Abstract
BACKGROUND:
Kawasaki disease (KD) is an acute, self‐limiting vasculitis of unknown etiology. The incidence of KD is increasing world wide. However, the epidemiological data for KD in Turkey has not been well described.
OBJECTIVE:
To describe the demographic, clinical, and laboratory features of children with KD who were diagnosed and managed in the American Hospital, Istanbul, Turkey.
METHOD:
Patients with KD were retrospectively identified from the hospital discharge records between 2002 and 2010. Atypical cases of KD were excluded. A standardized form was used to collect demographic data, clinical information, echocardiography and laboratory results.
RESULTS:
Thirty‐five patients with KD, with a mean age of 2.5±1.9 years, were identified. Eighty‐five point seven per cent of patients were under 5 years of age. A seasonal pattern favouring the winter months was noticed. In addition to fever and bilateral conjunctival injection, changes in the oral cavity and lips were the most commonly detected clinical signs in our cases. Coronary artery abnormalities were detected in nine patients. The majority of our patients had started treatment with intravenous immunoglobulin in the first 10 days of the onset of fever, and only one patient required systemic steroids for intravenous immunoglobulin‐resistant KD. The coronary artery abnormalities resolved in all nine patients within 8 months.
CONCLUSION:
This study is the most comprehensive series of children from Turkey with KD included in Medline. As adult‐onset ischemic heart disease may be due to KD in childhood, further prospective clinical investigations are needed to understand the epidemiology, management and long‐term follow‐up of the disease.
Keywords: Kawasaki disease, Coronary artery disease, Echocardiography, Steroids
INTRODUCTION
Kawasaki disease (KD) is an acute, self‐limiting systemic vasculitis of unknown etiology, which mainly affects children aged <5 years. It was first described by Tomisaku Kawasaki in 1967 in Japan.1 KD has now replaced rheumatic fever as the leading cause of acquired heart disease in childhood in the developed world, and is the second most common childhood vasculitis.2,3 Although the incidence of KD varies among countries, it is much higher in children from Asian countries.4,5 The clinical signs of KD are similar to those of many other childhood illnesses. The disease is often complicated by coronary artery abnormalities (CAA), including dilatation and/or aneurysms, and thus is a leading cause of acquired heart disease in children.6,7 Some clinical features other than the classic diagnostic criteria are intense irritability, cough, diarrhea, sterile pyuria, arthritis, arthralgia, redness and induration at the site of a Bacille–Calmette–Guerin (BCG) scar. Patients with prolonged fever and fewer than four of the other principal criteria are diagnosed as atypical or incomplete KD if CAA are present.8
The incentive for this research came from the remarkable lack of knowledge about the epidemiology and features of KD in Turkey. In this article we present the demographic, clinical and laboratory features of children with KD, who were diagnosed and managed in our hospital.
MATERIAL AND METHODS
Patients with KD were identified from hospital discharge records between 2002 and 2010. All the children were being followed up routinely at an outpatient clinic of the American Hospital, Istanbul, Turkey—a private hospital, generally relying on a high socioeconomic population from Istanbul. Diagnosis of KD was made according to American Heart Association guidelines.9 Table 1 shows the standard diagnostic criteria for KD. Medical charts of patients with KD were reviewed using a standardized form to collect demographic data, clinical information, and laboratory test results, retrospectively. All children diagnosed with atypical KD were excluded. Echocardiography was performed during hospitalization and follow‐up in all patients.
Table 1.
Diagnostic criteria for Kawasaki disease.
| Fever for 5 days or more |
| Presence of at least four of the following five conditions: |
| (1) Bilateral (non‐purulent) conjunctivitis |
| (2) Skin rash |
| (3) Changes in the lips and mouth |
| • reddened, dry or cracked lips |
| • strawberry tongue |
| • diffuse erythema of oral or pharyngeal mucosa |
| (4) Changes in the extremities |
| • erythema of palms or soles |
| • indurative edema of hands or feet |
| • desquamation of skin of hand, feet and perineum |
| (5) Cervical lymphadenopathy |
| • more than 1.5 cm in diameter |
| Differential diagnosis with a similar presentation: |
| Staphylococcal infection (such as scalded skin syndrome, toxic shock syndrome), streptococcal infection (such as scarlet fever, toxic shock‐like syndrome, carriage of group A streptococcus does not exclude the possibility of Kawasaki disease), measles and other viral exanthems (including rubella, enterovirus, Epstein–Barr virus, cytomegalovirus, human herpes virus 6, parvovirus), mycoplasma, Stevens–Johnson syndrome, drug reaction, juvenile rheumatoid arthritis. |
Definitions of CAA were based on the following criteria: for children aged <5 years, an internal lumen diameter (ILD) ≤3 mm was considered normal and for children aged ≥5 years, an ILD ≤4 mm was considered normal. An ILD of a coronary artery segment enlarged to <1.5 times the normal upper limit was defined as a dilatation, and an ILD enlarged to ≥1.5 times the normal upper limit was defined as an aneurysm. When a coronary artery was larger than normal (dilated) and without a segmental aneurysm, the vessel was considered ectasic.9 Echocardiography was usually repeated within 2 weeks of the onset of illness, during the fourth week, and thereafter depending on the initial findings. All patients underwent laboratory investigations for platelets, leukocyte (white blood cell) count, hemoglobin (Hb), C‐reactive protein (CRP), erythrocyte sedimentation rate (ESR), aspartate aminotransferase, alanine aminotransferase and underwent urine analysis.
Qualitative data are presented as frequencies with percentages and quantitative data as means with standard deviations (SD).
RESULTS
Thirty‐five patients with KD were treated in the Istanbul American Hospital during the 8‐year period. The mean age of the patients was 2.5±1.9 years (range 2 months to 7 years). Eighty per cent of the cases were diagnosed within the first 10 days, with the longest time to diagnosis being 15 days. The demographic and clinical characteristics of patients are summarized in Table 2. Eighty six per cent (30 cases) of patients were aged <5 years and 14% (five cases) of patients were aged >5 years at the time of diagnosis. Fever was the main clinical sign in all patients and mean body temperature was 39.4±0.9°C at diagnosis. Although intense irritability is not part of the classic diagnostic criteria, it was present in all patients. In one patient there was redness and induration at the site of the BCG scar. Three patients had arthritis and one patient had abdominal pain and diarrhea. Laboratory findings showed that anemia (Hb <11 g/dl) was present in 14 (40%) patients who were all a year old. Elevated serum transaminases were detected in 10 (28.6%) patients. Laboratory values of the patients at diagnosis are shown in Table 3. No positive culture (blood, urine and throat) was found in our patients. Sterile pyuria was present in two patients.
Table 2.
Demographic and clinical characteristics of patients at diagnosis.
| ‐ Age (months) | 30.4±22.4 (2–84) |
| ‐ Male (%) | 27 (77.1) |
| ‐ Female (%) | 8 (22.9) |
| ‐ Duration of fever (days) | 7.81±2.81 (4–15) |
| ‐ Clinical features (%) | |
| • Fever | 35 (100) |
| • Skin rash | 30 (85.7) |
| • Changes in oral cavity and lips | 34 (97.1) |
| • Bilateral conjuctival injection | 34 (97.1) |
| • Cervical lympadenopathy | 17 (48.6) |
| • Changes in extremities | 23 (65.7) |
Data are presented as mean ± SD (range) or number (%).
Table 3.
Laboratory values of patients at diagnosis.
| Value | Mean ± SD (range) |
| Hb (g/dl) | 11.1±0.9 (9.3±13.3) |
| WBC (/mm3) | 15896±6383 (3420–28490) |
| Platelet count (/mm3) | 496889±208503 (189000–933000) |
| CRP (mg/dl) | 65.4±52.4 (3.1–237) |
| ESR (mm/h) | 65.3±29.5 (16–132) |
| AST (IU/L) | 45.9±31.7 (17–208) |
| ALT (IU/L) | 52.4±52.8 (13–326) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C‐reactive protein; ESR. erythocyte sedimentation rate; Hb, hemoglobin; WBV, white blood cell count.
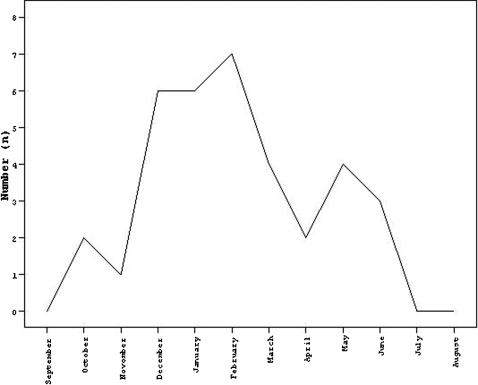
Patients were receiving antibiotic therapy at diagnosis, with the exception of two patients. During the 8‐year period, the monthly number of cases was lowest between July and September and peaked in the winter months (Fig 1). The highest incidence was seen in February, with seven patients.
Figure 1.
Monthly hospital admission of patients during 2002–2010.
CAA were detected in nine (25.7%) cases (six males, three female). One patient had ectasia in the right coronary artery and two patients in the left coronary artery. Six children had coronary artery aneurysms (one in both right and left coronary arteries with pericardial effusion, three had left coronary artery aneurysms and two had right coronary artery aneurysms with mitral valve regurgitation). All children were treated with intravenous immunoglobulin (IVIg) at a dose of 2 g/kg for a period of 10–12 h in addition to high‐dose aspirin (100 mg/kg) during the febrile period, according to current consensus guidelines.10 In one patient, after a second infusion of IVIg, high‐dose (30 mg/kg) methylprednisolone pulse therapy was carried out for a period of 2 h. Symptoms resolved in this child after two intravenous doses of steroids. There was no recurrence in any patient.
DISCUSSION
KD is an acute febrile vasculitis that has been reported with increasing incidence among all racial ethnic groups. While Japan has the highest and increasing annual incidence in the world (184.6 per 100,000 children aged <5 years between 2005 and 2006), the epidemiology of KD in Europe has not been well described.11,12 Likewise, the epidemiology of KD in Turkey is also not well known. A nationwide survey found that KD had an incidence of 9% of childhood vasculitides.13
There are many unconfirmed hypotheses about the cause of KD. Although clinical, laboratory and epidemiologic features strongly suggest an infectious cause, the etiology of KD still remains unknown. One possibility is that a viral repiratory infection (represented by a seasonal pattern), juxtaposed with a subsequent secondary bacterial colonization, precipitates a cascade of events that result in an exaggerated immunologic response.14,15 Our study confirms the winter peak of KD (Figure 1), but no clear relationship with respiratory pathogens was shown as virologic and immunologic data were lacking. KD is more common in boys than girls and 85% of cases occur in children aged <5 years.11 In our study, we detected a male predominance and age distribution resembling that of previous studies.16 Studies from the United States also show that KD is more common during the winter and that 76% of children are <5 years old.17
In KD, the fever is typically high‐spiking and remittent, with peak temperatures of >39°C and, in many cases, >40°C.18 Our patients' fever pattern was consistent with this classic finding, with a mean body temperature of 39.4±0.9°C at diagnosis. In addition to fever, bilateral conjunctival injection and changes in the oral cavity and lips were the most commonly detected clinical signs in our cases, as found in other studies from Turkey and other parts of the world.19-22 The occurrence of cervical lymphadenopathy in KD is variable. It is seen in only 50–75% of patients, whereas most of the other features are seen in approximately 90%.23 It is usually unilateral and confined to the anterior cervical triangle, and its classic criteria include ≥1 lymph node that is >1.5 cm in diameter.18 In our study, cervical lymphadenopathy was the least common of the principal clinical features (48.6%). The lymphadenopathies in our patients were typically classic, except for one patient who presented with massive cervical node enlargement.
Multiple clinical findings may be observed in patients with KD. Arthritis or arthralgia can occur in the first week of the illness and tends to affect multiple joints, including the small interphalangeal joints and large weight‐bearing joints.18 In our series, two patients had arthritis in their knees and one patient had arthritis in his ankle.
Children with KD are often more irritable than the children with other febrile ilnesses,18 and all the patients in this study were irritable. Gastrointestinal complaints, including diarrhea, vomiting and abdominal pain, occur in about one‐third of patients.24 Rarely, KD can present as an acute surgical abdomen.24 Contrary to previous studies, only one of our 35 patients had abdominal pain and diarrhea. Erythema and induration at the site of BCG vaccination is common in Japan, where BCG is used widely.25 As in Japan, BCG is routinely performed in Turkey, but only one patient had redness and induration at the site of the BCG scar.
KD is mainly a clinical diagnosis and there are no pathognomonic laboratory tests or findings. However, leukocytosis is typical during the acute stage of KD. Approximately 50% of patients had white blood cell counts >15,000/mm3.18 Similar to other reports,8,10,22 the mean leukocyte count of our study group was 15,896±6,383/mm3. Normocytic anemia may develop, particularly with more prolonged duration of active inflammation.8,18 Marked anemia (Hb <11 g/dl) on admission was noted in 14 (40%) patients who were all over 1 year old and who had prolonged fever for more than 7 days. Thrombocytosis is a characteristic feature of the later phases of the illness. It is rarely present in the first week of the illness, usually appearing in the second week, and peaking in the third week with a gradual return to normal by 4–8 weeks after onset in uncomplicated cases. Platelet counts are usually >450,000/mm3 in patients evaluated after day 7 of illness.18 In our series most of the patients were diagnosed in the second week and the mean platelet count was 496,889±208,503/mm3, which is consistent with other studies.18 Elevations of CRP and ESR are almost universal in KD, usually returning to normal values by 6–10 weeks after the onset of illness.18 Mean values of CRP and ESR were high in our patients. Burns et al.26 reported mild to moderate elevations in serum transaminases in ≤40% of patients. Although the mean values of transaminases were not high in our study group, the ratio of patients with high levels (28.6%) was consistent with this multicenter study.
Urine analysis showed intermittent mild to moderate sterile pyuria in approximately 33% of patients, suggesting urethritis.18 Urine cultures of two patients with sterile pyuria were normal.
As the principal clinical findings that fulfill the diagnostic criteria are not specific for KD, patients with other diseases (Table 1) with similar clinical features were excluded from our study.
The major sequelae of KD are related to the cardiovascular, and more specifically, the coronary arterial system. Therefore cardiac imaging by echocardiography is a critical part of the evaluation of all patients with suspected KD. For uncomplicated cases, echocardiography is recommended at the time of diagnosis, at 2 weeks, and at 6–8 weeks after the onset of disease.27 We followed the above recommendations and also repeated the echocardiography beyond 8 weeks for all patients with previously normal findings, as other abnormalities in the coronary artery vasculature (aneurysms) and aortic root (dilatation with or without regurgitation) may develop, even among patients with normal baseline echocardiograms.27,28 Additionally, echocardiography was performed with higher frequency for patients with pericardial effusion, mitral valve regurgitation and IVIg‐resistant KD, for closer evaluation and follow‐up. None of our patients with normal baseline echocardiography was shown to develop CAA on follow‐up echocardiograms beyond the 8 weeks. CAA develop in approximately 15–25% of untreated children with the disease and may lead to ischemic heart disease, myocardial infarction or sudden death.18 In our patients, CAA were detected in nine (25.7%) cases. None of them led to myocardial infarction or ischemic heart disease. A recent study from Turkey reported the CAA rate as 33.3% in 24 children with KD.22 Burns et al. described the emergence of the peak mortality as 15–45 days after the onset of fever; and during this time, well‐established coronary vasculitis occurs concomitantly with a marked elevation of the platelet count and a hypercoagulable state.28 The mean duration of fever in our cases was 7.8±2.8 days (range 4–15). Our patients had fewer adverse sequelae, as all were treated before the peak mortality days, thus suggesting the importance of early diagnosis and treatment. The opportunity for early management occurred as the patients were being routinely followed up in our outpatient clinic.
The current medical management of KD is IVIg and high‐dose aspirin. IVIg is very effective when given in the first ten days of illness. It reduces the risk of CAA from 20–25% to 1–2%.6,9 A subgroup of patients with KD will be resistant to IVIg therapy; these patients are at greatest risk of developing coronary artery aneurysms and long‐term sequelae of the disease.29 Data have demonstrated the usefulness and safety of systemic steroids in patients resistant to IVIg.30 In our series, the majority of our patients were given IVIg before the tenth day and only one patient was IVIg resistant, who was successfully treated with pulse methylprednisolone. Newburger et al. reported that 50–67% of aneurysms resolve within 1–2 years.31 Echocardiographic evaluation of the nine children with CAA in our study was normal within 8 months (three within 6 months, four within 7 months, and two within 8 months). All patients are alive and receiving annual echocardiographic follow‐up.
CONCLUSIONS
In summary, it is our belief that KD is not a rare disease in Turkey. This study is the most comprehensive series of children from Turkey with KD included in Medline. As adult‐onset ischemic heart disease may be due to KD in childhood,9,18,28 further prospective clinical investigations are needed to understand the epidemiology, management and long‐term follow‐up the disease in Turkey.
Conflict of interest
None.
REFERENCES
- 1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of fingers and toes in children. Arerugi. 1967;16:178–222. [PubMed] [Google Scholar]
- 2. Taubert KA, Rowley AH, Shulman ST. Nationwide survey of Kawasaki disease and acute rheumatic fever. J Pediatr. 1991;119:279–82. doi: 10.1016/s0022-3476(05)80742-5. 10.1016/S0022‐3476(05)80742‐5 [DOI] [PubMed] [Google Scholar]
- 3. Gardner‐Medwin JM, Dolezalova P, Cummins C, Southwood TR. Incidence of Henoch‐Schönlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet. 2002;360:1197–202. doi: 10.1016/S0140-6736(02)11279-7. 10.1016/S0140‐6736(02)11279‐7 [DOI] [PubMed] [Google Scholar]
- 4. Taubert KA. Epidemiology of Kawasaki disease in the United States and worldwide. Progress in Pediatric Cardiology. 1997;6:181–5. 10.1016/S1058‐9813(97)00188‐4 [Google Scholar]
- 5. Huang WC, Huang LM, Chang IS, Chang LY, Chiang BL, Chen PJ, et al. Epidemiologic feature of Kawasaki disease in Taiwan, 2003‐2006. Pediatrics. 2009;123:e401–5. doi: 10.1542/peds.2008-2187. 10.1542/peds.2008‐2187 [DOI] [PubMed] [Google Scholar]
- 6. Royle J, Burgner D, Curtis N. The diagnosis and management of Kawasaki disease. J Paediatr Child Health. 2005;41:87–93. doi: 10.1111/j.1440-1754.2005.00555.x. 10.1111/j.1440‐1754.2005.00555.x [DOI] [PubMed] [Google Scholar]
- 7. Pinna GS, Kafetzis DA, Tselkas OI, Skevaki CL. Kawasaki disease: an overview. Current Opin Infect Dis. 2008;21:263–70. doi: 10.1097/QCO.0b013e3282fbf9cd. 10.1097/QCO.0b013e3282fbf9cd [DOI] [PubMed] [Google Scholar]
- 8. Levy M, Koren G. Atypical Kawasaki disease: analysis of clinical presentation and diagnostic clues. Pediatr Infect Dis J. 1990;9:122–6. 10.1097/00006454‐199002000‐00010 [PubMed] [Google Scholar]
- 9. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long‐term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association, American Academy of Pediatrics. Circulation. 2004;110:2747–71. doi: 10.1161/01.CIR.0000145143.19711.78. 10.1161/01.CIR.0000145143.19711.78 [DOI] [PubMed] [Google Scholar]
- 10. Satou GM, Giamelli J, Gewitz MH. Kawasaki disease: diagnosis, management, and long‐term implications. Cardiol Rev. 2007;15:163–9. doi: 10.1097/CRD.0b013e31802ea93f. 10.1097/CRD.0b013e31802ea93f [DOI] [PubMed] [Google Scholar]
- 11. Nakamura Y, Yashiro M, Uehara R, Oki I, Watanabe M, Yanagawa H. Epidemiologic features of Kawasaki disease in Japan: results from the nationwide surveys in 2005‐2006. J Epidemiol. 2008;18:167–72. doi: 10.2188/jea.JE2008001. 10.2188/jea.JE2008001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harnden A, Mayon‐White R, Perera R, Yeates D, Goldacre M, Burgner D. Kawasaki disease in England: ethnicity, deprivation, and respiratory pathogens. Pediatr Infect Dis J. 2009;28:21–4. doi: 10.1097/inf.0b013e3181812ca4. 10.1097/INF.0b013e3181812ca4 [DOI] [PubMed] [Google Scholar]
- 13. Ozen S, Bakkaloglu A, Dusunsel R, Soylemezoglu O, Ozaltin F, Poyrazoglu H, et al. Childhood vasculitides in Turkey: a nationwide survey. Clin Rheumatol. 2007;26:196–200. doi: 10.1007/s10067-006-0266-6. [DOI] [PubMed] [Google Scholar]
- 14. Burgner D, Harnden A. Kawasaki disease: what is the epidemiology telling us about the etiology? Int J Infect Dis. 2005;9:185–94. doi: 10.1016/j.ijid.2005.03.002. 10.1016/j.ijid.2005.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shike H, Shimizu C, Kanegaye JT, Newburger JW, Sundel RP, Brown PO, et al. Adenovirus, adeno‐associated virus and Kawasaki disease. Pediatr Infect Dis J. 2005;24:1011–14. doi: 10.1097/01.inf.0000183769.31951.1e. 10.1097/01.inf.0000183769.31951.1e [DOI] [PubMed] [Google Scholar]
- 16. Burns JC, Kushner HI, Bastian JF, Shike H, Shimizu C, Matsubara T, et al. Kawasaki disease: a brief history. Pediatrics. 2000;106:E27. doi: 10.1542/peds.106.2.e27. 10.1542/peds.106.2.e27",-1,"xxx/2.e27 [DOI] [PubMed] [Google Scholar]
- 17. Holman RC, Curns AT, Belay ED, Steiner CA, Schonberger LB. Kawasaki syndrome hospitalizations in the United States, 1997 and 2000. Pediatrics. 2003;112:495–501. doi: 10.1542/peds.112.3.495. 10.1542/peds.112.3.495 [DOI] [PubMed] [Google Scholar]
- 18. Newburger J, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long‐term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–33. doi: 10.1542/peds.2004-2182. 10.1542/peds.2004‐2182 [DOI] [PubMed] [Google Scholar]
- 19. Royle JA, Williams K, Elliott E, Sholler G, Nolan T, Allen R, et al. Kawasaki disease in Australia, 1993‐95. Arch Dis Child. 1998;78:33–9. doi: 10.1136/adc.78.1.33. 10.1136/adc.78.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heaton P, Wilson N, Nicholson R, Doran J, Parsons A, Aiken G. Kawasaki disease in New Zealand. J Paediatr Child Health. 2006;42:184–90. doi: 10.1111/j.1440-1754.2006.00827.x. 10.1111/j.1440‐1754.2006.00827.x [DOI] [PubMed] [Google Scholar]
- 21. Dhillon R, Newton L, Rudd PT, Hall SM. Management of Kawasaki disease in the British Isles. Arch Dis Child. 1993;69:631–6. doi: 10.1136/adc.69.6.631. 10.1136/adc.69.6.631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ozdemir H, Ciftçi E, Tapisiz A, Ince E, Tutar E, Atalay S, et al. Clinical and epidemiological characteristics of children with Kawasaki disease in Turkey. J Trop Pediatr. 2010;56:260–2. doi: 10.1093/tropej/fmp110. 10.1093/tropej/fmp110 [DOI] [PubMed] [Google Scholar]
- 23. Singh S, Kawasaki T. Kawasaki disease‐an Indian perspective. Indian Pediatr. 2009;46:563–71. [PubMed] [Google Scholar]
- 24. Zulian F, Falcini F, Zancan L, Martini G, Secchieri S, Luzzatto C, et al. Acute surgical abdomen as presenting manifestation of Kawasaki disease. J Pediatr. 2003;142:731–5. doi: 10.1067/mpd.2003.232. 10.1067/mpd.2003.232 [DOI] [PubMed] [Google Scholar]
- 25. Kuniyuki S, Asada M. An ulcerated lesion at the BCG vaccination site during the course of Kawasaki disease. J Am Acad Dermatol. 1997;37:303–4. 10.1016/S0190‐9622(97)80376‐3 [PubMed] [Google Scholar]
- 26. Burns JC, Mason WH, Glode MP, Shulman ST, Melish ME, Meissner C, et al. Clinical and epidemiologic characteristics of patients referred for evaluation of possible Kawasaki disease. United States Multicenter Kawasaki Disease Study Group. J Pediatr. 1991;118:680–6. doi: 10.1016/s0022-3476(05)80026-5. 10.1016/S0022‐3476(05)80026‐5 [DOI] [PubMed] [Google Scholar]
- 27. McMorrow Tuohy AM, Tani LY, Cetta F, Lewin MB, Eidem BW, Van Buren P, et al. How many echocardiograms are necessary for follow‐up evaluation of patients with Kawasaki disease? Am J Cardiol. 2001;88:328–30. doi: 10.1016/s0002-9149(01)01655-1. 10.1016/S0002‐9149(01)01655‐1 [DOI] [PubMed] [Google Scholar]
- 28. Burns JC, Glode MP, Clarke SH, Wiggins J, Jr, Hathaway WE. Coagulopathy and platelet activation in Kawasaki syndrome: identification of patients at high risk for development of coronary artery aneurysms. J Pediatr. 1984;105:206–11. doi: 10.1016/s0022-3476(84)80114-6. 10.1016/S0022‐3476(84)80114‐6 [DOI] [PubMed] [Google Scholar]
- 29. Sundel RP, Burns JC, Baker A, Beiser A, Newberger J. Gamma globulin re‐treatment in Kawasaki disease. J Pediatr. 1993;123:657–9. doi: 10.1016/s0022-3476(05)80972-2. 10.1016/S0022‐3476(05)80972‐2 [DOI] [PubMed] [Google Scholar]
- 30. Wright DA, Newburger JW, Baker A, Sundel RP. Treatment of immune globulin‐resistant Kawasaki disease with pulsed doses of corticosteroids. J Pediatr. 1996;128:146–9. doi: 10.1016/s0022-3476(96)70447-x. 10.1016/S0022‐3476(96)70447‐X [DOI] [PubMed] [Google Scholar]
- 31. Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633–39. doi: 10.1056/NEJM199106063242305. 10.1056/NEJM199106063242305 [DOI] [PubMed] [Google Scholar]