Abstract
AIM: To investigate serotonergic Ca2+ signaling and the expression of 5-hydroxytryptamine (5-HT) receptors, as well as Ca2+ transporting proteins, in hepatic stellate cells (HSCs).
METHODS: The intracellular Ca2+ concentration ([Ca2+]i) of isolated rat HSCs was measured with a fluorescence microscopic imaging system. Quantitative PCR was performed to determine the transcriptional levels of 5-HT receptors and endoplasmic reticulum (ER) proteins involved in Ca2+ storage and release in cultured rat HSCs.
RESULTS: Distinct from quiescent cells, activated HSCs exhibited [Ca2+]i transients following treatment with 5-HT, which was abolished by U-73122, a phospholipase C inhibitor. Upregulation of 5-HT2A and 5-HT2B receptors, but not 5-HT3, was prominent during trans-differentiation of HSCs. Pretreatment with ritanserin, a 5-HT2 antagonist, inhibited [Ca2+]i changes upon application of 5-HT. Expression of type 1 inositol-5’-triphosphate receptor and type 2 sarcoplasmic/endoplasmic reticulum Ca2+ ATPase were also increased during activation of HSCs and serve as the major isotypes for ER Ca2+ storage and release in activated HSCs. Ca2+ binding chaperone proteins of the ER, including calreticulin, calnexin and calsequestrin, were up-regulated following activation of HSCs.
CONCLUSION: The appearance of 5-HT-induced [Ca2+]i response accompanied by upregulation of metabotropic 5-HT2 receptors and Ca2+ transporting/chaperone ER proteins may participate in the activating process of HSCs.
Keywords: Hepatic stellate cells, 5-hydroxytryptamine, Intracellular Ca2+ transient, Sarcoplasmic/endoplasmic reticulum Ca2+ ATPase, Inositol-5’-triphosphate receptor, Endoplasmic reticulum chaperone
INTRODUCTION
Hepatic stellate cells (HSCs), also known as “Ito cells” or “fat-storing cells”, localize between hepatocytes and sinusoids (space of Disse) in mammalian livers. In their healthy state, HSCs control retinoid homeostasis, sinusoidal blood flow, macromolecule transport, and potentially act as antigen-presenting cells in the liver[1,2]. However, in response to hepatic injury, HSCs undergo gross morphological and functional changes, transforming to a myofibroblast-like phenotype in a process called “activation” or “trans-differentiation”[3,4]. Manifestations of activated HSCs include: (1) the expression of contractile cytoskeletal proteins such as α-smooth muscle actin (α-SMA)[5,6]; (2) enhanced extracellular matrix synthesis[7,8]; (3) increased cell size and proliferation[9]; (4) decreased size of lipid droplets[8,10]; and (5) well developed endoplasmic reticulum (ER), Golgi bodies, and compacted microfilaments[11,12]. In particular, the deposition of cross-linked collagen during the activation process may result in cirrhotic changes accompanied by life-threatening hepatic dysfunction.
Serotonin [5-hydroxytryptamine (5-HT)] is a neurotransmitter that also acts as a multifunctional hormone in various tissues[13], where it modulates proliferation and differentiation of muscle, neurons, and mammary glands[14-16]. Serotonin released from platelets at sites of injury plays an important role in liver regeneration and fibrosis[17]. It has been reported that patients with cirrhosis of the liver and portal hypertension have increased plasma serotonin levels[18]. The expression levels of 5-HT2A and 5-HT2B are increased in the liver after hepatectomy as well as in activated HSCs[2,17]. Moreover, 5-HT2 receptor antagonists suppress cell proliferation and expression of key fibrogenic factors in activated HSCs[2,19]. Among the mammalian 5-HT receptors (5-HT1 to 5-HT7), the 5-HT2 receptor family is coupled to the Gq/11 protein and increases intracellular Ca2+ concentration ([Ca2+]i) mobilized from ER reservoirs[20].
As the major intracellular calcium storage site, the ER possesses various kinds of calcium regulatory proteins that participate in: (1) pumping Ca2+ into the ER lumen, such as the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA); (2) releasing Ca2+ into the cytosol, such as IP3 or ryanodine receptors; and (3) buffering Ca2+, such as calreticulin and calnexin, which are also known as chaperones. ER Ca2+ homeostasis is maintained by a balance between Ca2+ release and replenishment[21]. The free Ca2+ concentration in the ER ([Ca2+]ER) ranges from 60-400 μmol/L, and disturbances in [Ca2+]ER homeostasis can affect many of the functions of the ER including protein synthesis, secretion[22], protein folding[23], and sensitivity of cells to apoptosis[24]. Further, [Ca2+]ER homeostasis might be critically required for the activation process of HSCs in order to keep up with accelerated protein synthesis. However, until now, the compensatory changes in ER protein expression involved in Ca2+ homeostasis and chaperone function have not been clearly elucidated.
[Ca2+]i may be important for the activation of HSCs, primarily because [Ca2+]i regulates the transcription of genes critical for cell function[25], and secondly because contractile elements such as α-SMA respond sensitively to [Ca2+]i[26]. We hypothesized that serotonin, acting as an autocrine or paracrine mediator, can elicit a Ca2+ signal, and this signal might be involved in the activation of HSCs. Moreover, there may be an alteration in the ER function of HSCs such as Ca2+ release and protein folding. In this study, we isolated and cultured rat HSCs on plastic dishes in vitro, which has been widely accepted as an appropriate model for the study of activated HSCs[8,27]. Appearance of [Ca2+]i transients induced by 5-HT and the upregulation of 5-HT2 receptors and ER proteins were observed during HSC activation. These observed changes may participate in an activation signal as well as adaptive changes during the trans-differentiation of HSCs.
MATERIALS AND METHODS
Isolation of rat HSCs
HSCs were isolated from male Sprague-Dawley rats (150-250 g) by means of a collagenase/pronase perfusion and Nycodenz-gradient centrifugation, as previously described[28,29]. HSCs were cultured with DMEM containing fetal bovine serum (10%) and antibiotics-antimycotics (Invitrogen, Carlsbad, CA, USA) in a humidified incubator (5% CO2, 37°C). The purity of HSCs was > 95% as assessed by their typical microscopic morphology and positive immunocytochemical staining for desmin at 24 to 48 h after seeding.
Quantitative reverse transcription-polymerase chain reaction analysis
Total cellular RNA was isolated and purified from HSCs at different culture periods, and reverse transcription (RT) was performed with random hexamers. Quantitative real time PCR using SYBR Green PCR Master mix (Applied Biosystems, Foster City, CA, USA) was performed on an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). Sequence specific oligonucleotide primers for the genes of interest were designed based on rat sequences deposited in the GenBank database (Tables 1 and 2), and the amplification program included the activation of AmpliTaq Gold at 95°C for 10 min, followed by 45 cycles of a two-step PCR reaction with denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min. The constitutively expressed housekeeping gene glyceraldehydes-3-phosphate dehydrogenase (GAPDH) was selected as an endogenous control to correct for potential variation in RNA loading and efficiency of amplification reactions.
Table 1.
Primers for reverse transcription-polymerase chain reaction
| Name | Sequence | Accession code | Position | Product (bp) |
| 5-HT1A | ||||
| (+) | 5'-TCAGCTACCAAGTGATCACC-3' | NM_012585.1 | 98-117 | 211 |
| (-) | 5'-GTCCACTTGTTGAGCACCTG-3' | 308-289 | ||
| 5-HT1B | ||||
| (+) | 5'-TACACGGTCTACTCCACGGT-3' | NM_022225.1 | 610-629 | 258 |
| (-) | 5'-TCGCACTTTGACTTGGTTCAC-3' | 867-847 | ||
| 5-HT2A | ||||
| (+) | 5'-GTGTCCATGTTAACCATCCT-3' | NM_017254 | 446-465 | 376 |
| (-) | 5'-GTAGGTGATCACCATGATGG-3' | 821-802 | ||
| 5-HT2B | ||||
| (+) | 5'-CATGCATCTCTGTGCCATTTC-3' | NM_017250 | 652-672 | 352 |
| (-) | 5'-TGTTAGGCGTTGAGGTGGC-3' | 1003-985 | ||
| 5-HT3A | ||||
| (+) | 5'-TCCTCAACGTGGATGAGAAG-3' | NM_024394.1 | 553-572 | 352 |
| (-) | 5'-ATGTTGATGTCCTGGATGGT-3' | 904-885 | ||
| 5-HT3B | ||||
| (+) | 5'-AAGCCCATCCAGGTGGTCTC-3' | NM_022189.1 | 459-478 | 428 |
| (-) | 5'-GACATGTTGACCCTGAAGAC-3' | 886-867 | ||
| 5-HT4 | ||||
| (+) | 5'-TCATGGTGCTGGCCTATTAC-3' | NM_012853.1 | 640-659 | 377 |
| (-) | 5'-CTCATCATCACAGCAGAGGA-3' | 1016-997 | ||
| 5-HT5A | ||||
| (+) | 5'-GAACAGGAGGAAGGAAGAGA-3' | NM_013148 | 1535-1554 | 109 |
| (-) | 5'-TAAGTCTCCTTGGTGTGAGG-3' | 1643-1624 | ||
| 5-HT5B | ||||
| (+) | 5'-TTCACCGTACTCGTGGTAAC-3' | L10073.1 | 453-472 | 132 |
| (-) | 5'-GGTCGAGGCTACCAAGTTAT-3' | 584-565 | ||
| 5-HT6 | ||||
| (+) | 5'-CCTGAGAGTGTGCTGAATTG-3' | NM_024365.1 | 1716-1735 | 129 |
| (-) | 5'-AGCCACACTACACAAGCAAC-3' | 1844-1825 | ||
| 5-HT7 | ||||
| (+) | 5'-GTGTGTCCACTGTCAAATCC-3' | NM_022938 | 2072-2091 | 148 |
| (-) | 5'-TCACTCATCTCCAGTTACCG-3' | 2219-2200 |
5-HT: 5-hydroxytryptamine.
Table 2.
Primers for quantitative reverse transcription-polymerase chain reaction
| Name | Sequence | Accession code | Position | Product (bp) |
| 5-HT2A | ||||
| (+) | 5'-GGGTACCTCCCACCGACAT-3' | NM_ | 234-252 | 101 |
| (-) | 5'-TTTCCAGCAATGGTGAGAATAATC-3' | 17254 | 334-311 | |
| 5-HT2B | ||||
| (+) | 5'-CGCCATCCCAGTCCCTATTA-3' | NM_ | 781-800 | 101 |
| (-) | 5'-AGAGCATGAAACTGCCAAAGC-3' | 17250 | 881-861 | |
| IP3R 1 | ||||
| (+) | 5'-GCAGAAGCAGATTGGCTATG-3' | NM_ | 2072-2091 | 261 |
| (-) | 5'-GTCTCAATCAGGATGTCAGC-3' | 1007235 | 2332-2313 | |
| IP3R 2 | ||||
| (+) | 5'-CAAGAAGTTCAGAGACTGCC-3' | NM_ | 396-415 | 295 |
| (-) | 5'-ACGCATGGCATTCTTCTCCA-3' | 31046.3 | 690-671 | |
| IP3R 3 | ||||
| (+) | 5'-GATGTGGTGTTGCTGCAGAA-3' | NM_ | 390-409 | 137 |
| (-) | 5'-TTGTTGCTCTTCATGTGCAG-3' | 13138.1 | 526-507 | |
| RyR 1 | ||||
| (+) | 5'-CTGAATGTCTGCTCTCCAAG-3' | AC | 35577-35596 | 112 |
| (-) | 5'-GAAGGGCAGAGAGACAAGAT-3' | 165142.3 | 35688-35669 | |
| RyR 2 | ||||
| (+) | 5'-ATGTAGGCTTCTTCCAGAGC-3' | XR_ | 11405-11414 | 136 |
| (-) | 5'-TGCAGTACCTTCTCTCCTGA-3' | 8338.1 | 11540-11521 | |
| RyR 3 | ||||
| (+) | 5'-TACCTTGCCTGGTACACAAC-3' | XM_001080527.1 | 13957-13976 | 123 |
| (-) | 5'-AGTCACAGATGACAGGATCG-3' | 14079-14060 | ||
| SERCA 1 | ||||
| (+) | 5'-CCAAGGAGCCTCTTATCAGT-3' | NM_017254 | 2516-2535 | 111 |
| (-) | 5'-CCTCTGCATACAAGAACCAC-3' | 2626-2607 | ||
| SERCA 2 | ||||
| (+) | 5'-AGTTCATCCGCTACCTCATC-3' | M 23114 | 2297-2316 | 119 |
| (-) | 5'-CACCAGATTGACCCAGAGTA-3' | 2415-2396 | ||
| SERCA 3 | ||||
| (+) | 5'-CTCATGCAGAAGGAGTTCAC-3' | NM_172812 | 1563-1582 | 140 |
| (-) | 5'-CGCTCAATTACACTCTCAGG-3' | 1702-1683 | ||
| Calreticulin | ||||
| (+) | 5'-AGAAGACTGGGATGAACGAG-3' | NM_ | 683-701 | 109 |
| (-) | 5'-GTCCTCAGGCTTCTTAGCAT-3' | 22399.1 | 791-772 | |
| Calsequestrin-2 | ||||
| (+) | 5'-CAGATGGCTATGAGTTCCTG-3' | NM_ | 988-1007 | 118 |
| (-) | 5'-CAGTAAGCAACAAGCAGAGG-3' | 17131.2 | 1105-1086 | |
| Calnexin | ||||
| (+) | 5'-GTGTTTGCTACTGGTCCTTG-3' | NM_ | 21-40 | 146 |
| (-) | 5'-ATGGAGGAGTGCTGGTATCT-3' | 172008.1 | 166-147 | |
| TGF-β type 1 R | ||||
| (+) | 5'-ACCAGCTATTGCCCATAGAG-3' | L 26110 | 1011-1030 | 106 |
| (-) | 5'-GGCAGAATCATGTCTCACAG-3' | 1116-1097 | ||
| α-SMA | ||||
| (+) | 5'-GCAGAGCAAGAGGGATCCT-3' | X 06801 | 222-242 | 73 |
| (-) | 5'-CATGTCGTCCCAGTTGGTGAT-3' | 294-274 | ||
| Cav1.2 (α1c) | ||||
| (+) | 5'-GACCCGTAGGAGCACGTTTG-3' | NM_012517 | 2327-2346 | 71 |
| (-) | 5'-CCTCCCCGGTCAGGATCT-3' | 2397-2380 |
5-HT: 5-hydroxytryptamine; SERCA: Sarcoplasmic/endoplasmic reticulum Ca2+ ATPase; α-SMA: α-smooth muscle actin; RyR: Ryanodine receptor; TGF: Transforming growth factor.
Fluorescent [Ca2+]i measurement
HSCs at 3 d or 2 wk after isolation were seeded on glass coverslips and loaded with fura-2/AM (5 μmol/L) in a dark room for 30 to 60 min at room temperature. Dye-loaded cells were then washed and transferred to a perfusion chamber on a fluorescence microscope (IX-70, Olympus, Tokyo, Japan). The HSCs were alternately excited at 340 and 380 nm by a monochromatic light source (LAMDA DG-4; Sutter, Novato, CA, USA), and fluorescence images were captured at 510 nm with an intensified CCD camera (Cascade; Roper, Duluth, GA, USA). Images were analyzed using the Metafluor 6.1 software package (Universal Imaging Corporation, Downingtown, PA, USA).
Immunocytochemistry
HSCs cultured on coverslips were fixed in 4% paraformaldehyde and immunocytochemical staining was performed using an antibody for α-SMA (Sigma Chemical Co., St Louis, MO, USA). After incubating with a biotinylated secondary antibody, an avidin-conjugated peroxidase complex was added to the slides and 3-amino-9-ethylcarbazole (AEC) was used as the chromogen.
Electrophysiology
Whole-cell membrane currents were recorded using the gramicidin-perforated patch-clamp technique as described previously[28]. All experiments were performed at room temperature (20-24°C). The internal solution for the perforated patch clamp contained (in mmol/L): 140 KCl, 5 EGTA, 10 HEPES, 0.5 CaCl2, 5 NaCl, and gramicidin (50 μg/mL) (pH 7.2). The external solution contained (in mmol/L): 135 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES, and 10 glucose (pH 7.4).
Statistical analysis
Quantitative data are expressed as the mean ± SE. Statistical comparisons were made by the two-tailed Student’s t-test and ANOVA. Differences with P < 0.05 were considered to be significant. PCR from each cDNA sample was done in triplicate and n indicates the number of experiments. For quantitative comparisons, the expression level of each gene was normalized to that of GAPDH and presented as relative expression ratio (target/GAPDH) by applying the formula 2-ΔΔCt[30].
RESULTS
Serotonergic signaling and receptor expression during HSC activation
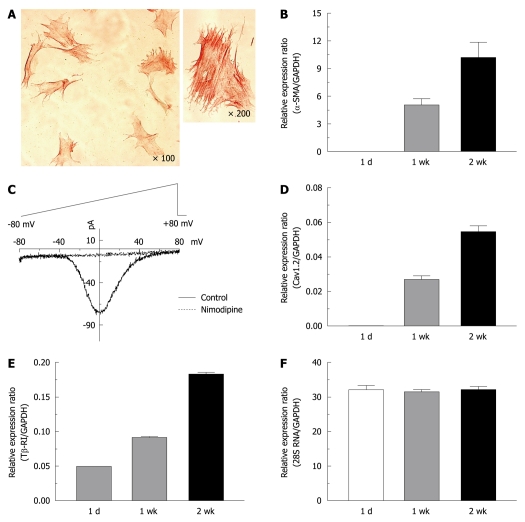
We isolated HSCs using density gradient-based separation with Nycodenz. Most of the harvested cells (> 95%) exhibited positive intra-cytoplasmic staining for desmin and glial fibrillary acidic proteins (GFAP). Expression of HSC trans-differentiation markers was tested at 1 d, 1 wk and 2 wk after isolation. In activated HSCs (2 wk after isolation), bundles of α-SMA were clearly observed as cytoskeletal fibers in immunocytochemical staining (Figure 1A), which was not evident in quiescent cells. In a voltage-clamp mode, nimodipine (10 μmol/L)-sensitive L-type Ca2+ currents were recorded only for activated HSCs (Figure 1C). The expression level of α-SMA and the L-type Ca2+ channel (Cav1.2) were proportional to the activation period elicited by culturing cells on plastic dishes (Figure 1B and D). Transforming growth factor-β1 (TGF-β1), an abundant isoform of TGF in both normal and cirrhotic liver, is known as the main profibrogenic cytokine[31]. We observed that the type I receptor for TGF-β1 (Tβ-RI) was also upregulated during activation (Figure 1E), while the expression of 28S RNA as well as GAPDH was not changed during the activation process of HSCs (Figure 1F).
Figure 1.
Expression of α-smooth muscle actin, L-type calcium channels and type 1 transforming growth factor-β receptors in activated rat hepatic stellate cells. A: Immunocytochemical staining for α-smooth muscle actin (α-SMA) was performed on hepatic stellate cells (HSCs) cultured for 1 wk; C: Whole cell Ca2+ currents in a voltage-clamp mode were recorded from 2 wk-cultured HSCs, and were completely blocked by nimodipine (10 μmol/L); Changes in the transcript levels of α-SMA (B), the α1c subunit of the L-type Ca2+ channel (Cav1.2) (D), the type 1 receptor of transforming growth factor-β (Tβ-RI) (E), and 28S RNA (F) during HSC culturing (1 d, 1 wk and 2 wk) were measured by quantitative real-time reverse transcription-polymerase chain reaction analysis. Expression levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and expressed as a relative expression ratio (target/GAPDH). Data are presented as the mean ± SE (n = 3).
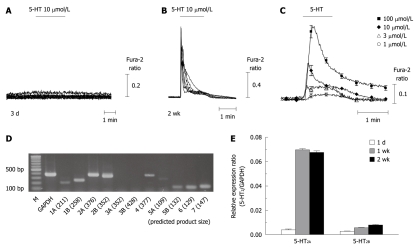
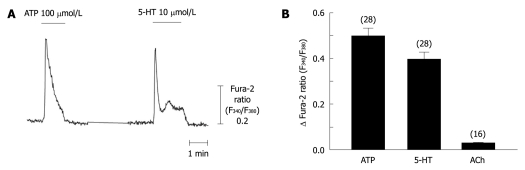
Serotonergic signaling has been suggested as a candidate for triggering activation of HSCs[2,17]. We focused on [Ca2+]i signaling in HSCs, which has been emphasized by previous work as having an important role in the activation process[26,32]. As shown in Figure 2A and B, strong [Ca2+]i transients followed by a slow plateau increase were recorded in response to 5-HT (10 μmol/L) application only from most of the activated HSCs (2 wk after isolation; 81 cells out of 92 cells), but not from quiescent cells (3 d after isolation; 0 out of 11 cells). The 5-HT-induced [Ca2+]i increase was dose-dependent in activated HSCs (Figure 2C). Consistent with a previous report[33], ATP also evoked [Ca2+]i transients in activated HSCs while acetylcholine did not (Figure 3).
Figure 2.
5-hydroxytryptamine-induced intracellular Ca2+ concentration changes and the expression of 5-hydroxytryptamine2 receptors in quiescent and activated hepatic stellate cells. A, B: 5-hydroxytryptamine (5-HT)-induced intracellular Ca2+ concentration ([Ca2+]i) transients were recorded from hepatic stellate cells (HSCs) at 3 d (A) and 2 wk (B) after isolation; C: Averages of [Ca2+]i changes (from 13-40 cells/each trace) in response to 5-HT (1-100 μmol/L) application to 2 wk-cultured HSCs are shown; D: Steady-state mRNA levels of the 5-HT receptor isotypes in 2 wk-cultured HSCs were compared using reverse transcription-polymerase chain reaction (RT-PCR); E: Using quantitative RT-PCR, the transcriptional changes in 5-HT2 receptors among 1 d-, 1 wk- and 2 wk-cultured HSCs were compared. Expression levels were normalized to GAPDH and expressed as a relative expression ratio (target/GAPDH, n = 3). Data are presented as the mean ± SE.
Figure 3.
Comparison of intracellular Ca2+ concentration responses to various metabotropic receptor agonists in activated hepatic stellate cells. Intracellular Ca2+ concentration changes following application of ATP (100 μmol/L), 5-hydroxytryptamine (5-HT) (10 μmol/L), or acetylcholine (ACh, 10 μmol/L) were measured in 2 wk-cultured hepatic stellate cells (n = 3-6, 16-28 cells). Data are presented as the mean ± SE.
Among the 5-HT receptors, 5-HT2 is known to release Ca2+ from the ER while 5-HT3 acts as a ligand-gated cation channel[20]. We estimated the steady-state mRNA levels of 5-HT receptor isotypes (5-HT1 to 5-HT7) using reverse transcription-polymerase chain reaction (RT-PCR) and found that the 5-HT2A and 5-HT2B receptors, but not 5-HT3, were abundantly transcribed (Figure 2D). Consistent with the observed changes in [Ca2+]i, the expression of 5-HT2A was increased by about 17-fold after 2 wk of isolation (5-HT2A/GAPDH; from 0.004 at 1 d to 0.067 at 2 wk). 5-HT2B was also found to be upregulated in activated HSCs (from 0.003 to 0.008) using quantitative RT-PCR (Figure 2E).
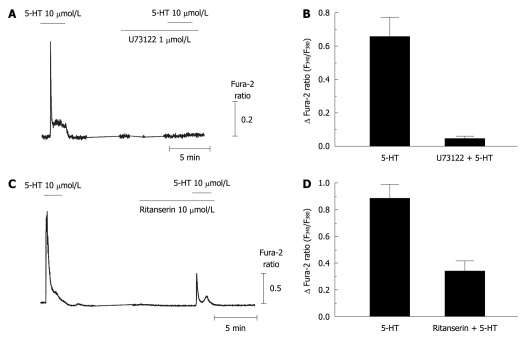
It has been recognized that 5-HT2 receptors are coupled with the Gq/11-phospholipase C pathway. Figure 4A and B show that the 5-HT-induced [Ca2+]i changes were abolished by pretreatment with 1 μmol/L U73122, a phospholipase C inhibitor (0.05 ± 0.05 peak changes of Fura-2 ratio from 0.66 ± 0.12, n = 13). We also observed that [Ca2+]i transients induced by 5-HT were not altered in extracellular Ca2+-free conditions (data not shown). These results suggest that 5-HT activates phospholipase C to produce IP3, which induces Ca2+ release from ER in activated HSCs. To confirm the receptor subtype, we tested blocking effects of a universal 5-HT2 antagonist, ritanserin, which does not discriminate among 5HT2 isotypes. 5-HT-induced [Ca2+]i responses were attenuated by pretreatment with 10 μmol/L ritanserin by 46.3% (0.34 ± 0.08 from 0.89 ± 0.10, n = 11).
Figure 4.
5-hydroxytryptamine-induced intracellular Ca2+ concentration transients via metabotropic 5-hydroxytryptamine2 receptor in activated hepatic stellate cells. A, B: 5-hydroxytryptamine (5-HT)-induced intracellular Ca2+ concentration ([Ca2+]i) transients were completely abolished by pretreatment with U73122 (1 μmol/L), a phospholipase C blocker (n = 3, 11 cells); C, D: Ritanserin (10 μmol/L), a 5-HT2 antagonist, inhibited the [Ca2+]i responses to 5-HT in activated hepatic stellate cells (2 wk-cultured cells; n = 3, 13 cells). Data are presented as the mean ± SE.
Upregulation of calcium transporting and binding proteins in the ER
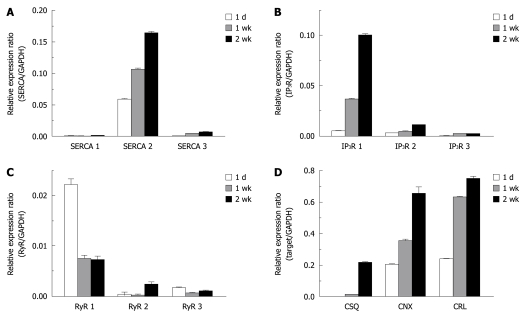
In mammalian cells, there are three major subtypes of the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA1, 2, and 3) which pump Ca2+ into the ER lumen. We observed SERCA2 to be the dominant subtype in HSCs. SERCA2, especially SERCA2b, is considered to be a house-keeping protein expressed constitutively in most kinds of cells; however, in HSCs, the expression of SERCA2 tends to increase during activation. Specifically, the relative expression ratio of SERCA2 (SERCA2/GAPDH) at 1 d after isolation was 0.058, and increased to 0.106 after 1 wk in culture and 0.164 after 2 wk in culture in vitro (Figure 5A). The expression of SERCA3 was also increased during culture (SERCA3/GAPDH; 0.4 × 10-3 at 1 d and 6.9 × 10-3 at 2 wk).
Figure 5.
Up-regulation of endoplasmic reticulum Ca2+ transporting and binding proteins in activated hepatic stellate cells. Changes in the expression level of 3 isoforms of the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) (A), inositol triphosphate receptor (IP3R) (B), ryanodine receptor (RyR) (C) and Ca2+ binding chaperones (D) during the culture periods (1 d, 1 wk and 2 wk) were measured by quantitative real-time reverse transcription-polymerase chain reaction analysis. Expression levels were normalized to GAPDH and expressed as a relative expression ratio (target/GAPDH). Data are presented as the mean ± SE (n = 3). CSQ: Calsequestrin, CNX: Calnexin, and CRL: Calreticulin.
Among the three isoforms (types 1 through 3) of the IP3 receptor, the type 1 IP3 receptor was the main subtype expressed in activated HSCs. We observed that the expression of the type 1 IP3 receptor increased by about 7-fold (IP3R 1/GAPDH = 0.037) after 1 wk of culture, and 20-fold (0.100) after 2 wk of culture compared to (0.005) levels 1 d after isolation (Figure 5B). In contrast, the expression level of ryanodine receptors, which are a family of Ca2+-releasing channel proteins expressed in the ER, either did not change or was decreased during the activation of HSCs (Figure 5C).
We investigated whether Ca2+ binding chaperones of the ER could be up-regulated following the activation process of HSCs. There were similar increases in the expression levels of calreticulin (calreticulin/GAPDH; from 0.204 at 1 d to 0.655 at 2 wk), calnexin (calnexin/GAPDH; from 0.240 at 1 d to 0.750 at 2 wk), and calsequestrin in HSCs. In the case of calsequestrin, the expression level in HSCs at 1 d after isolation was undetectable, but was markedly increased (calsequestrin/GAPDH; 0.217) after 2 wk of culturing (Figure 5D).
DISCUSSION
Trans-differentiation of HSCs is accompanied by marked increases in protein synthesis, including collagen, elastin, and glycoproteins[7]. It is well known that Ca2+ homeostasis in the ER is critical for the synthesis, folding, and secretion of protein[22,23]. In HSCs, the depletion of ER Ca2+ stores inhibits protein synthesis and increases intracellular degradation of collagen[34]. Maintaining a high Ca2+ gradient across the ER membrane (around 1000-fold) is accomplished by active Ca2+ transport by SERCAs. Among the three different isoforms of SERCAs, SERCA2 is considered to be a house-keeping protein expressed in the ER of most cell types, including HSCs[34]. We observed that SERCA2 was the main isotype in quiescent and activated HSCs (Figure 5A). During activation, the expression of SERCA2 (and also SERCA3) was increased, which likely helped to maintain appropriate ER Ca2+ concentrations.
Chaperone proteins in the ER facilitate the folding of newly synthesized proteins and glycoproteins. In particular, calreticulin and calnexin are important chaperones involved in a “quality control” system for protein synthesis[35]. In addition, these chaperones act as Ca2+ binding proteins in the ER. Overexpression of calreticulin increases the total amount of Ca2+ in intracellular stores, whereas calreticulin-deficient cells have reduced ER Ca2+ storage capacity[36]. Impaired collagen synthesis has been observed in cells derived from mice possessing genetic defects in ER chaperone proteins[37]. In this study, we observed for the first time that the expression of ER Ca2+ binding proteins was markedly increased during the activation process of HSCs, which might be an important adaptive change for trans-differentiation.
Upon stimulation from the extracellular space, ER Ca2+ is the main source for releasing Ca2+ and is responsible for enabling biologic signaling mediated by Ca2+. In addition, Ca2+ release from the ER stimulates store-operated Ca2+ entry into the cytosol, which eventually increases the refilling of the ER Ca2+ reservoir. It has been shown that cytosolic Ca2+ signaling is important for proliferation and differentiation of HSCs[25]. Similar to myofibroblast-like cells, activated HSCs can have a contractile response to [Ca2+]i changes, which may increase vascular resistance leading to portal hypertension in vivo[32]. During trans-differentiation, the expression of L-type calcium channels increases, which may contribute to cytosolic Ca2+ signaling in HSCs[26,38]. In the present study, we observed that 5-HT increased [Ca2+]i only in activated HSCs via a serotonergic receptor. Until now, 5-HT-induced [Ca2+]i changes have not been reported in HSCs. Physiologic concentrations of 5-HT in plasma are known to be less than 100 nmol/L, but those in cirrhotic patients are significantly elevated (3-4 fold) compared to controls[39]. Moreover, intrahepatic neighboring cells secrete 5-HT to act as an autocrine/paracrine regulator[40]. Thus, we hypothesize that local 5-HT concentration close to the releasing cells might be higher than the plasma level and repetitive exposure may have additive effects on [Ca2+]i-mediated changes in the process of HSC activation.
We observed that 5-HT elicited a [Ca2+]i response via the metabotropic 5-HT2 receptor in activated HSCs. This was demonstrated by the findings that 5-HT-induced [Ca2+]i transients were (1) completely blocked by a PLC inhibitor; (2) not altered by nominally Ca2+ free conditions; and (3) reduced by a 5-HT2 blocker. 5-HT2A is known to mediate mitogenic effects in fibroblasts[41], while 5-HT2B is involved in the development of the heart and enteric nervous system[42]. However, we did not discriminate whether the 5-HT2A and/or 5-HT2B receptor mediated the serotonergic Ca2+ signaling in activated HSCs. We also observed that the type I IP3 receptor (IP3R 1) is the main isoform expressed in activated HSCs, which is consistent with a recent report by Kruglov et al[32]. The expression level of IP3R 1 was increased during the activation process (Figure 5B).
Various ligands for Gq/11-coupled metabotropic receptors could be important extracellular stimuli, as they generate IP3 by activating phospholipase-C. Interestingly, it has been reported that the expression of the P2Y metabotropic purinoceptor (P2Y6) is rapidly upregulated following activation of HSCs, with a similar increase in ATP-induced [Ca2+]i transients[33]. The same study also reported that extracellular UDP increases the transcription of procollagen in activated HSCs via activation of the P2Y receptor, and this effect is partially inhibited by a P2Y receptor blocker. These results add further support to the hypothesis that Ca2+ signaling released from ER stores is associated with HSCs undergoing the process of activation. We also observed that ATP increased [Ca2+]i, which might be mediated by the metabotropic P2Y receptor (Figure 3). However, acetylcholine did not induce calcium changes, indicating that muscarinic acetylcholine receptors do not functionally exist in activated HSCs, even in the presence of machinery for ER Ca2+ release.
In this study, we observed the pronounced increase in serotonergic [Ca2+]i response related to the upregulation of metabotropic 5-HT2 receptors, type 1 inositol-5’-triphosphate receptor, type 2 sarcoplasmic/endoplasmic reticulum Ca2+ ATPase, and Ca2+ binding ER chaperone proteins following trans-differentiation of HSCs. These changes may be involved in the pathophysiologic (profibrotic) process of rat HSCs as well as being a compensatory mechanism for maintaining ER Ca2+ homeostasis and protein synthesis/maturation. Switching on and off of the serotonergic signaling pathway might be implicated in potential treatment for portal hypertension. Yet, the biological relevance of a 5-HT-induced [Ca2+]i transient in HSCs remains to be clarified. Moreover, it is not obvious whether simply switching-off this serotonergic signaling is an ideal target for developing treatments for liver cirrhosis. While there is evidence to suggest that 5-HT2 antagonists reduce proliferation and increase cell death of isolated HSCs[2,19], a recent study found that fibrotic changes induced by CCl4 are not ameliorated by a 5-HT2 antagonist[29,43]. Further studies to elucidate the detailed role of serotonergic signaling in HSCs are needed in order to develop therapeutic approaches to hepatic fibrosis.
COMMENTS
Background
Hepatic stellate cells (HSCs) are known to initiate hepatic fibrosis by trans-differentiating into myofibroblast-like cells. Changes in intracellular Ca2+ concentration ([Ca2+]i) have been suggested as a stimulus for the activation of HSCs.
Research frontiers
Recent data showed that activated HSCs responded to 5-hydroxytryptamine (5-HT) in a profibrogenic manner, which can be suppressed by 5-HT2 antagonists. In this study, the authors demonstrated that 5-HT generated [Ca2+]i transients released from endoplasmic reticulum (ER) in trans-differentiated HSCs, which was consistent with the upregulation of 5-HT2 receptors.
Innovations and breakthroughs
Serotonergic [Ca2+]i signaling has not been reported in HSCs, until now. It is also a novel finding that the expression of ER Ca2+ binding proteins was markedly increased during the activation process of HSCs.
Applications
The identification of [Ca2+]i signaling and the expressional changes of Ca2+ handling proteins in the process of HSC activation could help us to understand the pathophysiology and develop therapeutic approaches to hepatic fibrosis.
Terminology
IP3 receptor and sarcoplasmic/endoplasmic reticulum Ca2+ ATPase are ER proteins involved in Ca2+ release from, and refilling into, ER. Calsequestrin, calnexin, and calreticulin are ER Ca2+ binding chaperone proteins. Upregulation of all these proteins is important not only for [Ca2+]i signaling but also for maintaining ER Ca2+ levels needed for protein synthesis/maturation.
Peer review
The manuscript by Park et al reports the results of investigations on the serotonergic Ca2+ signaling, and the expression of 5-HT receptors and Ca2+ transporting proteins in rat HSCs. By employing reverse transcription-polymerase chain reaction, and fluorescent (fura-2) and electrophysiological techniques, as well as immunocytochemistry, the authors conclude that the increase in serotonergic [Ca2+]i responses accompanied by the upregulation in 5-HT2 receptors and Ca-transport proteins attests to their role in HSC activation. It is worthy of publication.
Footnotes
Supported by Grants from the Korean National Research Foundation (2010-0014617); the Myung Sun Kim Memorial Foundation (2009); and the Yonsei University Faculty Research Grant (2004)
Peer reviewer: Bronislaw L Slomiany, PhD, Professor, Research Center, C-875, UMDNJ-NJ Dental School, 110 Bergen Street, PO Box 1709, Newark, NJ 07103-2400, United States
S- Editor Sun H L- Editor Logan S E- Editor Zheng XM
References
- 1.Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruddell RG, Oakley F, Hussain Z, Yeung I, Bryan-Lluka LJ, Ramm GA, Mann DA. A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. Am J Pathol. 2006;169:861–876. doi: 10.2353/ajpath.2006.050767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gressner AM. Transdifferentiation of hepatic stellate cells (Ito cells) to myofibroblasts: a key event in hepatic fibrogenesis. Kidney Int Suppl. 1996;54:S39–S45. [PubMed] [Google Scholar]
- 4.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 5.Ramadori G, Veit T, Schwögler S, Dienes HP, Knittel T, Rieder H, Meyer zum Büschenfelde KH. Expression of the gene of the alpha-smooth muscle-actin isoform in rat liver and in rat fat-storing (ITO) cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59:349–357. doi: 10.1007/BF02899424. [DOI] [PubMed] [Google Scholar]
- 6.Rockey DC, Housset CN, Friedman SL. Activation-dependent contractility of rat hepatic lipocytes in culture and in vivo. J Clin Invest. 1993;92:1795–1804. doi: 10.1172/JCI116769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogawa K, Suzuki J, Mukai H, Mori M. Sequential changes of extracellular matrix and proliferation of Ito cells with enhanced expression of desmin and actin in focal hepatic injury. Am J Pathol. 1986;125:611–619. [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman SL, Rockey DC, McGuire RF, Maher JJ, Boyles JK, Yamasaki G. Isolated hepatic lipocytes and Kupffer cells from normal human liver: morphological and functional characteristics in primary culture. Hepatology. 1992;15:234–243. doi: 10.1002/hep.1840150211. [DOI] [PubMed] [Google Scholar]
- 9.Senoo H, Imai K, Matano Y, Sato M. Molecular mechanisms in the reversible regulation of morphology, proliferation and collagen metabolism in hepatic stellate cells by the three-dimensional structure of the extracellular matrix. J Gastroenterol Hepatol. 1998;13 Suppl:S19–S32. doi: 10.1111/jgh.1998.13.s1.19. [DOI] [PubMed] [Google Scholar]
- 10.Ramm GA, Britton RS, O'Neill R, Blaner WS, Bacon BR. Vitamin A-poor lipocytes: a novel desmin-negative lipocyte subpopulation, which can be activated to myofibroblasts. Am J Physiol. 1995;269:G532–G541. doi: 10.1152/ajpgi.1995.269.4.G532. [DOI] [PubMed] [Google Scholar]
- 11.Minato Y, Hasumura Y, Takeuchi J. The role of fat-storing cells in Disse space fibrogenesis in alcoholic liver disease. Hepatology. 1983;3:559–566. doi: 10.1002/hep.1840030414. [DOI] [PubMed] [Google Scholar]
- 12.Wanless IR, Belgiorno J, Huet PM. Hepatic sinusoidal fibrosis induced by cholesterol and stilbestrol in the rabbit: 1. Morphology and inhibition of fibrogenesis by dipyridamole. Hepatology. 1996;24:855–864. doi: 10.1002/hep.510240417. [DOI] [PubMed] [Google Scholar]
- 13.Veenstra-VanderWeele J, Anderson GM, Cook EH Jr. Pharmacogenetics and the serotonin system: initial studies and future directions. Eur J Pharmacol. 2000;410:165–181. doi: 10.1016/s0014-2999(00)00814-1. [DOI] [PubMed] [Google Scholar]
- 14.Fanburg BL, Lee SL. A new role for an old molecule: serotonin as a mitogen. Am J Physiol. 1997;272:L795–L806. doi: 10.1152/ajplung.1997.272.5.L795. [DOI] [PubMed] [Google Scholar]
- 15.Vitalis T, Parnavelas JG. The role of serotonin in early cortical development. Dev Neurosci. 2003;25:245–256. doi: 10.1159/000072272. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda M, Imaoka T, Vomachka AJ, Gudelsky GA, Hou Z, Mistry M, Bailey JP, Nieport KM, Walther DJ, Bader M, et al. Serotonin regulates mammary gland development via an autocrine-paracrine loop. Dev Cell. 2004;6:193–203. doi: 10.1016/s1534-5807(04)00022-x. [DOI] [PubMed] [Google Scholar]
- 17.Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–107. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 18.Beaudry P, Hadengue A, Callebert J, Gaudin C, Soliman H, Moreau R, Launay JM, Lebrec D. Blood and plasma 5-hydroxytryptamine levels in patients with cirrhosis. Hepatology. 1994;20:800–803. doi: 10.1002/hep.1840200405. [DOI] [PubMed] [Google Scholar]
- 19.Li T, Weng SG, Leng XS, Peng JR, Wei YH, Mou DC, Wang WX. Effects of 5-hydroxytamine and its antagonists on hepatic stellate cells. Hepatobiliary Pancreat Dis Int. 2006;5:96–100. [PubMed] [Google Scholar]
- 20.Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- 21.Putney JW Jr, McKay RR. Capacitative calcium entry channels. Bioessays. 1999;21:38–46. doi: 10.1002/(SICI)1521-1878(199901)21:1<38::AID-BIES5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook JF. The involvement of calcium in transport of secretory proteins from the endoplasmic reticulum. Cell. 1990;61:197–199. doi: 10.1016/0092-8674(90)90798-j. [DOI] [PubMed] [Google Scholar]
- 23.Corbett EF, Oikawa K, Francois P, Tessier DC, Kay C, Bergeron JJ, Thomas DY, Krause KH, Michalak M. Ca2+ regulation of interactions between endoplasmic reticulum chaperones. J Biol Chem. 1999;274:6203–6211. doi: 10.1074/jbc.274.10.6203. [DOI] [PubMed] [Google Scholar]
- 24.Demaurex N, Distelhorst C. Cell biology. Apoptosis--the calcium connection. Science. 2003;300:65–67. doi: 10.1126/science.1083628. [DOI] [PubMed] [Google Scholar]
- 25.Gallo EM, Canté-Barrett K, Crabtree GR. Lymphocyte calcium signaling from membrane to nucleus. Nat Immunol. 2006;7:25–32. doi: 10.1038/ni1295. [DOI] [PubMed] [Google Scholar]
- 26.Bataller R, Gasull X, Ginès P, Hellemans K, Görbig MN, Nicolás JM, Sancho-Bru P, De Las Heras D, Gual A, Geerts A, et al. In vitro and in vivo activation of rat hepatic stellate cells results in de novo expression of L-type voltage-operated calcium channels. Hepatology. 2001;33:956–962. doi: 10.1053/jhep.2001.23500. [DOI] [PubMed] [Google Scholar]
- 27.Rockey DC, Boyles JK, Gabbiani G, Friedman SL. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992;24:193–203. [PubMed] [Google Scholar]
- 28.Lee DH, Kong ID, Lee JW, Park KS. Changes in inward rectifier K+ channels in hepatic stellate cells during primary culture. Yonsei Med J. 2008;49:459–471. doi: 10.3349/ymj.2008.49.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baik SK, Jo HS, Suk KT, Kim JM, Lee BJ, Choi YJ, Kim HS, Lee DK, Kwon SO, Lee KI, et al. [Inhibitory effect of angiotensin II receptor antagonist on the contraction and growth of hepatic stellate cells] Korean J Gastroenterol. 2003;42:134–141. [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Breitkopf K, Haas S, Wiercinska E, Singer MV, Dooley S. Anti-TGF-beta strategies for the treatment of chronic liver disease. Alcohol Clin Exp Res. 2005;29:121S–131S. doi: 10.1097/01.alc.0000189284.98684.22. [DOI] [PubMed] [Google Scholar]
- 32.Kruglov EA, Correa PR, Arora G, Yu J, Nathanson MH, Dranoff JA. Molecular basis for calcium signaling in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G975–G982. doi: 10.1152/ajpgi.00401.2006. [DOI] [PubMed] [Google Scholar]
- 33.Dranoff JA, Ogawa M, Kruglov EA, Gaça MD, Sévigny J, Robson SC, Wells RG. Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G417–G424. doi: 10.1152/ajpgi.00294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stefanovic B, Stefanovic L, Schnabl B, Bataller R, Brenner DA. TRAM2 protein interacts with endoplasmic reticulum Ca2+ pump Serca2b and is necessary for collagen type I synthesis. Mol Cell Biol. 2004;24:1758–1768. doi: 10.1128/MCB.24.4.1758-1768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gelebart P, Opas M, Michalak M. Calreticulin, a Ca2+-binding chaperone of the endoplasmic reticulum. Int J Biochem Cell Biol. 2005;37:260–266. doi: 10.1016/j.biocel.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura K, Zuppini A, Arnaudeau S, Lynch J, Ahsan I, Krause R, Papp S, De Smedt H, Parys JB, Muller-Esterl W, et al. Functional specialization of calreticulin domains. J Cell Biol. 2001;154:961–972. doi: 10.1083/jcb.200102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagai N, Hosokawa M, Itohara S, Adachi E, Matsushita T, Hosokawa N, Nagata K. Embryonic lethality of molecular chaperone hsp47 knockout mice is associated with defects in collagen biosynthesis. J Cell Biol. 2000;150:1499–1506. doi: 10.1083/jcb.150.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oide H, Tateyama M, Wang XE, Hirose M, Itatsu T, Watanabe S, Ochi R, Sato N. Activated stellate (Ito) cells possess voltage-activated calcium current. Biochim Biophys Acta. 1999;1418:158–164. doi: 10.1016/s0005-2736(99)00018-8. [DOI] [PubMed] [Google Scholar]
- 39.Culafic DM, Mirkovic DS, Vukcevic MD, Rudic JS. Plasma and platelet serotonin levels in patients with liver cirrhosis. World J Gastroenterol. 2007;13:5750–5753. doi: 10.3748/wjg.v13.i43.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marzioni M, Glaser S, Francis H, Marucci L, Benedetti A, Alvaro D, Taffetani S, Ueno Y, Roskams T, Phinizy JL, et al. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology. 2005;128:121–137. doi: 10.1053/j.gastro.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Julius D, Huang KN, Livelli TJ, Axel R, Jessell TM. The 5HT2 receptor defines a family of structurally distinct but functionally conserved serotonin receptors. Proc Natl Acad Sci USA. 1990;87:928–932. doi: 10.1073/pnas.87.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nebigil CG, Choi DS, Dierich A, Hickel P, Le Meur M, Messaddeq N, Launay JM, Maroteaux L. Serotonin 2B receptor is required for heart development. Proc Natl Acad Sci USA. 2000;97:9508–9513. doi: 10.1073/pnas.97.17.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hauso O, Gustafsson BI, Nordrum IS, Waldum HL. The effect of terguride in carbon tetrachloride-induced liver fibrosis in rat. Exp Biol Med (Maywood) 2008;233:1385–1388. doi: 10.3181/0804-RM-137. [DOI] [PubMed] [Google Scholar]