Abstract
OBJECTIVES:
To evaluate the effects of Urtica dioica on hepatic ischemia‐reperfusion injury.
METHODS:
Thirty adult male Wistar albino rats were divided into three groups: sham group (group 1), control group (group 2), and Urtica dioica group (group 3). All the rats were exposed to hepatic ischemia for 60 min, followed by 60 min of reperfusion. In group 2, a total of 2 ml/kg 0.9% saline solution was given intraperitoneally. In group 3, a total of 2 ml/kg Urtica dioica was given intraperitoneally. At the end of the procedure, liver tissue and blood samples were taken from all rats. Serum aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, ceruloplasmin, catalase, paraoxonase, arylesterase, and lipid hydroperoxide levels were measured. Liver tissue histopathologies were also evaluated by light microscopy.
RESULTS:
Serum aspartate aminotransferase, alanine aminotransferase and lactate dehydrogenase levels were significantly higher in group 2 than in group 1, and significantly lower in group 3 than in group 2. Also, group 2 had higher serum lipid hydroperoxides and ceruloplasmin levels but lower catalase, paraoxonase, and arylesterase levels than group 1.
In group 3, serum lipid hydroperoxides and ceruloplasmin levels were significantly lower, and catalase, paraoxonase, and arylesterase levels were higher than those in group 2. Histopathological examination showed that liver tissue damage was significantly decreased in group 3 compared with group 2.
CONCLUSIONS:
Urtica dioica has a protective effect on the liver in hepatic ischemia‐reperfusion‐injured rats.
Keywords: Liver, Surgery, Ischemia, Reperfusion injury, Urtica dioica
INTRODUCTION
Ischemia‐reperfusion (IR) injury is cellular damage that causes the release of toxic metabolites and several inflammatory substances into the systemic circulation.1,2 When the ischemic tissue is reperfused, there may be more severe damage than the ischemic injury itself. An acute inflammatory response is the basis of the physiopathology of IR injury. Complex mechanisms such as emerging free oxygen radicals and leukocyte aggregation cause cellular death, organ dysfunction, and, finally, organ failure.3,4
Many studies of plants and drugs illustrate their protective antioxidant and anti‐inflammatory effects on IR injury. One such plant is Urtica dioica (UD)–a common green plant that grows all over the world. From long ago to the present day, it has been used in many different applications, such as alternative medicine, food, paint, fiber, manure and cosmetics.5 In addition, studies have shown that UD has antimicrobial, anti‐inflammatory, analgesic, and antioxidant effects.6-8
This study aimed to examine the biochemical and histopathological effects of UD on hepatic IR injury in rats.
METHODS
UD Ethereal Oil Isolation
UD seeds were collected by a botanist, converted to granules in an electrical grinder, and added to diethyl ether. After half an hour's extraction, the mixture was filtered. The filtrate was separated from the ether with a rotary evaporator under vacuum, and the etheric oil extract was collected. The extract was centrifuged at 4,000 rpm for 15 minutes, then the grounds at the bottom of the tube were removed, and the remaining clear fluid was used in this study.6,9,10
Animal Experiments
This study was approved by the local ethics board in Duzce University Faculty of Medicine, Experimental Animals Laboratory, in 2009. (DU Ethics Committee Number: 2009‐18). For the study, 30 adult male Wistar albino rats from the same colony, ranging in weight from 200 to 230 g were used. The rats were cared for in accordance with the Guide for the Care and Use of Laboratory Animals. Rats were given standard rat food and pipe water, with a 12 h light/12 h dark rhythm, in a room at a temperature of 22±2°C.
Rats were divided at random into three groups containing 10 rats each: a sham group (group 1), control group (group 2), and Urtica dioica group (group 3). All rats were anesthetized by the administration of ketamine hydrochloride (Ketalar; Parke Davis, Eczacibasi, Istanbul) 50 mg/kg intramuscularly and xylazine hydrochloride (Rompun; Bayer AG, Leverkusen, Germany) 3 mg/kg/ intramuscularly. During the operations, additional doses were administered if necessary.
The operation was performed with the rats in a supine position; the abdominal front wall was shaved and disinfected using povidone‐iodine 10%. Laparotomy was carried out on each rat with midline incision. Group 1 was given only laparotomy; the other groups were given hepatic ischemia and reperfusion. The hepatic artery, portal vein, and common bile duct were clamped with microvascular bulldog clamps in order to achieve ischemia. After 60 min ischemia, the clamps were opened, and reperfusion was allowed for 60 min. In group 2, a total of 2 ml/kg 0.9% saline solution (1 ml/kg before ischemia and 1 ml/kg after reperfusion) was given intraperitoneally. In group 3, a total of 2 ml/kg UD (1 ml/kg before ischemia and 1 ml/kg after reperfusion) was given intraperitoneally. At the end of the procedure, liver tissue samples and blood samples were collected from all the rats. Then, the rats were sacrificed by intraperitoneal administration of lethal ketamine hydrochloride.
Liver tissue samples were divided into two. One part was used for histopathological evaluation, and the other for the detection of catalase activity. The tissues, for catalase analysis, were homogenized in a homogenizer (Omni TH International, USA). After centrifugation at 10,000 g for about 60 min, the upper clear layer was taken. The homogenate was divided into aliquots. The homogenate aliquots and serum samples were stored for 3 months at −80°C until biochemically analyzed.
Biochemical Analysis
Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), and ceruloplasmin levels were measured in serum samples of rats using the commercial kit, Abbott Architect C–8000 Autoanalyzer (Abbott Laboratories, USA). The determination of ceruloplasmin is based on the specific immunoturbidimetric reaction which occurs between the anticeruloplasmin polyclonal antiserum and its corresponding antigen under optimal pH conditions and in the presence of polyethylene glycol. The turbidity of the immunocomplex is proportional to the concentration of the analyte in the sample.
Catalase activity in liver tissue was measured in the supernatant at 20°C according to the method of Aebi.11 A molar extinction coefficient of 43.6 M‐1 cm‐1 was used, and the rate of the first 30 s was used to calculate the activity. Catalase activity was expressed as U/mg protein. Protein was identified by the Bradford method.12
Serum paraoxonase (PON1) activity was determined by spectrophotometric measurement of the colored product 4‐nitrophenol, which was formed by enzymatic hydrolysis of paraoxonase used as substrate (O,O‐diethyl‐O‐p‐nitrophenyl phosphate; Sigma Co, London, UK). Arylesterase (ARE) activity was determined by spectrophotometric measurement of the colored phenol product formed by enzymatic hydrolysis of phenylacetate (Sigma).13 One unit (U) was defined as 1 nmol 4‐nitrophenol/L serum/min for PON1 activity and 1 µmol phenol/ml serum/min for ARE activity.
The tri‐iodide complex, which was formed by reaction between lipid hydroperoxides (LOOH) and iodide was spectrophotometrically evaluated at a wavelength of 365 nm. The tri‐iodide extinction coefficient (ε = 2.46 × 104 M‐1 cm‐1) was used to calculate the results. We used butylated hydroxytoluene (BHT) as an antioxidant according to Gorog et al.14
Spectrometric measurements were carried out with a Stat Fax spectrophotometer (Awareness Technology, USA).
Histopathological Evaluation
For histopathological examination, the liver tissues were dissected and the tissue samples fixed in Zenker solution for 24 h, processed using a graded ethanol series, and embedded in paraffin. The paraffin sections were cut into 4 µm thick slices and stained with hematoxylin and eosin for light microscopic examination. The sections were viewed and photographed using an Olympus light microscope (Olympus BX51, Tokyo, Japan) with an attached photographic machine (Olympus E–330, Olympus Optical Co. Ltd, Japan). Ten slides were prepared from each liver. All sections were evaluated for the degree of portal inflammation, necrosis, vacuolar degeneration, sinusoidal dilatation, and vascular congestion. Each liver slide was examined, and the severity of the changes observed was scored using a scale of none [0], mild [1], moderate [2], and severe [3] damage. Histopathological evaluation was made by an experienced pathologist.15
Statistical Analysis
Complete measured information was assessed using SPSS 11.5 (Statistical Package for Social Science). Data were given as mean±standard deviation. One way analysis of variance (post hoc Bonferroni test) was used in multiple group analysis, a t‐test was used to compare two independent groups, χ2 (Fisher's exact) test was used for categorical data analysis, and p<0.05 was accepted as statistically significant.
RESULTS
Biochemical Findings
Biochemical findings of the study are shown in Table 1. Serum AST, ALT, and LDH levels were significantly higher in group 2 than in group 1 (p<0.0001), and significantly lower in group 3 than in group 2 (p<0.0001).
Table 1.
Biochemical markers in groups.
| Groups | |||
| Parameters | Group 1 (sham) n = 10 | Group 2 (control) n = 10 | Group 3 (UD) n = 10 |
| LOOH (µmol/mL)* | 23.0±4.5 | 29.9±3.2 | 27.0±4.3 |
| Ceruloplasmin (mg/dL)† | 16.7±2.2 | 20.3±4.4 | 16.0±3.9 |
| Arylesterase (U/L)‡ | 168.3±19.4 | 87.5±47.5 | 133.7±45.6 |
| Paraoxonase (U/L)§ | 38.8±15.2 | 20.5±17.1 | 31.9±21.1 |
| Liver catalase (U/mg protein)¶ | 54.5±12.0 | 43.3±9.6 | 54.0±7.7 |
| AST (U/L)** | 125.4±19.9 | 907.2±205.1 | 444.2±78.8 |
| ALT (U/L)** | 79.8±12.9 | 636.8±139.7 | 246.5±87.5 |
| LDH (U/L)** | 481.8±78.5 | 4098.9±496.3 | 1760.7±978.3 |
Group 1 vs. group 2, p = 0.001; however, group 1 vs. group 3 and group 2 vs. group 3, NS.
Group 1 vs. group 2, p = 0.02, and group 2 vs. group 3, p = 0.04; however, group 1 vs. group 3, NS.
Group 1 vs. group 2, p<0.0001 and group 2 vs. group 3, p = 0.01; however, group 1 vs. group 3, NS.
Group 1 vs. group 2, p = 0.02 and group 2 vs. group 3, p = 0.03; however, group 1 vs. group 3, NS.
Group 1 vs. group 2, p = 0.02 and group 2 vs. group 3, p = 0.01; however, group 1 vs. group 3, NS.
Group 1 vs. group 2, group 1 vs. group 3, and group 2 vs. group 3, all p<0.0001.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; JDH, lactate dehydrogenase; LOOH, lipid hydroperoxides; UD, Urtica dioica.
Serum LOOH and ceruloplasmin levels in group 2 were significantly higher than in group 1 (respectively, p = 0.001 and p = 0.02). However, serum arylesterase, paraoxonase, and liver tissue catalase in group 2 activity were also significantly lower than in group 1 (p<0.0001, p = 0.02, and p = 0.02, respectively).
Serum LOOH and ceruloplasmin levels in group 3 were lower than in group 2; however, only ceruloplasmin was statistically significant (p = 0.04). A statistically significant increase was found in serum arylesterase, paraoxonase, and liver tissue catalase activity in group 3 in comparison with group 2 (p = 0.01, p = 0.03, and p = 0.01, respectively).
Histopathological Findings
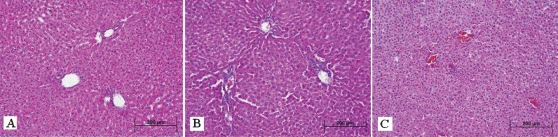
Histopathological findings of the study are shown in Table 2. This evaluation showed that there were no pathological changes in liver tissues of group 1. There was no necrosis, congestion, or inflammation findings (HE ×200) (Figure 1A). Hepatocyte necrosis and congestion of the sinusoids and central vein were evident in group 2 (HE ×200) (Figure 1B). There was minimal central vein congestion in hepatic lobules in group 3. Hepatocytes of group 3 appeared the same as those of group 1 (HE ×200) (Figure 1C). In addition, tissue damage in group 3 was lower than that in group 2.
Table 2.
Median histological injury scores* of liver tissue.
| Groups | |||
| Parameters | Group 1 (sham) n = 10 | Group 2 (control) n = 10 | Group 3 (UD) n = 10 |
| Portal inflammation | 0 | 2 | 0 |
| Necrosis | 0 | 3 | 0 |
| Vacuolar degeneration | 0 | 2 | 0 |
| Sinusoidal dilatation | 0 | 1 | 1 |
| Vascular congestion | 0 | 2 | 1 |
Scale: none [0], mild [1], moderate [2], and severe [3] damage.
UD, Urtica dioica.
Figure 1.
(A) In group 1, normal liver tissue was observed on histopathological examination (hematoxylin–eosin, original magnification ×200); (B) in group 2, hepatocyte necrosis and congestion of the sinusoids and central vein on liver tissue were seen (hematoxylin–eosin, original magnification ×200); (C) in group 3, minimal central vein congestion in hepatic lobules and significantly less tissue damage on hepatocytes were seen (hematoxylin–eosin, original magnification ×200).
DISCUSSION
This study underlines three points: (1) the emergence of free oxygen radicals that arise after hepatic IR and cause injury can be prevented by UD; (2) the increase in AST, ALT, and LDH—an indication of tissue damage in hepatic IR injury—is less after UD treatment; (3) in the early stages of hepatic IR injury, histopathological examination of liver tissue shows considerably less hepatocyte injury with UD treatment.
Free oxygen radicals have a marked mediator role in IR injuries of several organs, including the liver.16-18 Some research suggests that antioxidant molecules may provide protection from IR injury.16,19-21 UD is known to be a strong antioxidant, breaking up free radicals. For this reason, it is expected to be protective in hepatic IR injury of rats.6-8
Studies of UD have used different doses and methods of application. No standard dose has been agreed. UD is given internally in most of the studies.9,22 We used a total dose of 2 ml/kg administered intraperitoneally.6,9,10 To decrease IR injury, some studies have given the drug only before ischemia or after reperfusion, but others have given the drug for both ischemia and reperfusion.6,16,23 In our study we preferred to use 2 ml/kg UD (1 ml/kg before ischemia and 1 ml/kg after reperfusion). Our aim was to benefit from a prophylactic effect by attempting to reach a definite plasma level of UD before creating ischemia and to detect the protective effect on immediate damage that will occur after reperfusion.
Many studies on the effects of UD on the liver have been reported but most describe liver toxicity achieved by toxic substances.9,24 In this study, the protective effect of UD was examined after IR injury had occurred.
In IR injury of the liver during the reperfusion phase, emerging reactive oxygen radicals activate some mediators and can cause inflammatory response and tissue damage. For this reason AST, ALT, and LDH activities may increase.16,25 The increase of AST, ALT, and LDH activities in group 2 of our study supports this finding. In this study, it is shown that in group 3, ALT, AST, and LDH enzymes, markers of liver parenchymal injury, have decreased levels in comparison with group 2 (p<0.0001). This finding supports the protective effect of UD treatment on IR injury.
Oxidative stress occurs particularly in reperfusion after ischemia. Synthesis of proinflammatory cytokines and cell adhesion molecules is activated, and the inflammatory response is increased by oxidative stress. The antioxidant system has an important role in protection from the damage of oxidative stress.16,21,23,26 In our study, by measuring the liver tissue catalase, paraoxonase, arylesterase, ceruloplasmin, and lipid hydroperoxide levels, we obtained information about hepatic IR injury. Catalase is an antioxidant enzyme that catalyses the change from hydrogen peroxide into water. Paraoxonase and arylesterase are similar gene‐coded enzymes that have similar active centers. Paraoxonase enzyme shows antioxidant activity by protecting LDL from oxidation and neutralizing free radicals, including hydrogen peroxide.13,27,28 In this study, UD increased the activity of paraoxonase, arylesterase, and liver tissue catalase activity, supporting the protective effect of UD.
Ceruloplasmin, has a molecular mass of 125 kDa, and its antioxidant effect may depend on ferroxidase activity, ascorbate oxidase activity, O2 scavenging activity, and glutathione‐dependent peroxidase activity. Oxygen is directly reduced to water in a redox reaction and may be the mechanism by which ceruloplasmin inhibits superoxide‐induced lipid peroxidation. Therefore, elevated serum ceruloplasmin levels could signal abnormally high oxidant stress.29 In our study, the ceruloplasmin level was significantly elevated in group 2. These findings are evidence that effective hepatic IR injury and oxidative stress was created in group 2. In group 3, ceruloplasmin levels fell until they reached almost the same level as those of the control group, suggesting that treatment with UD reduced oxidative stress resulting in a decrease in ceruloplasmin levels.
Lipid hydroperoxide is an intermediate free radical oxidant that is synthesized during lipid peroxidation.30,31 In our study, lipid hydroperoxide levels were found to be significantly higher in group 2 than group 1. Also, it was found that treatment with UD in group 3 decreased the lipid hydroperoxide activity, indicating that the antioxidant effect of UD had prevented the emergence of an oxidant agent such as LOOH with creation of hepatic IR.
A histopathological examination detected no pathological changes in group 1. Particularly severe necrosis, moderate inflammation, vacuolar degeneration, and vascular congestion were seen in group 2. Examination of group 3 showed that the pathological changes existing in group 2 had almost completely disappeared. These results indicate that UD has a protective effect on hepatic damage created with IR.
This study had some limitations. Because of the study protocol, hepatic IR‐injured rats were sacrificed just after reperfusion in order to observe the early effects of UD. If we could have kept the rats alive, we could also have observed the long‐term effects of UD in hepatic IR injury. We expect that further studies on the long‐term effects of UD will increase the value of our positive findings. In addition, it is important to examine the possible ability of UD to reverse IR‐induced damage in liver tissue and its effects on levels of antioxidant enzymes.
In conclusion, it was found that UD increased the antioxidant ability and decreased oxygen free radicals in the early period of hepatic IR injury in rats. Also, evaluation of liver enzymes and histopathological findings of liver tissue indicated that UD had beneficial effects on the liver, so UD can be considered a preventive treatment agent in hepatic IR injury. As this study does not contain information about the long‐term results of UD treatment of hepatic IR injury, further experimental and clinical studies are needed. These views should be re‐examined in light of the results obtained from clinical phase 1 and phase 2 studies in which UD is administered to humans.
REFERENCES
- 1. Carden DL, Granger DN. Pathophysiology of ischemia reperfusion injury. J Pathol. 2000;190:255–66. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. 10.1002/(SICI)1096‐9896(200002)190:3<255::AID‐PATH526>3.0.CO;2‐6 [DOI] [PubMed] [Google Scholar]
- 2. Baykara B, Tekmen I. Ischemıa reperfusion injury in liver. Deutsch Med Faculty J. 2005;19:185–94. [Google Scholar]
- 3. Kaplowitz N. Mechanisms of liver injury. J Hepatol. 2000;32:39–47. doi: 10.1016/s0168-8278(00)80414-6. 10.1016/S0168‐8278(00)80414‐6 [DOI] [PubMed] [Google Scholar]
- 4. Jaeschke H. Molecular mechanisms of hepatic ischemia‐reperfusion injury and preconditioning. Am J Physiol‐Gastrointest Liver Physiol. 2003;284:15–26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 5. Ak A, Caliskan O, Cırak C. Economical importance of stinging nettle (Urtica spp.) and its cultivation. J Fac Agric OMU. 2006;21:357–63. [Google Scholar]
- 6. Terzi A, Yildiz F, Çoban S, Taşkin A, Bitiren M, Aksoy N. Protective effect of Urtica dioica on liver injury induced by hepatic ischemia reperfusion injury in rats. Düzce Med J. 2010;12:43–7. [Google Scholar]
- 7. Gülçin I, Küfrevioglu OI, Oktay M, Büyükokuroglu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.) J Ethnopharmacol. 2004;90:205–15. doi: 10.1016/j.jep.2003.09.028. 10.1016/j.jep.2003.09.028 [DOI] [PubMed] [Google Scholar]
- 8. Mittman P. Randomized, double‐blind study of freeze‐dried Urtica dioica in the treatment of allergic rhinitis. Planta Med. 1990;56:44–47. doi: 10.1055/s-2006-960881. 10.1055/s‐2006‐960881 [DOI] [PubMed] [Google Scholar]
- 9. Turkdogan MK, Ozbek H, Yener Z, Tuncer I, Uygan I, Ceylan E. The role of Urtica dioica and Nigella sativa in the prevention of carbon tetrachloride induced hepatotoxicity in rats. Phytother Res. 2003;17:942–6. doi: 10.1002/ptr.1266. 10.1002/ptr.1266 [DOI] [PubMed] [Google Scholar]
- 10. Kanter M, Meral I, Dede S, Cemek M, Ozbek H, Uygan I, et al. Effects of Nigella sativa L. and Urtica dioica L. on lipid peroxidation, antioxidant enzyme systems and some liver enzymes in CCl4‐treated rats. J Vet Med A Physiol Pathol Clin Med. 2003;50:264–8. doi: 10.1046/j.1439-0442.2003.00537.x. [DOI] [PubMed] [Google Scholar]
- 11. Aebi H. Catalase Bergmeyer H U, Methods of enzymatic analysis Weinheim: Verlag Chemie; 1974673–8. [Google Scholar]
- 12. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem. 1976;72:248. doi: 10.1006/abio.1976.9999. 10.1016/0003‐2697(76)90527‐3 [DOI] [PubMed] [Google Scholar]
- 13. Celik M, Gulcu F, Ozan G, Gursu MF. Paraoxonase and arylesterase activity levels in workers exposed to organic solvents. Turk J Biochem. 2005;30:194–9. 10.1016/j.tibs.2005.02.008 [Google Scholar]
- 14. Gorog P, Kotak DC, Kovacs IB. Simple and specific test for measuring lipid peroxides in plasma. J Clin Pathol. 1991;44:765–7. doi: 10.1136/jcp.44.9.765. 10.1136/jcp.44.9.765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coskun A, Gunal O, Sahin I, Aslaner A, Yildirim U, Yavuz O. Does l‐carnitine have any effect on cold preservation injury of non‐fatty liver in the University of Wisconsin solution? Hepatol Res. 2007;37:656–60. doi: 10.1111/j.1872-034X.2007.00088.x. 10.1111/j.1872‐034X.2007.00088.x [DOI] [PubMed] [Google Scholar]
- 16. Yildiz F, Coban S, Terzi A, Ates M, Aksoy N, Cakir H, et al. Nigella sativa relieves the deleterious effects of ischemia reperfusion injury on liver. World J Gastroenterol. 2008;14:5204–9. doi: 10.3748/wjg.14.5204. 10.3748/wjg.14.5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yuan GJ, Ma JC, Gong ZJ, Sun XM, Zheng SH, Li X. Modulation of liver oxidant‐antioxidant system by ischemic preconditioning during ischemia/reperfusion injury in rats. World J Gastroenterol. 2005;11:1825–8. doi: 10.3748/wjg.v11.i12.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang SJ, Shi JH, Tang Z, Wu Y, Chen S. Protective effects of glycine pretreatment on brain‐death donor liver. Hepatobiliary Pancreat Dis Int. 2005;4:37–40. [PubMed] [Google Scholar]
- 19. Hassan‐Khabbar S, Cottart CH, Wendum D, Vibert F, Clot JP, Savouret JF, et al. Postischemic treatment by trans‐resveratrol in rat liver ischemia‐reperfusion: a possible strategy in liver surgery. Liver Transpl. 2008;14:451–9. doi: 10.1002/lt.21405. 10.1002/lt.21405 [DOI] [PubMed] [Google Scholar]
- 20. Polat KY, Aydinli B, Polat O, Aydin U, Yazici P, Ozturk G, et al. The protective effect of aprotinin and alpha‐tocopherol on ischemia‐reperfusion injury of the rat liver. Transplant Proc. 2008;40:63–8. doi: 10.1016/j.transproceed.2007.11.047. 10.1016/j.transproceed.2007.11.047 [DOI] [PubMed] [Google Scholar]
- 21. Shen SQ, Zhang Y, Xiang JJ, Xiong CL. Protective effect of curcumin against liver warm ischemia/reperfusion injury in rat model is associated with regulation of heat shock protein and antioxidant enzymes. World J Gastroenterol. 2007;13:1953–61. doi: 10.3748/wjg.v13.i13.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keles MS, Demirci N, Yildirim A, Atamanalp SS, Altinkaynak K. Protective effects of N‐acetylcysteine and Ginkgo biloba extract on ischaemia‐reperfusion‐induced hepatic DNA damage in rats. Clin Exp Med. 2008;8:193–8. doi: 10.1007/s10238-008-0005-1. 10.1007/s10238‐008‐0005‐1 [DOI] [PubMed] [Google Scholar]
- 23. Sener G, Tosun O, Sehirli AO, Kacma A, Arbak S, Ersoy Y, et al. Melatonin and N‐acetylcysteine have beneficial effects during hepatic ischemia and reperfusion. Life Sci. 2003;72:2707–18. doi: 10.1016/s0024-3205(03)00187-5. 10.1016/S0024‐3205(03)00187‐5 [DOI] [PubMed] [Google Scholar]
- 24. Ozbek H, Tuncer I, Dulger H, Ugras S, Bayram I, Turkdogan K, et al. The effects of vitamin E, N‐acetyl cystein, penicillin‐G and Urtica dioica L. on the phalloidin toxicity. Van Med. J. 2005;12:16–21. [Google Scholar]
- 25. Chouker A, Martignoni A, Schauer RJ, Dugas M, Schachtner T, Kaufmann I, et al. Alpha‐gluthathione S‐transferase as an early marker of hepatic ischemia/reperfusion injury after liver resection. World J Surg. 2005;29:528–34. doi: 10.1007/s00268-004-7431-3. 10.1007/s00268‐004‐7431‐3 [DOI] [PubMed] [Google Scholar]
- 26. Tarpey MM, Wink DA, Grisham MB. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am J Physiol Regul Integr Comp Physiol. 2004;286:431–44. doi: 10.1152/ajpregu.00361.2003. [DOI] [PubMed] [Google Scholar]
- 27. Yildirim A, Aslan S, Ocak T, Yildirim S, Kara F, Sahin YN. Serum paraoxonase/arylesterase activities and malondialdehyde levels in trauma patients. Eurasian J Med. 2007;39:85–88. [Google Scholar]
- 28. Memisogullari R, Orhan N. Paraoxonase and cancer. Konuralp Tıp Dergisi. 2010;2:22–6. [Google Scholar]
- 29. Memişoğullari R, Bakan E. Levels of ceruloplasmin, transferrin, and lipidperoxidation in the serum of patients with type 2 diabetes mellitus. J Diabetes Complications. 2004;18:193–7. doi: 10.1016/S1056-8727(03)00032-1. 10.1016/S1056‐8727(03)00032‐1 [DOI] [PubMed] [Google Scholar]
- 30. Altan N, Dincel AS, Koca C. Diabetes mellitus and oxidative stress. Turk J Biochem. 2006;3:51–6. [Google Scholar]
- 31. Arab K, Steghens JP. Plasma lipid hydroperoxides measurement by an automated xylenol orange method. Anal Biochem. 2004;325:158–3. doi: 10.1016/j.ab.2003.10.022. 10.1016/j.ab.2003.10.022 [DOI] [PubMed] [Google Scholar]