Abstract
Some species in the polyphyletic fungal genus Acremonium are important opportunist pathogens. Determining the actual spectrum of species and their incidence in the clinical setting, however, has long been hampered because of the difficulties encountered in phenotypic species-level identification. The goal of this study was to re-identify a large number of clinical isolates morphologically and to confirm the identifications by comparing sequences of the internal transcribed spacer region of the rRNA gene of these isolates to those of type or reference strains of well-known Acremonium species. Of the 119 isolates referred to a United States reference laboratory under the name Acremonium, only 75 were identified morphologically as belonging to that genus. The remainder (44 isolates) were identified as belonging to other morphologically similar genera. The Acremonium clinical isolates were related to species of Hypocreales, Sordariales, and of an incertae sedis family of ascomycetes, Plectosphaerellaceae. A total of 50 of the 75 Acremonium isolates (67%) could be identified by molecular means, the prevalent species being Acremonium kiliense (15 isolates), A. sclerotigenum-A. egyptiacum (11 isolates), A. implicatum (7 isolates), A. persicinum (7 isolates), and A. atrogriseum (4 isolates). One of the most interesting findings of our study was that we identified several species among this large collection of clinical isolates that had not previously been reported from human infections, and we failed to confirm other Acremonium species, such as A. potronii, A. recifei, and A. strictum, that had been considered significant. The most common anatomic sites for Acremonium isolates were the respiratory tract (41.3%), nails (10.7%), and the eye (9.3%). Antifungal susceptibility testing demonstrated high MICs for all agents tested, except for terbinafine. Since numerous isolates could not be identified, we concluded that the list of opportunistic Acremonium species is far from be complete and that a considerable number of additional species will be discovered.
Acremonium is a large polyphyletic fungal genus that comprises approximately 150 species, most of them being saprobes in soil and pathogens of plants, insects, and other fungi. Some species are considered opportunists of humans and other mammals (5, 11, 18). Infections in humans typically develop following traumatic inoculation of the fungus, with keratitis and mycetoma being the most common, although more recently a significant role of Acremonium as a cause of onychomycosis has also been reported (13). Locally invasive infections such as osteomyelitis, sinusitis, arthritis, peritonitis, and less frequently central nervous system infections have also been reported. In recent years, the number of infections caused by Acremonium have increased considerably; aggressive modern medical techniques and new diseases involving the immune system have become important predisposing factors (4, 5, 11-13, 16, 18). The typical morphological features of Acremonium include slow-growing colonies, thin hyphae, and long, narrow, and tapered phialides formed singly; however, conidiophores with simple or verticillate branching may occur in some species. The phialides produce conidia which are small, mostly unicellular, in either slimy heads, chains, or both (5, 6, 8, 18). Numerous genera of ascomycetes have Acremonium or Acremonium-like anamorphs. Glenn et al. (10) and more recently Zare et al. (21), using molecular methods, demonstrated that Acremonium is polyphyletic, being associated with at least two ascomycetous orders, i.e., Hypocreales and Sordariales, and to the family Plectosphaerellaceae (incertae sedis, closely related to Microascales).
The species of Acremonium are morphologically very similar to each other and at best can only be distinguished on the basis of subtle differences, making their identification difficult. Therefore, in most of the clinical cases the etiological agent is reported only as an Acremonium sp., which drastically reduces the value of the report (11). This is the main reason that the real incidence of the different species of Acremonium in the clinical setting is unknown. Re-identification of available clinical isolates of Acremonium using modern DNA-based methods is essential for a critical evaluation of the reported cases. However, the major difficulty for the molecular identification of Acremonium isolates lies in the absence of reliable reference sequences in public databases for use in comparison.
To assess the incidence of different species of Acremonium in human infections, we studied a large set of clinical isolates referred from different regions of the United States to the Fungus Testing Laboratory, a fungal reference laboratory in San Antonio, for identification and/or antifungal susceptibility determination. It is important to note that we lack sufficient clinical information to ascertain whether any of these isolates was confirmed as a causal agent of infection. It is, however, likely that a significant proportion of them were causal agents, and the remainder may represent clinical contaminants, the identification of which will be a regular feature of clinical practice in the future. These isolates were identified using traditional morphological criteria (5, 6, 8, 9, 15, 18), and their internal transcribed spacer (ITS) sequences were compared to those available in GenBank and those of type or reference strains sequenced by us. The ITS sequences of authentic strains of the most relevant species of clinical interest generated in the present study have been deposited in GenBank.
MATERIALS AND METHODS
Fungal isolates.
A total of 119 clinical isolates, presumably belonging to Acremonium spp., received by the Fungus Testing Laboratory in the Department of Pathology at the University of Texas Health Science Center at San Antonio for identification or antifungal susceptibility determination, were included in the present study (Table 1) . In addition, 29 type strains (living cultures of the specimen used to describe a given species) or reference strains (living cultures of species identified by specialists and deposited in international collections) of Acremonium species provided by the Centraalbureau voor Schimmelcultures (CBS-KNAW; Utrecht, Netherlands) and the Mycothèque de l'Université Catholique de Louvain (MUCL; Belgium) were also tested. Seven ITS ribosomal DNA (rDNA) sequences of Acremonium species retrieved from GenBank were also included in the phylogenetic analyses (Table 1).
TABLE 1.
Clinical isolates, type or reference strains, and sequences of Acremonium spp. included in the study
| Isolatea | Originb | Morphological identification | Molecular identification | GenBank accession no. (ITS)c |
|---|---|---|---|---|
| UTHSC 01-194 | Blood, Washington | A. strictum | A. sclerotigenum-A. egyptiacum | |
| UTHSC 01-1246 | Eye, Louisiana | A. strictum | Acremoniumsp. | |
| UTHSC 01-1249 | Lung, California | A. persicinum | A. persicinum | FN706545 |
| UTHSC 01-1389 | Lung, Utah | A. persicinum | A. persicinum | FN706546 |
| UTHSC 01-1896 | Knee, Montana | A. persicinum | A. persicinum | |
| UTHSC 01-2238 | Left eye, Utah | A. kiliense | A. kiliense | |
| UTHSC 02-1892 | Sputum, Wisconsin | A. strictum | Acremoniumsp. | |
| UTHSC 02-1958 | Sputum, Texas | A. implicatum | A. implicatum | FN706540 |
| UTHSC 02-2054 | Tracheal aspirate, Ohio | A. alternatum | A. sclerotigenum-A. egyptiacum | |
| UTHSC 02-2429 | Pleural fluid, Utah | A. persicinum | A. persicinum | |
| UTHSC 02-2482 | BAL, Texas | A. hyalinulum | A. hyalinulum | |
| UTHSC 02-2564 | Leg, Alaska | A. strictum | Acremoniumsp. | |
| UTHSC 02-2890 | Olecrenon bursa, Wisconsin | A. strictum | A. sclerotigenum-A. egyptiacum | |
| UTHSC 03-2 | Sinus, California | A. sclerotigenum | A. sclerotigenum-A. egyptiacum | |
| UTHSC 03-986 | BAL aplastic anemia, Pennsylvania | A. atrogriseum | A. atrogriseum | |
| UTHSC 03-1930 | BAL, Utah | A. atrogriseum | A. atrogriseum | |
| UTHSC 03-2933 | Bronch wash, Michigan | A. implicatum | A. implicatum | |
| UTHSC 03-3197 | Vitreous fluid, Florida | A. kiliense | A. kiliense | |
| UTHSC 04-60 | Left foot mass bx, Wisconsin | A. kiliense | A. kiliense | |
| UTHSC 04-292 | Sputum, Colorado | A. alternatum | Acremoniumsp. | |
| UTHSC 04-721 | Vertebral disc, California | A. kiliense | A. kiliense | |
| UTHSC 04-956 | Sinus, Minnesota | A. implicatum | A. implicatum | |
| UTHSC 04-1034 | Right calf tissue, Florida | A. bactrocephalum | Acremoniumsp. | |
| UTHSC 04-1531 | Abscess scalp, Texas | A. alternatum | Acremoniumsp. | |
| UTHSC 04-2454 | Blood, Florida | A. kiliense | A. kiliense | FN691449 |
| UTHSC 04-3176 | CSF, Minnesota | A. sclerotigenum | A. sclerotigenum-A. egyptiacum | |
| UTHSC 04-3464 | Sputum, Texas | A. strictum | Acremoniumsp. | |
| UTHSC 05-104 | Unknown, California | A. egyptiacum | A. sclerotigenum-A. egyptiacum | |
| UTHSC 05-541 | Nonhealing wound leg, Minnesota | A. strictum | Acremoniumsp. | |
| UTHSC 05-1172 | BAL, Florida | A. strictum | A. sclerotigenum-A. egyptiacum | |
| UTHSC 05-1713 | Blood, Pennsylvania | A. kiliense | A. kiliense | |
| UTHSC 05-1929 | Hip fluid, Louisiana | A. bactrocephalum | Acremoniumsp. | |
| UTHSC 05-2022 | Scalp, Texas | A. kiliense | A. kiliense | |
| UTHSC 05-2270 | Blood, Utah | A. strictum | A. sclerotigenum-A. egyptiacum | |
| UTHSC 05-2310 | Contact lens, Texas | A. strictum | A. zeae | FN691452 |
| UTHSC 05-2451 | BAL, Massachusetts | A. persicinum | A. persicinum | |
| UTHSC 05-3311 | BAL, Texas | A. implicatum | Acremoniumsp. | |
| UTHSC 06-79 | Skin right thigh, Florida | A. strictum | Acremoniumsp. | |
| UTHSC 06-415 | Sputum, Minnesota | A. hyalinulum | A. hyalinulum | |
| UTHSC 06-482 | Right cornea, Virginia | A. kiliense | A. kiliense | |
| UTHSC 06-528 | Toe nail, South Carolina | A. hansfordii | Acremoniumsp. | |
| UTHSC 06-795 | Foot, Arkansas | A. persicinum | A. persicinum | |
| UTHSC 06-874 | Sputum, Hawaii | A. furcatum | Acremoniumsp. | |
| UTHSC 06-1454 | Toe nail, Florida | A. sclerotigenum | A. sclerotigenum-A. egyptiacum | |
| UTHSC 06-1476 | Right cornea, Colorado | A. kiliense | A. kiliense | FN691448 |
| UTHSC 06-2147 | Nail, Washington | A. atrogriseum | A. atrogriseum | |
| UTHSC 06-2849 | Bronch wash, Pennsylvania | A. kiliense | A. kiliense | |
| UTHSC 06-4335 | Sinus, Minnesota | A. griseoviride | Acremoniumsp. | |
| UTHSC 07-110 | Bone, California | A. implicatum | Acremoniumsp. | |
| UTHSC 07-550 | Blood, Arkansas | A. kiliense | A. kiliense | |
| UTHSC 07-646 | Bronch wash, Florida | A. fusidioides | A. fusidioides | |
| UTHSC 07-1181 | Sputum, Hawaii | A. glaucum | A. glaucum | FN691445 |
| UTHSC 07-1974 | CSF-seizures, Florida | A. kiliense | A. kiliense | |
| UTHSC 07-2604 | Sinus, Minnesota | A. kiliense | A. kiliense | FN691450 |
| UTHSC 07-3260 | Bone, Illinois | A. implicatum | A. implicatum | |
| UTHSC 07-3446 | Bronch wash, Texas | A. implicatum | Acremoniumsp. | |
| UTHSC 07-3667 | Bronch wash, Minnesota | A. implicatum | A. implicatum | |
| UTHSC 07-3739 | Toe nail, Minnesota | A. alternatum | A. sclerotigenum-A. egyptiacum | |
| UTHSC 08-661 | Forearm tissue, Minnesota | A. strictum | Acremoniumsp. | |
| UTHSC 08-844 (1) | BAL, Texas | A. implicatum | A. implicatum | |
| UTHSC 08-844 (2) | BAL, Texas | A. kiliense | A. kiliense | |
| UTHSC 08-1028 | Nail, Minnesota | A. polychromum | A. polychromum | FN706548 |
| UTHSC 08-1188 | BAL, Texas | A. fusidioides | A. fusidioides | FN706543 |
| UTHSC 08-1455 | Bronch wash, Texas | A. fusidioides | A. fusidioides | FN706544 |
| UTHSC 08-2284 | Toe nail, Utah | A. bactrocephalum | Acremoniumsp. | |
| UTHSC 08-3115 | BAL, Illinois | A. acutatum | Acremoniumsp. | |
| UTHSC 08-3180 | RUL BAL, Texas | A. implicatum | A. implicatum | FN706541 |
| UTHSC 08-3294 | Sputum, California | A. strictum | A. sclerotigenum-A. egyptiacum | |
| UTHSC 08-3421 | Nail-finger, South Carolina | A. persicinum | A. persicinum | |
| UTHSC 08-3639 | BAL, Texas | A. borodinense | Acremoniumsp. | |
| UTHSC 08-3693 | Nails-dermatitis, Missouri | A. pteridii | Acremoniumsp. | |
| UTHSC 09-384 | Eye, California | A. strictum | Acremoniumsp. | |
| UTHSC 09-597 | Tissue L4-5, Minnesota | A. atrogriseum | A. atrogriseum | |
| UTHSC R-3853 (1) | Sputum, California | A. kiliense | A. kiliense | |
| UTHSC R-3853 (2) | Sputum, California | A. hyalinulum | A. hyalinulum | |
| CBS 124.42 (T) | Dune sand, France | A. sclerotigenum | FN706552 | |
| CBS 136.33 (T) | Toe nail, Argentina | A. spinosum | ||
| CBS 158.61 | Maduromycosis, India | A. kiliense | FN691447 | |
| CBS 223.70 | Plaster, unknown | A. alternatum | U57674* | |
| CBS 270.86 | Toe nail, Nancy, France | A. sclerotigenum | FN706551 | |
| CBS 281.80 | Nail, Netherlands | A. sclerotigenum | FN706549 | |
| CBS 310.59 (T) | Coastal sand, France | A. persicinum | FN706554 | |
| CBS 346.70 (T) | Triticum aestivum, Germany | A. strictum | FN691453 | |
| CBS 379.70F | Skin lesion, Dolphin, Belgium | A. potronii | AY632655* | |
| CBS 406.66 | Wall in greenhouse, Germany | A. alternatum | ||
| CBS 407.66 | Hypoxylon deustum, Austria | A. alternatum | ||
| CBS 430.66 (T) | Wheat field soil, Germany | A. curvulum | ||
| CBS 485.77 | Man, India | A. recifei | ||
| CBS 545.89 | Blood culture, Netherlands | A. alternatum | ||
| CBS 560.86 | Bamboo living leaf, France | A. hyalinulum | ||
| CBS 604.67 (T) | Noodles, Ukraine | A. atrogriseum | ||
| CBS 654.96 | Eucalyptus leaf, Japan | A. strictum | ||
| CBS 749.69 (T) | Ustilago sp., Manitoba, Canada | A. bactrocephalum | ||
| CBS 774.97 | Urine, Germany | A. atrogriseum | ||
| CBS 796.69 (T) | Woolen overcoat, Solomon Islands | A. glaucum | FN691454 | |
| CBS 800.69 (T) | Zea mays stalk, Nebraska | A. zeae | FN691451 | |
| CBS 840.68 (T) | Antilope dung, Central African Republic | A. fusidioides | FN706542 | |
| CBS 881.73 | Dracaena, India | A. charticola | AJ621774* | |
| CBS 987.87 | Hypogymnia physodes, Luxembourg | A. antarcticum | DQ825970* | |
| CBS 993.69 | Skin, Netherlands | A. blochii | ||
| CBS 101148 (T) | Sugarcane field soil, Japan | A. borodinense | ||
| CBS 111360 | Fucus serratus, Germany | A. tubakii | AY632654* | |
| CBS 112868 (T) | Fucus serratus, Germany | A. fuci | AY632653* | |
| CBS 113360 (T) | Corucia zebrata, California | A. exuviarum | AY882946* | |
| CBS 114785 (T) | Ground, Egypt | A. egyptiacum | FN706550 | |
| MUCL 4112 | Soil, Georgia, United States | A. implicatum | FN706553 | |
| MUCL 9696 (T) | Mycetoma, Brazil | A. recifei | ||
| MUCL 9724 (T) | Skin, Germany | A. kiliense | FN691446 | |
| MUCL 9745 (T) | Sand, France | A. furcatum | ||
| MUCL 9834 (T) | Hevea brasiliensis bark, Indonesia | A. polychromum | FN706547 | |
| MUCL 30020 | Field soil, France | A. hyalinulum |
UTHSC, Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX; CBS, Centraalbureau voor Schimmelcultures, Utrecht, Netherlands; MUCL, Mycotheque de l'Universite Catholique de Louvain, Louvain, Belgium; (T), type strains.
BAL, bronchoalveolar lavage specimen; CSF, cerebrospinal fluid.
Accession numbers without an asterisk represent sequences newly generated in this study; *, sequences retrieved from the GenBank database.
Morphological study.
All isolates were initially cultured on malt extract agar 2% (MEA; Difco, Laboratories, Detroit, MI), potato dextrose agar (PDA; Difco), and oatmeal agar (OA; 30 g of filtered oat flakes, 20 g of agar, 1 liter of distilled water) media, which are the common culture media used to identify Acremonium species. However, since they grew and sporulated better on OA, this medium was used in the morphological study. Cultures were incubated at room temperature (25 ± 1°C), alternating light and darkness (12 h of each) for 7 to 14 days up to 1 month. The identification criteria were mainly based on the works of Gams (8, 9) and De Hoog et al. (5). Microscopic features were examined by making direct wet mounts with 85% lactic acid and lactophenol cotton blue and by slide cultures on OA using light microscopy. Photomicrographs were obtained with a Zeiss Axio-Imager M1 light microscope, using phase contrast and Nomarski differential interference.
Molecular study.
Isolates were grown on yeast extract sucrose (YES; yeast extract, 2%; sucrose, 15%; agar, 2%; water, 1 liter) for 3 to 5 days at 25 ± 1°C, and DNA was extracted using a PrepMan Ultra sample preparation reagent (Applied Biosystems, Foster City, CA), according to the manufacturer's protocol. The DNA was quantified using GeneQuantpro (Amersham Pharmacia Biotech, Cambridge, England). The ITS region of the nuclear rDNA was amplified and sequenced with the primer pair ITS5 and ITS4 according to the protocols described by Alvarez et al. (2).
Phylogenetic analysis.
Sequences of 112 taxa, including that of Boletus rubropunctus (accession no. FJ480433) as an outgroup and those downloaded from GenBank, were analyzed phylogenetically. The sequences generated in the present study have been deposited in GenBank/EMBL databases, and their accession numbers are indicated in Table 1. All sequences were aligned with the CLUSTAL X (version 1.81) computer program (20), followed by manual adjustments with a text editor. Phylogenetic analyses were performed with the software program MEGA 4.0 (19). The neighbor-joining method and the algorithm Kimura two-parameter were used to obtain the distance tree. Gaps were treated as pairwise deletions. Support for internal branches was assessed by a search of 1,000 bootstrapped pseudoreplicates of the data. Due to the high phylogenetic distances between the species tested and the high degree of variability of their ITS sequences, two types of approaches were performed: (i) an analysis of the 5.8S rRNA gene sequences of all of the isolates and (ii) analyses of the entire ITS sequences of the main clades obtained in the first analysis.
Antifungal susceptibility.
The in vitro activity of amphotericin B (AMB), natamycin (NAT), itraconazole (ITC), posaconazole (PSC), voriconazole (VRC), fluconazole (FLC), anidulafungin (ANID), caspofungin (CAS), micafungin (MICA), 5-fluorocytocine (5FC), and terbinafine (TRB) were determined according to methods outlined in the CLSI document M38-A2 (3). This method includes growing isolates on PDA at 35°C for 1 week prior to setup to ensure sufficient conidial formation. Slants were overlaid with sterile-distilled water, and then the surface was gently scraped to produce a conidial suspension. The tubes were permitted to sit for ∼10 min to allow large hyphal clumps to settle. Inoculum was standardized spectrophotometrically to an optical density at 530 nm (OD530) of 0.09 to 0.13 or 80 to 82% transmittance, diluted in Roswell Park Memorial Institute (RPMI) medium, and then 100 μl was added to each of the microtiter wells containing antifungal agents for a final inoculum concentration of 1 × 104 to 5 × 104 CFU/ml. The plates were incubated at 35°C for 48 h before reading. The MIC was defined as the lowest concentration that resulted in complete inhibition of growth for AMB, NAT, ITC, PSC, and VRC. The MIC for FLC, 5FC, and TRB was defined as the lowest concentration that resulted in an ca. 50% reduction in growth compared to the drug-free control well. For the candins, ANID, CAS, and MICA, the endpoint was defined as the minimum effective concentration (MEC), which was defined as the lowest concentration at which the appearance of small, compact hyphal growth was observed compared to the growth of healthy hyphae in the drug-free negative control well.
Nucleotide sequence accession numbers.
A representative number of the sequences that were newly generated in the study have been submitted to GenBank under accession numbers FN691445 to FN691454 and FN706540 to FN706554.
RESULTS
Using morphological features, 75 of 119 isolates were determined to belong to the genus Acremonium. The main characteristics included the production of flat to very thin cottony, whitish, yellowish, pinkish, or greenish colonies of moderate to slow growth; thin hyphae, producing awl-shaped and erect phialides with a septum at the base, formed singly or in very simple branching conidiophores; and conidia that were one celled, hyaline, subhyaline, or pigmented, arranged in slimy heads or in chains. The remaining isolates (n = 44) were only identified to the genus level and included Fusarium (13 isolates), Phaeoacremonium (10 isolates), Verticillium (7 isolates), Phialemonium (6 isolates), Lecanicillium (3 isolates), and one isolate each of Lecythophora, Monocillium, Paecilomyces, Pleurostomophora, and a Sagenomella-like strain. These latter non-Acremonium identifications were confirmed molecularly via a BLAST search of GenBank.
Sequences of the ITS region of the Acremonium isolates were compared to those available in GenBank by using a BLAST search. This analysis demonstrated that 67 isolates belonged to the order Hypocreales; four isolates were close to members of the Plectosphaerellaceae, and four others, identified as A. atrogriseum, were related to the Sordariales. No morphological features useful for distinguishing members of the respective above-mentioned taxonomic groups were found, except that phialides in the Sordariales are moderately inflated at the base, whereas only a few Acremonium species belonging to the Hypocreales and none of the Plectosphaerellaceae has such character.
Phylogenetic analysis of 5.8S rDNA sequences and morphological characterization.
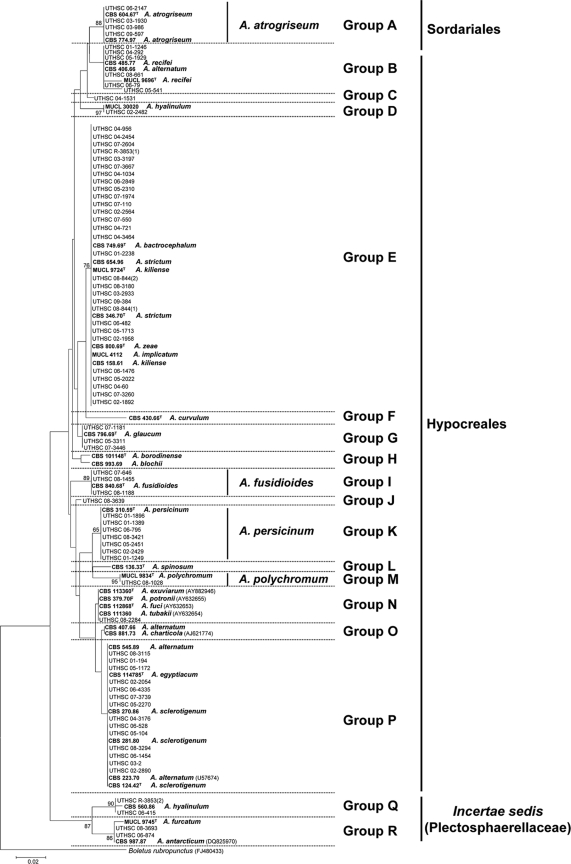
Figure 1 shows the neighbor-joining tree inferred from analysis of the 5.8S rRNA gene (169 bp) of a total of 112 sequences belonging to the 75 clinical isolates, 29 types or reference strains, and 7 sequences retrieved from GenBank for species of Acremonium that were morphologically similar to some of the clinical isolates tested. Eighteen groups (A to R) were identified among the in-group taxa, although the genetic distances among some of them were considerably high, and most of these groups were not supported by bootstrapping. Clinical isolates were nested in 14 (A to E, G, I to K, M, N, and P to R) of these 18 groups.
FIG. 1.
Neighbor-joining tree inferred from 5.8S rRNA gene sequences of the isolates listed in Table 1. Branch lengths are proportional to the distance. Sequences of the 5.8S rRNA gene obtained from the GenBank database are indicated with the accession number in parentheses. Type and reference strains are indicated in boldface. A superscript “T” indicates a type strain.
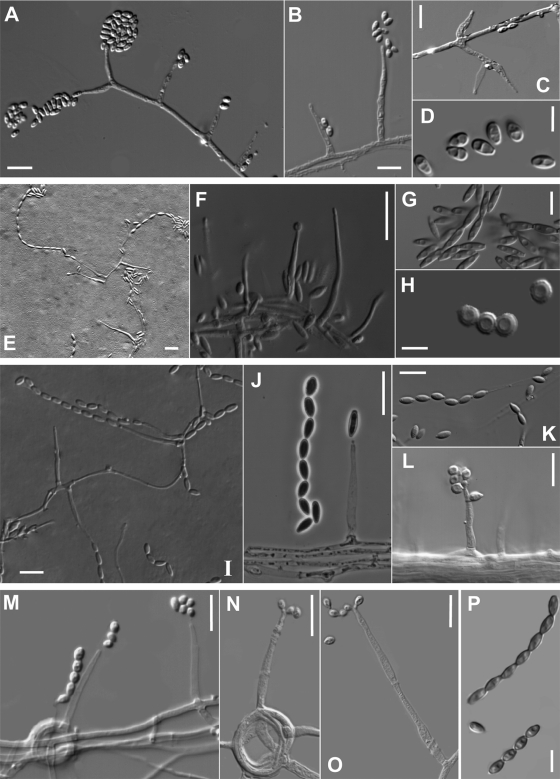
Group A received 88% of bootstrap support (bs) and included four clinical isolates morphologically identified as A. atrogriseum, including the type (CBS 604.67) and a reference strain (CBS 774.97) of this species. This species was characterized by powdery and brownish colonies, flask-shaped phialides, occasionally with an inflated base, and obovoid or ellipsoidal conidia arranged in slimy heads or chains (Fig. 2 A to D).
FIG. 2.
(A to D) Acremonium atrogriseum (CBS 604.67T); (A to C) flask-shaped phialides and conidia in slimy heads; (D) ellipsoidal or obovoid conidia. (E to H) Acremonium fusidioides (CBS 840.68T); (E and F) phialides and conidia in chains; (G) fusiform and smooth conidia; (H) spherical and warty conidia. (I to L) Acremonium persicinum (I to K, CBS 310.59T; L, UTHSC 06-795); (I and J) phialides and conidia in chains; (K) obovoid conidia; (L) phialide with conidia arranged in slimy heads. (M to P) Acremonium polychromum (MUCL 9834T); (M to O) phialides and conidia in chains; (P) obovate conidia with pointed base. Scale bars: A to C, E, F, I to L, and M to O, 10 μm; D, G, H, and P, 5 μm.
Group B was a morphologically heterogeneous assemblage that included six clinical isolates and the type or reference strains of A. recifei (MUCL 9696 and CBS 485.77) and A. alternatum (CBS 406.66).
Group C had only a single clinical isolate (UTHSC 04-1531). This isolate showed typical features of A. alternatum, except that its conidia were arranged in slimy heads, instead of in chains like those described for that species (8).
Groups D (97% bs) and Q (90% bs) comprised a total of three clinical isolates which were morphologically identified as A. hyalinulum. Both groups included respective reference strains of this species. The fact that two reference strains of A. hyalinulum were nested in two very distant groups is of concern, particularly since the type strain of that species is not available.
Group E, the largest group sampled, included 29 clinical isolates and the type or reference strains of A. kiliense, A. bactrocephalum, A. implicatum, A. strictum, and A. zeae. The 5.8S sequences were all identical and thus not useful for identifying the group E species, which were otherwise morphologically distinct from each other.
Group G included the type strain of A. glaucum (CBS 796.69) and three clinical isolates (UTHSC 07-1181, UTHSC 05-3311, and UTHSC 07-3446), but only strain UTHSC 07-1181 showed the typical morphological features of the mentioned species (see below).
Group I was statistically well supported (89% bs) and included the type strain of A. fusidioides (CBS 840.68) and three morphologically similar clinical isolates (Fig. 2E to H). The most distinctive character of this group was the presence of two types of conidia including: (i) those that were fusiform, smooth-walled and arranged in long dry chains, which were the predominant type, and (ii) those that were spherical, slightly warty, and organized in loose chains.
Group J contained a single isolate (UTHSC 08-3639), which produced two types of conidia: (i) ellipsoidal to ovoid and smooth-walled and (ii) spherical to ovoid and rugose. Although this clinical isolate was genetically distant from A. borodinense (15), it was morphologically similar.
Group K comprised seven clinical isolates morphologically identified as A. persicinum and the type strain of that species (CBS 310.59). The most remarkable morphological characters of this group were the production of whitish to ochre-brown colonies and smooth or occasionally rugose, obovoid conidia, with a protruding and slightly truncate base, arranged in chains or in masses (Fig. 2I to L).
Group M (95% bs) included the type strain of A. polychromum (MUCL 9834) and a clinical isolate (UTHSC 08-1028) identified morphologically as belonging to that species. These two isolates were characterized by olivaceous-brown obovoid conidia that were pointed at the base, and arranged in dry chains (Fig. 2M to P).
Group N contained a clinical isolate (UTHSC 08-2284) and four type or reference strains of Acremonium spp. considered anamorphs of the ascomycetous genus Emericellopsis: A. exuviarum (type strain, CBS 113360), A. fuci (type strain, CBS 112868), A. potronii (CBS 379.70F), and A. tubakii (CBS 110360). These fungi have been shown to form a coherent group, the Emericellopsis clade, in a more detailed phylogenetic study (22). The clinical isolate was different morphologically from the other species of the group by its cylindrical conidia with a slightly apiculate base, arranged in slimy heads, and by the absence of chlamydospores.
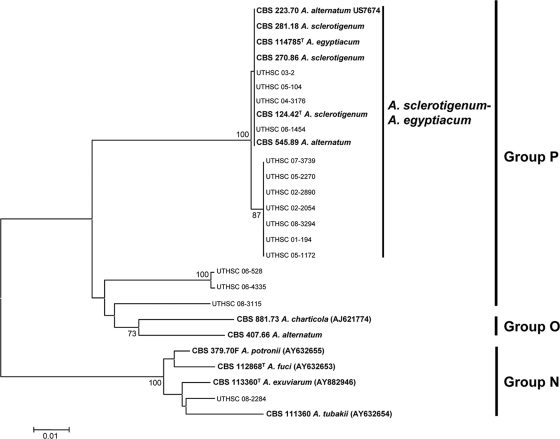
Group P contained the second highest number of clinical isolates (n = 14). Several type or reference strains of different species of Acremonium (i.e., A. alternatum, A. egyptiacum, and A. sclerotigenum) were also nested in this group, but without bootstrap support. It is worth mentioning that reference strains of A. alternatum were nested also in groups B and O.
Finally, group R (86% bs) was represented by two clinical isolates, the type strain of A. furcatum (MUCL 9745) and a reference sequence of A. antarcticum (CBS 987.87). A common feature of these isolates was the presence of schizophialides (i.e., phialides with very short aseptate branches near the apex). Since schizophialides were not described in A. antarcticum (14), the identification of the CBS 987.87 is doubtful.
Phylogenetic analysis of ITS1-5.8S-ITS2 sequences and morphological characterization.
The first phylogenetic analysis of the ITS region included members of groups A to D. The sequence alignment was 523 bp in length. Groups A (A. atrogriseum) and D (A. hyalinulum) received high statistical support (100% bs), whereas the other groups remained poorly supported (data not shown).
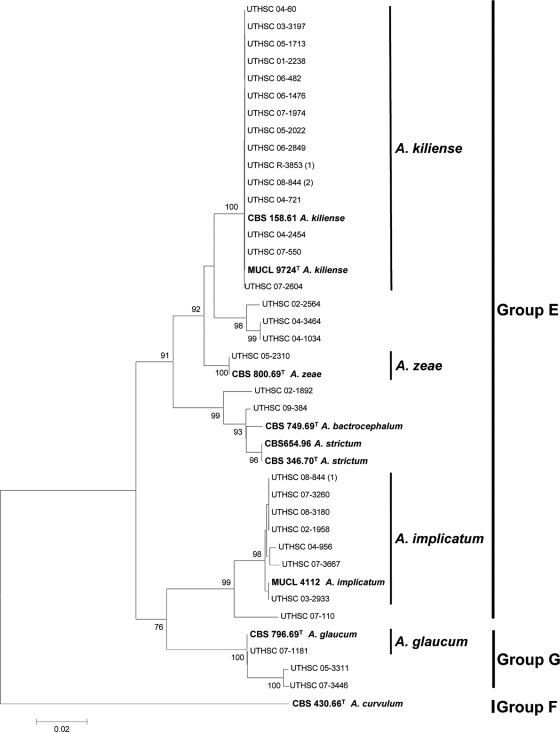
Figure 3 shows the phylogenetic tree inferred from the ITS region of 32 clinical isolates and the respective type or reference strains included in groups E, F, and G. The type strain of A. curvulum was used as the outgroup. The ITS alignment consisted of 496 bp. Eleven well-supported terminal clades were obtained. Four of these clades contained clinical strains together with type or reference strains of described species of Acremonium. These clades corresponded to A. kiliense (100% bs), where 15 clinical isolates were included; A. zeae (100% bs) and A. glaucum (100% bs), each with only one clinical isolate; and A. implicatum (98% bs) with 7 clinical isolates. The isolates belonging to A. kiliense can be easily identified since on OA they form flat or wrinkled, ropy or slightly cottony, and dirty white to pale orange colonies. The phialides are acicular (Fig. 4 A) and produce ellipsoidal to cylindrical conidia in slimy heads (Fig. 4B and C). This species also produces adelophialides (a reduced form of a phialide without a basal septum) (Fig. 4F) and chlamydospores (Fig. 4D and E), with both structures protruding from vegetative hyphae submerged in growth medium. The most distinctive morphological feature of A. zeae is the production of branched conidiophores with phialides, chromophilic at the base, arranged in whorls, and conidia in slimy heads (Fig. 4G to I). A. glaucum is characterized by bluish-green colonies and conidia arranged in long chains (Fig. 2J and K). A. implicatum is characterized by the production of fusiform conidia with sharply pointed ends disposed in dry chains (Fig. 4L and M). The two latter species do not produce chlamydospores. The rest of the clades, which contained a total of 8 clinical isolates, corresponded to unidentified or unknown species of Acremonium. It is worth mentioning, however, that the strains UTHSC 02-2564, UTHSC 04-3464, UTHS 04-1034, UTHSC 02-1892, and UTHSC 09-384 were morphologically very similar to A. strictum and that the strains UTHSC 07-110, UTHSC 05-3311 and UTHSC 07-3446 were morphologically very similar to A. implicatum.
FIG. 3.
Neighbor-joining tree constructed with the ribosomal ITS region sequences of the isolates included in the E, F, and G groups shown in Fig. 1. Branch lengths are proportional to distance. Examples of bootstrap support (1,000 replicates) above 70% are indicated on the nodes. Types and reference strains are indicated in boldface. A superscript “T” indicates a type strain.
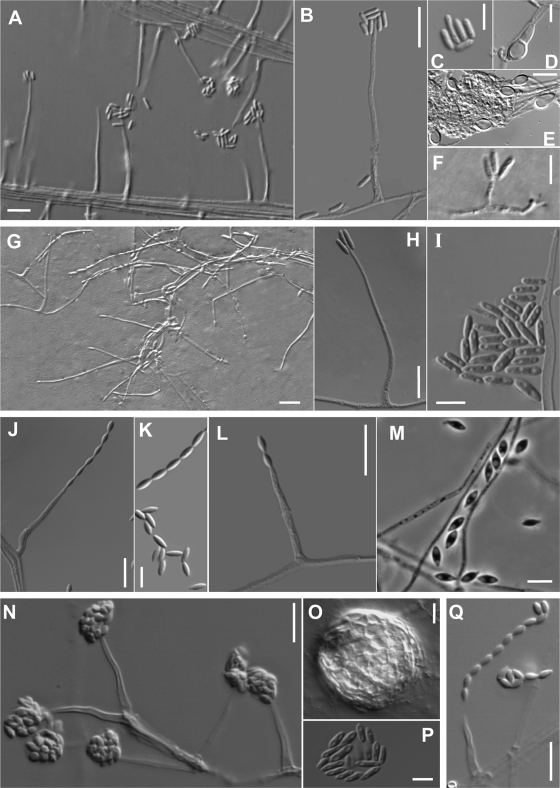
FIG. 4.
(A to F) Acremonium kiliense (MUCL 9724T); (A and B) phialides and conidia in slimy heads; (C) ellipsoidal to cylindrical conidia; (D and E) intercalary and terminal chlamydospores; (F) an adelophialide and straight or irregularly curved conidia. (G to I) Acremonium zeae (CBS 800.69T); (G and H) phialides and conidia; (I) cylindrical conidia with rounded ends. (J and K) Acremonium glaucum (CBS 796.69T); (J) phialide producing a conidial chain; (K) fusiform conidia with pointed ends. (L and M) Acremonium implicatum (MUCL 4112); (L) phialide-producing conidia; (M) fusiform conidia. (N to P) Acremonium sclerotigenum (CBS 124.42T); (N) phialides and conidia in slimy heads; (O) sclerotium; (P) conidia. (Q) Acremonium egyptiacum (CBS 114785T); phialide and conidial chain. Scale bars: A to E, G, H, J, L to O, and Q, 10 μm; F, I, K, and P, 5 μm.
Groups H to M, which included 12 clinical isolates, were also analyzed separately. The ITS alignment was 544 bp in length. The resulting tree showed a similar topology to that of the 5.8S tree. However, some of the groups, i.e., I (A. fusidioides), K (A. persicinum), and M (A. polychromum) received significant support (100% bs) (data not shown).
Figure 5 shows the phylogenetic tree inferred from neighbor-joining analysis of the 15 clinical isolates included in the groups N, O, and P. The ITS alignments consisted of 471 bp. Most of the clinical isolates (n = 11) grouped in a main clade (100% bs), together with the types or reference strains of A. alternatum, A. egyptiacum, and A. sclerotigenum. It should be noted that type strains are reliable indicators of the correct placement of taxonomic names, whereas reference strains may be misleading. Of the taxonomic names falling into this group, only A. egyptiacum and A. sclerotigenum are anchored by data obtained from extant type strains; A. alternatum may be misapplied. With the exception of UTHSC 05-104 and UTHSC 05-1172, which produced conidia in chains, the rest of the clinical isolates produced conidia in slimy heads morphologically resembling A. strictum. However, many of these isolates produced submerged sclerotia (Fig. 4O) in the OA after 2 to 4 weeks of incubation, a fungal structure absent in A. strictum and characteristic of some but not all isolates of A. sclerotigenum and A. egyptiacum. The last two species have traditionally been distinguished morphologically by their conidial arrangement, with slimy heads attributed to the former (Fig. 4N and P) and dry chains attributed to the latter (Fig. 4Q). The other related clinical isolates (n = 4) were nested in three different clades, showing no clear relationship with any of the reference strains included in the study. Morphologically, the strain UTHSC 06-528 was similar to A. hansfordii, the strain UTHSC 06-4335 was similar to A. griseoviride, the strain UTHSC 08-3115 was similar to A. acutatum, and the strain UTHSC 08-3115 was similar to A. bactrocephalum, but none of them was confirmed molecularly.
FIG. 5.
Neighbor-joining tree constructed with the ribosomal ITS region sequences of the isolates included in the N, O, and P groups shown in Fig. 1. Branch lengths are proportional to distance. Bootstrap values above 70% are indicated on the internodes. GenBank accession numbers are in parentheses. Type and reference strains are indicated in boldface. A superscript “T” indicates a type strain.
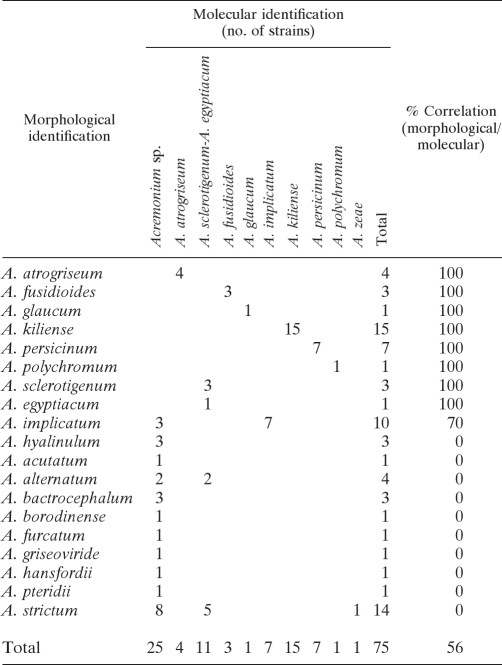
The correlation between morphological and molecular identification is shown in Table 2. Of the 75 clinical isolates of Acremonium identified morphologically, only 50 (67%), representing 9 species, could be confirmed molecularly by comparison with sequences of type strains or reference strains. The species identified were A. kiliense (15 isolates), A. sclerotigenum-A. egyptiacum (11 isolates), A. implicatum (7 isolates), A. persicinum (7 isolates), A. atrogriseum (4 isolates), A. fusidioides (3 isolates), A. glaucum (1 isolate), A. polychromum (1 isolate), and A. zeae (1 isolate).
TABLE 2.
Comparison of morphological and molecular identification based on ITS sequences of 75 Acremonium clinical isolates
Table 3 summarizes some relevant morphological features, based on OA cultures, useful for distinguishing the Acremonium species identified confidently in the present study. This table may be helpful in the identification of clinical isolates of Acremonium for laboratories that lack molecular facilities.
TABLE 3.
Distinctive morphological features, based on oatmeal cultures at 25°C after 7 days in alternating light/darkness of well-characterized Acremonium species found in clinical samples
| Order | Species | Colonies | Conidia | Other distinctive features |
|---|---|---|---|---|
| Sordariales | A. atrogriseum | Pale ochre-brown to brownish-black | Obovoid or ellipsoidal, smooth, subhyaline to dark gray, in slimy heads or in chains | Phialides flask-shaped, occasionally with a marked inflated base; sclerotia and chlamydospores absent |
| Hypocreales | A. fusidioides | White to ochraceous-brown, often reddish | Two types: (i) fusiform, smooth, and thin-walled, in long dry chains, more numerous, and (ii) spherical, thick and warty-walled, in loose chains | Sclerotia and chlamydospores absent |
| A. persicinum | Whitish to ochre brown | Obovoid, with slightly protruding and truncate base, usually smooth, hyaline, slimy heads or in chains | Sclerotia and chlamydospores absent | |
| A. polychromum | Ochre brown | Obovoid, with a pointed base, smooth to rugose, olivaceous-brown, in dry chains | Phialides often with incrusted pigment near the tip; sclerotia and chlamydospores absent | |
| A. kiliense | Dirty white to pale orange | Ellipsoidal to cylindrical, straight or curved, smooth-walled, hyaline, in slimy heads | Chlamydospores present after 7 days, globose to ellipsoidal, thick-walled, intercalary, or terminal; adelophialides present; sclerotia absent | |
| A. zeae | White to pink | Cylindrical with rounded ends, smooth, hyaline, in slimy heads | Conidiophores usually several times branched; sclerotia and chlamydospores absent | |
| A. glaucum | Intense gray-green to bluish-green | Fusiform, smooth-walled, weakly pigmented (grayish-green), in long dry chains | Sclerotia and chlamydospores absent | |
| A. implicatum | Whitish to pinkish | Fusiform with sharply pointed ends, hyaline, in long dry chains | Sclerotia and chlamydospores absent | |
| A. egyptiacum-A. sclerotigenum | Pale ochre to light pink | Obovoid, cylindrical or slighly fusiform, smooth, hyaline, or pale greenish, in chains or mostly collapsing in heads | Submerged sclerotia present after 2 to 4 weeks; chlamydospores occasionally produced |
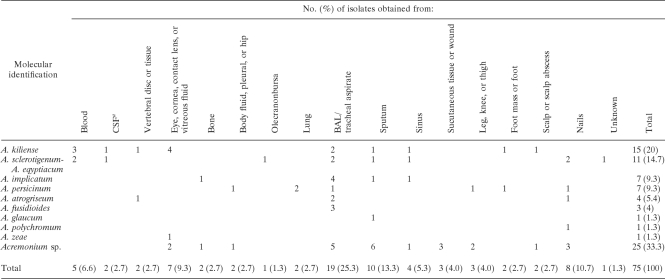
A listing of the anatomic sites yielding the isolates based upon the information available and cross-referenced by species is provided in Table 4. Again, we emphasize that these data do not necessarily signal confirmed infection.
TABLE 4.
Source and identification of U.S. Acremonium clinical isolates examined
a CSF, cerebrospinal fluid.
The results of antifungal susceptibility testing (Table 5) reveal an overall lack of activity for ANID, CAS, MICA, and ITC. All Acremonium isolates were resistant to 5FC and FLC, like most other genera of hyaline molds. Although there is a lack of breakpoint data for non-Candida species, the elevated MICs for AMB suggest poor activity of this agent. Endpoints for NAT have not been determined or proposed in the literature, but the MICs obtained ranging from 4 to 16 μg/ml most likely reflect achievable levels when treating topically for fungal keratitis. Although TRB has not been evaluated for potential cutoffs, the lowest MICs for all antifungal agents tested against all Acremonium species were obtained with this compound, followed by PSC and VRC.
TABLE 5.
Results of in vitro antifungal susceptibility testing
| Species (no. of isolates) | Drug concn (μg/ml)a |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMB |
ITC |
PSC |
VRC |
TRB |
NAT |
MICA |
ANID |
CAS |
||||||||||
| MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MEC50 | MEC90 | MEC50 | MEC90 | MEC50 | MEC90 | |
| A. kiliense (15) | 4 | 16 | >16 | >16 | 4 | >16 | 2 | 4 | 1 | 2 | 8 | 8 | >16 | >16 | >16 | >16 | >16 | >16 |
| A. sclerotigenum- | ||||||||||||||||||
| A. egyptiacum (11) | 4 | 16 | >16 | >16 | 1 | 2 | 2 | 2 | 0.125 | 0.5 | 8 | 16 | >16 | >16 | >16 | >16 | >16 | >16 |
| A. implicatum (7) | 2 | 4 | >16 | >16 | 2 | >16 | 2 | 8 | 0.25 | 1 | 4 | 8 | >16 | >16 | >16 | >16 | >16 | >16 |
| A. persicinum (7) | 2 | 8 | >16 | >16 | 1 | 8 | 2 | 8 | 0.125 | 0.25 | 4 | 8 | >16 | >16 | >16 | >16 | >16 | >16 |
| A. atrogriseum (4) | 1 | 2 | 4 | 8 | 1 | 2 | 2 | 2 | 0.125 | 0.125 | 2 | 4 | 0.125 | >16 | 0.5 | >16 | 0.5 | >16 |
| A. fusidioides (3) | 2 | 2 | 1 | >16 | 1 | 2 | 2 | 2 | 0.125 | 2 | 4 | 4 | 4 | >16 | 4 | >16 | 4 | >16 |
AMB, amphotericin B; ANID, anidulafungin; CAS, caspofungin; ITC, itraconazole; MICA, micafungin; NAT, natamycin; PSC, posaconazole; TRB, terbinafine; VRC, voriconazole.
DISCUSSION
This is the first study that has identified a large panel of clinical isolates of Acremonium to the species level using both phenotypic features and DNA sequencing. Although only a limited number of species could be characterized, the identification can be considered reasonably reliable since the sequences were compared to those of type strains, where available, or reference strains identified by specialists (8). Our results agree with Glenn et al. (10), who demonstrated that the genus Acremonium is highly polyphyletic comprising phylogenetically distant fungi. Up to now, except for limited groups (21, 22), the taxonomy of Acremonium has been almost exclusively based on morphological criteria. However, the present study has demonstrated that certain Acremonium species cannot be reliably identified using only phenotypic features. For example, reference strains of A. alternatum, the type species of the genus, grouped in more than one clade (Fig. 1). A. alternatum is a species of ambiguous typification, and the Acremonium database of one of us (R.C.S.) indicates there are at least six different taxa that are contenders to bear this name based on close congruence with dried type material, plus numerous other taxa that are morphologically similar and would be routinely identified under this name. Similarly, reference strains of A. hyalinulum were also nested in very distant clades. In contrast, authentic strains of species that were morphologically distinct, e.g., A. sclerotigenum, A. egyptiacum, and A. alternatum, were nested together in a closely homologous, well-supported clade (Fig. 5). Although morphological identification of Acremonium species is difficult; credible identification can be done to some extent even for experts using Gams' monograph (8) and a few additional studies (5, 6, 9, 18). In part because there are many undescribed species in Acremonium, the only medically important seen species that can be reliably identified morphologically are A. kiliense, A. implicatum, and A. persicinum. Isolates should be sequenced for identification if considered medically significant, and sequence-based identifications should be controlled for possible GenBank error with detailed morphological study.
Since the advent of useful molecular techniques and the availability of nucleotide sequences for clinical isolates, it might be expected that accurate identification of causal agents of Acremonium infections could be achieved in clinical laboratories. The present study revealed, however, that molecular identification of clinical isolates of Acremonium using a BLAST search of GenBank sequences is of limited utility due to the scarcity of GenBank sequences that have been verified. Thus, we have shown that sequenced strains identified in two recent clinical reports as A. strictum (12, 16) do not belong to this species but instead are related to the A. sclerotigenum-A. egyptiacum group.
Although cases of hyalohyphomycosis incited by Acremonium appear to be relatively rare compared to those caused by other common fungi, this could be a false perception created by the difficulties in the identification of the etiological agents. Older published cases commonly only reported the etiologic agent as Acremonium sp. Due to the morphological similarity of these fungi with Fusarium, especially when the latter does not produce characteristic macroconidia, it is likely that some cases of infections by Acremonium were erroneously reported as a Fusarium infection (1, 7, 11, 18). In the last review of Acremonium infections, 36 cases of localized or disseminated infections, other than mycetoma or ocular infections, were summarized (11). It is probable, however, that the actual number of Acremonium cases may be higher considering that the Fungus Testing Laboratory at San Antonio received at least 75 isolates from clinical samples across the United States over an 8-year period.
One of the most remarkable findings of the present study was that A. alabamense, A. blochii, A. curvulum, A. potronii, A, recifei, A. roseogriseum, A. spinosum, and A. strictum, which had been previously described as opportunistic species (5, 11, 18), were not represented in the isolates tested here. In a previous study, we indicated that the involvement of A. strictum in human infections was uncertain (11). The lack of isolates of A. recifei could be due to the fact that this species is mainly known from mycetoma cases, which were not represented in the present study. Also, A. recifei is mostly known from the tropics (18), so its absence among these mostly American isolates may be a sampling artifact. Related studies by one of us (R.C.S.) have shown that A. blochii and A. potronii are names not rooted by a type strain and that each name has had a wide range of mostly undescribed genetic species attributed to it (data not shown, except for a marine “A. potronii” isolate CBS 379.70F). The name A. potronii, however, is probably the oldest valid name for the most common genetic species in the A. sclerotigenum-A. egyptiacum complex, and other attributions of this name are strictly based on overlap between large numbers of morphologically simple Acremonium species. A neotype will need to be designated for A. potronii, along with detailed justification for the choice before this name becomes taxonomically well grounded.
This survey discovered species such as A. fusidioides, A. glaucum, A. implicatum, A. persicinum, A. polychromum, and A. zeae, which have not been referenced in any clinical reports. None of these species, however, has been formally demonstrated as a causal agent of disease. Isolations may simply indicate body surface contamination or contamination arising during the course of obtaining and processing clinical specimens. An important objective for future studies would be to elucidate the true clinical role of the species reported in the present study.
Another significant finding was the laboratory reporting of an Acremonium species in 44 isolates not belonging to this genus. In addition to strains of Fusarium being reported as Acremonium, several other morphologically similar isolates containing various degrees of melanization, such as Phialemonium, Lecythophora, Phaeoacremonium, and Pleurostomophora, were also reported as Acremonium species, further supporting the difficulties associated with phenotypic identification of morphologically similar genera.
In our study, the MICs for PSC and VRC were high compared to those found for other hyaline molds such as Aspergillus fumigatus, where MICs were 0.006 to 2.0 μg/ml for PSC and 0.12 to 4.0 μg/ml for VRC, and associated epidemiological cutoff values (ECVs) were reported as 1.0 μg/ml for VRC and 0.25 μg/ml for PSC (17). However, these cutoffs are similar to those of Fusarium species for which PSC and VRC are licensed, as there is some clinical benefit.
In conclusion, clinical Acremonium isolates are difficult to identify using morphological or molecular methods. However, a first step for facilitating this task has been completed since ITS reference sequences of species which appear to be the most clinically relevant, at least in the United States, have been deposited in GenBank for future reference. Further studies testing more clinical isolates, other species of the genus and the other genetic loci, which are more phylogenetically informative than the ITS, are required to determine the spectrum of well characterized Acremonium species involved in human infections. Once this has been done, animal models to evaluate the real pathogenic potential of these species and testing appropriate therapies for their treatment should be developed. For the clinically relevant species examined here, the MICs for all of the antifungal agents commonly used, except for terbinafine, were high.
Acknowledgments
We are indebted to the curators of the Centraalbureau voor Schimmelcultures (Utrecht, Netherlands) and the Mycotheque de l'Universite Catholique de Louvain (Belgium) for supplying many of the strains used in the study.
This study was supported by the Spanish Ministerio de Educación y Ciencia, grants CGL 2009-08698/BOS and CGL 2008-04226/BOS.
Footnotes
Published ahead of print on 10 November 2010.
REFERENCES
- 1.Ajello, L., A. A. Padhye, F. W. Chandler, M. R. McGinnis, L. Morganti, and F. Alberici. 1985. Fusarium moniliforme, a new mycetoma agent: restudy of European case. Eur. J. Epidemiol. 1:5-10. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez, E., A. Stchigel, J. Cano, D. Sutton, A. Fothergill, J. Chander, V. Salas, M. Rinaldi, and J. Guarro. 2010. Molecular phylogenetic diversity of the emerging mucoralean fungus Apophysomyces: proposal of three new species. Rev. Iberoam. Micol. 27:80-89. [DOI] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard, 2nd ed. Document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Das, S., R. Saha, S. A. Dar, and V. G. Ramachandran. 2010. Acremonium species: a review of the etiological agents of emerging hyalohyphomycosis. Mycopathologia doi 10.1007/s11046-010-9334-1. [DOI] [PubMed]
- 5.De Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, Netherlands/University Rovira i Virgili, Reus, Spain.
- 6.Domsch, K. H., W. Gams, and T.-H. Anderson. 2007. Acremonium, p. 30-38. In Compendium of soil fungi, vol. 2. IHW-Verlag, Eching, Germany. [Google Scholar]
- 7.Fincher, R. M., J. F. Fisher, R. D. Lovell, C. L. Newman, A. Espinel-Ingroff, and H. J. Shadomy. 1991. Infection due to the fungus Acremonium (Cephalosporium). Medicine 70:398-409. [DOI] [PubMed] [Google Scholar]
- 8.Gams, W. 1971. Cephalosporium-artige Schimmelpilze (hyphomycetes). Gustav Fischer Verlag, Stuttgart, Germany.
- 9.Gams, W. 1975. Cephalosporium-like hyphomycetes: some tropical species. Trans. Br. Mycol. Soc. 64:389-404. [Google Scholar]
- 10.Glenn, A. E., C. W. Bacon, R. Price, and R. T. Hanlin. 1996. Molecular phylogeny of Acremonium and its taxonomic implications. Mycologia 88:369-383. [Google Scholar]
- 11.Guarro, J., W. Gams, I. Pujol, and J. Gené. 1997. Acremonium species: new emerging opportunists—in vitro antifungal susceptibilities and review. Clin. Infect. Dis. 25:1222-1229. [DOI] [PubMed] [Google Scholar]
- 12.Guarro, J., A. Palacio, J. Gené, J. Cano, and C. Gómez. 2009. A case of colonization of a prosthetic mitral valve by Acremonium strictum. Rev. Iberoam. Micol. 26:146-148. [DOI] [PubMed] [Google Scholar]
- 13.Gupta, A., H. Jain, C. Lynde, P. Mcdonald, E. Cooper, and R. Summerbell. 2000. Prevalence and epidemiology of onychomycosis in patients visiting physicians' offices: a multicenter Canadian survey of 15,000 patients. J. Am. Acad. Dermatol. 43:244-248. [DOI] [PubMed] [Google Scholar]
- 14.Hawksworth, D. L. 1979. The lichenicolous Hyphomycetes. Bull. Br. Mus. Nat. Hist. (Bot.) 6:183-300. [Google Scholar]
- 15.Ito, T., I. Okane, A. Nakagiri, and W. Gams. 2000. Two species of Acremonium section Acremonium: A. borodinense sp. nov. and A. cavaraeanum rediscovered. Mycol. Res. 104:77-80. [Google Scholar]
- 16.Novicki, T. J., R. Geise, A. P. Limaye, K. Lafe, L. Bui, U. Bui, and B. T. Cookson. 2003. Genetic diversity among clinical isolates of Acremonium strictum determined during an investigation of a fatal mycosis. J. Clin. Microbiol. 41:2623-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfaller, M. A., D. J. Diekema, M. A. Ghannoum, J. H. Rex, B. D. Alexander, D. Andes, S. D. Brown, A. Espinel-Ingroff, C. L. Fowler, E. M. Johnson, C. C. Knapp, M. R. Moryl, L. Ostrosky-Zeichner, D. J. Sheehan, and T. Walsh. 2009. Wild type MIC distribution and epidemiological cutoff values for Aspergillus fumigatus and three triazoles as determined by the Clinical and Laboratory Standards Institute broth microdilution methods. J. Clin. Microbiol. 47:3142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Summerbell, R. C. 2003. Aspergillus, Fusarium, Sporothrix, Piedraia, and their relatives, p. 237-498. In D. H. Howard (ed.), Pathogenic fungi in humans and animals, 2nd ed. Marcel Dekker, New York, NY.
- 19.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 20.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zare, R., W. Gams, M. Starink-Willemse, and R. C. Summerbell. 2007. Gibellulopsis, a suitable genus for Verticillium nigrescens, and Musicillium, a new genus for V. theobromae. Nova Hedwigia 85:463-489. [Google Scholar]
- 22.Zuccaro, A., R. C. Summerbell, W. Gams, H.-J. Schroers, and Jl. Mitchell. 2004. A new Acremonium species associated with Fucus spp., and its affinity with a phylogenetically distinct marine Emericellopsis clade. Stud. Mycol. 50:283-297. [Google Scholar]