Abstract
We present evidence that both corepressors SMRT and N-CoR exist in large protein complexes with estimated sizes of 1.5–2 MDa in HeLa nuclear extracts. Using a combination of conventional and immunoaffinity chromatography, we have successfully isolated a SMRT complex and identified histone deacetylase 3 (HDAC3) and transducin (β)-like I (TBL1), a WD-40 repeat-containing protein, as the subunits of the purified SMRT complex. We show that the HDAC3-containing SMRT and N-CoR complexes can bind to unliganded thyroid hormone receptors (TRs) in vitro. We demonstrate further that in Xenopus oocytes, both SMRT and N-CoR also associate with HDAC3 in large protein complexes and that injection of antibodies against HDAC3 or SMRT/N-CoR led to a partial relief of repression by unliganded TR/RXR. These findings thus establish both SMRT and N-CoR complexes as bona fide HDAC-containing complexes and shed new light on the molecular pathways by which N-CoR and SMRT function in transcriptional repression.
Keywords: HDAC3/repression/SMRT and N-CoR corepressor complexes/TBL1
Introduction
In eukaryotic cells, genomic DNA is packaged with histones into nucleosomes, the primary structural units of chromatin. Packaging of DNA into chromatin strongly inhibits transcription, at least in part, by hindering the binding of transcription factors and the basal transcription machinery to their recognition sites (Wolffe, 1994). Numerous studies have linked the post-translational modifications of histones, particularly the acetylation and deacetylation of lysine residues in histone N-terminal tails, with the transcriptional capacity of chromatin (Hebbes et al., 1988; Turner and O’Neill, 1995). The acetylation of histones is catalyzed by histone acetyltransferases (HATs) and reversed by the activity of histone deacetylases (HDACs). The findings that many transcriptional coactivators including GCN5, PCAF, CBP/p300 and SRC-1/ACTR possess intrinsic HAT activity and that transcriptional corepressors associate with HDACs provide a direct molecular link between histone acetylation and transcription regulation (for reviews see Kouzarides, 1999; McKenna et al., 1999; Glass and Rosenfeld, 2000). Acetylation is believed to destabilize nucleosomes, at least in part, and thus facilitates the binding of transcription factors (Lee et al., 1993), whereas deacetylation is likely to stabilize nucleosomes and/or the higher order structure of chromatin further and thus reinforces the inhibitory effect of chromatin.
Nuclear receptors are a large group of structurally related transcription factors whose activities generally are regulated by ligands. Nuclear receptors such as thyroid hormone receptors (TRs) and retinoic acid receptors (RARs) have the capacity to bind to their target genes in the absence of their cognate ligands and actively repress transcription (Glass et al., 1989; Baniahmad et al., 1990). This repression activity appears to require accessory proteins termed corepressors (Casanova et al., 1994; Baniahmad et al., 1995). So far, several corepressor candidates, including SMRT (Chen and Evans, 1995), N-CoR (Horlein et al., 1995), Sun-CoR (Zamir et al., 1996) and Alien (Dressel et al., 1999), have been identified by using yeast two-hybrid screens based on their ability to interact with unliganded receptors. The recent cloning of full-length SMRT reveals a striking similarity between the SMRT and N-CoR proteins (Ordentlich et al., 1999; Park et al., 1999). Both SMRT and N-CoR appear to be essential for repression by unliganded TR and RAR (Horlein et al., 1995; Zhang et al., 1998). In addition, N-CoR and SMRT have also been implicated in the transcription repression by many other transcription factors such as BCL6/LAZ3 and ETO as well as the regulation of the Notch signaling pathway through interaction with CBF (Hong et al., 1997; Dhordain et al., 1998; Kao et al., 1998; Wang et al., 1998). Thus, N-CoR and SMRT are likely to play fundamental roles in development, differentiation and tumorigenesis through their ability to mediate transcription repression by a variety of transcription factors.
Strong evidence supports the functional importance of HDAC activity in repression mediated by unliganded TR/RXR (Heinzel et al., 1997; Nagy et al., 1997; Ciana et al., 1998; Wong et al., 1998). However, the molecular pathways that lead to the HDAC-dependent repression appear to be complicated. Earlier work indicates that both N-CoR and SMRT contain multiple transferable repression domains and are likely to recruit HDAC1/2 through interaction with mammalian homologs (mSin3A and mSin3B) of the yeast Sin3 protein (Heinzel et al., 1997; Nagy et al., 1997). These observations thus led to the proposal that unliganded nuclear receptors such as TR repress transcription at least in part through recruitment of mSin3–HDAC1/2 complex by N-CoR or SMRT. However, the purified mammalian mSin3A complex contains HDAC1/2, SAP18, SAP30, RbAp46 and RbAp48, but neither SMRT nor N-CoR (Zhang et al., 1997). The fact that both N-CoR and SMRT are not present in the purified mSin3A complex raises the question of whether repression pathways independent of mSin3 also exist with respect to N-CoR and SMRT. More recently, the class II HDACs including HDAC4, HDAC5, HDAC6 and HDAC7 have been shown to interact directly with N-CoR and SMRT, and thus could also contribute to the HDAC-dependent repression (Huang et al., 2000; Kao et al., 2000). However, it appears that only a small fraction of endogenous N-CoR or SMRT is associated with class II HDACs (Huang et al., 2000). Thus, it is unlikely that the recruitment of class II HDACs alone could account for the HDAC-dependent repression. In addition, pathway(s) independent of N-CoR and SMRT may also be involved in the repression by unliganded TR, as we have shown previously that another HDAC1/2-containing complex, NURD, also plays a role in the repression by unliganded TR/RXR (Xue et al., 1998).
Accumulating evidence indicates that transcriptional cofactors generally exist in and function as large protein complexes. To understand better the mechanism by which SMRT and N-CoR mediate transcription repression, we attempted to identify and characterize proteins associated with SMRT and N-CoR. Here we report the purification and identification of one SMRT complex that contains HDAC3 (Yang et al., 1997; Emiliani et al., 1998) and transducin (β)-like I (TBL1; Bassi et al., 1999). We further demonstrate that both SMRT and N-CoR are associated with HDAC3 and TBL1 in large protein complexes with a mass of 1.5–2 MDa. The association of N-CoR and SMRT with HDAC3 appears to be evolutionarily conserved since in Xenopus oocytes, xN-CoR and xSMRT also associate with xHDAC3 in large protein complexes with sizes of 1.5–2 MDa. Antibody injection experiments support the involvement and functional importance of xHDAC3 and xN-CoR/SMRT in repression mediated by unliganded TR/RXR in Xenopus oocytes.
Results
Both SMRT and N-CoR exist as large protein complexes
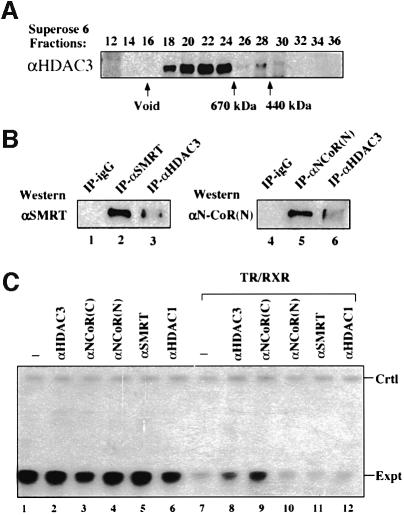
Although evidence exists for the interaction between N-CoR/SMRT and mSin3 (Alland et al., 1997; Heinzel et al., 1997; Nagy et al., 1997), the purified mammalian mSin3A complex contains HDAC1/2 and a number of other proteins but lacks N-CoR or SMRT (Zhang et al., 1997). This result prompted us to investigate whether N-CoR and SMRT also exist in large protein complexes. For this purpose, HeLa nuclear extract was fractionated with a Superose 6 gel filtration column and the presence of SMRT and N-CoR proteins was detected by western blotting analysis using a polyclonal antibody raised against N-CoR (2164–2441). This antibody, termed αN-CoR(C), actually detected both N-CoR and SMRT proteins (see Figure 2B). It should be pointed out that the full-length SMRT and N-CoR proteins are indistinguishable in size in SDS–PAGE (data not shown, see Figures 2B and 1). As shown in Figure 1, the collection of N-CoR and SMRT proteins was eluted immediately following the void fractions. The gel filtration profile of both SMRT and N-CoR proteins was also confirmed independently by western analyses using N-CoR- or SMRT-specific antibodies (data not shown; see also Figure 3A). As a size reference, the BRG1 protein, a key subunit of the mammalian SWI/SNF complex (Wang et al., 1996), was also analyzed by western blotting. The mammalian SWI/SNF complexes are known to exist as large protein complexes of ∼1.5–2 MDa (Wang et al., 1996). Since the peak fraction of N-CoR and SMRT (18–22) eluted approximately one fraction prior to the peak of the BRG1, we estimate that the size of the N-CoR and SMRT complexes is at least 1.5–2 MDa. Because both N-CoR and SMRT proteins are ∼275 kDa, this result indicates that the N-CoR and SMRT proteins in HeLa nuclear extracts exist in multisubunit protein complexes.
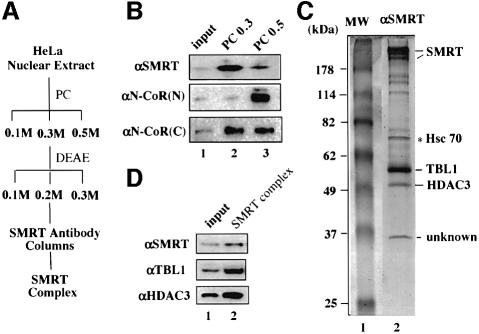
Fig. 2. Purification of a SMRT protein complex. (A) A diagram of the purification scheme for the SMRT complex. PC, phosphocellulose p11 resins; DEAE, DEAE–Sepharose Fast Flow resins. (B) Differential fractionation of SMRT and N-CoR proteins by a PC column. Note that αSMRT and αN-CoR(N) antibodies are specific for SMRT and N-CoR, respectively, whereas αN-CoR(C) antibody recognizes both SMRT and N-CoR. (C) A Coomassie blue-stained SDS–polyacrylamide gel showing the purified SMRT complex. The identity of the indicated subunits was determined by mass spectrometry and confirmed by western analyses as shown in (D). The Hsc70 protein was a contaminant because it also bound to the control IgG beads; 10% of the input (0.2 M DEAE fraction) was used in lane 1 in (D).
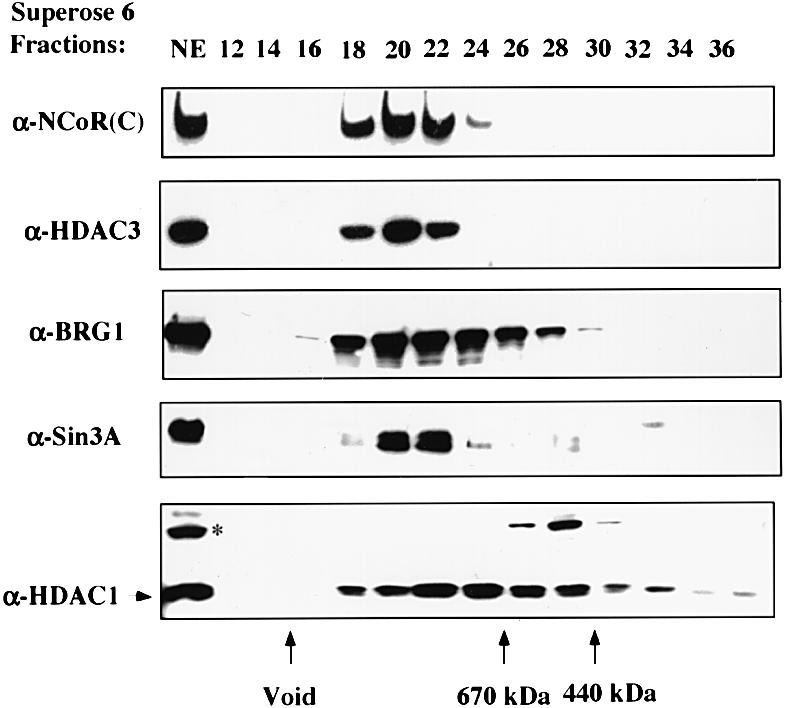
Fig. 1. SMRT and N-CoR proteins exist in large protein complexes with estimated sizes of 1.5–2 MDa. HeLa nuclear extract was fractionated with a Superose 6 gel filtration column. The indicated fractions were analyzed by western blotting using the antibodies indicated. The αN-CoR(C) antibody detected both SMRT and N-CoR proteins. The arrows at the bottom show the elution positions of calibration proteins of known molecular weights.
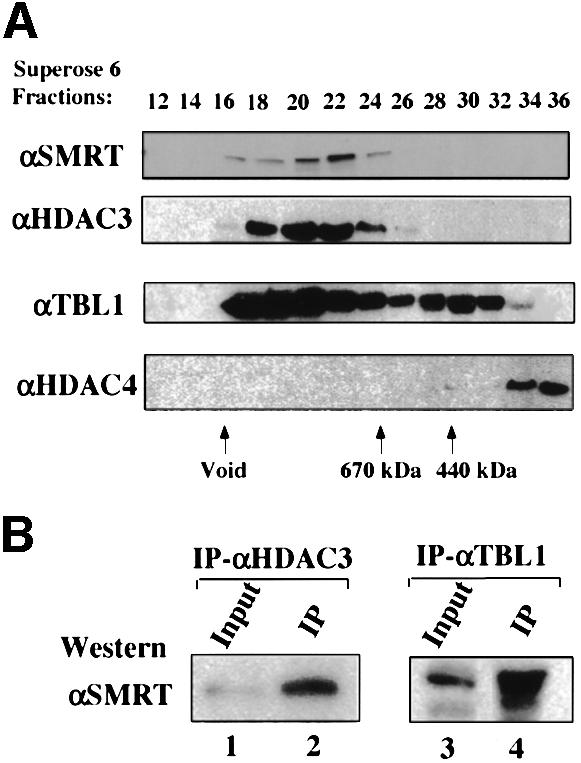
Fig. 3. Confirmation of the association of TBL1 and HDAC3 with SMRT complex. (A) HDAC3 and TBL1 co-fractionated with SMRT in gel filtration chromatography. HeLa nuclear extract was fractionated by using a Superose 6 gel filtration column as in Figure 1 and the indicated fractions were analyzed by western blotting using the antibodies indicated. (B) SMRT protein in HeLa nuclear extracts was co-immunoprecipitated by antibodies specific for HDAC3 and TBL1, respectively; 5% input HeLa nuclear extract was used in lanes 1 and 3.
In comparison, we also analyzed the gel filtration profiles of mSin3A and HDAC1. Consistent with the published data (Zhang et al., 1997), mSin3A behaved as a protein complex(s). The peak of mSin3A overlapped with the N-CoR/SMRT peak (Figure 1), presumably because they all exist in large protein complexes. HDAC1 was found in a broad range of fractions and exhibited several peaks, suggesting the existence of multiple HDAC1-containing protein complexes in HeLa nuclear extract. On the other hand, a protein that cross-reacted with the HDAC1 antibody (indicated by an asterisk) migrated as a sharp peak, indicating that the gel filtration worked well.
Isolation and characterization of an SMRT protein complex
We next attempted to purify N-CoR and SMRT complexes by a combination of conventional and immunoaffinity chromatography. A purification scheme for the SMRT complex is shown in Figure 2A. The SMRT proteins were eluted from the p11 phosphocellulose column primarily in the 0.3 M KCl fraction, whereas N-CoR proteins were eluted primarily in the 0.5 M KCl fraction (Figure 2B). In contrast to the specificity exhibited by αN-CoR(N) and αSMRT antibodies, the αN-CoR(C) antibody recognized both SMRT and N-CoR proteins (Figure 2B). The SMRT proteins in the 0.3 M fraction were fractionated further through a DEAE Fast Flow (Pharmacia) column, and the peak SMRT fraction from the 0.2 M elution was used for immunopurification of the SMRT complex. The resulting immunopurified SMRT complex is shown in Figure 2C. All strong bands except Hsc70 in lane 2 are likely candidates for the subunits of the SMRT complex, as they were absent in a control experiment using rabbit anti-mouse IgG as affinity resin (data not shown).
As expected, the top band was identified as SMRT by mass spectrometry. The 57 kDa protein was identified as TBL1 (Bassi et al., 1999), a transducin-β like protein containing six WD-40 repeats in the C-terminus. Interestingly, the deletion of the TBL1 gene in human has been shown recently to be associated with X-linked late-onset sensorineural deafness (Bassi et al., 1999), a phenotype reminiscent of TRβ gene knockout in mice (Forrest et al., 1996). The following evidence suggests that TBL1 is a genuine subunit of the SMRT complex. First, the presence of TBL1 in the purified SMRT complex is confirmed by western analysis using a TBL1-specific antibody (Figure 2D). Secondly, a substantial level of TBL1 proteins was found to co-fractionate with SMRT in gel filtration analysis (Figure 3A). Thirdly, the TBL1-specific antibody co-immunoprecipitated SMRT from HeLa nuclear extracts (Figure 3B). On the basis of these observations, we conclude that TBL1 is a bona fide subunit of the SMRT complex. The identity of the 37 kDa protein (unknown) and of several minor bands between SMRT and TBL1 has not yet been determined (Figure 2C).
HDAC3 is a subunit of the SMRT and N-CoR complexes
Most importantly, the 50 kDa protein co-purified with SMRT was identified by mass spectrometry as HDAC3. The association of HDAC3 with the purified SMRT complex was confirmed by western analysis using an HDAC3-specific antibody (Figure 2D). Although the HDAC1/2-containing complexes such as mSin3 and NURD as well as class II HDACs have been implicated in the repression mediated by unliganded nuclear receptors, the identification of HDAC3 as a subunit of the purified SMRT complex immediately suggests a new molecular pathway for HDAC-dependent repression mediated by SMRT. The association of HDAC3 with SMRT was supported further by the observation that HDAC3 co-fractionated with SMRT in gel filtration analysis (Figure 3A). In addition, an HDAC3-specific antibody was able to immunoprecipitate SMRT (Figure 3B). Taken together, we conclude that HDAC3 is a stable subunit of the SMRT complex.
Since N-CoR and SMRT are highly related proteins (Ordentlich et al., 1999; Park et al., 1999), we next examined whether HDAC3 is also present in the N-CoR complex. In a gel filtration assay as shown in Figure 1, HDAC3 actually co-fractionated with both SMRT and N-CoR, suggesting that HDAC3 could also associate with the N-CoR complex. To confirm this possibility, we also carried out immunoaffinity purification of the N-CoR complex using αN-CoR(N) and αN-CoR(C) antibodies. Although we have yet to obtain sufficient material for identification of N-CoR-associated proteins directly by mass spectrometry, it has allowed us to analyze the N-CoR-associated proteins by western analysis. As expected, N-CoR was found in the eluates from both antibody columns (Figure 4A). Importantly, HDAC3 was also highly enriched in both immunopurified N-CoR fractions (Figure 4A). The association of HDAC3 with N-CoR was confirmed further by co-immunoprecipitation (co-IP) of N-CoR with HDAC3 using the HDAC3-specific antibody (Figure 4B). The association of TBL1 with N-CoR was also confirmed by western blotting (Figure 4A) and by reciprocal IP of N-CoR proteins using the TBL1-specific antibody (Figure 4B). Thus, like the immunopurified SMRT complex, the N-CoR complex also contains HDAC3 and TBL1.
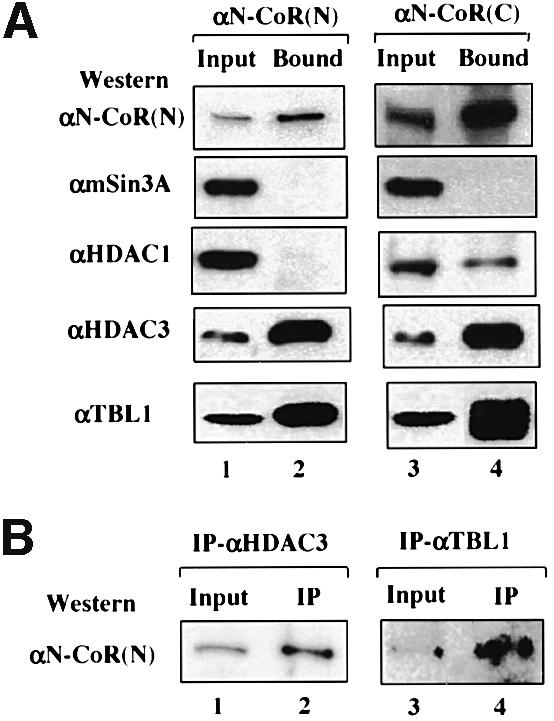
Fig. 4. Both TBL1 and HDAC3 are also associated with the N-CoR protein complex(es). (A) N-CoR complex(es) was affinity-purified by both αN-CoR(N) and αN-CoR(C) antibody affinity columns and the associated proteins were analyzed by western blotting; 5% input HeLa nuclear extract was used in lanes 1 and 3. (B) The N-CoR protein in HeLa nuclear extracts was also co-immunoprecipitated by antibodies specific for HDAC3 and TBL1, respectively; 5% input HeLa nuclear extract was used in lanes 1 and 3.
It is noteworthy that we could not detect the presence of mSin3A in either the immunopurified SMRT (data not show) or the N-CoR complex by western analyses (Figure 4). Interestingly, HDAC1 could be detected by western analysis using an HDAC1-specific antibody in the eluate fraction from the N-CoR(C) affinity column, but not in the eluate from the N-CoR(N) affinity column nor in the purified SMRT complex (data not show). The fact that HDAC1 was not detected in the eluate from the N-CoR(N) affinity column indicates that HDAC1 is likely to associate with only a subfraction of the N-CoR complex. Importantly, because mSin3A was not detected in the eluates from either affinity column, the association of HDAC1 with the subfraction of the N-CoR complex must be independent of the N-CoR–mSin3 interaction. Similarly, we could not detect a convincing association of HDAC4 with either SMRT or N-CoR. In fact, in the gel filtration experiments, the majority of HDAC4 did not appear to co-fractionate with N-CoR/SMRT (Figure 3D), indicating that HDAC4 is unlikely to be a constitutive component of the SMRT or N-CoR complexes.
The majority of SMRT and N-CoR complexes may contain HDAC3
The above results established that HDAC3 is associated with both SMRT and N-CoR complexes. We next wished to determine to what extent the N-CoR and SMRT complexes contain HDAC3. We tested whether an HDAC3-specific antibody could deplete N-CoR and SMRT proteins from HeLa nuclear extracts. As shown in Figure 5A, by western blotting using the αN-CoR(C) antibody that recognizes both SMRT and N-CoR, an increasing amount of anti-HDAC3 antibody in IP experiments depleted >50% of the SMRT/N-CoR proteins from the supernatants (compare lane 4 with lane 1) and led to simultaneously increased N-CoR/SMRT proteins in IP fractions. Western analyses using either the SMRT- or the N-CoR specific antibody confirmed that both SMRT and N-CoR were depleted to a similar extent (data not shown). We also found that the HDAC3 antibody could not completely deplete HDAC3 from the supernatants (Figure 5A), suggesting that some HDAC3 proteins were inaccessible to the antibody. Nevertheless, since >50% of SMRT/N-CoR proteins could be depleted by anti-HDAC3 antibody, we conclude that the majority of SMRT and N-CoR complexes appear to contain HDAC3.
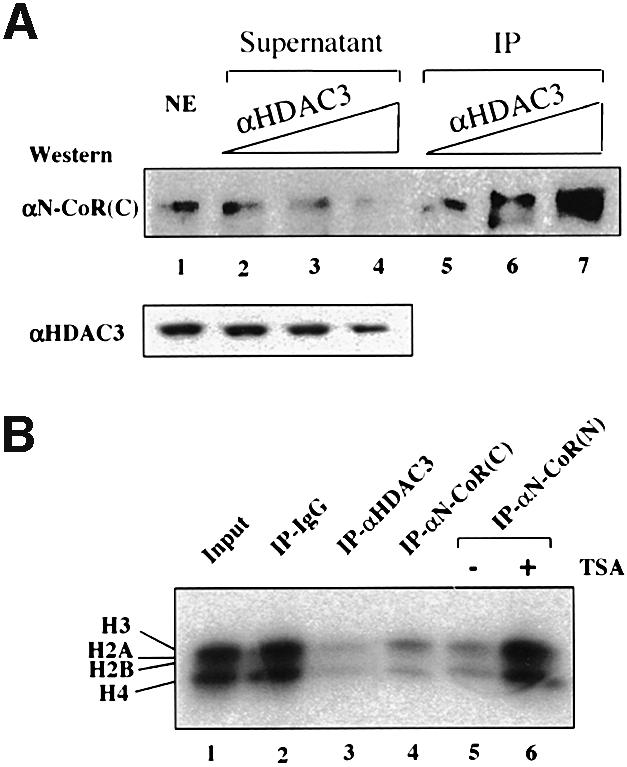
Fig. 5. The majority of the N-CoR and SMRT complexes are likely to contain HDAC3. (A) HeLa nuclear extracts were incubated with an increasing amount of HDAC3 antibody in an attempt to deplete HDAC3 from HeLa nuclear extracts. The levels of SMRT and N-CoR proteins in the supernatants or IP fractions were analyzed by western blotting using the αN-CoR(C) antibody, which detected both N-CoR and SMRT. The levels of HDAC3 in the supernatants are also shown, whereas the HDAC3 in IP fractions could not be determined by western analysis due to the overlapping of the signal from antibody heavy chain with that of HDAC3. (B) The immunopurified N-CoR complexes exhibited histone deacetylase activity. The N-CoR complex(es) or a mixture of SMRT and N-CoR complexes were pull-downed from HeLa nuclear extracts by using αN-CoR (N) and αN-CoR(C) affinity columns. The rabbit anti-rat IgG (lane 2) was used as negative control for IP, whereas αHDAC3 antibody served as a positive control. The purified calf thymus core histones were labeled with [3H]acetyl-CoA in vitro by using the purified recombinant p300 HAT domain and then used for measuring HDAC assay. In lane 6, TSA was added to a final concentration of 0.5 µM in the deacetylation assay.
The association of HDAC3 with N-CoR predicts that the N-CoR and SMRT complexes would contain HDAC activity. To test this prediction, we first immunoprecipitated the N-CoR complexes from HeLa nuclear extracts using both affinity-purified N-CoR(N) and N-CoR(C) antibodies. The core histone substrates used in the assay were labeled in vitro using [3H]acetyl-CoA and a recombinant p300 HAT domain protein purified from Escherichia coli (Gu and Roeder, 1997). As shown in Figure 5B, strong deacetylase activity was detected in both N-CoR(C) and N-CoR(N) IP fractions. As expected, strong deacetylase activity was also observed in the IP fraction of anti-HDAC3, whereas no significant deacetylase activity was detected in the IP fraction of the control IgG. Given that the eluate from N-CoR(N) affinity resins contained no detectable HDAC1 (Figure 2), the deacetylase activity observed in the IP fraction of N-CoR(N) antibody most likely reflects the HDAC3 activity co-immunoprecipitated with N-CoR. In addition, the HDAC activities in all IP fractions were sensitive to 0.5 µM trichostatin A (TSA) (see lane 6; and data not shown), consistent with the published results that the activity of HDAC3 is TSA sensitive (Emiliani et al., 1998). Similarly, the immunopurified SMRT complex also contained strong HDAC activity (data not shown).
HDAC3 may interact directly with N-CoR
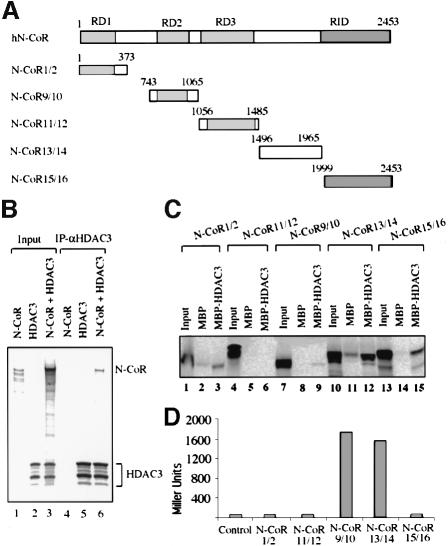
The above results demonstrate the presence of HDAC3 in both SMRT and N-CoR complexes. We next examined the possible direct interaction between HDAC3 and N-CoR by in vitro co-IP, pull-down and yeast two-hybrid assays. The interaction between N-CoR and HDAC3 was first demonstrated by co-IP of in vitro translated N-CoR with in vitro translated HDAC3 in an IP experiment using anti-HDAC3 antibody (Figure 6B). To define the region(s) in N-CoR responsible for HDAC3 interaction, the entire human N-CoR protein was divided into five fragments according to its known functional domains and tested for interaction with HDAC3 by in vitro pull-down and yeast two-hybrid assays. The maltose-binding protein (MBP)–HDAC3 fusion protein was used in pull-down assay instead of GST–HDAC3 because the MBP–HDAC3 has a better solubility in E.coli. As shown in Figure 6C, HDAC3 appears to interact most strongly with the N-CoR13/14 fragment (amino acids 1496–1965), although weak interactions with other regions could also be detected. In yeast two-hybrid assay, both the N-CoR13/14 and N-CoR9/10 fragments containing the previously mapped repression domain II (RDII) showed interaction with HDAC3 (Figure 6D). Thus, two different regions in N-CoR may interact with HDAC3, although only the N-CoR13/14 fragment was found to interact with HDAC3 in both in vitro and in vivo assays. Taken together, both in vitro and in vivo interaction assays indicate that N-CoR can interact directly with HDAC3. However, we would like to emphasize here that whether this interaction can account for the stable association of HDAC3 with the N-CoR complex is yet to be determined.
Fig. 6. HDAC3 may interact directly with N-CoR protein. (A) The schematic diagram illustrating the known functional domains of N-CoR protein and the five different N-CoR constructs. (B) In vitro translated N-CoR co-immunoprecipitated with the in vitro translated HDCA3. (C) The region between amino acids 1496 and 1965 of N-CoR interacted with MBP–HDAC3 in the in vitro pull-down assay. The indicated N-CoR fragments were translated and tested for binding to MBP–HDAC3; 5% input. (D) Yeast two-hybrid assay of the interactions between HDAC3 and different regions of the N-CoR protein. Yeast were transformed with the expression constructs for Gal4-HDAC3 and each of the N-CoR fragments fused with Gal4AD, and cell lysates were measured for activity of a β-galactosidase reporter.
The HDAC3-containing SMRT and N-CoR complexes are involved in the repression of transcription by unliganded TR/RXR
To probe whether the HDAC3-containing N-CoR and SMRT complexes could be involved in the repression by unliganded TR, we first examined whether the HDAC3-containing SMRT and N-CoR complexes in the HeLa nuclear extracts can bind directly to immobilized GST–TR(LBD) (Li et al., 2000). As shown in Figure 7, SMRT and N-CoR were readily detected in the GST–TR(LBD) but not in the control GST bead fraction (Figure 7, compare lane 5 with lane 3). Importantly, HDAC3 was also detected in the GST–TR(LBD) but not in the GST alone bead fraction. The binding of HDAC3 to GST–TR(LBD) is not a result of the direct interaction between HDAC3 and TR, since we failed to detect the binding of in vitro translated HDAC3 to the immobilized GST–TR(LBD) beads (data not shown). This result suggests that the HDAC3-containing SMRT/N-CoR complex can be recruited by unliganded TR and thus is likely to be involved in transcriptional repression by unliganded TR.
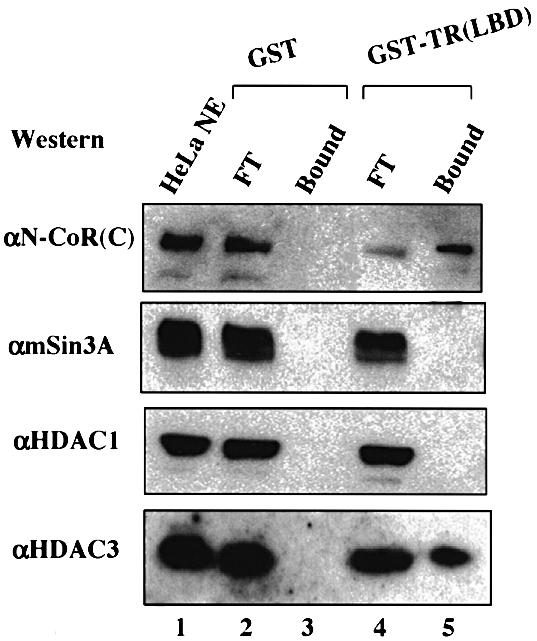
Fig. 7. The HDAC3-containing SMRT and N-CoR complexes interact with unliganded TR. HeLa nuclear extracts were incubated with the immobilized GST control or GST–TR fusion protein. The super natants and bound fractions were then fractionated by SDS–PAGE and analyzed by western blotting using the antibodies indicated. The HeLa NE (nuclear extract) in lane 1 and FT (supernatant) in lanes 2 and 4 were equivalent to 20% of the HeLa nuclear extracts used for pull-down.
Under our experimental conditions, we could not detect the binding of either mSin3A or HDAC1 to the GST–TR beads, although both mSin3A and HDAC1 were readily detected in the input (Figure 7, compare lane 5 with lane 4). The failure to detect Sin3A in the GST–TR pull-down assay is consistent with our findings that both purified SMRT and N-CoR complexes contain no mSin3A. The failure to detect HDAC1 in GST–TR pull-down assay further supports the idea that only a subfraction of the N-CoR complex may contain HDAC1.
We next wished to demonstrate the functional importance of HDAC3-containing SMRT and N-CoR complexes in mediating the repression by unliganded nuclear receptors. Using Xenopus oocytes as a model system, we demonstrated previously that repression by unliganded TR/RXR in the context of chromatin requires histone deacetylase activity (Wong et al., 1998). Since expression of SMRT or N-CoR has yet to be shown to facilitate repression by unliganded nuclear receptors, we tested the involvement of HDAC3-containing N-CoR and SMRT complexes in TR repression by testing whether injection of antibodies against HDAC3, N-CoR or SMRT could block the repression. Although Xenopus homologs of HDAC3, SMRT and N-CoR have not yet been cloned, we reasoned that all these proteins are likely to be highly conserved in evolution. Indeed, when Xenopus oocyte extracts were immunoprecipitated using an N-CoR-specific antibody, a protein (Figure 8B, lane 5) which co-migrated with the N-CoR in control HeLa nuclear extract (data not shown) was detected. Similarly, IP–western using the SMRT-specific antibody detected a protein in Xenopus oocyte extract (Figure 8B, lane 2) which co-migrated with the control SMRT (data not shown) from HeLa nuclear extracts. Together, these results suggest that they are the Xenopus homologs of the mammalian SMRT and N-CoR. Similarly, western analysis of Xenopus oocyte extracts using HDAC3-specific antibody also detected a protein with the same size as human HDAC3, thus likely to be the Xenopus HDAC3 protein (data not shown; see Figure 8A). When Xenopus oocyte extract was fractionated by a Superose 6 gel filtration column, the candidate Xenopus HDAC3 protein behaved as 1.5–2 MDa size protein complexes similar to the HDAC3 in HeLa nuclear extracts (Figure 8A). Importantly, IP of Xenopus oocyte extracts using anti-HDAC3 antibody co-precipitated both Xenopus SMRT and N-CoR candidate proteins (Figure 8B). These results thus indicate that HDAC3 in Xenopus oocytes is also associated with SMRT and N-CoR in large protein complexes and that HDAC3, N-CoR and SMRT proteins are likely to be highly conserved in evolution.
Fig. 8. The HDAC3-containing SMRT and N-CoR complexes are conserved during evolution and involved in the repression exerted by unliganded TR/RXR. (A) Gel filtration analysis of Xenopus oocyte extracts indicated that Xenopus HDAC3 also exists in large protein complexes with an estimated size of 1.5–2 MDa. (B) The association of Xenopus HDAC3 with Xenopus homologs of SMRT and N-CoR as revealed by co-immunoprecipitation experiments. Xenopus oocyte extracts were immunoprecipitated with a control rabbit anti-mouse IgG antibody (IgG), αSMRT, αN-CoR(N) and αHDAC3 antibodies as indicated, and the IP fractions were analyzed by western blotting. (C) Partial relief of the repression by unliganded TR/RXR through microinjection of antibodies against HDAC3 and SMRT/N-CoR. The reporter (pTRβA) was microinjected into Xenopus oocytes and the specific transcripts from this reporter were measured by primer extension analysis (Expt). The repression by unliganded TR/RXR was established by expression of TR and RXR in Xenopus oocytes by microinjection of their corresponding mRNAs. All antibodies used here were affinity-purified and injected at a concentration of 60 ng/µl (18.4 nl/oocyte). The control (Ctrl) was the primer extension product from the endogenous histone H4 mRNA using an H4 primer and served as a loading control.
The demonstration that antibodies raised against human HDAC3, N-CoR and SMRT can recognize their Xenopus homologs allowed us to test whether microinjection of these antibodies could block repression by unliganded TR/RXR. We first assembled a Xenopus TRβA promoter-driven reporter into transcriptionally permissive chromatin by microinjection of its plasmid DNA in double stranded form (dsDNA) (Wong et al., 1995) (Figure 8C, lane 1). Expression of TR/RXR heterodimers by microinjection of their in vitro synthesized mRNAs led to strong repression in the absence of T3 (Figure 8C, compare lane 7 with lane 1). Microinjection of affinity-purified antibody against HDAC3 led to a partial relief of repression (compare lane 8 with lane 7), whereas injection of an HDAC1-specific antibody did not affect the repression. This effect of HDAC3 antibody is specific since injection of HDAC3 antibody in the absence of TR/RXR did not affect the level of transcription. Although injection of both αN-CoR(N) and αSMRT antibodies did not appear to relieve repression, injection of αN-CoR(C) antibody led to a substantial relief of repression (compare lane 9 with lane 7). It should be pointed out that the αN-CoR(C) antibody recognizes both N-CoR and SMRT proteins and was raised against the C-terminus of the N-CoR protein. This region of N-CoR and SMRT is known to be responsible for interaction of N-CoR and SMRT with unliganded TR (Chen and Evans, 1995; Zamir et al., 1996). Thus, binding of αN-CoR(C) antibodies to the C-terminus of SMRT or N-CoR is likely to block the recruitment of N-CoR and SMRT complexes by unliganded TR/RXR. This result thus provides strong evidence for the functional importance of SMRT and N-CoR complexes in mediating the repression by unliganded TR/RXR in chromatin. It is unclear why both N-CoR(N) and SMRT antibodies fail to block the repression. One possible explanation is due to the functional redundancy of N-CoR and SMRT. An antibody specific for one of them will not block the repression as effectively as an antibody recognizing both SMRT and N-CoR. It is also formally possible that both antibodies can bind to their target proteins but fail to block the repression function. In essence, the ability of both antibodies against HDAC3 and N-CoR/SMRT to partially block the repression by unliganded TR/RXR strongly argues for the involvement and functional importance of HDAC3-containing SMRT and N-CoR complexes in repression by unliganded TR/RXR in the context of chromatin.
Discussion
SMRT and N-CoR complexes as a new class of HDAC-containing complexes
We present several lines of evidence showing that HDAC3 is a stable subunit of the mammalian SMRT and N-CoR complexes. First, HDAC3 was identified as a subunit of the immunoaffinity-purified SMRT complex by mass spectrometry (Figure 2) and was also demonstrated to associate with N-CoR by its co-purification with N-CoR (Figure 4). Secondly, an antibody specific for HDAC3 reciprocally precipitated both SMRT and N-CoR proteins (Figures 2 and 4). Thirdly, gel filtration experiments revealed that both SMRT and N-CoR in HeLa nuclear extracts exist as large protein complexes with estimated sizes of at least 1.5–2 MDa and that HDAC3 co-fractionated with both SMRT and N-CoR (Figures 1 and 3). Fourthly, the N-CoR complex immunoprecipitated from the HeLa nuclear extracts with both αN-CoR(N) and αN-CoR(C) antibodies contained HDAC activity (Figure 5A). In addition, the majority of N-CoR and SMRT complexes appeared to contain HDAC3, as the collection of N-CoR and SMRT could be substantially depleted from HeLa nuclear extracts by using an HDAC3 antibody (Figure 5B). Furthermore, the association of SMRT and N-CoR with HADC3 appears to be conserved during evolution, since HDAC3 in Xenopus oocytes was also found to associate with SMRT and N-CoR and exist in large protein complexes (Figure 8). Taken together, these results firmly establish both SMRT and N-CoR as a new class of histone deacetylase complexes containing HDAC3.
Molecular pathways for repression mediated by SMRT and N-CoR complexes
The identification of HDAC3 as a subunit of SMRT and N-CoR complexes immediately suggests a new molecular pathway for HDAC-dependent repression mediated through corepressors SMRT and N-CoR. Since the majority of SMRT/N-CoR complexes appear to contain HDAC3, HDAC3 is likely to be the primary HDAC responsible for the HDAC-dependent repression mediated through N-CoR and SMRT. Consistent with this idea, microinjection of an anti-HDAC3 antibody led to a partial block of the repression exerted by unliganded TR/RXR in Xenopus oocytes (Figure 8).
Despite numerous reports of the direct interaction of mSin3 with SMRT and N-CoR, we find no evidence for the association between mSin3A and SMRT or N-CoR in HeLa nuclear extracts. First, we find that mSin3A was absent from the immunopurified SMRT and N-CoR complexes. Secondly, mSin3A was not pull-downed by GST–TR in an in vitro pull-down assay that pull-downed both SMRT/N-CoR and HDAC3 (Figure 7). Although our results are in contradiction to some early observations, they are consistent with the biochemical purification of the mSin3A complex (Zhang et al., 1997), which contained neither SMRT nor N-CoR. One likely explanation is that free mSin3A protein may be able to interact with free N-CoR or SMRT proteins as shown by two-hybrid assays or co-IP assays when both mSin3A and SMRT or N-CoR proteins were overexpressed (Alland et al., 1997; Heinzel et al., 1997; Nagy et al., 1997). Yet, under normal circumstances, both mSin3A and N-CoR and SMRT exist in distinct large protein complexes that prevent their direct association. We also could not exclude the possibility that the mSin3A complex could associate unstably with N-CoR and SMRT complexes in vivo and that such an association is disrupted during nuclear extract preparation.
So far, N-CoR and SMRT have been implicated in the transcriptional repression by a variety of transcriptional factors including unliganded TR, RAR, Myc/Mad, PLZF, BCL6/LAZ3 and ETO, as well as the regulation of the Notch signaling pathway through interaction with CBF (Hong et al., 1997; Dhordain et al., 1998; Kao et al., 1998; Wang et al., 1998). Although the role of HDAC3 has yet to be tested in all those cases except unliganded TR, mSin3 complex appears to be required for repression by unliganded TR, RAR and Myc/Mad, as their repression can be abrogated through microinjection of neutralizing antibodies against mSin3 and HDAC1/2 (Heinzel et al., 1997). In all these cases, it is generally believed that mSin3 complex is recruited through interaction with SMRT and/or N-CoR (Heinzel et al., 1997; Nagy et al., 1997). Our finding that the mSin3 complex is unlikely to associate stably with SMRT or N-CoR complexes thus raises the question of how the mSin3 complex is involved in the above repression processes. One possibility is that SMRT or N-CoR complexes could be able to recruit the mSin3 complex through weak protein–protein interactions. A second possibility is that the mSin3 complex could be recruited into the repression processes in a repressor-dependent but SMRT- and N-CoR complex-independent manner. A third possibility is that the mSin3 complex may play an important role in repression through chromatin deacetylation in a non-targeting fashion. In accordance with this idea, it has been shown that RbAp46/48 proteins present in both mSin3 and NURD complexes can interact directly with core histones and thus have the potential to target mSin3A or NURD complexes to chromatin independently of any interaction with repressors or corepressors (Parthun et al., 1996).
Recently, class II HDACs including HDAC4, 5, 6 and 7 have been implicated to be involved in repression mediated through SMRT or N-CoR (Huang et al., 2000; Kao et al., 2000). Class II HDACs were shown to interact with the repression domain II of N-CoR or SMRT and thus contributed to the repression mediated by SMRT and N-CoR. Nevertheless, it appears that only a small fraction of SMRT or N-CoR complexes were found to associate with the class II HDACs such as HDAC4. Consistent with that result, we showed by gel filtration analysis that the majority of HDAC4 did not appear to co-fractionate with SMRT or N-CoR, although we have not yet tested for other class II HDACs. Thus, the class II HDACs may not associate constitutively with SMRT or N-CoR complexes as HDAC3 does, but could be recruited during the repression process. It remains to be seen whether SMRT and N-CoR complexes could simultaneously contain HDAC3 and class II HDACs.
Our finding that SMRT and N-CoR themselves exist as a large protein complex containing HDAC3 also raises the question as to why the HDAC1/2-containing mSin3 complex and perhaps class II HDACs would be needed for the repression mediated by N-CoR or SMRT. The answer to this question may lie in the difference in substrate specificity between HDAC1/2, HDAC3 and class II HDACs. So far, very little is known about the substrate specificity of the various HDACs. All HDACs seem capable of deacetylating all four core histones in vitro (Emiliani et al., 1998; Grozinger et al., 1999). However, whether they exhibit differential substrate specificity toward core histones and/or non-histone proteins in vivo is unclear at present. The second possibility is that the activity of the different HDACs may be utilized differentially dependent on the repressors involved and the promoter context. The ability to recruit multiple HDACs may be required to ensure an effective repression.
TBL1 as a subunit of both SMRT and N-CoR complexes
Interestingly, TBL1, a six WD-40 repeat-containing protein (Bassi et al., 1999), was identified as a subunit of the immunopurified SMRT complex. Although the exact stoichiometric ratio of TBL1 in purified SMRT complex is not known, more than one subunit of TBL1 appears to be present in each SMRT complex in comparison with HDAC3 (Figure 2C), which is likely to be present in the majority of the SMRT complexes (Figure 5A). We further demonstrate that TBL1 is also present in the N-CoR complex. The TBL1 gene was identified originally as a novel gene either partially or completely deleted in patients carrying Xp22.3 terminal deletions (Bassi et al., 1999). The deletion of TBL1 is postulated to be associated with the pathogenesis of the ocular albinism with late-onset sensorineural deafness phenotype (Bassi et al., 1999). Although it is not known whether the pathogenesis of TBL1 deletion patients has anything to do with the association of TBL1 with N-CoR or SMRT, it is intriguing that one phenotype of TRβ gene null mice is deafness (Forrest et al., 1996). In addition, TBL1 homologs exist in yeast and Drosophila (Bassi et al., 1999). In particular, the sequence identity between human TBL1 and the Drosophila protein is >80%, indicating that TBL1 is highly conserved in evolution. The Drosophila TBL1/ebi (Dong et al., 1999) appears to be involved in the epidermal growth factor receptor signaling pathways. Although the exact function of the WD-40 repeat motif is unclear, this motif is generally believed to be involved in protein–protein interactions, as in the case of transducin β-r complex. Thus, it is tempting to speculate that TBL1 may play a structural role in the formation of the SMRT and N-CoR complexes.
In conclusion, our finding that N-CoR itself exists as a large protein complex containing HDAC3 adds another layer of complexity to and also provides new insight into the molecular mechanisms by which the corepressors SMRT and N-CoR mediate repression by an increasing number of transcriptional repressors.
Materials and methods
Cloning and plasmid construction
The N-CoR expression constructs used for in vitro translation of N-CoR1/2 (1–373), N-CoR9/10 (743–1065), N-CoR11/12 (1056–1485), N-CoR13/14 (1496–1965) and N-CoR15/16 (1999–2453) were generated by PCR amplification of the corresponding regions of the human N-CoR cDNA and cloned into the expression vectors pRSET B and pGAD10 (Invitrogen). To generate recombinant proteins for raising antibodies against N-CoR, the cDNAs encoding N-CoR (1–373) and N-CoR (2164–2441) were cloned into pRSET B vector as His6-tagged proteins, and the cDNA region encoding SMRT (1165–1363) was cloned into the pMLc2 vector (New England Biolabs) as an MBP fusion protein. The expression construct for the MBP–HDAC3 fusion was generated by cloning the full-length HDAC3 cDNA into the pMLc2 vector. The HDAC3 construct for yeast two-hybrid assay was generated by cloning HDCA3 in-frame into the pAS1-CYH2 vector. The construction of GST–TR expression plasmid was as described (Li et al., 2000).
Purification of recombinant proteins and generation of antibodies
All MBP fusion proteins were purified according to the manufacturer’s instructions (New England Biolabs). Purification of GST and GST–TR was as described previously (Xue et al., 1998). Purification of his-tagged N-CoR (1–373) and N-CoR (2164–2440) was done using a kit from Novagen according to the manufacturer’s instructions. All rabbit polyclonal antibodies were generated in the Josman Laboratory by using the corresponding purified recombinant proteins as antigens.
Gel filtration, ion exchanger chromatography and affinity purification of SMRT complex
Gel filtration analysis of HeLa nuclear extracts was performed essentially as described (Wang et al., 1996). Proteins in each fraction (0.45 ml) were precipitated by trichloroacetic acid (TCA) and then separated by a 7.5% SDS–PAGE and analyzed by western blotting using different antibodies as indicated. The fractionation of SMRT and N-CoR complex by ion exchange chromatography is as illustrated in Figure 2A, and details are available upon request.
All antibody affinity resins were generated by binding and cross-linking of affinity-purified antibodies to protein A–Sepharose beads as described (Harlow and Lane, 1988). The affinity purification of the SMRT complex from the DEAE 0.2 M fraction was essentially as described (Wang et al., 1996). The protein identification by mass spectrometry was as described (Qin et al., 1997).
In vitro pull-down assay and yeast two-hybrid assay
Each N-CoR fragment was translated and labeled in vitro with [35S]methionine using a TNT T7 coupled transcription/translation kit from Promega. The in vitro translated proteins were examined for binding to either purified control MBP or MBP–HDAC3 according to the manufacturer’s instructions (New England Biolabs). Yeast two-hybrid assay was performed as described (Nawaz et al., 1999).
Histone deacetylase activity assay
The 3H-labeled acetylated core histones were prepared as described (Gu and Roeder, 1997). Immunoprecipitation of HeLa nuclear extracts using affinity-purified antibodies against N-CoR(N) and N-CoR(C), HDAC3 (Upstate) or rabbit anti-rat IgG and subsequent histone deacetylase assay were performed as described (Xue et al., 1998).
Microinjection of Xenopus oocytes
The preparation of Xenopus stage VI oocytes and the microinjection procedure were essentially as described (Wong et al., 1995). Each antibody was maintained in phosphate-buffered saline and injected (18.4 nl/oocyte) into the nuclei of the oocytes at a protein concentration of 60 µg/ml. After overnight incubation, the groups of oocytes were collected for RNA isolation and subsequent primer extension analysis to determine the level of transcription as described (Wong et al., 1995). In the result shown in Figure 8, both DNA and antibody were injected ∼1 h before the injection of TR/RXR mRNAs.
Acknowledgments
Acknowledgements
We thank Drs M.-J.Tsai, D.D.Moore, N.J.McKenna, R.Wu and F.A.Pereira for critical reading and comments of this manuscript. We are grateful Drs M.G.Guenther and M.A.Lazar for TBL1 antibody, Dr R.G.Roeder for p300 HAT domain expression plasmid, Dr W.Wang for BRG1 antibody and Dr X.Yang for HDAC4 antibody. This work was supported by NIH grant DK 56324 to J.W.
References
- Alland L., Muhle,R., Hou,H.,Jr, Potes,J., Chin,L., Schreiber-Agus,N. and DePinho,R.A. (1997) Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature, 387, 49–55. [DOI] [PubMed] [Google Scholar]
- Baniahmad A., Steiner,C., Kohne,A.C. and Renkawitz,R. (1990) Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell, 61, 505–514. [DOI] [PubMed] [Google Scholar]
- Baniahmad A., Leng,X., Burris,T.P., Tsai,S.Y., Tsai,M.J. and O’Malley,B.W. (1995) The tau 4 activation domain of the thyroid hormone receptor is required for release of a putative corepressor(s) necessary for transcriptional silencing. Mol. Cell. Biol., 15, 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi M.T. et al. (1999) X-linked late-onset sensorineural deafness caused by a deletion involving OA1 and a novel gene containing WD-40 repeats. Am. J. Hum. Genet., 64, 1604–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J. et al. (1994) Functional evidence for ligand-dependent dissociation of thyroid hormone and retinoic acid receptors from an inhibitory cellular factor. Mol. Cell. Biol., 14, 5756–5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.D. and Evans,R.M. (1995) A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature, 377, 454–457. [DOI] [PubMed] [Google Scholar]
- Ciana P., Braliou,G.G., Demay,F.G., von Lindern,M., Barettino,D., Beug,H. and Stunnenberg,H.G. (1998) Leukemic transformation by the v-ErbA oncoprotein entails constitutive binding to and repression of an erythroid enhancer in vivo. EMBO J., 17, 7382–7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhordain P., Lin,R.J., Quief,S., Lantoine,D., Kerckaert,J.P., Evans,R.M. and Albagli,O. (1998) The LAZ3(BCL-6) oncoprotein recruits a SMRT/mSIN3A/histone deacetylase containing complex to mediate transcriptional repression. Nucleic Acids Res., 26, 4645–4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Tsuda,L., Zavitz,K.H., Lin,M., Li,S., Carthew,R.W. and Zipursky,S.L. (1999) ebi regulates epidermal growth factor receptor signaling pathways in Drosophila. Genes Dev., 13, 954–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressel U., Thormeyer,D., Altincicek,B., Paululat,A., Eggert,M., Schneider,S., Tenbaum,S.P., Renkawitz,R. and Baniahmad,A. (1999) Alien, a highly conserved protein with characteristics of a corepressor for members of the nuclear hormone receptor superfamily. Mol. Cell. Biol., 19, 3383–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emiliani S., Fischle,W., Van Lint,C., Al-Abed,Y. and Verdin,E. (1998) Characterization of a human RPD3 ortholog, HDAC3. Proc. Natl Acad. Sci. USA, 95, 2795–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest D., Erway,L.C., Ng,L., Altschuler,R. and Curran,T. (1996) Thyroid hormone receptor β is essential for development of auditory function. Nature Genet., 13, 354–357. [DOI] [PubMed] [Google Scholar]
- Glass C.K., Lipkin,S.M., Devary,O.V. and Rosenfeld,M.G. (1989) Positive and negative regulation of gene transcription by a retinoic acid–thyroid hormone receptor heterodimer. Cell, 59, 697–708. [DOI] [PubMed] [Google Scholar]
- Glass C.K. and Rosenfeld,M.G. (2000) The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev., 14, 121–141. [PubMed] [Google Scholar]
- Grozinger C.M., Hassig,C.A. and Schreiber,S.L. (1999) Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc. Natl Acad. Sci. USA, 96, 4868–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W. and Roeder,R.G. (1997) Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell, 90, 595–606. [DOI] [PubMed] [Google Scholar]
- Harlow E. and Lane,D. (1988) Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Hebbes T.R., Thorne,A.W. and Crane-Robinson,C. (1988) A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J., 7, 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel T. et al. (1997) A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature, 387, 43–48. [DOI] [PubMed] [Google Scholar]
- Hong S.H., David,G., Wong,C.W., Dejean,A. and Privalsky,M.L. (1997) SMRT corepressor interacts with PLZF and with the PML-retinoic acid receptor α (RARα) and PLZF-RARα oncoproteins associated with acute promyelocytic leukemia. Proc. Natl Acad. Sci. USA, 94, 9028–9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlein A.J. et al. (1995) Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature, 377, 397–404. [DOI] [PubMed] [Google Scholar]
- Huang E.Y., Zhang,J., Miska,E.A., Guenther,M.G., Kouzarides,T. and Lazar,M.A. (2000) Nuclear receptor corepressors partner with class II histone deacetylases in a Sin3-independent repression pathway. Genes Dev., 14, 45–54. [PMC free article] [PubMed] [Google Scholar]
- Kao H.Y., Downes,M., Ordentlich,P. and Evans,R.M. (2000) Isolation of a novel histone deacetylase reveals that class I and class II deacetylases promote SMRT-mediated repression. Genes Dev., 14, 55–66. [PMC free article] [PubMed] [Google Scholar]
- Kao H.Y., Ordentlich,P., Koyano-Nakagawa,N., Tang,Z., Downes,M., Kintner,C.R., Evans,R.M. and Kadesch,T. (1998) A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Genes Dev., 12, 2269–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. (1999) Histone acetylases and deacetylases in cell proliferation. Curr. Opin. Genet. Dev., 9, 40–48. [DOI] [PubMed] [Google Scholar]
- Lee D.Y., Hayes,J.J., Pruss,D. and Wolffe,A.P. (1993) A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell, 72, 73–84. [DOI] [PubMed] [Google Scholar]
- Li J., O’Malley,B.W. and Wong,J. (2000) p300 requires its histone acetyltransferase activity and SRC-1 interaction domain to facilitate thyroid hormone receptor activation in chromatin. Mol. Cell. Biol., 20, 2031–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna N.J., Lanz,R.B. and O’Malley,B.W. (1999) Nuclear receptor coregulators: cellular and molecular biology. Endocrinol. Rev., 20, 321–344. [DOI] [PubMed] [Google Scholar]
- Nagy L., Kao,H.Y., Chakravarti,D., Lin,R.J., Hassig,C.A., Ayer,D.E., Schreiber,S.L. and Evans,R.M. (1997) Nuclear receptor repression mediated by a complex containing SMRT, mSin3A and histone deacetylase. Cell, 89, 373–380. [DOI] [PubMed] [Google Scholar]
- Nawaz Z., Lonard,D.M., Smith,C.L., Lev-Lehman,E., Tsai,S.Y., Tsai,M.J. and O’Malley,B.W. (1999) The Angelman syndrome-associated protein, E6-AP, is a coactivator for the nuclear hormone receptor superfamily. Mol. Cell. Biol., 19, 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordentlich P., Downes,M., Xie,W., Genin,A., Spinner,N.B. and Evans, R.M. (1999) Unique forms of human and mouse nuclear receptor corepressor SMRT. Proc. Natl Acad. Sci. USA, 96, 2639–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E.J., Schroen,D.J., Yang,M., Li,H., Li,L. and Chen,J.D. (1999) SMRTe, a silencing mediator for retinoid and thyroid hormone receptors—extended isoform that is more related to the nuclear receptor corepressor. Proc. Natl Acad. Sci. USA, 96, 3519–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthun M.R., Widom,J. and Gottschling,D.E. (1996) The major cytoplasmic histone acetyltransferase in yeast: links to chromatin replication and histone metabolism. Cell, 87, 85–94. [DOI] [PubMed] [Google Scholar]
- Qin J., Fenyo,D., Zhao,Y., Hall,W.W., Chao,D.M., Wilson,C.J., Young,R.A. and Chait,B.T. (1997) A strategy for rapid, high-confidence protein identification. Anal. Chem., 69, 3995–4001. [DOI] [PubMed] [Google Scholar]
- Turner B.M. and O’Neill,L.P. (1995) Histone acetylation in chromatin and chromosomes. Semin. Cell Biol., 6, 229–236. [DOI] [PubMed] [Google Scholar]
- Wang J., Hoshino,T., Redner,R.L., Kajigaya,S. and Liu,J.M. (1998) ETO, fusion partner in t(8;21) acute myeloid leukemia, represses transcription by interaction with the human N-CoR/mSin3/HDAC1 complex. Proc. Natl Acad. Sci. USA, 95, 10860–10865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. et al. (1996) Purification and biochemical heterogeneity of the mammalian SWI–SNF complex. EMBO J., 15, 5370–5382. [PMC free article] [PubMed] [Google Scholar]
- Wolffe A.P. (1994) Transcription: in tune with the histones. Cell, 77, 13–16. [DOI] [PubMed] [Google Scholar]
- Wong J., Patterton,D., Imhof,A., Guschin,D., Shi,Y.B. and Wolffe,A.P. (1998) Distinct requirements for chromatin assembly in transcriptional repression by thyroid hormone receptor and histone deacetylase. EMBO J., 17, 520–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J., Shi,Y.B. and Wolffe,A.P. (1995) A role for nucleosome assembly in both silencing and activation of the Xenopus TRβ A gene by the thyroid hormone receptor. Genes Dev., 9, 2696–2711. [DOI] [PubMed] [Google Scholar]
- Xue Y., Wong,J., Moreno,G.T., Young,M.K., Cote,J. and Wang,W. (1998) NURD, a novel complex with both ATP-dependent chromatin- remodeling and histone deacetylase activities. Mol. Cell, 2, 851–861. [DOI] [PubMed] [Google Scholar]
- Yang W.M., Yao,Y.L., Sun,J.M., Davie,J.R. and Seto,E. (1997) Isolation and characterization of cDNAs corresponding to an additional member of the human histone deacetylase gene family. J. Biol. Chem., 272, 28001–28007. [DOI] [PubMed] [Google Scholar]
- Zamir I., Harding,H.P., Atkins,G.B., Horlein,A., Glass,C.K., Rosenfeld,M.G. and Lazar,M.A. (1996) A nuclear hormone receptor corepressor mediates transcriptional silencing by receptors with distinct repression domains. Mol. Cell. Biol., 16, 5458–5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Guenther,M.G., Carthew,R.W. and Lazar,M.A. (1998) Proteasomal regulation of nuclear receptor corepressor-mediated repression. Genes Dev., 12, 1775–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Iratni,R., Erdjument-Bromage,H., Tempst,P. and Reinberg,D. (1997) Histone deacetylases and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell, 89, 357–364. [DOI] [PubMed] [Google Scholar]