Abstract
The release of cytochrome c from mitochondria results in the formation of an Apaf-1–caspase-9 apoptosome and induces the apoptotic protease cascade by activation of procaspase-3. The present studies demonstrate that heat shock protein 90 (Hsp90) forms a cytosolic complex with Apaf-1 and thereby inhibits the formation of the active complex. Immunodepletion of Hsp90 depletes Apaf-1 and thereby inhibits cytochrome c-mediated activation of caspase-9. Addition of purified Apaf-1 to Hsp90-depleted cytosolic extracts restores cytochrome c-mediated activation of procaspase-9. We also show that Hsp90 inhibits cytochrome c-mediated oligomerization of Apaf-1 and thereby activation of procaspase-9. Furthermore, treatment of cells with diverse DNA-damaging agents dissociates the Hsp90–Apaf-1 complex and relieves the inhibition of procaspase-9 activation. These findings provide the first evidence for a negative cytosolic regulator of cytochrome c-dependent apoptosis and for involvement of a chaperone in the caspase cascade.
Keywords: Apaf-1/apoptosis/caspase-9/cytochrome c/Hsp90
Introduction
The cellular response to genotoxic stress includes cell cycle arrest and activation of DNA repair. In the event of irreparable DNA damage, cells respond with induction of apoptosis. Apoptosis is a process resulting in characteristic morphological changes, such as internucleosomal DNA fragmentation, membrane blebbing and formation of apoptotic bodies (Martin and Green, 1995; Cryns and Yuan, 1998; Thornberry and Lazebnik, 1998). The induction of apoptosis by diverse stimuli is associated with activation of aspartate-specific cysteine proteases (caspases) (Martin and Green, 1995; Nicholson and Thornberry, 1997; Salvesen and Dixit, 1997) and cleavage of poly(ADP ribose) polymerase (PARP) (Kaufmann et al., 1993), protein kinase Cδ (Emoto et al., 1995) and diverse other proteins (Lazebnik et al., 1994; Nicholson et al., 1995; Tewari et al., 1995; Janicke et al., 1996; Orth et al., 1996; Song et al., 1996; Takahashi et al., 1996; Wang et al., 1996; Cohen, 1997; Kluck et al., 1997; Li et al., 1997; Enari et al., 1998; Shimizu et al., 1998). Caspases exist in cells as catalytically inactive zymogens or procaspases composed of three different subunits: a prodomain and two catalytic subdomains known as the large and small subunits (Alnemri et al., 1996).
Two pathways are known to be important for transducing a death signal to the apoptotic machinery. The ‘extrinsic’ pathway involves activation of death receptors, such as Fas/CD95 or TNF receptor 1, by binding of their respective ligands and thereby recruitment of caspase-8 (Huang et al., 1999). Caspase-8 can activate effector caspases directly (Boldin et al., 1996; Muzio et al., 1996) or indirectly by cleaving Bid and inducing the release of mitochondrial cyt c (Li et al., 1998; Luo et al., 1998; Gross et al., 1999). By contrast, the death receptor-independent, intrinsic, apoptotic pathway is activated directly by death stimuli and induces the release of mitochondrial cyt c into the cytosol (Liu et al., 1996; Kharbanda et al., 1997; Kim et al., 1997; Kluck et al., 1997; Yang et al., 1997; Bossy-Wetzel et al., 1998; Narula et al., 1999). Cytosolic cyt c binds to the CED-4 homolog Apaf-1 and induces caspase-9-dependent activation of caspase-3 (Li et al., 1997; Zou et al., 1997; Hu et al., 1998; Srinivasula et al., 1998). Formation of the cyt c–Apaf-1 complex is necessary for the activation of caspase-9 and is dependent on hydrolysis of dATP and/or ATP (Li et al., 1997; Zou et al., 1997). Recent studies have shown that Apaf-1 binds to procaspase-9 in the presence of dATP/cyt c and that oligomerization of Apaf-1 leads to caspase-9 activation (Li et al., 1997; Srinivasula et al., 1998; Adrain et al., 1999; Rodriguez and Lazebnik, 1999; Saleh et al., 1999; Zou et al., 1999). The finding that activated caspase-9 in turn cleaves and activates procaspase-3 has supported a model in which the Apaf-1–caspase-9 complex functions upstream of caspase-3 in this intrinsic apoptotic protease cascade (Li et al., 1997). Activation of the Apaf-1–caspase-9 complex appears to be a common death receptor-independent pathway employed by diverse stimuli, such as staurosporine, that induce apoptosis in mammalian cells (Chauhan et al., 1997; Kharbanda et al., 1997; Li et al., 1997; Fearnhead et al., 1998; Soengas et al., 1999; Sun et al., 1999). Mice deficient in Apaf-1 or caspase-9 exhibit severe developmental defects that lead to embryonic lethality, and their cells are resistant to death receptor-independent induction of apoptosis (Cecconi et al., 1998; Hakem et al., 1998; Yoshida et al., 1998).
The exposure of cells to a wide range of adverse conditions, such as increasing temperature or other environmental stress, is associated with the rapid and marked induction of heat shock protein (Hsp) expression. Many Hsps participate in an essential defense mechanism, conserved from bacteria to man, that consists of a stress response and acquired stress tolerance (Morimoto et al., 1990; Parsell and Lindquist, 1993; Chadli Ahmed et al., 1999). Studies have shown that Hsps act by preventing protein aggregation or by targeting protein to a proteolytic pathway (Gething and Sambrook, 1992; Ellis, 1996). Among the Hsps, the highly conserved and well studied Hsp90 is essential for viability of eukaryotic cells (Cutforth and Rubin, 1994). Hsp90, which constitutively comprises 1–2% of cytosolic proteins, is further stimulated when cells are exposed to high temperature (Yonehara et al., 1996; Chadli Ahmed et al., 1999). In eukaryotes, Hsp90 has dual chaperone functions, which are involved in the conformational maturation of nuclear hormone receptors and in the cellular stress response (Bohen and Yamamoto, 1994; Jacob and Buchner, 1994; Pratt and Welsh, 1994). These two processes have in common the ability of Hsp90 to prevent protein aggregation and to mediate the ATP-dependent refolding of heat-denatured proteins (Freeman and Morimoto, 1996; Schneider et al., 1996).
Overexpression of Hsp90 has been implicated in unregulated proliferation (Xu and Lindquist, 1993; Scheibel and Buchner, 1998). Moreover, the finding that Hsp90 is induced in response to diverse apoptotic stimuli, such as UV, doxorubicin and sodium arsenite, has supported its involvement in cell survival (Ferrarini et al., 1992; Maytin, 1992; Parsell and Lindquist, 1993; Ali et al., 1996; Bertram et al., 1996; Nayeem et al., 1997). However, the mechanisms responsible for the protective function of Hsp90 are for the most part unclear. The present studies demonstrate that Hsp90 inhibits activation of caspase-3 in vitro and in vivo. In contrast to caspases-3, -6, -8 and -9, Hsp90 binds to Apaf-1 and blocks activation of procaspase-3. The results also show that Hsp90 inhibits cyt c-mediated oligomerization of Apaf-1 and thereby activation of procaspase-9. Treatment of cells with cytotoxic agents dissociates the Hsp90–Apaf-1 complex and relieves inhibition of procaspase-9 activation. These findings provide the first evidence for a negative regulation of cyt c-induced formation of the Apaf-1–caspase-9 apoptosome and thereby cyt c-dependent induction of apoptosis.
Results and discussion
Elution of Hsp90 from cyt c column
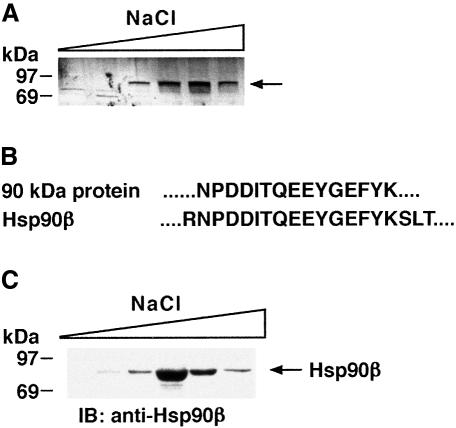
A cytochrome c affinity column was prepared to identify cytosolic proteins that interact with cyt c. S-100 extract from untreated U-937 cells was applied to the column and, after washing, proteins were eluted with a linear NaCl gradient. Analysis of the eluate by SDS–PAGE and silver staining revealed a band at ∼90 kDa (Figure 1A). Tryptic digestion and amino acid sequencing of the resulting peptides demonstrated identity with Hsp90β (Figure 1B). Immunoblot analysis of the eluate with anti-Hsp90β confirmed binding of Hsp90β to the cyt c column (Figure 1C). To assess whether Hsp90β and cyt c form a complex in cells, anti-cyt c immunoprecipitates were subjected to immunoblotting with anti-Hsp90β. As a control, immunoprecipitation with preimmune rabbit serum and immunoblot analysis of the precipitates showed no detectable Hsp90β (data not shown). The results demonstrate a constitutive association of Hsp90β and cyt c (Figure 2A). In the reciprocal experiment, analysis of anti-Hsp90β immunoprecipitates with anti-cyt c confirmed the presence of Hsp90β–cyt c complexes in cells (Figure 2B). Since Hsp90α demonstrates significant homology in sequence, structure and function to Hsp90β and the peptide that we have sequenced has only one amino acid difference, we also analyzed anti-Hsp90α immunoprecipitates by immunoblotting with anti-cyt c. The results demonstrate that Hsp90α also forms a complex with cyt c (data not shown). In addition, there was no detectable association of the heat-shock protein Hsp60 and cyt c (data not shown). To determine whether Hsp90 binds directly to cyt c, far-western analyses were performed in which purified cyt c was resolved by gel electrophoresis and transferred to nitrocellulose filters. The filters were incubated with purified Hsp90 and analyzed by immunoblotting with anti-Hsp90. The results demonstrate that Hsp90 fails to interact directly with cyt c (data not shown). Taken together, these results indicated that Hsp90 (α and/or β) exists in a cytoplasmic complex with cyt c through other intermediary protein(s).
Fig. 1. Detection of cytosolic proteins that associate with cyt c. (A) U-937 cells were disrupted in a Dounce homogenizer and the S-100 extract was applied to a cyt c bound to CNBr–sepharose beads column. After washing, elution was performed with a linear NaCl gradient (0–600 mM). The eluted proteins were separated by SDS–PAGE and analyzed by silver staining. (B) Following elution from cyt c, adsorbed proteins were separated by SDS–PAGE and then transferred to PVDF membranes. After localization of a 90 kDa protein by Ponceau staining, tryptic digestion and amino acid sequence of the resulting peptides was performed (Worcester Foundation for Biomedical Research, Shrewsbury, MA). Sequence analysis of the 90 kDa protein is compared with that of the Hsp90β protein. (C) Eluted fractions from the cyt c–CNBr–sepharose column were separated by 10% SDS–PAGE and analyzed by immunoblotting with anti-Hsp90β antibody.
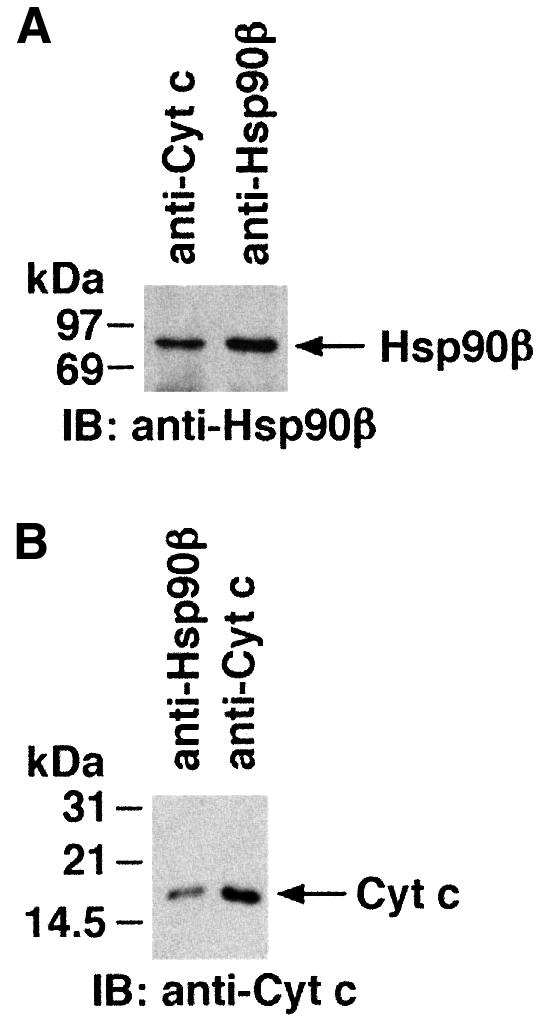
Fig. 2. (A) Lysates from U-937 cells were subjected to immunoprecipitation with anti-Hsp90β or anti-cyt c. The resulting protein precipitates were analyzed by immunoblotting with anti-Hsp90β. (B) Lysates from U-937 cells were subjected to immunoprecipitation with anti-Hsp90β or anti-cyt c and the precipit ates were analyzed by immunoblotting with anti-cyt c.
Immunodepletion of Hsp90 inhibits cyt c-dependent activation of procaspase-3
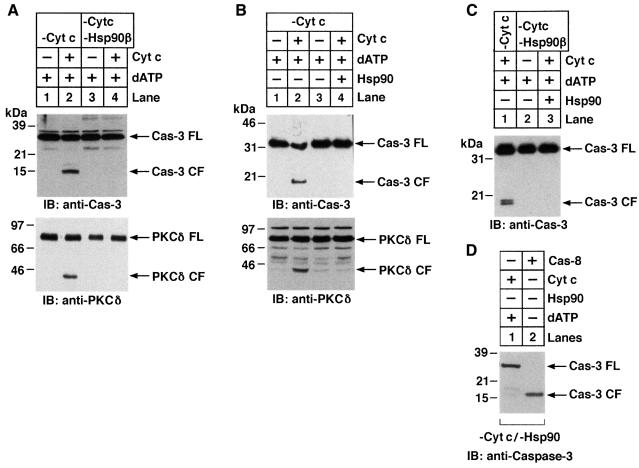
The functional significance of the interaction between Hsp90 and cyt c was assessed in a cell-free system of S-100 extracts prepared from untreated cells (Srinivasula et al., 1998; Narula et al., 1999; Saleh et al., 1999; Zou et al., 1999). S-100 extracts subjected to several immunoprecipitations with anti-cyt c were analyzed by immunoblotting with anti-cyt c to ensure complete depletion (data not shown). As reported previously (Liu et al., 1996), addition of purified cyt c and dATP to the cyt c-depleted extracts resulted in activation of caspase-3 and cleavage of the caspase-3 substrate, protein kinase Cδ (PKCδ) (Emoto et al., 1995; Ghayur et al., 1996) (Figure 3A, lanes 1 and 2). By contrast, following immunodepletion of both Hsp90β and cyt c, the addition of cyt c/dATP had no apparent effect on caspase-3 activation (Figure 3A, lanes 3 and 4). Similar results were obtained when S-100 extracts were immunodepleted of Hsp90α and cyt c (data not shown). To assess the role of Hsp90 in cyt c-mediated activation of caspase-3, we added cyt c/dATP and purified Hsp90 to the cyt c-depleted extracts. Whereas cyt c/dATP induced activation of caspase-3 and cleavage of PKCδ, the addition of cyt c/dATP and Hsp90 blocked these events (Figure 3B). To confirm these findings, cyt c- and Hsp90-immunodepleted U-937 cell S-100 extracts were incubated with cyt c/dATP and Hsp90, and then assessed for caspase-3 activity. The results demonstrate that addition of Hsp90 fails to restore caspase-3 activation by cyt c/dATP (Figure 3C). Other studies have shown that caspase-3 can be activated directly by caspase-8 in a cyt c-independent manner (Stennicke et al., 1998). To demonstrate further that the immunodepleted extracts were indeed competent to activate caspase-3 by other mechanisms, cyt c and Hsp90-immunodepleted cytosolic extracts were incubated with cyt c or caspase-8 and assayed for activation of caspase-3. The results demonstrate that following immunodepletion of Hsp90, addition of cyt c has no effect on caspase-3 activation. By contrast, the finding that caspase-3 is activated by caspase-8 in Hsp90-immunodepleted extracts supports a selective role for Hsp90 in cyt c-mediated activation of caspase-3 (Figure 3D). Moreover, immunodepletion of Hsp90 had no effect on the levels of caspase-8 (data not shown). Taken together, these results indicated that Hsp90 functions as a negative regulator of cyt c-mediated signaling and that an Hsp90-associated protein is required for cyt c-dependent activation of the caspase-3 cascade.
Fig. 3. Depletion of Hsp90β abrogates cyt c-mediated activation of caspase-3 in an in vitro cell-free system. (A) U-937 S-100 extracts were immunodepleted of cyt c (lanes 1–4) or Hsp90β (lanes 3 and 4). Aliquots (50 µg) were then incubated with buffer (lanes 1 and 3) or purified cyt c (1 µg, lanes 2 and 4) in the presence of 1 mM dATP in a final volume of 30 µl. After incubation at room temperature for 1 h, the proteins were separated by 10% SDS–PAGE and analyzed by immunoblotting with anti-caspase-3 (upper panel) or anti-PKCδ (lower panel). FL, full length; CF, cleaved fragment. (B) U-937 S-100 extracts were immunodepleted of cyt c (lanes 1–4). Aliquots were then incubated with buffer (lane 1), purified cyt c (lanes 2 and 4) or purified Hsp90 (1 µg; lanes 3 and 4) and analyzed by immunoblotting with anti-caspase-3 (upper panel) or anti-PKCδ (lower panel). (C) U-937 cell S-100 extracts were immunodepleted of cyt c (lanes 1–3) or Hsp90β (lanes 2 and 3). Aliquots (50 µg) were then incubated with purified cyt c (1 µg, lanes 1 and 3) or with Hsp90 (lane 3) in the presence of 1 mM dATP in a final volume of 30 µl. After incubation at room temperature for 1 h, the proteins were separated by 10% SDS–PAGE and analyzed by immunoblotting with anti-caspase-3 or anti-PKCδ. (D) U-937 cytosolic lysates were immunodepleted of cyt c and Hsp90β. Aliquots (50 µg) were then incubated with cyt c (1 µg) or purified recombinant caspase-8 in the presence of 1 mM dATP in a final volume of 30 µl. After incubation for 30 min at 30°C, the proteins were separated by SDS–PAGE and analyzed by immunoblotting with anti-caspase-3.
Hsp90 associates directly with Apaf-1
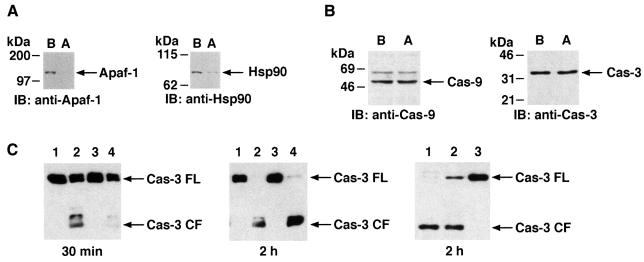
Recent studies have shown that Apaf-1 (human homolog of Caenorhabditis elegans CED-4) promotes both activation of caspase-9 and caspase-9-mediated activation of caspase-3 in mammalian cells (Li et al., 1997). These studies have further suggested that Apaf-1 functions as a docking protein for caspase-9 and cyt c/dATP in mediating cleavage of caspase-3 (Li et al., 1997). To determine whether Hsp90 inhibits cyt c-mediated activation of caspase-3 by binding to Apaf-1, procaspase-9 or procaspase-3, S-100 extracts were immunodepleted of Hsp90 and the extracts before and after immunodepletion were analyzed by immunoblotting with anti-Apaf-1, anti-Hsp90, anti-caspase-9 or anti-caspase-3 antibodies. The results demonstrate that, in contrast to extracts before Hsp90 immunodepletion, a significant decrease in the level of Apaf-1 was observed after immunodepletion of Hsp90 (Figure 4A). Moreover, levels of caspase-9 and caspase-3 were unchanged in extracts before and after immunodepletion of Hsp90 (Figure 4B). To determine whether the depletion of Apaf-1 contributes to inhibition of cyt c-dependent activation of procaspase-3, the Hsp90-immunodepleted S-100 extracts were analyzed by immunoblotting with anti-caspase-3 after the addition of purified cyt c/dATP. As expected, the results demonstrate significant inhibition of cyt c/dATP-dependent cleavage of procaspase-3 when the in vitro reaction was performed for 30 min (Figure 4C, left panel). Cleavage of procaspase-3 was restored when the reaction was performed for 2 h (Figure 4C, middle panel), indicating that the remaining Apaf-1 protein, unbound to Hsp90, is functional in procaspase-3 activation if incubated with cyt c/dATP for longer periods. Addition of Hsp90 protein to these immunodepleted extracts inhibited the function of remaining Apaf-1 and thereby activation of procaspase-3 (Figure 4C, right panel). Moreover, addition of purified Apaf-1 protein to these extracts restored cyt c/dATP-mediated cleavage of procaspase-3 (data not shown). These findings suggest that Hsp90 associates with Apaf-1 and inhibits cyt c/dATP-dependent activation of procaspase-3. To determine whether Hsp90 associates with Apaf-1 in cells, U-937 cell lysates were subjected to immunoprecipitation with anti-Apaf-1 and the immunoprecipitates were analyzed by immunoblotting with anti-Hsp90. The results demonstrate that, in contrast to caspase-9, Apaf-1 associates with Hsp90 (Figure 5A). In the reciprocal experiment, the finding that anti-Hsp90, but not anti-Hsp27, immunoprecipitates contain Apaf-1 provided further support for an interaction between these proteins (Figure 5B). To determine whether Hsp90 binds direcly to Apaf-1, far-western analyses were performed in which column-purified Apaf-1 was resolved by gel electrophoresis and transferred to nitrocellulose filters. The filters were incubated with purified Hsp90 and analyzed by immunoblotting with anti-Hsp90. As a control, filters were also incubated with Hsp60 protein and analyzed by immunoblotting with anti-Hsp60. The results demonstrate that, in contrast to Hsp60, Hsp90 interacts with Apaf-1 (Figure 5C). These findings indicate that binding of Apaf-1 and Hsp90 in cells is direct. To assess whether Apaf-1 protein refolds correctly on the membranes, we examined the capability of Apaf-1 to bind directly to cyt c and to hydrolyze ATP. The results demonstrate that Apaf-1 binds directly to cyt c and also hydrolyzes ATP (data not shown). Taken together, these findings indicated that both cyt c and Hsp90 bind directly to Apaf-1. While the region of Apaf-1 that is involved in binding with Hsp90 is not known, our results demonstrate that Hsp90 binds to Apaf-1 at a different site than that of cyt c (data not shown). Therefore, it is possible that Hsp90 exerts its protective function (such as inhibition of cyt c-mediated activation of caspase-3) by modulating the conformation of Apaf-1 or by inhibiting the affinity of Apaf-1 for binding to cyt c. This possibility might be physiologically significant since the association of monomeric Apaf-1 with Hsp90 in cells inhibits the affinity of Apaf-1 for binding to cyt c (data not shown).
Fig. 4. Hsp90 associates with Apaf-1. (A) U-937 cell S-100 extracts were immunodepleted of Hsp90. Lysates from lane B (before immunodepletion) and lane A (after immunodepletion) were subjected to SDS–PAGE and analyzed by immunoblotting with anti-Apaf-1 (left panel) or anti-Hsp90 (right panel). (B) Lysates from before and after immunodepletion were also subjected to SDS–PAGE and analyzed by immunoblotting with anti-caspase-9 (left panel) or anti-caspase-3 (right panel). (C) S-100 extracts were immunodepleted of cyt c (lanes 1–4) and Hsp90 (lanes 3 and 4). Aliquots (50 µg) were then incubated with buffer (lanes 1 and 3) or purified cyt c (1 µg, lanes 2 and 4) in the presence of 1 mM dATP in a final volume of 30 µl. After incubation at room temperature for 30 min (left panel) or 2 h (middle panel), the proteins were separated by 10% SDS–PAGE and analyzed by immunoblotting with anti-caspase-3. Right panel: S-100 extracts were immunodepleted of cyt c (lanes 1–3) and Hsp90β (lanes 2 and 3). Aliquots (50 µg) were then incubated with purified cyt c/dATP (lanes 1–3) and Hsp90 (lane 3) at room temperature for 2 h. Proteins were separated by 10% SDS–PAGE and analyzed by immunoblotting with anti-caspase-3.
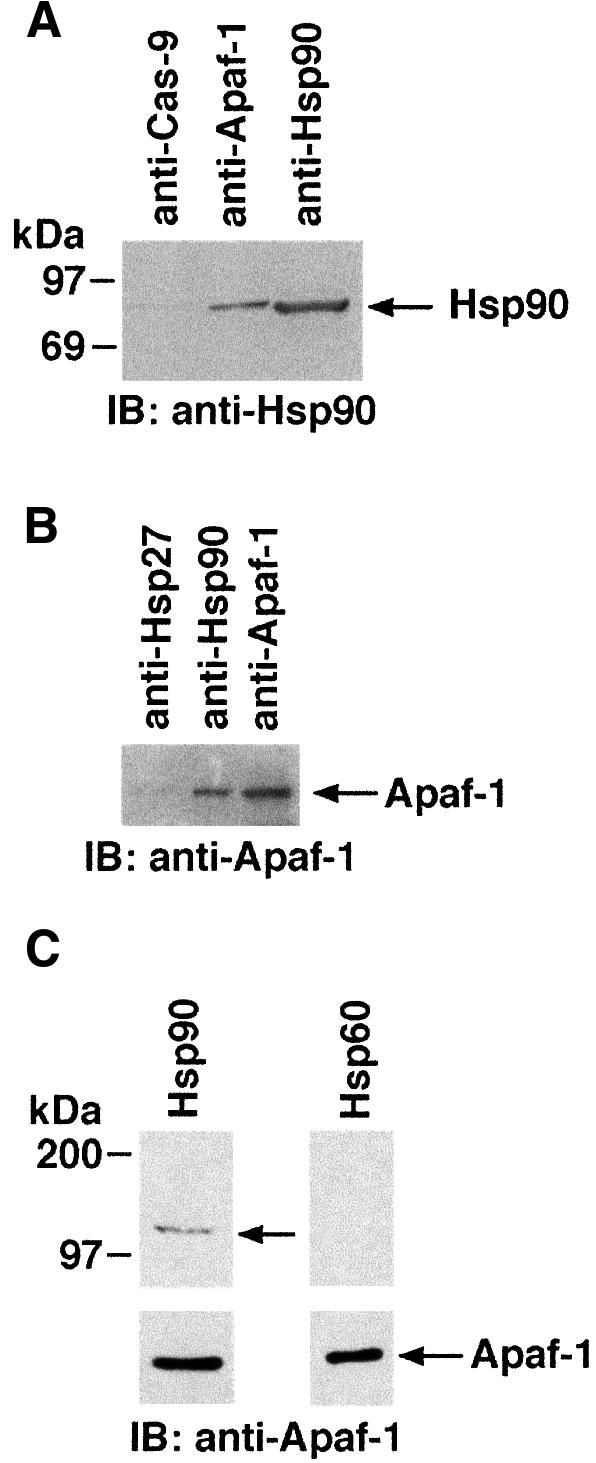
Fig. 5. (A) Lysates from U-937 cells were subjected to immunoprecipitation with anti-Hsp90, anti-caspase-9 or anti-Apaf-1. The resulting protein precipitates were then analyzed by immunoblotting with anti-Hsp90. (B) U-937 lysates were subjected to immunoprecipitation with anti-Hsp90, anti-Hsp27 or anti-Apaf-1 and analyzed by immunoblotting with anti-Apaf-1. (C) Column-purified Apaf-1 protein was resolved by SDS–PAGE and transferred to nitocellulose filters. The filters were incubated with either Hsp60 or Hsp90 protein. The filters were then analyzed by immunoblotting with anti-Hsp60 or anti-Hsp90 antibody (top panels). The same filters were also analyzed by immunoblotting with anti-Apaf-1 (bottom panels).
Apaf-1-mediated oligomerization of procaspase-9 is required for activation of caspase-9. Whereas Hsp90 and Apaf-1 are present in a complex in cells, we asked whether Hsp90 affects cyt c/dATP/Apaf-1-mediated activation of procaspase-9 in an in vitro cell-free system. Untreated U-937 S-100 extracts were incubated with in vitro-translated 35S-labeled recombinant procaspase-9 and cyt c/dATP in the presence and absence of purified Hsp90. After incubation, samples were subjected to SDS–PAGE and analyzed by autoradiography. The results demonstrate that endogeneous Apaf-1 promotes processing of procaspase-9 to produce 35 and 37 kDa fragments in the presence of cyt c/dATP, but not in the presence of Hsp90 and cyt c/dATP (Figure 6A). Since Apaf-1 binds to procaspase-9 in the presence of cyt c/dATP and Apaf-1-mediated oligomerization of procaspase-9 is required for activation of procaspase-9, we asked whether Hsp90 inhibits the association of Apaf-1 with procaspase-9. Column-purified Apaf-1 was incubated with in vitro-translated 35S-labeled procaspase-9 and cyt c/dATP in the presence of buffer or purified Hsp90. After incubation, anti-Apaf-1 immunoprecipitates were analyzed by autoradiography. The results demonstrate that Apaf-1 associates with procaspase-9 in the presence of Hsp90 in an in vitro system (Figure 6B). Previous studies have shown that the association between Apaf-1 and caspase-9 is mediated through the CARD sequences present in both proteins (Zou et al., 1997, 1999; Rodriguez and Lazebnik, 1999). Recent studies have demonstrated that cyt c/dATP-mediated oligomerization of Apaf-1 is required for its interaction with procaspase-9 (Cain et al., 1999; Rodriguez and Lazebnik, 1999; Saleh et al., 1999). Our results can not interpret the effect of Hsp90 on the association between Apaf-1 and procaspase-9 in vivo, since Hsp90 interferes with the oligomerization of Apaf-1, which is required for the interaction of Apaf-1 with procaspase-9. However, the finding that Hsp90 interacts with Apaf-1-CARD (data not shown) indicates that Hsp90 may not directly affect the CARD–CARD interaction between Apaf-1 and procaspase-9. Taken together, these findings support a model in which binding of Hsp90 with Apaf-1: (i) induces a potential conformational change in Apaf-1 and (ii) inhibits cyt c/dATP-mediated oligomerization of Apaf-1, and thereby exerts a dominant-negative effect on the function of Apaf-1 to activate procaspase-9. The functional significance of the interaction between Hsp90 and Apaf-1 was therefore further assessed in cytosolic extracts depleted of both cyt c and Hsp90. Addition of cyt c/dATP to the depleted extracts had no apparent effect on procaspase-9 (Figure 6C) or procaspase-3 (Figure 6D) activation. Significantly, addition of purified Apaf-1 and cyt c/dATP was associated with cleavage of procaspase-9 and procaspase-3 (Figure 6C and D). These findings demonstrate that Hsp90 functions as a negative regulator of cyt c-mediated signaling by sequestering Apaf-1 from the caspase cascade.
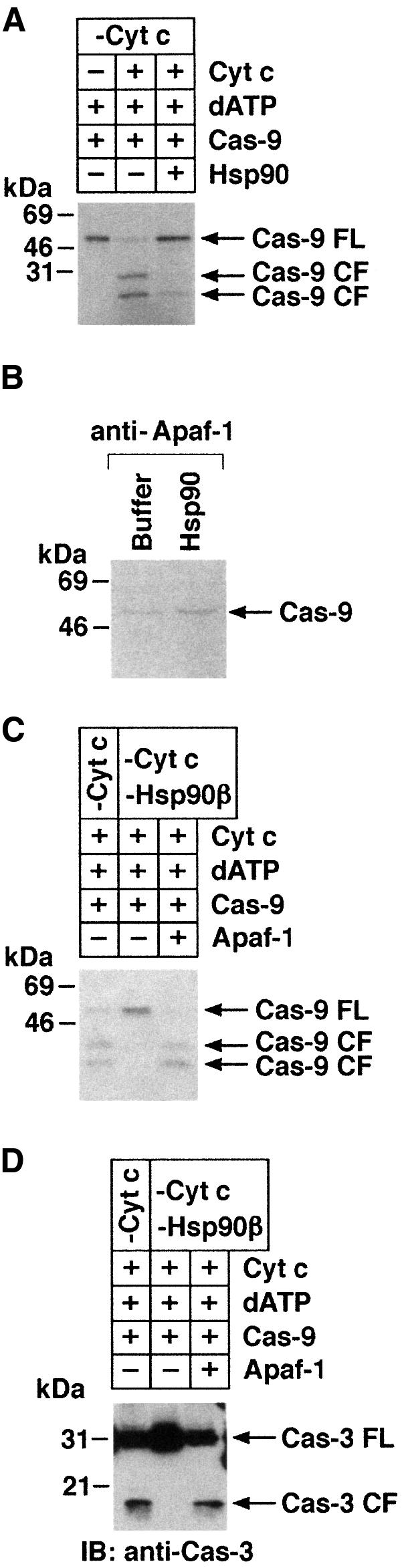
Fig. 6. (A) U-937 cytosolic lysates were immunodepleted of cyt c (lanes 1–3). Aliquots (50 µg) were then incubated with buffer (lane 1), cyt c (lanes 2 and 3) or Hsp90 (lane 3). In vitro-translated 35S-labeled caspase-9 (2 µl) was incubated in a final volume of 30 µl for 1 h at 30°C. After incubation, samples were separated by SDS–PAGE and analyzed by autoradiography. (B) Column-purified Apaf-1 was incubated with in vitro-translated 35S-labeled caspase-9 and cyt c/dATP in the presence of Hsp90 or buffer. After incubation, anti-Apaf-1 immuno precipitates were analyzed by autoradiography. (C and D) U-937 cell cytosolic lysates were immunodepleted of cyt c (lanes 1–3) or cyt c/Hsp90 (lanes 2 and 3). Aliquots (50 µg) were then incubated with cyt c/dATP (lanes 1–3), in vitro-translated 35S-labeled caspase-9 (D, lanes 1–3) in the presence (lane 3) or absence (lanes 1 and 2) of purified Apaf-1 L-WD13 in a final volume of 30 µl. After incubation at 30°C for 1 h, the proteins were separated by SDS–PAGE and analyzed by immunoblotting with anti-caspase-3 (C) or autoradiography (D).
Hsp90 inhibits oligomerization of Apaf-1 in vitro and in vivo
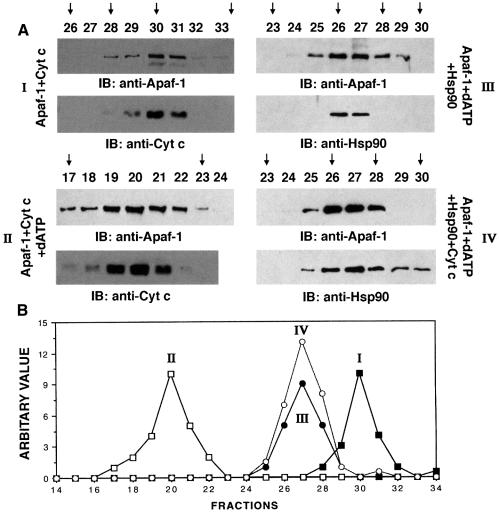
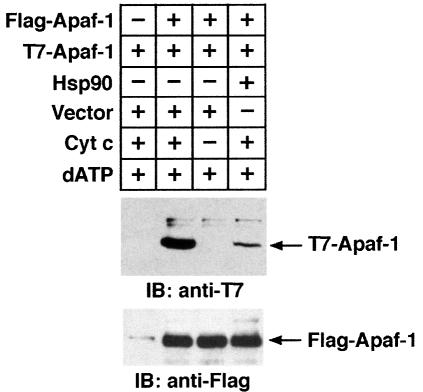
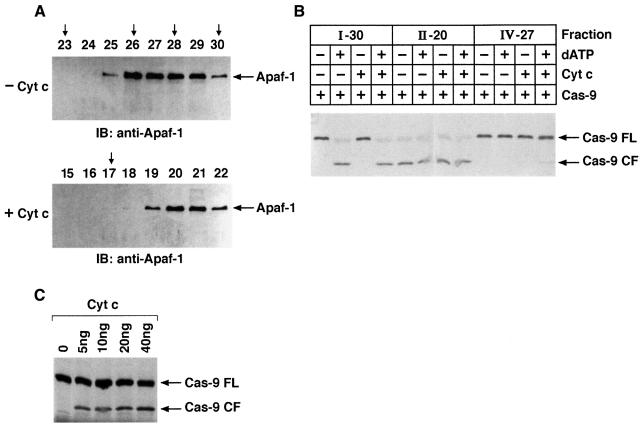
Recent observations indicate that cyt c/dATP-mediated oligomerization of Apaf-1 is critical in Apaf-1-dependent activation of procaspase-9 (Srinivasula et al., 1998; Hu et al., 1998; Adrain et al., 1999; Saleh et al., 1999; Zhou et al., 1999; Zou et al., 1999; Benedict et al., 2000). To further define the mechanism of Hsp90-mediated inhibition of procaspase-9 activation, purified recombinant Apaf-1 was incubated with cyt c/dATP in the presence or absence of Hsp90. The elution profile of Apaf-1 was assessed by FPLC gel-filtration using a Superose-6 column (Saleh et al., 1999). In the absence of cyt c and dATP, Apaf-1 eluted in peak fraction 33, corresponding to the size of the 125 kDa monomeric Apaf-1. When preincubated with cyt c alone, Apaf-1 eluted at fraction 30 (∼170 kDa) (Figure 7A and B, I). Preincubation of Apaf-1 with cyt c and dATP resulted in most of the Apaf-1 eluting in fraction 20 (∼1.4 MDa) (Figure 7A and B, II). This result and the finding that cyt c co-elutes with Apaf-1 in both fractions 30 and 20 indicated that cyt c associates with monomeric and oligomerized Apaf-1. In contrast to these findings, preincubation of Apaf-1 with Hsp90 in the presence or absence of cyt c resulted in elution of Apaf-1 at fraction 27 (∼350 kDa; Figure 7A and B, III and IV). The finding that Hsp90 co-elutes with Apaf-1 in fraction 27 indicated that Hsp90 associates with monomeric Apaf-1. Interestingly, the failure of cyt c to co-elute with Apaf-1 in fraction 27 suggested that Hsp90 competitively blocks binding of cyt c to Apaf-1 (not shown). To assess the effects of Hsp90 on oligomerization of Apaf-1 in vivo, we performed experiments using Flag- and T7-tagged Apaf-1 (Saleh et al., 1999). Lysates from cells cotransfected with Flag-Apaf-1, T7-Apaf-1 and Hsp90 or empty vector were incubated with or without cyt c/dATP. Immunoblot analysis of anti-Flag immunoprecipitates with anti-T7 demonstrated that, in contrast to vector, overexpression of Hsp90 significantly inhibited the interaction of Flag-Apaf-1 and T7-Apaf-1 (Figure 8). Taken together, these findings support a model in which binding of Hsp90 with Apaf-1 inhibits oligomerization of Apaf-1 and thereby negatively regulates its function.
Fig. 7. Hsp90 inhibits cyt c/dATP-mediated oligomerization of Apaf-1. (A) Purified Apaf-1 L-WD13 was incubated with cyt c (I), cyt c + dATP (II), dATP + Hsp90 (III) or dATP + cyt c + Hsp90 (IV). After incubation, the reaction mixtures were applied to Superose-6 FPLC column at a flow rate of 0.2 ml/min. Aliquots (45 µl) of the column fractions in each case were separated by SDS–PAGE and immunoblotted with anti-Apaf-1, anti-cyt c or anti-Hsp90. The calibration protein standards and their positions on the Superose-6 FPLC column are indicated by vertical arrows: fraction 17, 2 MDa; fraction 23, 669 kDa; fraction 26, 440 kDa, fraction 28, 232 kDa; fraction 30, 158 kDa; fraction 34, 66 kDa. (B) An elution profile of Apaf-1 L-WD13 under different conditions (I–IV) is shown.
Fig. 8. Hsp90 inhibits oligomerization of Apaf-1. 293T cells were transiently transfected with Flag-Apaf-1 (lanes 2–4) and T7-Apaf-1 (lanes 1–4) with (lane 4) or without (lanes 1–3) Hsp90. Cytosolic lysates were incubated with (lanes 1, 2 and 4) or without (lane 3) cyt c and subjected to immunoprecipitation with anti-Flag. The immuno precipitates were then analyzed by immunoblotting with anti-T7 (upper panel). Anti-Flag immunoprecipitates were also analyzed by immunoblotting with anti-Flag (lower panel).
Competition of Hsp90 and cyt c for binding to Apaf-1
Since both Hsp90 and cyt c bind directly to Apaf-1, we next examined whether these two proteins compete for binding to Apaf-1. Fractions 26 and 27 [Apaf-1 + Hsp90 + dATP (Figure 7A, III)] were pooled, concentrated and then incubated with an excess amount of purified cyt c. Following incubation for 1 h, the samples were loaded onto a Superose-6 column and the elution profile of Apaf-1 was assessed by FPLC followed by immunoblotting with anti-Apaf-1. The results demonstrate that incubation of Hsp90–Apaf-1 complexes with excess cyt c was associated with ∼40% oligomerization of Apaf-1 and elution in fraction 20 (Figure 9A). However, the remaining Apaf-1 protein was eluted in fraction 26 (data not shown). Detection of Hsp90 in fraction 26 further indicates that more cyt c is required to displace Hsp90 from Apaf-1 and thereby its oligomerization. Collectively, these findings indicate that cyt c and Hsp90 bind competitively to Apaf-1. Since our results demonstrate that Hsp90 fails to interact directly with cyt c, it is possible that the immobilized cyt c present in our affinity column functions differently than that present in solution. Immobilized cyt c may be able to bind to Apaf-1, but be kinetically unable to displace Hsp90 from Apaf-1. This property would favor the formation of a transitional tripartite complex of cyt c–Apaf 1–Hsp90 and thereby the detection of Hsp90 from cyt c affinity column.
Fig. 9. Hsp90 inhibits Apaf-1-mediated activation of caspase-9. (A) Fraction IV-26 and IV-27 from Apaf-1 + dATP + Hsp90 column eluate were pooled, concentrated and incubated at room temperature in the absence (–cyt c) or presence (+cyt c) of cyt c for 1 h. Following incubation, samples were applied to a Superose-6 FPLC column at a flow rate of 0.2 ml/min. Aliquots (45 µl) of the column fractions in each case were separated by SDS–PAGE and immunoblotted with anti-Apaf-1. (B) 35S-labeled caspase-9 was incubated with peak fractions (I-30, II-20 or IV-27) in the presence or absence of cyt c/dATP. Following incubation, samples were then analyzed by SDS–PAGE and autoradiography. Full-length caspase-9 (FL) and the p35 fragment of mature caspase-9 (CF) are shown. (C) Peak fraction from Apaf-1 + dATP + Hsp90 column eluate was incubated with 35S-labeled caspase-9 in the absence (lane 1) or presence (lanes 2–5) of increasing concentrations of cyt c. Following incubation, samples were analyzed by SDS–PAGE and autoradiography.
Hsp90 inhibits Apaf-1-mediated activation of procaspase-9
To assess activation of caspase-9, equal amounts of Apaf-1 from peak fractions (I-30, II-20 and IV-27) were incubated with 35S-labeled caspase-9 in the presence or absence of cyt c and dATP. The results demonstrate that, in contrast to oligomeric Apaf-1 (fraction II-20), the Apaf-1–Hsp90 complex (fraction IV-27) was ineffective in activating caspase-9 in the presence of cyt c/dATP (Figure 9B). Moreover, the finding that addition of an excess amount of cyt c to fraction IV-27 restored activation of caspase-9 (Figure 9C) provided further support for competitive binding of cyt c and Hsp90 to Apaf-1.
Hsp90 inhibits stress-induced oligomerization of Apaf-1 and activation of caspase-3
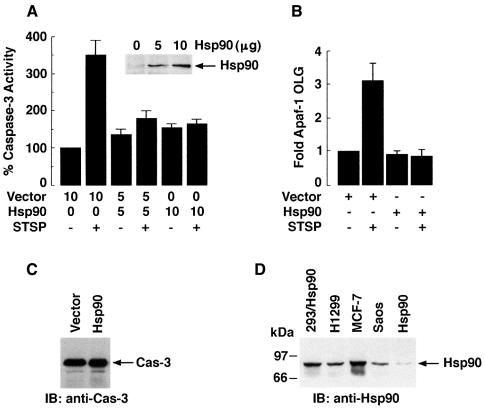
To further define the physiological role of Hsp90 in cyt c-dependent activation of caspase-3 in response to stress, L929 cells were cotransfected to express Hsp90 and pEGFP. Transfected cells were selected by fluorescence-activated cell sorting (FACS), stabilized in culture media for 24 h, and then treated with staurosporine (STSP) for 18 h. The results demonstrate that Hsp90 inhibits STSP-induced caspase-3 activity (Figure 10A). To further determine whether Hsp90 inhibits stress-induced oligomerization of Apaf-1, 293T cells transfected with T7-Apaf-1 and Flag-Apaf-1 in the presence or absence of Hsp90 were treated with STSP. Immunoblot analysis of anti-T7 precipitates with anti-Flag demonstrated that overexpression of Hsp90 inhibits STSP-induced oligomerization of Apaf-1 (Figure 10B). Moreover, overexpression of Hsp90 has no effect on caspase-3 levels (Figure 10C). In addition, the level of Hsp90 obtained with overexpression in 293T cells was comparable to that in tumor cell lines (Figure 10D). These findings indicate that the level of Hsp90 required to negatively regulate oligomerization of Apaf-1 is achieved in tumor cells. To demonstrate the physiological role of Hsp90 in cyt c-induced apoptosis, H1299 non-small cell lung carcinoma cells, which express high levels of Hsp90 [as compared with U-937 cells (Figure 11A)] were treated with STSP and S-100 cytosolic extracts were analyzed by immunoblotting with anti-cyt c and anti-caspase-3. The results demonstrate that treatment of H1299 cells with STSP is associated with accumulation of cyt c in the cytosol (Figure 11B). More importantly, treatment of H1299 cells with STSP is associated with significant inhibition in caspase-3 activation (Figure 11B). Similar results were obtained when SW2, small cell lung carcinoma cells, which express high levels of Hsp90, were treated with STSP (data not shown). In addition, H1299 and SW2 cells were also associated with the delay in STSP-induced apoptosis (data not shown). To further demonstrate the role of Hsp90 in cyt c-mediated activation of caspase-3, S-100 cytosolic extracts (without cyt c leakage) prepared from H1299 cells were incubated with different concentrations of purified cyt c. As control, S-100 extracts from U-937 cells were incubated separately with different concentrations of cyt c. Following incubation, lysates were analyzed by immunoblotting with anti-caspase-3. The results demonstrate that, in contrast to U-937 cell lysates, incubation of H1299 cell lysates with cyt c was associated with significant inhibition of caspase-3 activation (Figure 11C). Taken together, these findings demonstrate that Hsp90 inhibits cyt c-mediated activation of caspase-3 downstream of cyt c release.
Fig. 10. Hsp90 inhibits oligomerization of Apaf-1 and activation of caspase-3 in response to stress. (A) L929 cells were transfected with empty vector or 5 and 10 µg of vector expressing Hsp90. Cells were also cotransfected with pEGFP. Following transfection, GFP-positive cells were selected by FACS, stabilized in culture for 24 h and treated with 1 µg/ml STSP for 18 h. Total lysates from untreated and STSP-treated cells were analyzed for caspase-3 activity by measuring cleavage of an exogenous peptide substrate (DEVD-pNA). Results are expressed as percentage caspase-3 activity (mean ± SD) (normalized to untreated cells expressing vector) of four independent experiments. Total cell lysates were also analyzed by immunoblotting with anti-Hsp90 (insert). (B) 293T cells were cotransfected with T7-Apaf-1, Flag-Apaf-1 and empty vector or Hsp90. Following transfection, cells were treated with 1 µM STSP and harvested after 18 h. Total cell lysates were subjected to immunoprecipitation with anti-T7 and analyzed by immunoblotting with anti-Flag. Results are expressed as fold-Apaf-1 oligomerization (mean + SD) of three independent experiments. (C) 293T cells were transiently transfected with empty vector or Hsp90. Total cell lysates were analyzed by immunoblotting with anti-caspase-3. (D) 293T cells were transiently transfected with Hsp90. Total lysates from transfected 293T cells and lysates of H1299, MCF-7 and SAOS cells were analyzed by immunoblotting with anti-Hsp90. Hsp90-immunodepleted S-100 extract of U-937 cells was incubated with 1 µg Hsp90 and analyzed by immunoblotting with anti-Hsp90.
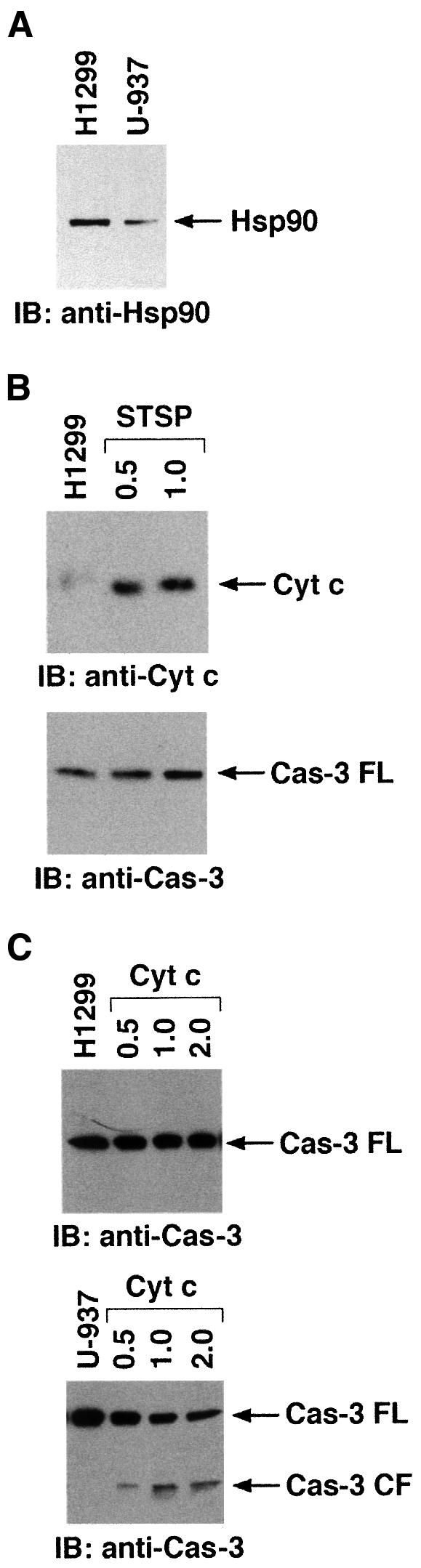
Fig. 11. (A) Total cell lysates from H1299 and U-937 cells were analyzed by immunoblotting with anti-Hsp90. (B) H1299 cells were treated with 0.5 or 1 µM STSP and harvested after 18 h. S-100 cytosolic extracts were prepared and analyzed by immunoblotting with anti-cyt c (upper panel) or anti-caspase-3 (lower panel). (C) S-100 cytosolic extracts from untreated H1299 (upper panel) or U-937 (lower panel) cells were incubated with 0, 0.25, 0.5 or 1 µg of purified cyt c in the presence of 1 mM dATP for 30 min at room temperature. Following incubation, proteins were separated by SDS–PAGE and analyzed by immunoblotting with anti-caspase-3.
Our results demonstrate that Hsp90 constitutively associates with Apaf-1 under non-apoptotic conditions (Figure 5), while Hsp90 and cyt c compete for binding to Apaf-1 in the setting of an apoptotic signal. Since an excess amount of cyt c is required to displace Hsp90 from Apaf-1 (Figure 9A), these findings indicate that the affinity of Hsp90 binding to Apaf-1 is greater than that of cyt c. Moreover, the finding that Apaf-1 is unable to oligomerize if Hsp90 is overexpressed provides a mechanistic explanation for why cells expressing high levels of Hsp90 are resistant to cyt c-mediated activation of caspase-3 and thereby to apoptosis.
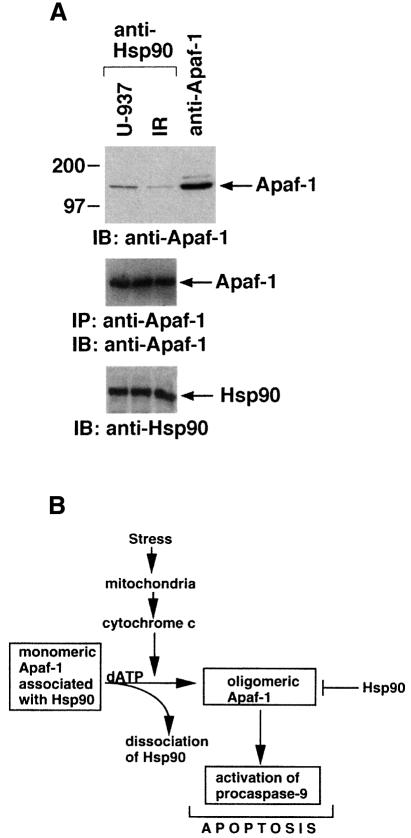
As treatment with cytotoxic agents is associated with release of cyt c (Liu et al., 1996; Kharbanda et al., 1997; Kluck et al., 1997; Narula et al., 1999), cells were exposed to ionizing radiation (IR) and analyzed for binding of Hsp90 and Apaf-1. The results demonstrate that IR exposure inhibits the interaction between Hsp90 and Apaf-1 (Figure 12A). Similar results were obtained when cells were treated with other cytotoxic agents, including ara-C, cis-platinum or STSP (data not shown). These results suggest that signals induced in response to stress regulate the interaction between Hsp90 and Apaf-1, and thereby relieve inhibition of cyt c-mediated oligomerization of Apaf-1. Furthermore, these findings suggest that, as a consequence of mitochondrial outer membrane rupture, stress-induced increases in cytosolic cyt c potentiate, at least in part, dissociation of Hsp90 from Apaf-1 and thereby stimulate its oligomerization. Our in vitro results indicate that higher concentrations of cyt c are required to dissociate Hsp90 from Apaf-1. However, in contrast to wild-type cells, STSP treatment of cells overexpressing Hsp90 was associated with a significant delay in apoptosis. Collectively, these findings indicate that cytosolic cyt c levels may be insufficient to dissociate Hsp90 from Apaf-1. Thus, other factors functioning downstream of cyt c might cooperate with cyt c to dissociate Hsp90 completely from Apaf-1. Another possibility is that post-translational modification of Hsp90 and/or Apaf-1 could be responsible for regulating the interaction. Alternatively, cleavage of Hsp90 following exposure of certain cell types to genotoxic stress (Prasad et al., 1998) could abrogate the inhibitory effect of Hsp90 on Apaf-1.
Fig. 12. (A) U-937 cells were exposed to 20 Gy IR and harvested at 6 h. Cytosolic lysates were immunoprecipitated with anti-Hsp90 and analyzed by immunoblotting with anti-Apaf-1 (upper panel). Anti-Apaf-1 immunoprecipitates were analyzed separately by immunoblotting with anti-Apaf-1 (middle panel). Total cell lysates were also analyzed by immunoblotting with anti-Hsp90 (lower panel). (B) Schematic representation of the role of Hsp90 in cyt c/dATP-dependent oligomerization and activation of Apaf-1.
The demonstration that Hsp90 is induced in response to diverse apoptotic stimuli, such as UV, doxorubicin and sodium arsenite, supports involvement of Hsp90 in an essential defense mechanism of stress tolerance (Ferrarini et al., 1992; Maytin, 1992; Parsell and Lindquist, 1993; Ali et al., 1996; Bertram et al., 1996; Nayeem et al., 1997). However, the mechanisms responsible for the protective function of Hsp90 are for the most part unclear. The present results demonstrate that Hsp90 inhibits cyt c-mediated oligomerization of Apaf-1 and thereby cleavage of caspase-9 and caspase-3 both in vitro and in response to stress (Figure 12B). The results also demonstrate that stress-induced signals relieve Hsp90-mediated inhibition of Apaf-1 oligomerization. These findings support a model in which Hsp90 functions as a physiological negative regulator of the cyt c-mediated apoptotic protease cascade.
Materials and methods
Cell culture and reagents
Human U-937 cells were grown in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (HI-FBS), 100 units/ml penicillin, 100 µg/ml streptomycin and 2 mM l-glutamine. 293T, L929, MCF-7, H1299 (non-small cell lung carcinova cell line), SW2 (small cell lung carcinoma cell line), Cos and Saos cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) with 10% HI-FBS and antibiotics. Cyt c was obtained from Sigma. Hsp90 and Hsp60 proteins were obtained from Stressgen, Victoria, BC, Canada. Recombinant caspase-8 and caspase-9 cDNA were provided by Robert Talanian, BASF, Worcester, MA.
Isolation of cytoplasmic fractions
Cells were washed twice with phosphate-buffered saline (PBS), and cell fractionation was performed as described (Kharbanda et al., 1997). In brief, after washing the cells with PBS, the pellet was suspended in 5 ml of ice cold buffer A [20 mM HEPES pH 7.5, 1.5 mM MgCl2, 10 mM KCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol (DTT) and 0.1 mM phenylmethylsulfonyl fluoride] with 10 µg each of aprotinin, pepstatin and leupeptin, containing 250 mM sucrose. The cells were then homogenized by douncing three times in a Dounce homogenizer with a sandpaper-polished pestle. After centrifugation for 5 min at 4°C, the supernatants were collected and centrifuged at 105 000 g for 30 min at 4°C. The resulting supernatant was used as the soluble S-100 extract.
Purification and characterization of the cytosolic cyt c-binding protein
Untreated U-937 cell S-100 extract was applied to a column prepared from cyt c bound to CNBr–sepharose beads (Sigma). After washing, the column was eluted a linear NaCl gradient (0–600 mM). The resultant fractions were then separated by SDS–PAGE and analyzed by silver staining. The protein band at ∼90 kDa was excised from the gels and identified by trypsin digestion and internal peptide sequence analysis.
Immunoprecipitations and immunoblot analysis
Immunoprecipitations were performed as described previously (Kharbanda et al., 1995). In brief, cell lysates were incubated with anti-Hsp90β (Santa Cruz Biotechnology, Santa Cruz, CA), anti-Hsp60 (Stressgen), anti-Flag (Kodak), anti-Apaf-1 (Zou et al., 1997), anti-Hsp27 (Santa Cruz), anti-Hsp90α (Stressgen), anti-T7 (Novagen, Madison, WI) or anti-cyt c (Kirken et al., 1995) antibodies. The resulting immunoprecipitates were analyzed by immunoblotting with anti-cyt c or anti-Hsp90. Anti-Hsp90 immunoprecipitates were also analyzed by immunoblotting with anti-Apaf-1, anti-caspase-9 (Li et al., 1997), anti-PKCδ (Santa Cruz) or anti-caspase-3 (Transduction Laboratories, Franklin Lakes, NJ) antibodies. Total cell lysates prepared from H1299 cells were analyzed by immunoblotting with anti-Hsp90 antibody (#SPA835; Stressgen).
Assay for caspase-3 and PKCδ activation
S-100 extracts were prepared as described (Kharbanda et al., 1997). Lysates were immunodepleted of cyt c and/or Hsp90 by multiple immunoprecipitations. Immunodepletion was confirmed by immunoblot analysis. The cyt c-depleted fractions were incubated with purified cyt c and/or Hsp90 protein in buffer B (20 mM HEPES pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT and 1 mM dATP) for 1 h at 30°C. The reactions were stopped by boiling in 6× SDS sample buffer. Proteins were separated by SDS–PAGE, transferred to nitocellulose filters and analyzed by immunoblotting with anti-caspase-3 or anti-PKCδ (Santa Cruz) antibodies. S-100 cytosolic extracts from untreated H1299 and U-937 cells were incubated with 0.5 and 1 µg of cyt c in the presence of 1 mM dATP for 30 min at room temperature. Following incubation, the proteins were separated on SDS–PAGE and analyzed by immunoblotting with anti-caspase-3.
Interactions of Apaf-1 with caspase-9 in vitro
Caspase-9 was in vitro translated and purified as described (Talanian et al., 1997). Column-purified Apaf-1 was incubated with in vitro-translated procaspase-9 in the presence of cyt c/dATP and in the presence or absence of Hsp90 for 2 h at 4°C. After incubation the reaction products were diluted with lysis buffer and subjected to immunoprecipitation with anti-Apaf-1. The immunoprecipitates were then analyzed by autoradiography.
Assay for caspase-9 activation
U-937 S-100 extracts were immunodepleted of cyt c and/or Hsp90. The depleted fractions were incubated with purified cyt c/dATP and in vitro-translated procaspase-9 with or without Hsp90 or column-purified Apaf-1 for 1 h at 30°C. The reaction was terminated by the addition of SDS sample buffer and boiling for 5 min. The proteins were separated by SDS–PAGE and analyzed by autoradiography.
Transient transfections
293T cells were transiently transfected with Flag-Apaf-1 and T7-Apaf-1 with or without Hsp90 by Lipofectamine as described (Pandey et al., 1999). Cytosolic S-100 lysates were incubated with or without cyt c and subjected to immunoprecipitation with anti-Flag (M2; IBI, Kodak). The immunoprecipitates were then analyzed by immunoblotting with anti-T7 (Novagen, Madison, WI). Anti-Flag immunoprecipitates were also analyzed by immunoblotting with anti-Flag. L929 cells were transfected with empty vector or 5 and 10 µg of vector expressing Hsp90. Cells were also cotransfected with pEGFP. Following transfection, GFP-positive cells were selected by FACS, stabilized in culture for 24 h and treated with 1 µM STSP for 18 h. Total lysates from untreated and STSP-treated cells were analyzed for caspase-3 activity by measuring cleavage of an exogenous peptide substrate (DEVD-pNA) (Promega caspase-3 kit, Promega, Madison, WI).
Oligomerization of Apaf-1
Oligomerization reactions were performed by incubating Apaf-1 (3 µg) at 4°C for 1 h in oligomerization buffer (25 mM HEPES pH 7.5, 50 mM NaCl, 10 mM KCl, 1.5 mM MgCl2, 10% glycerol, 0.1 mM DTT). The reaction mixtures were applied to Superose-6 FPLC column at a flow rate of 0.2 ml/min. Aliquots (45 µl) of the 500 µl fractions were fractionated by SDS–PAGE and analyzed by immunoblotting with anti-Apaf-1, anti-cyt c or anti-Hsp90 antibodies.
Acknowledgments
Acknowledgements
We thank Dr Lawrence Prochaska for providing the anti-cyt c antibody; Dr Xiaodong Wang for anti-Apaf-1 and anti-caspase-9 antibodies. We also thank Dr Robert Talanian for caspase-3 and caspase-9 cDNAs and for recombinant caspase-8; Dr Mike Robertson for anti-Fas. This investigation was supported by PHS Grant CA75216 (S.Kh.) awarded by the National Cancer Institute, DHHS; Charlette Geyer Foundation award (S.Kh.) and a Research Grant from Novartis Pharmaceutical Corporation (S.Kh. and D.K.).
References
- Adrain C., Slee,E., Harte,M. and Martin,S. (1999) Regulation of apoptotic protease activating factor-1 oligomerization and apoptosis by the WD-40 repeat region. J. Biol. Chem., 274, 20855–20860. [DOI] [PubMed] [Google Scholar]
- Ali A., Krone,P., Pearson,D. and Heikkila,J. (1996) Evaluation of stress-inducible hsp90 gene expression as a potential molecular biomarker in Xenopus laevis.Cell Stress Chaperones, 1, 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnemri E.S., Livingston,D.J., Nicholson,D.W., Salvesen,G., Thornberry,N.A., Wong,W.W. and Yuan,J. (1996) Human ICE/CED-3 protease nomenclature. Cell, 87, 171. [DOI] [PubMed] [Google Scholar]
- Benedict M., Hu,Y., Inohara,N. and Nunez,G. (2000) Expression and functional analysis of Apaf-1 isoforms. Extra WD-40 repeat is required for cytochrome c binding and regulated activation of procaspase-9. J. Biol. Chem., 275, 8461–8468. [DOI] [PubMed] [Google Scholar]
- Bertram J., Palfner,K., Hiddemann,W. and Kneba,M. (1996) Increase of P-glycoprotein-mediated drug resistance by hsp90 β. Anti-Cancer Drugs, 7, 838–845. [DOI] [PubMed] [Google Scholar]
- Bohen S.P. and Yamamoto,K.R. (1994) The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Boldin M., Goncharov,T., Goltsev,Y. and Wallach,D. (1996) Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell-death. Cell, 85, 803–815. [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E., Newmeyer,D. and Green,D. (1998) Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J., 17, 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain K., Brown,D.G., Langlais,C. and Cohen,G.M. (1999) Caspase activation involves the formation of the aposome, a large (approximately 700 kDa) caspase-activating complex. J. Biol. Chem., 274, 22686–22692. [DOI] [PubMed] [Google Scholar]
- Cecconi F., Bolado,G.A., Meyer,B.I., Roth,K. and Gruss,P. (1998) Apaf-1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell, 94, 727–737. [DOI] [PubMed] [Google Scholar]
- Chadli Ahmed M.L.M., Emile,B.E. and Grazia,C. (1999) Heat-induced oligomerization of the molecular chaperone Hsp90. J. Biol. Chem., 274, 4133–4139. [DOI] [PubMed] [Google Scholar]
- Chauhan D. et al. (1997) Cytochrome-C dependent and independent induction of apoptosis in multiple myeloma cells. J. Biol. Chem., 272, 29995–29997. [DOI] [PubMed] [Google Scholar]
- Cohen G.M. (1997) Caspases: the executioners of apoptosis. Biochem. J., 326, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryns V. and Yuan,J. (1998) Proteases to die for. Genes Dev., 12, 1551–1570. [DOI] [PubMed] [Google Scholar]
- Cutforth T. and Rubin,G. (1994) Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila.Cell, 77, 1027–1036. [DOI] [PubMed] [Google Scholar]
- Ellis R. (1996) Discovery of molecular chaperones. Cell Stress Chaperones, 1, 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto Y. et al. (1995) Proteolytic activation of protein kinase Cδ by an ICE-like protease in apoptotic cells. EMBO J., 14, 6148–6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enari M., Sakahira,H., Yokoyama,H., Okawa,K., Iwanatsu,A. and Natata,S. (1998) A caspase-activated DNase that degrades DNA during apoptosis and its inhibitor ICAD. Nature, 391, 43–50. [DOI] [PubMed] [Google Scholar]
- Fearnhead H., Rodriguez,J., Govek,E., Guo,W., Kobayashi,R., Hannon,G. and Lazebnik,Y. (1998) Oncogene-dependent apoptosis is mediated by caspase-9. Proc. Natl Acad. Sci. USA, 95, 13664–13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarini M., Heltai,S., Zocchi,M. and Rugarli,C. (1992) Unusual expression and localization of heat-shock proteins in human tumor cells. Int. J. Cancer, 51, 613–619. [DOI] [PubMed] [Google Scholar]
- Freeman B.C. and Morimoto,R.I. (1996) The human cytosolic molecular chaperones hsp90, hsp70 (hsc70) and hdj-1 have distinct roles in recognition of a non-native protein and protein refolding. EMBO J., 15, 2969–2979. [PMC free article] [PubMed] [Google Scholar]
- Gething M. and Sambrook,J. (1992) Protein folding in the cell. Nature, 355, 33–45. [DOI] [PubMed] [Google Scholar]
- Ghayur T. et al. (1996) Proteolytic activation of protein kinase C δ by an ICE/CED 3-like protease induces characteristics of apoptosis. J. Exp. Med., 184, 2399–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A., Yin,X.-M., Wang,K., Wei,M.C., Jockel,J., Milliman,C., Erdjument-Bromage,H., Tempst,P. and Kormeyer,S.J. (1999) Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-xL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem., 274, 1156–1163. [DOI] [PubMed] [Google Scholar]
- Hakem R. et al. (1998) Differential requirement for caspase-9 in apoptotic pathways in vivo. Cell, 94, 339–352. [DOI] [PubMed] [Google Scholar]
- Hu Y., Ding,L., Spencer,D. and Nunez,G. (1998) WD-40 repeat region regulates Apaf-1 self-association and procaspase-9 activation. J. Biol. Chem., 273, 33489–33494. [DOI] [PubMed] [Google Scholar]
- Huang D.C., Hahne,M., Schroeter,M., Frei,K., Fontana,A., Villunger,A., Newton,K., Tschopp,J. and Strasser,A. (1999) Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-x (L). Proc. Natl Acad. Sci. USA, 96, 14871–14876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob U. and Buchner,J. (1994) Assisting spontaneity: the role of Hsp90 and small Hsps as molecular chaperones. Science, 19, 205–211. [DOI] [PubMed] [Google Scholar]
- Janicke R.U., Walker,P.A., Lin,X.Y. and Porter,A.G. (1996) Specific cleavage of the retinoblastoma protein by an ICE-like protease in apoptosis. EMBO J., 15, 6969–6978. [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S.H., Desnoyers,S., Ottaviano,Y., Davidson,N.E. and Poirier,G.G. (1993) Specific proteolytic cleavage of poly (ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res., 53, 3976–3985. [PubMed] [Google Scholar]
- Kharbanda S., Saleem,A., Shafman,T., Emoto,Y., Weichselbaum,R., Woodgett,J., Avruch,J., Kyriakis,J. and Kufe,D. (1995) Ionizing radiation stimulates a Grb2-mediated association of the stress activated protein kinase with phosphatidylinositol 3-kinase. J. Biol. Chem., 270, 18871–18874. [DOI] [PubMed] [Google Scholar]
- Kharbanda S., Pandey,P., Schofield,L., Roncinske,R., Bharti,A., Yuan,Z., Weichselbaum,R., Nalin,C. and Kufe,D. (1997) Role for Bcl-xL as an inhibitor of cytosolic cytochrome C accumulation in apoptosis. Proc. Natl Acad. Sci. USA, 94, 6939–6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.N., Wang,X., Huang,Y., Ibrado,A.M., Liu,L., Fang,G. and Bhalla,K. (1997) Overexpression of Bcl-xL inhibits ara-C-induced mitochondrial loss of cytochrome c and other perturbations that activate the molecular cascade of apoptosis. Cancer Res., 57, 3115–3120. [PubMed] [Google Scholar]
- Kirken R., Lincoln,A., Fink,P. and Prochaska,L. (1995) High yield purification of a four subunit caa3-type cytochrome oxidase from the thermophilic bacterium Bacillus PS3 using fast protein liquid chromatography. Protein Exp. Purif., 6, 707–715. [DOI] [PubMed] [Google Scholar]
- Kluck R.M., Bossy-Wetzel,E., Green,D.R. and Newmeyer,D.D. (1997) The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science, 275, 1132–1136. [DOI] [PubMed] [Google Scholar]
- Lazebnik Y.A., Kaufmann,S.H., Desnoyers,S., Poirier,G.G. and Earnshaw,W.C. (1994) Cleavage of poly (ADP-ribose) polymerase by a proteinase with properties like ICE. Nature, 371, 346–347. [DOI] [PubMed] [Google Scholar]
- Li H., Zhu,H., Xu,C.-j. and Yuan,J. (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the fas pathway of apotosis. Cell, 94, 491–501. [DOI] [PubMed] [Google Scholar]
- Li P., Nijhawan,D., Budihardjo,I., Srinivasula,S.M., Ahmad,M., Alnemri,E.S. and Wang,X. (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell, 91, 479–489. [DOI] [PubMed] [Google Scholar]
- Liu X., Kim,C., Yang,J., Jemmerson,R. and Wang,X. (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell, 86, 147–157. [DOI] [PubMed] [Google Scholar]
- Luo X., Budiharjo,H., Zou,H., Slaughter,C. and Wang,X. (1998) Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell, 94, 481–490. [DOI] [PubMed] [Google Scholar]
- Martin S.J. and Green,D.R. (1995) Protease activation during apoptosis: death by a thousand cuts? Cell, 82, 349–352. [DOI] [PubMed] [Google Scholar]
- Maytin E. (1992) Differential effects of heat shock and UVB light upon stress protein expression in epidermal keratinocytes. J. Biol. Chem., 267, 23189–23196. [PubMed] [Google Scholar]
- Morimoto R., Tissieres,A. and Georgopoulos,C. (1990) Stress Proteins in Biology and Medicine. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 1–36. [Google Scholar]
- Muzio M. et al. (1996) FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell, 85, 817–827. [DOI] [PubMed] [Google Scholar]
- Narula J. et al. (1999) Apoptosis in heart failure: Release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc. Natl Acad. Sci. USA, 96, 8144–8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayeem M., Hess,M., Qian,Y., Loesser,K. and Kukreja,R. (1997) Delayed preconditioning of cultured adult rat cardiac myocytes: role of 70- and 90-kDa heat stress proteins. Am. J. Physiol., 273, 861–868. [DOI] [PubMed] [Google Scholar]
- Nicholson D.W. and Thornberry,N.A. (1997) Caspases: killer proteases. Trends Biochem. Sci., 257, 299–306. [DOI] [PubMed] [Google Scholar]
- Nicholson D.W. et al. (1995) Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature, 376, 37–43. [DOI] [PubMed] [Google Scholar]
- Orth K., Chinnaiyan,A.M., Garg,M., Froelich,C.J. and Dixit,V.M. (1996) The CED-3/ICE-like protease Mch2 is activated during apoptosis and cleaves the death substrate Lamin A. J. Biol. Chem., 271, 16443–16446. [PubMed] [Google Scholar]
- Pandey P. et al. (1999) Activation of p38 mitogen-activated kinase PYK2/Related adhesion focal tyrosine kinase-dependent mechanism. J. Biol. Chem., 274, 10140–10144. [DOI] [PubMed] [Google Scholar]
- Parsell D. and Lindquist,S. (1993) The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu. Rev. Genet., 27, 437–496. [DOI] [PubMed] [Google Scholar]
- Prasad S., Soldatenkov,V., Srinivasula,G. and Dritschilo,A. (1998) Identification of keratins 18, 19 and heat-shock protein 90 beta as candidate substrates of proteolysis during ionizing radiation-induced apoptosis of estrogen-receptor negative breast tumor cells. Int. J. Oncol., 13, 757–764. [DOI] [PubMed] [Google Scholar]
- Pratt W.B. and Welsh,M.J. (1994) Chaperone functions of the heat shock proteins associated with steroid receptors. Semin. Cell Biol., 5, 83–93. [DOI] [PubMed] [Google Scholar]
- Rodriguez J. and Lazebnik,Y. (1999) Caspase-9 and APAF-1 form an active holoenzyme. Genes Dev., 13, 3179–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A., Srinivasula,S., Acharya,S., Fishel,R. and Alnemri,E. (1999) Cytochrome c and dATP-mediated oligomerization of Apaf-1 is a prerequiste for procaspase-9 activation. J. Biol. Chem., 274, 17941–17945. [DOI] [PubMed] [Google Scholar]
- Salvesen G.S. and Dixit,V.M. (1997) Caspases: intracellular signaling by proteolysis. Cell, 91, 443–446. [DOI] [PubMed] [Google Scholar]
- Scheibel T. and Buchner,J. (1998) The Hsp90 complex—a super-chaperone machine as a novel drug target. Biochem. Pharmacol., 56, 675–682. [DOI] [PubMed] [Google Scholar]
- Schneider C., Sepp-Lorenzino,L., Nimmesgem,E., Ouerfelli,O., Danishefsky,S., Rosen,N. and Hartl,F.U. (1996) Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc. Natl Acad. Sci. USA, 93, 14536–14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Cao,C., Shao,R. and Pommier,Y. (1998) Lamin B phosphorylation by protein kinase Cα and proteolysis during apoptosis in human leukemia HL60 cells. J. Biol. Chem., 273, 8669–8674. [DOI] [PubMed] [Google Scholar]
- Soengas M., Alarcon,R., Yoshida,H., Giaccia,A., Hakem,R., Mak,T. and Lowe,S. (1999) Apaf-1 and caspase-9 in p53-dependent apoptosis tumor inhibition. Science, 284, 156. [DOI] [PubMed] [Google Scholar]
- Song Q. et al. (1996) DNA-dependent protein kinase catalytic subunit: a target for an ICE-like protease in apoptosis. EMBO J., 15, 3238–3246. [PMC free article] [PubMed] [Google Scholar]
- Srinivasula S., Ahmad,M., Alnemri,T. and Alnemri,E. (1998) Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol. Cell, 1, 949–957. [DOI] [PubMed] [Google Scholar]
- Stennicke H. et al. (1998) Pro-caspase-3 is a major physiologic target of caspase-8. J. Biol. Chem., 273, 27084–27090. [DOI] [PubMed] [Google Scholar]
- Sun X., Macfarlane,M., Zhuang,J., Wolf,B., Green,D. and Cohen,G. (1999) Distinct caspase cascades are inhibited in receptor-mediated and chemical-induced apoptosis. J. Biol. Chem., 274, 5053–5060. [DOI] [PubMed] [Google Scholar]
- Takahashi A. et al. (1996) Cleavage of lamin A by Mch2α but not CPP32: Multiple interleukin 1β-converting enzyme-related proteases with distinct substrate recognition properties are active in apoptosis. Proc. Natl Acad. Sci. USA, 93, 8395–8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talanian R.V., Quinlan,C., Trautz,S., Hackett,M.C., Mankovich,J.A., Banach,D., Ghayur,T., Brady,K.D. and Wong,W.W. (1997) Substrate specificities of caspase family proteases. J. Biol. Chem., 272, 9677–9682. [DOI] [PubMed] [Google Scholar]
- Tewari M., Quan,L.T., O’Rourke,K., Desnoyers,S., Zeng,Z., Beidler,D.R., Poirier,G.G., Salvesen,G.S. and Dixit,V.M. (1995) Yama/CPP32β, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly (ADP-ribose) polymerase. Cell, 81, 801–809. [DOI] [PubMed] [Google Scholar]
- Thornberry N. and Lazebnik,Y. (1998) Caspases: Enemies within. Science, 281, 1312–1316. [DOI] [PubMed] [Google Scholar]
- Wang X., Zelenski,N.G., Yang,J., Sakai,J., Brown,M.S. and Goldstein,J.L. (1996) Cleavage of sterol regulatory element binding proteins (SREBPs) by CPP32 during apoptosis. EMBO J., 15, 1012–1020. [PMC free article] [PubMed] [Google Scholar]
- Xu Y. and Lindquist,S. (1993) Heat shock protein Hsp90 governs the activity of v-src kinase. Proc. Natl Acad. Sci. USA, 90, 7074–7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Liu,X., Bhalla,K., Kaekyung Kim,C., Ibrado,A.M., Cai,J., Peng,T.I., Jones,D.P. and Wang,X. (1997) Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science, 275, 1129–1132. [DOI] [PubMed] [Google Scholar]
- Yonehara M., Minami,Y., Kwata,Y., Nagai,J. and Yahara,I. (1996) Heat-induced chaperone activity of Hsp90. J. Biol. Chem., 271, 2641–2645. [DOI] [PubMed] [Google Scholar]
- Yoshida H. et al. (1998) Apaf-1 is required for mitochondrial pathways in vivo. Cell, 94, 739–750. [DOI] [PubMed] [Google Scholar]
- Zhou P., Chou,J., Olea,S., Yuan,J. and Wagner,G. (1999) Solution structure of Apaf-1 CARD and its interaction with caspase-9 CARD: A structural basis for specific adaptor/caspase interaction. Proc. Natl Acad. Sci. USA, 96, 11265–11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H., Henzel,W.J., Liu,X., Lutschg,A. and Wang,X. (1997) Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell, 90, 405–413. [DOI] [PubMed] [Google Scholar]
- Zou H., Li,X., Liu,X. and Wang,X. (1999) An APAF-1 cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem., 274, 11549–11556. [DOI] [PubMed] [Google Scholar]