Abstract
Neurocysticercosis (NC), caused by the larval stage of Taenia solium, is one of the most common parasitic diseases of the central nervous system. The diagnosis of NC is mostly based on costly brain neuroimaging (computed tomography and/or nuclear magnetic resonance), which is rarely accessible in most affected areas. The most sensitive and specific tools for NC diagnosis are imagery techniques. The identification of specific antibodies and antigens is currently used only to support NC diagnosis due to their limited specificity and sensitivity. This study was performed to compare immunodiagnostic assays (antibody detection by enzyme-linked immunosorbent assay [ELISA] and enzyme-linked immunoelectrotransfer blotting [EITB] and HP10 antigen detection by ELISA) with the detection of parasite DNA by PCR amplification of a repetitive element of the parasite genome in the cerebrospinal fluid (CSF) of 121 radiologically and clinically characterized NC patients. Patients were divided into six groups according to the stage of the parasites and their localization. The CSF cellularity of each patient was also recorded. When all patients were considered, PCR exhibited the highest sensitivity (95.9%) and variable specificity (80% or 100%) depending on the controls used. The sensitivities of antibody detection by ELISA and EITB were not significantly different, and ELISA identified HP10 antigen mostly when vesicular cysticerci were located in the subarachnoideal basal cisterns. These results can help in the selection of different individual assays or combinations of assays to be used in NC diagnosis according to different requirements.
Neurocysticercosis (NC), caused by the larval stage of the cestode parasite Taenia solium, is one of the most common parasitic diseases of the central nervous system (CNS) (20, 39). This disease represents a major public health problem in areas of endemicity in Latin America, Africa, and Asia (18, 29, 33, 36) and has been diagnosed with increasing frequency in the United States, primarily as an imported disease (8). In Mexico, seroprevalence rates for human cysticercosis ranged from 3.7 to 12.2% (18), and computerized-tomography (CT)-based epidemiological studies performed on inhabitants of rural communities have found an NC prevalence as high as 10% (14, 17).
Most NC cases are asymptomatic and are caused by parasites established in the parenchyma. However, due to the high prevalence of CNS infection, symptomatic NC is also frequent. Symptomatic NC may adopt different forms, from a clinically mild to a severe, disabling disease (12). The most frequent clinical NC manifestations are seizures (in countries where NC is endemic, it is the most common cause of late-onset epilepsy [28, 35]), intracranial hypertension, neurological deficits, and mental changes (3). In these heterogeneous clinical presentations, parasite (location, size, number) and host (degree of immune and inflammatory reactions developed) factors are involved (13, 23). The lack of specificity of the neurological symptoms makes it impossible to diagnose the disease on clinical grounds alone. For this reason, in most cases, NC diagnosis is based on neuroimaging studies (CT and magnetic resonance imaging [MRI]). CT and MRI also provide information about the parasite stage and location (21, 31). Three main stages have been described: vesicular (viable), colloidal (degenerative), and calcified (inactive) (19). CT is the best radiological method for the detection of intraparenchymal calcification, while MRI is more sensitive for the identification of cysts in the ventricles (42). However, in some cases, particularly when parasites are located in the subarachnoid basal cisterns, neither CT nor MRI can detect the parasite. In these cases, NC diagnosis is supported by clinical, epidemiological, and serological data as well as by the response to cysticidal treatment (9).
Immunodiagnosis of NC by detecting an antigen (Ag) and/or antibodies (Ab) is an accessible, low-cost diagnostic tool in areas of endemicity. For Ab detection, the enzyme-linked immunosorbent assay (ELISA) and enzyme-linked immunoelectrotransfer blotting (EITB) employing total antigen or partially purified antigen preparations are the most widely used techniques (7, 10). Many authors report that Ab detection in sera identifies approximately 70% of NC patients; the missing cases are those with a single parasite (43) and parenchymal and/or damaged cysticerci (5, 15, 37, 42). It is also estimated that the specificity of Ab detection for identifying active infection is around 30%, mainly due to the persistence of antibodies months or years after the resolution of the infection (22). Extraneural cysticercosis and cross-reactions with other cestodes and helminths can also contribute to false-positive results obtained by using serum antibodies (22, 27). However, better Ab detection is reported when Ab are detected in the cerebrospinal fluid (CSF) of neurological patients (6). The detection of secreted cysticercal Ag by ELISA is highly sensitive and specific for the diagnosis of living cysticerci (vesicular) localized in the subarachnoideal space at the base of the skull (15, 16). More recently, methods based on the detection of cysticercal DNA have been increasingly explored. In one study, using highly repetitive elements of the parasite as probes, as little as 10 fg of T. solium DNA was detected by PCR in the CSF from 29 out of 30 patients (1). In addition, a seminested PCR based on HDP2 that can detect 0.174 fg of T. solium DNA has been reported (24, 25). Here a comparative study of immunological procedures plus the procedure described by Almeida et al. (1) were evaluated using CSF from neurological patients from Mexico and France.
MATERIALS AND METHODS
Patients and samples.
This study was performed on CSF obtained by lumbar puncture from 121 patients (66 men and 55 women) who were admitted to the Instituto Nacional de Neurología y Neurocirugía (INNN) in Mexico City, Mexico, between March 2004 and May 2008. Age at diagnosis ranged from 15 to 72 years (mean, 40.3 years; median, 40 years; interquartile range [IQR], 31 to 50 years). The stage (vesicular, colloidal, or calcified) and location (parenchyma and basal subarachnoid space or ventricle) of cysticerci were based on CT and/or MRI. CSF cellularity (considered increased when the concentration of white blood cells [WBC] exceeded 5 per μl) and hydrocephaly (clinically defined) were recorded. A total of 20 CSF samples from Mexican neurological patients without NC (mainly patients with epilepsy, tumors, demyelinating disease, headache, or congenital subarachnoideal cysts) and 49 CSF samples from non-NC patients (with toxoplasmosis, malaria, HIV, or candidosis) in the Parasitology-Mycology Laboratory at the Pitié-Salpêtrière hospital, Paris, France, were included.
Classification of neurocysticercosis cases.
All individuals included as NC patients were established and confirmed on the basis of radiological features, characteristics of CSF, clinical presentation and evolution, and response to treatment. Patients were classified as follows: NC patients with vesicular (group 1), colloidal (group 2), or calcified (group 3) cysticerci; patients for whom a doubt existed regarding the presence of a vesicular cyst (group 4); and patients for whom, at the moment of sampling, radiological studies failed to detect parasites but who were included after successful cysticidal treatment (group 5). Those patients with vesicular parasites (group 1) were classified according to parasite location: parenchyma or subarachnoid sulci (group 1a) versus subarachnoid basal cisterns or ventricles (group 1b). Group 4 corresponds mainly to patients with unilateral enlargement of a basal cistern but without direct evidence of parasites. In this location (subarachnoid basal cisterns), the radiological visualization of the parasite is often difficult, since the parasites exhibit a signal intensity similar to that of CSF; they generally do not exhibit enhancement after the use of gadolinium, and they commonly lack the scolex.
Detection of specific antibodies.
Anti-T. solium Ab levels were determined by an in-house ELISA. Vesicular fluid recovered as previously described (26) from T. solium cysticerci was used as the source of Ag. CSF samples were diluted (1/50), and 100 μl of each sample diluted in phosphate-buffered saline (PBS)-bovine serum albumin (BSA) buffer was used. Samples were run in duplicate and were considered positive if the mean of the optical density (OD) at 450 nm was higher than the cutoff (corresponding to the mean for 5 negative CSF samples + 2 standard deviations [SD], ranging from 0.06 to 0.10). Negative samples were from non-NC neurological patients at the INNN diagnosed by MRI (different from our control group). We also included as positive controls samples from NC patients at the INNN previously diagnosed on the basis of MRI, lumbar puncture, clinical examination, and follow-up.
EITB (LDBIO Diagnostics, Lyon, France) was also performed (40). The procedure recommended by the manufacturer was used with the following minor modifications for better reading of the strips. The detection of at least two bands was indicative of NC. The membrane strip was incubated for 5 min in buffer R2 before the addition of 50 μl of CSF samples. Strips were incubated on the rocking platform overnight (instead of 90 min) at room temperature. After a wash, strips were incubated for an additional 60 min with the anti-IgG conjugate at room temperature. After a wash, strips were incubated with nitroblue tetrazolium (NBT)-5-bromo-4-chloro-3-indolylphosphate (BCIP) substrate in the dark for 60 min (instead of 10 to 30 min). The reaction was stopped after aspiration of the liquid by the addition of distilled water. We used the positive control provided in the kit and the same negative controls from the INNN.
Detection of specific antigens.
Parasite HP10 Ag was detected by an in-house ELISA as previously described (16). Samples were run in duplicate. A sample was considered positive if the mean OD at 450 nm was greater than the cutoff value (corresponding to the mean for 5 negative CSF samples + 2 SD, ranging from 0.12 to 0.19). The cutoff value was estimated for each plate using five CSF samples from confirmed non-NC neurological patients at the INNN diagnosed by MRI (separate from our control group). A group of five additional samples from neurological patients confirmed as NC positive controls from the INNN was included.
Detection of Taenia solium DNA.
The presence of T. solium DNA was explored by PCR in each CSF sample. Primers designed to amplify the highly repetitive element pTsol9 of the genome were employed (GenBank accession no. U45987) (1, 4). This technique can detect 10 fg of DNA, as previously reported (1).
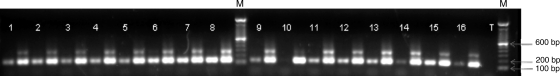
CSF samples were centrifuged for 10 min at 12,000 rpm. Supernatants were removed, and 100 μl of PBS was added. Genomic DNA (gDNA) was obtained using a spin column kit (DNeasy blood and tissue kit; Qiagen). Taenia solium gDNA obtained by the same procedure from cysticerci of naturally infected pigs was used as a positive control. Reactions were performed in a final volume of 50 μl containing 1.5 mM MgCl2, 0.5 μM each primer, 200 μM deoxynucleoside triphosphates (dNTPs) (Q-BIOgen), 1.25 U of AmpliTaq Gold DNA polymerase (Applied Biosystems), and 2 μl of DNA from the CSF sample. The primers used in the PCR to amplify pTsol9 were 5′-CAGGGTGTGACGTCATGG-3′ (forward primer; positions 21 to 38, 179 to 196, or 336 to 353) and 5′-GCTAGGCAACTGGCCTCCT-3′ (reverse primer; positions 122 to 140, 280 to 298, or 437 to 455). As an amplification control, we also used another tube with 1 μl of DNA from the CSF sample plus 1 μl of DNA from pig cysticerci. In the first cycle, DNA was denatured at 95°C for 5 min, followed by 38 cycles of denaturation at 95°C for 45 s, primer annealing at 57°C for 60 s, and elongation at 72°C for 45 s, plus one cycle at 72°C for 10 min. Ten microliters of each PCR amplification product was evaluated on a 2% agarose gel. A specific amplification product of 120 bp was expected for one repeat unit. When larger amounts of DNA were present, the amplification of 2 and 3 repeat units was also observed (Fig. 1). The presence of one band is sufficient to consider the PCR positive. This technique was also evaluated on negative controls from the Pitié-Salpêtrière hospital.
FIG. 1.
Amplicons from PCR after separation by electrophoresis in an agarose gel. Lanes: M, molecular weight markers; 1 to 16, samples of cerebrospinal fluid from patients; T, negative control. For each sample, two amplifications were performed: the first with 2 μl of DNA from the CSF sample and the second with 1 μl of DNA from the CSF sample plus 1 μl of DNA from pig cysticerci. In the second amplification, we observed 2 repeat units. All samples were positive except sample 10.
Statistical analysis.
Data were processed in Microsoft Excel 2008 and Stata/SE, version 9.0. The sensitivity and specificity of each method were determined with the 95% confidence interval. Data were compared using Pearson's χ2 test with Yates' correction when appropriate.
The concordance of methods was estimated by use of Cohen's kappa with the 95% confidence interval. If κ is 1, the concordance is perfect. Between 0.81 and 1, concordance is excellent; it is good between 0.61 and 0.8, moderate between 0.41 and 0.6, poor between 0.21 and 0.4, and null between 0 and 0.2.
Pearson's test of correlation was used to evaluate the correlation between the OD values from ELISAs and the numbers of cells. The Kruskal-Wallis test was used to determine the difference in the mean number of cells compared to the PCR and EITB results. The level of significance was less than 5%.
Ethical considerations.
The present study fulfils the requirements for research using human subjects set forth by Mexican laws and international regulations. It also complies with all ethical aspects of the General Rules of Health for Clinical Investigation. All participants volunteered to enter the study, donated a sample, and gave informed consent. The results were confidential. All patients received medical attention and the specific treatment required by a neurologist at the INNN.
RESULTS
Description of patients.
Table 1 summarizes the main clinical and biological characteristics of the patients included in this study. Of the 121 confirmed NC patients, 51 had vesicular cysticerci (group 1), 6 had colloidal cysticerci (group 2), and 31 had calcified cysticerci (group 3); for 19 of the individuals, there was a doubt concerning the presence of vesicular cysticerci (group 4); and 14 did not show cysticerci in the radiological studies at the moment of sampling but were included after adequate response to cysticidal treatment (group 5).
TABLE 1.
Principal characteristics of patients and controls included in the study
| Group | No. of individuals | No. male/female | Median age (yr) (range) | Median no. of cells/μl (range) | No. with hydrocephaly | No. with the indicated result by the following testa: |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ab-ELISA |
Ag-ELISA |
EITB |
PCR |
||||||||||
| POS | NEG | POS | NEG | POS | NEG | POS | NEG | ||||||
| Patients with confirmed NCb | 121 | 66/55 | 41 (19-72) | 9 (0-399) | 44 | 109 | 12 | 65 | 56 | 98 | 23 | 116 | 5 |
| Group 1 | 51 | 27/24 | 40 (19-72) | 19 (0-399) | 26 | 49 | 2 | 41 | 10 | 46 | 5 | 50 | 1 |
| Group 1a | 7 | 4/3 | 38 (22-56) | 4 (0-53) | 0 | 6 | 1 | 3 | 4 | 4 | 3 | 7 | 0 |
| Group 1b | 44 | 23/21 | 42 (19-72) | 25.5 (0-399) | 26 | 43 | 1 | 38 | 6 | 42 | 2 | 43 | 1 |
| Group 2 | 6 | 3/3 | 44.5 (28-51) | 6.5 (0-12) | 0 | 6 | 0 | 2 | 4 | 5 | 1 | 6 | 0 |
| Group 3 | 31 | 11/20 | 39 (21-67) | 3 (0-85) | 5 | 23 | 8 | 7 | 24 | 19 | 12 | 28 | 3 |
| Group 4 | 19 | 14/5 | 46 (28-69) | 24 (0-180) | 9 | 18 | 1 | 12 | 7 | 18 | 1 | 18 | 1 |
| Group 5 | 14 | 11/3 | 41 (23-59) | 4 (0-176) | 4 | 13 | 1 | 3 | 11 | 10 | 4 | 14 | 0 |
| Controls | |||||||||||||
| Mexico | 20 | 9/11 | 28 (15-59) | 2 (0-80) | ND | 2 | 18 | 0 | 20 | 0 | 20 | 4 | 16 |
| Paris | 49 | 32/17 | 49 (1-74) | ND | ND | ND | ND | ND | ND | 0 | 49 | 0 | 25 |
Ab-ELISA, antibody detection by ELISA; Ag-ELISA, antigen detection by ELISA; POS, positive; NEG, negative; ND, not done.
Group 1, vesicular parasites; group 1a, vesicular parasites in parenchyma or subarachnoid sulci; group 1b, vesicular parasites in subarachnoid basal cisterns or ventricles; group 2, colloidal parasites; group 3, calcified parasites; group 4, patients with doubts regarding the presence of a vesicular cyst; group 5, patients included after successful cysticidal treatment.
CSF cellularity (Table 1) was recorded for 117 confirmed NC samples and 11 controls. In NC patients, cellularity ranged from 0 to 399 cells/μl (mean, 30.6 ± 56.4 cells/μl; median, 9 cells/μl). Of these patients, 69 (59%) had inflammation (WBC count, >5/μl). In controls, cellularity ranged between 0 and 80 cells/μl (mean, 10.9 ± 26.0 cells/μl; median, 2 cells/μl) and was inflammatory for 2 individuals (22.2%). The number of cells in the CSF was missing for 2 patients with vesicular cysts, 1 with a calcification and 1 without distinguishable cysts in the radiological studies.
Sensitivity and specificity of each diagnostic tool.
Table 2 shows the sensitivity and specificity of each technique employed. For the diagnosis of vesicular parasites, the sensitivity of the Ag-ELISA was significantly lower than those of the Ab-ELISA and PCR. This difference disappeared when the samples were analyzed according to parasite location. The sensitivity of Ag-ELISA for the diagnosis of colloidal (group 2) and calcified (group 3) parasites or for the diagnosis of NC in patients for whom no parasites were distinguished by radiological studies after successful cysticidal treatment (group 5) was also lower than that of the other techniques. This difference was significant when this test was compared with the Ab-ELISA and PCR in cases when parasites were calcified or when no parasites were detectable. The sensitivity of PCR was higher than that of any other test for all groups of patients. Significant differences between PCR and EITB were observed, particularly when all patients, including those with calcified cysts, were considered.
TABLE 2.
Sensitivities and specificities of the diagnostic tools tested for the different patient categories
| Group | No. of individuals | Sensitivity or specificity (95% confidence interval)a |
Pb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ab-ELISA | Ag-ELISA | EITB | PCR | P1 | P2 | P3 | P4 | P5 | P6 | ||
| Patients with confirmed NCc | 121 | 90.1 (83.3-94.8) | 53.7 (44.4-62.8) | 81 (72.9-87.6) | 95.9 (90.6-98.6) | <0.0001 | 0.07 | 0.13 | <0.0001 | <0.0001 | 0.0006 |
| Group 1 | 51 | 96.1 (86.5-99.5) | 80.4 (66.9-90.2) | 90.2 (78.6-96.7) | 98 (89.6-100) | 0.03 | 0.43 | 1 | 0.26 | 0.01 | 0.21 |
| Group 1a | 7 | 85.7 (42.1-99.6) | 42.9 (9.9-81.6) | 57.1 (18.4-90.1) | 100 (59-100) | 0.27 | 0.56 | 1 | 1 | 0.07 | 0.19 |
| Group 1b | 44 | 97.7 (88-99.9) | 86.4 (72.6-94.8) | 95.5 (84.5-99.4) | 97.7 (88-99.9) | 0.12 | 1 | 0.47 | 0.27 | 0.12 | 1 |
| Group 2 | 6 | 100 (54.1-100) | 33.3 (4.33-77.7) | 83.3 (35.9-99.6) | 100 (54.1-100) | 0.06 | 1 | 1 | 0.24 | 0.06 | 1 |
| Group 3 | 31 | 74.2 (55.4-88.1) | 22.6 (9.59-41.1) | 61.3 (42.2-78.2) | 90.3 (74.2-98) | <0.0001 | 0.42 | 0.18 | 0.005 | <0.0001 | 0.02 |
| Group 4 | 19 | 94.7 (74-99.9) | 63.2 (38.4-83.7) | 94.7 (74-99.9) | 94.7 (74-99.9) | 0.04 | 1 | 1 | 0.04 | 0.04 | 1 |
| Group 5 | 14 | 92.9 (66.1-99.8) | 21.4 (4.66-50.8) | 71.4 (41.9-91.6) | 100 (76.8-100) | <0.0001 | 0.33 | 1 | 0.02 | <0.0001 | 0.09 |
| Controls | |||||||||||
| Mexico | 20 | 90 (68.3-98.8) | 100 (83.2-100) | 100 (83.2-100) | 80 (56.3-94.3) | 0.46 | 0.46 | 0.66 | 1 | 0.11 | 0.11 |
| Paris | 49 | 100 (97.2-100) | 100 (86.3-100) | 1 | |||||||
Values for patients with confirmed NC are sensitivities; values for controls are specificities.
P1, Ab-ELISA versus Ag-ELISA; P2, Ab-ELISA versus EITB; P3, Ab-ELISA versus PCR; P4, Ag-ELISA versus EITB; P5, Ag-ELISA versus PCR; P6, EITB versus PCR.
Group 1, vesicular parasites; group 1a, vesicular parasites in parenchyma or subarachnoid sulci; group 1b, vesicular parasites in subarachnoid basal cisterns or ventricles; group 2, colloidal parasites; group 3, calcified parasites; group 4, patients with doubts regarding the presence of a vesicular cyst; group 5, patients included after successful cysticidal treatment.
Although the sensitivity of Ab detection using ELISA was higher than that with EITB for all patient groups except one (the sensitivities were equal for group 4), this difference was not significant (P > 0.05). The specificities of the diagnostic tools ranged from 80% for PCR to 100% for Ag-ELISA and EITB, with no significant differences between techniques. Of the 20 controls, 4 were positive by PCR (presence of a specific band). Of these, one was also positive by the Ab-ELISA. This patient had multiple cavernomas. The PCR was also positive for a sample from a patient for whom, although a cyst was present upon brain imaging, the diagnosis of NC was discarded based on the lumbar puncture result, response to treatment, clinical and radiological evolution assessed by neurological examination, and repeated MRI. Another patient who was also positive by PCR had chronic hydrocephaly but no other clinical signs of NC. When PCR was tested on the non-NC French CSF samples, its specificity increased to 100%.
Concordance between diagnostic tools.
We evaluated the concordance between each pair of procedures for the different groups of patients (Table 3). The value of kappa ranged from 0.11 to 0.89. In Table 3, the best concordance found was that between the Ab-ELISA and the EITB test. Between these techniques, the concordance was excellent for groups 1, 1b, and 4; good for groups 2, 3, and 5; and moderate only for the group with vesicular parenchymal cysticerci (group 1a). The poorest concordance was that between Ag-ELISA and PCR, which was poor or null for 4 of the 7 patient categories, as shown in Table 3. The low concordance of the Ag-ELISA with the other techniques for groups 3 and 5 must be noted. In these cases, only 1 concordance was moderate; the other 5 were poor or null. The best concordance between each pair of diagnostic tools was observed for group 1b.
TABLE 3.
Concordance of tools estimated by Cohen's kappa with 95% confidence intervals
| Group | Cohen's kappa (95% confidence interval)a |
|||||
|---|---|---|---|---|---|---|
| Ab-ELISA vs Ag-ELISA | Ab-ELISA vs EITB | Ab-ELISA vs PCR | Ag-ELISA vs EITB | Ag-ELISA vs PCR | EITB vs PCR | |
| Patients with confirmed NC | 0.38 D (0.26-0.49) | 0.76 B (0.64-0.88) | 0.50 C (0.32-0.68) | 0.49 C (0.36-0.62) | 0.21 D (0.10-0.31) | 0.41 C (0.25-0.58) |
| Group 1b | 0.70 B (0.53-0.86) | 0.84 A (0.70-0.97) | 0.75 B (0.57-0.92) | 0.79 B (0.65-0.94) | 0.54 C (0.36-0.73) | 0.67 B (0.48-0.85) |
| Group 1a | 0.46 C (0.10-0.82) | 0.58 C (0.24-0.93) | 0.60 C (0.29-0.91) | 0.51 C (0-0.99) | 0.31 D (0.02-0.60) | 0.40 D (0.1-0.71) |
| Group 1b | 0.76 B (0.60-0.92) | 0.89 A (0.77-1) | 0.77 B (0.59-0.94) | 0.87 A (0.74-0.99) | 0.62 B (0.43-0.82) | 0.74 B (0.57-0.92) |
| Group 2 | 0.31 D (0-0.67) | 0.69 B (0.39-1) | 0.66 B (0.36-0.96) | 0.19 E (0-0.65) | 0.23 D (0-0.52) | 0.55 C (0.24-0.87) |
| Group 3 | 0.28 D (0.10-0.47) | 0.76 B (0.59-0.94) | 0.42 D (0.18-0.65) | 0.42 C (0.19-0.66) | 0.11 E (0-0.25) | 0.37 D (0.16-0.59) |
| Group 4 | 0.59 C (0.36-0.82) | 0.89 A (0.76-1) | 0.69 B (0.47-0.92) | 0.68 B (0.46-0.90) | 0.51 C (0.28-0.74) | 0.69 B (0.48-0.91) |
| Group 5 | 0.22 D (0-0.44) | 0.69 B (0.45-0.93) | 0.71 B (0.48-0.94) | 0.38 D (0.06-0.69) | 0.16 E (0-0.33) | 0.54 C (0.29-0.79) |
Levels of concordance: A, excellent (0.81-1); B, good (0.61-0.8); C, moderate (0.41-0.6); D, poor (0.21-0.4); E, null (0-0.2).
Group 1, vesicular parasites; group 1a, vesicular parasites in parenchyma or subarachnoid sulci; group 1b, vesicular parasites in subarachnoid basal cisterns or ventricles; group 2, colloidal parasites; group 3, calcified parasites; group 4, patients with doubts regarding the presence of a vesicular cyst; group 5, patients included after successful cysticidal treatment.
CSF cellularity and the sensitivity of diagnostic tests.
The number of cells (mainly lymphocytes) in the CSF correlated positively with the levels of antibody (r = 0.42; P < 0.001) and HP10 Ag (r = 0.24; P = 0.006). Also, PCR- and EITB-positive samples showed significantly higher numbers of cells than negative samples (P = 0.0007 and 0.0001, respectively). None of the CSF samples were hemorrhagic.
DISCUSSION
PCR based on pTsol9 amplification detected 90 to 100% of NC cases depending on the stage and location of the parasite. Unexpectedly, this technique detected 100% of parenchymal neurocysticerci. This observation is not in agreement with previous data indicating that access to the CSF is restricted for parenchymal cysticerci (15). The specificity of PCR was 80% for Mexican controls and 100% for French controls. The latter result is in accordance with those reported by Almeida et al. (1). The lower specificity obtained with Mexican samples was not expected, since this procedure is based on specific amplification of parasite DNA. One sample among the four CSF samples that were PCR positive was also positive for the detection of antibodies by ELISA. It is probable that these positive controls had or have had NC that was not diagnosed by radiological techniques. As we stated above, undetected infections could occur under conditions of endemicity without apparent symptoms or radiological signs. PCR could be useful for the diagnosis of NC cases when imagery techniques have failed.
In the study on HDP2 PCR, two uncertain NC cases were PCR positive (25). The authors suggest that the parasite DNA detected in these cases could be related to the perilesional edema occasionally observed with damaged cysticerci (30). Indeed, we have no information concerning the process leading to DNA release into the CSF. Further studies are required in order to better understand this process and also to evaluate the persistence of DNA in the CSF of patients.
The results obtained for the other immunological tools are in agreement with the findings of previous reports. A specificity of 100% was observed with the HP10 Ag-ELISA, accompanied by high sensitivity for the detection of vesicular parasites located in the subarachnoid space or in ventricles (86.4%). We also confirmed that the ability of the HP10 Ag-ELISA to detect vesicular parenchymal cysts is limited, though higher than that reported previously (42.9% versus 0%) (2). These contradictory results could be influenced by the low number of parenchymal neurocysticerci included in both studies. In addition, the possibility of radiological misdiagnosis of additional, undistinguishable subarachnoid cysts cannot be discarded. Particularly, in our study, 2 of the 3 positive patients showed inflammatory CSF, a feature seen mostly when parasites are localized in the subarachnoid basal space.
In general, samples found to be positive by different diagnostic tools were significantly more inflammatory than negative samples, showing the relevance of the presence of parasite markers in the genesis of the immune-inflammatory reaction associated. This was found in previous studies in which inflammatory CSF was found to be associated with positive levels of HP10 Ag, with odds ratios of 30 and 32 reported by Bobes et al. and Fleury et al., respectively (2, 16).
The results reported for the Ab-ELISA regarding the detection of vesicular parasites (sensitivity, 97.7% when parasites were located in the subarachnoid space or ventricles and 85.7% when they were located in the parenchyma) were similar to those of previous studies (15). It must also be noted that this technique effectively detects parasites independently of their viability.
The sensitivity and specificity of EITB were initially reported to be 98 and 100%, respectively (41). A subsequent evaluation of this test with 50 NC patients found a sensitivity of 91% for active NC and 88% for calcified cysticerci (43). Our results were not significantly different from those of the latter report (90.2% and 61.3%, respectively). In our study, although EITB exhibited higher specificity than the Ab-ELISA (100% versus 90%, respectively) and lower sensitivity (81% versus 90.1%, respectively), these differences were not statistically significant. Our result differs from those of some published studies (34, 38), although others are in agreement (11, 32). Since our Ab-ELISA is a simpler and less expensive tool than EITB for the immunological diagnosis of NC in the CSF, it seems more advisable for use in poor countries where NC is endemic, in order to consolidate the diagnosis of NC.
PCR of CSF samples for posttreatment follow-up remains to be evaluated. It will be also interesting to evaluate PCR of serum samples, because CSF sampling requires lumbar puncture. Serum samples are more readily available but contain large amounts of proteins and DNAs that can limit the specificity of PCR.
In summary, comparison of the procedures currently in use for NC diagnosis encourages the use of parasite DNA detection by PCR for diagnosis and points to the existence of possibly undiagnosed NC cases not detectable by available radiological or immunological tests.
Acknowledgments
This study was supported by ECOS Nord-ANUIES (project M06 SO1), the “French Ministry of Higher Education and Research,” Sanofi-Aventis, and the Paris-La Défense Rotary Club.
We thank Marisela Hernández for technical contributions to this study. We also thank Jeanne Cook-Moreau, who kindly agreed to correct the manuscript.
Footnotes
Published ahead of print on 10 November 2010.
REFERENCES
- 1.Almeida, C. R., E. P. Ojopi, C. M. Nunes, L. R. Machado, O. M. Takayanagui, J. A. Livramento, R. Abraham, W. F. Gattaz, A. J. Vaz, and E. Dias-Neto. 2006. Taenia solium DNA is present in the cerebrospinal fluid of neurocysticercosis patients and can be used for diagnosis. Eur. Arch. Psychiatry Clin. Neurosci. 256:307-310. [DOI] [PubMed] [Google Scholar]
- 2.Bobes, R. J., M. Hernandez, C. Marquez, G. Fragoso, E. Garcia, R. M. Parkhouse, L. J. Harrison, E. Sciutto, and A. Fleury. 2006. Subarachnoidal and intraventricular human neurocysticercosis: application of an antigen detection assay for the diagnosis and follow-up. Trop. Med. Int. Health 11:943-950. [DOI] [PubMed] [Google Scholar]
- 3.Carpio, A., A. Escobar, and W. A. Hauser. 1998. Cysticercosis and epilepsy: a critical review. Epilepsia 39:1025-1040. [DOI] [PubMed] [Google Scholar]
- 4.Chapman, A., V. Vallejo, K. G. Mossie, D. Ortiz, N. Agabian, and A. Flisser. 1995. Isolation and characterization of species-specific DNA probes from Taenia solium and Taenia saginata and their use in an egg detection assay. J. Clin. Microbiol. 33:1283-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung, J. Y., Y. Y. Bahk, S. Huh, S. Y. Kang, Y. Kong, and S. Y. Cho. 1999. A recombinant 10-kDa protein of Taenia solium metacestodes specific to active neurocysticercosis. J. Infect. Dis. 180:1307-1315. [DOI] [PubMed] [Google Scholar]
- 6.Corona, T., D. Pascoe, D. Gonzalez-Barranco, P. Abad, L. Landa, and B. Estanol. 1986. Anticysticercous antibodies in serum and cerebrospinal fluid in patients with cerebral cysticercosis. J. Neurol. Neurosurg. Psychiatry 49:1044-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deckers, N., and P. Dorny. 2010. Immunodiagnosis of Taenia solium taeniosis/cysticercosis. Trends Parasitol. 26:137-144. [DOI] [PubMed] [Google Scholar]
- 8.DeGiorgio, C., S. Pietsch-Escueta, V. Tsang, G. Corral-Leyva, L. Ng, M. T. Medina, S. Astudillo, N. Padilla, P. Leyva, L. Martinez, J. Noh, M. Levine, R. del Villasenor, and F. Sorvillo. 2005. Sero-prevalence of Taenia solium cysticercosis and Taenia solium taeniasis in California, U. S. A. Acta Neurol. Scand. 111:84-88. [DOI] [PubMed] [Google Scholar]
- 9.Del Brutto, O. H., V. Rajshekhar, A. C. White, Jr., V. C. Tsang, T. E. Nash, O. M. Takayanagui, P. M. Schantz, C. A. Evans, A. Flisser, D. Correa, D. Botero, J. C. Allan, E. Sarti, A. E. Gonzalez, R. H. Gilman, and H. H. Garcia. 2001. Proposed diagnostic criteria for neurocysticercosis. Neurology 57:177-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorny, P., J. Brandt, A. Zoli, and S. Geerts. 2003. Immunodiagnostic tools for human and porcine cysticercosis. Acta Trop. 87:79-86. [DOI] [PubMed] [Google Scholar]
- 11.Ferrer, E., M. M. Cortez, H. Perez, M. De la Rosa, B. A. de Noya, I. D'Avila, L. J. Harrison, M. Foster-Cuevas, R. M. Parkhouse, and A. Cabrera. 2002. Serological evidence for recent exposure to Taenia solium in Venezuelan Amerindians. Am. J. Trop. Med. Hyg. 66:170-174. [DOI] [PubMed] [Google Scholar]
- 12.Fleury, A., A. Dessein, P. M. Preux, M. Dumas, G. Tapia, C. Larralde, and E. Sciutto. 2004. Symptomatic human neurocysticercosis—age, sex and exposure factors relating with disease heterogeneity. J. Neurol. 251:830-837. [DOI] [PubMed] [Google Scholar]
- 13.Fleury, A., A. Escobar, G. Fragoso, E. Sciutto, and C. Larralde. 29 January 2010. Clinical heterogeneity of human neurocysticercosis results from complex interactions among parasite, host and environmental factors. Trans. R. Soc. Trop. Med. Hyg. 106:243-250. [DOI] [PubMed] [Google Scholar]
- 14.Fleury, A., T. Gomez, I. Alvarez, D. Meza, M. Huerta, A. Chavarria, R. A. Carrillo Mezo, C. Lloyd, A. Dessein, P. M. Preux, M. Dumas, C. Larralde, E. Sciutto, and G. Fragoso. 2003. High prevalence of calcified silent neurocysticercosis in a rural village of Mexico. Neuroepidemiology 22:139-145. [DOI] [PubMed] [Google Scholar]
- 15.Fleury, A., M. Hernandez, M. Avila, G. Cardenas, R. J. Bobes, M. Huerta, G. Fragoso, L. Uribe-Campero, L. J. Harrison, R. M. Parkhouse, and E. Sciutto. 2007. Detection of HP10 antigen in serum for diagnosis and follow-up of subarachnoidal and intraventricular human neurocysticercosis. J. Neurol. Neurosurg. Psychiatry 78:970-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleury, A., M. Hernandez, G. Fragoso, R. M. Parkhouse, L. J. Harrison, and E. Sciutto. 2003. Detection of secreted cysticercal antigen: a useful tool in the diagnosis of inflammatory neurocysticercosis. Trans. R Soc. Trop. Med. Hyg. 97:542-546. [DOI] [PubMed] [Google Scholar]
- 17.Fleury, A., J. Morales, R. J. Bobes, M. Dumas, O. Yanez, J. Pina, R. Carrillo-Mezo, J. J. Martinez, G. Fragoso, A. Dessein, C. Larralde, and E. Sciutto. 2006. An epidemiological study of familial neurocysticercosis in an endemic Mexican community. Trans. R Soc. Trop. Med. Hyg. 100:551-558. [DOI] [PubMed] [Google Scholar]
- 18.Flisser, A., E. Sarti, M. Lightowlers, and P. Schantz. 2003. Neurocysticercosis: regional status, epidemiology, impact and control measures in the Americas. Acta Trop. 87:43-51. [DOI] [PubMed] [Google Scholar]
- 19.Garcia, H. H., and O. H. Del Brutto. 2005. Neurocysticercosis: updated concepts about an old disease. Lancet Neurol. 4:653-661. [DOI] [PubMed] [Google Scholar]
- 20.Garcia, H. H., and O. H. Del Brutto. 2000. Taenia solium cysticercosis. Infect. Dis. Clin. North Am. 14:97-119. [DOI] [PubMed] [Google Scholar]
- 21.Garcia, H. H., O. H. Del Brutto, T. E. Nash, A. C. White, Jr., V. C. Tsang, and R. H. Gilman. 2005. New concepts in the diagnosis and management of neurocysticercosis (Taenia solium). Am. J. Trop. Med. Hyg. 72:3-9. [PubMed] [Google Scholar]
- 22.Garcia, H. H., R. H. Gilman, M. Catacora, M. Verastegui, A. E. Gonzalez, and V. C. Tsang. 1997. Serologic evolution of neurocysticercosis patients after antiparasitic therapy. Cysticercosis Working Group in Peru. J. Infect. Dis. 175:486-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia, H. H., A. E. Gonzalez, C. A. Evans, and R. H. Gilman. 2003. Taenia solium cysticercosis. Lancet 362:547-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison, L. J., J. Delgado, and R. M. Parkhouse. 1990. Differential diagnosis of Taenia saginata and Taenia solium with DNA probes. Parasitology 100(Pt. 3):459-461. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez, M., L. M. Gonzalez, A. Fleury, B. Saenz, R. M. Parkhouse, L. J. Harrison, T. Garate, and E. Sciutto. 2008. Neurocysticercosis: detection of Taenia solium DNA in human cerebrospinal fluid using a semi-nested PCR based on HDP2. Ann. Trop. Med. Parasitol. 102:317-323. [DOI] [PubMed] [Google Scholar]
- 26.Larralde, C., J. P. Laclette, C. S. Owen, I. Madrazo, M. Sandoval, R. Bojalil, E. Sciutto, L. Contreras, J. Arzate, M. L. Diaz, et al. 1986. Reliable serology of Taenia solium cysticercosis with antigens from cyst vesicular fluid: ELISA and hemagglutination tests. Am. J. Trop. Med. Hyg. 35:965-973. [DOI] [PubMed] [Google Scholar]
- 27.Larralde, C., R. M. Montoya, E. Sciutto, M. L. Diaz, T. Govezensky, and E. Coltorti. 1989. Deciphering Western blots of tapeworm antigens (Taenia solium, Echinococcus granulosus, and Taenia crassiceps) reacting with sera from neurocysticercosis and hydatid disease patients. Am. J. Trop. Med. Hyg. 40:282-290. [DOI] [PubMed] [Google Scholar]
- 28.Mac, T. L., D. S. Tran, F. Quet, P. Odermatt, P. M. Preux, and C. T. Tan. 2007. Epidemiology, aetiology, and clinical management of epilepsy in Asia: a systematic review. Lancet Neurol. 6:533-543. [DOI] [PubMed] [Google Scholar]
- 29.Mafojane, N. A., C. C. Appleton, R. C. Krecek, L. M. Michael, and A. L. Willingham III. 2003. The current status of neurocysticercosis in Eastern and Southern Africa. Acta Trop. 87:25-33. [DOI] [PubMed] [Google Scholar]
- 30.Nash, T. E., O. H. Del Brutto, J. A. Butman, T. Corona, A. Delgado-Escueta, R. M. Duron, C. A. Evans, R. H. Gilman, A. E. Gonzalez, J. A. Loeb, M. T. Medina, S. Pietsch-Escueta, E. J. Pretell, O. M. Takayanagui, W. Theodore, V. C. Tsang, and H. H. Garcia. 2004. Calcific neurocysticercosis and epileptogenesis. Neurology 62:1934-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osborn, A. G., and M. T. Preece. 2006. Intracranial cysts: radiologic-pathologic correlation and imaging approach. Radiology 239:650-664. [DOI] [PubMed] [Google Scholar]
- 32.Parija, S. C., and A. R. Gireesh. 2009. A serological study of cysticercosis in patients with HIV. Rev. Inst. Med. Trop. Sao Paulo 51:185-189. [DOI] [PubMed] [Google Scholar]
- 33.Pawlowski, Z., J. Allan, and E. Sarti. 2005. Control of Taenia solium taeniasis/cysticercosis: from research towards implementation. Int. J. Parasitol. 35:1221-1232. [DOI] [PubMed] [Google Scholar]
- 34.Prabhakaran, V., V. Rajshekhar, K. D. Murrell, and A. Oommen. 2004. Taenia solium metacestode glycoproteins as diagnostic antigens for solitary cysticercus granuloma in Indian patients. Trans. R Soc. Trop. Med. Hyg. 98:478-484. [DOI] [PubMed] [Google Scholar]
- 35.Preux, P. M., and M. Druet-Cabanac. 2005. Epidemiology and aetiology of epilepsy in sub-Saharan Africa. Lancet Neurol. 4:21-31. [DOI] [PubMed] [Google Scholar]
- 36.Rajshekhar, V., D. D. Joshi, N. Q. Doanh, N. van De, and Z. Xiaonong. 2003. Taenia solium taeniosis/cysticercosis in Asia: epidemiology, impact and issues. Acta Trop. 87:53-60. [DOI] [PubMed] [Google Scholar]
- 37.Ramos-Kuri, M., R. M. Montoya, A. Padilla, T. Govezensky, M. L. Diaz, E. Sciutto, J. Sotelo, and C. Larralde. 1992. Immunodiagnosis of neurocysticercosis. Disappointing performance of serology (enzyme-linked immunosorbent assay) in an unbiased sample of neurological patients. Arch. Neurol. 49:633-636. [DOI] [PubMed] [Google Scholar]
- 38.Scheel, C. M., A. Khan, K. Hancock, H. H. Garcia, A. E. Gonzalez, R. H. Gilman, and V. C. Tsang. 2005. Serodiagnosis of neurocysticercosis using synthetic 8-kD proteins: comparison of assay formats. Am. J. Trop. Med. Hyg. 73:771-776. [PubMed] [Google Scholar]
- 39.Sciutto, E., G. Fragoso, A. Fleury, J. P. Laclette, J. Sotelo, A. Aluja, L. Vargas, and C. Larralde. 2000. Taenia solium disease in humans and pigs: an ancient parasitosis disease rooted in developing countries and emerging as a major health problem of global dimensions. Microbes Infect. 2:1875-1890. [DOI] [PubMed] [Google Scholar]
- 40.Simac, C., P. Michel, A. Andriantsimahavandy, P. Esterre, and A. Michault. 1995. Use of enzyme-linked immunosorbent assay and enzyme-linked immunoelectrotransfer blot for the diagnosis and monitoring of neurocysticercosis. Parasitol. Res. 81:132-136. [DOI] [PubMed] [Google Scholar]
- 41.Tsang, V. C., J. A. Brand, and A. E. Boyer. 1989. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J. Infect. Dis. 159:50-59. [DOI] [PubMed] [Google Scholar]
- 42.White, A. C., Jr. 1997. Neurocysticercosis: a major cause of neurological disease worldwide. Clin. Infect. Dis. 24:101-113. [DOI] [PubMed] [Google Scholar]
- 43.Wilson, M., R. T. Bryan, J. A. Fried, D. A. Ware, P. M. Schantz, J. B. Pilcher, and V. C. Tsang. 1991. Clinical evaluation of the cysticercosis enzyme-linked immunoelectrotransfer blot in patients with neurocysticercosis. J. Infect. Dis. 164:1007-1009. [DOI] [PubMed] [Google Scholar]