Abstract
Intravesical therapy is the routine first-line treatment for effectively delaying or preventing the recurrence of bladder cancer. This route of drug administration has also shown tremendous promise in the treatment of interstitial cystitis/painful bladder syndrome (IC/PBS) and potentially overactive bladder to justify investments for further improvements. This review takes a bird’s eye view into the current status of intravesical therapy, with emphasis on liposomal nanoparticles, in diseases associated with lower urinary tract symptoms (LUTS). Ongoing efforts to advance the field of intravesical drug delivery include development of sustained-release drug implants and efforts to improve delivery of biotechnological products including large protein acting as neurotoxins and small interfering RNAs.
Key words: Intravesical therapy, Lower urinary tract symptoms, Bladder cancer, Interstitial cystitis, Painful bladder syndrome
Intravesical therapy continues to remain a first-line, effective treatment for delaying or preventing recurrence of superficial bladder cancer.1 It would be wise to apply the lessons learned over the decades in treatment of bladder cancer to improve the treatment of lower urinary tract symptoms (LUTS). The advertising slogan heard often in mass media for an over-the-counter (OTC) pharmaceutical, “Apply directly where it hurts,” will be apt for promoting wider acceptance of this line of therapy for lower urinary tract symptoms. Instillations of drugs into the bladder create a high concentration of drugs locally at the disease site without increasing systemic levels, which can explain the low risk of systemic side effects. The following review describes the status of intravesical drug delivery with respect to specific diseases and the latest developments in liposomal nanoparticles.
Bladder Cancer
Intravesical therapy is the routine first-line, effective treatment for delaying or preventing recurrence of bladder cancer.2 The standard of care, intravesical chemotherapy and immunotherapy, reduces tumor progression through either direct cytoablation or immunostimulation, which halts implantation of tumor cells after transurethral resection of bladder tumor and eradicates residual disease. Bacillus Calmette-Guérin (BCG) is the most commonly used first-line immunotherapeutic agent for prophylaxis and treatment of carcinoma in situ and high-grade bladder cancer.1 Other immunotherapeutic options include the interferons, interleukins 2 and 12, and tumor necrosis factor, all of which have activity in BCG refractory patients, although with low durable remission rates (Table 1).
Table 1.
Summary of Selected Novel Intravesical Agents
| Agent | Method of Action | Target Category | Trial Status |
| Docetaxel | Inhibits microtubule depolymerization | Nonmuscle invasive | Completed |
| Gemcitabine | Antimetabolite, inhibits DNA replication | High-grade papillary tumor with or without invasion of lamina propria | Completed |
| Nab-paclitaxel | Inhibits microtubule depolymerization | Nonmuscle invasive | Ongoing |
| IFN-α1α2/Syn3 | Adenovirus-mediated transfer of IFN-α1α2 gene therapy | Nonmuscle invasive | Ongoing |
| Mycobacterial cell wall-DNA complex | Immune stimulation/direct cytotoxicity | Carcinoma in situ | Ongoing |
IFN, interferon.
Interstitial Cystitis/Painful Bladder Syndrome
A large body of evidence supports the notion that symptoms of this painful pelvic disease emanate from underlying inflammation in the bladder.3 Studies on animal models of interstitial cystitis (IC)/painful bladder syndrome (PBS) have reported infiltration of neutrophils, enhanced activation of several inflammatory cytokines in the bladder, and increase in inflammatory gene expression.4,5 It is believed that activation of mast cells and disruptions in the bladder permeability barrier are the other key events in the bladder inflammation associated with IC/PBS.6
The intravesical route offers new and promising adjunctive therapies for immediate symptom relief during symptom flare up of IC/PBS (Table 2). Given the multifactorial nature of the disease, therapy is often tailored to improve therapeutic outcomes with multimodal treatment through pharmacological and nonpharmacological approaches such as hydrodistention acting via different mechanisms of action (MOA).
Table 2.
Summary of Current Intravesical Agents
| Intravesical Agent | Current Status |
| Dimethyl sulfoxide | FDA approved |
| Heparin | Clinical testing in progress |
| Hyaluronic acid | Clinical testing in progress |
| Chondroitin sulfate | Clinical testing in progress |
| Pentosan polysulfate | Case reports |
| Capsaicin/resiniferatoxin | Clinical testing in progress |
| Bacillus Calmette-Guérin | Abandoned |
| Oxybutinin | Clinical testing in progress |
| Lidocaine | Clinical testing in progress |
| Botulinum toxin | Clinical testing in progress |
| Neuromodulation | Clinical testing in progress |
| Liposomes | Clinical testing in progress |
Dimethyl Sulfoxide
Dimethyl sulfoxide (DMSO) (Rimso-50) is accepted as a moderately effective and safe treatment of relieving the symptoms of urgency and pain in the IC/PBS patient.7 Given the multitude of effects caused by DMSO on human cells, it may be acting on bladder cells with more than one MOA and in the process uniquely provide a multimodal treatment effect from a single drug. It has been shown that DMSO provides symptomatic relief via anti-inflammatory and mast cell-stabilizing effects.
Glycoaminoglycan Analogues
In a recent study, instillation of hyaluronic acid (HA) in patients with functional bladder capacity (< 200 mL) prolonged the beneficial effect of hydrodistention better than the other glycoaminoglycan (GAG) analogue heparin.8 After receiving bladder hydrodistention, 47 IC/PBS patients (aged 27–76 years) were split into 11 controls and the rest into 2 treatment arms. One arm of 20 patients received intravesical instillation of 40 mg HA weekly for the first month and monthly instillation thereafter for the subsequent 2 months. Sixteen patients received intravesical heparin of which 1 failed, and 2 failures were noted in the HA group. Three months after hydrodistention there was higher rate of improvement in the HA group, in contrast to no improvement in the control group. The effect in the HA group was significant at 6 and 9 months relative to the heparin group (50% vs 20%; P < .05). Improvement in voids per day (−1.8 ± 2.5; P < .01), visual analog scale (−0.9 ± 1.1; P < .01), and bladder capacity (16±18 mL; P<.01) was still significant in the HA group at 9 months relative to no improvement in the heparin group.
Drug Cocktails
Instead of combining instilled drugs with hydrodistention, Parsons attempted improvements in efficacy by instilling cocktails of 2 or more drugs acting via different MOA.9 Patients with newly diagnosed IC having significant frequency, urgency, and pain were treated with drug cocktails made by mixing different ratios of heparin with lidocaine. The treatment response evaluated at different time points showed reduction in the pain and urgency in most patients. Relief from symptoms 2 weeks after treatment suggested that efficacy lasted beyond the duration of the local anesthetic activity of lidocaine.
Liposomes
Liposomes are described as lipid vesicles composed of concentric phospholipid bilayers enclosing an aqueous interior.10 These lipid particulates have been widely studied as a vehicle for anti-neoplastic and antifungal agents.10,11 Liposomal delivery of chemotherapeutic agents allows prolonged duration of action and reduced toxicity profile.12,13 The success of liposomes in the clinic has been attributed to the nontoxic nature of the lipids used in their formulation.
The phospholipids composing empty liposomes themselves have been found to have pharmacological effects, which may explain their use as a topical healing agent in the treatment of dry eye.14,15 Existing therapy options for IC/PBS fail to completely address the significant bladder inflammation associated with IC/PBS. Local anesthetics like lidocaine, when used alone, can provide only short-term symptom relief by blocking the conduction from sensory nerves in the bladder without affecting the underlying bladder inflammation.
Recently, Chuang and colleagues assessed the clinical safety and efficacy of liposomes instilled in IC/PBS patients.16 An open-label, active comparator, prospective study compared the effect of intravesical liposomes against oral pentosan polysulfate sodium (PPS) on 24 IC/PBS patients. Patients divided into the 2 treatment arms each received either intravesical liposomes (80 mg/40 cc distilled water) once weekly or oral pentosan polysulfate sodium (100 mg) thrice daily for 4 weeks. Response to treatment was assessed by 10 measures at 4 and 8 weeks relative to baseline. Both liposomes and oral PPS significantly decreased the urinary frequency and nocturia to indicate comparable efficacy of liposomes. Urgency was profoundly reduced in the liposome group accompanied by significant decreases in pain and the O’Leary-Sant symptom score. There were no reports of urinary incontinence, retention, or infection due to liposome instillation, and there were no unanticipated adverse events and no significant worsening of symptoms during follow-up.
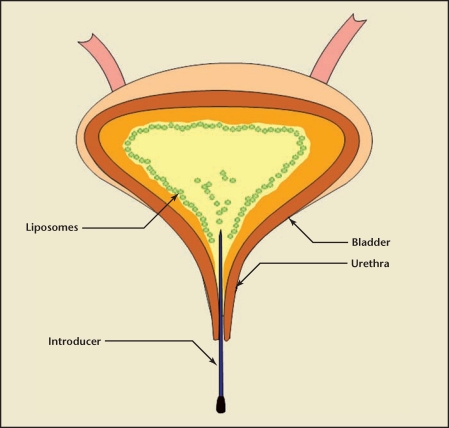
Intravesical instillation of liposomes was found to be safe for IC/PBS with potential improvement after a single course of therapy for up to 8 weeks. The results support liposomes as a promising new treatment of IC/PBS, and future large-scale, placebo-controlled studies are needed to confirm these results. The MOA for liposomes in IC/PBS bladder require mechanistic studies but a liposomal coating affording protection of urothelium from urine cannot be ruled out (Figure 1). Adherence of liposomes as tightly packed bilayers on bladder surface can contribute to the barrier property of the bladder. In addition, liposomes composed of bioactive lipids can exert anti-inflammatory effects by initiating local lipid signaling and affecting mast cell activity17,18 (Figure 1).
Figure 1.
The phospholipids of instilled liposomes have an innate affinity for lipids in cells lining the bladder surface, which facilitate the formation of a protective coating on the injured bladder lumen surface and help protect inflamed uroepithelium.
Overactive Bladder
Oral anticholinergic medications are the current standard of care for patients with overactive bladder (OAB) with limited benefits. The new therapeutic options are aimed at reducing the maximum symptoms, as well as the induced side effects. Intravesical delivery of anticholinergics is becoming a promising alternative for patients who fail oral therapies.
Intravesical Antimuscarinic
A recent study showed that intravesically administered anticholinergic drugs, apart from blocking muscarinic receptors in the bladder, may also be acting through blockade of bladdercooling reflex mediated by C-fibers in most patients with incomplete neurogenic lesion and detrusor overactivity. 19 Modified intravesical oxybutynin (1.25 mg/5 mL, twice daily) was shown to be an effective and relatively safe therapeutic option for children with neurogenic bladders who were either unresponsive to, or experienced intolerable side effects from, oral medications.20 Improvements in both cystometric bladder capacity and compliance were noted in all patients after 1 week, and detrusor overactivity was undetectable in 3 of 4 patients. At 1 year, there was further improvement in bladder compliance in 3 patients, and detrusor overactivity was not observed in 2 patients. The severity of incontinence was significantly improved, and none of the side effects of oral anticholinergics were observed in any of the patients. A single patient with vesicoureteral reflux discontinued the therapy after 2 months due to upper urinary tract infections (UTIs).
Botulinum Neurotoxin
In the field of neurourology, instillation of neurotoxins into the bladder is an accepted approach to achieve chemical neuromodulation of afferent neurotransmission underlying neurogenic bladder or OAB.21 Cystoscope-guided injections continue to remain the gold standard for administering botulinum neurotoxin (BoNT) to the bladder. Intravesical instillation of BoNT alone in animal models of bladder irritation has been evaluated in the past with mixed results.22 Several reasons have been surmised for the lack of efficacy from BoNT instillation such as protein degradation by proteases and proteinases in urine, dilution in urine, or poor uptake of the BoNT solution into the urothelium. Neuromodulation of bladder afferents by different intravesical neurotoxins is therefore limited by either solvent toxicity23 or degradation from proteases in urine.
Liposomes have been previously studied as a carrier of toxins to enhance their efficacy at lower doses.24 The lipid vesicles comprise either one or several aqueous compartments delineated by either one (unilamellar) or several (multilamellar) phospholipid bilayers.12 In the context of toxins instilled in the bladder, fat-soluble neurotoxins such as capsaicin can be integrated into the phospholipid bilayer25 and water-soluble neurotoxins such as botulinum can be protected inside the aqueous compartment(s) of liposomes delimited by the phospholipid bilayer(s)26 (Figure 2).
Figure 2.
Encapsulation of botulinum toxin (BoNT) in the aqueous core of liposomes permits liquid instillation of BoNT instead of cystoscope-guided injection. Following instillation, the liposomes encapsulating BoNT can adhere to the urothelium, whereby liposomes provide high concentration of BoNT at the site of binding for facilitated uptake in the bladder. BoNT slowly leached from adhered liposomes onto the luminal surface of the bladder is endocytosed where it then cleaves to the synaptosomal-associated protein-25 (SNAP-25) protein on membrane to halt the release of chemical mediators such as acetylcholine, and other neurotransmitters from their vesicles.
Liposomes can co-opt the native vesicular traffic ongoing in the bladder, necessary for periodic expansion of bladder lining during urine storage phases. As bladder lining expands, additional membrane material is added to its cells to help retain impermeability. 27 Therefore, vesicular trafficking may provide a favorable environment for drug delivery. Liposomes by themselves can mimic these vesicles and thereby aid in improving the delivery of cargo across the bladder permeability barrier. Moreover, biochemical studies have demonstrated that metalloproteolytic activity of the BoNT is strongly enhanced by the presence of lipid membranes.28 The BoNT entrapped inside liposomes is protected from urinary degradation without compromising efficacy in the rat model.26 Liposome-mediated transport of BoNT into urothelium was confirmed by immunohistochemical detection of its unique effect on neurotransmitters by proteolysis of synaptosomal-associated protein 25 (SNAP-25).26 Animal studies indicate that liposomes can restrict the BoNT delivery to detrusor muscle and avoid the risk of retention and incomplete bladder emptying.
Development of instillation as a mode for administering BoNT in patients may significantly decrease the treatment cost for bladder BoNT therapy. DMSO has recently been reported for liquid BoNT bladder instillation.29 However, DMSO does not afford the natural state of the BoNT protein and has to be formulated immediately prior to instillation with its attendant risks. Dissolving BoNT in DMSO may not be advisable owing to concerns of BoNT uptake into the systemic circulation of the patient being treated and potential repeated exposures with absorptions for the health care providers with dose accumulation.
Intravesical Antisense Therapeutics
Contrary to other approaches that aim to bind reversibly or irreversibly with target protein in the bladder, this approach is instead targeted toward sequence-specific silencing of target mRNA of that protein. The genesilencing approach (antisense or siRNA therapeutics) employs oligonucleotides (ODN) that are chemically modified stretches of single-strand DNA complementary to mRNA regions of a target gene which inhibit translation by forming RNA/DNA duplexes. In contrast to great progress made in translating antisense research into clinical therapies for oncology and ophthalmology,30 the applied research for lower urinary tract diseases has lagged behind. The primary hindrance for drug development of this approach has been inefficient intracellular delivery and cellular uptake of the ODN.
One ideal target for such silencing approach could be the increased production of nerve growth factor (NGF) in the bladder and bladder afferent pathways. NGF has been shown to be directly involved in the emergence of hyperexcitability of C-fiber bladder sensory pathways leading to the pathology of detrusor overactivity and OAB (Figure 3).31 Moreover, local suppression of NGF in the bladder can avoid the safety concerns such as paresthesia, hypoesthesia, and arthralgia noted with systemic administration of monoclonal human NGF antibodies (tanezumab). We previously demonstrated the proof of concept for the approach by suppression of bladder overactivity by local instillation of antisense against NGF based on peptide nucleic acid backbone. 32 Preliminary studies have shown that liposomes can serve as biocompatible effective carriers for local gene silencing in the bladder. The efficacy of liposome-delivered siRNA by intravesical route has been previously demonstrated in preclinical models of bladder cancer.33
Figure 3.
Comparison of antisense with antibody approach to knockdown nerve growth factor (NGF) overexpression in bladder. NGF is implicated as a chemical mediator of pathology-induced changes in C-fiber afferent nerve excitability linked to reflex bladder activity leading up to overactive bladder (OAB) symptoms. Antibodies physically block the receptor binding of NGF, whereas sequence-specific gene silencing blocks the NGF synthesis. Because NGF is a target (bladder) derived trophic protein (ie, synthesized in bladder), it is an ideal candidate for intravesical antisense therapeutics.
Apart from NGF gene, overexpression of angiogenic factors such as vascular endothelium growth factor (VEGF) and transforming growth factor (TGF-β1) can also be selectively targeted by this approach.5,34 These angiogenic factors contribute to the chronic inflammation associated with IC/PBS through endothelial proliferation or neovascularization (formation of new blood vessels).35,36 Local inhibition of VEGF gene in the bladder can be targeted to control neovascularization just as it is targeted by antisense eye drops for corneal angiogenesis. 37 The genes encoding chemokines from the CC family chemokine ligand 3 (CCL3) (macrophage inflammatory protein 1 α [MIP1α]) and CCL2/MCP-1 can be other alternative targets for intravesical antisense therapy in the management of IC/PBS. These chemokines have been identified as profibrotic mediators by their ability to recruit myofibroblasts, macrophages, and other key effector cells to sites of tissue injury.38
Advanced Delivery Options
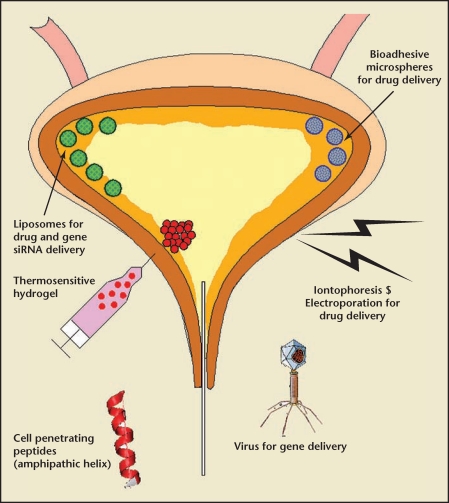
The urinary bladder lining has the most impermeable barrier in the human body.39,40 Various approaches have been attempted to improve bladder uptake of instilled drugs (Figure 4). In addition, most small molecule drugs can perform better after instillation if their pharmacokinetic half-life is extended because, unlike neurotoxins such as BoNT that are gifted with long-lasting duration of action because of their irreversible cleavage of target protein, small molecules have limited half-life and longer adhesion and exposure time would be a major benefit. It was demonstrated that increased bladder residence time translates into improvement in activity.41
Figure 4.
Schematic diagram to illustrate the advanced delivery options for intravesical drug or gene delivery. Anatomic location of bladder allows development of various drug delivery platforms such as virus, liposomes, microspheres, polymeric hydrogel, and cell penetrating peptides.
Electromotive Drug Administration
Electromotive drug administration (EMDA) involves the active transport of intravesically instilled ionized drugs across the urothelium by the transmural application of an electric current.42 Recently, patients suffering from urge syndrome with and without urge incontinence unresponsive to oral anticholinergic drugs underwent EMDA therapy using lidocaine, epinephrine, and dexamethasone.43 Performance of EMDA once in 4 weeks for a period of 3 months significantly improved urodynamic parameters, quality of life (QoL), and pad usage in patients. Following EMDA, a reduction of QoL impact evaluated using the Kings Health Questionnaire was observed from 11.8 ± 0.4 to 7.0 ± 0.3 (P < .001). The micturition chart over 48 h revealed reduction in mean daytime frequency from 14.1 ± 7.7 per day to 9.4 ± 6.2 per day (P < .0001), and in nocturia from 5.1 ± 5.1 per night before EMDA to 2.5 ± 2.4 per night (P = .035) after 2 EMDA sessions. The use of pads could be lowered from 4.5 ± 4.1 per 24 h to 1.8 ± 2.4 (P < .0074). The first desire to void volume assessed by urodynamics changed from 94.0±60.5 mL to 142.2±79.6 mL (P = .0064) and strong desire to void volume increased from 155.6±84.8 mL to 199.5 ± 97.3 mL (P = .001). Uninhibited detrusor contractions were reduced to 46.4% (P < .001) as well as patient-documented bladder capacity as micturition volume increased from 186.0 ± 108.7 mL to 234.2 ± 134.2 mL (P = .043). Complete withdrawal of symptoms was reported in 53.6% (45/84) of all patients and remarkable reduction was found in 28.6% (24/84). Only 10.7% (9/84) of patients did not continue therapy after 2 sessions, further emphasizing that previously reported risk from excess systemic absorption in older patients with inflamed bladder remains paramount.44
Indwelling Devices
Polymer technology represents the most widely studied option to extend the bladder residence time of drugs entrapped in polymeric devices or gel.45 Approaches focused on sustained release can obviate the need for frequent instillations of drug solutions into the bladder. Microdevices based on polymers can be fabricated into either resorbable elastomeric polymer-based devices or nonresorbable devices. However, both types of devices are dependent on surgical procedure of cystoscopy at the time of insertion and at the time of removal from patients.46 Fortunately, the elastomeric nature facilitates streamlining the shape of drug delivery devices for easy instillation. For example, a device in the shape of a tube allows it to elastically deform for ease of catheter insertion and the device can then reverse to a shape that is more retentive in the bladder.
Indwelling devices made from biodegradable polymers can act as a reservoir for drugs such as lidocaine upon bladder instillation. The drug to be delivered by the device is added in its solid form instead of solution phase to reduce the space requirement and the size of device to be inserted. Temporal kinetics of the diffusion-mediated release of the drug after insertion in the bladder is influenced by several factors including aqueous permeability of the polymer, rate of polymer degradation, and orifice size in the device that acts as site of drug release.47 Undoubtedly, a mechanical bladder drug delivery device is an attractive option; however, previous attempts using this approach reported high incidence of encrustration, stone formation, infection, irritation, obstruction, and hematuria in patients after bladder insertion. These adverse outcomes are probably related to the constant contact of a foreign object with urine inside the bladder, which becomes a source of irritation.
In a different approach, a drug reservoir in the bladder was created using the non-Newtonian fluid behavior of hydrogel polymeric matrix. We modified a temperature-sensitive biodegradable triblock polymer poly(ethylene glycol-b-[DL-lactic acid-coglycolic acid]-b-ethylene glycol) (PEG-PLGA-PEG)48 for bladder instillation.49 The aqueous solution of polymer not only allows simple dispersion of the drug but it also flows readily at room temperature. However, once inside the bladder at body temperature, the instilled polymer solution-containing drug into bladder converts into a gel.49 The gel formed inside the bladder acts as a drug depot that is degraded over a determined period of time. New advances in technology are essential to make indwelling devices a viable option for intravesical drug delivery.
Conclusions
Advances in the development of bladder coating with liposomes as well as drug delivery are expected to further improve the efficacy and safety of pharmacotherapy for bladder diseases in the future. Liposomes not only provide a biocompatible interface with affinity for bladder surface but can also facilitate absorption of high molecular weight drug and biologic agent by vesicular traffic. The latest developments in the field of nanotechnology can bring this mode of therapy as a new hope to the forefront of disease management for the lower urinary tract.
Main Points.
Intravesical therapy is the routine first-line treatment of delaying/preventing recurrence of bladder cancer. Intravesical chemotherapy and immunotherapy reduce tumor progression through either direct cytoablation or immunostimulation, halting implantation of tumor cells after transurethral resection of bladder tumor and eradicating residual disease.
Intravesical therapy offers new hope for immediate symptom relief during interstitial cystitis and painful bladder symptom flare up. Therapy is tailored to improve therapeutic outcomes with multimodal treatment through pharmacological and nonpharmacological approaches (eg, dimethyl sulfoxide, glycoaminoglycan analogues, liposomes, and drug cocktails).
Oral anticholinergic medications are the current standard therapy for overactive bladder with limited benefits. New therapeutic options are aimed at reducing the maximum symptoms, as well as the induced side effects. Intravesical delivery of anticholinergics is becoming a promising alternative for patients who fail oral therapies.
Advances in the development of bladder coating with liposomes as well as drug delivery are expected to further improve the efficacy and safety of pharmacotherapy for bladder diseases. Developments in the field of nanotechnology can bring this mode of therapy to the forefront of lower urinary tract disease management.
References
- 1.Cho DY, Bae JH, Moon DG, et al. The effects of intravesical chemoimmunotherapy with gemcitabine and Bacillus Calmette-Guérin in superficial bladder cancer: a preliminary study. J Int Med Res. 2009;37:1823–1830. doi: 10.1177/147323000903700618. [DOI] [PubMed] [Google Scholar]
- 2.Williams SK, Hoenig DM, Ghavamian R, Soloway M. Intravesical therapy for bladder cancer. Expert Opin Pharmacother. 2010;11:947–958. doi: 10.1517/14656561003657145. [DOI] [PubMed] [Google Scholar]
- 3.Parsons JK, Parsons CL. The historical origins of interstitial cystitis. J Urol. 2004;171:20–22. doi: 10.1097/01.ju.0000099890.35040.8d. [DOI] [PubMed] [Google Scholar]
- 4.Smaldone MC, Vodovotz Y, Tyagi V, et al. Multiplex analysis of urinary cytokine levels in rat model of cyclophosphamide-induced cystitis. Urology. 2009;73:421–426. doi: 10.1016/j.urology.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyagi P, Tyagi V, Yoshimura N, et al. Genderbased reciprocal expression of transforming growth factor-beta1 and the inducible nitric oxide synthase in a rat model of cyclophosphamide-induced cystitis. J Inflamm (Lond) 2009;6:23. doi: 10.1186/1476-9255-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen MC, Mudge CS, Klumpp DJ. Urothelial lesion formation is mediated by TNFR1 during neurogenic cystitis. Am J Physiol Renal Physiol. 2006;291:F741–F749. doi: 10.1152/ajprenal.00081.2006. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Chai TC. Effects of dimethyl sulphoxide and heparin on stretch-activated ATP release by bladder urothelial cells from patients with interstitial cystitis. BJU Int. 2002;90:381–385. doi: 10.1046/j.1464-410x.2002.02912.x. [DOI] [PubMed] [Google Scholar]
- 8.Shao Y, Shen ZJ, Rui WB, Zhou WL. Intravesical instillation of hyaluronic acid prolonged the effect of bladder hydrodistention in patients with severe interstitial cystitis. Urology. 2010;75:547–550. doi: 10.1016/j.urology.2009.09.078. [DOI] [PubMed] [Google Scholar]
- 9.Parsons CL. Successful downregulation of bladder sensory nerves with combination of heparin and alkalinized lidocaine in patients with interstitial cystitis. Urology. 2005;65:45–48. doi: 10.1016/j.urology.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 10.Gregoriadis G, Jain S, Papaioannou I, Laing P. Improving the therapeutic efficacy of peptides and proteins: a role for polysialic acids. Int J Pharm. 2005;300:125–130. doi: 10.1016/j.ijpharm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Gregoriadis G, Dapergolas G, Neerunjun ED. Penetration of target areas in the rat by liposome-associated agents administered parenterally and intragastrically. Biochem Soc Trans. 1976;4:256–259. doi: 10.1042/bst0040256. [DOI] [PubMed] [Google Scholar]
- 12.Sapra P, Tyagi P, Allen TM. Ligand-targeted liposomes for cancer treatment. Curr Drug Deliv. 2005;2:369–381. doi: 10.2174/156720105774370159. [DOI] [PubMed] [Google Scholar]
- 13.Gregoriadis G. Engineering liposomes for drug delivery: progress and problems. Trends Biotechnol. 1995;13:527–537. doi: 10.1016/S0167-7799(00)89017-4. [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Dausch S, Maierhofer G, Dausch D. A new therapy concept for the treatment of dry eye-the usefulness of phospholipid liposomes [in German] Klin Monatsbl Augenheilkd. 2004;221:825–836. doi: 10.1055/s-2004-813715. [DOI] [PubMed] [Google Scholar]
- 15.Dausch D, Lee S, Dausch S, et al. Comparative study of treatment of the dry eye syndrome due to disturbances of the tear film lipid layer with lipid-containing tear substitutes [in German] Klin Monatsbl Augenheilkd. 2006;223:974–983. doi: 10.1055/s-2006-927266. [DOI] [PubMed] [Google Scholar]
- 16.Chuang YC, Lee WC, Chiang PH. Intravesical liposome versus oral pentosan polysulfate for interstitial cystitis/painful bladder syndrome. J Urol. 2009;182:1393–1400. doi: 10.1016/j.juro.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Subbaiah PV, Sargis RM. Sphingomyelin: a natural modulator of membrane homeostasis and inflammation. Med Hypotheses. 2001;57:135–138. doi: 10.1054/mehy.2001.1336. [DOI] [PubMed] [Google Scholar]
- 18.Olivera A, Rivera J. Sphingolipids and the balancing of immune cell function: lessons from the mast cell. J Immunol. 2005;174:1153–1158. doi: 10.4049/jimmunol.174.3.1153. [DOI] [PubMed] [Google Scholar]
- 19.Van Meel TD, De Wachter S, Wyndaele JJ. The effect of intravesical oxybutynin on the ice water test and on electrical perception thresholds in patients with neurogenic detrusor overactivity. Neurourol Urodyn. 2010;29:391–394. doi: 10.1002/nau.20785. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi A, Saito M, Okada S, et al. Treatment with modified intravesical oxybutynin chloride for neurogenic bladder in children. J Pediatr Urol. 2007;3:438–442. doi: 10.1016/j.jpurol.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Chancellor MB, de Groat WC. Intravesical capsaicin and resiniferatoxin therapy: spicing up the ways to treat the overactive bladder. J Urol. 1999;162:3–11. doi: 10.1097/00005392-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Chuang YC, Yoshimura N, Huang CC, et al. Intravesical botulinum toxin a administration produces analgesia against acetic acid induced bladder pain responses in rats. J Urol. 2004;172(4 Pt 1):1529–1532. doi: 10.1097/01.ju.0000137844.77524.97. [DOI] [PubMed] [Google Scholar]
- 23.Byrne DS, Das A, Sedor J, et al. Effect of intravesical capsaicin and vehicle on bladder integrity in control and spinal cord injured rats. J Urol. 1998;159:1074–1078. doi: 10.1097/00005392-199803000-00150. [DOI] [PubMed] [Google Scholar]
- 24.Mandal M, Lee KD. Listeriolysin O-liposome-mediated cytosolic delivery of macromolecule antigen in vivo: enhancement of antigen-specific cytotoxic T lymphocyte frequency, activity, and tumor protection. Biochim Biophys Acta. 2002;1563:7–17. doi: 10.1016/s0005-2736(02)00368-1. [DOI] [PubMed] [Google Scholar]
- 25.Tyagi P, Chancellor MB, Li Z, et al. Urodynamic and immunohistochemical evaluation of intravesical capsaicin delivery using thermosensitive hydrogel and liposomes. J Urol. 2004;171:483–489. doi: 10.1097/01.ju.0000102360.11785.d7. [DOI] [PubMed] [Google Scholar]
- 26.Chuang YC, Tyagi P, Huang CC, et al. Urodynamic and immunohistochemical evaluation of intravesical botulinum toxin A delivery using liposomes. J Urol. 2009;182:786–792. doi: 10.1016/j.juro.2009.03.083. [DOI] [PubMed] [Google Scholar]
- 27.Truschel ST, Wang E, Ruiz WG, et al. Stretchregulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell. 2002;13:830–846. doi: 10.1091/mbc.01-09-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caccin P, Rossetto O, Rigoni M, et al. VAMP/synaptobrevin cleavage by tetanus and botulinum neurotoxins is strongly enhanced by acidic liposomes. FEBS Lett. 2003;542:132–136. doi: 10.1016/s0014-5793(03)00365-x. [DOI] [PubMed] [Google Scholar]
- 29.Petrou SP, Parker AS, Crook JE, et al. Botulinum a toxin/dimethyl sulfoxide bladder instillations for women with refractory idiopathic detrusor overactivity: a phase 1/2 study. Mayo Clin Proc. 2009;84:702–706. doi: 10.4065/84.8.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dadgostar H, Waheed N. The evolving role of vascular endothelial growth factor inhibitors in the treatment of neovascular age-related macular degeneration. Eye (Lond) 2008;22:761–767. doi: 10.1038/eye.2008.86. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimura N, Bennett NE, Hayashi Y, et al. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci. 2006;26:10847–10855. doi: 10.1523/JNEUROSCI.3023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tyagi P, Banerjee R, Basu S, et al. Intravesical antisense therapy for cystitis using TAT-peptide nucleic acid conjugates. Mol. Pharm. 2006;3:398–406. doi: 10.1021/mp050093x. [DOI] [PubMed] [Google Scholar]
- 33.Nogawa M, Yuasa T, Kimura S, et al. Intravesical administration of small interfering RNA targeting PLK-1 successfully prevents the growth of bladder cancer. J Clin Invest. 2005;115:978–985. doi: 10.1172/JCI23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheppudira BP, Girard BM, Malley SE, et al. Upregulation of vascular endothelial growth factor isoform (VEGF164) and receptors (VEGFR-2, Npn-1, Npn-2) in rats with cyclophosphamide (CYP)-induced cystitis. Am J Physiol Renal Physiol. 2008;295:F826–F836. doi: 10.1152/ajprenal.90305.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamaki M, Saito R, Ogawa O, et al. Possible mechanisms inducing glomerulations in interstitial cystitis: relationship between endoscopic findings and expression of angiogenic growth factors. J Urol. 2004;172:945–948. doi: 10.1097/01.ju.0000135009.55905.cb. [DOI] [PubMed] [Google Scholar]
- 36.van de Merwe JP, Yamada T, Sakamoto Y. Systemic aspects of interstitial cystitis, immunology and linkage with autoimmune disorders. Int J Urol. 2003;10(suppl):S35–S38. doi: 10.1046/j.1442-2042.10.s1.10.x. [DOI] [PubMed] [Google Scholar]
- 37.Cursiefen C, Bock F, Horn FK, et al. GS-101 antisense oligonucleotide eye drops inhibit corneal neovascularization: interim results of a randomized phase II trial. Ophthalmology. 2009;116:1630–1637. doi: 10.1016/j.ophtha.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 38.Qi W, Chen X, Polhill TS, et al. TGF-beta1 induces IL-8 and MCP-1 through a connective tissue growth factor-independent pathway. Am J Physiol Renal Physiol. 2006;290:F703–F709. doi: 10.1152/ajprenal.00254.2005. [DOI] [PubMed] [Google Scholar]
- 39.Eldrup J, Thorup J, Nielsen SL, et al. Permeability and ultrastructure of human bladder epithelium. Br J Urol. 1983;55:488–492. doi: 10.1111/j.1464-410x.1983.tb03354.x. [DOI] [PubMed] [Google Scholar]
- 40.Parsons CL, Boychuk D, Jones S, et al. Bladder surface glycosaminoglycans: an epithelial permeability barrier. J Urol. 1990;143:139–142. doi: 10.1016/s0022-5347(17)39897-x. [DOI] [PubMed] [Google Scholar]
- 41.Frangos DN, Killion JJ, Fan D, et al. The development of liposomes containing interferon alpha for the intravesical therapy of human superficial bladder cancer. J Urol. 1990;143:1252–1256. doi: 10.1016/s0022-5347(17)40248-5. [DOI] [PubMed] [Google Scholar]
- 42.Gürpinar T, Truong LD, Wong HY, Griffith DP. Electromotive drug administration to the urinary bladder: an animal model and preliminary results. J Urol. 1996;156:1496–1501. [PubMed] [Google Scholar]
- 43.Bach P, Wormland RT, Möhring C, Goepel M. Electromotive drug-administration: a pilot study for minimal-invasive treatment of therapyresistant idiopathic detrusor overactivity. Neurourol Urodyn. 2009;28:209–213. doi: 10.1002/nau.20624. [DOI] [PubMed] [Google Scholar]
- 44.Hinkel A, Pannek J. Transient ischemic attack after electromotive drug administration for chronic non-infectious cystitis: report of two similar cases. Neurourol Urodyn. 2004;23:180–182. doi: 10.1002/nau.10157. [DOI] [PubMed] [Google Scholar]
- 45.Elman NM, Patta Y, Scott AW, et al. The next generation of drug-delivery microdevices. Clin Pharmacol Ther. 2009;85:544–547. doi: 10.1038/clpt.2009.4. [DOI] [PubMed] [Google Scholar]
- 46.Giannantoni A, Di Stasi S, Chancellor M, et al. New frontiers in intravesical therapies and drug delivery. Eur Urol. 2006;50:1183–1193. doi: 10.1016/j.eururo.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 47.Farokhzad OC, Dimitrakov JD, Karp JM, et al. Drug delivery systems in urology-getting “smarter”. Urology. 2006;68:463–469. doi: 10.1016/j.urology.2006.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeong B, Bae YH, Kim SW. In situ gelation of PEG-PLGA-PEG triblock copolymer aqueous solutions and degradation thereof. J Biomed Mater Res. 2000;50:171–177. doi: 10.1002/(sici)1097-4636(200005)50:2<171::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 49.Tyagi P, Li Z, Chancellor M, et al. Sustained intravesical drug delivery using thermosensitive hydrogel. Pharm Res. 2004;21:832–837. doi: 10.1023/b:pham.0000026436.62869.9c. [DOI] [PubMed] [Google Scholar]