411. doi: 10.1177/153303460400300501. [DOI] [PubMed] [Google Scholar]
- 279.Adler HL, McCurdy MA, Kattan MW, et al. Elevated levels of circulating interleukin-6 and transforming growth factor-beta1 in patients with metastatic prostatic carcinoma. J Urol. 1999;161:182–187. [PubMed] [Google Scholar]
- 280.Lee LF, Louie MC, Desai SJ, et al. Interleukin-8 confers androgen-independent growth and migration of LNCaP: differential effects of tyrosine kinases Src and FAK. Oncogene. 2004;23:2197–2205. doi: 10.1038/sj.onc.1207344. [DOI] [PubMed] [Google Scholar]
- 281.Okamoto M, Lee C, Oyasu R. Interleukin-6 as a paracrine and autocrine growth factor in human prostatic carcinoma cells in vitro. Cancer Res. 1997;57:141–146. [PubMed] [Google Scholar]
- 282.Mizokami A, Gotoh A, Yamada H, et al. Tumor necrosis factor-alpha represses androgen sensitivity in the LNCaP prostate cancer cell line. J Urol. 2000;164(3 Pt 1):800–805. doi: 10.1097/00005392-200009010-00053. [DOI] [PubMed] [Google Scholar]
- 283.Vykhovanets EV, Shukla S, MacLennan GT, et al. Molecular imaging of NF-kappaB in prostate tissue after systemic administration of IL-1 beta. Prostate. 2008;68:34–41. doi: 10.1002/pros.20666. [DOI] [PubMed] [Google Scholar]
- 284.Palayoor ST, Youmell MY, Calderwood SK, et al. Constitutive activation of IkappaB kinase alpha and NF-kappaB in prostate cancer cells is inhibited by ibuprofen. Oncogene. 1999;18:7389–7394. doi: 10.1038/sj.onc.1203160. [DOI] [PubMed] [Google Scholar]
- 285.Suh J, Rabson AB. NF-kappaB activation in human prostate cancer: important mediator or epiphenomenon? J Cell Biochem. 2004;91:100–117. doi: 10.1002/jcb.10729. [DOI] [PubMed] [Google Scholar]
- 286.Lessard L, Karakiewicz PI, Bellon-Gagnon P, et al. Nuclear localization of nuclear factor-kappaB p65 in primary prostate tumors is highly predictive of pelvic lymph node metastases. Clin Cancer Res. 2006;12:5741–5745. doi: 10.1158/1078-0432.CCR-06-0330. [DOI] [PubMed] [Google Scholar]
- 287.Il’yasova D, Colbert LH, Harris TB, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]