Abstract
AIM: To study the relation between CYP1A1 Ile462Val polymorphism and colorectal cancer risk by meta-analysis.
METHODS: A meta-analysis was performed to investigate the relation between CYP1A1 Ile462Val polymorphism and colorectal cancer risk by reviewing the related studies until September 2010. Data were extracted and analyzed. Crude odds ratio (OR) with 95% confidence interval (CI) was used to assess the strength of relation between CYP1A1 Ile462Val polymorphism and colorectal cancer risk.
RESULTS: Thirteen published case-control studies including 5336 cases and 6226 controls were acquired. The pooled OR with 95% CI indicated that CYP1A1 Ile462Val polymorphism was significantly related with colorectal cancer risk (Val/Val vs Ile/Ile: OR = 1.47, 95% CI: 1.16-1.86, P = 0.002; dominant model: OR = 1.33, 95% CI: 1.01-1.75, P = 0.04; recessive model: OR = 1.49, 95% CI: 1.18-1.88, P = 0.0009). Subgroup ethnicity analysis showed that CYP1A1 Ile462Val polymorphism was also significantly related with colorectal cancer risk in Europeans (Ile/Val vs Ile/Ile: OR = 1.22, 95% CI: 1.05-1.42, P = 0.008; dominant model: OR = 1.24, 95% CI: 1.07-1.43, P = 0.004) and Asians (Val/Val vs Ile/Ile: OR = 1.40, 95% CI: 1.07-1.82, P = 0.01; recessive model: OR = 1.46, 95% CI: 1.12-1.89, P = 0.005).
CONCLUSION: CYP1A1 Ile462Val may be an increased risk factor for colorectal cancer.
Keywords: CYP1A1, Polymorphism, Colorectal cancer, Meta-analysis
INTRODUCTION
Colorectal cancer, one of the most prevalent cancers worldwide, ranks fourth in frequency in men and third in women[1]. In recent years, the incidence of colorectal cancer has increased in most countries but its prognosis is still poor. A number of researches have shown that colorectal cancer is possibly related with tobacco and alcohol consumption as well as other environmental sources[2-4]. It has been shown that inter-individual differences including single nucleotide polymorphism (SNP) may influence human susceptibility to colorectal cancer[5,6].
Metabolic enzymes including phase I and phase II enzymes are involved in activation and detoxification of xenobiotics, which play an important role in the pathogenesis of colorectal cancer[7]. Cytochrome P450, including family 1, subfamily A, polypeptide 1 (CYP1A1), is one of the phase I enzymes, metabolizing a large number of endogenous and exogenous substances, such as polycyclic aromatic hydrocarbons, heterocyclic amines, aromatic amines, and N-nitrosamines[8,9]. Thus, CYP1A1 plays an important role in human susceptibility to colorectal cancer due to various exogenous factors.
Non-synonymous SNP (rs1048943) leads to amino acid change in exon 7 of CYP1A1 from Ile to Val (nucleotides A-G) at codon 462, which may alter the protein activity and the human susceptibility to colorectal cancer. Since the first study on the relation between colorectal cancer and CYP1A1 Ile462Val polymorphism conducted by Sivaraman et al[10] in 1994, a large number of epidemiological studies on the relation between colorectal cancer and CYP1A1 Ile462Val polymorphism have been conducted, but their conclusions are different or even contradictory. In this study, a meta-analysis of the published case-control studies was performed to assess the relation between CYP1A1 Ile462Val polymorphism and colorectal cancer risk.
MATERIALS AND METHODS
Search strategy
Studies on the relation between CYP1A1 Ile462Val polymorphism and colorectal cancer risk were search from PubMed from 1994 to September 2010 using the key words “CYP1A1”, “colorectal cancer”, “colon cancer”, “rectum cancer”, and “polymorphism”. Related studies were also searched from the references of original papers or reviews. All studies were selected according to the following criteria: only case-control studies on the relation between CYP1A1 Ile462Val polymorphism and colorectal cancer risk, sufficient published data for estimating odds ratio (OR) with 95% confidence interval (CI), and selection of the largest or most recent studies when several publications reporting the same or overlapping data[11]. Only the data published in 2007 were selected from two studies by Kiss et al[12,13] who reported overlapping data in Hungarians. Finally, 13 case-control studies including 5336 patients with colorectal cancer and 6226 controls were enrolled in our meta-analysis.
Data extraction
Two investigators independently extracted the following data from the included publications, including name of the first author, publication data, country origin, source of control, racial descent of the study population, genotyping method, number of different genotypes, and Hardy-Weinberg equilibrium (HWE) in controls.
Statistical analysis
Crude OR with 95% CI was computed to assess the strength of relation between CYP1A1 Ile462Val polymorphisms and colorectal cancer risk. Codominant model (Val/Val vs Ile/Ile, Ile/Val vs Ile/Ile), dominant model [(Val/Val + Ile/Val) vs Ile/Ile] and recessive model [Val/Val vs (Ile/Val + Ile/Ile)] were evaluated. Subgroup statistical analysis of the relation between CYP1A1 Ile462Val polymorphism and colorectal cancer risk in Asians and Europeans was performed. Heterogeneity assumption was checked by chi-square based Q-test[14]. Pooled OR estimation of each study was calculated with the random-effect model (DerSimonian and Laird method) when P < 0.10[15]. Otherwise, the fixed-effect model (Mantel-Haenszel method) was selected[16]. The publication bias was evaluated with the funnel plot and linear regression asymmetry test as previously described[17]. Statistical analysis was performed using the STATA version 9.2 (Stata Corporation, College Station, TX) and Review Manager (version 4.2, Oxford, England), using two-sided P-values.
RESULTS
Study characteristics
Thirteen published case-control studies including 5336 patients with colorectal cancer and 6226 controls met the inclusion criteria for the meta-analysis[10,13,18-27]. The distribution of studies in different populations is listed in Table 1. The minor allele frequency of Val in controls ranged from 0.030 in Europeans[22] to 0.255 in Asians[23]. Genotyping methods included polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP), allele-specific PCR, TaqMan, MassARRAY system, microarray system, and APEX. The distribution of genotypes in controls of all studies was in agreement with HWE except for two studies[19,20].
Table 1.
Characteristics of case-control studies included in meta-analysis
| Author | Country /region | Racial descent | Source of controls | Case (n) | Control (n) |
Genotype distribution |
Genotying type | HWE | |||||
|
Case (n) |
Control (n) |
||||||||||||
| Ile/Ile | Ile/Val | Val/Val | Ile/Ile | Ile/Val | Val/Val | ||||||||
| Sivaraman et al[10], 1994 | USA | Mixed | Population control | 43 | 47 | 32 | 9 | 2 | 33 | 14 | 0 | Allele-specific PCR | 0.230 |
| Ishibe et al[18], 2000 | USA | European | Population control | 212 | 221 | 176 | 31 | 5 | 186 | 31 | 4 | PCR-RFLP | 0.057 |
| Sachse et al[19], 2002 | UK | European | Population control | 490 | 592 | 415 | 68 | 7 | 539 | 48 | 5 | TaqMan | < 0.01 |
| Slattery et al[20], 2004 | USA | European | Population control | 997 | 1170 | 910 | 82 | 5 | 1077 | 86 | 7 | Allele-specific PCR | < 0.01 |
| Slattery et al[20], 2004 | USA | European | Population control | 794 | 1010 | 722 | 66 | 6 | 920 | 85 | 5 | Allele-specific PCR | 0.052 |
| Landi et al[21], 2005 | Italy | European | Hospital control | 362 | 323 | 333 | 28 | 1 | 298 | 25 | 0 | Microarray and APEX | 0.469 |
| Little et al[22], 2006 | UK | European | Population control | 251 | 396 | 235 | 16 | 0 | 372 | 24 | 0 | PCR-RFLP | 0.534 |
| Kiss et al[13], 2007 | Hungary | European | Hospital control | 500 | 500 | 386 | 110 | 4 | 415 | 83 | 2 | Allele-specific PCR | 0.315 |
| Yeh et al[23], 2007 | China | Asian | Hospital control | 717 | 729 | 400 | 228 | 89 | 410 | 266 | 53 | PCR-RFLP | 0.280 |
| Yoshida et al[24], 2007 | Japan | Asian | Not report | 66 | 121 | 34 | 27 | 5 | 79 | 37 | 5 | PCR-RFLP | 0.800 |
| Pereira Serafim et al[25], 2008 | Brazil | Mixed | Population control | 114 | 114 | 14 | 97 | 3 | 81 | 33 | 0 | PCR-RFLP | 0.071 |
| Kobayashi et al[26], 2009 | Japan | Asian | Hospital control | 105 | 225 | 65 | 32 | 8 | 125 | 87 | 13 | MassARRAY system | 0.674 |
| Nisa et al[27], 2010 | Japan | Asian | Population control | 685 | 778 | 418 | 231 | 36 | 461 | 276 | 41 | PCR-RFLP | 0.970 |
HWE: Hardy-Weinberg equilibrium in control; PCR-RFLP: Polymerase chain reaction-restriction fragment length polymorphism; APEX: Arrayed primer extension.
Meta-analysis
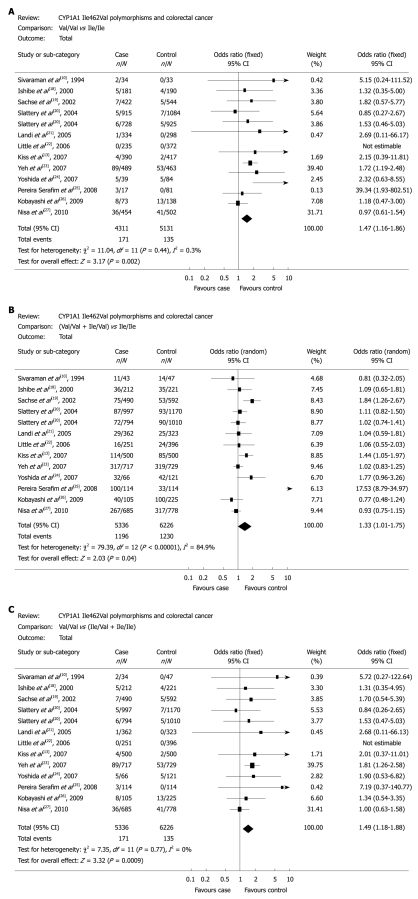
The results of meta-analysis and heterogeneity test are shown in Table 2. The colorectal cancer risk was significantly higher in individuals carrying the Val/Val genotype than in those carrying the Ile/Ile genotype (OR = 1.47, 95% CI: 1.16-1.86, P = 0.002, Pheterogeneity = 0.44, Figure 1A). The dominant and recessive models also showed that colorectal cancer risk was significantly related with the CYP1A1 Ile462Val polymorphism [(Val/Val + Ile/Val) vs Ile/Ile: OR = 1.33, 95% CI: 1.01-1.75, P = 0.04, Pheterogeneity < 0.01, Figure 1B; Val/Val vs (Ile/Val + Ile/Ile): OR = 1.49, 95% CI: 1.18-1.88, P = 0.0009, Pheterogeneity = 0.77, Figure 1C] in the total population. Subgroup race analysis showed that the CYP1A1 Ile462Val polymorphism was significantly related with colorectal cancer risk in Europeans [Ile/Val vs Ile/Ile: OR = 1.22, 95% CI: 1.05-1.42, P = 0.008, Pheterogeneity = 0.25; (Val/Val + Ile/Val) vs Ile/Ile: OR = 1.24, 95% CI: 1.07-1.43, P = 0.004, Pheterogeneity = 0.24] and in Asians [Val/Val vs Ile/Ile: OR = 1.40, 95% CI: 1.07-1.82, P = 0.01, Pheterogeneity = 0.23; Val/Val vs (Ile/Val + Ile/Ile): OR = 1.46, 95% CI: 1.12-1.89, P = 0.005, Pheterogeneity = 0.24].
Table 2.
Odds ratio and 95% confidence interval of CYP1A1 Ile462Val polymorphism and colorectal cancer risk
| Contrast | Racial descent | OR | 95% CI | Ph |
| Val/Val vs Ile/Ile | Total | 1.47 | 1.16-1.86 | 0.44 |
| European | 1.43 | 0.83-2.48 | 0.93 | |
| Asian | 1.40 | 1.07-1.82 | 0.23 | |
| Ile/Val vs Ile/Ile | Total | 1.28 | 0.96-1.72 | < 0.011 |
| European | 1.22 | 1.05-1.42 | 0.25 | |
| Asian | 0.91 | 0.79-1.05 | 0.19 | |
| (Val/Val + Ile/Val) vs Ile/Ile | Total | 1.33 | 1.01-1.75 | < 0.011 |
| European | 1.24 | 1.07-1.43 | 0.24 | |
| Asian | 0.98 | 0.96-1.13 | 0.17 | |
| Val/Val vs (Ile/Val + Ile/Ile) | Total | 1.49 | 1.18-1.88 | 0.77 |
| European | 1.39 | 0.80-2.41 | 0.94 | |
| Asian | 1.46 | 1.12-1.89 | 0.24 |
Estimates for random effects.
Ph: Test for heterogeneity; CYP1A1: Cytochrome P450, including family 1, subfamily A, polypeptide 1; OR: Odds ratio; CI: Confidence interval.
Figure 1.
Odds ratio of colorectal cancer associated with CYP1A1 Ile462Val for Val/Val vs Ile/Ile genotypes (A), Val/Val + Ile/Val vs Ile/Ile genotypes (B), and Val/Val vs Ile/Val + Ile/Ile genotypes (C).
Publication bias
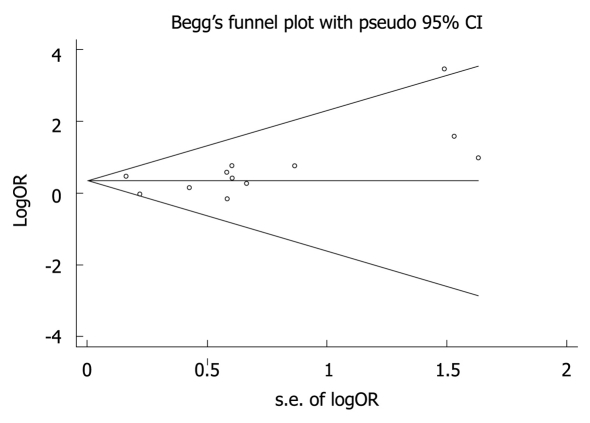
Funnel plot and Egger’s test were used to estimate the publication bias of studies. The funnel plots seemed symmetrical in all models (Val/Val vs Ile/Ile: P = 0.17, (Val/Val + Ile/Val) vs Ile/Ile: P = 0.17, Val/Val vs (Ile/Val + Ile/Ile): P = 0.39, Figure 2). No publication bias concerning the relation between CYP1A1 Ile462Val polymorphism and colorectal cancer risk was detected.
Figure 2.
Funnel plot analysis showing publication bias for Val/Val vs Ile/Ile genotypes. Each point represents a separate study for the indicated association.
DISCUSSION
CYP1A1, a phase I enzyme encoded by the CYP1A1 gene, has been mapped to chromosome 15q24.1. The CYP group of enzymes is involved in metabolic activation and detoxification of tobacco-derived carcinogen and other xenobiotics. It has been shown that alcohol intake and cigarette smoking are two important risk factors for colorectal cancer[20,25,27]. Reactive intermediates can bind to DNA when they are activated, resulting in adducts that cause mutations if not repaired, thereby initiating carcinogenesis[28]. Meanwhile, valine for isoleucine transition at codon 462 can lead to genetic disequilibrium from adenine to guanine mutation. It has been shown that CYP1A1 Ile462Val polymorphism can increase the activity of enzymes and activation of carcinogens may increases the risk of colorectal cancer[29,30]. At the same time, CYP1A1 Ile462Val polymorphisms in genotypes show considerable variations in their activities in different diseases and ethnics, as the variant Val, exhibiting an elevated breast caner risk in Caucasian[31], is a risk factor for esophageal cancer in Asians but not in Caucasians[32]. However, Ile/Val polymorphism is not related with the increased risk of prostate cancer[33].
The first study, published in 1994[10], did not reveal the relation between CYP1A1 Ile462Val polymorphism and colorectal cancer. To date, no consensus conclusion is available on the relation of CYP1A1 Ile462Val polymorphism and colorectal cancer. Pereira Serafim et al[25] demonstrated that the risk of colorectal cancer is 5-fold higher in Brazilians with the Val genotype (OR = 5.14, 95% CI: 3.15-10.80). Sachse et al[19] reported that the risk of colorectal cancer is about 2-fold higher in Europeans with the homozygous Val allele (OR = 2.15, 95% CI: 1.36-3.41). Kiss et al[13] and Yeh et al[23] also reported that the risk of colorectal cancer is similar to those reported by Pereira Serafim et al[25] and Sachse et al[19] in Hungarians and Asians with the Val genotype. However, other studies from USA and Europe showed that colorectal cancer risk is not significantly related with CYP1A1 Ile462Val polymorphism[10,18,20-22,24,26,27], but positively related with Val allele and smoking (OR = 2.5, 95% CI: 1.3-4.8) in Europeans[20]. The present meta-analysis of 13 eligible case-control studies including 5336 cases and 6226 controls showed that CYP1A1 Ile462Val polymorphism could contribute to colorectal cancer risk. The stratified analysis according to the ethnicity revealed that CYP1A1 Ile462Val polymorphism was positively related with colorectal cancer risk both in Asians and in Europeans. However, no report is available on the relation between CYP1A1 Ile462Val polymorphism and colorectal cancer risk in Africans. On the other hand, gender factor may change the risk of colorectal cancer sometimes. It was reported that the colorectal cancer risk is 3.1-fold higher in Chinese women with CYP1A1 Val/Val and XRCC3 Thr/Thr genotypes than in those with CYP1A1 Ile and XRCC3 Met alleles[23], suggesting that CYP1A1 Ile462Val polymorphism may be an important risk factor for colorectal cancer.
Heterogeneity is another problem found in our meta-analysis. A significant heterogeneity was observed in Ile/Val vs Ile/Ile and (Val/Val + Ile/Val) vs Ile/Ile. However, subgroup ethnicity analysis showed that the heterogeneity was removed apparently, indicating that the genetic background and environment are different in different ethnicities.
Several limitations in our meta-analysis need to be addressed. First, the results were obtained based on the unadjusted estimates and lacked of original data about the eligible studies, thus limiting the evaluation of effects of gene-gene and gene-environment interactions on the pathogenesis of colorectal cancer. Second, other single nucleotide polymorphisms of CYP1A1 were identified, but no linkage disequilibrium and haplotype analysis of these polymorphisms was performed. Third, the real relation between CYP1A1 Ile462Val polymorphism and colorectal cancer risk might have been influenced since the sample size was relatively small in this analysis, thus a further analysis of the relation between CYP1A1 polymorphism and colorectal cancer should be performed.
In conclusion, CYP1A1 Ile462Val polymorphism may contribute to colorectal cancer risk. Further study is needed with a large-scale case-control sample to validate the identified risk in our current meta-analysis, and potential gene-gene and gene-environment interactions should be taken into account when the relation between CYP1A1 Ile462Val polymorphism and colorectal cancer risk is further studied.
COMMENTS
Background
Colorectal cancer is one of the most prevalent malignances worldwide. CYP1A1 is one of the phase I enzymes. Ile to Val transition has been supposed as a risk factor for colorectal cancer. A large number of studies on the association between CYP1A1 and colorectal cancer risk have been conducted, but their conclusions are different or even contradictory.
Research frontiers
Many studies indicate that CYP1A1 Ile462Val polymorphism plays an important role in pathogenesis of esophageal cancer in Asians and breast caner in Caucasians. However, the relation between CYP1A1 Ile462Val polymorphism and colorectal cancer risk remains controversial and no meta-analysis has been conducted.
Innovations and breakthroughs
This meta-analysis systemically assessed the relation between CYP1A1 Ile462Val polymorphism and colorectal cancer risk, showing that the Val allele may be a risk factor for colorectal cancer in both Europeans and Asians.
Applications
The results of meta-analysis in this study show that the CYP1A1 Ile462Val polymorphism contributes the human susceptibility to colorectal cancer in both Europeans and Asians, which may help us to make early prevention and treatment of colorectal cancer.
Peer review
This is an interesting meta-analysis of the association between CYP1A1 Ile462Val polymorphism and colorectal cancer risk. The authors carefully reviewed the literature and collected the original data. The methods they used in meta-analysis are proper.
Footnotes
Peer reviewer: Luis Bujanda, PhD, Professor, Departament of Gastroenterology, CIBEREHD, University of Country Basque, Donostia Hospital, Paseo Dr. Beguiristain s/n, 20014 San Sebastián, Spain
S- Editor Sun H L- Editor Wang XL E- Editor Lin YP
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Storm HH, Engholm G, Hakulinen T, Tryggvadóttir L, Klint A, Gislum M, Kejs AM, Bray F. Survival of patients diagnosed with cancer in the Nordic countries up to 1999-2003 followed to the end of 2006. A critical overview of the results. Acta Oncol. 2010;49:532–544. doi: 10.3109/02841861003801148. [DOI] [PubMed] [Google Scholar]
- 3.Poynter JN, Haile RW, Siegmund KD, Campbell PT, Figueiredo JC, Limburg P, Young J, Le Marchand L, Potter JD, Cotterchio M, et al. Associations between smoking, alcohol consumption, and colorectal cancer, overall and by tumor microsatellite instability status. Cancer Epidemiol Biomarkers Prev. 2009;18:2745–2750. doi: 10.1158/1055-9965.EPI-09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zisman AL, Nickolov A, Brand RE, Gorchow A, Roy HK. Associations between the age at diagnosis and location of colorectal cancer and the use of alcohol and tobacco: implications for screening. Arch Intern Med. 2006;166:629–634. doi: 10.1001/archinte.166.6.629. [DOI] [PubMed] [Google Scholar]
- 5.Tomlinson IP, Webb E, Carvajal-Carmona L, Broderick P, Howarth K, Pittman AM, Spain S, Lubbe S, Walther A, Sullivan K, et al. A genome-wide association study identifies colorectal cancer susceptibility loci on chromosomes 10p14 and 8q23.3. Nat Genet. 2008;40:623–630. doi: 10.1038/ng.111. [DOI] [PubMed] [Google Scholar]
- 6.Abulí A, Bessa X, González JR, Ruiz-Ponte C, Cáceres A, Muñoz J, Gonzalo V, Balaguer F, Fernández-Rozadilla C, González D, et al. Susceptibility genetic variants associated with colorectal cancer risk correlate with cancer phenotype. Gastroenterology. 2010;139:788–796, 796.e1-e6. doi: 10.1053/j.gastro.2010.05.072. [DOI] [PubMed] [Google Scholar]
- 7.Nebert DW. Role of genetics and drug metabolism in human cancer risk. Mutat Res. 1991;247:267–281. doi: 10.1016/0027-5107(91)90022-g. [DOI] [PubMed] [Google Scholar]
- 8.Murtaugh MA, Sweeney C, Ma KN, Caan BJ, Slattery ML. The CYP1A1 genotype may alter the association of meat consumption patterns and preparation with the risk of colorectal cancer in men and women. J Nutr. 2005;135:179–186. doi: 10.1093/jn/135.2.179. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Chanock S, Tang D, Li Z, Jedrychowski W, Perera FP. Assessment of interactions between PAH exposure and genetic polymorphisms on PAH-DNA adducts in African American, Dominican, and Caucasian mothers and newborns. Cancer Epidemiol Biomarkers Prev. 2008;17:405–413. doi: 10.1158/1055-9965.EPI-07-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sivaraman L, Leatham MP, Yee J, Wilkens LR, Lau AF, Le Marchand L. CYP1A1 genetic polymorphisms and in situ colorectal cancer. Cancer Res. 1994;54:3692–3695. [PubMed] [Google Scholar]
- 11.Little J, Bradley L, Bray MS, Clyne M, Dorman J, Ellsworth DL, Hanson J, Khoury M, Lau J, O'Brien TR, et al. Reporting, appraising, and integrating data on genotype prevalence and gene-disease associations. Am J Epidemiol. 2002;156:300–310. doi: 10.1093/oxfordjournals.aje.a000179. [DOI] [PubMed] [Google Scholar]
- 12.Kiss I, Sándor J, Pajkos G, Bogner B, Hegedüs G, Ember I. Colorectal cancer risk in relation to genetic polymorphism of cytochrome P450 1A1, 2E1, and glutathione-S-transferase M1 enzymes. Anticancer Res. 2000;20:519–522. [PubMed] [Google Scholar]
- 13.Kiss I, Orsós Z, Gombos K, Bogner B, Csejtei A, Tibold A, Varga Z, Pázsit E, Magda I, Zölyomi A, et al. Association between allelic polymorphisms of metabolizing enzymes (CYP 1A1, CYP 1A2, CYP 2E1, mEH) and occurrence of colorectal cancer in Hungary. Anticancer Res. 2007;27:2931–2937. [PubMed] [Google Scholar]
- 14.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishibe N, Stampfer M, Hunter DJ, Hennekens C, Kelsey KT. A prospective study of cytochrome P450 1A1 polymorphisms and colorectal cancer risk in men. Cancer Epidemiol Biomarkers Prev. 2000;9:855–856. [PubMed] [Google Scholar]
- 19.Sachse C, Smith G, Wilkie MJ, Barrett JH, Waxman R, Sullivan F, Forman D, Bishop DT, Wolf CR. A pharmacogenetic study to investigate the role of dietary carcinogens in the etiology of colorectal cancer. Carcinogenesis. 2002;23:1839–1849. doi: 10.1093/carcin/23.11.1839. [DOI] [PubMed] [Google Scholar]
- 20.Slattery ML, Samowtiz W, Ma K, Murtaugh M, Sweeney C, Levin TR, Neuhausen S. CYP1A1, cigarette smoking, and colon and rectal cancer. Am J Epidemiol. 2004;160:842–852. doi: 10.1093/aje/kwh298. [DOI] [PubMed] [Google Scholar]
- 21.Landi S, Gemignani F, Moreno V, Gioia-Patricola L, Chabrier A, Guino E, Navarro M, de Oca J, Capellà G, Canzian F. A comprehensive analysis of phase I and phase II metabolism gene polymorphisms and risk of colorectal cancer. Pharmacogenet Genomics. 2005;15:535–546. doi: 10.1097/01.fpc.0000165904.48994.3d. [DOI] [PubMed] [Google Scholar]
- 22.Little J, Sharp L, Masson LF, Brockton NT, Cotton SC, Haites NE, Cassidy J. Colorectal cancer and genetic polymorphisms of CYP1A1, GSTM1 and GSTT1: a case-control study in the Grampian region of Scotland. Int J Cancer. 2006;119:2155–2164. doi: 10.1002/ijc.22093. [DOI] [PubMed] [Google Scholar]
- 23.Yeh CC, Sung FC, Tang R, Chang-Chieh CR, Hsieh LL. Association between polymorphisms of biotransformation and DNA-repair genes and risk of colorectal cancer in Taiwan. J Biomed Sci. 2007;14:183–193. doi: 10.1007/s11373-006-9139-x. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida K, Osawa K, Kasahara M, Miyaishi A, Nakanishi K, Hayamizu S, Osawa Y, Tsutou A, Tabuchi Y, Shimada E, et al. Association of CYP1A1, CYP1A2, GSTM1 and NAT2 gene polymorphisms with colorectal cancer and smoking. Asian Pac J Cancer Prev. 2007;8:438–444. [PubMed] [Google Scholar]
- 25.Pereira Serafim PV, Cotrim Guerreiro da Silva ID, Manoukias Forones N. Relationship between genetic polymorphism of CYP1A1 at codon 462 (Ile462Val) in colorectal cancer. Int J Biol Markers. 2008;23:18–23. doi: 10.1177/172460080802300103. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi M, Otani T, Iwasaki M, Natsukawa S, Shaura K, Koizumi Y, Kasuga Y, Sakamoto H, Yoshida T, Tsugane S. Association between dietary heterocyclic amine levels, genetic polymorphisms of NAT2, CYP1A1, and CYP1A2 and risk of colorectal cancer: a hospital-based case-control study in Japan. Scand J Gastroenterol. 2009;44:952–959. doi: 10.1080/00365520902964721. [DOI] [PubMed] [Google Scholar]
- 27.Nisa H, Kono S, Yin G, Toyomura K, Nagano J, Mibu R, Tanaka M, Kakeji Y, Maehara Y, Okamura T, et al. Cigarette smoking, genetic polymorphisms and colorectal cancer risk: the Fukuoka Colorectal Cancer Study. BMC Cancer. 2010;10:274. doi: 10.1186/1471-2407-10-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez FJ, Idle JR. Pharmacogenetic phenotyping and genotyping. Present status and future potential. Clin Pharmacokinet. 1994;26:59–70. doi: 10.2165/00003088-199426010-00005. [DOI] [PubMed] [Google Scholar]
- 30.Akiyama TE, Gonzalez FJ. Regulation of P450 genes by liver-enriched transcription factors and nuclear receptors. Biochim Biophys Acta. 2003;1619:223–234. doi: 10.1016/s0304-4165(02)00480-4. [DOI] [PubMed] [Google Scholar]
- 31.Sergentanis TN, Economopoulos KP. Four polymorphisms in cytochrome P450 1A1 (CYP1A1) gene and breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2010;122:459–469. doi: 10.1007/s10549-009-0694-5. [DOI] [PubMed] [Google Scholar]
- 32.Zhuo WL, Zhang YS, Wang Y, Zhuo XL, Zhu B, Cai L, Chen ZT. Association studies of CYP1A1 and GSTM1 polymorphisms with esophageal cancer risk: evidence-based meta-analyses. Arch Med Res. 2009;40:169–179. doi: 10.1016/j.arcmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Shaik AP, Jamil K, Das P. CYP1A1 polymorphisms and risk of prostate cancer: a meta-analysis. Urol J. 2009;6:78–86. [PubMed] [Google Scholar]