Abstract
OBJECTIVE:
This study provides the clinical pathological characteristics of 1301 cases of pediatric/adolescent lymphomas in patients from different geographic regions of Brazil.
METHODS:
A retrospective analyses of diagnosed pediatric lymphoma cases in a 10‐year period was performed. We believe that it represents the largest series of pediatric lymphomas presented from Brazil.
RESULTS:
Non‐Hodgkin lymphomas represented 68% of the cases, including those of precursor (36%) and mature (64%) cell origin. Mature cell lymphomas comprised 81% of the B‐cell phenotype and 19% of the T‐cell phenotype. Hodgkin lymphomas represented 32% of all cases, including 87% of the classical type and 13% of nodular lymphocyte predominant type. The geographic distribution showed 38.4% of the cases in the Southeast region, 28.7% in the Northeast, 16.1% in the South, 8.8% in the North, and 8% in the Central‐west region. The distribution by age groups was 15–18 years old, 33%; 11–14 years old, 26%; 6–10 years old, 24%; and 6 years old or younger, 17%. Among mature B‐cell lymphomas, most of the cases were Burkitt lymphomas (65%), followed by diffuse large B‐cell lymphomas (24%). In the mature T‐cell group, anaplastic large cell lymphoma, ALK‐positive was the most prevalent (57%), followed by peripheral T‐cell lymphoma, then not otherwise specified (25%). In the group of classic Hodgkin lymphomas, the main histological subtype was nodular sclerosis (76%). Nodular lymphocyte predominance occurred more frequently than in other series.
CONCLUSION:
Some of the results found in this study may reflect the heterogeneous socioeconomical status and environmental factors of the Brazilian population in different regions.
Keywords: Lymphomas, Pediatric, Childhood, Adolescent, Brazil, Hodgkin Lymphoma, Epidemiology, Geographic Region
INTRODUCTION
Lymphomas constitute about 10–12% of all malignancies in pediatric patients.1–3 About 7–10% are non‐Hodgkin lymphomas (NHLs), and 4–7% are Hodgkin lymphomas (HLs).2,4,5 There has been an overall increase in the incidence of NHLs in the Western population over the last two decades,5,6 primarily in adult patients. The incidence of NHLs among children younger than 15 years of age has remained fairly constant, with a slight increase in incidence for the 15‐ to 18‐year‐old population.7
Childhood NHL differs from adult NHL with respect to disease types, biological behavior, staging system, treatment, and outcome. Whereas NHL in adults is more often low to intermediate grade, the vast majority of NHLs in children are high‐grade lymphomas.8 There is considerable geographic variation in the incidence and distribution of subtypes of lymphomas. In Equatorial Africa, almost 50% of childhood cancers are lymphomas with a preponderance of Burkitt lymphoma (BL). In Western countries, approximately one‐third of childhood NHLs are lymphoblastic lymphomas/leukemia (LL), 40% BL, 10–20% diffuse large B‐cell lymphoma (DLBCL), and 10–20% anaplastic large cell lymphoma (ALCL); other types of NHLs are very rare.9,10–14
HL represents approximately one‐third of lymphomas in children.11,15,16 CHL is more prevalent, with few studies reporting findings on nodular lymphocyte predominance during childhood.17–21
Brazil is the fifth largest country in the world. It has a population of approximately 190 million people,22 and it is divided into five main large geographic regions (macroregions) with marked heterogeneity in socioeconomic development, population density, and climatic characteristics (http://countrystudies.us/brazil/).
The first report on cancer incidence among children and adolescents in Brazil, performed by the population‐based cancer registry (PBCR), was recently published and showed that the main groups of all cancers were leukemia (18–41%), lymphoma (13–24%), and central nervous system tumors (7–17%), considering different cancer based‐region registers.23,24
In Brazil there is a complete lack of comprehensive clinicopathological and epidemiological studies on pediatric lymphomas, especially regarding the relationship between geographic region and type of lymphoma. There are isolated regional data referring to the association with immunosuppression or socioeconomic status, viral infections, and different therapeutic protocols.24–34
Herein, we present the clinicopathological evaluation of what we believe to be the largest series (1301 cases) of lymphomas in the Brazilian pediatric population, with special consideration to histological subtype distribution in all geographic regions, which may reflect the heterogeneous socioeconomic and environmental status of the Brazilian population in the various regions.
MATERIAL AND METHODS
Description of characteristics of geographic regions
A general description of all five geographic regions in Brazil, including climate, population, and economic status, is given below.
North Region
The equatorial North, also known as the Amazon, is the country's largest region, covering 45.3% of the national territory. The climate is mainly humid tropical forest. The North region has the lowest population density. The per capita income is low.
Northeast Region
The Northeast region covers 18.3% of the national territory. Its principal biome is the semi‐arid region, which is subject to prolonged periodic droughts. The region has the country's largest concentration of rural population, and its living standards are the lowest in Brazil. The per capita income is very low.
Southeast Region
The Southeast region corresponds to 10.9% of the national territory. The region has the densest population in the country. It contains the mega‐cities of São Paulo and Rio de Janeiro. The region combines the highest living standards in Brazil with pockets of urban poverty. Originally, the principle biome in the Southeast was the Atlantic Forest, but less than 10% of the original forest remains.
South Region
The South region covers 6.8% of the national territory. In addition to the Atlantic Forest and pinewoods, the South contains Pampas grasslands. The per capita income is above average.
The Center‐West Region
The Center‐West region covers 18.9% of the national territory. Its main biome is the cerrado, the tropical savanna. It also includes the Pantanal wetlands in the West. The per capita income varies from area to area: it is low in most of the region, with the highest found in the area where the Federal District is located.
Case selection
All cases of lymphomas diagnosed in children and adolescents with available slides for review were retrieved retrospectively from the files of Consultoria em Patologia during the period of January 1999 and December 2009. Consultoria em Patologia is the largest reference consultation service in anatomic pathology in Brazil and is located in Botucatu, Sao Paulo State.
Hematoxylin and eosin and immunohistochemistry slides of each case were reviewed. Additional immunohistochemistry studies were performed as needed, in addition to the initial panel, to further classify lymphoma subtypes. Antibodies used are shown in Table 1. Epstein‐Barr virus status was evaluated by immunohistochemistry for LMP‐1 in BLs and HLs. In special cases, EBER in situ hybridization was also performed, mainly to confirm specific diagnosis. Human T‐cell leukemia virus‐1 was not evaluated in the study. Diagnosis criteria and lymphoma classification applied to this review were those established by the 2008 World Health Organization (WHO) classification.35 For practical purposes, the final diagnosis of each case was based on the specimens available for review from the files only. Information on blood cell count, flow cytometry, and bone marrow aspirate cytology were not usually available. Consequently, in cases of lymphoblastic malignant neoplasias, for instance, leukemia versus lymphoma, we were unable to discriminate between lymphoma and leukemia.
Table 1.
Primary antibodies used for immunohistochemical staining in paraffin sections.
| Antigen | Clone | Dilution | Antigen retrieval | Source |
| CD20 | L26 | 1∶1200 | MW; CB | Dako |
| CD3 | SP7 | 1∶200 | S; CB | Neomarkers |
| CD30 | BerH2 | 1∶100 | S; CB | Dako |
| Granzime | Gran‐B | 1∶40 | MW; CB | Novocastra |
| TIA‐1 | 2G9 | 1∶200 | MW, CB | Immunotech |
| Ki‐67 | MIB‐1 | 1∶4800 | PC; CB | Dako |
| LMP‐1 | CS1‐4 | 1∶2000 | S; CB | Dako |
| CD45RB | PD7 | 1∶500 | MW;CB | Dako |
| CD15 | Leu‐M1 | 1∶50 | MW; CB | Becton‐Dickinson |
| CD10 | 56C6 | 1∶100 | S; CB | Novocastra |
| BCL‐2 | 124 | 1∶400 | MW; CB | Dako |
| BCL‐6 | PG‐B6P | 1∶100 | T+ S; TRIS | Dako |
| IRF4/MUM‐1 | MUM1P | 1∶1200 | S; CB | Dako |
| ALK | ALK‐1 | 1∶200 | S; TRIS | Dako |
| EMA | E29 | 1∶800 | PC; CB | Dako |
| PAX‐5 | 24 | 1∶100 | S; CB | Becton‐Dickinson |
| CD25 | 4C9 | 1∶200 | S; EDTA | Novocastra |
| CD23 | SP23 | 1∶400 | MW; CB | Neomarkers |
| CD5 | 4C7 | 1∶1600 | S; CB | Novocastra |
| TDT | Polyclonal | 1∶600 | S; CB | Dako |
| Cyclin D1 | SP4 | 1∶100 | S; CB | Neomarkers |
| Ki‐67 | MIB‐1 | 1∶4800 | PC; CB | Dako |
DAKO, Carpinteria, CA, USA; Neomarkers, Fremont, CA, USA, Novocastra, United Kingdom; Abcam, San Francisco, CA, USA; RD, Minneapolis, MN, USA: Immunotech, Marseille, France; Becton‐Dickinson Biosciences, Mountain View, CA, USA. Heat‐induced epitope retrieval was employed. MW: microwave oven, PC: pressure cooker, S: steamer, T: trypsin, CB: citrate buffer.
Clinical data
The clinical information included gender and age at diagnosis (≤18 years old). The cases were categorized further into four groups according to age: 0–5, 6–10, 11–14, and 15–18 years of age. The anatomic location, stage, laboratory tests, treatment, and follow‐up, when available, were obtained from the referring hematologists/oncologists. All cases retrieved from the files were identified according to the five geographic regions of Brazil mentioned above.
Subtypes of lymphomas
The lymphomas' subtypes were defined according to the WHO 2008 Classification of Tumors of Hematopoietic and Lymphoid Tissues.35 For global analyses, the cases were divided into two main groups: NHL and HL. NHL was divided further according to the immunophenotype in B‐cell and T‐cell lineages, including the precursor and mature cell subtypes. HLs were subclassified into classical Hodgkin lymphoma (CHL) and nodular lymphocyte predominant (NLP) category. CHL was further subdivided into four subtypes: nodular sclerosis (NS), mixed cellularity (MC), lymphocyte rich, and lymphocyte depleted. All lymphoma types were correlated with age group, gender, and geographic regions.
RESULTS
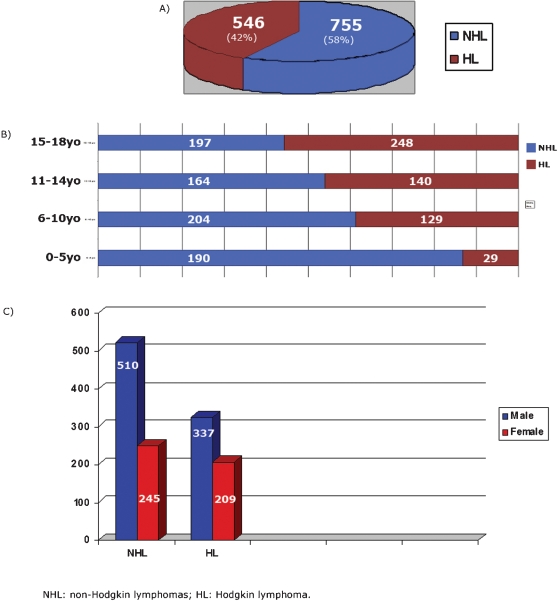
A total of 1301 cases of lymphoma involving pediatric/adolescent patients (≤18 years old) over an 11‐year period (1999–2009) were retrieved from the files of Consultoria em Patologia. All cases were seen in consultation, in general, for a second pathology diagnostic opinion. All geographic regions of Brazil were represented in this series. All the cases were divided into two major groups: NHL and HL. NHL included 755 cases (58%), whereas HL accounted for 546 cases (42%), being 476 cases of CHL and 70 cases of NLP. In the NHL group, 459 cases (60.8%) were B‐cell lymphomas, and 296 cases (39.2%) were T‐cell lymphomas, including precursor and mature‐cell origin in both lineages (Fig. 1A). The age distribution in the four groups is shown in Fig. 1B. Most of the cases belonged to the 15‐ to 18‐year‐old age group (445 cases, 33%), followed by the 6‐ to 10‐year‐old age group (333 cases, 25.6%) and the 11‐ to 14‐year‐olds (304 cases, 23.4%) with the 0 to 5‐year‐old age group having the lowest number of cases (219 cases, 17). Sixty‐six percent of all cases corresponded to patients 14 years old or younger. The male gender was predominant in all groups (Fig. 1C). The geographic distribution of all cases is described in Table 2. Thirty‐eight percent of the cases were from the Southeast region followed by 29% from the Northeast, 16% from the South, 8.8% in the North region, and 8% in the Central‐West region. The mean age varied from 9.7 years old in the North to 12.4 years old in the South. The male–female ratio varied from 2.25 in the North to 1.4 in the South.
Figure 1.
A, Global distribution of non‐Hodgkin and Hodgkin pediatric lymphoma cases in Brazil. B, Distribution of all cases of pediatric lymphoma by age groups. C, Distribution of all cases of pediatric lymphoma by gender.
Table 2.
Overview of all cases of pediatric lymphomas distributed by geographic region according to mean age, age range, gender, and lymphoma subtype.
| N 114 (8.8%) | NE 373 (28.7%) | CW 105 (8%) | S 209 (16.1%) | SE 500 (38.4%) | |
| Age | |||||
| Mean (y) | 9.7 | 10.9 | 11.2 | 12.4 | 10.9 |
| Range (y) | 1–18 | 1–18 | 3–18 | 1–18 | 1–18 |
| Gender | |||||
| Male | 79 | 255 | 61 | 123 | 328 |
| Female | 35 | 118 | 44 | 86 | 172 |
| Lymphoma type | |||||
| NHL | 57 (50%) | 212 (57%) | 62 (59%) | 138 (66%) | 286 (57%) |
| HL | 57 (50%) | 161 (43%) | 43 (41%) | 71 (34%) | 214 (43%) |
N: North; NE: Northeast; CW: Central‐West; S: South; SE: Southeast; NHL: non‐Hodgkin lymphoma; HL: Hodgkin lymphoma; y: years.
The histological distribution of the lymphomas subtypes according to the various regions showed that NHL accounted for 50% in the North and 66% in the South with about 58% in the other regions. HL varied from 50% in the North and 34% in the South compared with NHL. When the geographic distribution is evaluated in each group separately (NHL and HL) the highest frequency corresponded to the Southeast region (38%) and the lowest in the Central‐West region (8%), for both groups.
Non‐Hodgkin lymphomas
Based on the WHO's 2008 classification for hematolymphoid neoplasm,35 NHL was separated into two main groups: precursor and mature B‐cell and T‐cell hematolymphoid neoplasms.
Precursor lymphoid neoplasms
LLs accounted 272 (36%) of all NHL cases, with a male predominance (178M/94F, ratio 1.9/1). The distribution according to lineage revealed B‐cells in 69 cases (25.3%), T‐cell in 161 cases (59.2%), and unclassified in 42 cases (15.5%). Unclassified cases were defined by the absence of expression of lineage‐specific markers using immunohistochemistry methodology. The geographical distribution is shown in Table 3. Most of the cases were from the Southeastern region, followed by the Northeastern and the South regions (39%, 23.5%, and 23.2%, respectively). In almost all regions, the predominant gender was male, except for B‐cell LL in the South, where females were twice as frequent as males.
Table 3.
Precursor lymphoid neoplasms in pediatric patients classified according to cell lineage, gender, and geographical region distribution for each lineage group.
| Region | LL B cell | LL T cell | LL unclassified | ||||||
| Cases (%) | F | M | Total (%) | F | M | Total (%) | F | M | Total (%) |
| N 19 (7) | 1 | 2 | 3 (4.3) | 6 | 8 | 14 (8.7) | 0 | 2 | 2 (4.7) |
| NE 64 (23.5) | 6 | 10 | 16 (23.2) | 12 | 30 | 42 (26) | 4 | 2 | 6 (14.3) |
| CW 20 (7.3) | 2 | 2 | 4 (5.8) | 3 | 8 | 11 (6.8) | 3 | 2 | 5 (12) |
| S 63 (23.2) | 13 | 6 | 19 (27.5) | 10 | 18 | 28 (17.3) | 2 | 14 | 16 (38) |
| SE 106 (39) | 9 | 18 | 27 (39) | 18 | 48 | 66 (41) | 5 | 8 | 13 (31) |
| Total | 31 | 38 | 69 (100) | 49 | 112 | 161 (100) | 14 | 28 | 42 (100) |
N: North; NE: Northeast; CW: Central‐West; S: South; SE: Southeast; LL: lymphoblastic lymphoma; F: female; M: male.
Mature cell non‐Hodgkin lymphomas
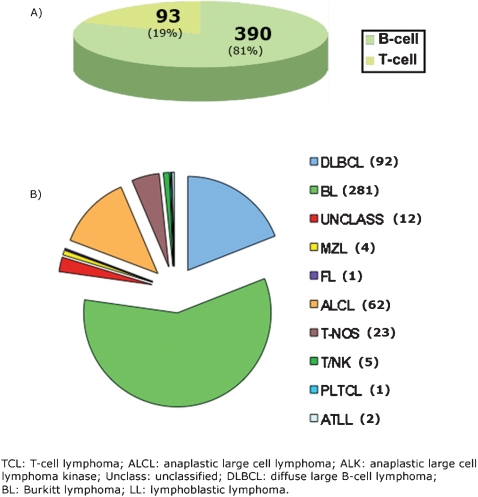
Mature cell NHLs corresponded to 483 (64%) of all NHL cases, including 390 (81%) of B‐cell and 93 (19%) cases of T‐cell. The distribution of mature NHL is shown in Fig. 2.
Figure 2.
Pediatric mature non‐Hodgkin lymphoid neoplasm. A, Global distribution according to cell lineage. B, Specific distribution according to main subtypes.
BL included 281 cases (58.2% of all mature lymphomas and 65% of all mature B‐cell lymphomas). It was by far the most common B‐cell lymphoma subtype, followed by DLBCL with 92 cases (19% of all mature, 23.6% of mature B). Seventy‐two cases were DLBCL, not otherwise specified (NOS), nine cases were T‐cell‐rich B‐cell lymphoma, and 11 cases were mediastinal B‐cell lymphoma variants. Twelve cases were considered intermediate between DLBCL and BL (2.5% of all mature cell lymphomas, 6.4% of B‐cell lymphomas). Only five cases were low‐grade B‐cell lymphomas (1.03%, 1.3%). In this low‐grade group, one case was follicular lymphoma, and four were extranodal marginal zone lymphoma (MALT type).
The geographical distribution of NHL revealed that 39% of the cases were from the Southeast followed by the Northeast with 29% of the patients, Central‐West with 15% and a similar frequency in the North and the South at 8% and 9%, respectively. The male–female ratio was 2.4/1 (278M/112F), (Table 4).
Table 4.
Mature B‐cell lymphoma in pediatric patients, distribution by gender and geographical areas for each subtype
| BL | DLBCL | Unclassif | MZL | ||||||||||
| F | M | T (%) | F | M | T (%) | F | M | T (%) | F | M | T (%) | Total | |
| N | 9 | 17 | 26 (9.3) | 2 | 3 | 5 (5.4) | 1 | 0 | 1 (8.3) | 0 | 0 | 0 | 32 (8.2) |
| NE | 18 | 65 | 83 (29.5) | 6 | 21 | 27 (29.3) | 2 | 0 | 2 (16.6) | 0 | 1 | 1 (25) | 114 (29.2) |
| CW | 11 | 15 | 26 (9.3) | 1 | 4 | 5 (5.4) | 0 | 1 | 1 (8.3) | 1 | 1 | 2 (50) | 34 (8.7) |
| S | 9 | 30 | 39 (13.9) | 7 | 8 | 15 (16.4) | 1 | 3 | 4 (33.3) | 0 | 0 | 0 | 58 (14.9) |
| SE | 28 | 79 | 107 (38) | 12 | 28 | 40 (43.5) | 3 | 1 | 4 (33.3) | 1 | 0 | 1 (25) | 152 (39) |
| Total | 75 | 206 | 281 (100) | 28 | 64 | 92 (100) | 7 | 5 | 12 (100) | 2 | 2 | 4 (100) | 390 (100) |
N: North; NE: Northeast; CW: Central‐West; S: South; SE: Southeast; F: female; M: male; BL: Burkitt lymphoma; DLBCL: diffuse large B‐cell lymphoma; MZL: marginal zone lymphoma; the only case of follicular lymphoma was a boy from NE region.
Mature T and T/NK‐cell lymphoma
ALCL was the most common subtype in this group: 53 cases of ALCL ALK positive (11% of all mature NHL and 57% of all T‐cell ones) cases and nine cases of ALCL ALK negative. Males were predominant. The mean age was 11.8 years old for ALCL‐ALK‐positive and 16.3 years old for ALCL‐ALK‐negative cases.
The other subtypes of mature T/NK cell lymphomas included 23 cases of T‐cell NOS (4% of all mature NHL and 25% of all T‐cell lineage), five cases of T/NK lymphoma, nasal type, one subcutaneous panniculitis‐like T‐cell lymphoma, and two cases of T‐cell lymphoma associated with HTLV‐1 virus.
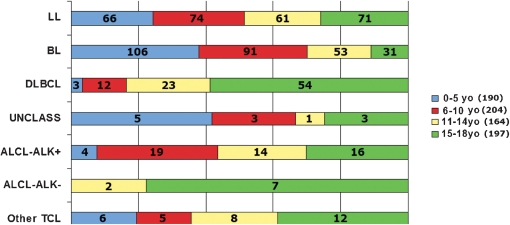
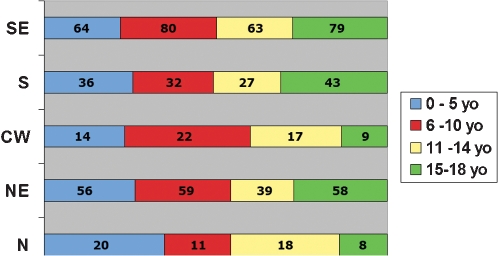
Table 5 shows the gender and geographic distribution for the different T‐cell NHL subtypes. T‐cell lymphomas presented with a more similar gender distribution than the mature B‐cell lymphomas. The geographic distribution of the T‐cells seemed to be more homogeneous than B‐cell lymphomas with no predominance of a specific geographic region. A global overview of the distribution of all NHL, for both precursor cells and mature cells derived by age group, is shown in Fig. 3, and the frequency of age groups affected in each geographic region is shown in Fig. 4.
Table 5.
Geographic distribution of mature T‐cell lymphomas in pediatric patients
| ALCL‐ALK+ | ALCL‐ALK‐ | PTLC‐NOS | T/NK | Other | ||||||||||||
| Region | F | M | T (%) | F | M | T (%) | F | M | T (%) | F | M | T (%) | F | M | T (%) | Total |
| N | 0 | 2 | 2 (3.8) | 0 | 1 | 1 (11.1) | 1 | 1 | 2 (8.7) | 0 | 1 | 1 (20) | 0 | 0 | 0 | 6 (6.4) |
| NE | 6 | 13 | 19 (35.8) | 0 | 2 | 2 (22.2) | 3 | 3 | 6 (26) | 0 | 2 | 2 (40) | 1 | 1 | 2 (66.6) | 31(33.3) |
| CW | 2 | 2 | 4 (7.6) | 0 | 0 | 0 | 1 | 2 | 3 (13) | 0 | 0 | 0 | 0 | 1 | 1 (33.3) | 8 (8.6) |
| S | 5 | 5 | 10 (18.9) | 3 | 1 | 4 (44.4) | 3 | 1 | 4 (17.4) | 1 | 0 | 1 (20) | 0 | 0 | 0 | 19 (20.4) |
| SE | 9 | 9 | 18 (33.9) | 0 | 2 | 2 (22.2) | 3 | 5 | 8 (34.8) | 1 | 0 | 1 (20) | 0 | 0 | 0 | 29 (31.2) |
| Total | 22 | 31 | 53 (100) | 3 | 6 | 9 (100) | 11 | 12 | 23 (100) | 2 | 3 | 5 (100) | 1 | 2 | 3 (100) | 93 (100) |
N: North; NE: Northeast; CW: Central‐West; S: South; SE: Southeast; F: female; M: male; ALCL‐ALK+ anaplastic large cell lymphoma; ALK‐ positive; ALCL‐ALK‐: anaplastic large cell lymphoma ALK‐ negative; PTCL‐NOS: peripheral T‐cell lymphoma, no‐otherwise specified; T/NK: T/NK cell‐ lymphoma nasal type.
Figure 3.
Comparative distribution of all types of pediatric non‐Hodgkin lymphomas separated by age group. TCL: T‐cell lymphoma; ALCL: anaplastic large cell lymphoma; ALK: anaplastic large cell lymphoma kinase; Unclass: unclassified; DLBCL: diffuse large B‐cell lymphoma; BL: Burkitt lymphoma; LL: lymphoblastic lymphoma.
Figure 4.
Comparative distribution of all cases of pediatric lymphomas by age groups in each geographic region. N: North; NE: Northeast; CW: Central‐West; S: South; SE: Southeast.
Hodgkin lymphoma
HLs corresponded to 546 cases with 476 CHL (87%) and 70 (13%) NLP. The comparative frequency of CHL and NLP is shown in Fig. 5. The geographic distribution for all cases is shown in Table 6: the frequency of CHL increased from North to South and the frequency of NLP was the reverse. The Southeast was the most prevalent region with 194 cases, followed by the Northeast with 136 cases, the South with 63 cases, 45 cases in the North, and 38 cases in the Central‐West region, as shown in Table 5.
Figure 5.
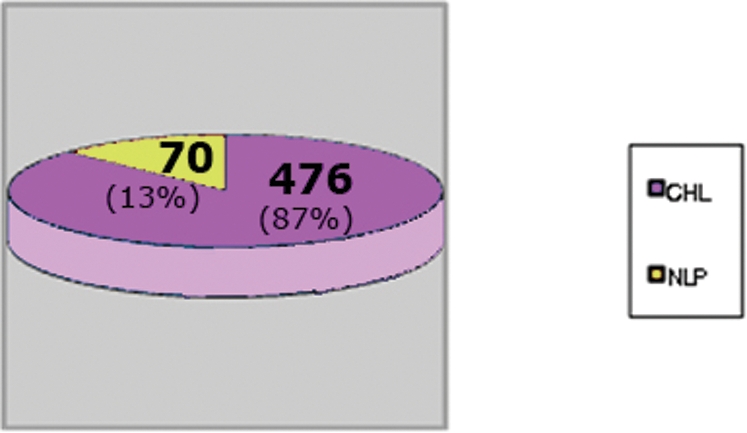
Hodgkin lymphoma distribution. CHL: classic Hodgkin lymphoma; NLPHL: nodular lymphocyte predominant Hodgkin lymphoma.
Table 6.
Geographic distribution of pediatric Hodgkin lymphoma.
| CHL | NLP | All cases HL | |
| N | 45 (79%) | 12 (21%) | 57 (100%) |
| NE | 136 (84%) | 25 (16%) | 161 (100%) |
| CO | 38 (88%) | 5 (12%) | 43 (100%) |
| S | 63 (89%) | 8 (11%) | 71 (100%) |
| SE | 194 (91%) | 20 (9%) | 214 (100%) |
CHL: classical Hodgkin lymphoma; NLP: nodular lymphocyte predominant Hodgkin lymphoma; HL: Hodgkin lymphoma; N: North; NE: Northeast; CW: Central‐West; S: South; SE: Southeast.
In the group of CHL, most of the cases were of the NS subtype (361 cases, 75.8%) with the remaining as follows: MC (92 cases, 19.3%), lymphocyte‐rich classical (five cases, 1.1%), lymphocyte depleted (3 cases, 0.6%), and unclassified (15 cases, 3.2%) and the distribution of the morphologic subtypes is shown in Table 7. The gender distribution showed 198 female patients and 278 male patients (ratio 1.5/1).
Table 7.
Geographic distribution of CHL morphologic subtypes.*
| NS | MC | LD | LR | |||||||||
| Region | F | M | S/T | F | M | S/T | F | M | S/T | F | M | S/T |
| N | 9 | 23 | 32 | 4 | 6 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| NE | 43 | 55 | 98 | 9 | 19 | 28 | 1 | 2 | 3 | 0 | 2 | 2 |
| CW | 16 | 15 | 31 | 2 | 2 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| S | 28 | 22 | 50 | 3 | 9 | 12 | 0 | 0 | 0 | 0 | 0 | 0 |
| SE | 76 | 74 | 150 | 3 | 35 | 38 | 0 | 0 | 0 | 2 | 1 | 3 |
| T | 172 | 189 | 361 | 21 | 71 | 92 | 1 | 2 | 3 | 2 | 3 | 5 |
15 unclassified cases are not included in the table. NS: nodular sclerosis; MC: mixed cellularity; LD: lymphocyte depletion; LR: lymphocyte rich; M: male; F: female; S/T: subtotal of each subgroup; T: total; N: North; NE: Northeast; CW: Central‐West; S: South; SE: Southeast.
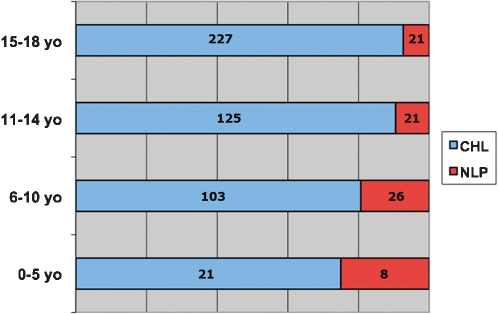
The mean age was 13.3 years old (female 14 years old and male 12.8 years old). The results grouped by age for each geographic region are shown in Fig. 6. The distribution of the four subtypes of CHL is shown in Table 8.
Figure 6.
Classical Hodgkin lymphoma and nodular predominance lymphocyte Hodgkin lymphoma distribution in all age groups.
Table 8.
Distribution of pediatric classical Hodgkin morphologic subtypes according to age.
| Age (years) | NS (%) | MC (%) | LD (%) | LR (%) | U (%) | Total (%) |
| 0–5 | 14 (66.6) | 6 (28.6) | 0 | 0 | 1 (4.8) | 21 (100) |
| 6–10 | 63 (61.2) | 30 (29.2) | 1 (0.9) | 2 (1.9) | 7 (6.8) | 103 (100) |
| 11–14 | 91 (72.8) | 27 (21.6) | 1 (0.8) | 2 (1.7) | 4 (3.2) | 125 (100) |
| 15–18 | 193 (85) | 29 (12.8) | 1 (0.4) | 1 (0.4) | 3 (1.2) | 227 (100) |
| Total | 361 (75.8) | 92 (19.3) | 3 (0.6) | 5 (1) | 15 (3.2) | 476 (100) |
NS: nodular sclerosis; MC: mixed cellularity; LD: lymphocyte depletion; LR: lymphocyte rich; U: unclassified.
NLP was diagnosed in 70 cases, 11 female and 59 male (ratio 6/1). The mean age was 11.2 years old (10.4 years old for female and 11.4 years old for male). The geographic region with the largest number of cases was the Northeast with 25 cases followed by 20 cases in the Southeast. The proportion of CHL/NLP varied among the regions. CHL was 8–10 times more frequent than NLP in the Southeast, the South, and in the Central‐West regions but only three to five times more frequent in the North and Northeast regions. NLPHL showed an inverse relationship between age and frequency, as depicted in Fig. 6. When the population of each region is taken in to consideration, both CHL and NLP have a higher incidence in the North.
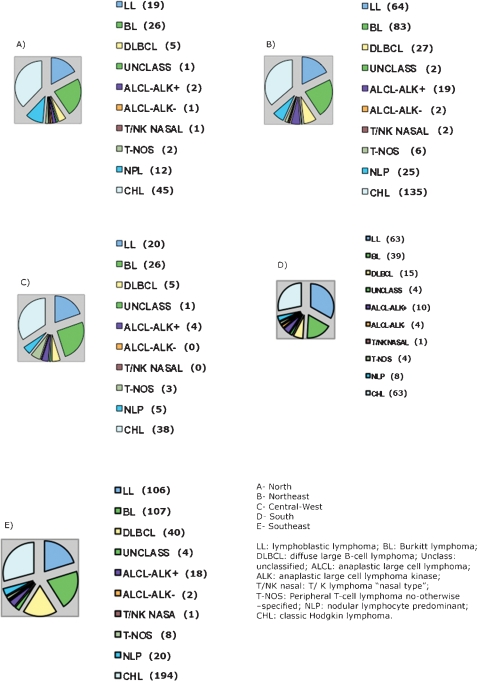
Comparing all types of lymphomas in all the regions, CHL and BL were the most prevalent lymphomas, except in the South where LL was the most frequent (Fig. 7).
Figure 7.
Distribution of all cases of pediatric lymphomas according to geographic regions. A: North, B: Northeast, C: Central‐West, D: SouthE, Southeast. LL: lymphoblastic lymphoma; BL: Burkitt lymphoma; DLBCL: diffuse large B‐cell lymphoma; Unclass: unclassified; ALCL: anaplastic large cell lymphoma; ALK: anaplastic large cell lymphoma kinase; T/NK nasal: T/K lymphoma “nasal type”; T‐NOS: peripheral T‐cell lymphoma not otherwise specified; NLP: nodular lymphocyte predominant; CHL: classic Hodgkin lymphoma.
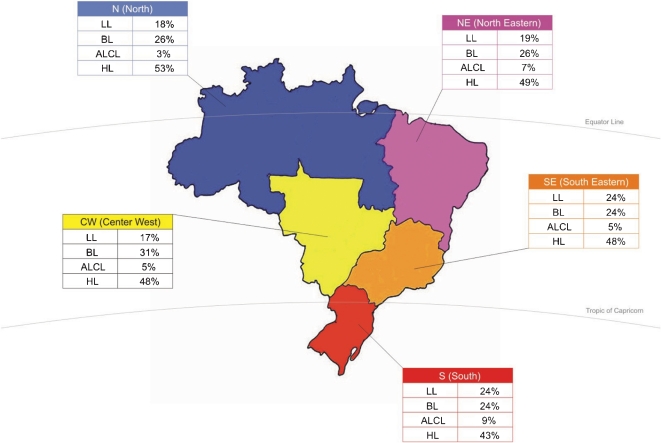
The overall distribution of the most frequent pediatric lymphomas according to the main geographic regions in Brazil is shown in Fig. 8; the percentages were calculated based on the most frequent types of lymphomas for each region.
Figure 8.
Overall distribution of the most frequent pediatric lymphomas according to main geographic regions in Brazil.
Footnote: LL: lymphoblastic lymphoma; BL: Burkitt lymphoma; ALCL: anaplastic large cell lymphoma; HL: Hodgkin lymphoma.
DISCUSSION
Overall, lymphomas are the third most common childhood malignancies after acute leukemia and brain tumors, constituting 10–12% of childhood cancers.1 In older adolescents, lymphomas surpass brain tumors in incidence, largely because of the increased frequency of HL in this age group. NHLs constitute approximately 7–10% of all cancers in children and adolescents younger than 20 years of age in the USA.2,5,11 Although more than 90% of pediatric NHL are of high‐grade histology, over 80% of patients achieve long‐term, event‐free survival with current treatment protocols.
In the Surveillance, Epidemiology, and End Results (SEER) database, the National Cancer Institute in the USA reported the NHL incidence per 1 000 000 pediatric patients in different age groups from 1975 to 2000.23 Patients younger than 5 years showed an incidence of 2.8 (66 cases), those 5–9 years old an incidence of 8.8 (147 cases), 10–14 years old showed an incidence of 14.3 (290 cases), and for those aged 15 to 19, the incidence was 21.8 (413 cases).36 For the period 2001–3, the incidence of lymphoma adjusted by age in the USA, according to the SEER data, was 27.84 per million for boys 19 years old and younger and 20.25 for girls of the same age.23 Comparatively, in Europe for the same age range, the highest rate was reported in Italy with 69 and 64 cases per million for boys and girls, respectively. Germany and Spain have reported a lower incidence with 14 and 6 per million for boys and girls, respectively.13
Brazil is a large country with marked heterogeneity of socioeconomic development among its different regions.23 The population distribution in the five main geographic regions is as follows: the North, approximately 14 million (9%) inhabitants; the Northeast, 51 million (28%); the Southeast, 77 million (42%); the South, 26 million (16%); and the Central‐West, 13 million (8%). As a developing index, the Minimum National Social Data Set (MNSDS) evaluated by the Instituto Brasileiro de Geografia e Estatistica of Brazil22,37 indicated a childhood mortality rate of 34.8 per 1000 children under 5 years of age. In the Northeast region of Brazil, a region of lower socioeconomic status, this rate reaches 53 per 1000, showing the great heterogeneity of the country. Also, Brazil is a country where undernourishment and endemic infections are common public health problems,37 especially in some geographic areas such as the Northeast and the North.
In 2009, the Brazilian National Cancer Institute (INCA) registry data indicated that patients younger than 19 years represented 2.8% of all types of cancer in all ages. The types of malignancy in this age group were distributed as follows: carcinomas (28%), leukemias (15%), malignant bone tumors (14%), lymphomas (10%), soft tissue (7.3%), renal neoplasias (6.2%), and central nervous system tumors (6%).24 In the Brazilian study performed by the population‐base cancer registires,23 the global incidence of lymphomas for males under 19 years old reached the highest rate in the Southeast region with 69.4 cases per million and the lowest in the North region with 17.5 cases per million. In females in this age group, the highest rate was 32.8 cases per million in the Northeast region, and the lowest was 6.2 cases per million in the North region. These incidence rates included both HLs and NHLs along with histiocytic neoplasia. In these studies, however, there was no discrimination of lymphoma subtypes.
We need to stress that the current study is not a formal epidemiology study, and may show a significant case selection bias depending on the referral patterns to our laboratory from each region in Brazil. Nonetheless, we believe it probably represents the most reliable data on pediatric lymphomas in Brazil. In the present study of 1301 cases of lymphoma, the global distribution of lymphoma subtypes was 68% NHL and 32% HL. Comparing the frequency of each group with other studies, high‐grade B‐cell lymphomas are by far the most common, as has been seen in the USA and European countries.8,10
The population of Brazil, formed by extensive admixture between Amerindians, Europeans, and Africans, is one of the most variable in the world, as a result of five centuries of interethnic crossing between peoples from three continents. A recent study using ancestry‐informative molecular markers concluded that in Brazil at an individual level, color, as determined by physical evaluation, was a poor predictor of genomic ancestry.38 Thus, these parameters were not considered in our analysis.
In reference to different lymphoma subtypes, LL accounts for approximately 20–30% of all NHL in childhood.4,7,8 Lymphoblastic lymphomas are usually positive for TdT, with more than 75% having a T‐cell immunophenotype and the remainder having a precursor B‐cell phenotype.7,8,39 Azevedo‐Silva et al.40 showed in a Brazilian epidemiology study of three cities that the frequency of lymphoblastic disease varies from 15% to 35% among all malignant diseases in pediatric patients.40 In our study, LL represented 36% of all NHLs (272 cases), with 65% being male (male–female ratio of 1.8/1.0). The T‐cell phenotype was the most prevalent at 60%, 25% were of the B‐cell phenotype, and the remaining 15% were unclassified. These results are coincident with those observed in Western countries: 75% of the LL having T‐ or null‐cell phenotype and the remaining cases showing the B‐cell phenotype.8,10
In the present series, 483 cases corresponded to mature cell lymphomas, with 81% belonging to the B‐cell and 19% the mature T‐cell lineage. The global results obtained in our series of pediatric lymphomas mirror the data in the population‐based registry.23 BL is the most prevalent mature B‐cell lymphoma in pediatric/adolescent patients.12,31,37,41 In our series, this corresponded to 281 cases (37% of all NHL). In a previous study, our group found that BL in Brazilian patients, including both children and adults, shows intermediate features between endemic and epidemic cases and predominates in the Northeast region.31
In the study by Sandlund et al.,8 BL corresponded to 40% of NHL in pediatric patients. Lones et al.12 found 47% of BL in a series of mature B‐cell lymphomas in children/adolescents belonging to three reference centers (USA, France, and the United Kingdom). Considering only the mature B‐cell lymphomas in the present series, BL corresponded to 72%, a much higher frequency than the Lones series.12 On the other hand, we only found 20% of DLBCL compared with 34% in their study. We considered 3% of the cases unclassified with features intermediate between BL and DLBCL. Lones et al.12 reported 11% of Burkitt‐like lymphoma, but we cannot assume all these cases were unclassified as proposed by the present WHO classification.35 Ferreira et al.26 found 78.2% of the lymphomas in children from the Northeast region of Brazil corresponded to BL.
The geographic distribution of our cases of BL showed a higher frequency in the Southeast region (38%), followed by the Northeast (30%), and 14%, 9%, and 9% for the South, the Central‐West, and the North regions, respectively. Male gender predominated with a rate of 2.7/1, similar to previously reported data.8,12,26 Considering all these data, we can speculate that BL may have a higher incidence in the Northeast than in other geographic regions, which may be associated to region‐specific environmental factors. Furthermore, these data underscore the need for epidemiological studies involving different regions of the country.
DLBCLs are quite infrequent in the pediatric population. In this series, they represented 7% of all lymphomas and 20% of mature B‐cell lymphomas; 24% of primary mediastinal B‐cell lymphoma were included with DLBCL‐NOS.42–44 Considering that the representation of the global population in Brazil is 28% and 42% for the Northeast and the Southeast regions, respectively, it seems that the DLBCL frequency related to the global population is probably the same in both regions. Nine cases of DLBCL were diagnosed as histiocytic/T‐cell rich B‐cell lymphomas. It is worth noting that this type is infrequently reported in children.32,45–49
Low‐grade B‐cell lymphomas are extremely rare in children and adolescents.10,50–54 We found only five cases, one follicular lymphoma and four extranodal marginal zone lymphomas, corresponding to 1.2% of mature B‐cell lymphomas and 0.6% of all NHL. The Follicular lymphoma was nodal and the four marginal zone lymphomas were extranodal, one intestinal and three gastric.
Most of the cases of mature T‐cell NHL described in children are ALCL‐ALK‐positive, with other types rare.8 In our series of mature T‐cell NHL, 57% (53 cases) corresponded to ALCL ALK‐positive, and 10% (9 cases) corresponded to the ALK–negative type. The second, more frequent mature T‐cell lymphoma in our series was peripheral T‐cell lymphoma, NOS, with 23 cases (25% of the cases) found. Only five cases of T/NK nasal‐type lymphomas were diagnosed in this series. This frequency is somewhat high compared with series in Western countries.10,33,55,56 Unfortunately, information about HTLV‐1 serological status was not usually available in our study.
HL is an uncommon disorder with an annual incidence of 2–3/100 000 in Europe and the USA.7 In developed countries, the onset of HL shows a bimodal distribution with a first peak in young adults and a second peak after 50 years of age. In underdeveloped countries or developing countries, the first peak occurs predominantly in infancy, and its incidence seems to decrease with age.17,23 In the pediatric population as well as in adults, most of the cases are CHL. It has been demonstrated that childhood HL displays characteristic epidemiological, clinical, and pathological features according to various geographic areas, particularly according to the socioeconomic level of a given country.18 In emerging countries there is a higher male–female ratio, a younger age of presentation, a high proportion of advanced stages, and MC as the most common subtype.22,25,55,57,58
Our series of 546 HLs included 476 (87%) CHLs and 70 (13%) NLPHLs in all geographic regions of Brazil. We recognized 476 cases of CHL, corresponding to 87% of HL and 37% of all lymphomas (HL and NHL). Nodular lymphocyte predominance corresponded to 70 cases (13% of HL and 7% of all lymphomas), having a higher frequency in the Northeast and the North areas of the country. The frequency in the South and the Southeast is similar to that reported in Western countries.59 To the best of our knowledge, this is the highest number of pediatric/adolescent NLPHL reported in one series in the English literature. In Brazil, HL seems to be frequent in children,27,29,57 and its association with EBV is well‐established.23,34,60 There are no studies reporting exclusively on pediatric HL Brazilian cases that included the entire country. Epidemiological data in two studies,30,60 including one with a large number of cases from Sao Paulo State, which has a higher socioeconomic level than the rest of the country, showed similar characteristics as industrialized countries. These studies included adult patients.30 In other reports comparing two regions—one with a high and the other with a low socioeconomic status—the difference was significant in terms of EBV infection and histologic subtypes.60
In 323 patients under 20 years with HL from the Southeast region,30 304 (94%) were CHL and 21 were NLP (6%). The male–female ratio was 1.5∶1 for CHL and 3∶1 for NLP. Our series of 546 HL included 476 cases (87%) of CHL and 70 cases (13%) of NLPHL in all geographic regions of Brazil. CHL distribution among all the different regions showed that the Southeast had 194 cases (41%), and the Northeast had 136 cases (29%), while the South, North and Central‐West regions had 13%, 9%, and 8% of the cases, respectively. On the other hand, a higher number of cases of NLPHL was observed in the Northeast region with 365, while the Southeast had 29% of the cases. When this is considered for each region separately, the frequency for both main groups of HL showed that the Southeast region had 91% of CHL and 9% of NLP, and the Northeast region had 84% of CHL and 16% of NLP. Even a higher relative frequency of NLPHL was observed in the North region with 22.2% of the cases and 77.8% of CHL, which shows an important difference in the NLPHL frequency between the North/Northeastern areas of the country in relation to the Southern region. As EBV is usually not associated with NLPHL, it may be possible that another epidemiological factor is involved to explain the differences between the North of the country and the Southeast/South; this raises a new question that may be answered in future studies. The gender distribution for both main types of HL demonstrated an important variation in the male–female ratio with NLPHL showing a male–female ratio of 6∶1, with CHL only 1.4∶1.
In general, it has been described that MC is the most prevalent CHL subtype in children in underdeveloped countries.61,62 We found NS to be the most frequent subtype (361 cases, 76.9%). Considering only the 124 cases in 10‐year‐olds or younger, NS constituted 62% of this group, and MC constituted 30%. On the other hand, older patients (older than 11 years old) comprised 80.7% of NS but only 15.9% of MC. Mixed cellularity HL is the second most common subtype in pediatric patients in Brazil, affecting mainly the younger population.
It has been described that NLPHL and MCHL subtypes are more common in the prepubertal child, accounting for 10–12% and 30–35% of HL, respectively. Nodular sclerosis occurs in 45–50% of children, in contrast to 70–80% of young adults and adolescents.61,63 We found almost 50% of the cases of NLPHL in patients 10 years old or younger, in contrast to 26% of CHL. Nodular lymphocyte predominance occurred more frequently in children with a male–female ratio of approximately 5∶1.62,63 Araujo et al.,34 in a series of pediatric HL from the Bahia state (Northeast region), found a marked predominance of MC. Elgui et al.60 described a high prevalence of MC in children and adults from the North compared with the Southeast, where the distribution of MC and NS was similar. Vassallo et al.30 studying patients from the Southeast region only, including both adults and children, found a 70% incidence of the NS subtype. Chabay et al.16 described similar results in a pediatric group from Rio de Janeiro (Southeast region).
In Uruguay64 and Chile,65 NS is the predominant subtype in both adults and pediatric patients. It is worth mentioning that in all these studies on HL, the number of cases of NLPHL in pediatric patients is very low. Sandoval et al.62 described a large series of NLPHL in patients under 21 years old. Eight‐five percent of their cases were male, and the mean age was 10.5 years old. In our series of 70 NLPHL cases, 84.5% of the cases were male, and the mean age was 10.8 years old. Almost half of our cases (34/70, 48%) were under 10 years old, while in the study of Sandoval et al.62 there were 19/51 cases (37%) under 10 years old.
In conclusion, this study, provides objective information on histological subtypes of a large number of pediatric lymphomas giving an overview of these diseases in the whole country according to the histopathological WHO 2008 classification of hematopoietic neoplasms.35
REFERENCES
- 1. Birch JM, Alston RD. Incidence of malignant disease by morphological type in young persons aged 12–24 years in England, 1979–1997. European J Cancer. 2003;39:2622–31. doi: 10.1016/j.ejca.2003.08.006. 10.1016/j.ejca.2003.08.006 [DOI] [PubMed] [Google Scholar]
- 2. Chiu BC, Weisenburger DD. An update of the epidemiology of non‐Hodgkin lymphoma. Clin Lymphoma. 2003;4:161–8. doi: 10.3816/clm.2003.n.025. 10.3816/CLM.2003.n.025 [DOI] [PubMed] [Google Scholar]
- 3. Heerema NA, Bernheim A, Lim MS, Look AT, Pasqualucci L, Raetz E, et al. State of the art and future needs in cytogenetic/molecular genetics/arrays in childhood lymphoma: summary report of workshop at the First International Symposium on childhood and adolescent non‐Hodgkin lymphoma, April 9, 2003, New York City, NY. Pediatr Blood Cancer. 2005;45:616–22. doi: 10.1002/pbc.20552. 10.1002/pbc.20552 [DOI] [PubMed] [Google Scholar]
- 4. Burkhardt B, Zimmermann M, Oschlies I, Niggli F, Mann G, Parwaresch R, et al. The impact of age and gender on biology, clinical features and treatment outcome of non‐Hodgkin lymphoma in childhood and adolescence. Br J Haematol. 2005;131:39–49. doi: 10.1111/j.1365-2141.2005.05735.x. 10.1111/j.1365‐2141.2005.05735.x [DOI] [PubMed] [Google Scholar]
- 5. Clarke CA, Glaser SL. Changing incidence of non‐Hodgkin lymphoma in the United States. Cancer. 2002;94:2015–23. doi: 10.1002/cncr.10403. 10.1002/cncr.10403 [DOI] [PubMed] [Google Scholar]
- 6. Liu S, Semenciw R, Mao Y. Increasing incidence of non‐Hodgkin lymphoma in Canada, 1970–1996, age‐period‐cohort analysis. Hematol Oncol. 2003;21:57–66. doi: 10.1002/hon.703. 10.1002/hon.703 [DOI] [PubMed] [Google Scholar]
- 7.Percy CL, Smith MA, Linet M, et al. Gloeckler Ries LA, Friedman D L. Lymphomas and reticuloendothelial neoplasms. In: Ries L A, Smith M A, Gurney J G, editors. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. Bethesda, Md: National Cancer Institute, SEER Program, 1999. NIH Pub.No. 99–4649., pp 35–50. Also available online. Last accessed May 24, 2010; In: eds. [Google Scholar]
- 8. Sandlund JT, Downing JR, Crist WM. Non‐Hodgkin's lymphoma in childhood. N Engl J Med. 1996;334:1238–48. doi: 10.1056/NEJM199605093341906. 10.1056/NEJM199605093341906 [DOI] [PubMed] [Google Scholar]
- 9. Cairo MS, Raetz E, Lim MS, Davenport V, Perkins SL. Childhood and adolescent non‐Hodgkin lymphoma: new insights in biology and critical challenges for the future. Pediatr Blood Cancer. 2005;45:753–69. doi: 10.1002/pbc.20342. 10.1002/pbc.20342 [DOI] [PubMed] [Google Scholar]
- 10. Gross TG, Termuhlen AM. Pediatric non‐Hodgkin lymphomas. Curr Oncol Reports. 2007;9:459–65. doi: 10.1007/s11912-007-0064-6. 10.1007/s11912‐007‐0064‐6 [DOI] [PubMed] [Google Scholar]
- 11. Jaglowski S, Linden E, Termuhlen AM, Flynn JM. Lymphoma in adolescent and young adults. Semin Oncol. 2009;36:381–418. doi: 10.1053/j.seminoncol.2009.07.009. 10.1053/j.seminoncol.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 12. Lones MA, Raphael M, Perkins SL, Wotherspoon A, Auperin A, Terrier‐Lacombe MJ, et al. Mature B‐cell lymphoma in children and adolescents. international group pathologist consensus correlates with histology technical quality. J Pediatr Hematol Oncol. 2006;28:568–74. doi: 10.1097/01.mph.0000212980.67114.a5. 10.1097/01.mph.0000212980.67114.a5 [DOI] [PubMed] [Google Scholar]
- 13. Li J, Thompson T, Miller J, Pollack L, Stewart S. Cancer incidence among children and adolescents in the United States, 2001–2003. Pediatrics. 2008;121:1470–7. doi: 10.1542/peds.2007-2964. 10.1542/peds.2007‐2964 [DOI] [PubMed] [Google Scholar]
- 14.Curado MP, Eduards B, Shin H. Cancer incidence in five continents [Monograph on the internet] Lyon: Agency for Research on Cancer; 2007. vol IX. http://www‐dep.iarc.fr/ [Google Scholar]
- 15. Belgaumi A, Al‐Kofide A, Joseph N, Jamil‐Malik R, Khafaga Y, Sabbah R. Hodgkin lymphoma in very young children: clinical characteristics and outcome of treatment. Leuk Lymphoma. 2008;49:910–6. doi: 10.1080/10428190801947492. 10.1080/10428190801947492 [DOI] [PubMed] [Google Scholar]
- 16. Chabay PA, Barros MH, Hassan R, De Matteo E, Rey G, Carrico MK, et al. Pediatric Hodgkin lymphoma in two South American series: a distinctive epidemiologic pattern and lack of association of Epstein‐Barr virus with clinical outcome. J Pediatr Hematol Oncol. 2008;30:285–91. doi: 10.1097/MPH.0b013e3181647bc3. 10.1097/MPH.0b013e3181647bc3 [DOI] [PubMed] [Google Scholar]
- 17. Thomas RK, Re D, Zander T, Wolf J, Diehl V. Epidemiology and etiology of Hodgkin's lymphoma. Ann Oncol. 2002;13 Suppl 4:147–52. doi: 10.1093/annonc/mdf652. [DOI] [PubMed] [Google Scholar]
- 18. Dinand V, Arya LS. Epidemiology of childhood Hodgkins disease: is it different in developing countries? Indian Pediatr. 2006;43:141–7. [PubMed] [Google Scholar]
- 19. Woolf KM, Wei MC, Link MP, Arber DA, Warnke RA. Nodular lymphocyte‐predominant Hodgkin lymphoma presenting as fulminant hepatic failure in a pediatric patient: a case report with pathologic, immunophenotypic, and molecular findings. Appl Immunohistochem Mol Morphol. 2008;16:196–201. doi: 10.1097/PAI.0b013e3180cc3211. 10.1097/PAI.0b013e3180cc3211 [DOI] [PubMed] [Google Scholar]
- 20. Hall GW, Katzilakis N, Pinkerton CR, Nicolin G, Ashley S, McCarthy K, et al. Outcome of children with nodular lymphocyte predominant Hodgkin lymphoma: a Children's Cancer and Leukaemia Group report. Br J Haematol. 2007;138:761–8. doi: 10.1111/j.1365-2141.2007.06736.x. 10.1111/j.1365‐2141.2007.06736.x [DOI] [PubMed] [Google Scholar]
- 21. Karayalcin G, Behm FG, Gieser PW, Kung F, Weiner M, Tebbi CK, et al. Lymphocyte predominant Hodgkin disease: clinico‐pathologic features and results of treatment‐‐the Pediatric Oncology Group experience. Med Pediatr Oncol. 1997;29:519–25. doi: 10.1002/(sici)1096-911x(199712)29:6<519::aid-mpo1>3.0.co;2-n. 10.1002/(SICI)1096‐911X(199712)29:6<519::AID‐MPO1>3.0.CO;2‐N [DOI] [PubMed] [Google Scholar]
- 22.Instituto Brasileiro de Geografia e Estatística. Available at: http://www.ibge.gov.br/english. Accessed March 12, 2010. [Google Scholar]
- 23. de Camargo B, de Oliveira Santos M, Rebelo MS, de Souza Reis R, Ferman S, Noronha CP, et al. Cancer inicidence among children and adolescents in Brazil: first report of 14 population‐based cancer registries. Int J Cancer. 2009;126:715–20. doi: 10.1002/ijc.24799. 10.1002/ijc.24799 [DOI] [PubMed] [Google Scholar]
- 24.Epidemiologia do câncer em criancas. Instituto Nacional de Câncer. Rio de Janeiro; 2008. [Google Scholar]
- 25. Pedrosa MF, Pedrosa F, Lins MN, Pontes NT, Hanois Falbo G. Non‐Hodgkin's lymphoma in childhood: clinical and epidemiological characteristics and survival analysis at a single center in Northeast Brazil. J Pediatr. (Rio J) 2007;83:547–54. doi: 10.2223/JPED.1726. 10.2223/JPED.1726 [DOI] [PubMed] [Google Scholar]
- 26. Braga P, Latorre M, Curado M. Cancer na infancia: analise comparativa da incidencia mortalidad e sobrevida em Goiania (Brasil) e outros paises. Cad Saude Publica. 2002;18:33–44. doi: 10.1590/s0102-311x2002000100004. 10.1590/S0102‐311X2002000100004 [DOI] [PubMed] [Google Scholar]
- 27. Hsu SC, Metzger ML, Hudson MM, Pedrosa F, Lins M, Pedrosa M, et al. Comparison of treatment outcomes of childhood Hodgkin lymphoma in two US centers and a center in Recife, Brazil. Pediatr Blood Cancer. 2007;49:139–44. doi: 10.1002/pbc.20883. 10.1002/pbc.20883 [DOI] [PubMed] [Google Scholar]
- 28. Faria SL, Vassallo J, Cosset JM, Brandalise SR. Childhood Hodgkin disease in Campinas, Brazil. Med Pediatr Oncol. 1996;26:90–4. doi: 10.1002/(SICI)1096-911X(199602)26:2<90::AID-MPO4>3.0.CO;2-P. 10.1002/(SICI)1096‐911X(199602)26:2<90::AID‐MPO4>3.0.CO;2‐P [DOI] [PubMed] [Google Scholar]
- 29. Oliveira BM, Viana MB, Cunha KCCM. Hodgkin's disease in childhood: a sixteen years experience in a single institution. J Pediatr. (Rio J) 2000;76:281–6. doi: 10.2223/jped.11. [DOI] [PubMed] [Google Scholar]
- 30. Vassallo J, Paes RP, Soares FA, Menezes Y, Aldred V, Ribeiro Kde C, et al. Histological classification of 1025 cases of Hodgkin's lymphoma from the state of Sao Paulo, Brazil. Sao Paulo Med J. 2005;123:134–6. doi: 10.1590/S1516-31802005000300009. 10.1590/S1516‐31802005000300009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Queiroga EM, Gualco G, Weiss LM, Dittmer DP, Araujo I, Klumb CE, et al. Burkitt lymphoma in Brazil is characterized by geographically distinct clinicopathologic features. Am J Clin Pathol. 2008;130:946–56. doi: 10.1309/AJCP64YOHAWLUMPK. 10.1309/AJCP64YOHAWLUMPK [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gualco G, Weiss LM, Harrington WJ, Jr, Bacchi CE. Nodal diffuse large B‐cell lymphomas in children and adolescents: immunohistochemical expression patterns and c‐MYC translocation in relation to clinical outcome. Am J Surg Pathol. 2009;33:1815–22. doi: 10.1097/PAS.0b013e3181bb9a18. 10.1097/PAS.0b013e3181bb9a18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gualco G, Chioato L, Weiss LM, Harrington WJ, Jr, Bacchi CE. Analysis of human T‐cell lymphotropic virus in CD25+ anaplastic large cell lymphoma in children. Am J Clin Pathol. 2009;132:28–33. doi: 10.1309/AJCP6Q7QMUVGMVMF. 10.1309/AJCP6Q7QMUVGMVMF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Araujo I, Bittencourt AL, Barbosa HS, Netto EM, Mendonça N, Foss HD, et al. The high frequency of EBV infection in pediatric Hodgkin lymphoma is related to the classical type in Bahia, Brazil. Virchows Arch. 2006;449:315–9. doi: 10.1007/s00428-006-0244-z. 10.1007/s00428‐006‐0244‐z [DOI] [PubMed] [Google Scholar]
- 35.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. (Eds.). [Google Scholar]
- 36. O'Leary M, Krailo M, Anderson JR, Reaman GH. Children's Oncology Group. Progress in childhood cancer: 50 years of research collaboration, a report from the Children's Oncology Group. Semin Oncol. 2008;35:484–93. doi: 10.1053/j.seminoncol.2008.07.008. 10.1053/j.seminoncol.2008.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Klumb CE, Hassan R, De Oliveira DE, De Resende LM, Carriço MK, De Almeida Dobbin J, et al. Geographic variation in Epstein‐Barr virus‐associated Burkitt's lymphoma in children from Brazil. Int J Cancer. 2004;108:66–70. doi: 10.1002/ijc.11443. 10.1002/ijc.11443 [DOI] [PubMed] [Google Scholar]
- 38. Pimenta JR, Zuccherato LW, Debes AA, Maselli L, Soares RP, Moura‐Neto RS, et al. Color and genomic ancestry in Brazilians: a study with forensic microsatellites. Hum Hered. 2006;62:190–5. doi: 10.1159/000096872. 10.1159/000096872 [DOI] [PubMed] [Google Scholar]
- 39. Neth O, Seidemann K, Jansen P, Mann G, Tiemann M, Ludwig WD, et al. Precursor B‐cell lymphoblastic lymphoma in childhood and adolescence: clinical features, treatment, and results in trials NHL‐BFM 86 and 90. Med Pediatr Oncol. 2000;35:20–7. doi: 10.1002/1096-911x(200007)35:1<20::aid-mpo4>3.0.co;2-l. 10.1002/1096‐911X(200007)35:1<20::AID‐MPO4>3.0.CO;2‐L [DOI] [PubMed] [Google Scholar]
- 40. Azevedo‐Silva F, Reis R de S, Santos M de O, Luiz RR, Pombo‐de‐Oliveira MS. Evaluation of childhood acute leukemia incidence and underreporting in Brazil by capture‐recapture methodology. Cancer Epidemiol. 2009;33:403–5. doi: 10.1016/j.canep.2009.09.004. 10.1016/j.canep.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 41. Pizza M, Bruniera P, Luporini SM, Marcelino da Silva HR, Borsato ML, de Castro HC, et al. Detection of Epstein‐Barr virus in children and adolescents with Burkitt's lymphoma by in situ hybridization using tissue microarrays. Hematology. 2008;13:114–8. doi: 10.1179/102453308X315942. 10.1179/102453308X315942 [DOI] [PubMed] [Google Scholar]
- 42. Miles RR, Raphael M, McCarthy K, Wotherspoon A, Lones MA, Terrier‐Lacombe MJ, et al. Pediatric diffuse large B‐cell lymphoma demonstrates a high proliferation index, frequent c‐Myc protein expression, and a high incidence of germinal center subtype: report of the French‐American‐British (FAB) international study group. Pediatr Blood Cancer. 2008;51:369–74. doi: 10.1002/pbc.21619. 10.1002/pbc.21619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seidemann K, Tiemann M, Lauterbach I, Mann G, Simonitsch I, Stankewitz K, et al. Primary mediastinal large B‐cell lymphoma with sclerosis in pediatric and adolescent patients: treatment and results from three therapeutic studies of the Berlin‐Frankfurt‐Munster Group. J Clin Oncol. 2003;21:1782–9. doi: 10.1200/JCO.2003.08.151. 10.1200/JCO.2003.08.151 [DOI] [PubMed] [Google Scholar]
- 44. Oschlies I, Klapper W, Zimmermann M, Krams M, Wacker HH, Burkhardt B, et al. Diffuse large B‐cell lymphoma in pediatric patients belongs predominantly to the germinal‐center type B‐cell lymphomas: a clinicopathologic analysis of cases included in the German BFM (Berlin‐Frankfurt‐Munster) Multicenter Trial. Blood. 2006;107:4047–52. doi: 10.1182/blood-2005-10-4213. 10.1182/blood‐2005‐10‐4213 [DOI] [PubMed] [Google Scholar]
- 45. Ozgonenel B, Savasan S, Rabah R, Mohamed AN, Cushing B. Pediatric EBV‐positive T‐cell/histiocyte‐rich large B‐cell lymphoma with clonal cells in the bone marrow without overt involvement. Leuk Lymphoma. 2005;46:465–9. doi: 10.1080/10428190400018463. 10.1080/10428190400018463 [DOI] [PubMed] [Google Scholar]
- 46. Wang J, Sun NC, Chen YY, Weiss LM. T‐cell/histiocyte‐rich large B‐cell lymphoma displays a heterogeneity similar to diffuse large B‐cell lymphoma: a clinicopathologic, immunohistochemical, and molecular study of 30 cases. Appl Immunohistochem Mol Morphol. 2005;13:109–15. doi: 10.1097/01.pai.0000132199.47017.35. 10.1097/01.pai.0000132199.47017.35 [DOI] [PubMed] [Google Scholar]
- 47. Lones MA, Cairo MS, Perkins SL. T‐cell‐rich large B‐cell lymphoma in children and adolescents: a clinicopathologic report of six cases from the Children's Cancer Group Study CCG‐5961. Cancer. 2000;88:2378–86. doi: 10.1002/(sici)1097-0142(20000515)88:10<2378::aid-cncr24>3.0.co;2-q. 10.1002/(SICI)1097‐0142(20000515)88:10<2378::AID‐CNCR24>3.0.CO;2‐Q [DOI] [PubMed] [Google Scholar]
- 48. Camilleri‐Bröet S, Molina T, Audouin J, Tourneau AL, Diebold J. Morphological variability of tumour cells in T‐cell‐rich B‐cell lymphoma. A histopathological study of 14 cases. Virchows Arch. 1996;429:243–8. doi: 10.1007/BF00198340. [DOI] [PubMed] [Google Scholar]
- 49. Stenhammar L, Masreliez V. T‐cell‐rich B‐cell lymphoma in a child with celiac disease. J Pediatr Gastroenterol Nutr. 1993;17:337–8. doi: 10.1097/00005176-199310000-00021. 10.1097/00005176‐199310000‐00021 [DOI] [PubMed] [Google Scholar]
- 50. Claviez A, Meyer U, Dominic K, Beck Jf, Rister M, Tiemann M. Malt lymphomas in children: a report from the NHL‐BFM Study group. Pediatr Blood Cancer. 2006;47:210–4. doi: 10.1002/pbc.20575. 10.1002/pbc.20575 [DOI] [PubMed] [Google Scholar]
- 51. Agrawal R, Wang J. Pediatric follicular lymphoma: a rare clinicopathologic entity. Arch Pathol Lab Med. 2009;133:142–6. doi: 10.5858/133.1.142. [DOI] [PubMed] [Google Scholar]
- 52. Elenitoba‐Johnson KS, Kumar S, Lim MS, Kingma DW, Raffeld M, Jaffe ES. Marginal zone B‐cell lymphoma with monocytoid B‐cell lymphocytes in pediatric patients without immunodeficiency. A report of two cases. Am J Clin Pathol. 1997;107:92–8. doi: 10.1093/ajcp/107.1.92. [DOI] [PubMed] [Google Scholar]
- 53. Mo JQ, Dimashkieh H, Mallery SR, Swerdlow SH, Bove KE. MALT Lymphoma in children: case report and review of the literature. Pediatr Dev Pathol. 2004;7:407–13. doi: 10.1007/s10024-003-3025-6. 10.1007/s10024‐003‐3025‐6 [DOI] [PubMed] [Google Scholar]
- 54. Winberg CD, Nathwani BN, Bearman RM, Rappaport H. Follicular (nodular) lymphoma during the first two decades of life: a clinicopathologic study of 12 patients. Cancer. 1981;48:2223–35. doi: 10.1002/1097-0142(19811115)48:10<2223::aid-cncr2820481018>3.0.co;2-t. 10.1002/1097‐0142(19811115)48:10<2223::AID‐CNCR2820481018>3.0.CO;2‐T [DOI] [PubMed] [Google Scholar]
- 55. Shukla N, Trippett T. Non‐Hodgkin lymphomas in children and adolescents. Curr Oncol Rep. 2006;8:387–94. doi: 10.1007/s11912-006-0062-0. 10.1007/s11912‐006‐0062‐0 [DOI] [PubMed] [Google Scholar]
- 56. Di Cataldo A, Bertuna G, Mirabile E, Munda S, Tettoni K, Notarangelo LD, et al. Natural killer lymphoma/leukemia: an uncommon pediatric case with indolent course. Leuk Lymphoma. 2004;45:1687–9. doi: 10.1080/10428190410001683714. 10.1080/10428190410001683714 [DOI] [PubMed] [Google Scholar]
- 57. Razzouk B, Gan Y, Mendoça C, Jenkins JJ, Liu Q, Hudson M, et al. Epstein‐Barr virus in pediatric Hodgkin disease: age and histiotype are more predictive than geographic region. Med Pediatr Oncol. 1997;28:248–54. doi: 10.1002/(sici)1096-911x(199704)28:4<248::aid-mpo2>3.0.co;2-i. 10.1002/(SICI)1096‐911X(199704)28:4<248::AID‐MPO2>3.0.CO;2‐I [DOI] [PubMed] [Google Scholar]
- 58. Olweny C, Katongole‐Mbidde E, Kiire C, Lwanga SK, Magrath I, Ziegler JL. Childhood Hodgkin's disease in Uganda. Cancer. 1978;42:787–92. doi: 10.1002/1097-0142(197808)42:2<787::aid-cncr2820420251>3.0.co;2-4. 10.1002/1097‐0142(197808)42:2<787::AID‐CNCR2820420251>3.0.CO;2‐4 [DOI] [PubMed] [Google Scholar]
- 59. Mauz‐Körholz C, Gorde‐Grosjean S, Hasenclever D, Shankar A, Dörffel W, Wallace WH, et al. Resection alone in 58 children with limited stage, lymphocyte‐predominant Hodgkin lymphoma‐experience from the European network group on pediatric Hodgkin lymphoma. Cancer. 2007;110:179–85. doi: 10.1002/cncr.22762. 10.1002/cncr.22762 [DOI] [PubMed] [Google Scholar]
- 60. Elgui de Oliveira D, Bacchi M, Abreu E, Niero‐Merlo L, Bacchi C. Hodgkin disease in adult and juvenile groups from two different geographic regions clinicopathologic aspects and relationship with Epstein‐Barr virus infection. Am J Clin Pathol. 2002;118:25–30. doi: 10.1309/QFCB-PY52-BYR8-CGFC. 10.1309/QFCB‐PY52‐BYR8‐CGFC [DOI] [PubMed] [Google Scholar]
- 61. Schwartz CL. Special issues in pediatric Hodgkin's disease. Eur J Haematol Suppl. 2005l;66:55–62. doi: 10.1111/j.1600-0609.2005.00456.x. 10.1111/j.1600‐0609.2005.00456.x [DOI] [PubMed] [Google Scholar]
- 62. Sandoval C, Venkateswaran L, Billups C, Slim M, Jayabose S, Hudson M. Lymphocyte‐predominat Hodgkin disease in children. J Ped Hematol Oncol. 2002;24:269–73. doi: 10.1097/00043426-200205000-00010. 10.1097/00043426‐200205000‐00010 [DOI] [PubMed] [Google Scholar]
- 63. CLeary DF, Donaldson SS. Hodgkin's disease in the very young. Int J Radiat Oncol Biol Phys. 1994;28:77–83. doi: 10.1016/0360-3016(94)90143-0. 10.1016/0360‐3016(94)90143‐0 [DOI] [PubMed] [Google Scholar]
- 64. Gualco G, Ortega V, Musto M. Analisis de 511 casos de linfomas en Uruguay. Arch Med Int. 2007;29:37–41. [Google Scholar]
- 65. Vargas L. Cáncer infantil en Chile. Diez años del programa infantil. Rev Chil Pediatr. 1998;69:270–5. [Google Scholar]