Abstract
AIM: To evaluate the efficacy and acceptability of magnesium citrate and a single dose of oral sodium phosphate (45 mL) solution for morning colonoscopy bowel preparation.
METHODS: A total of 159 patients were randomly assigned to receive two split doses of 90 mg of sodium phosphate (Group I, n = 79) or magnesium citrate (250 mL, the day before the procedure) followed by 45 mL of sodium phosphate (the day of procedure, Group II, n = 80). The quality of bowel cleansing and the acceptability of each regimen were compared, including the satisfaction, taste, willing to repeat and adverse effects of each regimen.
RESULTS: The quality of bowel cleansing of Group II was as good as that of Group I (An Aronchick scale score of good or excellent: 70.9% vs 81.0%, respectively, P = 0.34; the Ottawa system score: 4.4 ± 2.6 vs 3.8 ± 3.0, respectively, P = 0.76). There was no statistically significant difference between both groups with regard to acceptability, including the satisfaction, taste and willingness to repeat the regimen. A significantly greater number of older patients (over 65 years old) in Group II graded the overall satisfaction as satisfactory (48.1% vs 78.1%, respectively; Group I vs Group II, P = 0.01). There were no significant adverse reactions.
CONCLUSION: Magnesium citrate and a single dose of sodium phosphate was as effective and tolerable as the conventional sodium phosphate regimen and is a satisfactory option.
Keywords: Colonoscopy, Bowel preparation, Efficacy, Acceptability, Magnesium citrate, Sodium phosphate
INTRODUCTION
The worldwide introduction of screening colonoscopy programs has led to a dramatic increase in the number of colonoscopies. Despite the development of techniques and instruments, the difficulties in bowel preparation for a complete colonoscopic examination still remain a challenge to the physician and the examinee. These difficulties may result in significant noncompliance and thus suboptimal preparation, which subsequently may lead to missed lesions[1], an increased procedure time and the need for repeat colonoscopy[2], as well as reluctance to undergo follow-up examinations.
Sodium phosphate is a low-volume laxative, and it is known to be superior to 4l polyethylene glycol (PEG) solution, both for acceptability by the patient and for the quality of bowel cleansing[3-6]. However, a significant proportion of patients complain that its bad taste is unacceptable and there are adverse reactions such as nausea, vomiting and headache. Moreover, more potential adverse reactions associated with hyperphosphatemia may theoretically develop under any conditions that increase absorption or decreased elimination of phosphate[7]. Unfortunately, poor compliance with the instructions about the dosage or interval of oral sodium phosphate and undetected chronic illness, particularly in the elderly, may result in a higher possibility of unexpected adverse events. Recent reports of renal failure associated with sodium phosphate have raised increasing concerns[8-12] in spite of some reports demonstrating its safety in a series of clinical trials[13,14].
In this study, we wanted to find a better and easier preparation regimen for a complete colonoscopic examination. We have experienced that the usual bowel preparation for sigmoidoscopy in our hospital using magnesium citrate the day before the procedure scheduled in the next morning was able to debulk the colon of solid fecal material without much patient discomfort. Therefore, we hypothesized that taking magnesium citrate the day before the procedure and then taking a single dose of sodium phosphate (45 mL) on the day of procedure would be as effective a regimen as the conventional two doses of sodium phosphate and more tolerable for morning colonoscopy.
MATERIALS AND METHODS
Study design, patient enrollment and the end outcomes
This was a prospective, randomized, endoscopist-blinded, controlled study. All consecutive patients referred to the Outpatient Department of Gastroenterology and Surgery at Daehang Hospital (Seoul, Korea) between September 2009 and December 2009 for colonoscopy in the morning were enrolled in this clinical study. The exclusion criteria were as follows: ileus or suspected bowel obstruction, significant gastroparesis or gastric outlet obstruction, the presence of serious medical conditions such as severe cardiac, renal, hepatic or metabolic diseases, pregnancy, lactation and a history of prior colon or rectal surgery. Written informed consent was obtained from each patient. No patient refused to participate. The primary end outcomes in this study were the efficacy of bowel preparation according to the Aronchick scale and the Ottawa Bowel Preparation Scale (OBPS), the patients’ tolerance, the overall satisfaction, the taste and the willingness to repeat the same preparation regimen, if necessary. The secondary end outcomes included assessment of the OBPS components (i.e. the right vs middle vs left colon, and the fluid score) and a subgroup assessment according to age, gender, a prior bowel cleansing regimen and so forth.
Colon bowel preparation
A total of 159 patients were randomly assigned to receive two split doses of 90 mg of oral sodium phosphate (Group I, n = 79) or 250 mL of magnesium citrate (magnesium carbonate 4.3 g and anhydrous citric acid 7.8 g/100 mL) (the day before the procedure) and this was followed by 45 mL of sodium phosphate (the day of the procedure, Group II, n = 80). Group I ingested a single dose (45 mL) of sodium phosphate starting at 8:00 PM on the day before the procedure and then they ingested the remaining 45 mL of sodium phosphate at 5:00 AM on the day of colonoscopy. Group II ingested magnesium citrate (one bottle; 250 mL) at 8:00 PM on the preceding day and a single dose (45 mL) of sodium phosphate at 5:00 AM on the procedure day. Both groups took one tablet of bisacodyl as pretreatment 30 min before each laxative on the day before the procedure. All the patients who had taken oral sodium phosphate were instructed to drink an additional 1-1.5 L of water. Both groups had a thick liquid diet for dinner on the day before the procedure, and they took nothing further by mouth after 6:00 PM. All the colonoscopy procedures were performed between 8:30 AM and 11:30 AM. The quality of bowel cleansing, the tolerability, the satisfaction, the willing to repeat the procedure and the adverse effects of each regimen were compared.
Evaluation of bowel preparation
Efficacy of bowel cleansing: The endoscopists, who were blinded to the form of preparation, performed the colonoscopy exams. To standardize and minimize interobserver variation, we had a meeting before the start of this study and an investigator gave a standardized explanation for assessing the quality of bowel preparation during the meeting. A poster with endoscopic photos that demonstrated the scoring system was placed in each endoscopy room as a reminder. The endoscopists were also requested to take pictures of each colon segment before cleansing the segments with saline during the procedure.
Bowel cleansing was assessed at the end of the colonoscopy using the OBPS and the Aronchick scale. The OBPS has a potential score ranging from 0 (excellent preparation, no fluid) to 14 (inadequate in all segments with a large amount of fluid) (Table 1). The Aronchick scale was as follows: excellent (a small volume of clear liquid or greater than 95% of the surface was seen); good (a large volume of clear liquid covering 5% to 25% of the surface but greater than 90% of the surface was seen); fair (some semisolid stool that could be suctioned or washed away but greater than 90% of the surface was seen); and poor (semisolid stool that could not be suctioned or washed away and less than 90% of the surface was seen).
Table 1.
Summary of the scoring system for the Ottawa Bowel Preparation Scale
| Cleanliness |
| Excellent (0): mucosal detail clearly visible. If fluid present, it is clear. Almost no stool residue |
| Good (1): some turbid fluid or stool residue but mucosal detail still visible. Washing and suctioning not necessary |
| Fair (2): turbid fluid or stool residue obscuring mucosal detail and contour. However, mucosal detail becomes visible with suctioning. Washing not necessary |
| Poor (3): presence of stool obscuring mucosal detail and contour. However, with suctioning and washing, a reasonable view is obtained |
| Inadequate (4): solid stool obscuring mucosal detail and contour despite aggressive washing and suctioning |
| Fluid: small (0), moderate (1), large (2) |
There were ten colonoscopists who performed colonoscopies in this study. All the colonoscopists were experts who had experienced at least 1000 cases of colonoscopy after their gastrointestinal or coloproctologic fellowship. To check the interobserver variation for rating the efficacy of bowel preparation, all the colonoscopy pictures were reviewed by two experienced endoscopists (Choi YS and Suh JP) who were blinded to the preparation methods.
Patient acceptability
All the patients completed a questionnaire before colonoscopy. It was used to assess the past medical history, compliance, discomfort symptoms during preparation and the overall satisfaction. The investigated symptoms were nausea or vomiting (present = 1, absent = 0), abdominal pain or discomfort (present = 1, none = 0) and abdominal distension (present = 1, none = 0). The overall satisfaction with the preparation was rated on a 3 scale (satisfactory, fair and unsatisfactory). The willingness to repeat the same preparation method in the future, if necessary, was also evaluated with a “yes” or “no” question.
Statistical analysis
All the continuous variables are presented as means and standard deviations or as medians and ranges, as appropriate. The χ2 test was used for comparisons between categorical variables and Student’s t test was used for comparisons between continuous variables. P values less than 0.05 were regarded as statistically significant.
RESULTS
Baseline characteristics
A total of 180 consecutive patients were enrolled and randomized into two groups (90 patients in each group). Twenty-one patients were excluded because the procedure was postponed or canceled. Finally, 159 patients were included in the analysis. There was no significant difference in the baseline characteristics between both groups (Table 2). In all cases, cecal intubation was successful. There were no serious complications or adverse events immediately after the procedure.
Table 2.
Baseline characteristics (mean ± SD) n (%)
| Variables | Group I (n = 79) | Group II (n = 80) | P value |
| Age (yr), median (range) | 59 (46-77) | 60 (48-78) | NS |
| Male | 40 (50.6) | 50 (62.5) | NS |
| Cecal intubation time (s) | 300.6 ± 171.9 | 314 ± 190.5 | NS |
| Weight (kg) | 61.5 ± 15.6 | 64.1 ± 15.3 | NS |
| Prior colonoscopy | 45 (56.9) | 44 (55.0) | NS |
| With PEG | 11 (13.9) | 14 (17.5) | |
| With Sodium phosphate | 34 (43.0) | 30 (37.5) | |
| Hypertension | 12 (15.2) | 15 (18.8) | NS |
| Diabetes | 8 (10.1) | 5 (6.3) | NS |
| Constipation (< 3/wk) | 12 (15.2) | 14 (17.6) | NS |
PEG: Polyethylene glycol; NS: Not significant.
Assessment of the quality of bowel cleansing
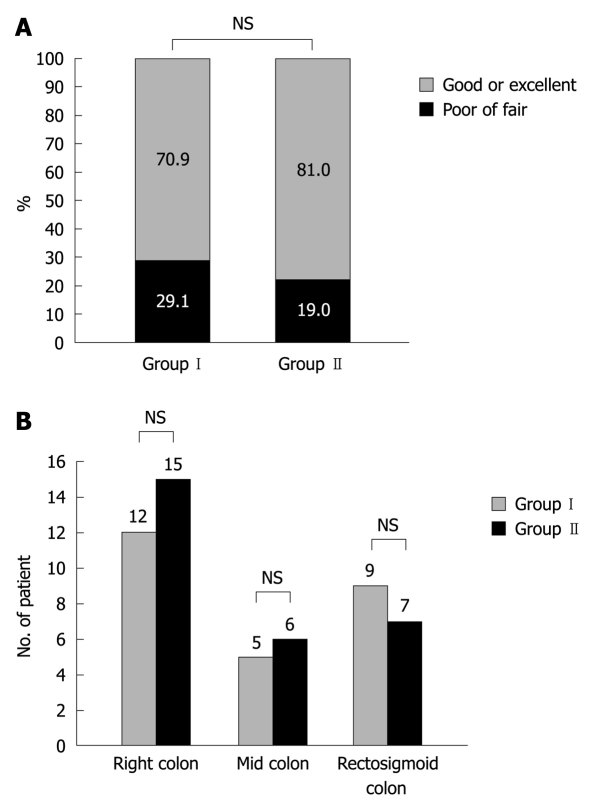
There was no significant difference in bowel cleansing quality as evaluated by the Aronchick scale between both bowel preparation regimens. Group I had 56 satisfactory preparations (defined as excellent or good) of 79 (70.9%) compared with 62 of 80 (81.0%) for Group II (P = 0.34) (Figure 1A).
Figure 1.
Quality of bowel cleansing. A: Quality of bowel cleansing. The patients are graded as good or excellent quality vs fair or poor quality with the conventional sodium phosphate regimen (Group I) or the magnesium citrate and a single dose sodium phosphate regimen (Group II); B: Segmental quality of bowel cleansing. The patients are scored with colon segment grades of poor or inadequate with the conventional sodium phosphate regimen (Group I) or the magnesium citrate and a single dose sodium phosphate regimen (Group II). NS: Not significant.
When bowel preparation was assessed by the Ottawa scale, the preparation quality was not significantly different as well (Table 3). The mean score was not significantly different between both groups for the right colon (P = 0.54), the mid colon (P = 0.47), the rectosigmoid colon (P = 0.97) or the total amount of fluid (P = 0.48). In both groups, the patients with colon segment grades of poor or inadequate were 12 (15.2%) vs 15 (18.8%), 5 (6.3%) vs 6 (8.0%) and 9 (11.4%) vs 7 (9.0%) for the right colon, the mid colon and the rectosigmoid colon, respectively (Figure 1B). There was no significant difference of the segmental bowel preparation quality either.
Table 3.
Efficacy of bowel cleansing by Ottawa scoring system (mean ± SD)
| Variables | Group I (n = 79) | Group II (n = 73) | P value |
| Right colon | 1.4 ± 1.1 | 1.3 ± 1.1 | NS |
| Mid colon | 1.0 ± 0.9 | 0.8 ± 0.9 | NS |
| Rectosigmoid colon | 1.3 ± 0.9 | 1.2 ± 1.0 | NS |
| Fluid present | 0.7 ± 0.6 | 0.4 ± 0.6 | NS |
| Overall score | 4.4 ± 2.6 | 3.8 ± 3.0 | NS |
NS: Not significant.
Assessment of patient acceptability (overall satisfaction, taste and willing to repeat)
The percentage of patients with symptoms such as abdominal distension with the two preparation regimens was 23% vs 34%, respectively, nausea or vomiting 45% vs 41%, respectively, and abdominal pain 15% vs 13%, respectively.
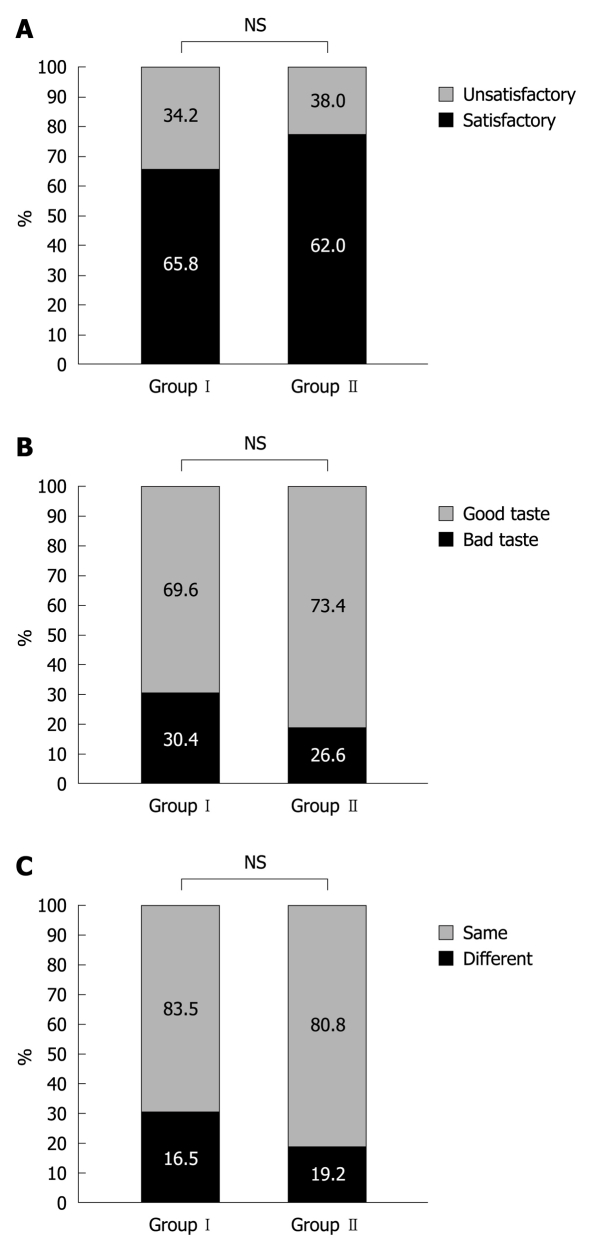
When patients were asked whether they were satisfied with the bowel preparation regimen, 65.8% (52 of 79) in Group I and 82.0% (62 of 80) in Group II replied that they were “satisfied” or it was “fair” (Figure 2A). When patients were asked how the laxatives tasted, the patients who responded “good or tolerable” were not significantly different between Group I (69.6%) vs Group II (73.4%) (Figure 2B). When patients were asked whether they were willing to repeat the same preparation regimen if necessary, the percentage of patients who responded “yes” was not significantly different between Group I (83.5%) vs Group II (80.8%) (Figure 2C).
Figure 2.
Patient acceptability. A: For overall satisfaction, 65.8% in Group I and 82.0% in Group II replied that they were “satisfied” or it was “fair”; B: For assessing taste, the percentage of patients who responded “good or tolerable” was not significantly different between Group I (69.6%) and Group II (73.4%); C: For the willingness to repeat the regimen, the percentage of patients who responded “yes” was not significantly different between Group I (83.5%) and Group II (80.8%). NS: Not significant.
Subgroup assessment of the bowel cleansing quality and patient acceptability
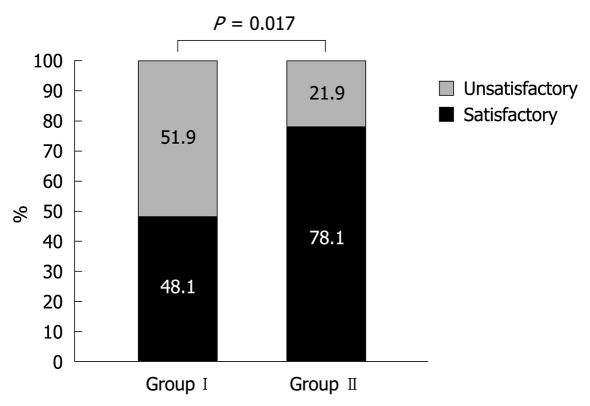
When the patients over 65 years old were asked whether they were satisfied with the bowel preparation regimen, 48.1% (13 of 27) in group I and 78.1% (25 of 32) in group II replied that they were “satisfied” or it was “fair” (P = 0.017) (Figure 3). The question about taste and willingness to repeat revealed no significant differences between both groups.
Figure 3.
Overall satisfaction with bowel preparation in the elderly (> 65 years). 48.1% in Group I and 78.1% in Group II replied that they were “satisfied” or it was “fair” (P = 0.017).
When only the patients who had previously undergone colonoscopy and had taken PEG or oral sodium phosphate were asked about patient acceptability, the prior sodium phosphate users generally had a preference for this new regimen rather than the new PEG users concerning overall satisfaction (80.0% vs 64.3%, respectively, P = 0.26), taste tolerance (80% vs 71.4%, respectively, P = 0.52) and willing to repeat (90.0% vs 61.3%, respectively, P = 0.04).
DISCUSSION
The early detection of colonic neoplasm via colonoscopy and its removal by polypectomy is popular and increasing, yet many people avoid screening colonoscopy because they fear the preparation required for colonoscopy. The two most commonly-used regimens for colonoscopic bowel preparation are currently a large volume of osmotically balanced PEG solution and a small volume of an osmotically active agent, oral sodium phosphate. Four liters of PEG solution is known to be safe and effective, but poorly tolerated. In contrast, sodium phosphate is known to be well-tolerated and as effective as PEG solution. Oral sodium phosphate has been shown to be very safe in a large series of clinical trials[13,14]. Its administration is known to be associated with only an asymptomatic, mild increase in serum phosphate (mean increase: 3 mg/dL) and a minimal decrease in serum calcium (mean decrease: 0.3 mg/L); these changes are readily reversible[10]. Clinically significant adverse reactions, including severe hyperphosphatemia, hypocalcemia, seizure, tetany, hypovolaemia and acute renal failure are thought to be extremely rare, with a reported incidence of 1.44 per million patients[10,15]. Under normal conditions, phosphate is absorbed in the small intestine and eliminated by the kidney as calcium phosphate[15]. The conventional dosage of oral phosphate for bowel cleansing is one bottle (45 mL) the evening before the procedure and another bottle the next morning. Any condition that can increase absorption or decrease elimination of phosphate such as repeated dosing, dehydration, the use of diuretics and ACE inhibitor, or a preexisting illness that may impair phosphate homeostasis, for example, hyperparathyroidism, renal insufficiency or delayed intestinal transit can be a risk factor. Indeed, fatal cases have been observed among the patients with a history of renal dysfunction[16-18], ischemic colitis[17], cirrhosis[18] and in elderly patients with normal renal function[19,20]. The maximum safe dose of sodium phosphate is 90 mL[21]. Several studies on the adverse effects of high doses of sodium phosphate have suggested that these high doses should be avoided[13-15]. If the recommended dose of 60 g (90 mL) is surpassed, or if the interval between doses is < 5 h, severe hyperphosphatemia could develop[10,15,18,22,23]. Fine et al[24] have found that the mortality rate was 33%, and that the risk of death was high if the serum phosphate level increased beyond 32.69 mg/dL (10.56 mmol/L). Most of the deaths reported in the literature have been caused by arrhythmia or heart attack associated with electrolyte changes and dehydration[25]. Therefore, adequate fluid intake is necessary. Markowitz et al[10] has suggested that patients must be encouraged to drink eight cups of fluids (1920 mL) and Rex et al[2] have promoted taking 3.6 L of clear fluids. However, it was reported that the standard dose of sodium phosphate could induce the development of hyperphosphatemia even in low-risk and well-hydrated patients[7].
In this study, we attempted to debulk the colon of solid fecal material with magnesium citrate at first and thereby decrease the volume of oral sodium phosphate required for bowel cleansing without compromising the quality of the bowel preparation. Rather, we achieved superior overall satisfaction in the elderly patients who were pretreated with magnesium citrate. It is practically significant to find that this new regimen is better tolerated by elderly patients since the potential for complications associated with bowel preparation may be relatively increased in elderly patients due to poor compliance with the recommended fluid intake. In addition, elderly patients have a greater possibility of having an underlying chronic illness that may be undetected. Therefore, we think a lower volume of sodium phosphate can be safer and better suited for everybody. The patients who had previously taken two doses of sodium phosphate for bowel preparation had a tendency to prefer this new regimen rather than the PEG solution, suggesting that this regimen can be an alternative option for the intolerant patients.
We found that overall rates of adequate bowel cleansing in both groups were relatively high (Group I, 70.9% and Group II, 81%), which suggested that bisacodyl as pretreatment and procedure time scheduled for the morning had influenced the overall quality of bowel cleansing. Both regimens were scheduled for morning because afternoon colonoscopies have higher failure rates than morning colonoscopies, and the suggested scheduling of all outpatient colonoscopies is preferentially in the morning to avoid suboptimal procedures[26]. The dosing time of magnesium citrate was determined as 9 h before taking the oral sodium phosphate. In a previous study that used magnesium citrate as pretreatment to decrease the volume of PEG solution, magnesium citrate was taken at 1-2 h before ingesting 2 L of PEG solution[27,28]. However, we expect that a 9 h time interval would be optimal, with reference to the usual interval time for the split-dosed PEG solution regimen or the oral sodium phosphate regimen.
Our study has several limitations. First, the safety profiles, including electrolytes, hemoglobin and the BUN/creatinine change, were not evaluated to prove the safety advantages of this new regimen compared to the conventional sodium phosphate regimen, although the volume of sodium phosphate was reduced by half. Second, we excluded the patients with medical conditions that are currently contraindicated for conventional sodium phosphate use such as heart failure, cirrhosis, renal disease, very old age patients or those taking diuretics or ACE inhibitors and so forth. Thus, the findings in our study need to be confirmed in a large study with various groups of patients. Third, there were ten colonoscopists involved in this study. Although all the endoscopists were trained in using the scale with photographs illustrating each point on the OBPS, and the degree of agreement was acceptable according to a κ coefficient of greater than 0.4, the study could have been more powerful if all the colonoscopies had been performed by a single colonoscopist. Fourth, there was a discrepancy between “better” overall satisfaction and a “similar” propensity of willingness to repeat the bowel preparation regimen among the elderly patients. Further tailored evaluation of a large group of elderly patients is needed to verify this.
As mentioned above, the use of the conventional dose of sodium phosphate is theoretically limited for patients with any condition that can increase the absorption or decrease the elimination of phosphate. If we think a lower volume of sodium phosphate might be safer, then this must be verified in individual cases since the mechanism of an adverse reaction to sodium phosphate and its risk factors has not been fully discovered. Further evaluation of the safety profile is mandatory.
In conclusion, a regimen of magnesium citrate and a single dose of oral sodium phosphate was as effective as the conventional two doses of sodium phosphate, and our new regimen was well tolerated. Therefore, this regimen could be a good option to routinely prepare for morning colonoscopy.
COMMENTS
Background
Oral sodium phosphate is a hyperosmolar, low-volume laxative for colonoscopy bowel preparation, and it is known to be as effective as polyethylene glycol solution. It is generally better tolerated, but the bad taste and uncomfortable abdominal symptoms such as nausea and vomiting frequently lead to poor compliance with the oral sodium phosphate regimen, and all this can subsequently cause incomplete bowel cleansing. Moreover, potential adverse reactions associated with hyperphosphatemia may develop.
Innovations and breakthroughs
This is the first clinical study to evaluate the efficacy and tolerability of magnesium citrate and a single dose of oral sodium phosphate for bowel preparation prior to morning colonoscopy. The findings demonstrated this modified regimen, in which the volume of sodium phosphate was reduced by half was as effective and tolerable as the conventional sodium phosphate regimen.
Applications
The study results suggest that magnesium citrate and a single dose of oral sodium phosphate can be an effective and satisfactory option to routinely prepare for morning colonoscopy.
Terminology
Magnesium citrate acts as an osmotic laxative which is often used for bowel preparation
Peer review
In this well designed paper the author has proposed a combination of magnesium citrate the day before the procedure and sodium phosphate early in the morning of the day of the procedure, associated with one tablet of bisacodyl as compared to standard cleansing using two doses of sodium phosphate, with one tablet of bisacodyl. The paper is interesting and the design is adequate, as are the results and comments.
Footnotes
Peer reviewer: Josep M Bordas, MD, Department of Gastroenterology IMD, Hospital Clinic”, Llusanes 11-13 at, Barcelona 08022, Spain
S- Editor Sun H L- Editor O’Neill M E- Editor Zheng XM
References
- 1.Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76–79. doi: 10.1067/mge.2003.294. [DOI] [PubMed] [Google Scholar]
- 2.Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol. 2002;97:1696–1700. doi: 10.1111/j.1572-0241.2002.05827.x. [DOI] [PubMed] [Google Scholar]
- 3.Cohen SM, Wexner SD, Binderow SR, Nogueras JJ, Daniel N, Ehrenpreis ED, Jensen J, Bonner GF, Ruderman WB. Prospective, randomized, endoscopic-blinded trial comparing precolonoscopy bowel cleansing methods. Dis Colon Rectum. 1994;37:689–696. doi: 10.1007/BF02054413. [DOI] [PubMed] [Google Scholar]
- 4.Frommer D. Cleansing ability and tolerance of three bowel preparations for colonoscopy. Dis Colon Rectum. 1997;40:100–104. doi: 10.1007/BF02055690. [DOI] [PubMed] [Google Scholar]
- 5.Hsu CW, Imperiale TF. Meta-analysis and cost comparison of polyethylene glycol lavage versus sodium phosphate for colonoscopy preparation. Gastrointest Endosc. 1998;48:276–282. doi: 10.1016/s0016-5107(98)70191-9. [DOI] [PubMed] [Google Scholar]
- 6.Golub RW, Kerner BA, Wise WE Jr, Meesig DM, Hartmann RF, Khanduja KS, Aguilar PS. Colonoscopic bowel preparations--which one? A blinded, prospective, randomized trial. Dis Colon Rectum. 1995;38:594–599. doi: 10.1007/BF02054117. [DOI] [PubMed] [Google Scholar]
- 7.Casais MN, Rosa-Diez G, Pérez S, Mansilla EN, Bravo S, Bonofiglio FC. Hyperphosphatemia after sodium phosphate laxatives in low risk patients: prospective study. World J Gastroenterol. 2009;15:5960–5965. doi: 10.3748/wjg.15.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sica DA, Carl D, Zfass AM. Acute phosphate nephropathy--an emerging issue. Am J Gastroenterol. 2007;102:1844–1847. doi: 10.1111/j.1572-0241.2007.01047.x. [DOI] [PubMed] [Google Scholar]
- 9.Desmeules S, Bergeron MJ, Isenring P. Acute phosphate nephropathy and renal failure. N Engl J Med. 2003;349:1006–1007. doi: 10.1056/NEJM200309043491020. [DOI] [PubMed] [Google Scholar]
- 10.Markowitz GS, Stokes MB, Radhakrishnan J, D'Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16:3389–3396. doi: 10.1681/ASN.2005050496. [DOI] [PubMed] [Google Scholar]
- 11.Carl DE, Sica DA. Acute phosphate nephropathy following colonoscopy preparation. Am J Med Sci. 2007;334:151–154. doi: 10.1097/MAJ.0b013e318156c529. [DOI] [PubMed] [Google Scholar]
- 12.Markowitz GS, Radhakrishnan J, D'Agati VD. Towards the incidence of acute phosphate nephropathy. J Am Soc Nephrol. 2007;18:3020–3022. doi: 10.1681/ASN.2007101073. [DOI] [PubMed] [Google Scholar]
- 13.Hookey LC, Depew WT, Vanner S. The safety profile of oral sodium phosphate for colonic cleansing before colonoscopy in adults. Gastrointest Endosc. 2002;56:895–902. doi: 10.1067/mge.2002.129522. [DOI] [PubMed] [Google Scholar]
- 14.Belsey J, Epstein O, Heresbach D. Systematic review: oral bowel preparation for colonoscopy. Aliment Pharmacol Ther. 2007;25:373–384. doi: 10.1111/j.1365-2036.2006.03212.x. [DOI] [PubMed] [Google Scholar]
- 15.Tan HL, Liew QY, Loo S, Hawkins R. Severe hyperphosphataemia and associated electrolyte and metabolic derangement following the administration of sodium phosphate for bowel preparation. Anaesthesia. 2002;57:478–483. doi: 10.1046/j.0003-2409.2001.02519.x. [DOI] [PubMed] [Google Scholar]
- 16.Martin RR, Lisehora GR, Braxton M Jr, Barcia PJ. Fatal poisoning from sodium phosphate enema. Case report and experimental study. JAMA. 1987;257:2190–2192. [PubMed] [Google Scholar]
- 17.Ullah N, Yeh R, Ehrinpreis M. Fatal hyperphosphatemia from a phosphosoda bowel preparation. J Clin Gastroenterol. 2002;34:457–458. doi: 10.1097/00004836-200204000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Azzam I, Kovalev Y, Storch S, Elias N. Life threatening hyperphosphataemia after administration of sodium phosphate in preparation for colonoscopy. Postgrad Med J. 2004;80:487–488. doi: 10.1136/pgmj.2003.017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farah R. Fatal acute sodium phosphate enemas intoxication. Acta Gastroenterol Belg. 2005;68:392–393. [PubMed] [Google Scholar]
- 20.Aydogan T, Kanbay M, Uz B, Kaya A, Isik A, Bozalan R, Erkman M, Akcay A. Fatal hyperphosphatemia secondary to a phosphosoda bowel preparation in a geriatric patient with normal renal function. J Clin Gastroenterol. 2006;40:177. doi: 10.1097/01.mcg.0000196408.60851.cf. [DOI] [PubMed] [Google Scholar]
- 21.US Food and Drug Administration. Science backgrounder: Safety of sodium phosphates oral solution. 2001. Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm173897.htm.
- 22.US Food and Drug Administration. Science background paper: Acute phosphate nephropathy and renal failure associated with the use of oral sodium phosphate bowel cleansing products. Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm161581.htm.
- 23.Rex DK. Phosphate nephropathy. Am J Gastroenterol. 2008;103:807. doi: 10.1111/j.1572-0241.2007.01612_11.x. [DOI] [PubMed] [Google Scholar]
- 24.Fine A, Patterson J. Severe hyperphosphatemia following phosphate administration for bowel preparation in patients with renal failure: two cases and a review of the literature. Am J Kidney Dis. 1997;29:103–105. doi: 10.1016/s0272-6386(97)90015-9. [DOI] [PubMed] [Google Scholar]
- 25.Rex DK. Dosing considerations in the use of sodium phosphate bowel preparations for colonoscopy. Ann Pharmacother. 2007;41:1466–1475. doi: 10.1345/aph.1K206. [DOI] [PubMed] [Google Scholar]
- 26.Sanaka MR, Shah N, Mullen KD, Ferguson DR, Thomas C, McCullough AJ. Afternoon colonoscopies have higher failure rates than morning colonoscopies. Am J Gastroenterol. 2006;101:2726–2730. doi: 10.1111/j.1572-0241.2006.00887.x. [DOI] [PubMed] [Google Scholar]
- 27.Sharma VK, Steinberg EN, Vasudeva R, Howden CW. Randomized, controlled study of pretreatment with magnesium citrate on the quality of colonoscopy preparation with polyethylene glycol electrolyte lavage solution. Gastrointest Endosc. 1997;46:541–543. doi: 10.1016/s0016-5107(97)70011-7. [DOI] [PubMed] [Google Scholar]
- 28.Sharma VK, Chockalingham SK, Ugheoke EA, Kapur A, Ling PH, Vasudeva R, Howden CW. Prospective, randomized, controlled comparison of the use of polyethylene glycol electrolyte lavage solution in four-liter versus two-liter volumes and pretreatment with either magnesium citrate or bisacodyl for colonoscopy preparation. Gastrointest Endosc. 1998;47:167–71. doi: 10.1016/s0016-5107(98)70351-7. [DOI] [PubMed] [Google Scholar]