Abstract
A female patient presented with episodes of fever and pain in the lower right abdomen after hysteroscopic removal of an intrauterine device 2 months earlier. Pelvic actinomycosis originating from a tubo-ovarian abscess was diagnosed with Propionibacterium propionicum, formerly known as Arachnia propionica, as causative agent.
CASE REPORT
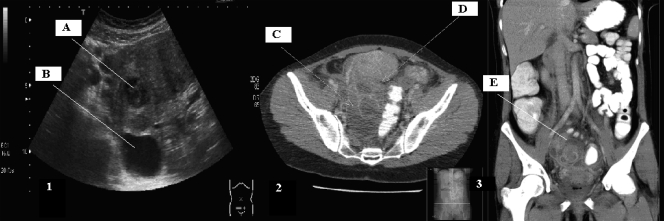
A 38-year-old woman presented at the emergency department with complaints of periodic fever and pain in the lower right abdomen 2 months after hysteroscopic removal of an intrauterine device (IUD). The temperature was 38°C. At gynecological examination, tenderness of the right lower abdomen was noticed with a bulging swelling in the recto-uterine pouch. Laboratory examinations showed a leukocytosis (13.4 × 109/liter; normal value = 4.0 × 109 to 10.0 × 109/liter), an elevated C-reactive protein level (129 mg/liter; normal value = 1 to 10 mg/liter), an elevated erythrocyte sedimentation rate (45 mm; normal value = 0 to 25 mm), and a microcytic anemia (hemoglobin [Hb] = 11.4 g/dl; normal value = 12 to 16.1 g/dl), indicating the presence of a chronic infection. An abdominal-pelvic ultrasonography showed an enlarged right adnexum measuring 9 cm in diameter with a multilocular circumscribed fluid collection with a small echodense area and a larger transonic area with some free fluid in the recto-uterine pouch (Fig. 1, panel 1, A and B).
FIG. 1.
(1) Abdominal-pelvic ultrasonography showing an enlarged right adnexum with a multilocular circumscribed fluid collection with an echodense area (A) and a transonic area (B). (2) CT scan showing a fluid collection of 9 by 5 cm in the recto-uterine pouch formed by a big collection (C) and a smaller one (D) with coloring of the walls indicating infection. (3) The same fluid collection (E) as in panel 2 from a frontal point of view.
Pelvic inflammatory disease with a tubo-ovarian abscess was suspected, and antibiotic treatment consisting of doxycycline and metronidazole was administered. There was no clinical improvement after 2 days of treatment, and subsequently vaginal puncture of the abscess was performed. Gram stains of the abscess material showed a few Gram-positive, filamentous rods with possible branching, positive cocci in chains, and many polymorphonuclear leukocytes. Based on the results of the Gram stain, doxycycline was switched to penicillin to treat a possible actinomycosis.
After 2 days of incubation, smooth white colonies of branching Gram-positive rods and small colorless colonies of Gram-positive cocci were observed on the anaerobic medium, Columbia agar with 5% sheep blood (bioMérieux Benelux B.V., Boxtel, Netherlands). The Gram-positive rods were catalase and oxidase negative, and biochemical evaluation on the basis of the API Rapid ID 32 A test (bioMérieux Benelux B.V., Boxtel, Netherlands) identified the organism as Propionibacterium propionicum, formerly known as Arachnia propionica. The Gram-positive cocci were identified as Parvimonas micra and a Peptostreptococcus species using the same API Rapid ID 32 A test (bioMérieux Benelux B.V., Boxtel, Netherlands). The P. propionicum and Peptostreptococcus species were confirmed with 16S rRNA sequencing, and both showed >99.9% homology with representatives in GenBank. Sequence analysis of the P. propionicum strain resulted in 1,061 bp of the 5′ end from the 16S rRNA gene, which was given the GenBank accession number HQ413290. The highest homology was observed with the partial 16S rRNA sequence of P. propionicum with accession number AF285117.
Two days after the antibiotic switch to penicillin, no clinical improvement was observed. A computed tomography (CT) scan showed a multilocular lesion of 9 cm in diameter in the recto-uterine pouch (Fig. 1, panel 2, C and D, and panel 3, E).
An open laparoscopic procedure was performed and showed an enlarged right ovary of approximately 9 by 5 cm that was located behind the uterus and reached into the recto-uterine pouch. Several adhesions between the uterus and right adnexum were laparoscopically removed. The fallopian tube was also enlarged, and during manipulation pus was released from the right ovary. The left adnexum was partly visible due to adhesions but was considered normal. The abscess of the right ovary was drained, and a drain was left in situ in the recto-uterine pouch.
Gram stains of the abscess material again showed rare Gram-positive, filamentous rods with possible branching suggestive of actinomycosis, positive cocci in chains, and many polymorphonuclear leukocytes. Culture grew no aerobic organisms, but after 2 days there was growth of smooth white colonies of branching Gram-positive rods and small colorless colonies of Gram-positive cocci on the anaerobic medium, Columbia agar with 5% sheep blood (bioMérieux Benelux B.V., Boxtel, Netherlands). Results of identification by the API Rapid ID 32 A test (bioMérieux Benelux B.V., Boxtel, Netherlands) revealed P. propionicum, P. micra, and Peptostreptococcus species.
The patient showed a rapid clinical improvement after the operation and was released from the hospital 1 week later. All the cultured isolates were penicillin susceptible as determined by penicillin Etest (AB Biodisk, Solna, Sweden). The MICs for penicillin were 0.094 μg/ml for P. propionicum, 0.047 μg/ml for the Peptostreptococcus species, and 0.012 μg/ml for P. micra. The patient was treated for 4 weeks with 24 million units of penicillin intravenously, after which the treatment was continued with amoxicillin for 6 months orally. Follow-up of the right adnexum was performed with ultrasonography. After 6 months, the patient was well and ultrasonography and gynecologic investigation showed a normal aspect of both adnexa.
Discussion.
We report a patient with an actinomycosis-like pelvic process originating from a tubo-ovarian abscess 2 months after hysteroscopic removal of an IUD that was in situ for approximately 8 years. P. propionicum, P. micra, and Peptostreptococcus species were found as causative agents. Of these three, P. propionicum is the most pathogenic species, causing actinomycosis-like disease. P. propionicum has been isolated from patients with lacrimal canaliculitis, osteomyelitis, brain abscess, endodontic infections, chronic tympanomastoiditis, renal abscess, pulmonary abscess, and cervico-facial actinomycosis (2, 4, 6, 10, 21, 23, 30, 33); in one case of chronic granulomatous disease with multiple episodes of chest trauma and microabscesses and granulomatous inflammation in lung tissue (26); and in one case of disseminated actinomycosis with a hepatic abscess (14). This case report represents the first finding of P. propionicum in association with pelvic disease.
Propionibacteria belong to the family of Propionibacteriaceae and are pleomorphic, slow-growing, non-spore-forming, Gram-positive, anaerobic bacteria. Species of propionibacteria can be found as members of commensal flora, especially on the skin, in the mouth, and in the gastrointestinal tract. They are generally nonpathogenic but become pathogenic in patients with implantation of foreign bodies, with immunosuppression, and after surgery or trauma. Four clinically relevant species of propionibacteria have been recognized: Propionibacterium acnes, Propionibacterium avidum, Propionibacterium granulosum, and Propionibacterium propionicum (20).
P. propionicum was first described by Pine and Hardin in 1959. At that time the organism was identified as Actinomyces israelii (27). Subsequent work by Buchanan and Pine suggested that this organism represented a new species, Actinomyces propionicus, on the basis of differences in metabolism, physiology, and cell wall composition (7). The organism was later reclassified again and placed in a new genus, Arachnia, under the name of Arachnia propionica (2). Further research later classified the organism in the genus Propionibacterium, under the name of P. propionicum (8, 11, 35).
P. propionicum differs from Actinomyces species by production of propionic acid from glucose and by the presence of diaminopimelic acid in the cell wall that resembles those of other Propionibacterium species (15, 25). Differentiation between the two different species can also be performed with 16S rRNA sequencing (1, 28).
P. propionicum and A. israelii are difficult to distinguish because both bacteria are microaerophilic to anaerobic and grow optimally under anaerobic conditions. The most pronounced differences of P. propionicum and A. israelii are differences in growth rate and colony morphology on Columbia agar with 5% sheep blood (bioMérieux Benelux B.V., Boxtel, Netherlands) incubated anaerobically. P. propionicum grows after 18 to 24 h of incubation as very small smooth white colonies with branches originating from a single point; after 7 to 14 days of incubation, the colonies get bigger and then resemble the colonies of A. israelii with its rough breadcrumb or molar structure. In contrast to P. propionicum, A. israelii starts to grow only after 7 to 10 days of incubation and its colony morphology immediately looks like a molar tooth.
P. micra and Peptostreptococcus species were also found in the actinomycosis-like pelvic lesions due to P. propionicum. We consider their presence to be that of “companion microbes.” These concomitant bacteria may serve as copathogens enhancing the progress of the disease. Actinobacillus actinomycetemcomitans, Eikenella corrodens, Fusobacterium spp., Bacteroides spp., Capnocytophaga spp., Staphylococcus spp., Streptococcus spp., and Enterobacteriaceae have been commonly isolated in various combinations depending on the site of infection (13, 16, 24, 29, 31, 32). The coisolation of P. micra and Peptostreptococcus sp. in this case of an actinomycosis-like disease due to P. propionicum therefore is not unusual. We consider P. propionicum to be the main pathogen, because in this case it is the only isolate that can cause an actinomycosis-like disease (2, 4, 6, 10, 14, 21, 23, 26, 30, 33). The contribution of P. micra and Peptostreptococcus sp. to pathogenesis, however, is difficult to assess, and it seems reasonable to consider them as being copathogens when designing the therapeutic regimen. Therefore, it is important to perform the identification and to determine the antimicrobial susceptibility of the concomitant bacteria in cases of actinomycosis or actinomycosis-like disease.
The first two cases of pelvic actinomycosis associated with a contraceptive device were reported by Brenner and Gehring in 1967 (5) and Henderson in 1973, who reported a tubo-ovarian abscess associated with an IUD (17). There are several studies reporting that pelvic actinomycosis is associated with prolonged use of an IUD (3, 18, 19, 34). In general the risk of acquiring pelvic actinomycosis is significantly higher in women who use an IUD for a prolonged period, especially ≥5 years (9, 12).
To our knowledge, only one study reported two cases of actinomycosis in association with an IUD which were considered to be associated with P. propionicum. Diagnosis was confirmed by immunofluorescence, but no positive cultures were obtained (22). A literature search for Arachnia propionica and Actinomyces propionicus causing actinomycosis associated with an IUD delivered no results. This case report thus represents the first finding of P. propionicum causing pelvic actinomycosis associated with an IUD.
Nucleotide sequence accession number.
The 1,061-bp sequence of the 5′ end of the 16S rRNA gene from the P. propionicum isolate was given the GenBank accession number HQ413290.
Footnotes
Published ahead of print on 10 November 2010.
REFERENCES
- 1.Aas, J. A., A. L. Griffen, S. R. Dardis, A. M. Lee, I. Olsen, F. E. Dewhirst, E. J. Leys, and B. J. Paster. 2008. Bacteria of dental caries in primary and permanent teeth in children and young adults. J. Clin. Microbiol. 46:1407-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albright, L., S. Toczek, V. J. Brenner, and A. K. Ommaya. 1974. Osteomyelitis and epidural abscess caused by Arachnia propionica. Case report. J. Neurosurg. 40:115-119. [DOI] [PubMed] [Google Scholar]
- 3.Barres, A., J. L. Criscuolo, F. Vilde, and R. Taurelle. 1990. Tubo-ovarian actinomycosis. Rev. Fr. Gynecol. Obstet. 85:479-482. [PubMed] [Google Scholar]
- 4.Brazier, J. S., and V. Hall. 1993. Propionibacterium propionicum and infections of the lacrimal apparatus. Clin. Infect. Dis. 17:892-893. [DOI] [PubMed] [Google Scholar]
- 5.Brenner, R. W., and S. W. Gehring. 1967. Pelvic actinomycosis in the presence of an endocervical contraceptive device. Report of a case. Obstet. Gynecol. 29:71-73. [PubMed] [Google Scholar]
- 6.Brock, D. W., L. K. Georg, J. M. Brown, and M. D. Hicklin. 1973. Actinomycosis caused by Arachnia propionica: report of 11 cases. Am. J. Clin. Pathol. 59:66-77. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan, B. B., and L. Pine. 1962. Characterization of a propionic acid producing actinomycete, Actinomyces propionicus, sp. nov. J. Gen. Microbiol. 28:305-323. [DOI] [PubMed] [Google Scholar]
- 8.Charfreitag, O., and E. Stackebrandt. 1989. Inter- and intrageneric relationships of the genus Propionibacterium as determined by 16S rRNA sequences. J. Gen. Microbiol. 135:2065-2070. [DOI] [PubMed] [Google Scholar]
- 9.Charonis, G., and P. G. Larsson. 2009. Prolonged use of intrauterine contraceptive device as a risk factor for tubo-ovarian abscess. Acta Obstet. Gynecol. Scand. 88:680-684. [DOI] [PubMed] [Google Scholar]
- 10.Conrad, S. E., J. Breivis, and M. A. Fried. 1978. Vertebral osteomyelitis, caused by Arachnia propionica and resembling actinomycosis. Report of a case. J. Bone Joint Surg. Am. 60:549-553. [PubMed] [Google Scholar]
- 11.Cummins, C. S., and C. W. Moss. 1990. Fatty acid composition of Propionibacterium propionicum (Arachnia propionica). Int. J. Syst. Bacteriol. 40:307-308. [DOI] [PubMed] [Google Scholar]
- 12.Curtis, E. M., and L. Pine. 1981. Actinomyces in the vaginas of women with and without intrauterine contraceptive devices. Am. J. Obstet. Gynecol. 140:880-884. [DOI] [PubMed] [Google Scholar]
- 13.Fiorino, A. S. 1996. Intrauterine contraceptive device-associated actinomycotic abscess and Actinomyces detection on cervical smear. Obstet. Gynecol. 87:142-149. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Martos, P., J. Candau, M. Benjumeda, F. de la Rubia, M. J. Soria, and R. Perez. 1992. Disseminated actinomycosis and hepatic abscess caused by Arachnia propionica. Enferm. Infecc. Microbiol. Clin. 10:123-124. [PubMed] [Google Scholar]
- 15.Gerencser, M. A., and J. M. Slack. 1967. Isolation and characterization of Actinomyces propionicus. J. Bacteriol. 94:109-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghafghaichi, L., S. Troy, I. Budvytiene, N. Banaei, and E. J. Baron. 2010. Mixed infection involving Actinomyces, Aggregatibacter, and Fusobacterium species presenting as perispinal tumor. Anaerobe 16:174-178. [DOI] [PubMed] [Google Scholar]
- 17.Henderson, S. R. 1973. Pelvic actinomycosis associated with an intrauterine device. Obstet. Gynecol. 41:726-732. [PubMed] [Google Scholar]
- 18.Hwang, J. H., J. H. Hong, and J. K. Lee. 2009. Ovarian and vesical actinomycosis: a case report and literature review. Arch. Gynecol. Obstet. 279:591-593. [DOI] [PubMed] [Google Scholar]
- 19.Joshi, C., R. Sharma, and Z. Mohsin. 2010. Pelvic actinomycosis: a rare entity presenting as tubo-ovarian abscess. Arch. Gynecol. Obstet. 281:305-306. [DOI] [PubMed] [Google Scholar]
- 20.Könönen, E., and W. G. Wade. 2007. Propionibacterium, Lactobacillus, Actinomyces and other non-spore-forming anaerobic gram-positive rods, p. 872-888. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed., vol. 1. ASM Press, Washington, DC. [Google Scholar]
- 21.Miglets, A. W., and D. Branson. 1983. Arachnia propionica (Actinomyces propionicus) as an unusual agent in tympanomastoiditis. Arch. Otolaryngol. 109:410-412. [DOI] [PubMed] [Google Scholar]
- 22.Nayar, M., M. Chandra, K. Chitraratha, D. S. Kumari, and C. G. Rai. 1985. Incidence of actinomycetes infection in women using intrauterine contraceptive devices. Acta Cytol. 29:111-116. [PubMed] [Google Scholar]
- 23.Novak, A., and P. Brutsch. 1980. Case report of actinomycosis caused by Arachnia propionica. Infection 8(Suppl. 2):S209-S211. [DOI] [PubMed] [Google Scholar]
- 24.Ochiai, K., T. Kurita-Ochiai, Y. Kamino, and T. Ikeda. 1993. Effect of co-aggregation on the pathogenicity of oral bacteria. J. Med. Microbiol. 39:183-190. [DOI] [PubMed] [Google Scholar]
- 25.O'Donnell, A. G., D. E. Minnikin, M. Goodfellow, J. H. Parlett, G. M. Schofield, and K. P. Schaal. 1985. Lipid and wall amino acid composition in the classification and identification of Arachnia propionica. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 260:300-310. [DOI] [PubMed] [Google Scholar]
- 26.Pasic, S., D. Savic, I. Milovic, Z. Vasiljevic, and S. Djuricic. 2004. Propionibacterium propionicus infection in chronic granulomatous disease. Clin. Infect. Dis. 38:459. [DOI] [PubMed] [Google Scholar]
- 27.Pine, L., H. Hardin, L. Turner, and S. S. Roberts. 1960. Actinomycotic lacrimal canaliculitis. A report of two cases with a review of the characteristics which identify the causal organism, Actinomyces israelii. Am. J. Ophthalmol. 49:1278-1288. [PubMed] [Google Scholar]
- 28.Preza, D., I. Olsen, J. A. Aas, T. Willumsen, B. Grinde, and B. J. Paster. 2008. Bacterial profiles of root caries in elderly patients. J. Clin. Microbiol. 46:2015-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulverer, G., H. Schutt-Gerowitt, and K. P. Schaal. 2003. Human cervicofacial actinomycoses: microbiological data for 1997 cases. Clin. Infect. Dis. 37:490-497. [DOI] [PubMed] [Google Scholar]
- 30.Riley, T. V., and A. K. Ott. 1981. Brain abscess due to Arachnia propionica. Br. Med. J. (Clin. Res. Ed.) 282:1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma, M., L. E. Briski, and R. Khatib. 2002. Hepatic actinomycosis: an overview of salient features and outcome of therapy. Scand. J. Infect. Dis. 34:386-391. [DOI] [PubMed] [Google Scholar]
- 32.Simpson, A. J., S. S. Das, and I. J. Mitchelmore. 1996. Polymicrobial brain abscess involving Haemophilus paraphrophilus and Actinomyces odontolyticus. Postgrad. Med. J. 72:297-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siqueira, J. F., Jr., and I. N. Rocas. 2004. Polymerase chain reaction-based analysis of microorganisms associated with failed endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 97:85-94. [DOI] [PubMed] [Google Scholar]
- 34.Sparks, R. A., B. G. Purrier, P. J. Watt, and M. Elstein. 1981. Bacteriological colonisation of uterine cavity: role of tailed intrauterine contraceptive device. Br. Med. J. (Clin. Res. Ed.) 282:1189-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Summanen, P. 1993. Recent taxonomic changes for anaerobic gram-positive and selected gram-negative organisms. Clin. Infect. Dis. 16(Suppl. 4):S168-S174. [DOI] [PubMed] [Google Scholar]