Abstract
OBJECTIVES:
A duplex ultrasound study was performed to investigate morphological and hemodynamic patterns of carotid stenoses treated by endarterectomy with patch closure versus stenting.
MATERIALS AND METHOD:
Twenty‐nine carotid stenoses were treated with stenting and 65 with patch closure. Duplex ultrasound parameters (luminal diameter, mm; peak systolic velocity and end‐diastolic velocity, cm/s) were measured 24 hours after the procedures and also at 12 months post‐procedure. Residual stenoses (immediately post‐procedure) and restenoses (within 12 months of procedure) were defined as narrowings of ≥50% on duplex ultrasound examination.
RESULTS:
In stented patients, the luminal diameter of the proximal internal carotid artery increased in the interval between the 24‐hour and 12‐month post‐procedure studies, while in the patch closure patients, the diameter decreased. Carotid hemodynamics normalized immediately after both patching and stenting and remained relatively stable thereafter up to 12 months. No statistically elevated flow velocities (in the absence of residual stenosis or restenosis) were observed in the patched or stented carotid arteries. No significant differences in residual stenosis rates were observed between the stenting group (3 cases, 10.34%) and the patch closure group (1 case, 1.53%, P = 0.08). At 12 months, 2 stenting patients (6.88%) and 2 patch closure patients (3.07%) had ≥50% restenosis (P = 0.58). One case of late stroke due to restenosis was observed in the stenting group; the patient died 12 months postoperatively, before receiving new intervention.
CONCLUSION:
Measurements over time in luminal diameter signalized differences in arterial remodeling mechanisms between patched and stented carotids. Both stenting and patch closure were associated with carotid patency and flow restoration. This study does not support a general approach to new velocity criteria indiscriminately applied to stented or patched carotids.
Keywords: Bovine pericardial patch angioplasty, Carotid artery stenosis, Carotid endarterectomy, Carotid stenting, Duplex ultrasound
INTRODUCTION
Carotid endarterectomy with patch closure and carotid artery stenting with embolic protection are therapeutic approaches for the prevention of stroke and transient ischemic attack (TIA) in patients with moderate to severe symptomatic and asymptomatic carotid artery stenosis.1-9 The efficacy of both patch closure and carotid artery stenting is highly dependent on low procedural morbidity and producing a durable repair with a low incidence of recurrent stenosis or occlusion.
As duplex ultrasound (DUS) provides detailed information of the extracranial carotid bifurcation, many vascular laboratories have adopted a policy of DUS surveillance to confirm carotid patency and grade residual stenosis, and restenosis severity after patch closure and stenting. In general, DUS examination is largely based on analysis of the peak systolic velocity (PSV) and the end‐diastolic velocity (EDV).10-14 However, there is no consensus regarding the threshold criteria for the interpretation of DUS results after patching and stenting, and the performance of carotid DUS varies considerably from laboratory to laboratory. Some groups,15-17 including our own, have used a DUS algorithm validated for native (nonoperated) carotid arteries in the follow‐up of patch closure and carotid artery stenting, whereas other groups18-28 have emphasized that patched and stented carotids are best evaluated by revised velocity criteria.
Previous clinical studies reported flow disturbances in the internal carotid artery (ICA) in patients undergoing patch closure or carotid artery stenting,26,29,30 supporting the idea that velocity thresholds for patched and stented carotids may need revision. However, many of the hemodynamic studies following patch closure and carotid artery stenting have been performed on patients in diverse post‐procedural periods. Therefore, it is possible that velocity profiles related to treated site arterial remodeling and/or biomechanical changes present distinct alterations as a function of time, thereby potentially biasing the results. Furthermore, in the studies comparing velocity profiles and morphological measurements, the analyses could not be performed across the full spectrum of the degree of restenosis. DUS parameters of patched and stented carotids and how they relate to patient outcome are not yet well defined.
In this study, our group investigated the effects of stenting and patching on carotid morphological and hemodynamic DUS parameters measured 24 hours post‐procedure and at 12 months post‐procedure. In addition, our experience using previously validated criteria for native carotids and its application to patch closure and carotid artery stenting is detailed in this study. New clinical events were recorded during and after the procedures (20‐month surveillance period), as were deaths and the causes of death. DUS and clinical outcomes obtained for stenting were compared with patch closure.
MATERIALS AND METHOD
Patient population
Between May 2005 and May 2007, 131 patients undergoing 139 carotid procedures (patch closure or stenting) were prospectively entered into a nonrandomized study. Our protocol complied with Declaration of Helsinki and was approved by the Institutional Ethics Committee, and written informed consent was obtained from all patients.
Of the 139 carotids, 94 with asymptomatic (≥70%) or symptomatic (≥50%) carotid bifurcation atherosclerotic stenoses were admitted in this protocol. The severity of carotid bifurcation stenosis was evaluated on DUS validated against subtraction angiography, computed tomographic angiography or magnetic resonance angiography. Patients facing additional myocardial revascularization and who had carotids with radiation‐induced or fibromuscular dysplasia, or recurrent stenoses were not included in the study. Patients were excluded if they did not undergo postoperative DUS because of the unavailability of the vascular radiologist (all examinations were performed by a single vascular radiologist), or because of technical difficulties.
Carotid artery stenting was offered to 29 patients. The selection of 18 patients from our practice was based on the presence of either anatomic high risk [carotid bifurcation above C2 level, n = 8; previous cervical surgery, n = 3; concomitant intracranial stenosis, n = 1; common carotid artery (CCA) plaque, n = 1] or high medical risk (coronary artery disease, n = 2; peripheral arterial disease, n = 2; chronic obstructive pulmonary disease, n = 1). Eleven normal‐risk patients in the stenting group were referred from other physicians specifically for endovascular management. The mean age of stenting patients was 68.9 ± 8.25 years, and there was a higher number of male patients (25 men and 4 women). Twenty‐two cases were asymptomatic and 7 were symptomatic.
Sixty‐five carotid bifurcation stenoses in 39 asymptomatic and 26 symptomatic patients were treated with patch closure (50 men, 15 women; mean age, 69.7 ± 10.33 years).
Patient demographics, carotid bifurcation status, risk factors and clinical symptoms at the time of procedure are shown in Table 1. The patch closure and stenting groups were similar in all aspects with the exception that there were significantly more patients with coronary artery disease (65.51%) and peripheral arterial disease (62.06%) in the carotid artery stenting group than in the patch closure group (43.07% and 29.23%, respectively).
Table 1.
Baseline characteristics of patients according to treatments.
| Variable | Stenting group | Patch closure group | P‐value |
| n° of procedures | 29 | 65 | |
| n° asymptomatic patients | 22 (75.86) | 39 (60) | 0.20 |
| n° symptomatic patients | 07 (20.75) | 26 (40) | 0.20 |
| male/female | 25/4 | 50/15 | 0.40 |
| mean age ± SD (range) | 68.9 ± 8.25 (57‐94) | 69.7 ± 10.33 (44‐88) | 0.73 |
| statin | 15 (51.42) | 27 (41.53) | 0.48 |
| Risk factors | |||
| coronary artery disease | 19 (65.51) | 28 (43.07) | 0.00* |
| chronic renal disease | 03 (10.34) | 03 (4.61) | 0.36 |
| diabetes mellitus | 11 (37.93) | 13 (20.0) | 0.11 |
| dyslipidemia | 22 (75.86) | 40 (61.53) | 0.26 |
| peripheral arterial disease | 18 (62.06) | 19 (29.23) | 0.00* |
| nicotine abuse | 18 (62.06) | 36 (55.38) | 0.70 |
| systemic arterial hypertension | 26 (89.65) | 58 (89.23) | 0.76 |
| Carotid status | 0.80 | ||
| 50‐69% stenosis | 05 (17.24) | 14 (21.53) | ‐ |
| 70‐99% stenosis | 17 (58.62) | 36 (55.38) | ‐ |
| ICA near‐occlusion | 05 (17.24) | 13 (20) | ‐ |
| calcific shadow | 02 (6.80) | 02 (3.07) | ‐ |
| Clinical symptoms | |||
| amaurosis fugax | 0 | 02 (3.07) | 1 |
| contralateral ICA stenosis (≥50%) | 17 (58.62) | 27 (41.53) | 0.19 |
| stroke | 04 (13.79) | 15 (23.07) | 0.40 |
| transient ischemic attack | 03 (10.34) | 09 (13.84) | 0.74 |
Categorical data, except male/female, are expressed as count (percentage)
ICA, internal carotid artery; SD, standard deviation, n°, number;
, highly significant
Protected‐carotid artery stenting
Carotid artery stenting was performed by a single team of vascular surgeons and interventional radiologists using a standardized protocol. All stentings were performed under local anesthesia and via a retrograde access from the common femoral artery. Anticoagulation was assured by the administration of heparin (100 UI/kg). Protective filters (EPI filter EZ, Boston Scientific, Natick, MA, USA) were used in all cases. A self‐expanding Wallstent® (Boston Scientific) was deployed in all cases in the ICA with extension into the CCA (the diameter and length of the most frequently used stent was 7 mm × 40 mm). Deployment was followed by in‐stent dilatation using a balloon catheter (Boston Scientific). No heparin reversal was performed. All patients received combined platelet inhibition with aspirin (100 mg) and clopidogrel (75 mg) for at least 1 week preoperatively and for 3 months postoperatively.
Carotid endarterectomy with bovine patch closure
Carotid endarterectomy was performed by a single vascular surgeon using a standardized method (longitudinal arteriotomy). All patients were administered general anesthesia. Selective shunting (Edwards Lifesciences LLC, Irvine, CA, USA) was used in 24 operations (37%). The shunt criteria were: presence of contralateral severe stenosis (>60%) or occlusion (11 cases, 46%); history of recent stroke (6 cases, 25%); anatomical variations resulting in abnormal physiology of the Willis circle (3 cases, 12.5%); technical difficulties for the performance of the surgical procedures (2 cases, 8.3%); requirement of extension of the proximal or distal arteriotomy (use of balloon shunt) (2 cases, 8.3%).
In all cases, intravenous heparin was administered before carotid clamping and activated clotting time was maintained in the range of 200 to 250 seconds throughout the operation. The arteriotomy was closed with a bovine pericardial patch angioplasty with a continuous suture technique. All patches were 8 mm wide and manufacturer‐designed (Braile Biomédica Ind. e Com. Rep. S/A, São José do Rio Preto, São Paulo, Brazil). Preoperative imaging data concerning the vessel dimensions, manufacturer specifications and technical data, and observations from previous experience, were all considered when standardizing the patch diameter to 8 mm. Here, standardization contributed to reducing the bias in the postoperative DUS measurements. The systemic heparinization was reversed with protamine chloride. Preoperatively, all patients received 100 mg aspirin daily which was then continued for life.
Color‐coded DUS
All carotids were examined with a Philips/ATL HDI 5000 scanner (Philips, Bothell, WA, USA) operated by a single registered vascular radiologist. Morphological (luminal diameter, mm) and hemodynamic (PSV and EDV, cm/s) parameters were measured 1 day before the procedure and at 24 hours and 12 months post‐procedure. All DUS examinations were performed using an algorithm validated for preoperative carotid arteries.14 Stenoses of ≥50% were defined by a PSV of ≥125 cm/s and an EDV of 40 to 100 cm/s. Peak systolic velocities of ≥230 cm/s and an EDV of ≥100 indentified ≥70% stenosis. Lesions that generated an EDV of ≥140 cm/s were interpreted as ≥80% stenosis.14 Additional information derived from B‐mode imaging and spectral broadening was used to supplement the velocity criteria to determine stenosis severity.
The examinations included insonation of CCA, proximal ICA (p‐ICA) and distal ICA (d‐ICA), which were studied in the sagital and transverse planes. The p‐ICA was defined as a 2 cm segment beginning at the origin of the external carotid artery. In the d‐ICA, the postoperative DUS parameters were measured immediately distal to the patch or stent, except when the area of transition between the native d‐ICA and the distal end of the stent was not visualized on B‐mode. In these cases, the DUS measurements were performed at the level of the most distal signal of blood flow. The angle of insonation used to obtain B‐mode images was near 90 degrees. The Doppler curve was obtained with an angle of insonation of 60 degrees or less when using angle correction.
Residual stenoses (immediately post‐procedure) and restenoses (within 12 months of procedure) were defined as narrowings of ≥50% on DUS examination.31-34 Greater than 70% restenoses were confirmed using subtraction angiography.
Clinical evaluation
New clinical events were recorded during and after the procedures (20‐month surveillance period), as were deaths and causes of death. Stroke was defined as an acute disturbance of focal neurological function with symptoms lasting longer than 24 hours and TIA was defined as a new neurological event that lasted less than 24 hours. All cases with clear or suspected neurological symptoms were evaluated by an independent neurologist. When a neurological deficit was encountered, computed tomography was used to define the cause of the event. Patients were instructed to inform the physician when any new symptoms occurred after hospital discharge. All patients were followed‐up at 3 and 6 months postoperatively, and at 6‐month intervals thereafter.
Statistical analysis
Continuous values are presented as mean ± standard deviation (SD) and nominal data as count and percentages. For comparisons of clinical outcomes, the Chi‐square test with Yates correction and Fisher's exact test were used. The Kaplan‐Meier method was used to estimate patient survival after the procedures. Short‐term morphologic and hemodynamic parameters of both carotid artery stenting and patch closure were assessed by comparing DUS results obtained immediately postoperatively with preoperative DUS. Similarly, late DUS parameters were assessed by comparing the results from postoperative day 1 with 12 months postoperative. All comparisons between two DUS were done using paired sampled t‐tests. The tests were performed at a significance level of <0.05. All calculations and statistical comparisons were performed using Minitab 12™ (State College, PA, USA) and MedCalc 9.3 (MedCalc Inc, Mariakerke, Belgium).
RESULTS
24‐hour DUS results
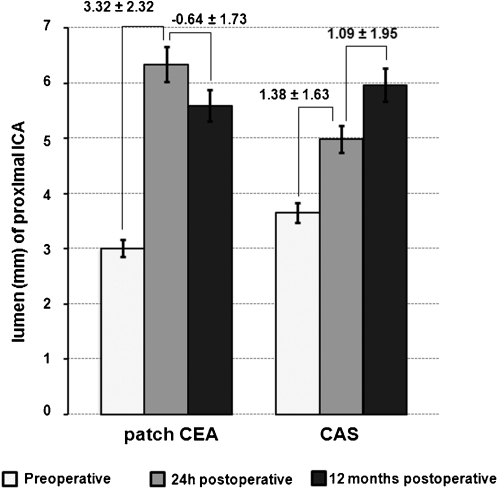
Twenty‐four hours after the procedures, the two groups of this study showed significant increase in luminal diameter of p‐ICA versus baseline (Fig. 1). The mean luminal diameter of p‐ICA increased from 3.65 ± 2.36 mm to 4.98 ± 1.36 mm in the carotid artery stenting group (P = 0.00) and from 3.00 ± 2.09 mm to 6.33 ± 1.17 mm in the patch closure group (P = 0.00).
Figure 1.
Luminal diameter (mm) of p‐ICA, 1 day before and 24 hours and 12 months after carotid endarterectomy (CEA) and carotid artery stenting (CAS). Numbers above the bars indicate the quantitative changes ± standard deviations.
p‐ICA: proximal internal carotid artery
Before stenting, a mean PSV of 244.64 ± 109.22 cm/s and a mean EDV of 97.30 ± 72.10 cm/s were measured in the p‐ICA. After stenting, both PSV and EDV of p‐ICA were significantly decreased to the normal range [(mean PSV = 71.64 ± 24.08 cm/s; P = 0.00) (mean EDV = 22.07 ± 10.68 cm/s; P = 0.00)]. In patients undergoing patch closure, mean PSV and EDV of p‐ICA also normalized 24 hours postoperatively {[PSV = 285.59 ± 123.14 cm/s (pre) vs 68.86 ± 26.66 cm/s (post); P = 0.00] [EDV = 149.31 ± 371.35 cm/s (pre) vs 20.57 ± 16.37 cm/s (post); P = 0.00]}.
Table 2 shows that no significant differences in luminal diameter (mm) and PSV (cm/s) measured in CCA and d‐ICA were found between preoperative and postoperative DUS.
Table 2.
Short‐term DUS results: Comparison of preoperative and 24h postoperative.
| Carotid artery stenting group (n = 29) | Patch closure group (n = 65) | |||||||||||
| 95%CI | 95%CI | |||||||||||
| DUS parameters | MD | SD | t | P‐value | lower | upper | MD | SD | t | P‐value | lower | upper |
| Proximal ICA | ||||||||||||
| luminal diameter (mm) | ‐1.38 | 1.63 | ‐4.32 | 0.00* | ‐1.96 | ‐0.70 | ‐3.32 | 2.32 | ‐10.42 | 0.00* | ‐3.97 | ‐2.68 |
| PSV (cm/s) | 173.00 | 110.17 | 8.30 | 0.00* | 130.27 | 215.72 | 216.75 | 124.38 | 13.61 | 0.00* | 184.89 | 248.60 |
| EDV (cm/s) | 75.23 | 0.05 | 5.46 | 0.00* | 47.61 | 102.84 | 128.74 | 72.23 | 2.74 | 0.00* | 34.99 | 222.48 |
| Distal ICA | ||||||||||||
| luminal diameter (mm) | ‐0.07 | 0.41 | ‐0.93 | 0.35 | ‐0.20 | ‐0.08 | ‐0.16 | 0.76 | ‐1.63 | 0.10 | ‐0.35 | ‐0.03 |
| PSV (cm/s) | 8.14 | 31.23 | 1.37 | 0.17 | ‐3.96 | 20.25 | 5.27 | 7.37 | 1.34 | 0.18 | ‐2.41 | 12.95 |
| EDV (cm/s) | 0.35 | 7.69 | ‐0.24 | 0.80 | ‐3.34 | 2.62 | ‐0.68 | 13.52 | ‐0.38 | 0.69 | ‐4.21 | 2.84 |
| CCA | ||||||||||||
| luminal diameter (mm) | 0.11 | 0.55 | 1.10 | 0.28 | ‐0.09 | 0.32 | ‐0.05 | 1.09 | ‐0.36 | 0.71 | ‐0.32 | 0.22 |
| PSV (cm/s) | 10.96 | 45.98 | 1.28 | 0.21 | ‐6.52 | 28.45 | ‐2.09 | 25.35 | ‐0.65 | 0.51 | ‐8.48 | 4.29 |
| EDV (cm/s) | 11.91 | 32.53 | 2.17 | 0.03† | 0.96 | 22.85 | ‐2.20 | 21.58 | ‐0.81 | 0.41 | ‐7.59 | 3.18 |
CCA, common carotid artery; CI, confidence interval; DUS, duplex ultrasound; EDV, end‐diastolic velocity; ICA, internal carotid artery; MD, mean difference; PSV, peak systolic velocity; SD, standard deviation; t, value of the paired t‐test.
, highly significant;
, significant.
Among carotid artery stenting patients, the mean EDV of CCA reduced from 26.28 ± 28.95 cm/s to 14.37 ± 5.28 cm/s (P = 0.03) after the procedures.
No significant differences in residual stenosis rates were observed between the stenting group (3 cases, 10.34%) and the patch closure group (1 case, 1.53%, P = 0.08). One carotid artery stenting patient presented with 50‐69% residual stenosis in ipsilateral ICA, which had a PSV of 158 cm/s and an EDV of 45 cm/s. In two stenting patients, the luminal diameter of the stented carotid was narrowest at 24 hours (identified on B‐mode imaging), indicating residual stenosis caused by the presence of calcified plaque and the suboptimal stent expansion. In the patch closure group, one patient presented with a distal flap in p‐ICA, which was identified with a PSV of 170 cm/s and an EDV of 65 cm/s; this lesion was repaired with carotid artery stenting 30 hours postoperatively.
12‐month DUS results
The two groups of this study showed significant differences in luminal diameter of p‐ICA when the 24‐hour and 12‐month duplex imaging data were compared (Fig. 1). The p‐ICA lumen significantly increased from 4.98 ± 1.36 mm to 5.95 ± 1.29 mm in the stenting group (P = 0.00). In contrast, in the patch closure group the lumen of p‐ICA reduced from 6.33 ± 1.77 mm to 5.59 ± 1.19 mm (P = 0.00).
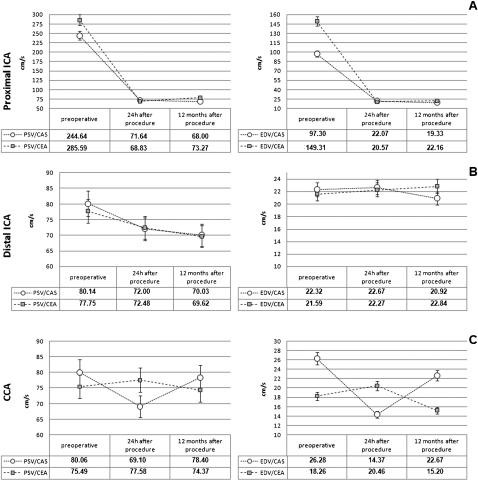
Figure 2 (a‐c) shows the mean velocities (hemodynamic patterns) through the p‐ICA, d‐ICA and CCA over the follow‐up period. Table 3 shows that the mean velocities were not significantly different when 24‐hour and 12‐month studies were compared.
Figure 2.
Mean duplex‐velocities (PSV and EDV, cm/s) through the p‐ICA (a), d‐ICA (b) and CCA (c) over the follow‐up period.
PSV: peak systolic velocity; EDV: end diastolic velocity; p‐ICA: proximal internal carotid artery; d‐ICA: distal internal carotid artery; CCA: common carotid artery; CAS: carotid artery stenting; CEA: carotid endarterectomy.
Table 3.
DUS results: Comparison of postoperative day 1 and 12 months.
| Carotid artery stenting group (n = 29) | Patch closure group (n = 65) | |||||||||||
| 95%CI | 95%CI | |||||||||||
| DUS parameters | MD | SD | t | P‐value | lower | upper | MD | SD | t | P‐value | lower | upper |
| Proximal ICA | ||||||||||||
| luminal diameter (mm) | ‐1.09 | 1.95 | ‐2.85 | 0.00* | ‐1.88 | ‐0.30 | 0.64 | 1.73 | 2.80 | 0.00* | 0.18 | 1.10 |
| PSV (cm/s) | 1.62 | 34.38 | 0.24 | 0.80 | ‐11.97 | 15.23 | ‐9.15 | 60.78 | ‐1.15 | 0.25 | ‐22.99 | 6.68 |
| EDV (cm/s) | 2.14 | 18.21 | 0.61 | 0.54 | ‐5.05 | 9.35 | ‐0.54 | 26.56 | ‐0.15 | 0.81 | ‐7.46 | 6.38 |
| Distal ICA | ||||||||||||
| luminal diameter (mm) | 0.00 | 0.56 | 0.03 | 0.97 | ‐0.21 | 0.22 | 0.10 | 0.69 | 1.16 | 0.24 | ‐0.07 | 0.29 |
| PSV (cm/s) | 0.92 | 30.85 | 0.15 | 0.87 | 11.28 | 13.13 | 1.55 | 29.05 | 0.41 | 0.68 | ‐6.01 | 9.13 |
| EDV (cm/s) | 1.07 | 9.44 | 0.59 | 0.56 | ‐2.66 | 4.80 | 0.93 | 2.11 | 0.47 | 0.63 | ‐2.13 | 3.27 |
| CCA | ||||||||||||
| luminal diameter (mm) | 0.24 | 1.34 | 0.93 | 0.36 | ‐0.29 | 0.77 | 0.07 | 1.49 | 0.39 | 0.69 | ‐0.31 | 0.47 |
| PSV (cm/s) | ‐9.77 | 26.22 | ‐1.93 | 0.06 | ‐20.15 | 0.59 | 3.72 | 30.56 | 0.92 | 0.35 | ‐4.31 | 11.76 |
| EDV (cm/s) | ‐8.22 | 23.41 | ‐1.82 | 0.07 | ‐17.49 | 1.03 | 3.93 | 17.07 | 1.76 | 0.08 | ‐0.51 | 8.38 |
CCA, common carotid artery; CI, confidence interval; DUS, duplex ultrasound; EDV, end‐diastolic velocity; ICA, internal carotid artery; MD, mean difference; PSV, peak systolic velocity; SD, standard deviation; t, value of the paired t‐test.
, highly significant.
During the follow‐up period, restenoses developed in two stenting sites (6.88%). One of these was identified with a PSV of 156 cm/s and an EDV of 46 cm/s; the other representing the progression of a residual lesion was identified with a PSV of 410 cm/s and an EDV of 140 cm/s (the patient died of a stroke before receiving new intervention). Similarly, two patients in the patch closure group (3.07%, P = 0.58) also presented with restenosis during the follow‐up period: one patient developed ≥80% restenosis in p‐ICA (PSV of 328 cm/s and EDV of 120 cm/s) and underwent subsequent reintervention with stenting; the other patient presented with a lesion in the CCA (PSV of 180 cm/s and EDV of 60 cm/s), which was associated with progression of a 40% residual stenosis.
Clinical outcomes
There were no significant differences in ipsilateral stroke rates between the stenting group and the patch closure group (6.88% vs 0%, P = 0.09). However, the number of new TIAs in the stenting group was significantly higher than the number of new TIAs in the patch closure group (10.36% vs 0%, P = 0.02). Specifically, two stenting patients suffered ipsilateral stroke; one of these during the endovascular procedure, the other 12 months postoperatively (the latter was associated with a 90% restenosis). Three stenting patients experienced TIA. In one of 3 cases, TIA appeared during the procedure; the other two cases appeared after a symptom‐free interval of 4‐ and 9 hours, respectively. In all cases with TIA, the 24 hour DUS examination indicated carotid patency, PSV <125 cm/s and EDV <40 cm/s.
In one stenting patient, a pseudoaneurysm of the common femoral artery was discovered during follow‐up and required surgical treatment. In the patch closure group, one patient developed a neck hematoma that did not require evacuation.
At 20 months, 2 stenting patients (6.89%) had myocardial infarction versus 3 patch closure patients (4.61%, P = 0.64).
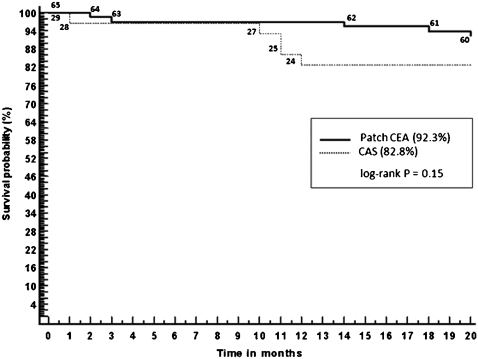
Kaplan‐Meier survival curves show that stenting patients appeared proportionately more likely to die during follow‐up (Fig. 3). Estimated 20‐month survival was 82.2% in the stenting group and 92.3% in the patch closure group; however, this apparent trend was not statistically significant (P = 0.15). Five stenting patients died after the procedure; one of these deaths occurred during the perioperative period (within 30 days) and was not related to the procedure (cause of death was pulmonary infection). The other deaths occurred during the late postoperative period: two because of myocardial infarction (at 10 and 11 months, respectively), one due to lung cancer (11 months), and one because of ipsilateral stroke caused by restenosis (12 months). In the patch closure group there were no perioperative deaths but there were five postoperative deaths: one due to contralateral stroke (3 months), three because of myocardial infarction (2, 14 and 18 months, respectively), and one caused by pneumonia (20 months).
Figure 3.
Kaplan‐Meier survival curves. The survival probability at 20 months was 82.8% among patients who received stent (n = 29) versus 92.3% among those who underwent patch closure (n = 65) (P = 0.15, 95%CI = 0.0949 to 1.4458). Numbers on the graphed lines indicate the number of patients. CAS: carotid artery stenting; CEA: carotid endarterectomy.
DISCUSSION
As the clinical application of carotid artery stenting expands, its safety and durability continues to be evaluated and compared with carotid endarterectomy which is the gold‐standard treatment.1-5 Unfortunately, in major trials comparing carotid artery stenting with endarterectomy, only the technique for the performance of stenting has been defined; the technique for endarterectomy has usually been the surgeon's customary approach. At present, few studies have specifically set out to compare stenting and routine patch closure, although patching is now thought to be part of the optimal care of patients undergoing traditional endarterectomy. Randomized prospective trials and meta‐analyses have reported improved rates of perioperative and mid‐term stroke prevention, as well as reduced rates of restenosis for patches compared with primary arterial closure; and these favorable results have been attributed to arterial widening, with a reduction in the effect of intimal hyperplasia.6-8,35-40 Our group's preference is for biological patches, such as bovine pericardium, because they offer the benefits of ‘off‐the‐shelf’ availability, durability and biocompatibility.6,7,35 Bovine pericardial patches have shown significantly decreased intraoperative suture line bleeding compared with prosthetic patches.41 Also, when compared with outcomes after the use of prosthetic patches, bovine pericardial patches show a lower incidence of restenosis (4% vs 7.6%, P <0.05).42
The use of bovine pericardium for the patch material (as used in this study) permits excellent quality DUS, because both Dacron® and polytetrafluoroethylene materials cause acoustical shadowing and prevent a complete examination.43 Previous studies report that patients with carotid patching with broadened lumen at the bulb presented statistically elevated turbulent flow disturbances with increased flow velocity in the ICA, which may adversely affect the accuracy of DUS scanning.18,30 However, in the present study, no measurable flow disturbances in the absence of a residual stenosis or restenosis were observed. Mean DUS parameters (luminal diameter, PSV and EDV) obtained 24 hours and 12 months after patch closure were indicative of arterial widening and hemodynamics restoration. We believe that the hemodynamic effect from patching is directly related to the size of the dilated patched vessel, and bovine 8 mm wide pericardial patch angioplasty (as used in this study) permits optimization of vessel geometry and blood flow. In agreement with this, Fietsam et al44 demonstrated that only a large patch (≥10 mm wide) relative to native vessel dimensions might create marked flow disturbances throughout the cardiac cycle. Literature reports, preoperative imaging data, manufacturer data and observations from previous experience were considered when standardizing the patch diameter at 8 mm. There were no occurrences of long‐term complications of patches such as infection or pseudoaneurysm formation in our study.
As a consequence of arterial geometrical remodeling, in patched carotids, the luminal diameter of p‐ICA decreased in the interval between the 24‐hour and 12‐month studies. In contrast, in stented patients, DUS revealed that Wallstents had expanded after implantation by exerting steady radial pressure against the arterial wall and that further increase in luminal diameter might occur over time. A recent DUS study29 demonstrated that Wallstents expand over 2 years and that this expansion is most pronounced in soft plaques, less so in fibrous plaques and least pronounced in calcified plaques. The implications of this continuous self‐expansion of the Wallstent on late clinical outcome remain undetermined. In this self‐expansion period, our group has recommended a policy of DUS surveillance and antiplatelet therapy for prevention of thromboembolic events.
Willfort‐Ehringer et al29 reported that arterial remodeling after stenting most commonly produced an increase in PSV from day 1 (75 ± 27 cm/s) to 12 months (101 ± 37 cm/s, P <0.001) post‐procedure, indicating a segmental arterial narrowing secondary to neointimal hyperplasia. However, the results from our study indicate that arterial hemodynamics (mean PSVs) normalized immediately after stenting and remained fairly stable, thereafter, up to 12 months. In this work, stenting was associated with patency and no statistical evidence of neointimal proliferation exceeding stent expansion or yielding increased PSV was observed at 12 months post‐procedure. These data allow us to speculate that alterations in the physical properties of the vessel wall after self‐expanding stent placement have no relevant effect on DUS derived PSV in normal stented carotids.45 However, the real effect of stenting on DUS‐velocity remains to be determined in larger scale clinical comparison of various stent types, as the PSV may be influenced differently according to the device used, as previously described.46
Interestingly, stenting was associated with a significant reduction in EDV of CCA [26.28 ± 28.95 cm/s (pre) vs 14.37 ± 5.28 cm/s (post); P = 0.03] in this study, which could be secondary to alterations in vessel geometry or arterial wall biomechanics after stenting. To the best of our knowledge, no previous clinical study has shown similar results; therefore the real significance of this hemodynamic alteration remains under investigation.
This study did not support a general approach to new velocity criteria indiscriminately applied to patched or stented carotids. Our group has used a native carotid validated DUS algorithm14 to confirm carotid patency and grade residual stenosis and restenosis severity after carotid artery stenting and patch closure. This algorithm has been associated with infrequent neurological events. For example, B‐mode imaging and spectral broadening have routinely been used to supplement our velocity thresholds to determine stenosis severity. Obviously, data obtained by individual vascular laboratories will vary due to differences in equipment, abilities and the consistency of vascular technician, and the interpretation of the results obtained. Each vascular laboratory, therefore, must adopt a method that conforms to the equipment used and validate their method when performing DUS surveillance after stenting or patch closure. In this study, all carotids were examined with a Philips/ATL HDI 5000 scanner (Bothell, WA, USA), operated by a single registered vascular radiologist.
Regarding DUS examination, no significant differences in residual stenosis (immediately post‐procedure) and restenosis (within of 12 months of procedure) were observed between the stenting and patch closure groups (P levels = 0.08 and 0.58, respectively). It should be mentioned that in‐stent stenosis was estimated at 6.88%; however, restenoses in self‐expanding stents are reported nearly exclusively during the first year29 and we expect better results with a longer follow‐up.
In this study, one single stenting patient suffered late stroke, which was associated with a 90% restenosis identified at 12 month DUS examination. Four other neurologic events (1 stroke and 3 TIA) were reported for the stenting group, which occurred before the 24 hour DUS examination; two of these four events (1 stroke and 1 TIA) occurred during the endovascular procedure, despite the use of a cerebral protection filter. In all stenting cases with TIA, the DUS examination indicated carotid patency and hemodynamic restoration. It is possible that these events are affected by the indications for stenting: a factor that should be carefully evaluated by our Service. In the early stages of this study, new lesions were influenced by anatomical findings that now represent relative contraindications for endovascular management, including the angulation of the aortic arch and severe calcification of the target carotid stenosis.
There was no evidence of statistically significant differences in the rate of strokes, myocardial infarctions and deaths between the groups studied. The number of new TIAs in the stenting group was significantly higher than the number of new TIAs in the patch closure group (P = 0.02); however, this study was not large enough to rule out any advantage or disadvantage of one treatment over the other. Only large controlled trials or large cohort studies might to prove an equivalency between stenting and patch closure (CEA) with sufficient scientific evidence.
One limitation of this work is that it was restricted to a nonrandomized single‐center study; however, the advantage is that it was prospective. The number of cases may have been too small to reveal differences in residual stenosis, restenosis and clinical outcomes between stenting and patch closure; however, the sample size was robust enough to demonstrate quantitative alterations in morphological and hemodynamic DUS parameters after stenting and patch closure.
CONCLUSION
In stented patients, the luminal diameter of the p‐ICA increased in the interval between the 24‐hour and 12‐month post‐procedure studies, while in the patch closure patients, the p‐ICA diameter decreased; signaling differences in arterial remodeling mechanisms. Carotid hemodynamics normalized immediately after both patching and stenting and remained relatively stable, thereafter, up to 12 months. As no statistically elevated flow velocities in the absence of residual stenosis or restenosis were observed in this study, our data did not support a general approach to new velocity criteria indiscriminately applied to stented or patched carotids.
ACKNOWLEDGEMENTS
C.E. Piccinato is a researcher from Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico (CNPq Proc. 306452‐2006‐5), Brazil.
There is no conflict of interest that would prejudice the impartiality of this study.
REFERENCES
- 1. Lin PH, Brashes NR, Annambhotla S, Huynh TT. Prospective randomized trials of carotid artery stenting versus carotid endarterectomy: an appraisal of the current literature. Vasc Endovasc Surg. 2008;42:5–11. doi: 10.1177/1538574407312654. 10.1177/1538574407312654 [DOI] [PubMed] [Google Scholar]
- 2. Mansour MA. Carotid artery stenting in the SPACE and EVA‐3S Trials: Analysis and update. Perspect Vasc Surg Endovasc Ther. 2008;20:11–4. doi: 10.1177/1531003507313219. 10.1177/1531003507313219 [DOI] [PubMed] [Google Scholar]
- 3. Rudarankanchana N, Dialynas M, Halliday A. Asymptomatic Carotid Surgery Trial‐2 (ACST): Rationale for a randomized clinical trial comparing carotid endarterectomy with carotid artery stenting in patients with asymptomatic carotid artery stenosis. Eur J Vasc Endovasc Surg. 2009;38:239–42. doi: 10.1016/j.ejvs.2009.05.010. 10.1016/j.ejvs.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 4. Naylor AR, Bolia A, Abbott RJ, Pye JF, Smith J, Lennard N, et al. Randomized study of carotid angioplasty and stenting versus carotid endarterectomy: a stopped trial. J Vasc Surg. 1998;2:326–34. doi: 10.1016/s0741-5214(98)70182-x. 10.1016/S0741‐5214(98)70182‐X [DOI] [PubMed] [Google Scholar]
- 5. Mas JL, Chatellier G, Beysen B, Branchereau A, Moulin T, Becquemin JP, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;16:1660–71. doi: 10.1056/NEJMoa061752. 10.1056/NEJMoa061752 [DOI] [PubMed] [Google Scholar]
- 6. Matsagas MI, Bali C, Arnaoutoglou E, Papakostas JC, Nassis C, Papadoupoulus G, et al. Carotid endarterectomy with bovine pericardium patch angioplasty: mid‐term results. Ann Vasc Surg. 2006;20:614–19. doi: 10.1007/s10016-006-9102-3. 10.1007/S10016‐006‐9102‐3 [DOI] [PubMed] [Google Scholar]
- 7. Kim GE, Kwon TW, Cho YP, Kim DK, Kim HS. Carotid endarterectomy with bovine patch angioplasty: a preliminary report. Cardiovasc Surg. 2001;9:458–62. doi: 10.1016/s0967-2109(01)00042-4. 10.1016/S0967‐2109(01)00042‐4 [DOI] [PubMed] [Google Scholar]
- 8. Bond R, Rerkasem K, Naylor AR, Aburahma AF, Rothwell PM. Systematic review of randomized controlled trials of patch angioplasty versus primary closure and different types of patch materials during carotid endarterectomy. J Vasc Surg. 2004;40:1126–35. doi: 10.1016/j.jvs.2004.08.048. 10.1016/j.jvs.2004.08.048 [DOI] [PubMed] [Google Scholar]
- 9. Liapis CD, Bell SPRF, Mikhiailidis D, Sivenius J, Nicolaides A, Fernandes e Fernandes J, et al. ESVS Guidelines. Invasive Treatment for Carotid Stenosis: Indications, Techniques. Eur J Vasc Endovasc Surg. 2009;37:S1–S19. doi: 10.1016/j.ejvs.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 10. Moneta GL, Edwards JM, Chitwood RW, Taylor JM, Jr, Lee RW, Cummings CA, et al. Correlation of North American Symptomatic Carotid Endarterectomy Trial (NASCET) angiographic definition of 70% to 99% internal carotid artery stenosis with duplex scanning. J Vasc Surg. 1993;17:152–9. doi: 10.1067/mva.1993.42888. 10.1067/mva.1993.42888 [DOI] [PubMed] [Google Scholar]
- 11. Faught WE, Mattos MA, van Bemmelen PS, Hordgson KJ, Barkemeuer LD, Ramsey DE, et al. Color‐flow duplex scanning of carotid arteries: new velocity criteria based on receiver operator characteristic analysis for threshold stenoses used in the symptomatic and asymptomatic carotid trials. J Vasc Surg. 1994;19:818–28. doi: 10.1016/s0741-5214(94)70006-0. [DOI] [PubMed] [Google Scholar]
- 12. Moneta GL, Edwards JM, Papanicolaou G, Hatsukami T, Taylos JM, Jr, Strandness DE, Jr, et al. Screening for asymptomatic internal carotid artery stenosis: duplex criteria for discriminating 60% to 99% stenosis. J Vasc Surg. 1995;21:989–94. doi: 10.1016/s0741-5214(95)70228-8. 10.1016/S0741‐5214(95)70228‐8 [DOI] [PubMed] [Google Scholar]
- 13. Carpenter JP, Lexa FJ, Davis JT. Determination of sixty percent or greater carotid artery stenosis by duplex Doppler ultrasonography. J Vasc Surg. 1995;22:697–705. doi: 10.1016/s0741-5214(95)70060-9. 10.1016/S0741‐5214(95)70060‐9 [DOI] [PubMed] [Google Scholar]
- 14. Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: gray‐scale and Doppler US diagnosis – society of radiologists in ultrasound consensus conference. Radiology. 2003;229:340–6. doi: 10.1148/radiol.2292030516. 10.1148/radiol.2292030516 [DOI] [PubMed] [Google Scholar]
- 15. McCabe DH, Pereira AC, Clifton A, Bland JM, Brown MM. FRCP on behalf of the CAVATAS investigation. Restenosis after carotid angioplasty, stenting, or endarterectomy in the carotid and vertebral artery transluminal angioplasty study (CAVATAS) Stroke. 2005;36:281–86. doi: 10.1161/01.STR.0000152333.75932.fe. 10.1161/01.STR.0000152333.75932.fe [DOI] [PubMed] [Google Scholar]
- 16. Cao P, De Rango P, Verzini F, Maselli A, Morgiolini L, Giordano G. Outcome of carotid stenting versus endarterectomy: A case control‐study. Stroke. 2006;37:1221–29. doi: 10.1161/01.STR.0000217435.21051.60. 10.1161/01.STR.0000217435.21051.60 [DOI] [PubMed] [Google Scholar]
- 17. De Borst GJ, Meijer R, Lo RH, Ackerstaff RG, Moll FL. Effect of Carotid Angioplasty and Stenting on Duplex Velocity Measurements in a Porcine Model. J Endovasc Ther. 2008;15:672–79. doi: 10.1583/08-2500.1. 10.1583/08‐2500.1 [DOI] [PubMed] [Google Scholar]
- 18. AbuRahma AF, Stone P, Deem S, Deam LS, Keiffer T, Deem E. Proposed duplex velocity criteria for carotid restenosis following carotid endarterectomy with patch closure. J Vasc Surg. 2009;50:286–91. doi: 10.1016/j.jvs.2009.01.065. 10.1016/j.jvs.2009.01.065 [DOI] [PubMed] [Google Scholar]
- 19. AbuRahma AF, Abu‐Halimah S, Bensenhaver J, Dean LS, Keiffer T, Emmet M, et al. Optimal carotid duplex velocity criteria for defining the severity of carotid in‐stent restenosis. J Vasc Surg. 2008;48:589–94. doi: 10.1016/j.jvs.2008.04.004. 10.1016/j.jvs.2008.04.004 [DOI] [PubMed] [Google Scholar]
- 20. Kuntz KM, Polak JF, Whittemore AD, Skillman JJ, Kent KC. Duplex ultrasound criteria for the identification of carotid stenosis should be laboratory specific. Stroke. 1997;28:597–602. doi: 10.1161/01.str.28.3.597. [DOI] [PubMed] [Google Scholar]
- 21. Ringer AJ, German JW, Guterman LR, Hopkins LN. Follow‐up of stented carotid arteries by Doppler ultrasound. Neurosurgery. 2002;51:639–43. 10.1097/00006123‐200209000‐00007 [PubMed] [Google Scholar]
- 22. Lal BK, Hobson RW, Goldstein J, Chakhtoura EY, Durán WN. Carotid artery stenting: is there a need to revise ultrasound velocity criteria? J Vasc Surg. 2004;39:58–66. doi: 10.1016/j.jvs.2003.10.043. 10.1016/j.jvs.2003.10.043 [DOI] [PubMed] [Google Scholar]
- 23. Peterson BG, Longo GM, Kibbe MR, Matsumura JS, Blackburn D, Astleford P, et al. Duplex ultrasound remains a reliable test even after carotid stenting. Ann Vasc Surg. 2005;19:793–97. doi: 10.1007/s10016-005-7976-0. 10.1007/s10016‐005‐7976‐0 [DOI] [PubMed] [Google Scholar]
- 24. Stanziale SF, Wholey MH, Boules TN, Selzer F, Makaroun MS. Determining in‐stent stenosis of carotid arteries by duplex ultrasound criteria. J Endovasc Ther. 2005;12:346–53. doi: 10.1583/04-1527.1. 10.1583/04‐1527.1 [DOI] [PubMed] [Google Scholar]
- 25. Chi YW, White CJ, Woods TC, Goldman CK. Ultrasound velocity criteria for carotid in‐stent restenosis. Catheter Cardiovasc Interv. 2007;69:349–54. doi: 10.1002/ccd.21032. 10.1002/ccd.21032 [DOI] [PubMed] [Google Scholar]
- 26. Chahwan S, Miller MT, Pigott JP, Whalen RC, Jones L, Comerota AJ. Carotid artery velocity characteristics after carotid artery angioplasty and stenting. J Vasc Surg. 2007;45:523–26. doi: 10.1016/j.jvs.2006.11.044. 10.1016/j.jvs.2006.11.044 [DOI] [PubMed] [Google Scholar]
- 27. Lal BK, Hobson RW, 2nd, Tofighi B, Kapadia I, Cuadra S, Jamil Z. Duplex ultrasound velocity criteria for the stented carotid artery. J Vasc Surg. 2008;47:63–73. doi: 10.1016/j.jvs.2007.09.038. 10.1016/j.jvs.2007.09.038 [DOI] [PubMed] [Google Scholar]
- 28. Zhou W, Felkai DD, Evans M. Ultrasound criteria for severe in‐stent restenosis following carotid artery stenting. J Vasc Surg. 2008;47:74–80. doi: 10.1016/j.jvs.2007.09.031. 10.1016/j.jvs.2007.09.031 [DOI] [PubMed] [Google Scholar]
- 29. Willfort‐Ehringer A, Ahmadi R, Gruber D, Gschwandtner ME, Haumer A, Haumer M, et al. Arterial remodeling and hemodynamics in carotid stents: A prospective duplex ultrasound study over 2 years. J Vasc Surg. 2004;39:728–34. doi: 10.1016/j.jvs.2003.12.029. 10.1016/j.jvs.2003.12.029 [DOI] [PubMed] [Google Scholar]
- 30. Hirschl M, Bernt RA, Hirschl MM. Carotid endarterectomy of the internal carotid artery with and without patch angioplasty: comparison of hemodynamical and morphological parameters. Int Angiol. 1989;8:10–15. [PubMed] [Google Scholar]
- 31. Kastrati A, Mehilli J, Dirschinger J, Pache J, Uln K, Schühlen H, et al. Restenosis after coronary placement of various stent types. Am J Cardiol. 2001;87:34–9. doi: 10.1016/s0002-9149(00)01268-6. 10.1016/S0002‐9149(00)01268‐6 [DOI] [PubMed] [Google Scholar]
- 32. Gray WA, Hopkins LN, Yadav S, Davis T, Wholwy M, Atkinson R, et al. Protected carotid stenting in high‐surgical‐risk patients: The archer results. J Vasc Surg. 2006;44:258–68. doi: 10.1016/j.jvs.2006.03.044. 10.1016/j.jvs.2006.03.044 [DOI] [PubMed] [Google Scholar]
- 33. Gray WA, Yadav JS, Verta P, Sacli A, Faorman R, Wholey M, et al. The capture registry: results of carotid stenting with embolic protection in the post approval setting. Catheter Cardiovasc Interv. 2007;69:341–48. doi: 10.1002/ccd.21050. 10.1002/ccd.21050 [DOI] [PubMed] [Google Scholar]
- 34. Sadek M, Cayne NS, Shen HJ, Turnbull IC, Marin ML, Faries PL. Safety and efficacy of carotid angioplasty and stenting for radiation associated carotid artery stenosis: Analysis of restenosis and embolic potential. J Vasc Surg. 2009;50:1308–13. doi: 10.1016/j.jvs.2009.07.015. 10.1016/j.jvs.2009.07.015 [DOI] [PubMed] [Google Scholar]
- 35. Muto A, Nishibe T, Dardik H, Dardik A. Patches for artery endarterectomy: Current materials and prospects. J Vasc Surg. 2009;50:206–213. doi: 10.1016/j.jvs.2009.01.062. 10.1016/j.jvs.2009.01.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bond R, Rerkasem K, Naylor AR, Aburahma AF, Rothwell PM. Systematic review of randomized controlled trials of patch angioplasty versus primary closure and different types of patch materials during carotid endarterectomy. J Vasc Surg. 2004;40:1126–35. doi: 10.1016/j.jvs.2004.08.048. 10.1016/j.jvs.2004.08.048 [DOI] [PubMed] [Google Scholar]
- 37. Dirrenberger RA, Sundt TM., Jr Carotid endarterectomy. Temporal profile of the healing process and effects of anticoagulation therapy. J Neurosurg. 1978;48:201–19. doi: 10.3171/jns.1978.48.2.0201. 10.3171/jns.1978.48.2.0201 [DOI] [PubMed] [Google Scholar]
- 38. Byrne J, Feustel P, Darling RC., 3rd Primary closure, routine patching and eversion endarterectomy: what is the current state of the literature supporting use of these techniques? Semin Vasc Surg. 2007;20:226–35. doi: 10.1053/j.semvascsurg.2007.10.006. 10.1053/j.semvascsurg.2007.10.006 [DOI] [PubMed] [Google Scholar]
- 39. Kresowik TF, Bratzler D, Karp HR, Hemann RA, Hendel ME, Grund SL, et al. Multistate utilization, processes, and outcomes of carotid endarterectomy. J Vasc Surg. 2001;33:227–34. doi: 10.1067/mva.2001.111881. 10.1067/mva.2001.111881 [DOI] [PubMed] [Google Scholar]
- 40. Teso D, Frattini JC, Dardik A. Improved outcomes of carotid endarterectomy: the critical role of vascular surgeons. Semin Vasc Surg. 2004;17:214–8. doi: 10.1016/s0895-7967(04)00051-1. 10.1016/S0895‐7967(04)00051‐1 [DOI] [PubMed] [Google Scholar]
- 41. Marien BJ, Raffetto JD, Seidman CS, LaMorte WW, Menzoian JO. Bovine pericardium vs Dacron for patch angioplasty after carotid endarterectomy: a prospective randomized study. Arch Surg. 2002;137:785–8. doi: 10.1001/archsurg.137.7.785. 10.1001/archsurg.137.7.785 [DOI] [PubMed] [Google Scholar]
- 42. Neuhauser B, Oldenburg WA. Polyester vs bovine pericardial patching during carotid endarterectomy: early neurologic events and incidence of restenosis. Cardiovasc Surg. 2003;11:465–70. doi: 10.1016/S0967-2109(03)00109-1. 10.1016/S0967‐2109(03)00109‐1 [DOI] [PubMed] [Google Scholar]
- 43. Flanighan DP, Flanighan ME, Dorne AL, et al. Long‐term results of 442 consecutive standardized carotid endarterectomy procedures in standard risk and high‐risk patients. J Vasc Surg. 2007;46:876–882. doi: 10.1016/j.jvs.2007.06.045. 10.1016/j.jvs.2007.06.045 [DOI] [PubMed] [Google Scholar]
- 44. Fietsam R, Ranval T, Cohn S, Brown OW, Bendick P, Glover JL. Hemodynamic effects of primary closure versus patch angioplasty of the carotid artery. Ann Vasc Surg. 1992;6:443–49. doi: 10.1007/BF02007000. 10.1007/BF02007000 [DOI] [PubMed] [Google Scholar]
- 45. Vernhet H, Jean B, Laroche JP. Wall mechanisms of the stented extracranial carotid artery. Stroke. 2003;34:c222–c224. doi: 10.1161/01.STR.0000092490.21761.7C. 10.1161/01.STR.0000092490.21761.7C [DOI] [PubMed] [Google Scholar]
- 46. Spies C, Doshie R, Spoon J, Snell J. Carotid artery type influences duplex ultrasound derived peak systolic velocity: Findings of an in‐vitro model. Peripheral Vascular Disease. 2007;70:309–15. doi: 10.1002/ccd.21224. [DOI] [PubMed] [Google Scholar]