Abstract
Objective
Suicide rates are very high in old age, and the contribution of cognitive risk factors remains poorly understood. Suicide may be viewed as an outcome of an altered decision process. We hypothesized that impairment in a component of affective decision-making – reward/punishment-based learning – is associated with attempted suicide in late-life depression. We expected that suicide attempters would discount past reward/punishment history, focusing excessively on the most recent rewards and punishments. Further, we hypothesized that this impairment could be dissociated from executive abilities such as forward planning.
Method
We assessed reward/punishment-based learning using the Probabilistic Reversal Learning task in 65 individuals aged 60 and older: suicide attempters, suicide ideators, non-suicidal depressed elderly, and non-depressed controls. We used a reinforcement learning computational model to decompose reward/punishment processing over time. The Stockings of Cambridge test served as a control measure of executive function.
Results
Suicide attempters but not suicide ideators showed impaired probabilistic reversal learning compared to both non-suicidal depressed elderly and to non-depressed controls, after controlling for effects of education, global cognitive function, and substance use. Model-based analyses revealed that suicide attempters discounted previous history to a higher degree, compared to controls, basing their choice largely on reward/punishment received on the last trial. Groups did not differ in their performance on the Stockings of Cambridge.
Conclusions
Older suicide attempters display impaired reward/punishment-based learning. We propose a hypothesis that older suicide attempters make overly present-focused decisions, ignoring past experiences. Modification of this ‘myopia for the past’ may have therapeutic potential.
Keywords: suicide, suicide, attempted, cognition, adaptation, psychological, frontal lobe, prefrontal cortex, depressive disorder, aged
Worldwide, suicide is more common in the elderly than in any other age group (1). Suicide attempts in late life are more lethal than in mid-life (2), with up to one-half ending in death in older men (3). Though cognitive decline is prevalent in old age and may contribute to the heightened suicide risk, little is known about the role of cognitive factors. Initial evidence links late-life suicidal behavior to poor performance on screening measures of cognitive control (4, 5).
Evidence from younger suicide attempters indicates impaired performance on tests of cognitive control (6–9) and poor problem-solving (10). To the extent that findings from younger adults generalize to late-life suicide, they suggest a deficit in cognitive abilities relevant to dealing with life’s problems and making decisions. Yet, cognitive mechanisms that underpin these relationships remain unclear. In a pioneering study, Jollant and colleagues found impaired decision-making in otherwise cognitively-intact euthymic younger suicide attempters on the Iowa Gambling Task (11). Indeed, since a suicide attempt may be viewed as an outcome of a non-optimal decision process, it is plausible that impaired decision-making is a causal cognitive factor in suicidal behavior. However, the mechanisms of impairment on the Iowa Gambling Task are unknown, since the typically reported “net score” variable (total number of risky choices minus total number of safe choices) does not easily allow for dissection of underlying components. Suicide attempters may fail to represent the reinforcement contingencies on the task, or may be deficient in adapting a decision-making strategy, or may have a genuine preference for high-risk choices (12–14).
In this study, we sought to identify the components of decision-making that are associated with attempted suicide in old age. We selected a probabilistic reversal learning task, which requires one to ‘map’ an uncertain and changing environment by choosing between two stimuli variably associated with reward/punishment. Poor performance on this task can reflect a problem in mentally representing the environment, such as (1) a representation which is overly static in time (slow reinforcement learning) leading to perseverative responses, or (2) an unstable representation which changes too rapidly based on recent feedback and discounts past reinforcement history, leading to excessive switches in choice, or (3) abnormal sensitivity to rewards or punishments. Alternatively, it may result from a sub-optimal choice process, regardless of the nature of the representation. Previous research found that depressed people switch excessively in response to non-contingent punishment, suggesting that their representations are either unstable and/or overemphasize punishment (15, 16). Behavioral and neural responses during decision-making on this task may be described by Bayesian computational models, which predict the activity of the ventromedial prefrontal cortex (20) and the striatum (21).
To determine whether impaired reward/punishment-based learning is specifically related to attempted suicide, we attempted to dissociate the performance on the probabilistic learning task from executive abilities which do not involve reward or punishment. We hypothesized that suicide attempters would show impaired probabilistic reversal learning, reflecting unstable representations that discount past history and are overly focused on recent events. One may call such a view of one’s past history ‘myopic’, akin to ‘myopia for the future’ linked by Bechara and colleagues to ventral prefrontal lesions (22). This ‘myopia for the past’ could be related to habitually decreased consideration of one’s personal past in the face of recent events (23) during a suicidal crisis. We predicted that this ‘myopia for the past’ would be distinct from over-sensitivity to punishments. Further, we hypothesized that the suicide attempters’ executive abilities would appear intact on a test of forward planning and working memory. Another limitation of the existing literature is that studies that contrast suicide attempters with non-suicidal individuals (7, 11) cannot determine whether cognitive markers are specific for suicidal behavior or whether they also extend to suicidal ideation – a distinction of great clinical importance. To more strongly link decision-making with suicidal behavior, we included a comparison group of elderly with serious suicidal ideation but no history of attempt.
METHODS
Participants
Between May of 2006 and October of 2008, we recruited 65 participants aged 60 and older: 15 depressed suicide attempters, 12 depressed suicide ideators, 24 non-suicidal depressed elderly, and 14 non-depressed, non-suicidal elderly control subjects. Suicide attempters, suicide ideators, and non-suicidal depressed elderly were diagnosed with major depression without psychotic features by Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Axis I Disorders (24, 25) (SCID/DSM-IV). To exclude individuals with clinical dementia and to ensure that participants could engage in computerized assessments, all were required to have a score of ≥24 on the Mini-Mental State Exam (26). We excluded elderly with sensory disorders that precluded cognitive testing, mental retardation, delirium, neurologic disorders, bipolar disorder, schizophrenia, schizoaffective disorder, and exposure to electroconvulsive therapy in the previous 6 months.
All participants provided written informed consent. The University of Pittsburgh Institutional Review Board approved the study.
Suicide attempters had made a suicide attempt; 9/15 made their first suicide attempt after age 60; all were inpatients. These participants displayed a high level of suicidal intent during their attempts and expressed severe suicidal ideation (Table 1); 5/15 made repeat attempts; 6/15 made an attempt within a month of assessment and 9/15, within a year. Suicide attempt history was verified by a psychiatrist, using the interview, medical records, information from the treatment team, and information from family or friends. We excluded participants with significant discrepancies between these sources. None of the suicide attempters had experienced head injuries directly related to attempt, however we assessed potential anoxic-ischemic or toxic brain injury, based on the Beck Lethality Scale (27), medical records and the clinical interview. A study psychiatrist (AYD) identified any attempts with a score of >4 on the Beck Lethality Scale and any history of systemic hypotension >5 minutes or asphyxia or neurotoxic ingestion, 1/15. For 2/15 participants it was impossible to rule out brain injury during past attempts Thus, we excluded these 3 participants from sensitivity analyses.
Table 1.
Demographic, clinical, and cognitive group characteristics
| Non-Depressed Controls (C) N=14 | Non-Suicidal Depressed (D) N=24 | Suicidal Ideators (I) N=12 | Suicide Attempters (A) N=15 | P | Post-hoc, Tukey HSD | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | SD | M | N | % | SD | M | N | % | SD | M | N | % | SD | M | |||
| Age | 4.9 | 65.6 | 7.4 | 70 | 12 | 5.6 | 68.8 | 15 | 7.8 | 66.8 | 0.22 | NS | ||||||
| % Men | 12 | 86 | 11 | 46 | 7 | 58 | 6 | 40 | 0.06 | - | ||||||||
| % White | 13 | 93 | 20 | 83 | 11 | 83 | 11 | 73 | 0.67 | |||||||||
| % Married | 10 | 71 | 11 | 46 | 9 | 75 | 4 | 27 | 0.2 | |||||||||
| Years of Education (N=59) | 2.6 | 15.7 | 2.7 | 14.3 | 2.6 | 15.7 | 2.8 | 12.9 | 0.06 | NS | ||||||||
| Burden of physical illness (Cumulative Illness Rating Scale adapted for Geriatrics) (N=60) | 2.5 | 6.9 | 3.5 | 9.5 | 0 | 6.6 | 3.4 | 9.3 | 0.022 | NS | ||||||||
| Mini-mental status examination (N=56) | 1.8 | 29 | 1.6 | 28.3 | 2.1 | 28.3 | 1.8 | 28.7 | 0.76 | NS | ||||||||
| Dementia rating scale (N=58) | 3.4 | 138.4 | 3.9 | 133.7 | 6 | 133.5 | 5.7 | 132.3 | 0.011 | A, I, D<C | ||||||||
| Depressive Severity (HRS16) (N=64) | 1.7 | 3 | 3.7 | 18.5 | 4.7 | 20.2 | 4.7 | 19.2 | <.01 | A, I, D<C | ||||||||
| Lifetime substance use disorders | 0 | 0 | 2 | 9 | 3 | 25 | 25 | 8 | 53 | 53 | <.01 | - | ||||||
| Current substance use disorders | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 40 | ||||||||||
| Intensity of Antidepressant Pharmacotherapy During Current Episode, Antidepressent Treatment History Form Score (N=41) | - | 1.4 | 1.4 | 2.1 | 2 | 1.2 | 1.9 | 0.5 | NS | |||||||||
| % Receiving Sedatives/Hynotics (N=48) | - | 9 | 39 | 8 | 67 | 7 | 54 | 0.29 | - | |||||||||
| & Receiving Anticholinergic Agents (N=48) | - | 6 | 26 | 4 | 33 | 6 | 46 | 0.47 | - | |||||||||
| % Receiving Opioid Analgesics (N=48) | - | 6 | 26 | 4 | 33 | 2 | 15 | 0.58 | - | |||||||||
| Scale for Suicidal Ideation | - | - | 6.9 | 15.3 | 8.7 | 19.6 | 0.24 | - | ||||||||||
| Suicidal Intent Scale | - | - | - | 5.3 | 17 | - | - | |||||||||||
Suicide ideators had thoughts of suicide with specific plan, serious enough to precipitate an inpatient admission (10/12) or an increase in the level of outpatient care (2/12) and no lifetime history of suicide attempt. Suicidal ideation was also very severe in this group (Table 1).
Non-suicidal depressed elderly were included in the study to detect an association between decision-making and suicidal behavior above and beyond cognitive effects of depression. These participants had no current or lifetime history of suicide attempts or suicidal ideation as established by clinical interview, review of medical records, SCID/DSMIV, and Scale for Suicidal Ideation (lifetime); 2/22 were inpatients. Participants were excluded from this group if they had indirect self-destructive behaviors.
Non-depressed control subjects were included as the benchmark group. They had to have no lifetime history of any psychiatric disorder as determined by SCID/DSMIV.
Procedures
Our study was conducted at a university psychogeriatric inpatient unit and a specialty outpatient clinic for late-life depression. Since we aimed to capture decision-making in a state similar to a suicidal crisis, we assessed participants with major depression during an acute depressive episode. Participants were tested within two weeks of inpatient admission or at the beginning of outpatient treatment. Depressed participants continued to receive psychotropic medications as clinically indicated (Table 1). We ensured that none were intoxicated or had withdrawal symptoms at the time of testing. Neuropsychological testing took place in one to two sessions over 1–3 days. Assessors were blind to clinical history and ratings.
Assessments
Cognitive and clinical characterization
Current global cognitive function was assessed with the Dementia Rating Scale (DRS) (28). Depression severity was measured with the 17-item Hamilton Rating Scale for Depression (HRSD) (29). Burden of physical illness was assessed with the Cumulative Illness Rating Scale adapted for Geriatrics (CIRS-G) (30). We obtained medication lists from pharmacy records. We measured the intensity of pharmacotherapy for the current episode of depression with the cumulative strength score from the Antidepressant Treatment History Form (31), based on antidepressant trial duration, the dose, and the use of augmenting agents. In order to capture exposure to psychotropic medications not included in this score, we additionally assessed exposure to sedatives/hypnotics, anticholinergic drugs, and opioid analgesics. Intra-class correlation coefficients measuring interrater reliability among our assessors were 0.95 for HRSD, 0.97 for the Cumulative Illness Rating Scale, and 0.99 for the Dementia Rating Scale.
Probabilistic reversal learning
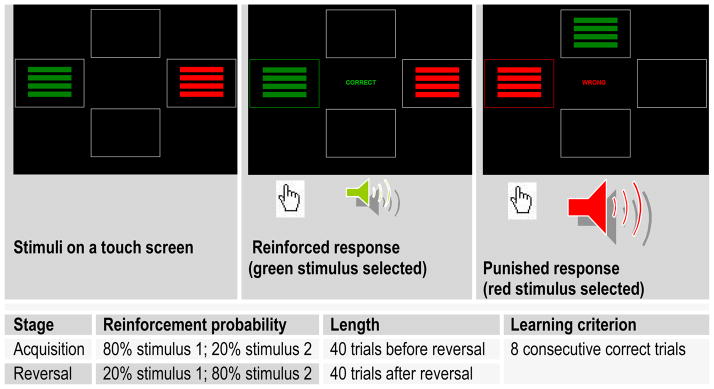
The probabilistic reversal learning task requires participants to learn to choose one of two colored rectangles in each of 80 trials. In an initial 40-trial acquisition stage, on 80% of the trials the subject is rewarded for selecting stimulus 1 and punished for selecting stimulus 2. On 20% of the trials, false feedback is delivered such that the subject is punished for selecting stimulus 1 (non-contingent or ‘probabilistic’ punishment), or rewarded for selecting stimulus 2. In the second 40-trial reversal stage, the probabilities are reversed (see Fig. 1). Our version of the task used symbolic reward (green frame around the stimulus, green ‘CORRECT’ display, high-frequency tone) and punishment (red frame around the stimulus, red ‘WRONG’ display, low-frequency tone). Prior studies of probabilistic reversal learning have found similar behavioral and neural responses to symbolic reward and punishment (19), monetary reward and loss (20), and angry and happy faces (32). Subjects were considered to ‘pass’ a stage if they reached a pre-determined learning criterion of eight consecutive correct trials. It is difficult to achieve this learning criterion by chance, without mastering the task, since at least one or two trials are likely to include non-contingent punishment. In addition, we used the number of trials before achieving the learning criterion in each stage as a parametric measure of performance.
Figure 1.
Probabilistic reversal learning task
To perform well on this task, one needs to trade off ‘staying’ (i.e. choosing the previously reinforced stimulus despite occasional non-contingent punishment) and ‘switching’ (i.e. reversing the preferred choice after punishment is received). One strategy is to integrate the reinforcement across a number of trials. The tendency to ‘stay’ while ignoring punishment feedback leads to perseverative errors, which result from selecting the previously correct stimulus after the reversal of probabilities. Conversely, the tendency to ‘switch’ too easily results in a large number of probabilistic switch errors, when one switches away from the preferentially reinforced stimulus in response to non-contingent punishment.
Control measures: forward planning and spatial working memory
To determine whether suicidal elderly demonstrate deficits that are specific to decision-making and can be dissociated from other aspects of cognition, we employed a test of forward planning and spatial working memory, the Stockings of Cambridge, a modification of the Tower of London task (33).
Statistical analysis
We used SPSS 15.0 (SPSS Inc., Chicago, IL) and MATLAB 7.6 (The MathWorks Inc., Natick, MA). All tests were two-sided. We first compared groups on demographic and clinical characteristics using F-tests and chi-square tests. For these and all subsequent F-tests, we examined post-hoc contrasts using the Tukey HSD test. We then used the chi-square test to compare proportions of participants in each group passing acquisition and reversal stages of the probabilistic reversal learning task. In our follow-up contrasts, we compared each of the two suicidal groups to control subjects and to non-suicidal depressed subjects. We then examined the performance pattern in the reversal stage by group using multinomial regression with group as dependent variable and two alternative markers of poor performance – perseverative errors and probabilistic switch errors – as covariates. Our sensitivity analyses used binary logistic regression with passing/failing the reversal stage as the dependent variable. The predictors included group status (dummy-coded) and potential confounding factors: global cognitive functioning, years of education, lifetime or current substance use disorders, and gender.
Computational model
To test our hypothesis that poor decision-making in suicide attempters is explained by abnormally high discounting of past rewards and punishments, we employed a computational modeling procedure in which the degree to which subjects incorporate the past history into their decisions (‘memory’) is estimated based on their responses. We adapted a reinforcement learning model common in studies of reinforcement/punishment-based learning (e.g. (20, 34, 35)). The model, described in detail in the Data Supplement, used a Rescorla-Wagner learning rule to choose parameters that afforded the strongest match between choices produced by the model and subjects’ actual choices, based on the reinforcement history experienced by each subject. The four free parameters were memory, learning rate from rewards, learning rate from punishments, and exploration. Memory reflected the effect of the prior reinforcement history on the choice. Learning rate from rewards and learning rate from punishments reflected the impact of reward or punishment on the last trial on the subject’s subsequent choice. Exploration reflected whether choice was random vs. determined by feedback.
RESULTS
Group characteristics
The groups were similar in their demographic characteristics (Table 1). Global cognitive functioning measured by the Dementia Rating Scale was lower in the three depressed groups compared to non-depressed control subjects. Suicide attempters had a higher lifetime prevalence of substance use disorders, compared to non-suicidal depressed participants (Table 1; χ2(1)=1615, p=0.001); 6/15 suicide attempters and none of the participants in the other groups had current substance use disorders. Psychotropic exposure was similar across the three depressed groups.
Probabilistic reversal learning
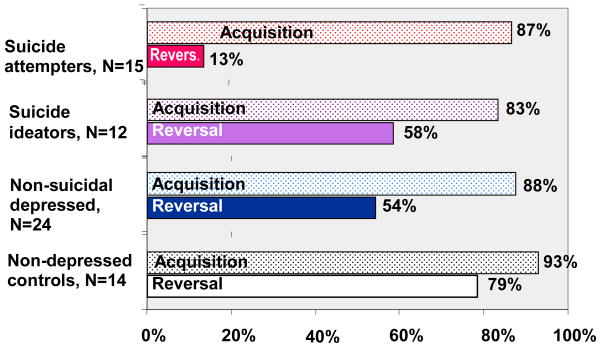
Groups did not differ in their performance in the acquisition stage, suggesting similar overall learning ability. On the other hand, significant differences in performance emerged in the reversal stage (Fig. 2). After the reinforcement contingencies were reversed, suicide attempters were less likely to achieve a learning criterion than non-depressed control subjects (χ2(1)=12.5, p<0.001) and than non-suicidal depressed participants (χ2(1)=6.5, p=0.011). Suicide ideators did not differ from the two control groups (χ2(1) ≤1.2, p≤0.26). These results were mirrored on trials needed to achieve the learning criterion in the acquisition (F[3,61]=1.4, p=0.24) and reversal stages (F[3,61]=5.3, p=0.003).
Figure 2. Overall probabilistic reversal learning performance: proportion of participants reaching the learning criterion in each stage.
The bars display the proportion of participants in each group who reached a pre-defined 8-trial learning criterion. All groups demonstrated similar probabilistic learning ability in the acquisition stage (chi-square test: χ2(3)=0.57, p=0.90), but performance in the reversal stage differed considerably between groups (χ2(3)=13.1, p=0.004)
Measures of excessive switching (probabilistic switch errors) and perseveration in the reversal stage differentiated the groups (Table 2). Examination of suicide attempters’ performance revealed two patterns: most attempters made multiple probabilistic switch errors, switching away from the newly reinforced stimulus after non-contingent punishment. Another, smaller, subgroup of suicide attempters made multiple perseverative errors. Suicide ideators did not differ from other groups.
Table 2.
Decision-making performance: behavioral indices and meta-learning parameters
| Non-depressed controls (C) N=14 | Non-suicidal depressed (D) N=24 | Suicide ideators (I) N=12 | Suicide Attempters (A) N=15 | X2 | p | Group Contrasts | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SD | M | SD | M | SD | M | SD | M | ||||
| Excessive switching in response to non-contingent negative feedback: probabilistic switch errors (lose-switch errors) | 0.8 | 1 | 1.5 | 1.9 | 1.5 | 1.5 | 2.3 | 2.5 | 12.6* | 0.006 | A>C p=0.004 |
| Perseveration: perseverative errors | 3.1 | 3.4 | 7.5 | 6.7 | 10.2 | 9.2 | 14.1 | 11.1 | 13.2† | 0.004 | A>C p=0.023 |
Multinomial logistic regression with group as dependent variable, covarying for perseverative errors.
Multinomial logistic regression with group as dependent variable, covarying for probabilistic switch errors.
Reinforcement learning model-based analyses
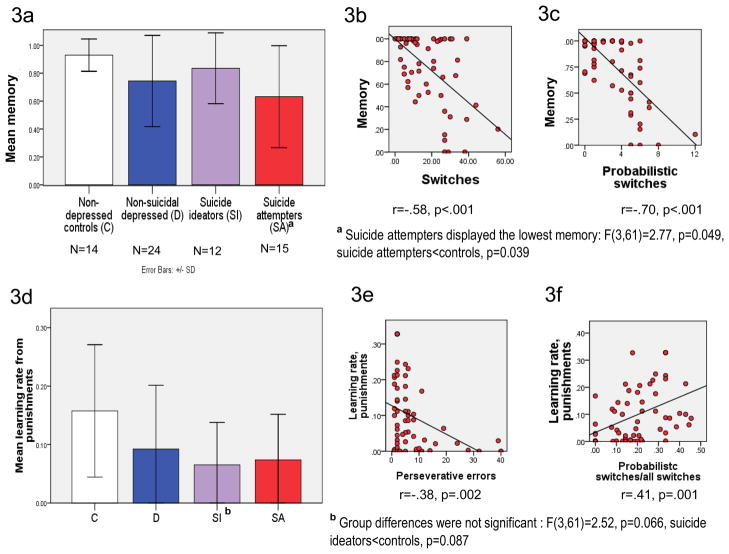
Suicide attempters displayed lower memory compared to non-depressed controls (omnibus ANOVA F(3,61)=2.77, p=0.049, Tukey HSD post-hoc: suicide attempters<controls, p=0.039; Figure 3a). That is, in their choice, attempters relied less on their previous reinforcement history and more on feedback on the last trial. As expected, participants with lower memory made more total switches in their choice (3b) and more probabilistic switches (3c). While the three depressed groups, particularly suicide ideators, tended to have a lower learning rate from punishments, group differences were not significant (F(3,61)=2.52, p=0.066, suicide ideators<controls, p=0.087; 3d.). This seemingly surprising trend was due to perseveration in the depressed groups (Table 2): participants who were less likely to switch their choice following punishment made more perseverative errors (3e). Learning rate from punishments was also positively correlated with the proportion of probabilistic switches among all switches (3f). Learning rate from rewards and exploration did not differ between groups (Data Supplement, Results, page s5).
Figure 3. Model-based analyses of probabilistic reversal learning: reliance on past reinforcement history (decay) and learning from punishments.
3a, 3d: model-derived meta-learning parameters by group. 3b, 3c, 3e, 3f: correlations with behavioral indices illustrate the meaning of meta-learning parameters. Memory: attention to prior reinforcement history vs. last trial. Learning rate from punishments: impact of punishment on the last trial on subsequent choice.
3a. Analyses based on the fit-parameters of the computational model revealed a lower memory among suicide attempters, compared to non-depressed controls (omnibus ANOVA F(3,61)=2.77, p=0.049, Tukey HSD post-hoc: suicide attempters<controls, p=0.039). That is, attempters relied less on their previous reinforcement history in making their decisions and more on feedback on the last trial, compared to non-depressed controls. As expected, memory was negatively correlated with the total number of switches in subjects’ choice (3b) and with the number of probabilistic switches (switches following non-contingent negative feedback, 3c). 3d. While the three depressed groups, particularly suicide ideators, tended to have a lower learning rate from punishments, group differences were not significant: F(3,61)=2.52, p=0.066, suicide ideators<controls, p=0.087. This seemingly surprising trend was due to perseverative errors in the three depressed groups (means shown in Table 2): learning rate from punishments was negatively correlated with the number of perseverative errors (3e), i.e. subjects who did not switch their choice in response to punishment made multiple perseverative errors. Learning rate from punishments was positively correlated with the proportion of switches in response to non-contingent punishment (probabilistic switches) among all switches (3f).
Forward planning and spatial working memory
On the Stockings of Cambridge test, groups did not differ in the number of problems solved in minimum moves, the number of moves used to solve the problems, adjusting for the level of difficulty, or deliberation times (Data Supplement, Results, Forward Planning and Working Memory, Fig. s2).
Sensitivity analyses: global cognitive function, substance use, age of first attempt, possible brain injury, medication exposure, and gender
The groups still differed in achieving the learning criterion in the reversal stage (Wald(1)=5.8, p=0.015) after controlling for global cognitive functioning measured by the Dementia Rating Scale (Wald(1)=1.5, p=0.22), years of education (Wald(1)=5.8, p=0.016), and for the effects of lifetime history of substance use disorders (Wald(1)=2.4, p=0.12). After all participants with lifetime substance use disorders were excluded, suicide attempters were still least likely to pass the reversal stage (χ2=11.6, p=0.009, N=52; suicide attempters vs. non-suicidal depressed: χ2=5.6, p=0.018, N=29). The same was true after excluding participants with current substance use disorders and after limiting the attempter group to 9/15 with first attempt after age 60 (Data Supplement, p. s6). Similarly, the difference between suicide attempters and non-suicidal depressed remained after excluding 1/15 suicide attempter with potential anoxic brain injury from the suicide attempt and 2/15 for whom brain injury could not be ruled out (χ2=10.2, p=0.001). Gender was not related to performance in the reversal stage (χ2=1.85, p=0.17).
DISCUSSION
We found that in depressed elders, a deficit in a component of decision-making – probabilistic reversal learning – is associated with attempted suicide, but not with suicidal ideation. Suicide attempters discounted their reinforcement history to a high degree compared to controls, basing their choices largely on reward/punishment received on the last trial. Besides, some suicide attempters also made multiple perseverative errors. This impairment was not explained by lower global cognitive function, effects of lifetime substance use disorders, or possible brain injury from suicide attempts. Furthermore, it was dissociated from cognitive abilities engaged outside the context of punishment and reward – forward planning and working memory.
Time and decision-making in suicidal behavior
Our results extend earlier findings of impaired decision-making in younger suicide attempters with affective disorders (11, 36) to a group of depressed elders with a history of suicide attempt. Suicide attempters in our study showed unstable decision-making, which has been described in mid-life depression (15, 16), only to a more extreme degree. Further, decreased reliance on past history was dissociated from abnormal sensitivity to rewards or punishments. Thus, in counterpoint to the prevailing view that suicidal individuals’ representations of reality are distorted in the valence domain (negative cognitive biases; e.g. (37)), our findings indicate distortions in the time domain. This notion is supported by early empirical findings of altered time perception (38–41) and by self-report evidence that future orientation is weakened in older suicide ideators and attempters (42). A similar process could have contributed to the decaying performance of suicide attempters on the Iowa Gambling Task (11); that is, they may have failed to integrate reinforcement history from a deck over a number of trials. Assuming that these laboratory observations are representative of individuals’ lives, they suggest that people vulnerable to suicidal behavior, faced with change and uncertainty, fail to access their past experiences, making decisions based largely on their present state.
Decision-making and possible neural substrates of suicidal behavior
Human lesion (13, 17, 18, 43) and imaging (19, 20) studies implicate the ventral prefrontal cortex in reward/punishment-based learning. Thus, our findings converge with evidence of ventral prefrontal pathology from post-mortem [reviewed in (44)] and imaging studies of attempted suicide in younger persons (45–47). Further, the suicide attempters’ intact performance on the Stockings of Cambridge test could indicate that forward planning and working memory associated with the dorsolateral prefrontal cortex (48) are relatively preserved.
Strengths, limitations, and future directions
Detailed clinical characterization, the inclusion of suicide ideators, and the use of a control task add confidence in our findings. Many cognitive studies of suicide are difficult to interpret, since they often combine individuals with major depression, bipolar disorder (11), and schizophrenia (9). Unipolar depression is the most common antecedent of late-life suicide (49–51), and thus our study focused on elders with major depression. It is unclear whether our findings can be generalized to other psychiatric disorders, although in mid-life the association between impaired decision-making and attempted suicide appears to transcend diagnostic boundaries (36). Further, we cannot assume that impaired reward/punishment-based probabilistic learning is unique to attempted suicide. While in our small sample it was not related to substance use, similar impairments have been described in pathological states characterized by impulsivity, such as pathological gambling (52) and behavioral toxicity of dopamine agonists (53, 54). Small group sizes and case-control design comprise the main limitations of this study. The lack of effect of depression on forward planning and working memory test raises questions about the sensitivity of the Stockings of Cambridge as a control test – a concern partly mitigated by the presence of robust test difficulty effects. Further, suicide attempters paid less attention to their reinforcement history than controls, but did not differ significantly from depressed elders. This observation cannot rule out an alternative hypothesis that distortions in the time domain are associated with depression and not specifically with suicidal behavior. Finally, our reinforcement learning model is limited in the extent to which it captures human decision-making. More complex formalisms, such as hidden Markov models (20) or models based on production rule learning (55), may be needed to characterize decision-making in suicidal behavior.
In summary, our study presents preliminary case-control evidence of dissociable deficits in older suicide attempters’ ability to learn from the experience of rewards and punishments under uncertainty. We propose a hypothesis that older suicide attempters make overly present-focused decisions, ignoring past experiences.
Supplementary Material
Acknowledgments
Supported by: NIMH grants K23 MH070471 (to Dr. Szanto) and R01MH072947 (to Dr. Butters), the American Foundation for Suicide Prevention Young Investigator award (to Dr. Szanto), and the John A. Hartford Foundation. The authors would like to thank Charles F. Reynolds, 3rd, M.D., (Univ. of Pittsburgh) for editorial comments and administrative support.
Footnotes
All authors report no competing interests.
References
- 1.World Health Organization. Suicide prevention. 2005 Available from: http://www.who.int/mental_health/prevention/suicide/suicideprevent/en/
- 2.Dombrovski AY, Szanto K, Duberstein P, Conner KR, Houck PR, Conwell Y. Sex differences in correlates of suicide attempt lethality in late life. Am J Geriatr Psychiatry. 2008;16:905–913. doi: 10.1097/JGP.0b013e3181860034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedmann H, Kohn R. Mortality, or probability of death, from a suicidal act in the United States. Suicide Life Threat Behav. 2008;38:287–301. doi: 10.1521/suli.2008.38.3.287. [DOI] [PubMed] [Google Scholar]
- 4.Dombrovski AY, Butters MA, Reynolds CF, 3rd, Houck PR, Clark L, Mazumdar S, et al. Cognitive Performance in Suicidal Depressed Elderly: Preliminary Report. Am J Geriatr Psychiatry. 2008;16:109–115. doi: 10.1097/JGP.0b013e3180f6338d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King DA, Conwell Y, Cox C, Henderson RE, Denning DG, Caine ED. A neuropsychological comparison of depressed suicide attempters and nonattempters. Journal of Neuropsychiatry & Clinical Neurosciences. 2000;12:64–70. doi: 10.1176/jnp.12.1.64. [DOI] [PubMed] [Google Scholar]
- 6.Keilp JG, Gorlyn M, Oquendo MA, Burke AK, Mann JJ. Attention deficit in depressed suicide attempters. Psychiatry Research. 2008;159:7–17. doi: 10.1016/j.psychres.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keilp JG, Sackeim HA, Brodsky BS, Oquendo MA, Malone KM, Mann JJ. Neuropsychological dysfunction in depressed suicide attempters. American Journal of Psychiatry. 2001;158:735–41. doi: 10.1176/appi.ajp.158.5.735. [DOI] [PubMed] [Google Scholar]
- 8.Marzuk PM, Hartwell N, Leon AC, Portera L. Executive functioning in depressed patients with suicidal ideation. Acta Psychiatr Scand. 2005;112:294–301. doi: 10.1111/j.1600-0447.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- 9.Raust A, Slama F, Mathieu F, Roy I, Chenu A, Koncke D, et al. Prefrontal cortex dysfunction in patients with suicidal behavior. Psychol Med. 2007;37:411–9. doi: 10.1017/S0033291706009111. [DOI] [PubMed] [Google Scholar]
- 10.Pollock LR, Williams JM. Problem-solving in suicide attempters. Psychol Med. 2004;34:163–7. doi: 10.1017/s0033291703008092. [DOI] [PubMed] [Google Scholar]
- 11.Jollant F, Bellivier F, Leboyer M, Astruc B, Torres S, Verdier R, et al. Impaired decision making in suicide attempters. American Journal of Psychiatry. 2005;162:304–10. doi: 10.1176/appi.ajp.162.2.304. [DOI] [PubMed] [Google Scholar]
- 12.Fellows LK, Farah MJ. Dissociable elements of human foresight: A role for the ventromedial frontal lobes in framing the future, but not in discounting future rewards. Neuropsychologia. 2005;43:1214–1221. doi: 10.1016/j.neuropsychologia.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131:1311–22. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers RD, Blackshaw AJ, Middleton HC, Matthews K, Hawtin K, Crowley C, et al. Tryptophan depletion impairs stimulus-reward learning while methylphenidate disrupts attentional control in healthy young adults: implications for the monoaminergic basis of impulsive behaviour. Psychopharmacology. 1999;146:482–491. doi: 10.1007/pl00005494. [DOI] [PubMed] [Google Scholar]
- 15.Murphy FC, Michael A, Robbins TW, Sahakian BJ. Neuropsychological impairment in patients with major depressive disorder: the effects of feedback on task performance. Psychol Med. 2003;33:455–67. doi: 10.1017/s0033291702007018. [DOI] [PubMed] [Google Scholar]
- 16.Taylor Tavares JV, Clark L, Furey ML, Williams GB, Sahakian BJ, Drevets WC. Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage. 2008;42:1118–26. doi: 10.1016/j.neuroimage.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–7. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- 18.Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry. 1994;57:1518–24. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:4563–7. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hampton AN, Bossaerts P, O’Doherty JP. The role of the ventromedial prefrontal cortex in abstract state-based inference during decision making in humans. Journal of Neuroscience. 2006;26:8360–8367. doi: 10.1523/JNEUROSCI.1010-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samejima K, Ueda Y, Doya K, Kimura M. Representation of action-specific reward values in the striatum. Science. 2005;310:1337–40. doi: 10.1126/science.1115270. [DOI] [PubMed] [Google Scholar]
- 22.Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40:1690–705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- 23.Williams JM, Broadbent K. Autobiographical memory in suicide attempters. Journal of abnormal psychology. 1986;95:144–9. doi: 10.1037//0021-843x.95.2.144. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. DSM-IV. [Google Scholar]
- 25.First MSR, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P) 1995. Version 2.0 ed. [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Beck R, Kovacs M. Classification of suicidal behaviors: I. Quantifying intent and medical lethality. Am J Psychiatry. 1975;132:285–7. doi: 10.1176/ajp.132.3.285. [DOI] [PubMed] [Google Scholar]
- 28.Mattis S. Dementia Rating Scale (DRS) Psychological Assessment Resources; Odessa, FL: 1988. [Google Scholar]
- 29.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–48. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 31.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62 (Suppl 16):10–7. [PubMed] [Google Scholar]
- 32.Kringelbach ML, Rolls ET. Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage. 2003;20:1371–83. doi: 10.1016/S1053-8119(03)00393-8. [DOI] [PubMed] [Google Scholar]
- 33.Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- 34.Yechiam E, Busemeyer JR, Stout JC, Bechara A. Using cognitive models to map relations between neuropsychological disorders and human decision-making deficits. Psychol Sci. 2005;16:973–8. doi: 10.1111/j.1467-9280.2005.01646.x. [DOI] [PubMed] [Google Scholar]
- 35.Samejima K, Doya K. Multiple representations of belief states and action values in corticobasal ganglia loops. Ann N Y Acad Sci. 2007;1104:213–28. doi: 10.1196/annals.1390.024. [DOI] [PubMed] [Google Scholar]
- 36.Malloy-Diniz LF, Neves FS, Abrantes SS, Fuentes D, Correa H. Suicide behavior and neuropsychological assessment of type I bipolar patients. J Affect Disord. 2009;112:231–6. doi: 10.1016/j.jad.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Beck AT, Kovacs M, Weissman A. Hopelessness and suicidal behavior. An overview. Jama. 1975;234:1146–9. [PubMed] [Google Scholar]
- 38.Brockopp GW, Lester D. Time competence and suicidal history. Psychological Reports. 1971;28:80. doi: 10.2466/pr0.1971.28.1.80. [DOI] [PubMed] [Google Scholar]
- 39.Greaves G. Temporal orientation in suicidal patients. Perceptual & Motor Skills. 1971;33:1020. doi: 10.2466/pms.1971.33.3.1020. [DOI] [PubMed] [Google Scholar]
- 40.Neuringer C, Harris RM. The perception of the passage of time among death-involved hospital patients. Life-Threatening Behavior. 1974;4:240–54. [PubMed] [Google Scholar]
- 41.Yufit RI, Benzies B, Fonte ME, Fawcett JA. Suicide potential and time perspective. Archives of General Psychiatry. 1970;23:158–63. doi: 10.1001/archpsyc.1970.01750020062008. [DOI] [PubMed] [Google Scholar]
- 42.Hirsch JK, Duberstein PR, Conner KR, Heisel MJ, Beckman A, Franus N, et al. Future orientation and suicide ideation and attempts in depressed adults ages 50 and over. Am J Geriatr Psychiatry. 2006;14:752–7. doi: 10.1097/01.JGP.0000209219.06017.62. [DOI] [PubMed] [Google Scholar]
- 43.Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, et al. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–39. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 44.Arango V, Underwood MD, Mann JJ. Postmortem findings in suicide victims. Implications for in vivo imaging studies. Ann N Y Acad Sci. 1997;836:269–87. doi: 10.1111/j.1749-6632.1997.tb52365.x. [DOI] [PubMed] [Google Scholar]
- 45.Jollant F, Lawrence NS, Giampietro V, Brammer MJ, Fullana MA, Drapier D, et al. Orbitofrontal Cortex Response to Angry Faces in Men With Histories of Suicide Attempts. Am J Psychiatry. 2008;165:740–748. doi: 10.1176/appi.ajp.2008.07081239. [DOI] [PubMed] [Google Scholar]
- 46.Monkul ES, Hatch JP, Nicoletti MA, Spence S, Brambilla P, Lacerda AL, et al. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Molecular psychiatry. 2007;12:360–6. doi: 10.1038/sj.mp.4001919. [DOI] [PubMed] [Google Scholar]
- 47.Oquendo MA, Placidi GP, Malone KM, Campbell C, Keilp J, Brodsky B, et al. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge and lethality of suicide attempts in major depression. Arch Gen Psychiatry. 2003;60:14–22. doi: 10.1001/archpsyc.60.1.14. [DOI] [PubMed] [Google Scholar]
- 48.Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, et al. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–39. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- 49.Waern M, Runeson BS, Allebeck P, Beskow J, Rubenowitz E, Skoog I, et al. Mental disorder in elderly suicides: a case-control study. American Journal of Psychiatry. 2002;159:450–5. doi: 10.1176/appi.ajp.159.3.450. [DOI] [PubMed] [Google Scholar]
- 50.Beautrais AL. A case control study of suicide and attempted suicide in older adults. Suicide Life Threat Behav. 2002;32:1–9. doi: 10.1521/suli.32.1.1.22184. [DOI] [PubMed] [Google Scholar]
- 51.Conwell Y, Lyness JM, Duberstein P, Cox C, Seidlitz L, DiGiorgio A, et al. Completed suicide among older patients in primary care practices: a controlled study. J Am Geriatr Soc. 2000;48:23–9. doi: 10.1111/j.1532-5415.2000.tb03024.x. [DOI] [PubMed] [Google Scholar]
- 52.de Ruiter MB, Veltman DJ, Goudriaan AE, Oosterlaan J, Sjoerds Z, van den Brink W. Response Perseveration and Ventral Prefrontal Sensitivity to Reward and Punishment in Male Problem Gamblers and Smokers. Neuropsychopharmacology. 2008;34:1027–1038. doi: 10.1038/npp.2008.175. [DOI] [PubMed] [Google Scholar]
- 53.Cools R, Barker RA, Sahakian BJ, Robbins TW. L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson’s disease. Neuropsychologia. 2003;41:1431–41. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 54.Mehta MA, Swainson R, Ogilvie AD, Sahakian J, Robbins TW. Improved short-term spatial memory but impaired reversal learning following the dopamine D(2) agonist bromocriptine in human volunteers. Psychopharmacology (Berl) 2001;159:10–20. doi: 10.1007/s002130100851. [DOI] [PubMed] [Google Scholar]
- 55.Anderson JR, Lebiere C. The atomic components of thought. Lawrence Erlbaum; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.