Abstract
Delayed orthopedic joint prosthesis infections (DOJP-Is) due to staphylococci frequently result in prosthetic revision. Specific and noninvasive diagnostic tests are unavailable, and DOJP-Is are commonly diagnosed at advanced stages of disease. An enzyme-linked immunosorbent assay (ELISA) was developed to detect serum antibodies against staphylococcal slime polysaccharide antigens. Using a cutoff of 0.35 ELISA units, the test showed a specificity of 95.1% (95% confidence interval [CI], 85.4 to 98.7%) and a sensitivity of 89.7% (71.5 to 97.3%) on a sample of 90 individuals.
Joint replacement surgery is the most common procedure in people with damaged joints. Prosthetic joint replacements of the hip and knee are generally successful operations, but a significant proportion—in the range of 0.5 to 3%—lead to infection. Infections are more likely to occur in surgical revision than in primary joint replacement (up to 40%) (3). Prosthetic joint infection represents the most devastating complication, implying protracted hospitalization, patient risk complications, and renewed disability, together with substantial costs (1, 10).
Staphylococci are the leading cause of infection related to implanted medical devices. This is directly related to their capability to establish multilayered, highly structured biofilms on artificial surfaces. Polysaccharides, and to a lesser extent nucleic acids and proteins, are the main constituents of the microbial biofilm matrix and play a major role in bacterial resistance against antimicrobial agents and host defenses in delayed (>1 year from implant) orthopedic joint prosthetic infections (DOJP-Is) (5, 10).
DOJP-Is are often characterized by a clinically subacute onset, with unpredictable and scarce local inflammatory signs and late bone erosion. Since specific and noninvasive laboratory tests to diagnose orthopedic implant-related infections are unavailable, diagnosis is often made only at an advanced stage, with relevant implications on therapeutic outcomes and survival (2).
An innovative enzyme-linked immunosorbent assay (ELISA), previously described to detect serum antibodies to staphylococcal slime polysaccharide antigens (SSPA) in late-onset infections of synthetic vascular grafts, was here applied to diagnose DOJP-Is (9) (U.S. patent application/control number 10/135,827; 2010).
We compared the titers of immunoglobulin M (IgM) antibodies against SSPA in the sera of 90 subjects recruited between 2003 and 2009. The sample included 29 subjects with ongoing staphylococcal DOJP-Is (16 hip, 12 knee, and one shoulder prosthesis) (group A), 34 subjects with orthopedic joint prostheses implanted at least 1 year previously without infection (20 hip and 14 knee prostheses) (group B), and 27 subjects not previously operated on for orthopedic implants, attending the hospital for noninfectious diseases (group C). Exclusion criteria for groups A and B were time from implant of <1 year and any known rheumatologic disease or concomitant infection. All subjects in group A underwent surgical removal of the infected prosthesis, and staphylococcal infection had been microbiologically confirmed by intraoperative cultures. All subjects in group B were followed for an additional 2 years after serum sampling, in order to exclude the onset of delayed prosthetic infection.
All subjects provided written informed consent, and the procedures were in accordance with the ethical standards of the responsible committee on human experimentation and with the Declaration of Helsinki (1975; revised in 1983).
SSPA from Staphylococcus strain SA1545 (a slime-producing clinical isolate) was prepared as previously reported, with important modifications to achieve higher repeatability, antigen stability, and a laboratory scale-up of antigen production (9). In particular, in order to standardize the chemically defined growth medium (4), it was prepared starting from a premixed combination of chemicals. The final 6-liter solution was filter sterilized using a 0.22-μm 1-liter filter unit (Millipore Stericup). Every lot of antigen preparation originated from a single clone scaled up to a 6-liter culture for 5 days at 37°C. Each preparation was then lyophilized using the same Stericup bottle, closed by a fiber glass cap. The ELISA was prepared as previously reported (9). For orthopedic applications, we adopted a cutoff value of 0.35 ELISA units (EU). Each serum sample was tested in triplicate in two different assays, and values were expressed as means ± standard deviations (SD) (Table 1). The test specificity and sensitivity were computed using several EU cutoffs, and only the most relevant ones have been shown to avoid redundancy. Ninety-five-percent confidence intervals (CI) for specificity and sensitivity were computed according to the efficient-score method (corrected for continuity) described by Newcombe (6).
TABLE 1.
Comparison of titers of IgM antibodies against SSPA, expressed as ELISA units, in sera from subjects with an ongoing staphylococcal late-onset infection of orthopedic prostheses and in controls
| Group (no. of samples) | Mean (SD) IgM titer (EU) | % (no.) of positive tests for an IgM titer: |
|
|---|---|---|---|
| ≥0.35 EU | ≥0.40 EU | ||
| Prosthesis infectiona (29) | 0.72 (0.55) | 89.7 (26) | 69.0 (20) |
| Prosthesis, no infection (34) | 0.21 (0.09)b | 8.8 (3) | 5.9 (2) |
| No prosthesis, no infection (27) | 0.20 (0.05)b | 0 (0) | 0 (0) |
| All controls (61) | 0.21 (0.07)b | 4.9 (3) | 3.3 (2) |
Prosthesis infection is defined by subjects with an ongoing prosthetic infection caused by Staphylococcus epidermidis (n = 15), Staphylococcus aureus (n = 8), coagulase-negative staphylococci other than S. epidermidis (n = 2), and mixed infection by one or more staphylococcal species plus enterococci, Pseudomonas aeruginosa, or Escherichia coli (n = 4).
P < 0.001 versus infected subjects (Kruskal-Wallis test).
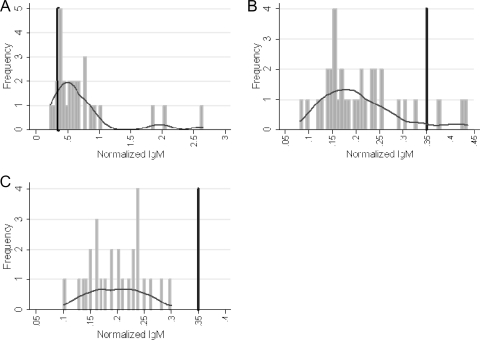
The mean titers of IgM against SSPA were 0.72 ± 0.55 in subjects with ongoing staphylococcal DOJP-Is and 0.21 ± 0.07 in controls (P < 0.001), and a plot of the values for the two subject groups revealed very little overlap (Table 1 and Fig. 1). Only 4.9% and 3.3% of all controls showed EU values higher than 0.35 and 0.40, respectively, while 89.7% and 69.0% of the subjects with prosthesis infection were above such thresholds (Table 1).
FIG. 1.
Frequency distribution of the IgM antibody titers against SSPA in the sample. Group A, individuals with prosthesis and staphylococcal infection (n = 29); group B, controls with prosthesis and no history of infection (n = 34); group C, controls with no prosthesis and no history of infection (n = 27). The vertical dark bars indicate an IgM antibody titer against SSPA of 0.35 EU (cutoff). The horizontal curves indicate Kernel density plots.
As shown in Table 2, the best combination of sensitivity and specificity was observed at a cutoff value of 0.35 EU: a mean value of IgM titers against SSPA that was higher than 0.35 EU was able to diagnose 89.7% of the infections, with a specificity of 95.1% (95% CI, 85.4 to 98.7%). A higher cutoff value—0.40 EU—slightly increased the specificity to 96.7%, but the sensitivity dropped to 69.0%. Overall, the area under the receiver operating characteristics (ROC) curve of the IgM test with the 0.35 EU cutoff was 0.97 (CI, 0.94 to 1.00).
TABLE 2.
Discriminatory power of the ELISA for each subject population based on two different cutoffs in the diagnosis of staphylococcal DOJP-Isa
| EU | % of infections diagnosed |
% specificity (range) | % sensitivity (range) | |||
|---|---|---|---|---|---|---|
| TP | FP | TN | FN | |||
| ≥0.35 | 89.7 | 10.3 | 95.1 | 4.9 | 95.1 (85.4-98.7) | 89.7 (71.5-97.3) |
| ≥0.40 | 90.9 | 9.1 | 86.8 | 13.2 | 96.7 (87.6-99.4) | 69.0 (49.0-84.0) |
TP, true-positive subjects; FP, false-positive subjects; TN, true-negative subjects; FN, false-negative subjects.
Diagnosis of implant-associated infections by classical tools of microbiological analysis is insensitive. To date, attempts to design new serology diagnostic assays to diagnose bacterial biofilm infections of orthopedic implants early have failed (7, 8). This study shows that high anti-SSPA IgM levels may provide for noninvasive detection of the immune response elicited by biofilm colonization on artificial orthopedic implants, improving patient monitoring with respect to infectious complications.
We observed the best combination of sensitivity and specificity at the antibody titer cutoff value of 0.35 EU. It must be noted, however, that although all results were strongly significant, the 95% confidence intervals for both specificity and sensitivity were not narrow due to the relatively limited sample size. Thus, the choice of the best cutoff may be refined in future studies with larger samples, which are warranted to confirm the present findings and evaluate whether the present ELISA should be included in the clinical monitoring of all subjects with orthopedic prostheses.
We did not evaluate IgG titers, because they were not associated with current infection both in a previous study by our group (9) and in a preliminary analysis on five cases and eight controls (data not shown).
The performance of IgM and IgG response depends on the chemical characteristics of the antigen: SSPA is a mixture of purified polysaccharides eliciting a thymus-independent humoral response which is an expression of innate immunity (7). Antigens of this type elicit IgM antibodies synthesized by a particular subpopulation of B lymphocytes (B1) corresponding to splenic marginal-zone B cells. This response is maintained as long as the antigenic stimulus is present and does not shift to IgG production (11). This peculiar behavior offers a diagnostic advantage since the monitoring of IgMs could also be useful to observe the evolution of a device infection after treatment.
Measurement of serum IgM antibodies against SSPA could be a simple, sensitive, and noninvasive laboratory test useful in the diagnosis of staphylococcal DOJP-Is. The SSPA preparation described here proved effective in detecting antibodies in DOJP-Is caused by different staphylococcal species. Identification of conserved antigens produced in biofilms by the various causative agents could be used to develop a panel of immunoassays useful for diagnosis of all types of DOJP-Is. To our knowledge, this is the only IgM-based ELISA able to monitor staphylococcal DOJP-Is with an acceptable sensitivity and specificity.
Acknowledgments
We thank E. Mileto and A. Cellini for precious technical assistance.
This study was not funded.
Footnotes
Published ahead of print on 10 November 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Campoccia, D., L. Montanaro, and C. R. Arciola. 2006. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 27:2331-2339. [DOI] [PubMed] [Google Scholar]
- 2.Costerton, J. W. 2005. Biofilm theory can guide the treatment of device-related orthopaedic infections. Clin. Orthop. Relat. Res. 437:7-11. [DOI] [PubMed] [Google Scholar]
- 3.Davis, J. S. 2005. Management of bone and joint infections due to Staphylococcus aureus. Intern. Med. J. 35(Suppl. 2):S79-S96. [DOI] [PubMed] [Google Scholar]
- 4.Hussain, M., J. G. Hastings, and P. J. White. 1991. A chemically defined medium for slime production by coagulase-negative staphylococci. J. Med. Microbiol. 34:143-147. [DOI] [PubMed] [Google Scholar]
- 5.Matthews, P. C., A. R. Berendt, M. A. McNally, and I. Byren. 2009. Diagnosis and management of prosthetic joint infection. BMJ 338:b1773. [DOI] [PubMed] [Google Scholar]
- 6.Newcombe, R. G. 1998. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat. Med. 17:857-872. [DOI] [PubMed] [Google Scholar]
- 7.Sadovskaya, I., S. Faure, D. Watier, D. Leterme, A. Chokr, J. Girard, H. Migaud, and S. Jabbouri. 2007. Potential use of poly-N-acetyl-beta-(1,6)-glucosamine as an antigen for diagnosis of staphylococcal orthopedic-prosthesis-related infections. Clin. Vaccine Immunol. 14:1609-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selan, L., L. Kofonow, G. L. Scoarughi, T. Vail, J. G. Leid, and M. Artini. 2009. Use of immunodiagnostics for the early detection of biofilm infections, p. 219-237. In M. Shirtliff and J. G. Leid (ed.), The role of biofilms in device-related infections, vol. 3. Springer, Berlin, Germany. [Google Scholar]
- 9.Selan, L., C. Passariello, L. Rizzo, P. Varesi, F. Speziale, G. Renzini, M. C. Thaller, P. Fiorani, and G. M. Rossolini. 2002. Diagnosis of vascular graft infections with antibodies against staphylococcal slime antigens. Lancet 359:2166-2168. [DOI] [PubMed] [Google Scholar]
- 10.Trampuz, A., and W. Zimmerli. 2005. Prosthetic joint infections: update in diagnosis and treatment. Swiss Med. Wkly. 135:243-251. [DOI] [PubMed] [Google Scholar]
- 11.Weller, S., M. C. Braun, B. K. Tan, A. Rosenwald, C. Cordier, M. E. Conley, A. Plebani, D. S. Kumararatne, D. Bonnet, O. Tournilhac, G. Tchernia, B. Steiniger, L. M. Staudt, J. L. Casanova, C. A. Reynaud, and J. C. Weill. 2004. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood 104:3647-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]