Abstract
There is currently no consensus method for the active screening of Acinetobacter baumannii. The use of swabs to culture nostrils, pharynx, and skin surface of various anatomical sites is known to yield less-than-optimal sensitivity. In the present study, we sought to determine whether the use of sterile sponges to sample large areas of the skin would improve the sensitivity of the detection of A. baumannii colonization. Forty-six patients known to be colonized with A. baumannii, defined by a positive clinical culture for this organism as defined by resistance to more than two classes of antimicrobials, participated in the study. The screening sites included the forehead, nostrils, buccal mucosa, axilla, antecubital fossa, groin, and toe webs with separate rayon swabs and the forehead, upper arm, and thigh with separate sponges. Modified Leeds Acinetobacter medium (mLAM) agar plates that contained vancomycin and either aztreonam or ceftazidime were used as the selective medium. An enrichment culture grown overnight substantially increased the sensitivity for most sites. The sensitivity ranged between 69.6 and 82.6% for individual sponge sites and 21.7 to 52.2% for individual swab sites when mLAM plates with ceftazidime were inoculated after a 24-h enrichment period. The sponge and swab sites with the best sensitivity were the leg and the buccal mucosa, respectively (82.6% and 52.2%; P = 0.003). The combined sensitivity for the upper arm and leg with a sponge was 89.1%. The novel screening method using sterile sponges was easy to perform and achieved excellent sensitivity for the detection of A. baumannii colonization.
Acinetobacter baumannii is increasingly becoming a common pathogen in the hospital environment, especially in intensive care units (ICUs). According to recent data from the National Healthcare Safety Network, A. baumannii is the third most common pathogen implicated in ventilator-associated pneumonia after Staphylococcus aureus and Pseudomonas aeruginosa, causing 8.4% of these infections (6). An estimated 60% of A. baumannii strains are resistant to multiple classes of antimicrobials in the United States, leaving few therapeutic options for those who are infected (8). Once A. baumannii is in the hospital, its eradication can be difficult, even with the implementation of appropriate infection control procedures. Several factors, including its resistance to both antimicrobials and desiccation, play a role in the persistence of A. baumannii in the hospital environment (13). Cohorting of infected or colonized patients is the main measure to prevent its spread. At least two studies have evaluated the role of the universal active screening of inpatients to identify A. baumannii carriers, with mixed results (1, 10). A major problem with active screening is the lack of data on how to achieve clinically acceptable sensitivity for the detection of A. baumannii colonization. For example, a study examining the yield of swab cultures of six body sites gave a sensitivity of 55% when all sites were combined (11). In that study, the best single site was endotracheal aspirate, with a sensitivity of 29%. Other studies of skin colonization of patients and healthy volunteers using swab cultures have also shown low rates of colonization with A. baumannii (2, 4, 14). This low yield has been attributed to the low colonization density of Acinetobacter species, including A. baumannii, on body surfaces (3). One study comparing the yields of various skin sampling methods showed that the use of moist sponges in lieu of swabs resulted in an approximately 100-fold increase in the number of colonies of aerobic bacteria obtained (5). We therefore hypothesized that a higher sensitivity will be achieved for the detection of A. baumannii colonization by sampling larger areas of the body surface with sponges, coupled with the use of medium to promote the selective growth of this organism.
MATERIALS AND METHODS
Study design.
The study was approved by the Institutional Review Board of the University of Pittsburgh, and informed consent was obtained for all participating patients. Patients who were admitted to the University of Pittsburgh Medical Center between June 2009 and April 2010 and who had at least one clinical culture growing A. baumannii which was resistant to three or more classes of antimicrobials commonly used against this organism were recruited for the study (12). Patients had to be enrolled in the study within 10 days of the date at which the positive clinical culture was taken.
Sample collection.
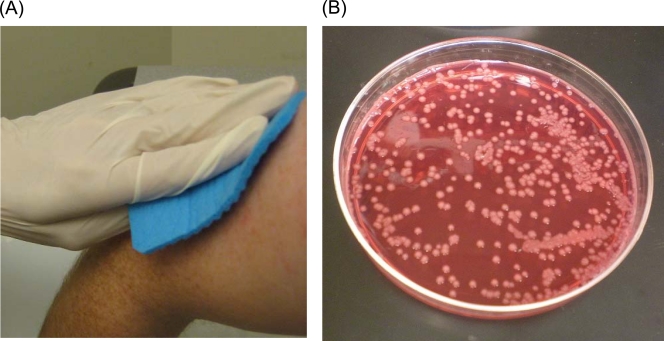
Seven rayon swabs (BBL CultureSwab Plus; BD, Sparks, MD) and three sponges (Polywipe premoistened sponge swab; Medical Wire & Equipment, Wiltshire, England) were used to collect samples from each patient. For the swabs, the following sites were rubbed, each with a new swab, in a rotating manner to cover up to a 2- by 2-in. area whenever feasible: the forehead, nostrils, buccal mucosa, antecubital fossa, axilla, groin, and toe web. These are body sites where A. baumannii has been recovered in previous studies (2, 4, 14). The swabs were then stabbed into the transport medium provided with the swab. For the sponges, the forehead, upper arm, and thigh were swiped down approximately 5 in., once each using both sides, and each with a new sponge and gloves (Fig. 1 A). The upper arm and thigh were surrogate sites for the axilla and groin, since the latter sites were difficult to reach with the sponge. After sampling, each sponge was dropped into a sterile container provided with the sponge. The specimens were then transported to the research laboratory for immediate processing.
FIG. 1.
(A) Sampling of the upper arm using a sponge. (B) A. baumannii colonies on modified Leeds Acinetobacter medium.
mLAM.
Leeds Acinetobacter medium (LAM) was previously proposed as a selective medium for the culturing of Acinetobacter spp. (7). Acinetobacter spp. typically produce circular, convex, smooth, pink colonies with a mauve background after 24 h of incubation in this medium. The original LAM contains vancomycin, cefsulodin, and cephradine. For the modified LAM (mLAM) used in the present study, cefsulodin and cephradine were replaced with either 4 μg/ml aztreonam or 8 μg/ml ceftazidime. All A. baumannii strains isolated at our hospital have been resistant to aztreonam regardless of their resistance profiles. On the other hand, only isolates that are resistant to multiple classes of antimicrobials have typically been resistant to ceftazidime. Therefore, we hypothesized that mLAM with aztreonam would have a higher sensitivity for the detection of A. baumannii than that with ceftazidime but possibly at the cost of more nonspecific growth of other organisms.
Processing of the specimens.
Each swab was suspended in 20 ml of nutrient broth (BD) and incubated at 37°C for enrichment. Each container with a sponge was filled with 100 ml of nutrient broth to soak the sponge and incubated likewise. Since it was suggested previously that an overnight enrichment process enhances the sensitivity of A. baumannii screening cultures (11), enrichment periods of both 1 h and 24 h were examined. After 1 or 24 h of enrichment culture, 100 μl of the broth was taken from each specimen and inoculated onto two mLAM plates, one with aztreonam and one with ceftazidime. A sheep blood agar plate was also inoculated as a control. The plates were then incubated at 37°C for 24 h before reading.
Identification of A. baumannii.
At least one smooth, pink colony with a mauve background consistent with A. baumannii was subjected to biochemical identification for each patient using API 20NE (bioMerieux, Durham, NC). A colony was also selected from all plates with growth consistent with A. baumannii and subjected to susceptibility testing using the disk method to ensure concordance with the representative colony, which was biochemically identified. The concordance of susceptibility was also verified between the surveillance isolates and the clinical isolate for each patient.
Statistical methods.
The characteristics of the study population were described by using proportions for categorical variables and medians and ranges for continuous variables. Since all of the patients were known to be positive for A. baumannii, the sensitivity of each method was calculated as the percentage of tests that were positive. McNemar's test for paired data was used to compare the sensitivities of two methods based on samples from the same patients. Under the assumption that the composite sensitivities of the swab culture method and the sponge culture method were 55% and 80%, respectively, this study was designed to enroll 45 patients in order to achieve 80% power for detecting a difference between the two sensitivities with a two-sided alpha of 0.05. The analyses were performed by using SAS, version 9.2 (SAS Institute Inc., Cary, NC), and a P value of 0.05 was considered statistically significant.
RESULTS
Patient population.
A total of 46 patients were enrolled in the study. Of them, 25 (54.3%) were male and 40 (87.0%) were Caucasian. The median age was 57 years (range, 26 to 90 years). Twenty-one (45.7%) patients were in an intensive care unit, and 22 (47.8%) were mechanically ventilated. The sources of the positive clinical culture were as follows: 17 sputum (37.0%), 4 bronchoalveolar lavage/tracheal aspirate (8.7%), 13 wound/drainage (28.3%), 9 urine (19.6%), 2 blood (4.3%), and 1 catheter tip (2.2%). Sixteen patients (34.8%) were considered to have infection by A. baumannii, whereas 30 patients (65.2%) were felt to be colonized only. The median and mean times from collection of the positive clinical culture to the collection of the study specimens were 4 days and 4.5 days, respectively (range, 2 to 8 days). Six patients (13.0%) were on antimicrobial therapy active against the particular isolate in the meantime. These agents included ampicillin-sulbactam, meropenem, colistimethate, and tigecycline.
Sensitivities of the culture methods.
The overall results are shown in Table 1. For both mLAM plates with aztreonam and those with ceftazidime, 24-h enrichment provided a statistically higher sensitivity for the detection A. baumannii than 1-h enrichment for all three sponge culture sites and some swab culture sites (antecubital fossa, groin, and forehead [ceftazidime only]) (Table 1). mLAM plates with aztreonam gave higher sensitivities than those with ceftazidime for the thigh when 1-h enrichment was used, whose difference became negligible when 24-h enrichment was used. The results for other sites were comparable between aztreonam and ceftazidime. A. baumannii isolates from all of the positive specimens were resistant to three or more classes of antimicrobials.
TABLE 1.
Sensitivity of detection of A. baumannii from colonized patients
| Collection method | Site | No. of patients | ATMa |
CAZb |
||||
|---|---|---|---|---|---|---|---|---|
| % sensitivity |
P value | % sensitivity |
P value | |||||
| 1 hc | 24 hc | 1 hc | 24 hc | |||||
| Sponge | Forehead | 46 | 19.6 | 67.4 | <0.0001 | 21.7 | 69.6 | <0.0001 |
| Upper arm | 46 | 26.1 | 76.1 | <0.0001 | 21.7 | 76.1 | <0.0001 | |
| Thigh | 46 | 34.8 | 82.6 | <0.0001 | 19.6 | 82.6 | <0.0001 | |
| Swab | Forehead | 46 | 4.3 | 23.9 | 0.03 | 2.1 | 23.9 | 0.002 |
| Nostrils | 46 | 23.9 | 32.6 | 0.10 | 26.1 | 32.6 | 0.18 | |
| Buccal mucosa | 46 | 43.5 | 50.0 | 0.32 | 43.5 | 52.2 | 0.21 | |
| Antecubital fossa | 46 | 0 | 19.6 | 0 | 21.7 | |||
| Axilla | 46 | 10.9 | 26.1 | 0.02 | 10.9 | 26.1 | 0.02 | |
| Groin | 45 | 17.8 | 48.9 | 0.001 | 15.6 | 48.9 | 0.001 | |
| Toe web | 45 | 11.1 | 22.2 | 0.10 | 6.7 | 22.2 | 0.03 | |
ATM indicates modified Leeds Acinetobacter medium with 10 μg/ml vancomycin and 4 μg/ml aztreonam.
CAZ indicates modified Leeds Acinetobacter medium with 10 μg/ml vancomycin and 8 μg/ml ceftazidime.
Enrichment period.
(i) Swabs.
Among the seven swab sites investigated, the buccal mucosa had the highest sensitivity for the detection of A. baumannii colonization with both 1-h and 24-h enrichments (43.4 and 52.2%, respectively, with ceftazidime). With the 1-h enrichment, the buccal mucosa yielded the highest sensitivity across all sites, including sponge sites. With the 24-h enrichment, the groin yielded a sensitivity similar to that for the buccal mucosa (48.9%), followed by the nostrils, axilla, antecubital fossa, forehead, and toe web. The sensitivity for buccal mucosa was even higher (71.4% after 24-h enrichment) among patients who were in an intensive care unit (Table 2). This likely reflected the fact that the majority of these patients (18 of 21; 85.7%) were enrolled in the study due to a positive respiratory culture. The two swab culture sites yielding the best combined sensitivity were the buccal mucosa and groin after the 24-h enrichment period (sensitivity of 73.3 to 77.8%) (Table 3).
TABLE 2.
Sensitivity of detection of A. baumannii from colonized patients in ICUs
| Collection method | Site | No. of patients | ATMa |
CAZb |
||||
|---|---|---|---|---|---|---|---|---|
| % sensitivity |
P value | % sensitivity |
P value | |||||
| 1 hc | 24 hc | 1 hc | 24 hc | |||||
| Sponge | Forehead | 21 | 19.0 | 71.4 | 0.002 | 23.8 | 76.2 | 0.001 |
| Upper arm | 21 | 33.3 | 81.0 | 0.004 | 42.9 | 81.0 | 0.005 | |
| Thigh | 21 | 23.8 | 85.7 | 0.0003 | 9.5 | 85.7 | <0.0001 | |
| Swab | Forehead | 21 | 4.8 | 28.6 | 0.03 | 0 | 28.6 | |
| Nostrils | 21 | 42.9 | 47.6 | 0.56 | 42.9 | 52.4 | 0.32 | |
| Buccal mucosa | 21 | 61.9 | 71.4 | 0.41 | 61.9 | 71.4 | 0.41 | |
| Antecubital fossa | 21 | 0 | 28.6 | 0 | 28.6 | |||
| Axilla | 21 | 23.8 | 38.1 | 0.18 | 23.8 | 33.3 | 0.32 | |
| Groin | 21 | 14.3 | 42.9 | 0.09 | 14.3 | 47.6 | 0.02 | |
| Toe web | 20 | 15.0 | 20.0 | 0.65 | 10.0 | 20.0 | 0.41 | |
ATM indicates modified Leeds Acinetobacter medium with 10 μg/ml vancomycin and 4 μg/ml aztreonam.
CAZ indicates modified Leeds Acinetobacter medium with 10 μg/ml vancomycin and 8 μg/ml ceftazidime.
Enrichment period.
TABLE 3.
Composite sensitivities of the most sensitive sponge and swab sites
| Enrichment time (h) | mLAM antibiotica | Composite sensitivity (%) |
P value | |
|---|---|---|---|---|
| Sponge site | Swab site | |||
| Upper arm and/or thigh | Nostril and/or buccal mucosa | |||
| 1 | ATM | 43.5 | 50.0 | 0.49 |
| CAZ | 37.0 | 50.0 | 0.13 | |
| 24 | ATM | 89.1 | 56.5 | 0.0006 |
| CAZ | 89.1 | 58.7 | 0.001 | |
| Upper arm and/or thigh | Nostril and/or groin | |||
| 1 | ATM | 44.4 | 42.2 | 0.80 |
| CAZ | 37.8 | 42.2 | 0.56 | |
| 24 | ATM | 88.9 | 64.4 | 0.002 |
| CAZ | 88.9 | 66.7 | 0.004 | |
| Upper arm and/or thigh | Buccal mucosa and/or groin | |||
| 1 | ATM | 44.4 | 53.3 | 0.35 |
| CAZ | 37.8 | 51.1 | 0.11 | |
| 24 | ATM | 88.9 | 73.3 | 0.052 |
| CAZ | 88.9 | 77.8 | 0.13 | |
ATM, aztreonam; CAZ, ceftazidime.
(ii) Sponges.
All three sponge culture sites (forehead, upper arm, and thigh) had sensitivities higher than that for buccal mucosa, which was the most sensitive swab site, after the 24-h enrichment (Table 1). Among them, the thigh provided the highest sensitivity of 82.6%, which was significantly higher than those of the buccal mucosa (P ≤ 0.003) and the groin (P = 0.0003), the two most sensitive swab culture sites. In practice, the upper arm can be swiped first and the thigh can then be swiped using one sponge. Therefore, we calculated the composite sensitivity of the upper arm and thigh and compared it with that of various combinations of the most sensitive swab culture sites (nostrils, buccal mucosa, and groin). As depicted in Table 3, the combination of the upper arm and thigh with the sponge and with the 24-h enrichment yielded a significantly higher sensitivity (88.9 to 89.1%) than that of the combination of the nostril and buccal mucosa (56.5 to 58.7%) or nostril and groin (64.4 to 66.7%) and a trend toward a higher sensitivity than that with the combination of the buccal mucosa and groin (73.3 to 77.8%).
(iii) Effect of the infection/colonization status and prior therapy.
The sensitivities between patients who had infection and those who were only colonized by A. baumannii were compared by using various composite sensitivities, including ≥1 positive sponge culture, ≥1 positive swab culture, positive cultures for upper arm and/or thigh, and positive cultures for buccal mucosa and/or groin. There was a trend toward a higher composite sensitivity of the upper arm and thigh for patients who had infection (16/16 [100%] for infection and 25/30 [83.3%] for colonization with 24-h enrichment [P = 0.15]; the same results were found with aztreonam and ceftazidime). Otherwise, no statistically significant difference in composite sensitivity was observed.
The sensitivities were then compared between patients who were on effective therapy between the time of clinical culture collection and study sample collection in the same manner. Patients who were on effective therapy had lower composite sensitivities for the buccal mucosa and groin than those who were not (2/6 [33.3%] for the effective-therapy group and 31/39 [79.5%] for the no-therapy group with 24-h enrichment and aztreonam [P = 0.04] and 3/6 [50.0%] for the effective-therapy group and 32/39 [82.1%] for the no-therapy group with 24-h enrichment and ceftazidime [P = 0.11]). On the other hand, all 6 patients on effective therapy were positive by sponge specimens from the upper arm and thigh by either selective agent.
Growth of organisms other than A. baumannii.
A. baumannii formed smooth, pink colonies with a mauve background, as described in the original report of the use of LAM (Fig. 1B) (7). Ceftazidime-aztreonam-resistant Enterobacteriaceae, especially Klebsiella pneumoniae and Enterobacter spp., occasionally grew on mLAM plates, but they formed yellow colonies and were easily distinguished from A. baumannii. Pseudomonas aeruginosa grew in 7 cases, most of them from the buccal mucosa. P. aeruginosa formed pink colonies that were more flat and more translucent than A. baumannii and could be distinguished visually after experiencing several cases. Colonies indistinguishable from A. baumannii were observed in 10 cases, including colonies of Pseudomonas luteola (3 cases), Burkholderia cepacia (2 cases), Acinetobacter lwoffii, Pasteurella pneumotropica, Pseudomonas putida, Sphingomonas paucimobilis, and Vibrio metschnikovii (1 case each). The majority of them grew from the buccal mucosa, with only 2 cases growing them from any sponge site.
DISCUSSION
A. baumannii has become a major hospital pathogen in recent years, disproportionately affecting patients who are immunocompromised as well as those in intensive care units (13). The economic impact of A. baumannii colonization and infection in hospitals is also substantial. A mathematical model of ICU patients colonized with this organism suggests that the additional cost to the hospital due to colonization may be in excess of $44,000 per case, assuming that 50% of them will progress to infection (9). A. baumannii is a pathogen that is transmitted among hospitalized patients, most likely by health care workers (13). The prevention of transmission therefore requires the early identification of colonized patients and the institution of contact isolation for those who are colonized. This would be particularly important in epidemic scenarios. However, unlike other common hospital pathogens such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and Clostridium difficile, the screening method for A. baumannii has not been studied in detail. A recent study reported a combined sensitivity of 55% using six swabs targeting various anatomical sites (11). While that study was very well executed, we felt that 55% sensitivity was still too low and that the swabbing of six sites ranging from the nares to the rectum was not clinically feasible. The present study was therefore designed and conducted with the goal of significantly improving the sensitivity of screening for A. baumannii colonization.
The results of our study have several salient points. First, an enrichment culture grown overnight was necessary before culturing on selective media to achieve clinically acceptable sensitivity. This is consistent with the findings reported previously by Marchaim et al. (11) and is likely due to the low starting inoculum in the screening specimens. The exception was the buccal mucosa, where similar sensitivities were obtained with and without enrichment overnight. The distribution of A. baumannii at this site is probably dichotomous, with high colonization densities for some patients, especially those who are intubated, and no colonization at all for others.
Second, the use of a sponge to sample a large area of the skin, particularly the upper arm and thigh, proved to be easy to perform and effective in significantly improving the sensitivity compared with that with the use of a swab. Although the three sponge sites were sampled separately for the purpose of this study, one could easily sample the upper arm and thigh using the same sponge in clinical practice, which would give a theoretical sensitivity as high as 89.1%. On the other hand, it would not be feasible to use one swab to sample, for example, the buccal mucosa and groin, making it difficult to improve the sensitivity of swabs without increasing the number of specimens to be processed.
Third, the sponge method performed well in detecting colonization in patients who were on effective antimicrobial therapy against A. baumannii, whereas half or more of them were missed by the swab method. This could be a potential advantage of the sponge method for patients where lower colonization density due to ongoing therapy could be overcome by sampling a larger area.
Fourth, mLAM, a medium selective for A. baumannii, performed well in selectively growing this organism. mLAM with aztreonam was designed to grow all A. baumannii strains, and that with ceftazidime was designed to grow A. baumannii strains with resistance to three or more classes of antimicrobials. However, culture results were similar for both mLAM plates, presumably since only patients with A. baumannii with resistance to three or more classes of antimicrobials were enrolled in the study. There were several other species that grew on mLAM and were difficult to distinguish from A. baumannii. The majority of them grew from swab samples taken from the buccal mucosa, whereas such nonspecific growth occurred from sponge specimens in only two cases. The observed higher specificity is potentially another advantage of using a sponge over a swab. While standard identification procedures are still recommended when colonies consistent with A. baumannii are observed, the use of mLAM has the potential to substantially streamline the plate-reading process in the clinical microbiology laboratory. One unanticipated benefit of using mLAM was that ceftazidime-resistant Enterobacteriaceae (such as Klebsiella pneumoniae producing KPC-type carbapenemase) grew distinct yellow colonies. This may provide additional helpful information from an infection control standpoint in certain epidemiological circumstances.
One limitation of the screening method described here is that the protocol requires a total of 3 days until final identification is obtained. There is little room for improving the turnaround time as long as the method is culture based, as the enrichment process was crucial in improving sensitivity. While only 1-h and 24-h enrichment periods were tested in the present study, an enrichment period between these two time points may provide adequate sensitivity without causing a 1-day delay in the reporting of the results, which will require further investigation. Alternatively, nucleic acid testing (NAT)-based approaches may have the potential to significantly reduce the turnaround time while maintaining sensitivity. However, the cost-effectiveness of these approaches needs to be considered carefully since the prevalence of A. baumannii is generally lower than those of other organisms for which routine screening may be indicated, such as MRSA and VRE. In addition, the sponge used in this study costs approximately $2.50 each (prepackaged in a specimen container), which is more expensive than a swab.
In conclusion, screening for A. baumannii by use of a sponge is easy to perform and significantly improves the sensitivity for the detection of this organism compared with that of screening using a swab.
Acknowledgments
This study was funded by the Pennsylvania Department of Health (grant 4100047864) and by National Institute of Allergy and Infectious Diseases research career awards (K22AI80584 [Y.D.] and K24AI52788 [L.H.H.]).
Footnotes
Published ahead of print on 27 October 2010.
REFERENCES
- 1.Apisarnthanarak, A., U. Pinitchai, K. Thongphubeth, C. Yuekyen, D. K. Warren, and V. J. Fraser. 2008. A multifaceted intervention to reduce pandrug-resistant Acinetobacter baumannii colonization and infection in 3 intensive care units in a Thai tertiary care center: a 3-year study. Clin. Infect. Dis. 47:760-767. [DOI] [PubMed] [Google Scholar]
- 2.Berlau, J., H. Aucken, H. Malnick, and T. Pitt. 1999. Distribution of Acinetobacter species on skin of healthy humans. Eur. J. Clin. Microbiol. Infect. Dis. 18:179-183. [DOI] [PubMed] [Google Scholar]
- 3.Chu, Y. W., C. M. Leung, E. T. Houang, K. C. Ng, C. B. Leung, H. Y. Leung, and A. F. Cheng. 1999. Skin carriage of acinetobacters in Hong Kong. J. Clin. Microbiol. 37:2962-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffith, M. E., J. M. Ceremuga, M. W. Ellis, C. H. Guymon, D. R. Hospenthal, and C. K. Murray. 2006. Acinetobacter skin colonization of US Army soldiers. Infect. Control Hosp. Epidemiol. 27:659-661. [DOI] [PubMed] [Google Scholar]
- 5.Hambraeus, A., J. Hoborn, and W. Whyte. 1990. Skin sampling—validation of a pad method and comparison with commonly used methods. J. Hosp. Infect. 16:19-27. [DOI] [PubMed] [Google Scholar]
- 6.Hidron, A. I., J. R. Edwards, J. Patel, T. C. Horan, D. M. Sievert, D. A. Pollock, and S. K. Fridkin. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections. Annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect. Control Hosp. Epidemiol. 29:996-1011. [DOI] [PubMed] [Google Scholar]
- 7.Jawad, A., P. M. Hawkey, J. Heritage, and A. M. Snelling. 1994. Description of Leeds Acinetobacter medium, a new selective and differential medium for isolation of clinically important Acinetobacter spp., and comparison with Herellea agar and Holton's agar. J. Clin. Microbiol. 32:2353-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kallen, A. J., A. I. Hidron, J. Patel, and A. Srinivasan. 2010. Multidrug resistance among Gram-negative pathogens that caused healthcare-associated infections reported to the National Healthcare Safety Network, 2006-2008. Infect. Control Hosp. Epidemiol. 31:528-531. [DOI] [PubMed] [Google Scholar]
- 9.Lee, B. Y., S. M. McGlone, Y. Doi, R. R. Bailey, and L. H. Harrison. 2010. Economic impact of Acinetobacter baumannii infection in the intensive care unit. Infect. Control Hosp. Epidemiol. 31:1087-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maragakis, L. L., S. E. Cosgrove, X. Song, D. Kim, P. Rosenbaum, N. Ciesla, A. Srinivasan, T. Ross, K. Carroll, and T. M. Perl. 2004. An outbreak of multidrug-resistant Acinetobacter baumannii associated with pulsatile lavage wound treatment. JAMA 292:3006-3011. [DOI] [PubMed] [Google Scholar]
- 11.Marchaim, D., S. Navon-Venezia, D. Schwartz, J. Tarabeia, I. Fefer, M. J. Schwaber, and Y. Carmeli. 2007. Surveillance cultures and duration of carriage of multidrug-resistant Acinetobacter baumannii. J. Clin. Microbiol. 45:1551-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paterson, D. L. 2006. The epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species. Clin. Infect. Dis. 43(Suppl. 2):S43-S48. [DOI] [PubMed] [Google Scholar]
- 13.Peleg, A. Y., H. Seifert, and D. L. Paterson. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21:538-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seifert, H., L. Dijkshoorn, P. Gerner-Smidt, N. Pelzer, I. Tjernberg, and M. Vaneechoutte. 1997. Distribution of Acinetobacter species on human skin: comparison of phenotypic and genotypic identification methods. J. Clin. Microbiol. 35:2819-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]