Abstract
Polyamines have been implicated in a wide range of biological processes, including growth and development in bacteria and animals, but their function in higher plants is unclear. Here we show that the Arabidopsis ACAULIS5 (ACL5) gene, whose inactivation causes a defect in the elongation of stem internodes by reducing cell expansion, encodes a protein that shares sequence similarity with the polyamine biosynthetic enzymes spermidine synthase and spermine synthase. Expression of the recombinant ACL5 protein in Escherichia coli showed that ACL5 possesses spermine synthase activity. Restoration of the acl5 mutant phenotype by somatic reversion of a transposon-induced allele suggests a non-cell-autonomous function for the ACL5 gene product. We also found that expression of the ACL5 cDNA under the control of a heat shock gene promoter in acl5 mutant plants restores the phenotype in a heat shock-dependent manner. The results of the experiments showed that polyamines play an essential role in promotion of internode elongation through cell expansion in Arabidopsis. We discuss the relationships to plant growth regulators such as auxin and gibberellins that have related functions.
Keywords: ACAULIS5/Arabidopsis/internode elongation/polyamines/spermine
Introduction
The external appearance of flowering plants is determined by temporal and spatial control of the formation of leaves, flowers and stem internodes. Following germination, the vegetative shoot meristem generates leaf primordia on its periphery. Upon receiving the appropriate developmental signals, it is transformed into an inflorescence meristem, which gives rise to flowers. The transition to the reproductive phase is often accompanied by drastic changes in the branching pattern and in the elongation growth of stem internodes (Weberling, 1989). In sunflowers (Helianthus annuus), for example, the shoot meristem produces leaves in unison with elongating internodes at the vegetative stage. After floral transition, it generates an inflorescence composed of hundreds of individual flowers, which is known as a head. In contrast, in the case of the model plant Arabidopsis thaliana, little internode elongation occurs during vegetative growth and a characteristic rosette is formed. On transition to reproductive development, flowers are formed on the flanks of the shoot, and rapid elongation of internodes occurs between the last 2–3 leaf nodes. Therefore, the mature flowering plant bears flowers on an elongated shoot above the rosette. On the other hand, the dandelion (Taraxacum officinale) is an example of a plant with rosette leaves, a single elongated internode and a head inflorescence. These varieties of shoot architecture suggest the existence of a developmental programme for internode elongation unique to individual species and also provide important criteria for their relationships.
Numerous studies based on identification and characterization of mutants with defects in internode elongation have revealed that some of these mutants accumulate very low levels of bioactive gibberellic acid (GA) and are defective in one of the steps of the GA biosynthetic pathway (for a review see Hedden and Kamiya, 1997). Recent genetic strategies using Arabidopsis mutants have led to the isolation of a series of genes involved in GA biosynthesis. Furthermore, isolation and analysis of suppressor mutants for GA-requiring dwarfs in Arabidopsis have led to the identification of transcription factors belonging to the plant-specific VHIID family (Peng et al., 1997; Silverstone et al., 1998). Of these suppressors, which are recessive and are thought to be loss-of-function mutations, one has been shown to be allelic to a GA-insensitive (gai) mutant, which represents a semi-dominant gain-of-function allele and shows a dwarf phenotype. It remains unclear, however, how their functions are inactivated by GA. Transcription factors of different classes have been identified from other GA-insensitive dwarf mutants, tiny (Wilson et al., 1996) and short internode (shi) (Fridborg et al., 1999). These two mutants also represent semi-dominant gain-of-function alleles because they were isolated by a transposon tagging strategy using a Dissociation (Ds) element with a cauliflower mosaic virus (CaMV) 35S promoter, which causes overexpression of the flanking plant gene (Wilson et al., 1996; Long et al., 1997). These findings suggest the presence of a regulatory mechanism that represses inappropriate elongation of stem internodes in Arabidopsis. Genes under the control of these transcription factors remain to be determined.
Because GA appears to participate in many aspects of plant development, including seed germination, flower induction and anther development, as well as internode elongation, it has been suggested that GA functions may be specified by its interaction with other phytohormones such as auxin and brassinosteroid and/or the presence of downstream signalling molecules. However, we have little genetic information on such interactions and signalling cascades during the internode elongation. Furthermore, in order to unravel the cellular basis for elongation growth, it is also important to identify actual molecules that play a critical role in the process of cell elongation, which underlies the internode elongation. Nicol et al. (1998) identified one such gene, KORRIGAN (KOR), which encodes a membrane-anchored member of the endo-1,4-β-d-glucanase. The kor mutants are defective in organ elongation, suggesting a role for KOR in the correct assembly of the cellulose–hemicellulose network in the expanding cell wall.
To study further the control of shoot architecture, we have focused on Arabidopsis mutants named acaulis5 (acl5). Recessive mutations in the ACL5 gene result in a severe reduction in the length of stem internodes and cause a reduction in the number of flowers due to early proliferative arrest of apical inflorescence meristems (Hanzawa et al., 1997). The mutant plants show little or no morphological defects in other organs, unlike those defective in biosynthesis or perception of phytohormones, and the phenotype is not rescued by the addition of exogenous phytohormones. The transcript levels of the GA5 gene encoding a GA 20-oxidase and other GA-related genes are affected in acl5 plants, suggesting that ACL5 represents a novel regulator of the shoot development and functions downstream of GA response pathways. In the present study, we found that the ACL5 gene encodes spermine synthase, one of the polyamine biosynthetic enzymes. Our results showing that introduction of the heat shock promoter–ACL5 gene fusion into acl5 plants leads to heat shock-dependent complementation of the phenotype may allow genetic manipulation of the shoot architecture, which is a long-standing goal in cultivated plants.
Results
Isolation of the ACL5 gene
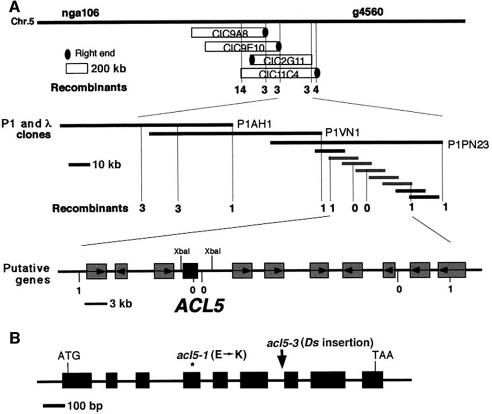
To understand the molecular function of the ACL5 gene product, we cloned the gene by positional cloning (Figure 1A). Genetic mapping experiments placed the ACL5 locus between the right end of a yeast artificial chromosome (YAC) clone, CIC9F10, and the left end of a YAC clone, CIC2G11, on chromosome 5 (Schmidt et al., 1997). An Arabidopsis genomic library constructed in bacterial P1 plasmids was screened with these YAC end probes, and three positive clones were used to construct a λ phage library. Further genetic mapping experiments using restriction fragment length polymorphisms (RFLPs) delimited the ACL5 locus to a 54 kb region covered by five overlapping phage clones derived from a P1 clone, PN23. The nucleotide sequence of this region was determined and computer analysis identified 11 putative open reading frames (ORFs). Sequence determination of the ORFs of the mutant acl5-1 identified a single base pair substitution in only one of these ORFs (Figure 1B). The acl5-2 allele carried an identical mutation. Nine cDNAs that hybridized to this ORF were isolated, and they were all approximately the same length. Analysis of their sequence demonstrated that the gene consists of nine exons and eight introns. The mutant phenotype was fully corrected by introducing a 3.8 kb genomic fragment that contains a complete coding sequence of this gene into the acl5-1 mutant (Figure 2).
Fig. 1. Cloning and sequence analysis of the ACL5 gene. (A) Summary of positional cloning of the ACL5 gene. Molecular markers in the region and the number of recombinations between the marker and the ACL5 locus are shown. Grey boxes represent putative ORFs around the ACL5 locus (black box), whose direction of transcription is indicated by arrows. Restriction sites (XbaI) used for Ti-plasmid construction for complementation are indicated. (B) Genomic structure of the ACL5 gene (AF184093, AF184094). The nine exons indicated by black boxes were determined by comparison between genomic and cDNA sequences. Positions mutated in acl5-1 and acl5-3 alleles are indicated.
Fig. 2. Complementation of the acl5-1 allele by the wild-type ACL5 gene. Thirty-five-day-old plants of wild-type Ler, acl5-1 and transgenic acl5-1 with the wild-type ACL5 genomic fragment are shown from left to right. Transgenic acl5-1 plants in the Col-0 background were backcrossed to the original acl5-1 mutant in the Ler background three times.
ACL5 has a non-cell-autonomous function
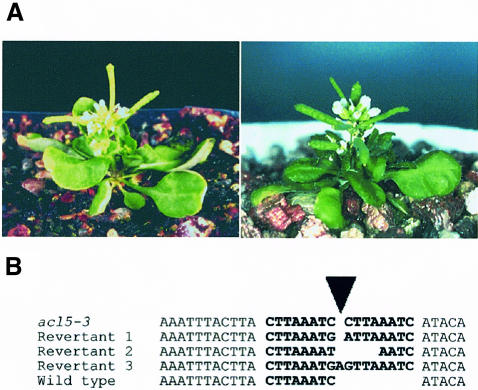
In parallel, we identified another mutant allele, acl5-3, caused by a Ds transposon insertion (Figure 3A) (Long et al., 1993, 1997). The Ds element was inserted into the sixth intron of the same gene carrying the mutation in acl5-1 (Figure 1B). Excision of the Ds element in the progeny of mutant plants by crossing with plants carrying the Ac transposase (TPase) gene caused one stable mutant allele (acl5-4) and revertant alleles that restored the wild-type phenotype (see Materials and methods). DNA fragments containing Ds excision sites in the revertant alleles were isolated by PCR, and the footprint sequences present after excision of Ds were determined (Figure 3B). Genomic DNA and RNA gel blot analyses revealed that the stable mutant allele, acl5-4, carried a large deletion of the locus and produced no detectable ACL5 transcript (see below), indicating that it represents a null allele. Plants homozygous for the acl5-4 allele showed the phenotype identical to that of acl5-1 and acl5-3 (Figure 3B).
Fig. 3. acl5 mutants caused by transposon insertion. (A) Phenotypes of acl5-3 and acl5-4 mutant alleles. The acl5-3 allele (left) was caused by a Ds insertion into the ACL5 gene. acl5-4 (right) was identified as a deletion allele of the ACL5 locus from the progeny of the cross between acl5-3 and plants with an Ac transposase. (B) Sequences adjacent to the Ds element in the acl5-3 allele and the footprints after excision of the Ds element in revertant alleles. The Ds element is represented as a triangle.
In addition, in the F2 generation from the cross between plants homozygous for acl5-3 and those carrying a source of TPase, we obtained a remarkably low frequency of plants that carried the TPase construct and showed the acl5 phenotype (only 3/613 plants, excluding the deletion of acl5-4 allele, while a ratio of 3/16 would be expected by Mendelian segregation), and these mutant plants only produced wild-type progeny that also contained the TPase. This strongly suggests that the somatic excision of the Ds element from acl5-3, which is driven by TPase, corrects non-cell-autonomously the mutant phenotype of acl5-3 to the wild-type phenotype so that mutants carrying TPase are exceedingly rare.
ACL5 encodes a polyamine biosynthetic gene
The predicted protein product encoded by the ACL5 gene contains 339 amino acids and shows sequence similarity (32–36% identity) to spermidine synthase (EC 2.5.1.16) and 24–28% identity to spermine synthase (EC 2.5.1.22) from several organisms (Figure 4). Both of these enzymes have major roles in polyamine biosynthesis. Polyamines, low molecular weight polycationic molecules ubiquitously present in living organisms, have been implicated in a wide range of growth and developmental processes. The diamine putrescine is converted into spermidine and spermine through the consecutive activity of spermidine synthase and spermine synthase using decarboxylated S-adenosyl methionine (dcSAM) as an aminopropyl donor. BLAST searches indicated that both enzymes share 25–30% amino acid sequence identity with each other in animals and lower eukaryotes. While genes encoding spermidine synthase have been cloned from several plant species (Hashimoto et al., 1998; Alabadí et al., 1999), cloning of the plant spermine synthase gene has not been reported. The deduced protein sequence of the spermidine synthase gene of A.thaliana is 87% identical to a second putative spermidine synthase identified among expressed sequence tag clones (DDBJ/EMBL/GenBank accession No. AC005990) and 32% identical to ACL5. The amino acid substitution of glutamate at position 123 to lysine in the acl5-1 allele occurs in a potential dcSAM-binding site (Posfai et al., 1989) and probably disables dcSAM binding (Figure 4). Southern hybridization with ACL5 probes under low stringency conditions suggested that there is no gene closely related to ACL5 in the Arabidopsis genome (data not shown).
Fig. 4. Alignment of the deduced amino acid sequences of ACL5, Arabidopsis spermidine synthase (AtSPDS) (Hashimoto et al., 1998) and yeast (Saccharomyces cerevisiae) spermine synthase (Hamasaki-Katagiri et al., 1998). The potential decarboxylated S-adenosylmethionine-binding motifs (Posfai et al., 1989) are double underlined. Identities and similarities among the different proteins are indicated by black and grey boxes, respectively. The asterisk indicates the glutamate mutated in the acl5-1 allele.
ACL5 possesses spermine synthase activity
In order to define the enzyme activity of the ACL5 gene product, the wild-type ACL5 protein was produced in Escherichia coli as a fusion protein with maltose-binding protein (MBP). High-performance liquid chromatography (HPLC) analysis of polyamines extracted from cells carrying the fusion construct revealed that ACL5 possessed spermine synthase activity, which is normally undetectable in E.coli cells (Figure 5). Conversion of radiolabelled spermidine into spermine in E.coli cells was also detected in feeding experiments (Table I). The mutated ACL5 was also produced by using a cDNA fragment derived from the acl5-1 allele, and we confirmed that it had no spermine synthase activity (data not shown).

Fig. 5. ACL5 has spermine synthase activity. HPLC analysis of polyamine products in E.coli cells expressing ACL5. Extracts from cells carrying a pMAL empty vector (A) or the ACL5 fusion construct with MBP (B) were analysed by HPLC following the method of Flores and Galston (1982).
Table I. Radioactivities of the fractions corresponding to putrescine, spermidine and spermine in E.coli extracts.
| Plasmid | Putrescine (d.p.m.) | Spermidine (d.p.m.) | Spermine (d.p.m.) |
|---|---|---|---|
| pMAL | 49 | 2753 | 31 |
| 52 | 3215 | 83 | |
| pMAL/ACL5 | 56 | 469 | 1824 |
| 45 | 352 | 1810 |
Feeding experiments with [14C]spermidine were performed twice for E.coli cells carrying each construct (see Materials and methods).
Expression analysis
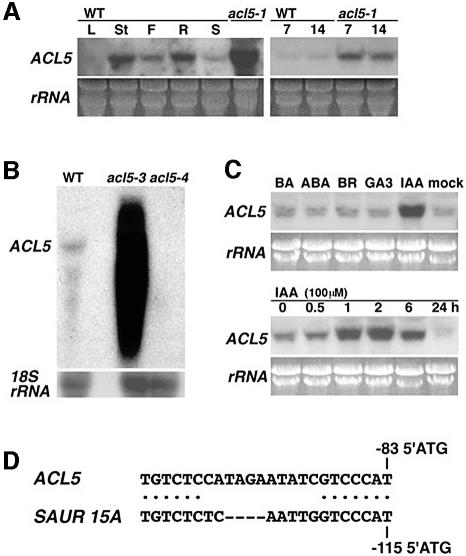
RNA gel blot analysis showed that the ACL5 transcript accumulated to high levels in stem internodes of adult flowering plants and also in root tissue (Figure 6A). This is in agreement with the effect of the mutation on elongation of the shoot and its slight effect on root elongation. Much lower levels of the transcript were observed in young seedlings before flowering and in rosette leaves, correlating with the lack of discernible phenotypes during vegetative growth of the mutant. Interestingly, ACL5 transcript levels were much higher in the acl5-1 mutant than in the wild-type, suggesting that ACL5 expression may be under negative feedback control. The ACL5 transcript could not be detected in the acl5-4 allele carrying a large deletion of the locus (Figure 6B). Smeared intense signals in acl5-3 may represent chimeric transcripts with the Ds sequence, truncated transcripts driven by the CaMV 35S promoter located in the Ds element (Long et al., 1997) and their degradation products.
Fig. 6. ACL5 expression patterns. (A) Organ-specific expression of ACL5. Northern blots were performed using 10 µg of total RNA per lane. Wild-type (WT) RNA samples in the left panels were prepared from leaves (L), stem internodes (St), flowers with apical meristems (F) and root tissue (R) of 35-day-old plants and 10-day-old seedlings (S). RNA of acl5-1 was prepared from whole tissue of 35-day-old mutant plants. RNA samples in the right panels were prepared from 7- and 14-day-old seedlings of wild-type and acl5-1 plants. (B) Expression of ACL5 in acl5-3 and acl5-4 alleles. RNA samples were prepared from primary shoots of flowering plants. (C) Phytohormone-induced expression of ACL5. RNA samples were prepared from wild-type seedlings grown for 10 days in liquid culture media and treated with 100 µM each of phytohormones for 2 h (upper panels) or with 100 µM IAA for the indicated periods (lower panels). Northern blots were performed using 10 µg of total RNA per lane. (D) Putative auxin-responsive cis-elements (AuxREs) in the ACL5 promoter region. The upstream sequence of the ACL5 gene is compared with that of the soybean SAUR 15A gene (Li et al., 1994). Two putative AuxREs (TGTCTC and GTCCCAT) (Guilfoyle et al., 1998) are indicated by dots.
The effects of phytohormones on polyamine metabolism have been implicated in some aspects of plant development. We therefore investigated whether ACL5 transcript levels in wild-type seedlings are changed following hormone application. Auxin treatment with 100 µM indole-3-acetic acid (IAA) increased ACL5 transcript levels (Figure 6C). Similar effects on ACL5 expression were obtained after treatment with 1 µM IAA. Treatment with 100 µM benzyl aminopurine (BA), abscisic acid (ABA), brassinolid (BR) and gibberelic acid (GA3) had no influence on ACL5 expression (Figure 6C). The upstream region of the ACL5 gene appeared to contain putative auxin-responsive cis-acting elements (Figure 6D), which have been suggested to be a recognition site of transcription factors referred to as auxin response factors (Guilfoyle et al., 1998).
Manipulation of the internode length by transgenic ACL5 expression
To understand further the roles of the ACL5 gene during the elongation of stem internodes, we generated transgenic acl5-1 plants carrying the full-length ACL5 cDNA fused with a heat shock-inducible promoter, and we examined the effect of heat shock-dependent overexpression of the ACL5 gene on various stages of plant development. Plants homozygous for acl5-1 (Col-0 background) were transformed directly with a T-DNA construct containing the Arabidopsis HSP18.2 gene promoter–ACL5 cDNA fusion gene. When young seedlings of transgenic acl5-1 lines were treated with heat shock, they showed no inducible phenotype and were indistinguishable from non-treated mutant plants. The treatment did not affect the mutant phenotype after flowering. When treated with heat shock after flowering, however, these transgenic plants dramatically restored the phenotype (Figure 7A). We detected a tight correlation between the frequency of heat shock treatments and the degree of restoration in the length of stem internodes.
Fig. 7. Transgenic expression of the ACL5 cDNA. (A) Heat shock-dependent complementation of the phenotype in transgenic acl5-1 plants. The full-length ACL5 cDNA was cloned into a binary vector, pTT101 (Matsuhara et al., 2000), which contains the heat shock-inducible Arabidopsis HSP18.2 gene promoter, and it was introduced into the acl5-1 mutant. Plants were heat shocked at 37°C for 30 min every third day after flowering. The frequencies of heat shock treatment are indicated. (B) Phenotypes caused by antisense ACL5 expression. The T-DNA construct for antisense expression of the full-length ACL5 cDNA under the control of the CaMV 35S promoter was introduced into wild-type (Col-0) plants. Wild-type plants (WT) and the T2 progeny plants from transgenic lines #1 and #5 are shown.
We also introduced a T-DNA construct for antisense expression of the ACL5 cDNA under the control of the CaMV 35S promoter into wild-type plants and obtained five transformants. In one of the transgenic lines, #5, all of the progeny plants that were resistant to kanamycin exhibited a reduction in the length of stem internodes (Figure 7B). Although less frequently, the other three lines also showed a similar semi-dwarf phenotype in their progeny plants. Northern blot analysis revealed that these plants expressed a greatly reduced level of the ACL5 transcript (data not shown).
Discussion
Identification of a novel component of shoot development
Recessive mutations in the Arabidopsis ACL5 gene result in plants showing a severe defect that is restricted to cell elongation after transition to the reproductive stage. The results of our previous study suggested that the wild-type ACL5 gene product represents a novel and essential regulator of the inflorescence development in flowering plants (Hanzawa et al., 1997). In the present study, we isolated the ACL5 gene by positional cloning and revealed that it encodes a polyamine biosynthetic enzyme. Polyamines, a class of aliphatic amines, have been implicated in a wide range of growth and developmental processes in bacteria, animals and plants. Although it is well known that polyamines are involved in cell proliferation of both prokaryotes and eukaryotes, their exact role has yet to be firmly established. In animals, the gene encoding ornithine decarboxylase (EC 4.1.1.17), by which ornithine is converted to putrescine, has been suggested to be a proto-oncogene (Auvinen et al., 1992). In addition to the involvement of polyamines in DNA, RNA and protein synthesis, specific interactions with certain potassium channels and glutamate receptors have been reported (Ficker et al., 1994; Fakler et al., 1995; Williams, 1997). In higher plants, increases in polyamine biosynthetic activities have been observed in a wide range of developmental processes (Evans and Malmberg, 1989). Tobacco mutants resistant to the inhibitor of SAM decarboxylase (EC 4.1.1.50) exhibit abnormal patterns of floral organ development (Malmberg and McIndoo, 1983). The analysis of transgenic plants with engineered sense and antisense genes for polyamine biosynthetic enzymes indicates that changes in internal polyamine levels can affect stem elongation, leaf morphology and root growth (Kumar et al., 1996; Masgrau et al., 1997). However, there have been no definitive conclusions on the physiological role of polyamines in plant growth and development because of the lack of mutants that are defective in a single polyamine biosynthetic gene (Watson et al., 1998).
The high similarity between ACL5 protein and spermidine synthase or spermine synthase from other organisms suggests that ACL5 functions as a key enzyme in the polyamine biosynthesis pathway in plant cells. This suggestion is supported by results from expression of the ACL5 cDNA in E.coli cells. ACL5 protein catalyses the pathway that converts spermidine into spermine, indicating that ACL5 can act as a spermine synthase in plants. This result is consistent with the fact that the exogenous addition of dl-α-difluoromethylornithine (DFMO) to wild-type plants causes a severe defect in the internode elongation, which is similar to the acl5 mutant phenotype (data not shown). DFMO is an inhibitor of ornithine decarboxylase and is thought to reduce the endogenous level of putrescine, a precursor of spermidine and spermine. However, the possibility that ACL5 exhibits broad amine substrate specificities and may also be involved in the synthesis of other polyamines in plant tissues cannot be excluded. Although many uncommon polyamines, which differ from the common aliphatic polyamines in the number of methylenic moieties between the amine groups, have been discovered, especially in plants and thermophilic bacteria, their biosynthetic enzymes remain largely unidentified (Phillips and Kuehn, 1991).
Sequence analysis of the acl5-1 allele revealed that the mutation caused amino acid substitution (E to K) in a highly conserved residue within a potential dcSAM-binding site. When expressed in E.coli, the mutated ACL5 protein derived from the acl5-1 allele had no spermine synthase activity. Taken together with the fact that the phenotype of acl5-1 plants is identical to that of the deletion allele, acl5-4, it is likely that the acl5-1 allele represents a complete loss-of-function mutation. Further biochemical analyses of recombinant ACL5 proteins by site-directed mutagenesis are currently underway and should help to elucidate specific amino acids necessary for spermine synthase activity.
Control of internode elongation by ACL5
Isolation of the ACL5 gene provides the first genetic evidence confirming that polyamines play an important role in plant development. Although expression of the ACL5 gene occurs mainly in those tissues in which the mutation has its greatest effect, it is also likely that ACL5 can act non-cell-autonomously like phytohormones to regulate shoot elongation. This is suggested by the results of experiments in which plants homozygous for acl5-3, the allele induced by insertion of the Ds element, were crossed to those carrying a source of the TPase. The extremely low frequency of mutant plants carrying the TPase in the F2 families strongly suggests that somatic excision of Ds from acl5-3 is sufficient to restore the phenotype and that polyamines produced by ACL5 can be transported. This is in good agreement with recent biochemical and physiological data suggesting that polyamines can be transported over distances through xylem and phloem (Caffaro et al., 1993; Antognoni et al., 1998). In animal systems, it has been shown that polyamines are moved via specific transporters and that the transport is energy dependent (Seiler and Dezeure, 1990). Regardless of whether or not polyamines can be defined as phytohormones, identification of such transport systems is expected to provide new insights into the actions of polyamines in plant cells. Although less likely, the possibility that the mutant phenotype is rescued by the movement of the ACL5 protein itself from cell to cell in acl5-3 mutants carrying the TPase cannot be ruled out.
Application of exogenous spermine failed to rescue the acl5 mutant phenotype in our feeding experiments (data not shown). One possible explanation for this is that additional polyamine products may be produced by ACL5 in wild-type Arabidopsis tissue and, therefore, that spermine alone cannot restore normal growth. Alternatively, exogenous spermine might not be transported to its proper intracellular targets in a physiologically active form. This is partly because of the existence of the cell wall, in which interactions of spermine with negatively charged pectic substances can block its uptake by cells (Bagni and Pistocchi, 1991). Polyamine-oxidizing enzymes tightly bound to the cell wall (Slocum, 1991) might also eliminate supplied spermine. As a further complication, plant polyamines exist predominantly in conjugated forms with hydroxycinnamic acids (Flores and Martin-Tanguy, 1991). Even if such conjugates are physiologically active, it remains unknown whether or not exogenous spermine can be integrated into their biosynthetic pathways.
RNA gel blot analysis revealed that expression of the ACL5 gene is up-regulated by auxin. In addition, we identified a putative auxin-responsive cis-acting element in the upstream region of the ACL5 gene. Considering the critical requirement for auxin in plant cell division and expansion, it is possible that early proliferative arrest of apical inflorescence meristems and/or reduced cell length in stem internodes in acl5 mutants are caused by a defect in an ACL5-mediated response to auxin. Alternatively, we have shown previously that the acl5 mutation affects expression levels of GA-regulated genes in stem internodes (Hanzawa et al., 1997), suggesting ACL5 involvement in GA signal transduction or GA biosynthesis. These data raise the possibility that interactions of auxin and GA in shoot development, on which there have been numerous reports, may be mediated in part by polyamines. Further study with phytohormone-related mutants should help to clarify the relationships between auxin, GA and polyamines. It would be interesting to examine whether the morphological phenotypes of such mutants can be partially restored by ACL5 overexpression.
Our observations showing that acl5-1 mutants have increased levels of ACL5 transcripts compared with that of the wild-type suggest the presence of a negative feedback mechanism for ACL5 expression. Similar observations have been reported for genes involved in GA biosynthesis (Phillips et al., 1995). It remains to be determined whether the acl5 mutation also affects expression of other genes in polyamine biosynthetic pathways. To date, there has been little information on expression profiles of genes related to polyamine biosynthesis and those responsive to polyamines. Experiments to address this issue might be possible by using transgenic acl5-1 plants carrying a heat shock-inducible ACL5 cDNA. It should be noted that the acl5 phenotype is restored dramatically by heat shock treatments of these transgenic plants. Taking into account that heat shock treatments of young seedlings have no effect and that repeated heat shocks of flowering plants are more effective for the restoration of the phenotype than a single heat shock pulse, it is thought that the internode elongation may be triggered by rapid and transient action of the ACL5 gene product in transgenic mutant plants. Finally, this approach using an inducible promoter will not only provide a new opportunity to determine the precise mode of action of polyamines during internode elongation but also open the way for manipulation of the shoot architecture of flowering plants by genetic engineering.
Materials and methods
Plant material and growth conditions
Isolation of the acl5-1 and acl5-2 mutant alleles in the Landsberg erecta (Ler) ecotype has been described previously (Hanzawa et al., 1997). Sequence determination of the acl5-2 allele (see below) revealed that it contained the same mutation as the acl5-1 allele, and it is therefore likely that they were not independently induced mutations. Another ecotype, Columbia (Col-0), was used as the wild-type for genetic experiments. Plants were grown in a growth chamber at 22°C under continuous fluorescent light. For RNA preparation from wild-type seedlings treated with phytohormones, seeds were surface sterilized, and sown and grown in an aerated solution of MS salts with 2% sucrose. The acl5-3 allele was identified in a transposon-tagged mutant screen by using a two-component Activator/Dissociation (Ac/Ds) strategy described previously (Long et al., 1993, 1997). The stable deletion allele of acl5 (acl5-4) was identified among the F2 population from the cross between the acl5-3 plants and plants carrying the CaMV 35S promoter::TPase gene construct. Eight F1 plants were self-fertilized, generating eight separate F2 families. The segregation ratio of phenotypically wild-type plants to acl5 mutant plants was nearly 15:1 (the combined ratio in all families was 613 wild-type:32 mutants). Of the 32 mutants, only four carried the TPase construct, which can easily be determined because 35S::TPase also carries a bacterial β-glucuronidase (GUS) gene (Long et al., 1993, 1997). One of these four plants was shown to carry a stable deletion allele of acl5 (acl5-4), which gave rise to a mutant phenotype in the presence of the TPase, by Southern blot hybridization. For the Southern hybridization, wild-type and acl5-4 DNA were cleaved with XbaI and hybridized to the ACL5 cDNA, which detected a strongly hybridizing fragment of 3.8 kb in the wild-type and a weakly hybridizing fragment of 1.4 kb in acl5-4 (data not shown). Revertant alleles from acl5-3 were isolated by crossing the acl5-3 plants with plants homozygous for both the 35S::TPase construct and the acl5-4 allele. In the F2 progeny with the wild-type phenotype, three plants that did not carry the TPase gene construct were identified.
Cloning and sequencing of ACL5
Genetic crosses were performed as described previously (Hanzawa et al., 1997). DNAs from F2 acl5-1 homozygous plants were prepared for simple sequence length polymorphisms (SSLPs) (Bell and Ecker, 1994) and cleaved–amplified polymorphic sequences (CAPS) (Konieczny and Ausubel, 1993) analysis. F2 acl5-1 homozygous plants with recombination break points in the nga106-ACL5 region (28 recombinants) or in the ACL5-ttg region (180 recombinants) were used to map SSLP, CAPS and RFLP molecular markers relative to the ACL5 locus by Southern blot hybridization or PCR. For further fine mapping, the ends of YAC clones CIC9A8, CIC9F11, CIC2G11 and CIC11C4 were amplified by tail-PCR and used as RFLP markers. The right end of CIC9F10 and the left end of CIC2G11 were used as probes to isolate P1 clones (Research Institute of Innovative Research for the Earth and Mitsui Plant Biotechnology Research Institute). A genomic library was then constructed in λ-EMBL3 (Stratagene) phages by using restriction fragments of the P1 clone, PN23. Contiguous phage clones were used to identify RFLPs between two ecotypes and delimit the ACL5 locus to a 54 kb region. DNASIS version 2.0 (Hitachi Software) was used to identify ORFs. All of the putative ORFs were PCR amplified and sequenced from the acl5-1 allele with gene-specific primers. A labelled DNA fragment containing the ACL5 gene was used as a probe to screen an Arabidopsis cDNA library constructed in λ ZAPII (Clontech). Protein databases were searched using the BLAST program.
For isolating plant DNA flanking a Ds insertion from the acl5-3 allele, inverse PCR was performed as described previously (Wilson et al., 1996).
Plant transformation
For complementation, the bacterial GUS gene in the binary Ti-vector, pBI101 (Clontech), was replaced with a 3.8 kb XbaI genomic fragment containing a complete coding sequence of the ACL5 gene (Figure 1A). For heat shock-inducible ACL5 expression, a BamHI–SalI fragment of the full-length ACL5 cDNA was cloned into the heat shock cassette Ti-vector pTT101 (Matsuhara et al., 2000). The CaMV 35S promoter::antisense ACL5 construct was made by replacing the GUS gene in pBI121 (Clontech) with a SacI–XbaI fragment of the full-length ACL5 cDNA. The resulting constructs were introduced into Agrobacterium C58C1 strain and used to transform homozygous acl5-1 or wild-type plants in the Col-0 background by the vacuum infiltration method (Bechtold et al., 1993). Transgenic seedlings were selected by resistance to kanamycin and transplanted to soil.
Expression in E.coli
The PCR fragment of the wild-type ACL5 cDNA was cloned into the pMAL-c2 vector (New England Biolabs). The resulting construct was introduced into E.coli TB1 strain. The MBP–ACL5 fusion protein was induced by addition of 0.3 mM isopropyl-β-d-thiogalactopyranoside (IPTG) to culture media for 4 h. Polyamines were extracted according to the method of Flores and Galston (1982). In brief, cells in 2.0 ml of culture medium were harvested and homogenized with 0.4 ml of trichloracetic acid (5% w/w) on ice. The homogenate was centrifuged (10 000 g, 10 min), and 0.2 ml of the supernatant was mixed with 0.2 ml of saturated Na2CO3 and 0.4 ml of dansyl chloride (5 mg/ml acetone) and incubated in darkness overnight. The fluorescent polyamines extracted were analysed by HPLC. For feeding experiments, 10 µl of [14C]spermidine (4.14 Gbp/mmol) was added to the culture media with IPTG. The fractions corresponding to putrescine, spermidine and spermine were collected and counted using a scintillation counter.
RNA gel blot analysis
Total RNA was prepared by the SDS–phenol extraction method. For northern hybridization, 10 µg of total RNA was separated on formaldehyde-denatured agarose gels, blotted onto GeneScreen nylon membranes (New England Nuclear) and hybridized to 32P-labelled ACL5 cDNA probes. Hybridizations and washes were performed according to the manufacturer’s instructions.
Acknowledgments
Acknowledgements
We thank Drs Satoshi Naito and Takashi Hashimoto for technical advice and Drs Nello Bagni, Kazuei Igarashi and Subhash C.Minocha for their comments and discussion. The P1 clones used in this work were provided by the Research Institute of Innovative Research for the Earth and the Mitsui Plant Biotechnology Research Institute. This work was supported by grants from the Japan Society for the Promotion of Science and the Ministry of Education, Science and Culture of Japan. Y.H. acknowledges additional support from the Naito Foundation.
References
- Alabadí D. and Carbonell,J. (1999) Differential expression of two spermidine synthase genes during early fruit development and in vegetative tissues of pea. Plant Mol. Biol., 39, 933–943. [DOI] [PubMed] [Google Scholar]
- Antognoni F., Fornale,S., Grimmer,C., Komor,E. and Bagni,N. (1998) Long-distance translocation of polyamines in phloem and xylem of Ricinus communis L. plants. Planta, 204, 520–527. [Google Scholar]
- Auvinen M., Paasinen,A. and Hölttä,E. (1992) Ornithine decarboxylase activity is critical for cell transformation. Nature, 360, 355–358. [DOI] [PubMed] [Google Scholar]
- Bagni N. and Pistocchi,R. (1991) Uptake and transport of polyamines and inhibitors of polyamine metabolism in plants. In Slocum,R.D. and Flores,H.E. (eds), Biochemistry and Physiology of Polyamines in Plants. CRC Press, London, pp. 105–120. [Google Scholar]
- Bechtold N., Ellis,J. and Pelletier,G. (1993) In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C.R. Acad. Sci. Paris, 316, 1194–1199. [Google Scholar]
- Bell C.J. and Ecker,J.R. (1994) Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics, 19, 137–144. [DOI] [PubMed] [Google Scholar]
- Caffaro S., Scaramagli,S., Antognoni,F. and Bagni,N. (1993) Polyamine content and translocation in soybean plants. J. Plant Physiol., 141, 563–568. [Google Scholar]
- Evans P.T. and Malmberg,R.L. (1989) Do polyamines have roles in plant development? Annu. Rev. Plant Physiol., 40, 235–269. [Google Scholar]
- Fakler B., Brändle,U., Glowatzki,E., Weidemann,S., Zenner,H.-P. and Ruppersberg,J.P. (1995) Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell, 80, 149–154. [DOI] [PubMed] [Google Scholar]
- Ficker E., Taglialatela,M., Wible,B.A., Henly,C.M. and Brown,A.M. (1994) Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science, 266, 1068–1072. [DOI] [PubMed] [Google Scholar]
- Flores H.E. and Galston,A.W. (1982) Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol., 69, 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores H.E. and Martin-Tanguy,J. (1991) Polyamines and plant secondary metabolites. In Slocum,R.D. and Flores,H.E. (eds), Biochemistry and Physiology of Polyamines in Plants. CRC Press, London, pp. 57–76. [Google Scholar]
- Fridborg I., Kuusk,S., Moritz,T. and Sundberg,E. (1999) The Arabidopsis dwarf mutant shi exhibits reduced gibberellin responses conferred by overexpression of a new putative zinc finger protein. Plant Cell, 11, 1019–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle T., Hagen,G., Ulmasov,T. and Murfatt,J. (1998) How does auxin turn on genes? Plant Physiol., 118, 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki-Katagiri N., Katagiri,Y., Tabor,C.W. and Tabor,H. (1998) Spermine is not essential for growth of Saccharomyces cerevisiae: identification of the SPE4 gene (spermine synthase) and characterization of a spe4 deletion mutant. Gene, 210, 195–201. [DOI] [PubMed] [Google Scholar]
- Hanzawa Y., Takahashi,T. and Komeda,Y. (1997) ACL5: an Arabidopsis gene required for internodal elongation after flowering. Plant J., 12, 863–874. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Tamaki,K., Suzuki,K. and Yamada,Y. (1998) Molecular cloning of plant spermidine synthases. Plant Cell Physiol., 39, 73–79. [DOI] [PubMed] [Google Scholar]
- Hedden P. and Kamiya,Y. (1997) Gibberellin biosynthesis: enzymes, genes and their regulation. Annu. Rev. Plant Physiol. Plant Mol. Biol., 48, 431–460. [DOI] [PubMed] [Google Scholar]
- Konieczny A. and Ausubel,F.M. (1993) A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J., 4, 403–410. [DOI] [PubMed] [Google Scholar]
- Kumar A., Taylor,M.A., Mad Arif S.A. and Davirs,H. (1996) Potato plants expressing antisense and sense S-adenosylmethionine decarboxylase (SAMDC) transgenes show altered levels of polyamines and ethylene: antisense plants display abnormal phenotypes. Plant J., 9, 147–158. [Google Scholar]
- Li Y., Liu,Z.B., Shi,X., Hagen,G. and Guilfoyle,T.J. (1994) An auxin-inducible element in soybean SAUR promoters. Plant Physiol., 106, 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D. Martin,M., Sundberg,E., Swinburne,J., Puangsomlee,P. and Coupland,G. (1993) The maize transposable element system Ac/Ds as a mutagen in Arabidopsis: identification of an albino mutation induced by Ds insertion. Proc. Natl Acad. Sci. USA, 90, 10370–10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D., Goodrich,J., Wilson,K., Sundberg,E., Martin,M., Puangsomlee,P. and Coupland,G. (1997) Ds elements on all five Arabidopsis chromosomes and assessment of their utility for transposon tagging. Plant J., 11, 145–148. [DOI] [PubMed] [Google Scholar]
- Malmberg R.L. and McIndoo,J. (1983) Abnormal floral development of a tobacco mutant with elevated polyamine levels. Nature, 305, 623–625. [Google Scholar]
- Masgrau C., Altabella,T., Farräs,R., Flores,D., Thompson,A.J., Besford,R.T. and Tiburcio,A.F. (1997) Inducible overexpression of oat arginine decarboxylase in transgenic tobacco plants. Plant J., 11, 465–473. [DOI] [PubMed] [Google Scholar]
- Matsuhara S., Jingu,F., Takahashi,T. and Komeda,Y. (2000) Heat-shock-tagging: a simple method for expression and isolation of plant genome DNA flanked by T-DNA insertions. Plant J., 22, 79–86. [DOI] [PubMed] [Google Scholar]
- Nicol F., His,I., Jauneau,A., Vernhettes,S., Canut,H. and Höfte,H. (1998) A plasma membrane-bound putative endo-1,4-β-d-glucanase is required for normal wall assembly and cell elongation in Arabidopsis. EMBO J., 17, 5563–5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Carol,P., Richards,D.E., King,K.E., Cawling,R.J., Murphy,G.P and Harberd,N.P. (1997) The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev., 11, 3194–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A.L., Ward,D.A., Uknes,S., Appleford,N.E.J., Lange,T., Huttly,A.K., Gaskin,P., Graebe,J.E. and Hedden,P. (1995) Isolation and expression of three gibberellin 20-oxidase cDNA clones from Arabidopsis. Plant Physiol., 108, 1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G.C. and Kuehn,G.D. (1991) Uncommon polyamines in plants and other natural sources. In Slocum,R.D. and Flores,H.E. (eds), Biochemistry and Physiology of Polyamines in Plants. CRC Press, London, pp. 121–136. [Google Scholar]
- Posfai J., Bhagwat,A.S., Posfai,G. and Robert,R.J. (1989) Predictive motifs derived from cytosine methyltransferase. Nucleic Acids Res., 17, 2421–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R., Love,K., West,J., Lenehan,Z. and Dean,C. (1997) Description of 31 YAC contigs spanning the majority of Arabidopsis thaliana chromosome 5. Plant J., 11, 563–572. [DOI] [PubMed] [Google Scholar]
- Seiler N. and Dezeure,F. (1990) Polyamine transport in mammalian cells. Int. J. Biochem., 22, 211–218. [DOI] [PubMed] [Google Scholar]
- Silverstone A.L., Ciampaglio,C.N. and Sun,T.-P. (1998) The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell, 10, 155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocum R.D. (1991) Tissue and subcellular localization of polyamines and enzymes of polyamine metabolism. In Slocum,R.D. and Flores,H.E. (eds), Biochemistry and Physiology of Polyamines in Plants. CRC Press, London, pp. 93–104. [Google Scholar]
- Watson M.B., Emory,K.K., Piatak,R.M. and Malmberg,R.L. (1998) Arginine decarboxylase (polyamine synthesis) mutants of Arabidopsis thaliana exhibit altered root growth. Plant J., 13, 231–239. [DOI] [PubMed] [Google Scholar]
- Weberling F. (1989) Morphology of Flowers and Inflorescences. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Williams K. (1997) Modulation and block of ion channels: a new biology of polyamines. Cell Signal., 9, 1–13. [DOI] [PubMed] [Google Scholar]
- Wilson K., Long,D., Swinburne,J. and Coupland,G. (1996) A Dissociation insertion causes a semidomonant mutation that increases expression of TINY, an Arabidopsis gene related to APETALA2. Plant Cell, 8, 659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]