Abstract
Compared to the incidence in adults, cryptococcosis is inexplicably rare among children, even in sub-Saharan Africa, which has the highest prevalence of coinfection with HIV and Cryptococcus neoformans. To explore any mycological basis for this age-related difference in the incidence of cryptococcosis, we investigated isolates of C. neoformans recovered from pediatric and adult patients during a 2-year period in South Africa. From reports to the Group for Enteric, Respiratory, and Meningeal Disease Surveillance in South Africa (GERMS-SA), we reviewed all cases of cryptococcosis in 2005 and 2006. We analyzed one isolate of C. neoformans from each of 82 pediatric patients (<15 years of age) and determined the multilocus sequence type (ST), mating type, ploidy, and allelic profile. This sample included isolates of all three molecular types of serotype A or C. neoformans var. grubii (molecular types VNI, VNII, and VNB) and one AD hybrid. Seventy-seven (94%) of the strains possessed the MATα mating type allele, and five were MATa. Seventy-five (91%) were haploid, and seven were diploid. A total of 24 different STs were identified. The ratios of each mating type and the proportion of haploids were comparable to those for the isolates that were obtained from 86 adult patients during the same period. Notably, the most prevalent pediatric ST was significantly associated with male patients. Overall, these pediatric isolates exhibited high genotypic diversity. They included a relatively large percentage of diploids and the rarely reported MATa mating type.
The latest estimates indicate that two-thirds of all people infected with HIV and almost three-fourths of all AIDS-related deaths occurred in sub-Saharan Africa, where HIV/AIDS is the leading cause of death. In South Africa alone, an estimated 5.7 million people, approximately 12% of the population, are seropositive for HIV (64, 65). Many of these deaths are related to AIDS-defining infections, particularly tuberculosis and cryptococcosis (12, 26, 28, 57, 59). Both infections are acquired by inhalation, but cryptococcosis is not contagious. More than 90% of cases of cryptococcosis among HIV-infected persons are caused by isolates of Cryptococcus neoformans var. grubii, which possess capsular serotype A (42).
C. neoformans is neurotropic, and the most common clinical presentation of cryptococcal disease is meningoencephalitis. The introduction of antiretroviral therapy (ART) has significantly reduced the incidence of cryptococcosis in developed countries; however, resource-limited countries continue to be burdened with high morbidity and mortality, even in regions with access to ART (25, 27, 41, 45, 61).
Compared to the number of adults, the number of children who acquire cryptococcosis has always been unaccountably low, even in sub-Saharan Africa, which is home to almost 90% of all children with HIV, most of whom were infected by maternal transmission (1, 4, 22, 34, 60). One of the largest, published case series to date was reported from a 2-year surveillance program in Gauteng Province, South Africa (48). From 2002 through 2004, a total of 2,753 cases of cryptococcosis were documented, and 24 patients (0.9%) were less than 15 years old (48). Earlier studies estimated a similar prevalence of about 1% for childhood coinfections with HIV and Cryptococcus (1, 22). However, an 8-year summary of cases in Chiang Mai, Thailand, conducted from 1994 to 2001, reported 707 hospitalized patients with HIV and cryptococcal meningitis, and 21 (2.97%) were children (34). Similarly, the incidence of cryptococcosis among Colombian children (≤16 years) with AIDS from 1997 to 2005 was 2.7% (40). Overall, most reported pediatric cases have involved older children, aged 6 to 12 years (1, 22).
Molecular-based methods, such as multilocus sequence typing (MLST) or detection of amplified fragment length polymorphisms (AFLPs), have enabled the identification and epidemiological tracking of etiological strains of Cryptococcus (53). In these studies, it is useful to determine the degree of relatedness among a sample of isolates (24). Genetic variation among the isolates provides useful data for comparing strains that may vary in virulence, drug resistance, and type of host response, as well as their ecology and geographic distribution (66). This information may provide insight into the evolution of a pathogen and the dynamics of its pathogenicity, as well as identify the source of the infection and indicate strategies to impede its spread or limit human exposure.
On the basis of MLST and AFLP genotyping, three distinct subpopulations or molecular types of serotype A have been identified, VNI, VNII, and VNB (5, 39, 50, 53). Isolates of the other variety, C. neoformans var. neoformans, possess serotype D and are identified as molecular type VNIV. In addition, AD hybrids cluster separately as molecular type VNIII (5, 39, 42, 50, 53). Most isolates are haploid, but diploid strains, typically AD hybrids, have also been isolated from patients and environmental samples (37, 67). Isolates of C. neoformans may possess either of two mating type alleles, but among the dominant variety of serotype A, the MATa mating type allele is extraordinarily rare everywhere except in sub-Saharan Africa (38). To characterize pediatric isolates of C. neoformans, we analyzed one isolate from each of 82 selected pediatric cases reported to the Group for Enteric, Respiratory, and Meningeal Disease Surveillance in South Africa (GERMS-SA) during 2005 and 2006 (18, 19). All the isolates were serotype A. MLST and other molecular markers were used to determine the sequence type (ST), mating type, and ploidy of each isolate. We also compared the results of analyses of the pediatric isolates with similar data from isolates from adults with cryptococcosis.
MATERIALS AND METHODS
Pediatric cases and isolates.
National, population-based surveillance for laboratory-confirmed cryptococcosis was initiated in South Africa on 1 January 2005. Cryptococcosis was confirmed at the participating laboratories by positive India ink stains, cryptococcal antigen tests, and/or cultures. Pediatric cases were defined as children <15 years of age who met the laboratory case definition. Clinical isolates and demographic/clinical data from cases were submitted to the National Institute for Communicable Diseases. We selected pediatric cases submitted from 1 January 2005 through 31 December 2006. During this 2-year period, 199 pediatric cases were detected through national surveillance. We were able to analyze only 82 strains (from 82 unique cases) that had remained viable following long-term storage at −70°C, and these are listed in Table 1 . In addition, DNA sequences of the following reference strains of C. neoformans were included in the molecular typing analyses: VNI reference strains H99 and WM148 (50); VNB strains bt63, bt85, bt88, and bt89 (38, 39); VNII reference strains WM626 (50), MMRL1246, MMRL1310, and MMRL1320 (Duke Medical Mycological Research Laboratory); and VNIV reference strain JEC21 (46). The mating type tester strains were H99 (MATα) and bt63 (MATa). For comparison with the isolates from pediatric patients, we also analyzed selected strains of C. neoformans that were submitted to GERMS-SA from 86 adult patients during the same surveillance period. As detailed below, both pediatric and adult isolates were analyzed for molecular type, mating type, and ploidy, but only the pediatric isolates were sequence typed. All strains were culture purified and grown for 2 to 3 days on yeast extract potato dextrose (YPD) agar prior to use.
TABLE 1.
Pediatric isolates of Cryptococcus neoformansa
| RSA strain no. | Province | Specimen | Patient characteristic |
Molecular type | ST | Ploidy | Mating type | |
|---|---|---|---|---|---|---|---|---|
| Gender | Age | |||||||
| 110 | MP | CSF | M | 6 yr | VNI | 8 | n | α |
| 164 | GA | CSF | F | 8 yr | VNI | 13 | n | α |
| 438 | EC | Blood | F | 8 yr | VNI | 2 | 2n | α/α |
| 491 | GA | Blood | M | 6 yr | VNI | 15 | n | α |
| 530 | NW | CSF | F | 11 yr | VNI | 12 | n | α |
| 617 | KZ | CSF | M | 5 yr | VNI | 8 | n | α |
| 984 | KZ | Blood | F | 9 yr | VNI | 9 | n | α |
| 1040 | EC | CSF | M | 10 yr | VNI | 2 | n | α |
| 1111 | GA | CSF | F | 11 yr | VNI | 9 | n | α |
| 1162 | KZ | CSF | F | 11 yr | VNI | 9 | n | α |
| 1171 | KZ | CSF | F | 4 yr | VNI | 16 | n | α |
| 1190 | KZ | Blood | F | 7 yr | VNI | 8 | n | α |
| 1195 | KZ | CSF | M | 9 yr | VNI | 8 | n | α |
| 1234 | MP | CSF | M | 3 yr | VNI | 2 | n | α |
| 1322 | EC | CSF | F | 13 yr | VNI | 8 | n | α |
| 1640 | MP | CSF | M | 5 mo | VNI | 4 | n | α |
| 1678 | FS | CSF | M | 12 yr | VNI | 1 | n | α |
| 1848 | GA | CSF | M | 8 yr | VNI | 2 | n | α |
| 1852 | WC | Blood | M | 5 yr | VNI | 2 | n | α |
| 1857 | NW | CSF | M | 2 days | VNI | 9 | n | α |
| 2278 | FS | CSF | M | 10 yr | VNI | 9 | n | α |
| 2390 | MP | CSF | F | 10 yr | VNI | 7 | n | α |
| 2449 | WC | CSF | M | 4 yr | VNI | 4 | n | α |
| 2516 | GA | CSF | F | 8 yr | VNI | 2 | n | α |
| 2634 | GA | CSF | F | 8 yr | VNI | 1 | n | α |
| 2663 | KZ | CSF | M | 6 yr | VNI | 8 | n | α |
| 2668 | KZ | CSF | M | 8 yr | VNI | 8 | n | α |
| 2792 | MP | CSF | F | 4 mo | VNI | 4 | n | α |
| 2806 | KZ | CSF | M | 6 yr | VNI | 8 | n | α |
| 2893B | GA | CSF | M | 2 yr | VNI | 2 | 2n | α/α |
| 2933 | KZ | Blood | F | 6 yr | VNI | 16 | n | α |
| 2941 | KZ | CSF | F | 9 yr | VNI | 5 | n | α |
| 2998 | ND | CSF | F | 10 yr | VNI | 9 | n | α |
| 3162 | MP | CSF | M | 9 yr | VNI | 4 | n | α |
| 3432 | GA | CSF | F | 5 mo | VNI | 17 | n | α |
| 3671 | KZ | CSF | M | 5 yr | VNI | 8 | n | α |
| 3689 | EC | CSF | M | 9 yr | VNI | 13 | 2n | α/α |
| 3741 | MP | CSF | F | 6 yr | VNI | 2 | n | α |
| 3765 | GA | CSF | M | 8 yr | VNI | 8 | n | α |
| 3906 | EC | CSF | M | 8 yr | VNI | 8 | n | α |
| 3934 | GA | CSF | M | 11 yr | VNI | 16 | n | α |
| 3944 | KZ | CSF | F | 7 yr | VNI | 6 | n | a |
| 4164 | KZ | CSF | M | 13 yr | VNI | 8 | n | α |
| 4270 | GA | Blood | M | 6 yr | VNI | 11 | n | α |
| 4573 | FS | CSF | F | 13 yr | VNI | 3 | n | α |
| 4579 | KZ | CSF | M | 11 yr | VNI | 8 | n | α |
| 4687 | MP | CSF | M | 11 yr | VNI | 17 | n | α |
| 4724 | NW | CSF | F | 12 yr | VNI | 12 | n | α |
| 4749 | ND | Blood | F | 7 yr | VNI | 9 | n | α |
| 4777 | KZ | CSF | F | 12 yr | VNI | 8 | n | α |
| 4836B | KZ | CSF | M | 28 mo | VNI | 17 | n | α |
| 4929 | KZ | Blood | F | 11 yr | VNI | 13 | n | α |
| 5093 | NW | CSF | F | 11 yr | VNI | 10 | n | a |
| 5163 | ND | CSF | M | 17 mo | VNI | 8 | n | α |
| 5184 | EC | CSF | M | 5 yr | VNI | 8 | n | α |
| 5257 | LP | CSF | M | 6 yr | VNI | 15 | n | α |
| 5556 | MP | CSF | F | 6 yr | VNI | 2 | n | α |
| 5858 | GA | Blood | M | 2 mo | VNI | 14 | 2n | a/a |
| 6030 | LP | CSF | M | 5 yr | VNI | 4 | 2n | α/α |
| 6105 | KZ | CSF | M | 7 yr | VNI | 8 | n | α |
| 6186 | KZ | CSF | M | 7 yr | VNI | 8 | n | α |
| 6306 | KZ | CSF | M | 10 yr | VNI | 9 | n | α |
| 6310 | KZ | CSF | M | 10 yr | VNI | 9 | n | α |
| 6328 | KZ | CSF | M | 7 yr | VNI | 8 | n | α |
| 6420 | EC | CSF | F | 8 yr | VNI | 1 | n | α |
| 6509 | KZ | CSF | M | 7 yr | VNI | 1 | n | α |
| 6656 | WC | CSF | F | 10 yr | VNI | 2 | 2n | α/α |
| 110 | MP | CSF | M | 6 yr | VNI | 8 | n | α |
| 107 | MP | CSF | M | 4 yr | VNB | 21 | n | α |
| 364 | LP | CSF | M | 10 yr | VNB | 18 | n | a |
| 576 | GA | Sputum | M | 11 yr | VNB | 21 | n | α |
| 703 | EC | CSF | F | 3 yr | VNB | 20 | n | α |
| 1949 | NW | CSF | F | 6 yr | VNB | 19 | n | α |
| 2980 | FS | CSF | F | 9 yr | VNB | 20 | n | α |
| 3894 | GA | Blood | F | 9 yr | VNB | 21 | n | α |
| 4905 | GA | CSF | F | 9 yr | VNB | 21 | n | α |
| 526 | KZ | CSF | M | 11 yr | VNII | 22 | n | α |
| 1052 | KZ | CSF | M | 11 yr | VNII | 22 | n | α |
| 1746 | KZ | CSF | F | 10 yr | VNII | 23 | n | α |
| 2651 | KZ | CSF | F | 9 yr | VNII | 22 | n | α |
| 2992B | KZ | CSF | F | 10 yr | VNII | 22 | n | α |
| 3976 | MP | CSF | F | 11 yr | VNII | 24 | n | α |
| 5272 | GA | CSF | M | 10 yr | VNIII | ND | 2n | Aa/Dα |
Data are for 82 isolates. Abbreviations: RSA, Republic of South Africa; M, male; F, female; CSF, cerebrospinal fluid; ND, not determined. Abbreviations for provinces: EC, Eastern Cape; FS, Free State; GA, Gauteng; KZ, KwaZulu-Natal; LP, Limpopo; MP, Mpumalanga; NW, North West; and WC, Western Cape.
DNA extraction, PCR, and sequencing.
Multilocus STs were determined using nuclear DNA sequence data from 10 previously described, independent loci and 1 new locus (see Table S1 in the supplemental material) (39). These loci include five of the seven MLST consensus markers (GPD1, LAC1, PLB1, SOD1, and IGS1) (49); the omitted loci (CAP59 and URA59) are more informative for typing the sibling species, Cryptococcus gattii. DNA was extracted from each strain and purified using a MasterPure yeast DNA purification kit (Epicentre Biotechnologies, Madison, WI), following the manufacturer's protocol. Each 25-μl PCR mixture contained 1× standard Taq reaction buffer, 2 mM MgCl2, 0.1 mM deoxynucleoside triphosphates, 1 μM each primer, and 1.25 units Taq polymerase (New England Biolabs, Inc., Ipswich, MA). Primer sequences and their respective annealing temperatures are listed in Table S1 in the supplemental material. All PCRs were run for 30 cycles using standard cycling times and temperatures for denaturation of DNA and extension of primers (39). Amplicons were treated enzymatically with exonuclease I and shrimp alkaline phosphatase (SAP; USB Corporation, Cleveland, OH). A 10 μl-portion of each PCR product was treated with 5 units exonuclease 1, 0.5 units SAP, and 2 μl SAP dilution buffer or sterilized distilled water, followed by gentle, thorough mixing by pipetting. Each reaction mixture was then incubated for 30 min at 37°C, followed by 15 min at 80°C. Purified amplicons were sequenced using the Sanger method with a BigDye Terminator (version 3.1) cycle sequencing kit (Applied Biosystems, Inc., Foster City, CA) and standard cycling parameters. Sequencing products were analyzed by capillary electrophoresis with an Applied Biosystems 3730xl DNA analyzer.
Contig assembly, alignment, and gene partitioning.
Sequences for each locus were assembled into contigs using the Sequencher (version 4.6) program (Gene Codes Corporation, Ann Arbor, MI), and the contigs were aligned with the MUSCLE (version 3.7) program (15). Using the MacClade (version 4.06) program (43), each alignment was visually inspected, manually edited, and partitioned into its respective exons and introns, and codon positions were calculated for each exon. One exception for partitioning was the IGS1 locus, which has no introns. Exon-intron partitioning was based on the genomic sequence of strain JEC21 (serotype D) because it is the most completely annotated C. neoformans genome in GenBank. Since our isolates were serotype A, the positions of each exon and intron for each locus in JEC21 were verified for the same regions in the genomic sequence of serotype A (strain H99). This confirmation was achieved by aligning the sequences of both serotypes and manually inspecting each partition at every locus. The models of nucleotide substitution that best fit the data were estimated for each locus using the MrModeltest (version 2.3) program (56); this information was required for the phylogenetic analyses (see below). Evolutionary models were estimated for exons and introns separately, as they are known to have different substitution properties (62).
Data analysis.
Bayesian inference analyses were conducted independently for each locus using MrBayes (version 3.1.2) software (58). Default prior settings and the models of nucleotide substitution estimated separately for introns and exons by the MrModeltest (version 2.3) program (56) were used for each Bayesian analysis that was run. By default in MrBayes, two independent metropolis-coupling Markov chain Monte Carlo (MCMC) analyses were run simultaneously for each locus. Run settings were 4.375 million generations, four chains, a burn-in of the first 4,375 generations, and a sampling frequency of every 250 generations. The 50% majority-rule consensus tree was estimated using the remaining 13,125 trees not discarded as burn-in.
To combine the data from all loci into a single analysis, we used the software program BUCKy (Bayesian untangling of concordance knots) to conduct a Bayesian concordance analysis (3). This method takes into account discordant gene tree topologies but makes no assumption about the source of discordance, such as recombination, lateral gene transfer, or incomplete lineage sorting. The primary concordance tree estimated by BUCKy represents the dominant evolutionary history of the strains. A BUCKy metropolis-coupled MCMC analysis was run for 1.1 million generations, with the initial 100,000 generations being discarded as burn-in. The analysis consisted of two independent four-chain runs at α (the α priori discordance factor) equal to 10,000 and no thinning of the samples.
Sequence typing.
Our sequence typing approach was based on the method of Maiden et al. (44). Contigs with identical sequences were sorted into groups using the Sequencher (version 4.6) program. Each group represented a unique allele and was assigned an arbitrary ST number. Isolates were scored on the basis of their grouping. The loci were processed in this way to determine the allelic profile of each isolate. The number of STs equaled the number of different allelic profiles among the isolates.
Ploidy.
Ploidy was determined for each isolate by measuring cellular DNA content, using a modified protocol for flow cytometry (36, 63). Control strains were the haploid H99 strain and our AD hybrid diploid strain RSA5272 (Table 1). Cells were grown overnight on YPD agar, washed in 1 ml phosphate-buffered saline buffer, and fixed in 70% ethanol overnight at 4°C. Fixed cells were washed with 1 ml NS buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA [pH 8.0], 1 mM MgCl2, 0.1 mM CaCl2, 0.01 mM ZnCl2) and then stained with propidium iodide (10 mg/ml) in a solution containing 180 μl NS buffer and 20 μl RNase A (1 mg/ml). Stained cells were incubated overnight at 4°C in the dark, and then a 50-μl portion was diluted with 2 ml 50 mM Tris-HCl for flow cytometry of 10,000 cells using the FL1 channel on a FACScan instrument (Becton Dickinson).
Mating type.
To identify the mating type allele(s) of each isolate, a PCR-based method that amplified a region of the STE gene in the mating type locus was used. Primers were both serotype (A or D) and mating type (MATα or MATa) specific, allowing each of the four serotype-mating type combinations to be identified (i.e., Aα, Aa, Dα, or Da) (55). The mating type of each isolate with the MATa allele was verified by coculturing with tester strains possessing either the MATα (strain H99) or MATa (strain bt63) allele. Controls consisted of the two tester strains cultured together. Fresh cells were streaked on V-8 solid medium, and plates were incubated in the dark at room temperature (30). Over a period of 1 to 3 weeks, plates were regularly checked for hyphal growth and the development of basidiospores, indicating fertile sexual reproduction.
Statistical tests.
Since nearly half (n = 38) of the 82 isolates possessed one of the three most prevalent STs, ST2 (n = 10), ST8 (n = 19), and ST9 (n = 9), we compared the STs with patient data. One testable trend was identified: the high frequency of male patients infected with ST8. Exact binomial and Yates' corrected chi-square (χ2) tests were conducted to assess any correlation between ST8 and the biased gender ratio observed (68). For the expected gender ratio, we used the known genders of all the pediatric cases in 2005 and 2006 (n = 122). As this sample comprised children who were immunocompromised, we also included the expected gender frequencies for healthy children of similar ages. For this parameter, we used the 2009 age-structure estimates for South African males and females 0 to 14 years of age, which took into account the impact of HIV/AIDS (7), and we tested whether each expected group (infected or healthy children) came from a population with a 1:1 gender ratio (68). Acceptance of the null hypothesis for both tests would confirm the expected frequency of a 1:1 gender ratio for testing the observed gender frequencies of ST8.
Nucleotide sequence accession numbers.
The DNA sequences at each locus for all strains have been deposited in the GenBank database under accession numbers FN996013 to FN996904.
RESULTS
Pediatric cases and isolates.
During 2005 and 2006, 10,991 cases of cryptococcosis were reported to GERMS-SA. Of the 9,952 (91%) cases with a known age, 199 (2%) were children. Isolates of C. neoformans were submitted from 123 (61%) of these pediatric patients from cerebrospinal fluid (n = 109), blood (n = 13), and the respiratory tract of 1 patient. There were 55 girls, 67 boys, and 1 child for whom the gender was unknown. The 82 viable isolates that we analyzed are shown in Table 1. The patients ranged in age from 2 days to 14 years, and their age distribution was as follows: <1 year, 4 cases; 1 to 3 years, 5 cases; 4 to 6 years, 20 cases; 7 to 9 years, 24 cases; 10 to 12 years, 26 cases; 13 or 14 years, 3 cases. For patients whose HIV status was recorded, 96% (43/45) were HIV infected. Each isolate represented a different patient. For comparison, we analyzed isolates of C. neoformans selected from the same surveillance period and geographic regions from 86 adult patients.
Molecular and sequence typing.
The molecular types and STs of the pediatric isolates are listed in Table 1. The number of alleles per locus ranged from 4 (URE1) to 15 (ISC1), yielding a total of 24 different STs (Table 2). Of the 24 STs, 17 belonged to the VNI molecular type, 4 were VNB, and 3 were VNII. For these isolates, the URE1 alleles were sufficient to identify the three molecular types (VNI, VNB, and VNII). When the pediatric sample was compared with a temporally and spatially similar sample of adult isolates, no significant differences in the percentages of molecular types, mating types, or diploids were observed (Table 3). Neither collection included an isolate of serotype D (VNIV) (i.e., C. neoformans var. neoformans).
TABLE 2.
Sequence types, allelic profiles, and molecular types based on the DNA sequence analyses of pediatric isolates of C. neoformans var. grubii from the Republic of South Africa (2005 and 2006)a
| Sequence type | No. of alleles per locus |
No. of isolates | Molecular type | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| URE1 | TOP1 | SOD1 | CAP10 | TEF1 | ISC1 | MPD1 | PLB1 | LAC1 | GPD1 | IGS1 | |||
| 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 3 | 4 | 1 | 1 | 4 | VNI |
| 2 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 3 | 2 | 1 | 1 | 10 | VNI |
| 3 | 1 | 1 | 1 | 1 | 7 | 2 | 1 | 1 | 11 | 5 | 1 | 1 | VNI |
| 4 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 3 | 8 | 1 | 1 | 5 | VNI |
| 5 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 3 | 10 | 1 | 1 | 1 | VNI |
| 6 | 1 | 1 | 1 | 2 | 1 | 10 | 1 | 1 | 4 | 8 | 1 | 1 | VNI |
| 7 | 1 | 1 | 1 | 2 | 3 | 13 | 1 | 1 | 4 | 6 | 1 | 1 | VNI |
| 8 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 19 | VNI |
| 9 | 1 | 3 | 1 | 1 | 3 | 4 | 1 | 2 | 3 | 2 | 1 | 9 | VNI |
| 10 | 1 | 4 | 1 | 1 | 3 | 10 | 1 | 10 | 2 | 8 | 1 | 1 | VNI |
| 11 | 1 | 4 | 1 | 1 | 4 | 1 | 5 | 2 | 1 | 1 | 8 | 1 | VNI |
| 12 | 1 | 4 | 1 | 1 | 7 | 2 | 1 | 1 | 4 | 5 | 6 | 2 | VNI |
| 13 | 1 | 4 | 1 | 1 | 8 | 2 | 1 | 6 | 1 | 6 | 1 | 3 | VNI |
| 14 | 1 | 4 | 1 | 2 | 11 | 11 | 1 | 8 | 1 | 1 | 5 | 1 | VNI |
| 15 | 1 | 4 | 4 | 1 | 4 | 1 | 1 | 2 | 1 | 7 | 5 | 2 | VNI |
| 16 | 1 | 5 | 1 | 1 | 3 | 1 | 4 | 2 | 1 | 1 | 2 | 3 | VNI |
| 17 | 1 | 5 | 1 | 1 | 4 | 7 | 1 | 2 | 7 | 1 | 1 | 3 | VNI |
| 18 | 2 | 1 | 6 | 6 | 9 | 8 | 6 | 7 | 9 | 3 | 9 | 1 | VNB |
| 19 | 2 | 1 | 7 | 5 | 9 | 12 | 2 | 7 | 5 | 3 | 10 | 1 | VNB |
| 20 | 2 | 6 | 1 | 5 | 5 | 9 | 2 | 4 | 5 | 3 | 3 | 2 | VNB |
| 21 | 2 | 6 | 3 | 4 | 5 | 6 | 2 | 4 | 5 | 3 | 3 | 4 | VNB |
| 22 | 3 | 7 | 2 | 3 | 6 | 5 | 3 | 5 | 6 | 4 | 4 | 4 | VNII |
| 23 | 3 | 7 | 2 | 7 | 6 | 15 | 3 | 5 | 6 | 4 | 7 | 1 | VNII |
| 24 | 4 | 8 | 5 | 3 | 10 | 14 | 3 | 9 | 6 | 9 | 11 | 1 | VNII |
| Total | 4 | 8 | 7 | 7 | 11 | 15 | 6 | 10 | 11 | 9 | 11 | 81 | |
Column totals equal the number of alleles per locus, and row totals equal the number of isolates assigned a given sequence type.
TABLE 3.
Comparison of molecular analyses of isolates of C. neoformans from pediatric and adult patients with cryptococcosis in the Republic of South Africaa
| Isolate | No. (%) of isolates by: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Serotype, molecular type |
MAT allele |
Ploidy |
|||||||
| A, VNI | A, VNB | A, VNII | AD, VNIII | D, VNIV | MATα | MATa | n | 2nb | |
| Pediatric (n = 82) | 67 (82) | 8 (10) | 6 (7) | 1 (1) | 0 | 77 (94) | 5 (6) | 75 (91) | 7 (9) |
| Adult (n = 86) | 68 (79) | 9 (10) | 6 (7) | 3 (4) | 0 | 82 (95) | 4 (5) | 75 (87) | 11 (13) |
Isolates and clinical data were obtained by GERMS-SA, NICD, in 2005 and 2006 (17, 18). Among children (≤14 years of age) and adults (>15 years of age) for whom HIV status was known, 96% (43/45) and 97% (73/75) were HIV infected, respectively.
Diploids included serotype A diploids (AA) and hybrids (AD).
For the Bayesian inference analyses, the models of nucleotide substitution were estimated separately for exons and introns at each locus, and the results are shown in Table S2 in the supplemental material. Table S3 in the supplemental material shows the regions included in the alignments for each locus, along with the results for the partitioning of the alignments into their respective exons and introns, including the total number of partitions for each locus. Figure S1 in the supplemental material is a representative Bayesian phylogeny of one locus, and Fig. 1 shows the concordance analysis of all the loci.
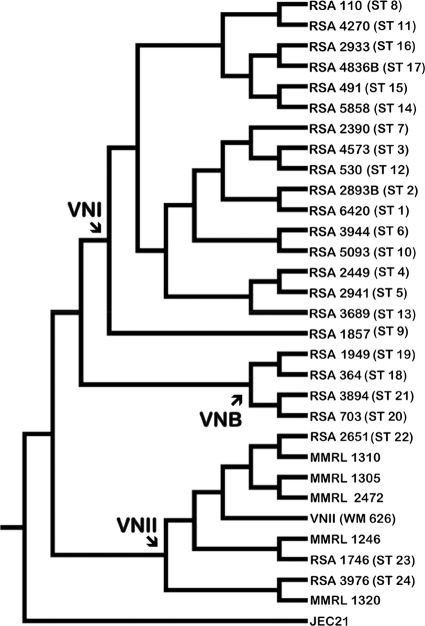
FIG. 1.
Pediatric isolates from the Republic of South Africa (RSA) were analyzed using Bayesian concordance analysis (3). The nuclear DNA sequence data were obtained from 10 unlinked loci: CAP10, GPD1, IGS1, ISC1, LAC1, MPD1, PLB1, SOD1, TEF1, and TOP1 (see Table S1 in the supplemental material). The 31 strains represented in this Bayesian primary concordance tree include one strain of each of the 24 pediatric sequence types and seven reference strains. The pediatric isolates are clustered in distinct clades that represent the three molecular types of serotype A (VNI, VNII, and VNB). The reference strains are the sequenced VNIV molecular type of serotype D (JEC21), which served as the outgroup, and reference strains for VNI (WM148) and VNII (WM626, MMRL2472, MMRL1246, MMRL1305, MMRL1310, and MMRL1320). The VNB genotypes were identified from separate Bayesian inference analyses (56) that included several VNB reference strains (bt63, bt85, bt88, and bt89).
Ploidy and mating types.
Seven of the 82 pediatric isolates were diploid; 6 were A/A diploids of VNI and 1 was an AD hybrid (Tables 1 and 3). All loci of the six homozygous diploids could be sequenced, and the sequences were all characteristic of serotype A isolates. Not surprisingly, only 3 of the 11 loci could be sequenced for the AD hybrid, and the sequenced loci were specific for either serotype A or D, depending on the locus. Thus, we assigned no ST to the hybrid, and this isolate is not included in Table 2. The overall percentages of diploids were similar in the pediatric and adult samples: 7% and 9%, respectively (Table 3). However, the STs of the six VNI diploids indicated a considerable bias, as three (50%) were strains of ST2 (Table 1).
The majority of pediatric isolates possessed the MATα allele (Table 3). The five isolates with the rare MATa allele included one AaAa diploid and the AaDα hybrid (Table 1). Except for the AD hybrid, all of the MATa strains mated with the tester strain possessing the MATα allele (H99), and they did not mate with the MATa tester strain (bt63).
Association of ST8 strains and pediatric males.
Because the two groups (HIV-infected versus HIV-uninfected children) that were tested to establish expected gender frequencies did not differ significantly from a 1:1 ratio, an equal gender ratio was used as the null hypothesis for testing the preponderance of male patients associated with ST8. The data in Table 1 indicate that most STs were equally distributed among the boys and girls. Excluding ST8, there were 48 infections with STs of VNI, 23 in boys and 25 in girls. Similarly, six boys and eight girls were infected with strains of VNB or VNII (i.e., ST18 to ST24). However, boys were significantly more likely to be infected with ST8 (VNI) than girls (16/19 [84%] versus 3/19 [16%]; P < 0.01).
Geographical distribution.
The 10 isolates of ST2 were comparably distributed in four South African provinces (see Table S4 in the supplemental material). However, 13 of 19 isolates of ST8 and 4 of 9 isolates of ST9 came from the province of KwaZulu-Natal (see Table S4 in the supplemental material).
DISCUSSION
From 2005 through 2008, preliminary surveillance data from the GERMS-SA program have suggested an increase in the incidence of cryptococcosis (18-20). This increase may reflect a rise in the number of HIV/AIDS cases, greater access to health care and diagnostic tests, and/or an increase in awareness and reporting. Notably, the expanding access to antiretroviral therapy has not reduced the incidence or mortality of cryptococcosis (25, 31, 32). Because the pathogenesis of cryptococcal disease may involve latent infections that were established as early as childhood, it is instructive to analyze isolates from pediatric cases.
Of all cases of C. neoformans reported to GERMS-SA in 2005 and 2006, 2% occurred among children. Although this proportion of pediatric cases is consistent with other reports, our sample of C. neoformans isolates from 82 pediatric patients is the largest published to date. These isolates are highly diverse, and they include the major haploid subpopulations of C. neoformans (VNI, VNB, and VNII), as well as a relatively high percentage (9%) of diploid isolates of VNI (Table 3). Multilocus analyses of the 75 haploid pediatric isolates identified 24 unique STs. The 67 VNI isolates were characterized by ST1 through ST17, the 8 VNB isolates had four different STs (ST18 to ST21), and the 6 VNII strains had three STs (ST22 to ST24). In this sample, the four alleles of the URE1 marker distinguished all three molecular types (Table 2).
One of the seven diploids was an AaDα hybrid, and the other six were serotype A and homozygous at the mating type locus. Five were AαAα strains, and one was AaAa. To the best of our knowledge, this is the first report of a clinical isolate with the AaAa genotype. Homozygous diploids of serotype A have been reported in other studies, and they most likely arose from same-sex mating (35, 36).
Six percent of the South African isolates possessed the MATa allele. An earlier report from neighboring Botswana found that approximately 10% of clinical isolates were also MATa (38). The presence of both mating types in this region should provide the opportunity for sexual reproduction. Indeed, the possibility of recombination was supported by analyses conducted with the software program Genetic Algorithm Recombination Detection (GARD) (29). Separate analyses of each alignment with the GARD program revealed evidence of recombination within one locus (SOD1) of the pediatric isolates (unpublished data). Whether a population is clonal, sexually reproductive, or both has important epidemiological implications because recombining pathogens are predictably more genetically diverse, evolve more quickly, and thus respond more rapidly to changing environments and growth conditions (52).
Previous epidemiological studies have noted that many mycotic diseases exhibit differences in prevalence that are associated with age, gender, or both. Overall, cryptococcosis, as well as the endemic mycoses (blastomycosis, coccidioidomycosis, histoplasmosis, and paracoccidioidomycosis), occurs more often among adult males; however, the prevalence of latent infections is comparable in both sexes (14, 33, 51). Surveys of delayed-type hypersensitivity and other immunological evidence have demonstrated that for endemic mycoses, the gender disparity is not due to sex-related differences in exposure or infection rates (51). It is more likely that hormonal differences in host defenses either render males more susceptible to disease or render females more resistant.
Cryptococcosis is similar, in that infection may or may not progress to disease. Data from the pre- and post-AIDS eras in developed countries indicate that approximately 60% to 85% of symptomatic cases of C. neoformans occurred among adult males (2, 6, 9, 10, 14, 16, 23, 40, 54). Most studies of C. neoformans in children involved small numbers of patients and reported a higher prevalence in males (>60%) (22) or a lack of gender bias (1, 13, 23, 34, 60). In the province of Gauteng from 2002 to 2004, the overall incidence rate for cryptococcosis in HIV-infected patients (>95% of whom were older than 15 years) was comparable for males and females (47). According to the GERMS-SA report for 2005 and 2006, when gender and age were known, women and children (<15 years) accounted for 56% and 2% of cryptococcal cases, respectively (21).
Of the 82 pediatric patients reported here, 55% were males, which was similar to the 49% previously reported for cryptococcosis in males in South Africa (48). The relatively equal percentages of boys and girls with cryptococcosis in South Africa may reflect the overall higher proportion of women with HIV/AIDS. In 2008, approximately one-third of the global population living with HIV were women, but in sub-Saharan Africa, it was estimated that about 60% of HIV-infected persons were women (65). In South Africa from 2005 to 2007, the estimates of the proportions of HIV-infected males and females who were 15 to 24 years of age were <5% and ca. 17%, respectively (64).
In contrast, 84% (16/19) of our pediatric patients infected with isolates of ST8 were boys. Assuming that the number of HIV-infected girls is equal to or larger than the population of boys at risk, this finding that the most prevalent sequence type (ST8) occurred more frequently in males suggests that the infecting strain has a significant impact on the development of cryptococcal disease. Sex-related differences in exposure to C. neoformans are unlikely to explain this result because the rate of cryptococcosis due to the other STs is similar for boys and girls. The allelic profile of ST8 reveals the presence of unique polymorphisms at two loci, and a third allele is found in only one other ST: in Table 2, the alleles of ST8 at TOP1, TEF1, and IGS1 are 2,2,2, and no other ST has this pattern at these three loci. Thus, it should be possible to use one or more of these markers to screen other isolates for this high-frequency ST in future molecular epidemiological studies. We plan to evaluate the relative virulence and other clinically relevant phenotypes of ST8 strains.
This investigation also confirmed the compelling observation that in South Africa as well as globally, the prevalence of cryptococcosis and HIV/AIDS is vastly lower among children than adults. For example, in Gauteng Province from 2002 to 2004, the rate of cryptococcosis per 100,000 cases with HIV infection was 2.5 times higher in adults than in children (48). However, most cases of cryptococcal disease manifest as meningitis, and the extent of subclinical infections among South African children is unknown. Antibodies to C. neoformans have been detected in healthy children and adults, a finding that documents the occurrence of latent, asymptomatic infections (8, 11, 17). The duration of latency from infection with C. neoformans to the manifestation of meningitis may be protracted and extend from childhood to adulthood in most patients, even those with HIV. Consequently, the few children who develop cryptococcal disease may represent the low end of the normal age distribution for cryptococcosis.
Once again, our results imply another explanation. The infecting strain may be as important as the age, gender, and immune status of the patient. We found no difference between children and adults in the prevalence of the major subpopulations of serotype A (i.e., VNI, VNII, and VNB) (Table 3). However, the more discriminating MLST analyses, which distinguish individual strains within these subpopulations, revealed that at least one dominant sequence type (ST8) was more prevalent in boys.
In summary, we analyzed clinical isolates of C. neoformans obtained in 2005 and 2006 from 82 pediatric patients in South Africa. These diverse isolates included representatives of the three major subpopulations of serotype A (molecular types VNI, VNII, and VNB) and a relatively high number of unique sequence types (n = 24), diploids, and isolates possessing the globally rare MATa mating type allele.
Supplementary Material
Acknowledgments
We thank Anastasia P. Litvintseva for strains, primers, protocols, advice, and encouragement and Edmund J. Byrnes III for technical advice regarding the protocol for determining ploidy by fluorescence-activated cell sorter analysis.
This project was supported by U.S. Public Health Service grant R01 AI 025783 from the National Institute of Allergy and Infectious Diseases. From 2005 through 2006, the GERMS-SA surveillance program was partially funded by the U.S. Agency for International Development's Antimicrobial Resistance Initiative, transferred via a cooperative agreement between the National Institute for Communicable Diseases and the U.S. Centers for Disease Control and Prevention (U60/CCU022088). Surveillance was also partially supported by the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention (NCHHSTP) and the Global AIDS Program (GAP), transferred via an NICD-CDC cooperative agreement (U62/PSO022901).
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC.
Members of the Group for Enteric, Respiratory, and Meningeal Disease Surveillance in South Africa, 2005 and 2006: Sandeep Vasaikar (Eastern Cape); Peter Smith, Nolan Janse van Rensburg, Andre Moller, and Anne-Marie Pretorius (Free State); Pyu-Pyu Sein, Anwar Hoosen, Ruth Lekalakala, Donald Ngwira, Olga Perovic, Charles Feldman, Alan Karstaedt, Mike Dove, Kathy Lindeque, Linda Meyer, Jeannette Wadula, Khatija Ahmed, and Gerhard Weldhagen (Gauteng); Trusha Vanmali, Wim Sturm, Prathna Bhola, Prashini Moodley, Sharona Seetharam, Sindisiwe Sithole, and Halima Dawood (KwaZulu-Natal); Stan Harvey and Pieter Jooste (Northern Cape); Danie Cilliers (North West); Ken Hamese (Limpopo); Keith Bauer, Greta Hoyland, Charles Mutanda, and Jacob Lebudi (Mpumalanga); Andrew Whitelaw, Rena Hoffman, Lynne Liebowitz, and Elizabeth Wasserman (Western Cape); Adrian Brink (Ampath Laboratories); Claire Heney (Lancet Laboratories); Marthinus Senekal (PathCare); Anne Schuchat and Stephanie Schrag (CDC); and Keith Klugman, Anne von Gottberg, Linda de Gouveia, Karen Keddy, John Frean, Vanessa Quan, Cheryl Cohen, Elizabeth Prentice, Kerrigan McCarthy, Arvinda Sooka, Leigh Dini, Susan Gould, Jay Patel, Susan Meiring, Mireille Cheyip, and Nelesh P. Govender (NICD).
Footnotes
Published ahead of print on 27 October 2010.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Abadi, J., S. Nachman, A. B. Kressel, and L.-A. Pirofski. 1999. Cryptococcosis in children with AIDS. Clin. Infect. Dis. 28:309-313. [DOI] [PubMed] [Google Scholar]
- 2.Amornkul, P. N., D. J. Hu, S. Tansuphaswadikul, S. Lee, B. Eampokalap, S. Likanonsakul, R. Nelson, N. L. Young, R. A. Hajjeh, K. Limpakarnjanarat, and T. D. Mastro. 2003. Human immunodeficiency virus type 1 subtype and other factors associated with extrapulmonary cryptococcosis among patients in Thailand with AIDS. AIDS Res. Hum. Retroviruses 19:85-90. [DOI] [PubMed] [Google Scholar]
- 3.Ané, C., B. R. Larget, D. A. Baum, S. D. Smith, and A. Rokas. 2007. Bayesian estimation of concordance among gene trees. Mol. Biol. Evol. 24:412-426. [DOI] [PubMed] [Google Scholar]
- 4.Becquet, R., and M. L. Newell. 2007. Prevention of postnatal HIV infection: infant feeding and antiretroviral interventions. Curr. Opin. HIV AIDS 2:361-366. [DOI] [PubMed] [Google Scholar]
- 5.Bovers, M., F. Hagen, E. E. Kuramae, and T. Boekhout. 2008. Six monophyletic lineages identified within Cryptococcus neoformans and Cryptococcus gattii by multi-locus sequence typing. Fungal Genet. Biol. 45:400-421. [DOI] [PubMed] [Google Scholar]
- 6.Casadevall, A., and J. R. Perfect. 1999. Cryptococcus neoformans. ASM Press, Washington, DC.
- 7.Central Intelligence Agency. 2009. The WorldFact Book. Central Intelligence Agency, Washington, DC. https://www.cia.gov/library/publications/the-world-factbook/geos/sf.html.
- 8.Chen, L.-C., D. L. Goldman, T. L. Doering, L.-A. Pirofski, and A. Casadevall. 1999. Antibody response to Cryptococcus neoformans proteins in rodents and humans. Infect. Immun. 67:2218-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng, Y. C., J. F. Ling, F.-C. Chang, S. J. Wang, J. L. Fuh, S. S. Chen, M. M. Teng, and C. Y. Chang. 2003. Radiological manifestations of cryptococcal infection in central nervous system. J. Chin. Med. Assoc. 66:19-26. [PubMed] [Google Scholar]
- 10.Chottanapund, S., P. Singhasivanon, J. Kaewkungwal, K. Chamroonswasdi, and W. Manosuthi. 2007. Survival time of HIV-infected patients with cryptococcal meningitis. J. Med. Assoc. Thai. 90:2104-2111. [PubMed] [Google Scholar]
- 11.Davis, J., W. Y. Zheng, A. Glatman-Freedman, J. A. Ng, M. R. Pagcatipunan, H. Lessin, A. Casadevall, and D. L. Goldman. 2007. Serologic evidence for regional differences in pediatric cryptococcal infection. Pediatr. Infect. Dis. J. 26:549-551. [DOI] [PubMed] [Google Scholar]
- 12.Dore, G. J., Y. Li, A. McDonald, and J. M. Kaldor. 2001. Spectrum of AIDS-defining illnesses in Australia, 1992 to 1998: influence of country/region of birth. J. Acquir. Immune Defic. Syndr. 26:283-290. [DOI] [PubMed] [Google Scholar]
- 13.Dromer, F., S. Mathoulin, B. Dupont, and A. Laporte. 1996. Epidemiology of cryptococcosis in France: a 9-year survey (1985-1993). French Cryptococcosis Study Group. Clin. Infect. Dis. 23:82-90. [DOI] [PubMed] [Google Scholar]
- 14.Dromer, F., S. Mathoulin-Pélissier, O. Launay, and O. Lortholary. 2007. Determinants of disease presentation and outcome during cryptococcosis: the cryptoA/D study. PLoS Med. 4:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edgar, R. C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman, G. D., F. W. Jeffrey, N. V. Udaltsova, and L. B. Hurley. 2005. Cryptococcosis: the 1981-2000 epidemic. Mycoses 48:122-125. [DOI] [PubMed] [Google Scholar]
- 17.Goldman, D. L., H. Khine, J. Abadi, D. J. Lindenberg, L.-A. Pirofski, R. Niang, and A. Casadevall. 2001. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 107:e66. [DOI] [PubMed] [Google Scholar]
- 18.Govender, N. P., and C. Cohen. 2007. GERMS-SA (Group for Enteric, Respiratory and Meningeal Disease Surveillance in South Africa). 2006. Annual report 2006. National Institute for Communicable Diseases, Johannesburg, South Africa.
- 19.Govender, N. P., and C. Cohen. 2007. GERMS-SA (Group for Enteric, Respiratory and Meningeal Disease Surveillance in South Africa). 2007. Annual report 2007, p. 1-26. National Institute for Communicable Diseases, Johannesburg, South Africa.
- 20.Govender, N. P., and C. Cohen. 2008. GERMS-SA (Group for Enteric, Respiratory and Meningeal Disease Surveillance in South Africa). 2008. Annual report 2008. National Institute for Communicable Diseases, Johannesburg, South Africa.
- 21.Govender, N. P., C. Cohen, S. Meiring, V. Quan, J. Patel, H. Dawood, A. S. Karstaedt, Y. M. Coovadia, A. Hoosen, O. Perovic, and K. M. McCarthy. 2008. Surveillance for cryptococcosis in South Africa, 2005-2007, p. 101, abstr. no. P-B-32. 7th Int. Conf. Cryptococcus Cryptococcosis.
- 22.Gumbo, T., G. Kadzirange, J. Mielke, I. T. Gangaidzo, and J. G. Hakim. 2002. Cryptococcus neoformans meningoencephalitis in African children with acquired immunodeficiency syndrome. Pediatr. Infect. Dis. J. 21:54-56. [DOI] [PubMed] [Google Scholar]
- 23.Hajjeh, R. A., L. A. Conn, D. A. Stephens, W. S. Baughman, R. J. Hamill, E. A. Graviss, P. G. Pappas, C. Thomas, A. L. Reingold, G. A. Rothrock, L. C. Hutwagner, A. Schuchat, M. E. Brandt, R. W. Pinner, and Cryptococcal Disease Active Surveillance Group. 1999. Cryptococcosis: population-based multistate active surveillance and risk factors in human immunodeficiency virus-infected persons. J. Infect. Dis. 179:449-454. [DOI] [PubMed] [Google Scholar]
- 24.Hall, B. G., and M. Barlow. 2006. Phylogenetic analysis as a tool in molecular epidemiology of infectious diseases. Ann. Epidemiol. 16:157-169. [DOI] [PubMed] [Google Scholar]
- 25.Jarvis, J. N., A. Boulle, A. Loyse, T. Bicanic, K. Rebe, A. Williams, T. S. Harrison, and G. A. Meintjes. 2009. High ongoing burden of cryptococcal disease in Africa despite antiretroviral roll out. AIDS 23:1182-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarvis, J. N., T. S. Harrison, E. L. Corbett, R. Wood, and S. D. Lawn. 2010. Is HIV-associated tuberculosis a risk factor for the development of cryptococcal disease? AIDS 24:612-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kambugu, A., D. B. Meya, J. Rhein, M. O'Brien, E. N. Janoff, A. R. Ronald, M. R. Kamya, H. Mayanja-Kizza, M. A. Sande, P. R. Bohjanen, and D. R. Boulware. 2008. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin. Infect. Dis. 46:1694-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan, J. E., D. Hanson, M. S. Dworkin, T. Frederick, J. Bertolli, M. L. Lindegren, S. Holmberg, and J. L. Jones. 2000. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin. Infect. Dis. 30(Suppl. 1):S5-S14. [DOI] [PubMed] [Google Scholar]
- 29.Kosakovsky Pond, S. L., D. Posada, M. B. Gravenor, C. H. Woelk, and S. D. Frost. 2006. Automated phylogenetic detection of recombination using a genetic algorithm. Mol. Biol. Evol. 23:1891-1901. [DOI] [PubMed] [Google Scholar]
- 30.Kwon-Chung, K. J., J. E. Bennett, and J. C. Rhodes. 1982. Taxonomic studies on Filobasidiella species and their anamorphs. Antonie Van Leeuwenhoek 48:25-38. [DOI] [PubMed] [Google Scholar]
- 31.Lawn, S. D., A. D. Harries, X. Anglaret, L. Myer, and R. Wood. 2008. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS 22:1897-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawn, S. D., A. D. Harries, and R. Wood. 2010. Strategies to reduce early morbidity and mortality in adults receiving antiretroviral therapy in resource-limited settings. Curr. Opin. HIV AIDS 5:18-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leal, A. L., J. Faganello, A. M. Fuentefria, J. T. Boldo, M. C. Bassanesi, and M. H. Vainstein. 2008. Epidemiological profile of cryptococcal meningitis patients in Rio Grande do Sul, Brazil. Mycopathology 166:71-75. [DOI] [PubMed] [Google Scholar]
- 34.Likasitwattanakul, S., B. Poneprasert, and V. Sirisanthana. 2004. Cryptococcosis in HIV-infected children. Southeast Asian J. Trop. Med. Public Health 35:935-939. [PubMed] [Google Scholar]
- 35.Lin, X., C. M. Hull, and J. Heitman. 2005. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature 434:1017-1021. [DOI] [PubMed] [Google Scholar]
- 36.Lin, X., S. Patel, A. P. Litvintseva, A. Floyd, R. Hicks, T. G. Mitchell, and J. Heitman. 2009. Diploids in the Cryptococcus neoformans serotype A population homozygous for the α mating type originate via unisexual mating. PLoS Pathog. 5:e1000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Litvintseva, A. P., L. Kestenbaum, R. J. Vilgalys, and T. G. Mitchell. 2005. Comparative analysis of environmental and clinical populations of Cryptococcus neoformans. J. Clin. Microbiol. 43:556-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Litvintseva, A. P., R. E. Marra, K. Nielsen, J. Heitman, R. J. Vilgalys, and T. G. Mitchell. 2003. Evidence of sexual recombination among Cryptococcus neoformans serotype A isolates in sub-Saharan Africa. Eukaryot. Cell 2:1162-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Litvintseva, A. P., R. Thakur, R. J. Vilgalys, and T. G. Mitchell. 2006. Multilocus sequence typing reveals three genetic subpopulations of Cryptococcus neoformans var. grubii (serotype A), including a unique population in Botswana. Genetics 172:2223-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lizarazo, J., M. Linares, C. de Bedout, A. Restrepo, C. I. Agudelo, and E. Castañeda. 2007. Results of nine years of the clinical and epidemiological survey on cryptococcosis in Colombia, 1997-2005. Biomédica 27:94-109. [PubMed] [Google Scholar]
- 41.Lortholary, O., G. Poizat, V. Zeller, S. Neuville, A. Boibieux, M. Alvarez, P. Dellamonica, F. Botterel, F. Dromer, and G. Chene. 2006. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS 20:2183-2191. [DOI] [PubMed] [Google Scholar]
- 42.Ma, H., and R. C. May. 2009. Virulence in Cryptococcus species. Adv. Appl. Microbiol. 67:131-190. [DOI] [PubMed] [Google Scholar]
- 43.Maddison, D. R., and W. P. Maddison. 2005. MacClade: analysis of phylogeny and character evolution (4.06). Sinauer Associates, Sunderland, MA.
- 44.Maiden, M. C. J., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. U. S. A. 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manfredi, R., L. Calza, and F. Chiodo. 2003. AIDS-associated Cryptococcus infection before and after the highly active antiretroviral therapy era: emerging management problems. Int. J. Antimicrob. Agents 22:449-452. [DOI] [PubMed] [Google Scholar]
- 46.Marra, R. E., J. C. Huang, E. Fung, K. Nielsen, J. Heitman, R. J. Vilgalys, and T. G. Mitchell. 2004. A genetic linkage map of Cryptococcus neoformans variety neoformans serotype D (Filobasidiella neoformans). Genetics 167:619-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCarthy, K. M., C. Cohen, H. Schneider, S. M. Gould, M. E. Brandt, and R. A. Hajjeh. 2008. Cryptococcosis in Gauteng: implications for monitoring of HIV treatment programmes. S. Afr. Med. J. 98:452-454. [PubMed] [Google Scholar]
- 48.McCarthy, K. M., J. Morgan, K. A. Wannemuehler, S. A. Mirza, S. M. Gould, N. Mhlongo, P. Moeng, B. R. Maloba, H. H. Crewe-Brown, M. E. Brandt, and R. A. Hajjeh. 2006. Population-based surveillance for cryptococcosis in an antiretroviral-naive South African province with a high HIV seroprevalence. AIDS 20:2199-2206. [DOI] [PubMed] [Google Scholar]
- 49.Meyer, W., D. M. Aanensen, T. Boekhout, M. Cogliati, M. R. Diaz, M. C. Esposto, M. C. Fisher, F. Gilgado, F. Hagen, S. Kaocharoen, A. P. Litvintseva, T. G. Mitchell, S. P. Simwami, L. Trilles, M. A. Viviani, and K. J. Kwon-Chung. 2009. Consensus multi-locus sequence typing scheme for Cryptococcus neoformans and Cryptococcus gattii. Med. Mycol. 47:561-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meyer, W., A. Castañeda, S. Jackson, M. Huynh, and E. Castañeda. 2003. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg. Infect. Dis. 9:189-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitchell, T. G. 2004. Systemic fungi, p. 2363-2381. In J. Cohen and W. G. Powderly (ed.), Infectious diseases, 2nd ed. Mosby, London, United Kingdom.
- 52.Mitchell, T. G. 2010. Population genetics of pathogenic fungi in humans and other animals, p. 139-158. In J. Xu (ed.), Microbial population genetics. Horizon Scientific Press, Hethersett, United Kingdom.
- 53.Mitchell, T. G., and A. P. Litvintseva. 2010. Typing species of Cryptococcus and epidemiology of cryptococcosis, p. 167-190. In H. R. Ashbee and E. M. Bignell (ed.), Pathogenic yeasts. Springer-Verlag, Berlin, Germany.
- 54.Mora, D. J., A. L. Pedrosa, V. Rodrigues, C. M. L. Maffei, L. Trilles, M. S. Lazéra, and M. L. Silva-Vergara. 2010. Genotype and mating type distribution within clinical Cryptococcus neoformans and Cryptococcus gattii isolates from patients with cryptococcal meningitis in Uberaba, Minas Gerais, Brazil. Med. Mycol. 48:561-569. [DOI] [PubMed] [Google Scholar]
- 55.Nielsen, K., G. M. Cox, P. Wang, D. L. Toffaletti, J. R. Perfect, and J. Heitman. 2003. The sexual cycle of Cryptococcus neoformans variety grubii and virulence of congenic a and α isolates. Infect. Immun. 71:4831-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nylander, J. A. A. 2004. MrModeltest. Program distributed by the author. Evolutionary Biology Centre, Uppsala University, Uppsala, Sweden.
- 57.Park, B. J., K. A. Wannemuehler, B. J. Marston, N. P. Govender, P. G. Pappas, and T. M. Chiller. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525-530. [DOI] [PubMed] [Google Scholar]
- 58.Ronquist, F., and J. P. Huelsenbeck. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572-1574. [DOI] [PubMed] [Google Scholar]
- 59.Schutte, C. M., C. H. van der Meyden, and D. S. Magazi. 2000. The impact of HIV on meningitis as seen at a South African Academic Hospital (1994 to 1998). Infection 28:3-7. [DOI] [PubMed] [Google Scholar]
- 60.Severo, C. B., M. O. Xavier, A. F. Gazzoni, and L. C. Severo. 2009. Cryptococcosis in children. Paediatr. Respir. Rev. 10:166-171. [DOI] [PubMed] [Google Scholar]
- 61.Sloan, D., S. Dlamini, N. Paul, and M. Dedicoat. 2008. Treatment of acute cryptococcal meningitis in HIV infected adults, with an emphasis on resource-limited settings. Cochrane Database Syst. Rev. CD005647. [DOI] [PubMed]
- 62.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: Molecular Evolutionary Genetic Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka, R., H. Taguchi, K. Takeo, M. Miyaji, and K. Nishimura. 1996. Determination of ploidy in Cryptococcus neoformans by flow cytometry. J. Med. Vet. Mycol. 34:299-301. [PubMed] [Google Scholar]
- 64.UNAIDS. 2008. Report on the global AIDS epidemic 2008, p. 1-362. World Health Organization, Geneva, Switzerland.
- 65.UNAIDS. 2009. AIDS epidemic update: December, 2009, p. 1-100. World Health Organization, Geneva, Switzerland.
- 66.van Belkum, A., M. Struelens, A. de Visser, H. A. Verbrugh, and M. Tibayrenc. 2001. Role of genomic typing in taxonomy, evolutionary genetics, and microbial epidemiology. Clin. Microbiol. Rev. 14:547-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Viviani, M. A., M. Cogliati, M. C. Esposto, K. Lemmer, K. Tintelnot, M. F. Valiente, D. Swinne, A. Velegraki, and R. Velho. 2006. Molecular analysis of 311 Cryptococcus neoformans isolates from a 30-month ECMM survey of cryptococcosis in Europe. FEMS Yeast Res. 6:614-619. [DOI] [PubMed] [Google Scholar]
- 68.Zar, J. H. 2010. Biostatistical analysis. Pearson, Upper Saddle River, NJ.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.