Abstract
In this study, we characterized the intratumoral expression of IL-17 and CD8+ TILs in gastric adenocarcinoma patients after resection and determined the correlation between the survival probability of gastric adenocarcinoma patients and the expression of IL-17 in tumor. Expression of IL-17 and CD8 was assessed by immunohistochemistry, and the prognostic effects of intratumoral IL-17 expression and CD8+ TILs were evaluated by Cox regression and Kaplan-Meier analysis. Immunohistochemical detection revealed the presence of IL-17 and CD8+ cells in gastric adenocarcinoma tissue samples (90.6%, 174 out of 192 patients and 96.9%, 186 out of 192 patients, respectively). We have also found that intratumoral IL-17 expression was significantly correlated with age (p=0.004) and that the number of CD8+ TILs was significantly correlated with UICC staging (p=0.012) and the depth of tumor invasion (p=0.022). The five-year overall survival probability among patients intratumorally expressing higher levels of IL-17 was significantly better than those expressing lower levels of IL-17 (p=0.036). Multivariate Cox proportional hazard analyses revealed that intratumoral IL-17 expression (HR: 0.521; 95% CI: 0.329-0.823; p=0.005) was an independent factor affecting the five-year overall survival probability. We conclude that low levels of intratumoral IL-17 expression may indicate poor prognosis in gastric adenocarcinoma patients.
Keywords: IL-17, intratumoral expression, gastric carcinoma, prognostic indicator
Introduction
Gastric carcinoma is one of the most common types of cancer. Recently, incidence and mortality rates of gastric carcinoma have declined overall. However, in Asian countries, such as Japan, China, and other developing countries, gastric carcinoma remains the most common form of cancer. Traditional therapies, such as surgery, chemotherapy, and radiotherapy, play an important role in treating different kinds and stages of gastric carcinoma 1, 2. In spite of this, determining the prognosis for a patient with gastric carcinoma has not yet been well studied. It is generally agreed that there is a correlation between the infiltration of immune cells within a tumor and tumor development. Therefore, the distribution of various types of immune cells detected in gastric carcinoma may provide useful information for a patient's prognosis.
Interleukin-17 (IL-17) is a CD4 T cell-derived proinflammatory cytokine. Many studies have revealed that IL-17 plays an active role in the development of inflammation, GVHD and autoimmune diseases 3-5. The relationship between cancer and IL-17 has been investigated. An increase in the number of IL-17-postive cells in tumors was detected in prostate cancer, ovarian cancer, and hepatocellular cancer 6-9. Furthermore, some researchers have shown that the number of IL-17-postive cells correlates with tumor development and patient prognosis. Although IL-17 mRNA has been detected in tumors in patients with gastric cancer 10, the distribution of IL-17-postive cells within the tumor and the relationship between IL-17 and prognosis have not yet been investigated. In this report, we examined the intratumoral expression of IL-17 and CD8 by immunohistochemistry, and correlated it with the clinical data of gastric adenocarcinoma patients. We evaluated the prognostic effects of intratumoral IL-17low or high expression and CD8+ TILs (tumor-infiltrating lymphocytes) by Cox regression and Kaplan-Meier analysis.
Material and Methods
Patients and tissue specimens
Paraffin-embedded samples were obtained from 192 gastric adenocarcinoma patients who underwent surgical operations at the Sun Yat-sen University Cancer Center, Guangzhou, China, between 2002 and 2005. Patients with autoimmune diseases were excluded. None of the patients had received anticancer treatment prior to surgery. There were 129 male and 63 female patients with a median age of 58 years (range, 17-85 years). The follow-up dates of the patients in this study are available and complete. The median follow-up for the entire cohort was 61 months (range 0.3-81.6 months). There were 79 cases of stage I-II and 113 cases of stage III-IV cancer according to the TNM classification for gastric cancer pTNM staging system (International Union Against Cancer, UICC). Each lesion was graded histologically according to the WHO classification criteria. Overall survival (OS) was defined as the interval between the date of surgery and date of death or the last known follow-up. All samples were coded anonymously in accordance with local ethical guidelines (as stipulated by the Declaration of Helsinki).Written informed consent was obtained, and the protocol was approved by the Review Board of the Sun Yat-sen University Cancer Center.
Immunohistochemistry
Formalin-fixed, paraffin-embedded samples were cut to a thickness of 5 μm. Each tissue section was deparaffinized and rehydrated with graded ethanol. For antigen retrieval, the slides were boiled in EDTA (1 mM; pH 8.0) for 15 min in a microwave oven. Endogenous peroxidase activity was blocked with a 0.3% hydrogen peroxide solution for 10 min at room temperature. After rinsing with PBS, slides were incubated overnight at 4°C with respective primary antibodies which include goat anti-human IL-17 polyclonal antibody (R&D systems; dilution 1/300) and mouse anti-human CD8 monoclonal antibody (Zhongshan Golden Bridge Biotech, Beijing, China; dilution 1/100). After three washes in PBS, sections were incubated with biotinylated anti-goat or anti-mouse secondary antibody (Zhongshan Golden Bridge Biotech, Beijing, China) respectively for 30 min at room temperature. Immunostaining was performed using the Envision System with diaminobenzidine (DakoCytomation, Glostrup, Denmark). Finally, the signal was developed with 3,3'-diaminobenzidine tetrahydrochloride (DAB), and all of the slides were counterstained with hematoxylin. Data were obtained by manually counting positively stained cells in five separate areas of intratumoral regions under 400× high-power magnification. Densities were determined by computing the mean number of positively stained cells per high power microscopic field (HPF). Mouse IgG1 (DAKO) and normal goat IgG (Santa) were used as negative control stains .
Statistical analysis
Descriptive statistics were expressed as the mean ± SD or median (range). The median value of immunohistochemically detected variables of IL-17 and CD8 was used as cut-off for defining the TIL subgroups in our results as previously described 11. Chi-squared tests or Fisher exact tests were used to assess the relationship between IL-17 and CD8 and clinic pathological features. Correlations between IL-17 and CD8 were determined by the Pearson correlation coefficient. Prognostic factors were examined by bivariable and multivariable analyses using Kaplan-Meier methodology and Cox proportional hazards model. A two-sided p value < 0.05 was considered statistically significant. All statistical analyses were performed with SPSS software (version 16.0; SPSS Inc., Chicago, IL, USA).
Results
Study population
The patients' characteristics are presented in Table 1. Of the 192 patients examined, 82(42.8%) were dead before the end of the observation period. The median age of the study population was 58 years (range17-85 years). The majority of patients (146) presented with serous histology (76.1%). The median follow-up for the entire cohort was 61 months (range 0.3-81.6 months). The 5-year survival for the entire study population was 57.2%.
Table 1.
Clinical characteristics of 192 patients with gastric adenocarcinoma
| Characteristics | Number (%) |
|---|---|
| Age, years | |
| median | 58 |
| range | 17-85 |
| Gender | |
| male | 129(67.2) |
| female | 63(32.8) |
| Tumor (T) stage | |
| T1 | 3(1.6) |
| T2 | 38(19.7) |
| T3 | 145(75.1) |
| T4 | 6(3.1) |
| Lymphoid Nodal (N) status | |
| N0 | 51(26.4) |
| N1 | 92(47.7) |
| N2 | 40(20.7) |
| N3 | 9(4.7) |
| Distant metastasis (M) status | |
| M0 | 176(91.2) |
| M1 | 16(8.3) |
| TNM stage | |
| Ⅰ | 8(4.1) |
| Ⅱ | 71(36.8) |
| Ⅲ | 94(48.7) |
| Ⅳ | 19(9.8) |
| Death | |
| no | 110(57.2) |
| yes | 82(42.8) |
| Histologic grade | |
| well | 6(3.1) |
| moderate | 40(20.8) |
| poor | 146(76.1) |
| Recurrence | |
| no | 175(90.7) |
| yes | 17(8.8) |
Immunohistochemical characteristics
The occurrences of IL-17 and CD8 positive cells were 90.6% (174 of 192) and 96.9% (186 of 192), respectively. IL-17 immunostaining was mostly localized to the cytoplasm whereas CD8 positive staining was mostly observed at the plasma membrane. CD8 positive staining is characteristic of cytotoxic T lymphocytes (Figure 1 and Table 2).
Figure 1.
Intratumoral expression of IL-17 and CD8+ TIL detection. Consecutive sections were used for immunohistochemical analysis of CD8+ TIL (A, B) and IL-17 expression (C, D) (A, C 200× magnification; B, D 400×magnification).
Table 2.
Descriptive statistics of immunohistochemical variables
| Variables | Mean | SE | Median | Range |
|---|---|---|---|---|
| CD8+ TIL | 15.53 | 0.78 | 13 | 0-60 |
| Intratumoral IL-17-producing cells | 3.42 | 0.238 | 2.5 | 0-21 |
Relationship between CD8+ TIL, intratumoral IL-17 expression and clinic pathological features of patients with gastric adenocarcinoma
CD8+ TILs were significantly related to UICC staging (p=0.012) and depth of tumor invasion (p=0.022) (Table 3). IL-17 expression was related to age (p=0.004) but not to gender, histologic grade, lymph node metastasis, depth of invasion, or UICC staging (Table 3).
Table 3.
Correlations between CD8+ TILs, intratumoral IL-17 expression and clinic pathological features of patients with gastric adenocarcinoma
| Variables | CD8+ T cells | p value | IL-17-postive cells | p value | ||
|---|---|---|---|---|---|---|
| low | high | low | high | |||
| Gender | ||||||
| male | 68 | 61 | 63 | 66 | ||
| female | 27 | 36 | 0.221 | 29 | 34 | 0.760 |
| Age | ||||||
| ≥60 | 46 | 39 | 51 | 34 | ||
| <60 | 49 | 58 | 0.309 | 41 | 66 | 0.004a |
| Tumor size | ||||||
| ≥4 cm | 72 | 66 | 69 | 69 | ||
| <4 cm | 23 | 31 | 0.263 | 23 | 31 | 0.422 |
| Depth of invasion | ||||||
| T1-T2 | 27 | 14 | 20 | 21 | ||
| T3-T4 | 68 | 83 | 0.022a | 72 | 79 | 1.000 |
| UICC staging | ||||||
| Ⅰ-Ⅱ | 47 | 66 | 65 | 58 | ||
| Ⅲ-Ⅳ | 48 | 31 | 0.012a | 37 | 42 | 0.883 |
| Lymph node metastasis | ||||||
| no | 28 | 23 | 23 | 28 | ||
| yes | 67 | 74 | 0.415 | 69 | 72 | 0.744 |
| Histologic grade | ||||||
| well | 3 | 3 | 4 | 2 | ||
| moderate | 26 | 14 | 17 | 23 | ||
| poor | 66 | 80 | 0.085 | 71 | 75 | 0.510 |
ap value<0.05
Correlation between intratumoral IL-17 expression, CD8+ TILs and patient survival
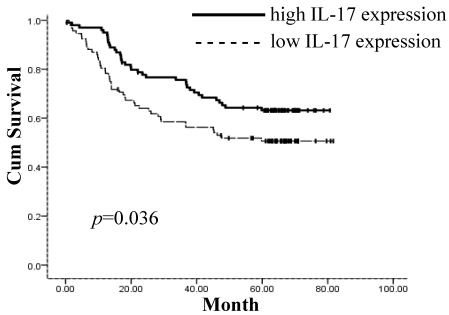
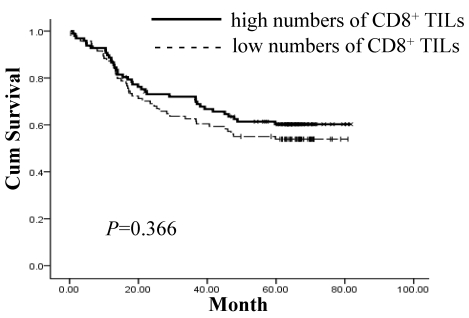
The prognostic value of IL-17 expression and CD8+ TILs on gastric adenocarcinoma patient survival was evaluated between patients with high and low IL-17 expression and CD8+ TIL numbers. Using a Kaplan-Meier curve assessment, we found that low IL-17 expression in tumor tissue was an independent predictor of poor prognosis in gastric adenocarcinoma patients. The five-year overall survival probability among patients expressing higher levels of IL-17 was significantly better than those expressing lower levels of IL-17 (p=0.036) (Figure 2). There was no significant correlation between the number of CD8+ TILs and patient survival (p=0.366) (Figure 3).
Figure 2.
Patients expressing higher levels of IL-17 intratumorally show significantly better five-year overall survival (p=0.036). Survival curves of 192 gastric adenocarcinoma patients with different IL-17 expression are shown. Kaplan-Meier survival curves for high intratumoral expression of IL-17 group were significantly different (p=0.036, log-rank test) from low IL-17 expression group in 192 gastric adenocarcinoma patients.
Figure 3.
Lack of significant correlation between the number of CD8+ TILs and patient survival (p=0.366). Survival curves of 192 gastric adenocarcinoma patients with various numbers of CD8+ TILs are shown. Kaplan-Meier survival curves for larger number of CD8+ TIL group were not significantly different (p=0.366, log-rank test) from smaller number of CD8+ TIL group in 192 gastric adenocarcinoma patients.
Univariate and multivariate analysis of prognosis variables in gastric adenocarcinoma patients
To identify the variables of potential prognostic significance in all patients with gastric adenocarcinoma, univariate and multivariate analyses were carried out using the Cox proportional hazard model to compare the impact of the expression levels of IL-17 and CD8+ TILs and other clinical pathological parameters on the prognosis of 192 gastric adenocarcinoma patients. Univariate analysis showed that IL-17 expression, tumor size, and recurrence were significant prognostic factors (Table 4). Multivariate analysis determined that IL-17 expression was an independent predictor of survival (p=0.005), as was recurrence (p=0.000). The relative risk in patients with low levels of IL-17 was 0.521 times greater than that of patients with higher expression of IL-17 (Table 4).
Table 4.
Univariate analyses of variables associated with survival and recurrence
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| CD8+ TIL (low v high) | 0.818 | 0.529-1.266 | 0.367 | |||
| Intratumoral IL-17-postive cells (low v high) | 0.628 | 0.405-0.974 | 0.038a | 0.521 | 0.329-0.823 | 0.005a |
| Age, years (≥60 v <60) | 1.326 | 0.857-2.050 | 0.205 | |||
| Gender (male v female) | 0.984 | 0.622-1.556 | 0.945 | |||
| Tumor size (≥4cm v <4cm) | 2.190 | 1.230-3.898 | 0.008a | 1.755 | 0.974-3.163 | 0.061 |
| Lymph node metastasis (no v yes) | 1.019 | 0.620-1.676 | 0.940 | |||
| Depth of invasion ( T1-T2 v T3-T4) | 0.968 | 0.573-1.635 | 0.903 | |||
| Recurrence (no v yes) | 5.142 | 2.899-9.119 | 0.000a | 5.585 | 3.035-10.779 | 0.000a |
| Histologic grade(well/moderate/poor) | 0.817 | 0.557-1.198 | 0.302 | |||
| HR Hazard ratio, CI confidence interval | ||||||
ap value<0.05
Correlation analysis between intratumoral expression of IL-17 and CD8+ TILs
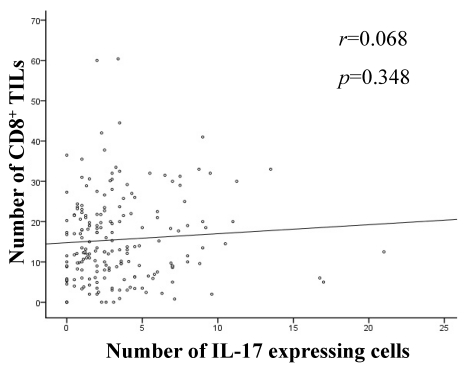
Through correlation analysis, we found no significant correlation between intratumoral expression of IL-17 and CD8+ TILs (Figure 4).
Figure 4.
Correlation analyses between intratumoral expression of IL-17 and CD8+ TILs. Linear regression analysis showed no correlation between intratumoral expression of IL-17 and CD8+ TILs (r=0.068, p=0.348).
Discussion
Gastric cancer is one of the most common cancers in Asia, including China. Despite the reduction in mortality rates due to both earlier detection and improved therapies, gastric cancer still poses a major threat to human health. It would therefore be valuable to identify a molecular target that could provide prognostic information.
IL-17 is a novel CD4 T cell-derived pro-inflammation factor that plays a potential role in inflammation, GVHD and autoimmune diseases 12-14. Recent study has shown that this effector T cell subset is also involved in tumor immunology. An increase in IL-17-positive cells has been observed in prostate cancer and hepatocellular cancers, and there are correlations between IL-17 and patient survival in ovarian cancer and small cell lung cancer 15-17. In this report, we examined the expression of IL-17 within gastric adenocarcinoma tumors and the relationship between IL-17-postive cells and gastric adenocarcinoma patient prognosis. This is the first report on the potential for IL-17 to serve as a prognostic indicator in gastric cancer. We used immunohistochemistry to characterize the intratumoral IL-17-postive cells and CD8+ cytotoxic T lymphocytes in gastric adenocarcinoma patients after resection and analyzed the association between prognosis and the detection of IL-17-postive cells and CD8+ TILs.
For the present study, we collected 192 gastric adenocarcinoma samples; 90.6% (174/192) expressed IL-17, which was distributed throughout the cytoplasm. In the CD8+ positive cells, CD8 was extensively expressed on the membrane. We further analyzed the relationship between intratumoral IL-17 expression, CD8+ TILs and the clinic pathologic features in patients with gastric adenocarcinoma. IL-17 expression was significantly correlated with age (p=0.004); the expression of IL-17 is significantly higher in younger patients. There was no significant association between the expression of IL-17 and other clinic pathologic features. Of note, we found no statistically significant correlation between the expression of IL-17 and UICC staging (p=0.883) or histologic grade (p=0.510), even though some other studies have shown that an increase in Th17 cells was associated with clinical stage in blood 10 and in tumor tissue 18.
Kaplan-Meier survival analysis showed that the five-year overall survival probability among patients with higher levels of IL-17 was significantly better than those with lower levels of IL-17 expression (p=0.036). This is consistent with a recent report on human ovarian cancer 8. However, in HCC patients, Zhang 19 et al. observed that increased intratumoral IL-17-postive cells correlate with poor survival. The different types of tumors and immunological statuses may contribute to different results. While there was no significant correlation between the level of CD8+ TILs and patient survival (p=0.366), CD8+ T lymphocytes are thought to be at the forefront fighting against tumors, and the number of CD8+ TILs was significantly correlated with UICC staging (p=0.012) in this study. However, they have no prognostic value in many cancer types. Our results are consistent with several previous reports 20-21.
Through correlation analysis, we found no significant correlation between the expression of IL-17 and CD8+ TILs (p=0.348, Figure 4). Studies regarding the mechanisms by which IL-17 mediates tumor immunity have shown that IL-17 can recruit Th1-related chemokines, such as CXCL9 and CXCL10, which promote the migration of effector T cells to the tumor site. Levels of CXCL9 and CXCL10 directly correlate with the number of tumor-infiltrating CD8+ T cells. IL-17 plays an indirect role in antitumor immunity by promoting effector CD8+ T cells 8. Since our study did not show significant correlation between CD8 T cells and IL-17, the results of our report may suggest that intratumoral expression of IL-17 may involve other mechanisms and anti-tumor effector cells alternative to the presence of CD8 T cells. While He D et al 22 observed that IL-17R deficiency caused an increase in CD8 T cell infiltration in an IL-17R-deficient mice model, the infiltration of myeloid-derived suppressor cells (MDSCs) are reduced in tumors. These controversial data about IL-17 in tumor development have reflected the complication in tumor microenvironment. Many factors, including the types of tumor and the microenvironment of the tumor itself , may lead to different endpoints.
In summary, our results show that IL-17 and CD8+ TILs are generally present in gastric adenocarcinoma. We also found that IL-17 expression is correlated with age (p=0.004) and that CD8+ TILs are correlated with UICC staging (p=0.012) and depth of tumor invasion (p=0.022). Multivariate Cox proportional hazard analyses revealed that IL-17 and recurrence are independent factors affecting the five-year overall survival probability. Our study suggests that the expression levels of IL-17 in the tumor can be an independent prognostic indicator in gastric adenocarcinoma patients. The molecular mechanisms for this correlation, which may help us better understand the role of IL-17 in the development of gastric adenocarcinoma, remain to be elucidated.
Acknowledgments
This work was supported by POTEN BIOMEDICAL TECHNOLOGY DEVELOPMENT CO., LTD, China, and supported in part by the Gillson Longenbaugh Foundation, USA.
References
- 1.Jemal A, Siegel R, Ward E. et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43– 66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Parkin D M, Bray F, Ferlay J. et al. Global cancerstatistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.ParkH LiZ, Yang XO Chang SH, Nurieva R Wang YH. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 5.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 7.Kryczek I. et al. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumour microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 8.Kryczek I. et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumour environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sfanos KS. et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B, Rong G, Wei H. et al. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008 Sep 26;374(3):533–7. doi: 10.1016/j.bbrc.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 11.Yi-Lan Zhang, Jiang Li, Hao-Yuan Mo. et al. Different subsets of tumor infiltrating lymphocytes correlate with NPC progression in different ways. Mol Cancer. 2010 Jan 10;9:4. doi: 10.1186/1476-4598-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awasthi A, Kuchroo VK. Th17 cells: from precursors to players in inflammation and infection. Int Immunol. 2009 May;21(5):489–98. doi: 10.1093/intimm/dxp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlson MJ, West ML, Coghill JM. et al. In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood. 2009 Feb 5;113(6):1365–74. doi: 10.1182/blood-2008-06-162420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oukka M. Th17 cells in immunity and autoimmunity. Ann Rheum Dis. 2008;67(Suppl 3):iii26–9. doi: 10.1136/ard.2008.098004. [DOI] [PubMed] [Google Scholar]
- 15.Koyama K. et al. Reciprocal CD4 T -cell balance of effector CD62 Llow CD4+ and CD62 Lhigh CD25+ CD4+ regulatory T cells in small cell lung cancer reflects disease stage. Clin Cancer Res. 2008;14:6770–6779. doi: 10.1158/1078-0432.CCR-08-1156. [DOI] [PubMed] [Google Scholar]
- 16.Charles KA. et al. The tumour-promoting actions of TNF-α involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011–3023. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyahara Y. et al. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci. 2008;105:15505–15510. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maruyama T, Kono K, Mizukami Y, Kawaguchi Y. et al. Distribution of Th17 cells and FoxP3(+) regulatory T cells in tumor-infiltrating lymphocytes, tumor-draining lymph nodes and peripheral blood lymphocytes in patients with gastric cancer. Cancer Sci. 2010 doi: 10.1111/j.1349-7006.2010.01624.x. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang JP, Yan J, Xu J. et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50(5):980–9. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 20.Grabenbauer GG, Lahmer G, Distel L. et al. Tumor-infiltrating cytotoxic T cells but not regulatory T cells predict outcome in anal squamous cell carcinoma. Clin Cancer Res. 2006;12(11 Pt 1):3355–60. doi: 10.1158/1078-0432.CCR-05-2434. [DOI] [PubMed] [Google Scholar]
- 21.Nakano O, Sato M, Naito Y. et al. Proliferative activity of intratumoral CD8+ T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61(13):5132–6. [PubMed] [Google Scholar]
- 22.He D, Li H, Yusuf N, Elmets CA. et al. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol. 2010;184(5):2281–8. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]