Abstract
KIF18A, a molecular motor, is an essential component in the regulation of orderly chromosome congression by attenuation of the kinetochore oscillation amplitude at the midzone during mitosis in vertebrate cells. Here we report that KIF18A depletion resulted in mitotic arrest which was accompanied by the presence of unaligned chromosomes in HeLa cells. This resembles the phenotype induced by an impaired function of CENP-E, also a mitotic kinesin essential for the formation of the mitotic spindles. Our further analysis showed that KIF18A depletion caused specific downregulation of CENP-E. Downregulation of CENP-E as the result of KIF18A silencing was not due to reduced transcription but primarily due to the enhanced protein degradation. Co-immunoprecipitation revealed that KIF18A physically interacted with CENP-E and BubR1 during mitosis. Ectopic expression of the wild-type tail domain of CENP-E, but not a corresponding mutant, significantly suppressed chromosome congression defects in mitotic cells. Together, our studies strongly suggest that chromosome congression defects as the result of KIF18A depletion is at least in part mediated through destabilizing kinetochore CENP-E.
Keywords: KIF18A, kinesin, mitosis, chromosome congression, protein stability
Introduction
Mitotic chromosome movements are driven by both microtubule-based motors and the dynamic properties of the mitotic spindles. KIF18A and CENP-E are microtubule-based motors in mammalian cells that localize at/or around kinetochores during early mitosis. KIF18A encodes a molecular motor protein of the kinesin-8 family.1–3 It is essential for chromosome congression during mitosis because it regulates proper assembly and positioning of the spindles.1–4 Microtubule dynamics facilitates chromosomal congression to the spindle equator before their synchronized segregation at the onset of anaphase. Before all chromosomes are aligned at the equator, chromosomes oscillate along the mid-zone. This appears to be a necessary step for proper alignment of paired chromosomes or sister chromatids, and for attachment by kinetochore microtubules. During mitosis, KIF18A is concentrated with a gradient at the plus ends of microtubules, facilitating microtubule depolymerization as a loss of its function results in the formation of elongated microtubules.2 KIF18A reduces the amplitude of preanaphase oscillations and negatively controls the movement of chromosomes toward the spindle poles during anaphase.1
Mitotic regulators including CENP-E and Sgo1 also affect chromosome alignment and segregation because disruption of their functions causes the appearance of unaligned chromosomes and induces chromosome missegregation.5–8 CENP-E is a plus end-directed motor protein, functioning in stabilizing kinetochore-microtubule capture during chromosome congression.9,10 Genetic studies indicate that CENP-E is essential for mammalian development.11 CENP-E also plays an important role in the regulation of the spindle checkpoint, probably by regulating BubR1 activities.12–15 Reduced CENP-E levels or impaired CENP-E functions can cause chromosome congression defects, leading to chromosome missegregation and aneuploidy.11,13,16 Time-lapse microscopy suggests that CENP-E may positively control chromosome congression by translocating unaligned chromosomes to the midzone along kinetochore microtubules.17 CENP-E levels are regulated during the cell cycle. It accumulates during late G2, peaks at mitosis, and is degraded at mitotic exit.18 This suggests that reduction in CENP-E may not cause major defects other than chromosome congression and spindle checkpoint activation during early mitosis.
Despite extensive studies on the individual roles of CENP-E and KIF18A during mitosis, it remains unclear if there are any physical and functional interactions between mitotic kinesins such as KIF18A and CENP-E during chromosome congression and mitotic progression. We observed that KIF18A depletion via RNAi resulted in decreased signals of kinetochore CENP-E, primarily due to its enhanced degradation. We also observed that depletion of either motor proteins caused chromosome congression defects. Given that KIF18A physically interacted with CENP-E during mitosis and that ectopic expression of a functional CENP-E tail domain, but not its mutant, suppressed chromosome congression defects, we propose that the mitotic roles of KIF18A is at least partially mediated by CENP-E.
Results
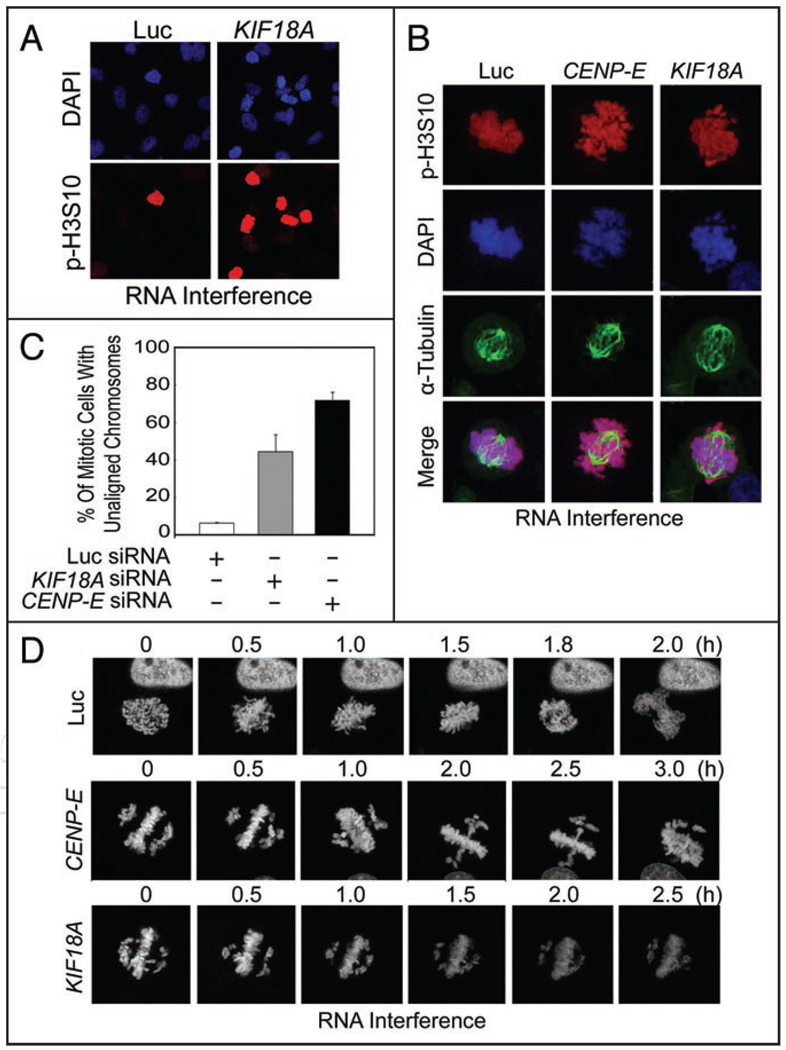
KIF18A is enriched at the plus end of kinetochore microtubules, regulating kinetochore microtubule dynamics and chromosome oscillation during mitosis. As the first step to studying the molecular mechanism by which KIF18A controls mitotic progression, we depleted KIF18A in HeLa cells via RNAi. Transfection of KIF18A siRNA significantly enriched cells positive for phospho-histone H3 (Fig. 1A), suggesting that downregulation of KIF18A induces mitotic arrest. Fluorescence microscopy revealed that a significant fraction of mitotic cells induced as the result of KIF18A depletion contained unaligned chromosomes (Fig. 1B and C). Consistent with early reports,6,16,19 CENP-E depletion also caused mitotic arrest which was accompanied by the presence of unaligned or lagging chromosomes (Fig. 1B and C). Time-lapse confocal microscopy revealed that cells transfected with KIF18A siRNA experienced a difficulty in completing chromosome congression at the equator (Fig. 1D). These cells contained clusters of chromosomes that surrounded the spindle poles (Fig. 1D). A majority of these cells eventually undergoing mitotic catastrophe (data not shown). Similar to that of KIF18A depletion, cells transfected with CENP-E siRNA also had mitotic defects, manifesting as having unaligned chromosomes that were unable to move to the equator (Fig. 1D).
Figure 1.
Defective chromosome congression after depletion of KIF18A or CENP-E. (A) HeLa cells cultured in chamber slides were transfected with KIF18A or luciferase siRNAs for 24 h were fixed and stained with antibodies to phosphorylated-serine 10 of histone H3 (p-H3S10). DNA was stained with DAPI. The stained cells were then examined by fluorescence microscopy. Representative images were shown. (B) HeLa cells were transfected with KIF18A, CENP-E or luciferase siRNAs for 24 h were fixed and stained with antibodies to p-H3S10 (red) and α-tubulin (green). DNA was stained with DAPI (blue). The stained cells were examined by fluorescence microscopy. Representative images were shown. (C) HeLa cells were transfected with KIF18A, CENP-E or luciferase siRNAs for 24 h were fixed and stained with the antibody to p-H3S10. The percentage of mitotic cells with unaligned chromosomes was summarized from three independent experiments. (D) HeLa cells stably expressing GFP-histone H2b were transfected with KIF18A, CENP-E or luciferase siRNAs for 24 h were directly analyzed by time-lapse microscopy. Representative green fluorescence time-lapse images from each treatment are shown.
We then examined expression of CENP-E after KIF18A silencing. After transfection of KIF18A siRNA for one day, the levels of CENP-E, were greatly reduced (Fig. 2A). Fluorescence microscopy showed that among mitotic cells induced by transfection of KIF18A siRNA, CENP-E staining at the kinetochores was either absent or significantly weakened in comparison with those transfected with luciferase siRNA (Fig. 2B and C), thus consistent with the immuno-blotting data. Moreover, the weakened kinetochore CENP-E was more apparent for chromosomes aligned at the equator (Fig. 2B).
Figure 2.
KIF18A depletion results in reduced CENP-E levels. (A) HeLa cells transfected with KIF18A, Sgo1 or luciferase siRNAs for 24 were collected and lysed. Equal amounts of proteins were blotted for KIF18A, CENP-E or β-actin. (B) HeLa cells were transfected with KIF18A siRNAs for 24 h and were fixed and stained with antibodies to CENP-E (green). DNA was stained with DAPI (blue). The stained cells were examined by fluorescence microscopy. Representative cells with no or low CENP-E staining are shown. (C) HeLa cells were transfected with KIF18A or luciferase siRNAs for 24 h were fixed and stained with antibodies to CENP-E (green). The stained cells were examined by fluorescence microscopy. The percentage of cells with no or low kinetochore CENP-E was summarized from three independent experiments.
Downregulation of CENP-E after KIF18A silencing could be due to a reduced level of CENP-E transcripts. Quantitative RT-PCR analysis revealed that whereas transfection of KIF18A siRNA greatly reduced its cellular transcript levels, it affected neither CENP-E nor SGO1 mRNA levels. On the other hand, transfection of CENP-E siRNA greatly reduced its mRNA levels (Fig. 3A). These results suggest that the reduction of CENP-E protein levels in KIF18A depleted cells was not at the transcription level. We then examined whether CENP-E protein could be stabilized in cells transfected with KIF18A siRNA by blocking the proteasome-mediated protein degradation. Whereas depletion of KIF18A via RNAi significantly down-regulated CENP-E, the treatment with MG132, a proteasome inhibitor, largely prevented CENP-E reduction (Fig. 3B and C).
Figure 3.
The reduced CENP-E levels in KIF18A-depleted cells are due to enhanced protein degradation. (A) HeLa cells transfected with KIF18A, SGO1, CENP-E or luciferase siRNAs for 48 h were collected for total RNA extraction. Equal amounts of RNA from each treatment, along with RNA from untreated cells, were subjected to quantitative reverse transcriptase-mediated PCR (RT-PCR). CENP-E and KIF18A mRNA levels summarized from three independent experiments are shown. (B) HeLa cells transfected with KIF18A or luciferase siRNAs for 24 h were treated with MG132 or the vehicle (DMSO) for 3 or 16 h. Cells were then collected and lysed. Equal amounts of cell lysates were blotted for KIF18A, CENP-E or β-actin. (C) The percentage of CENP-E changes after various treatments as shown in (B) were quantified by densitometric scanning.
To further confirm that KIF18A depletion destabilized CENP-E due to enhanced protein degradation, we examined the half-life of CENP-E and BubR1 in cells transfected with KIF18A siRNA as well as luciferase siRNA. The half-life of CENP-E and BubR1 in control cells was about 18 h in cells transfected with luciferase siRNA; after depletion of KIF18A, the half-life of CENP-E was less than 8 h, significantly shorter than that in the control cells (Fig. 4). We also observed that BubR1 had a significantly shortened half-life after KIF18A depletion (Fig. 4).
Figure 4.
The reduced CENP-E half-life in cells with KIF18A depletion. HeLa cells transfected with KIF18A or luciferase siRNAs for 24 h were treated with cycloheximide (CHX) for various times. Equal amounts of protein lysates collected at various times post CHX treatment were blotted for CENP-E, BubR1, KIF18A and α-tubulin.
To study whether or not there was physical interaction between KIF18A and CENP-E, we performed co-immunoprecipitation experiments. Although the KIF18A antibody was capable of precipitating KIF18A in both interphase and mitotic cell lysates, it brought down CENP-E in mitotic cells (Fig. 5A), suggesting the physical interaction between these two kinesins during mitosis. Consistent with the early observation that BubR1 interacts with CENP-E,14,20 we also detected CENP-E from BubR1 immunoprecipitates in mitotic cells (Fig. 5B). Fluorescence microscopy revealed that transfection of KIF18A siRNA affected BubR1 subcellular localization. BubR1 was strong in the kinetochores of chromosomes aligned at the equator in control cells (Fig. 5C). However, BubR1 signals were significantly weakened in congressed kinetochores in cells transfected with KIF18A siRNA; the reduced BubR1 staining in centromeric kinetochores was also observed in cells transfected with CENP-E siRNA (Fig. 5C). These results are consistent with the notion that there is a functional connection among KIF18A, CENP-E and BubR1 given the known physical interaction between CENP-E and BubR1.
Figure 5.
KIF18A interacts with CENP-E. (A) Equal amounts of interphase (I) and mitotic (M) HeLa cell lysates were immunoprecipitated with KIF18A IgGs or with control IgGs. Immunoprecipitates, along with lysate inputs, were blotted for KIF18A and CENP-E, respectively. (B) Equal amounts of interphase (I) and mitotic (M) HeLa cell lysates were immunoprecipitated with BubR1 IgGs or with control IgGs. Immunoprecipitates, along with lysate inputs, were blotted for BubR1 and CENP-E, respectively. (C) HeLa cells were transfected with KIF18A, CENP-E or luciferase siRNAs for 24 h and were fixed and stained with antibodies to BubR1 (red) and α-tubulin (green). DNA was stained with DAPI (blue). The stained cells were examined by fluorescence microscopy. Representative images were shown.
The motorless fragment of CENP-E is capable of constitutively activating BubR1 and the spindle checkpoint.14 The carboxyl-terminal fragment of CENP-E (amino acids 1,958–2,701), commonly referred as the tail domain, is also necessary and sufficient for its kinetochore localization.21 Moreover, a recent study shows that CENP-E tail is capable of binding to kinetochores whereas mutations in the SUMO-interaction motif (SIM) of the tail domain significantly abolished its binding.22 We next asked whether the chromosome congression defect in cells depleted of KIF18A could be affected by the enforced expression of CENP-E tail or its SIM mutant. Both the CENP-E tail domain and the SIM mutant were efficiently expressed in HeLa cells after transfection (Fig. 6A). Interestingly, ectopic expression of the wild-type CENP-E tail domain greatly suppressed the fraction of mitotic cells with unaligned chromosomes after KIF18A depletion; on the other hand, transfection of the SIM tail mutant failed to significantly reduce the percentage of cells with chromosome congression defects after KIF18A depletion compared with that in vehicle-transfected cells (Fig. 6B and C).
Figure 6.
Wild-type CENP-E tail domain partially rescues the chromosome congression defect induced by KIEF18A depletion. (A) HeLa cells were transfected with the plasmid vector alone or with a plasmid expressing the wild-type CENP-E tail domain (CENP-E-Tail) or a corresponding mutant defective in SUMO-interaction (CENP-E-TailMut) for 24 h. Equal amounts of cell lysates were blotted for CENP-E (both endogenous and recombinant ones) and β-actin. (B) HeLa cells were co-transfected with KIF18A siRNA and the plasmid expressing the wild-type CENP-E tail domain or the tail domain with SIM mutation for 48 h. HeLa cells transfected with luciferase siRNA for 48 h were used as control. The transfected cells were fixed and stained with antibodies to BubR1 (green) and CREST (red). DNA was stained with DAPI (blue). Representative images were shown. (C) The percentage of mitotic cells with unaligned chromosomes in various treatments was summarized from three independent experiments.
Discussion
KIF18A is enriched at the plus end of kinetochore microtubules.1,2 It is conceivable that KIF18A depletion would significantly affect the integrity of the kinetochores. The defective kinetochores due to a lack of KIF18A function would have a severe consequence on chromosome congression and segregation. Supporting this, nearly 50% of cells that are depleted of KIF18A contain unaligned/misaligned chromosomes. Chromosome congression defects inevitably cause a mitotic delay because these cells would be expected to correct the defect. We have also observed that KIF18A functions are essential for normal mitosis because most of these cells depleted of KIF18A would undergo mitotic catastrophe (data not shown). Phenotypically speaking, depletion of CENP-E, also a plus-end motor protein,5 results in the same mitotic defects as those seen in cells without KIF18A functions, including the presence of unaligned chromosomes, mitotic arrest and mitotic catastrophe.11,13,16 The close resemblance in cells depleted of KIF18A or CENP-E prompted us to speculate that these two proteins may be in the same regulatory hierarchy during early mitosis. Supporting this notion, we have demonstrated that KIF18A depletion causes downregulation of CENP-E and that KIF18A is physically associated with CENP-E during mitosis. More importantly, the chromosome congression defect is partially rescued by ectopic expression of the wild-type, but not the mutant, tail domain of CENP-E. We propose that CENP-E is downstream of KIF18A in the regulation of chromosome congression during mitosis. It is intriguing that CENP-E depletion induces the chromosome congression defect in a higher percentage of mitotic cells than that caused by KIF18A depletion. This could be explained by the fact that KIF18A depletion does not result in a complete elimination of CENP-E. It is also likely that KIF18A may not be the sole upstream regulator of CENP-E during mitosis.
A recent study demonstrates that KIF18A plays a role in chromosome congression by suppressing chromosome oscillation magnitudes.1 However, the molecular mechanism by which KIF18A controls chromosome congression by regulating the dynamic instability of microtubule plus-ends of the mitotic spindles remains unclear. Given the physical and functional interaction between KIF18A and CENP-E, we propose that KIF18A may function to maintain CENP-E levels at kinetochores during chromosome congression. Supporting this, KIF18A concentrates as a gradient on kinetochore microtubules which is dependent on its motor activity.2 Given the observed physical interaction between KIF18A and CENP-E and between CENP-E and BubR1, it is conceivable that KIF18A can stabilize the CENP-E-BubR1 complex at the kinetochores during early mitosis. In the absence of KIF18A, the CENP-E-BubR1 complex would be less stable at the kinetochores, especially the ones aligned at the metaphase plate. Unbound CENP-E and BubR1 could be more labile and accessible by the degradation system of the proteasome. Therefore, KIF18A levels are likely to be important for appropriate assembly of the kinetochores and the maintenance of the spindle checkpoint activation. This is consistent with the observation that both KIF18A and CENP-E levels are high during mitosis.18 An alternative model would be that KIF18A functions to provide the mitotic kinetochores with a constant flow of kinetochore proteins including CENP-E and BubR1. CENP-E, as well as BubR1, can be the cargos of KIF18A motor or that a major cargo molecule of KIF18A plays an important role in controlling BubR1 and CENP-E stability. In fact, it has been demonstrated that during prometaphase, spindle checkpoint proteins are very dynamic at the kinotochores and that there is only a small fraction of cellular checkpoint proteins that binds to the unattached kinetochores.23
Although the molecular mechanism by which KIF18A depletion causes instability of CENP-E remains unclear, the motor protein may keep CENP-E confined to the kinetochores during early mitosis, thus preventing the access of negative regulator(s). Alternatively, KIF18A may carry an unknown factor(s) that positively regulates or stabilizes CENP-E at the kinetochores. For example, among KIF18A cargos can be a factor that regulates phosphorylation and sumoylation of CENP-E because CENP-E phosphorylation and sumoylation are essential for the maintenance of its activity or kinetochore localization.22,24 A recent study shows that SUMO-2/3 modification of, as well as its binding to, CENP-E controls its kinetochore localization.22 It is conceivable that sumoylation stabilizes CENP-E by competing for lysine residues that are otherwise the targets of polyubiquitination followed by proteosomal degradation. KIF18A may interact with sumoylation modification enzyme(s) that stabilizes CENP-E given existing lines of supporting experimental evidence. (1) Mouse genetic studies indicate that sumoylation is essential for chromosome congression and mitotic progression.25 (2) Inhibition of the proteasome activity by MG132 treatment stabilizes CENP-E in cells depleted of KIF18A. (3) Ectopic expression of CENP-E tail defective in sumoylation and SUMO-interaction is incapable of rescuing the chromosome congression defect due to KIF18A depletion.
Materials and Methods
Cell culture
The HeLa cell line was obtained from the American Type Culture Collection. Cells were cultured under 5% CO2 in dishes or on Lab-Tek II chamber slides (Fisher Scientific) in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics (100 µg of penicillin and 50 µg of streptomycin sulfate per ml).
Flow cytometric analysis
HeLa cells of various treatments were first fixed in 75% ethanol at 4°C overnight. DNA was subsequently stained with propidium iodide (PI). Cell cycle distributions of various treatments were analyzed on a Beckman Coulter® Epics XL-MCL™ Flow Cytometer. Cell cycle distributions were analyzed using Multicycle software (Phoenix Flow System).
Reverse transcriptase-mediated polymerase chain reaction (RT-PCR)
Total RNA was extracted from HeLa cells transfected with KIF18A, SOG1 or luciferase siRNA for various times. Reverse transcription was carried out using Invitrogen reverse transcriptase. Specific primers (sequences) were designed for detecting expression of β-actin, CENP-E and KIF18A, respectively. CENP-E primers are with the following sequences: forward, 5'CAT CCT CAC CCT GAA CTA CCC3', and reverse, 5'TCA CTG CCT GCA AGA TCA AC3'; KIF18A primers are with the following sequences: 5' GGA GGG AGG AGG AAT TGA AG3', and reverse, CAG TCT GCC TGT GTT GCT GT; β-actin primers are with the following sequences: 5'CAT CCT CAC CCT GAA CTA CCC3', and reverse, AGC CTG GAT AGC AAC GTA CAT3'.
Western blotting
HeLa cells transfected with KIF18A, SGO1 or control (luciferase) siRNAs for various times. Rounded-up (mitotic) cells were collected by shake-off. The adherent fraction of cells was collected by trypsinization. Equal amounts (50 µg) of protein lysates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by immunoblotting with antibodies to KIF18A (Bethyl, 1:1,000), CENP-E (Sigma, 1:1,000), KIF18A or β-actin (Sigma, 1:1,500). Specific signals were detected with horseradish peroxidase-conjugated rabbit secondary antibodies (Cell Signaling) and enhanced chemiluminescence reagents (Pierce Biotechnology).
Fluorescence microscopy
HeLa cells cultured on chamber slides with various treatments were quickly washed with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde for 10 min at room temperature. Fixed cells were treated with 0.1% Triton X-100 in PBS for 10 min and then washed three times with ice-cold PBS. After blocking with 2% bovine serum albumin (BSA) in PBS for 15 min on ice, cells were incubated for 1 h at room temperature with antibodies against CENP-E, BubR1, KIF18A or α-tubulin with Rhodamine red X-conjugated anti-rabbit (or anti-human) IgGs and/or fluorescein isothiocyanate-conjugated anti-mouse IgGs (Molecular probe) at room temperature for 1 h in the dark. Cells were finally stained with 4'6'-diamidino-2-phenylindole (DAPI) (Fluka, 1 µg/ml) for 5 min. Fluorescence microscopy was performed and images were captured by Leica TCS SP5 confocal microscope using LAS AF software.
RNA interference
Small interfering RNAs (siRNAs) of human KIF18A were synthesized from Dharmacon which corresponded to the following sequences: 5'ACC AAC AAC AGT GCC ATA AA3' (designated as hKIF18A siRNA-1), 5'ACA GAT TCG TGA TCT CTT A3' (hKIF18A siRNA-2). These sequences are capable of silencing human KIF18A.2 Briefly, cells seeded at 60% confluency in an antibiotic-free culture medium were transfected with siRNA duplexes at a final concentration of 100 pM for 24 h (unless otherwise specified). The negative controls were cells transfected with 100 pM siRNA duplex targeting firefly (Photinus pyralis) luciferase (5'UUC CTA CGC TGA GTA CTT CGA3', GL-3 from Dharmacon).
Live cell time-lapse imaging
The HeLa cells were stably transfected with GFP-Histone H2b. HeLa GFP-Histone H2B cells grown in a 35 mm glass bottom dish (MatTek) for 24 hours at 60% confluence were transfected with 100 nM luciferase siRNA or human KIF18A or CENP-E siRNA. Cell culture medium was changed to CO2-independent medium (Invitrogen) supplemented with 10% FBS and 10 mM glutamine during live cell time-lapse imaging. From 18 hours to 36 hours after transfection, confocal GFP fluorescence and DIC time-lapse sequences were collected on a Leica TCS SP5 microscope equipped with a heated incubation chamber, a motorized Z-positioning device and 60xNA1.4 or 40xNA1.25 DIC optics. One DIC image and a stack of five fluorescent images (0.5 µm steps) were simultaneously acquired at 30 second intervals.
Statistical analysis
Data were expressed as mean ± s.d. The statistical differences were analyzed using Student’s t-test.
Acknowledgements
We thank Drs. Tim Yen and Michael Matunis for providing CENP-E expression plasmids and Xiaoqi Lia for the HeLa GFP-Histone H2B cells. This study was supported in part by US Public Service Awards to W.D. (CA090658 and CA113349) and by the National Nature Science Foundation to 2GW (3053039).
References
- 1.Stumpff J, von Dassow G, Wagenbach M, Asbury C, Wordeman L. The kinesin-8 motor Kif18A suppresses kinetochore movements to control mitotic chromosome alignment. Dev Cell. 2008;14:252–262. doi: 10.1016/j.devcel.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stumpff J, Wordeman L. Chromosome congression: the kinesin-8-step path to alignment. Curr Biol. 2007;17:326–328. doi: 10.1016/j.cub.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luboshits G, Benayahu D. MS-KIF18A, new kinesin; structure and cellular expression. Gene. 2005;351:19–28. doi: 10.1016/j.gene.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Zhu C, Zhao J, Bibikova M, Leverson JD, Bossy-Wetzel E, Fan JB, et al. Functional analysis of human microtubule-based motor proteins, the kinesins and dyneins, in mitosis/cytokinesis using RNA interference. Mol Biol Cell. 2005;16:3187–3199. doi: 10.1091/mbc.E05-02-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim Y, Heuser JE, Waterman CM, Cleveland DW. CENP-E combines a slow, processive motor and a flexible coiled coil to produce an essential motile kinetochore tether. J Cell Biol. 2008;181:411–419. doi: 10.1083/jcb.200802189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanudji M, Shoemaker J, L’Italien L, Russell L, Chin G, Schebye XM. Gene silencing of CENP-E by small interfering RNA in HeLa cells leads to missegregation of chromosomes after a mitotic delay. Mol Biol Cell. 2004;15:3771–3781. doi: 10.1091/mbc.E03-07-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang Z, Sun Y, Harley SE, Zou H, Yu H. Human Bub1 protects centromeric sister-chromatid cohesion through Shugoshin during mitosis. Proc Natl Acad Sci USA. 2004;101:18012–18017. doi: 10.1073/pnas.0408600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salic A, Waters JC, Mitchison TJ. Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell. 2004;118:567–578. doi: 10.1016/j.cell.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Espeut J, Gaussen A, Bieling P, Morin V, Prieto S, Fesquet D, et al. Phosphorylation relieves autoinhibition of the kinetochore motor Cenp-E. Mol Cell. 2008;29:637–643. doi: 10.1016/j.molcel.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Putkey FR, Cramer T, Morphew MK, Silk AD, Johnson RS, McIntosh JR, Cleveland DW. Unstable kinetochore-microtubule capture and chromosomal instability following deletion of CENP-E. Dev Cell. 2002;3:351–365. doi: 10.1016/s1534-5807(02)00255-1. [DOI] [PubMed] [Google Scholar]
- 11.Weaver BA, Silk AD, Montagna C, Verdier-Pinard P, Cleveland DW. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell. 2007;11:25–36. doi: 10.1016/j.ccr.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Abrieu A, Kahana JA, Wood KW, Cleveland DW. CENP-E as an essential component of the mitotic checkpoint in vitro. Cell. 2000;102:817–826. doi: 10.1016/s0092-8674(00)00070-2. [DOI] [PubMed] [Google Scholar]
- 13.Weaver BA, Bonday ZQ, Putkey FR, Kops GJ, Silk AD, Cleveland DW. Centromere-associated protein-E is essential for the mammalian mitotic checkpoint to prevent aneuploidy due to single chromosome loss. JCell Biol. 2003;162:551–563. doi: 10.1083/jcb.200303167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao Y, Desai A, Cleveland DW. Microtubule capture by CENP-E silences BubR1-dependent mitotic checkpoint signaling. JCell Biol. 2005;170:873–880. doi: 10.1083/jcb.200505040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan GK, Jablonski SA, Sudakin V, Hittle JC, Yen TJ. Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. JCell Biol. 1999;146:941–954. doi: 10.1083/jcb.146.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEwen BF, Chan GK, Zubrowski B, Savoian MS, Sauer MT, Yen TJ. CENP-E is essential for reliable bioriented spindle attachment, but chromosome alignment can be achieved via redundant mechanisms in mammalian cells. MolBiolCell. 2001;12:2776–2789. doi: 10.1091/mbc.12.9.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapoor TM, Lampson MA, Hergert P, Cameron L, Cimini D, Salmon ED, et al. Chromosomes can congress to the metaphase plate before biorientation. Science. 2006;311:388–391. doi: 10.1126/science.1122142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown KD, Coulson RM, Yen TJ, Cleveland DW. Cyclin-like accumulation and loss of the putative kinetochore motor CENP-E results from coupling continuous synthesis with specific degradation at the end of mitosis. J Cell Biol. 1994;125:1303–1312. doi: 10.1083/jcb.125.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McEwen BF, Chan GK, Zubrowski B, Savoian MS, Sauer MT, Yen TJ. CENP-E is essential for reliable bioriented spindle attachment, but chromosome alignment can be achieved via redundant mechanisms in mammalian cells. Mol Biol Cell. 2001;12:2776–2789. doi: 10.1091/mbc.12.9.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao Y, Abrieu A, Cleveland DW. Activating and silencing the mitotic checkpoint through CENP-E-dependent activation/inactivation of BubR1. Cell. 2003;114:87–98. doi: 10.1016/s0092-8674(03)00475-6. [DOI] [PubMed] [Google Scholar]
- 21.Chan GK, Schaar BT, Yen TJ. Characterization of the kinetochore binding domain of CENP-E reveals interactions with the kinetochore proteins CENP-F and hBUBR1. JCell Biol. 1998;143:49–63. doi: 10.1083/jcb.143.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang XD, Goeres J, Zhang H, Yen TJ, Porter AC, Matunis MJ. SUMO-2/3 modification and binding regulate the association of CENP-E with kinetochores and progression through mitosis. Mol Cell. 2008;29:729–741. doi: 10.1016/j.molcel.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howell BJ, Moree B, Farrar EM, Stewart S, Fang G, Salmon ED. Spindle checkpoint protein dynamics at kinetochores in living cells. Curr Biol. 2004;14:953–964. doi: 10.1016/j.cub.2004.05.053. [DOI] [PubMed] [Google Scholar]
- 24.Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, et al. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2 and Cenp-E to kinetochores. JCell Biol. 2003;161:267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nacerddine K, Lehembre F, Bhaumik M, Artus J, Cohen-Tannoudji M, Babinet C, et al. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev Cell. 2005;9:769–779. doi: 10.1016/j.devcel.2005.10.007. [DOI] [PubMed] [Google Scholar]