Abstract
An epidemic of infections by rapidly growing mycobacteria related to surgical procedures between 2004 and 2008 in Brazil was caused by a unique strain showing the Mycobacterium abscessus type 2 pattern when it was analyzed by the molecular method of PCR-restriction enzyme analysis of the hsp65 gene (PRA-hsp65). In order to investigate the diversity of M. abscessus type 2 clinical isolates and to assess whether this epidemic strain was present in specimens from nonsurgical patients, we studied 52 isolates from 38 patients showing this characteristic PRA-hsp65 pattern obtained between 2005 and 2009. All isolates were identified by sequencing of region V of the rpoB gene and typed by pulsed-field gel electrophoresis (PFGE) using two restriction enzymes, DraI and AseI. Seven isolates obtained from sputum, bronchoalveolar lavage fluid, and urine in three different Brazilian states showed rpoB sequences 100% similar to the rpoB sequence of epidemic strain INCQS 594 and PFGE patterns highly related to the patterns of isolates, evidencing the presence of the epidemic strain in isolates from patients not associated with the surgical epidemic. The remaining isolates showed diverse rpoB sequences, with the highest similarities being to the corresponding sequences of M. massilienseT CIP 108297 (21 isolates), M. bolletiiT CIP 108541 (19 isolates), or M. abscessusT ATCC 19977 (5 isolates). Two additional clusters could be detected by PFGE. PFGE showed 100% typeability and reproducibility and discriminatory powers, calculated by Simpson's index of diversity, of 0.978 (DraI) and 0.986 (AseI), confirming its suitability for the discrimination of M. abscessus type 2 isolates.
Outbreaks of infections caused by rapidly growing mycobacteria (RGM) related to surgical and cosmetic procedures have concerned Brazilian health care authorities since 2003 (5, 12, 19, 22-24, 28). More than 2,000 notifications received between 2004 and 2008 by the Brazilian National Health Surveillance Agency (ANVISA) reported cases of RGM surgical-site infections after laparoscopic, arthroscopic, or plastic surgeries (4, 19). Most isolates recovered from surgical patients studied so far showed three common features: an uncommon pattern when they were submitted to PCR-restriction enzyme analysis of the hsp65 gene (PRA-hsp65), called Mycobacterium abscessus type 2 by Devallois et al. (10) and others (19); two characteristic substitutions [C(2683)T and T(2874)C] in the sequence of region V of the rpoB gene (2, 19, 28); and two highly similar patterns with one band difference when they were typed by pulsed-field gel electrophoresis (PFGE) using DraI and AseI restriction enzymes (5, 12, 19, 28). The Brazilian National Institute for Health Quality Control (INCQS) deposited one of these isolates under number INCQS 594.
This PRA-hsp65 pattern was ascribed to two recently described members of the Mycobacterium chelonae-M. abscessus group: Mycobacterium massiliense and Mycobacterium bolletii (1, 3). Extensive phenotypic and genotypic analysis of members of the M. chelonae-M. abscessus group has recently demonstrated that M. massiliense and M. bolletii cannot be separated from M. abscessus, supporting the proposition that the three taxa represent a single species, namely, M. abscessus. Two subspecies, “M. abscessus subsp. abscessus” (which includes former M. abscessus isolates) and “M. abscessus subsp. massiliense” (which includes isolates identified as M. massiliense and M. bolletii) (18), were also proposed. Eventually, the name M. abscessus subsp. massiliense was corrected to M. abscessus subsp. bolletii, in accordance with the rules of the International Code of Nomenclature of Bacteria (1990 revision) (11, 16, 17a). According to this new arrangement, the strain associated with most surgical outbreak cases in different states in Brazil, identified here as the epidemic strain, is ascribed to M. abscessus subsp. bolletii.
Few publications have described clinical isolates in Brazil showing the M. abscessus type 2 PRA-hsp65 pattern, and the real incidence of these infections is not known (27). In two separate studies, Da Silva Rocha et al. (8, 9) analyzed 43 and 356 clinical isolates using PRA-hsp65 and found 1 and 8 isolates showing the M. abscessus type 2 pattern, respectively. Suffys et al. (25) detected this PRA-hsp65 pattern in 8 of 110 clinical isolates. Chimara et al. (6) analyzed 439 nontuberculous mycobacterial (NTM) isolates received in a reference laboratory between 2000 and 2001 and found only two isolates with the M. abscessus type 2 pattern. None of these isolates was analyzed by rpoB sequencing or PFGE.
The aim of this study was to investigate the diversity of clinical isolates showing the M. abscessus type 2 pattern and to estimate the presence of the epidemic strain among clinical isolates not recognized to be epidemiologically related to outbreaks. This information can add to the current knowledge about the possible source(s) of these surgical infections in Brazil. In addition, we evaluated the efficiency of PFGE for the discrimination of M. abscessus type 2 isolates.
MATERIALS AND METHODS
Mycobacterial isolates.
The study included clinical mycobacterial isolates received for identification between 2005 and 2009 at the Universidade Federal do Rio de Janeiro, Rio de Janeiro, Rio de Janeiro State (RJ), Brazil, and at the Instituto Adolfo Lutz, Sao Paulo, São Paulo State (SP), Brazil (Table 1). The inclusion criteria for this study were that isolates showed the M. abscessus type 2 PRA-hsp65 pattern and were not received in the laboratories as isolates from surgical-site infections. Isolates with other PRA-hsp65 patterns were excluded from this study. Clinical and epidemiological data for the patients were obtained from laboratory records or from notifications received by local surveillance services as part of routine monitoring and were analyzed anonymously.
TABLE 1.
Rapidly growing mycobacterial clinical isolates presenting the M. abscessus type 2 pattern included in this study
| Patient | Isolatea | No. of isolates per patientb | Yr of isolation | Stated |
|---|---|---|---|---|
| 1 | IAL 001 | 1 | 2005 | CE |
| 2 | IAL 002 | 1 | 2005 | CE |
| 3 | IAL 003 | 1 | 2005 | CE |
| 4 | IAL 004 | 1 | 2006 | CE |
| 5 | IAL 005 | 1 | 2006 | CE |
| 6 | IAL 006 | 1 | 2006 | CE |
| 7 | IAL 007 | 1 | 2008 | DF |
| 8 | IAL 008 | 1 | 2007 | MS |
| 9 | IAL 009 | 1 | 2006 | MS |
| 10 | IAL 010 | 1 | 2007 | MT |
| 11 | IAL 011 | 1 | 2007 | PI |
| 12 | IAL 012 | 1 | 2005 | SP |
| 13 | IAL 013 | 1 | 2005 | SP |
| 14 | IAL 014 | 1 | 2006 | SP |
| 15 | IAL 015 | 1 | 2006 | SP |
| 16 | IAL 016 | 1 | 2007 | SP |
| 17 | IAL 017 | 1 | 2007 | SP |
| 18 | IAL 018 | 1 | 2007 | SP |
| 19 | IAL 019 | 1 | 2007 | SP |
| 20 | IAL 020c | 1 | 2006 | SP |
| 21 | IAL 021 | 3 | 2006 | SP |
| 22 | IAL 024 | 4 | 2005, 2006, 2007 | SP |
| 23 | IAL 028 | 1 | 2008 | SP |
| 24 | IAL 029 | 5 | 2005, 2006, 2007 | SP |
| 25 | CRM 375 | 3 | 2008 | RJ |
| 26 | CRM 409 | 2 | 2008 | RJ |
| 27 | CRM 473 | 2 | 2009 | RJ |
| 28 | CRM 512 | 2 | 2009 | RJ |
| 29 | CRM 573 | 1 | 2009 | RJ |
| 30 | CRM 642 | 1 | 2009 | RJ |
| 31 | CRM 270c | 1 | 2007 | RJ |
| 32 | CRM 273c | 1 | 2007 | RJ |
| 33 | CRM 622c | 1 | 2009 | RJ |
| 34 | CRM 552c | 1 | 2009 | RJ |
| 35 | IAL 034c | 1 | 2006 | SP |
| 36 | IAL 035 | 1 | 2007 | SP |
| 37 | IAL 036c | 1 | 2006 | CE |
| 38 | IAL 037 | 1 | 2006 | SP |
Sources: IAL 001 to CRM 273, sputum; CRM 622 to IAL 034, bronchoalveolar lavage fluid; IAL 035, blood; IAL 036, urine; IAL 037, parotid nodule.
Data for only one isolate per patient are shown.
Isolates showing rpoB sequences 100% similar to the sequence of the epidemic strain and PFGE patterns highly similar to those of isolates of the epidemic strain.
Abbreviations for Brazilian states: CE, Ceará; DF, Distrito Federal; MS, Mato Grosso do Sul; MT, Mato Grosso; PI, Piauí; RJ, Rio de Janeiro; SP, São Paulo.
M. abscessusT ATCC 19977, M. chelonaeT ATCC 35752, M. immunogenumT ATCC 700505, M. massilienseT CCUG 48898, M. bolletiiT CCUG 50184, two outbreak isolates (INCQS 594 and B52) obtained from surgical patients, and one unrelated isolate from a postinjection abscess (B67) previously analyzed by our group during the outbreak of surgical-site infections in Belém, Pará (PA), Brazil, were included for comparison (28).
Isolates were cultivated in Middlebrook 7H9 liquid medium (Becton Dickinson and Company, Sparks, MD) or Middlebrook 7H10 agar (Becton Dickinson and Company) plates supplemented with oleic acid, albumin, dextrose, and catalase (OADC; Becton, Dickinson and Company) at 37°C. Frozen stocks were stored in 7H9 liquid medium-OADC and 15% glycerol at −80°C.
Species identification.
Species identification was confirmed by sequencing of region V of the rpoB gene, as described by Adékambi et al. (2). Other conserved regions in the mycobacterium genome, such as the hsp65, gyrA, and gyrB genes and the 16S-23S internal transcribed sequence (ITS), were sequenced to confirm the identification of isolates showing discordant results by PRA-hsp65 and rpoB sequencing.
Sequence similarity analysis.
Sequences were analyzed for their similarity with sequences in the GenBank database using the Basic Local Alignment Tool (BLAST; http://www.ncbi.nlm.nih.gov/BLAST).
The alignment of the rpoB gene sequences was performed using ClustalX (version 2.0) software (17). Phylogenetic trees were constructed using M. tuberculosis strain H37Rv (ATCC 27294) as an outgroup. The evolutionary history was generated using the neighbor-joining method. The evolutionary distances were computed using the Kimura two-parameter model and bootstrap analysis with 1,000 replications. Phylogenetic analysis was performed using MEGA4 software (26).
PFGE.
Isolates were typed by PFGE, as described by Leao et al. (19). Briefly, single colonies were grown at 37°C in Mueller-Hinton broth supplemented with 0.1% Tween 80 until an optical density of 0.64 at 650 nm was reached. Plugs of bacterial cells were prepared in 1% low-melt preparative-grade agarose (Bio-Rad Laboratories Inc., Hercules, CA). DNA was isolated from the plug molds and digested with 30 U DraI (Promega, Madison, WI) or AseI (Fermentas, Vilnius, Lithuania) at 37°C overnight. Plugs were loaded into 1% pulsed-field-certified agarose (Bio-Rad) in 0.5% TBE buffer (45 mM Tris-HCl, 45 mM boric acid, 1 mM EDTA), and electrophoresis was carried out in a CHEF-DR III system (Bio-Rad) at 14°C for 21 h at 6 V/cm with a switch time of 1.6 to 21.3 s. Bacteriophage lambda ladder PFG Marker (New England Biolabs, Ipswich, MA) was used as a molecular size standard. PFGE gel images were analyzed with the BioNumerics (version 5.1) program (Applied Maths, Sint-Martens-Latem, Belgium). The band-based Dice unweighted-pair group method using average linkages was used to prepare dendrograms of PFGE profiles, based on 2% optimization and position tolerance.
The efficiency of the PFGE typing method for M. abscessus type 2 isolates was estimated by the quantification of typeability, reproducibility, and discriminatory power, evaluated by calculating the numerical index of discrimination (D), based on Simpson's index of diversity, as previously described (14).
Nucleotide sequence accession numbers.
The rpoB, hsp65, gyrA, gyrB, and 16S-23S ITS sequences of the isolates included in this study were deposited in the GenBank database under accession numbers FJ859893, FJ859894, and HQ404261 to HQ404300.
RESULTS
Patients' data.
Among the 38 patients included in this study, 32 (84.2%) had isolates obtained from sputum (Table 1). Twenty-one of these 32 patients had suggestive clinical and/or radiological findings and more than two sputum isolates, meeting the diagnostic criteria for NTM lung disease according to the official ATS/IDSA statement (13). Not all isolates from these patients were included in this study, because some were retrieved outside the study period and others were processed in local laboratories. Eleven patients had only one sputum sample, but three were previously treated for pulmonary tuberculosis and were again under investigation for lung disease, suggesting probable disease. Among the 32 patients with sputum isolates, previous tuberculosis treatment was documented in 12 and cystic fibrosis was documented in 3. The mean age of these patients was 51 ± 22.5 years, and the median age was 52 years. Eighteen patients were male and 14 were female. For the remaining six patients, local surveillance services were not notified. Three isolates were obtained with the use of bronchoscopes. Two isolates were obtained from sterile sites (blood and a parotid nodule biopsy specimen) and one was obtained from urine from a symptomatic patient, with a total of 10 urine samples being positive for NTM and no other causative agent being identified, suggesting that they might be clinically relevant, indicating probable disease.
Species identification.
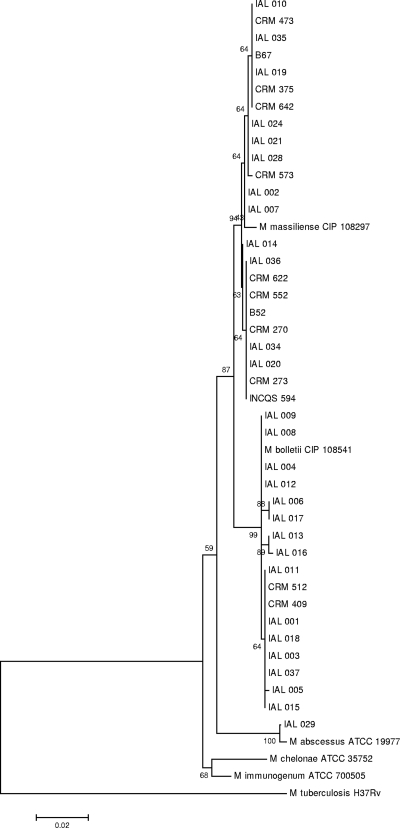
Fifty-two isolates from 38 patients fulfilled the inclusion criteria and were subjected to rpoB gene sequencing. Seven patients (patients 21, 22, 24, 25, 26, 27, and 28 in Table 1) had more than one isolate studied, and in all cases, the rpoB sequences from the same patient showed 100% similarity. BLAST analysis revealed that the rpoB sequences from seven patients' isolates (IAL 020, CRM 270, CRM 273, CRM 622, CRM 552, IAL 034, and IAL36) were 100% similar to the corresponding sequence of the epidemic strain, INCQS 594 (GenBank accession number EU117207). The rpoB sequences of 20 individual isolates and from the 3 isolates from PA used for comparison showed the highest similarities to the rpoB sequence of M. massiliense CIP 108297 (GenBank accession number AY593981.2). The sequences of 17 isolates showed the highest similarity to the rpoB sequence of M. bolletii CIP 108541 (accession number AY859692), and the sequences of the isolates from one patient (patient 24) showed the highest similarity to the rpoB sequence of M. abscessus ATCC 19977 (GenBank accession number AY147164) The close relationship found in comparing the rpoB nucleotide sequences is shown in a phylogenetic tree (Fig. 1).
FIG. 1.
Neighbor-joining tree based on rpoB gene sequences. Relationships between 46 taxa, including one isolate from each of the 38 patients from this study, three isolates from PA (INCQS 594, B52, and B67), and the type strains of M. abscessus, M. chelonae, M. immunogenum, M. massiliense, and M. bolletii are shown. M. tuberculosis H37Rv was included as an outgroup.
The results of PRA-hsp65 and rpoB gene sequencing of the five independent isolates from patient 24 (Table 1) were incongruent. The isolates showed the M. abscessus type 2 PRA-hsp65 pattern, which would be consistent with the identification of M. abscessus subsp. bolletii, but the rpoB sequences showed the highest similarity (99.71%) with the corresponding sequence of the M. abscessus ATCC 19977 type strain. Additional sequence analysis of the hsp65, gyrA, and gyrB genes and 16S-23S ITS confirmed the identification of these isolates as M. abscessus subsp. bolletii (data not shown).
Molecular typing.
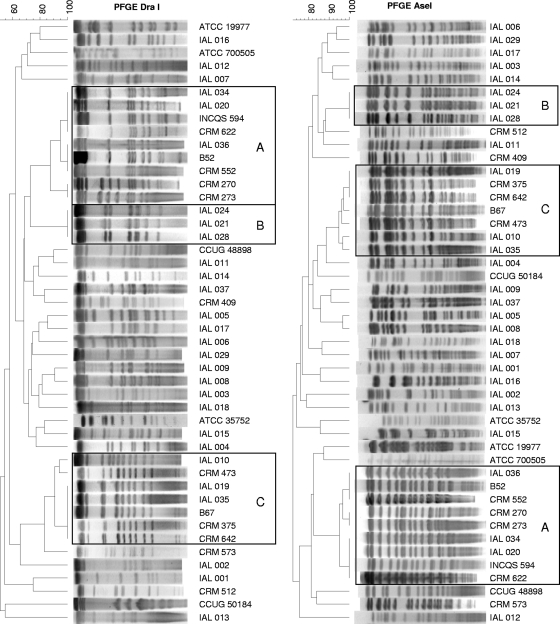
All patterns generated by PFGE with DraI and AseI endonucleases were interpretable, resulting in 100% typeability. Dendrograms obtained with DraI and AseI restriction enzymes generated similar clustering patterns (Fig. 2).
FIG. 2.
Dendrograms of DraI and AseI PFGE patterns of single isolates from 38 patients, M. abscessusT ATCC 19977, M. chelonaeT ATCC 35752, M. immunogenumT ATCC 700505, M. massilienseT CCUG 48898, M. bolletiiT CCUG 50184, and three previously studied isolates, INCQS 594, B52, and B67 (19, 28). Dendrograms were prepared using the BioNumerics (version 5.1) program by the Dice unweighted-pair group method with arithmetic means, based on 2% optimization and position tolerance. Isolates with highly similar (up to three band differences) PFGE patterns are boxed. Boxes A, isolates showing high degrees of similarity to surgical epidemic isolates; boxes B, indistinguishable patterns for isolates from three patients from different cities in SP; boxes C, isolates highly similar to a postinjection isolate from PA. Numbers at the upper left are percent similarity.
The seven isolates whose rpoB sequences were 100% similar to the corresponding sequence of the epidemic strain showed PFGE patterns that grouped with two highly related DraI and AseI PFGE patterns previously observed with the surgical outbreak isolates, which differ by only one fragment of approximately 50 kb (represented by isolates INCQS 594 and B52 in Fig. 2, box A) (12, 28). The PFGE patterns of two isolates (IAL 036, CRM 552) were 100% similar to those of outbreak isolate B52, the PFGE patterns of three isolates (IAL 020, IAL 034, CRM 622) were 100% similar to those of isolate INCQS 594, and two isolates (CRM 270, CRM 273) showed patterns highly similar (91.45% similarity in DraI and AseI PFGE cluster analyses) to the PFGE patterns of the epidemic strain (Fig. 2, boxes A).
Patients from three different cities in SP (Presidente Prudente, São Paulo, and Jundiai) were infected by the same strain. The isolates showed identical rpoB sequences and PFGE patterns (Fig. 1 and Fig. 2, boxes B).
The PFGE patterns of the isolates collected from sputum or blood from six patients (IAL 010, IAL 019, IAL 035, CRM 473, CRM 375, and CRM 642) in states distant geographically (SP, Mato Grosso [MT], and RJ) grouped with isolate B67, the only postinjection abscess isolate from our previous study with isolates from PA (28). Their PFGE patterns were closely related (90.89% similarity with DraI and 94.78% with AseI) (Fig. 2, boxes C). Moreover, the rpoB sequences of these isolates were identical (Fig. 1).
Between two and five independent clinical isolates were obtained from seven patients. In each case, rpoB sequences and PFGE patterns were maintained in independent isolates from each patient (Fig. 3). Exceptions were one additional band in the AseI PFGE pattern of isolate IAL 025 compared to the patterns of isolates IAL 024, IAL 026, and IAL 027 from the same patient and a one-band polymorphism in the DraI PFGE patterns in two isolates (CRM 409 and CRM 416) from patient 26 (Fig. 3). These results were confirmed in two different experiments.
FIG. 3.
Reproducibility of PFGE patterns among multiple isolates from seven patients. Between two and five isolates from each patient were analyzed by PFGE with DraI and AseI. The patterns obtained for isolates from the same patient were indistinguishable, except for the two polymorphisms indicated by arrows.
The remaining isolates showed diverse PFGE patterns, pointing to the existence of a high degree of diversity in M. abscessus subsp. bolletii isolates. This diversity was congruent with the distinct rpoB sequences (sequevars).
The efficiency of the PFGE method for discrimination of M. abscessus type 2 isolates was evaluated by comparison of the patterns for 41 isolates, consisting of 1 isolate from each of the 38 patients, the postinjection abscess isolate from PA, M. massiliense CCUG 48898, and M. bolletii CCUG 50184. No epidemiological correlation was detected among the patients. Both the typeability and reproducibility of PFGE were 100%. In the evaluation of the discriminatory power of PFGE, 31 and 33 different PFGE banding profiles were discriminated by Dice analysis after digestion with DraI and AseI, respectively, and the calculated D values were 0.978 for DraI and 0.986 for AseI.
DISCUSSION
Fifty-two M. abscessus type 2 isolates from 38 patients presumably not related to the Brazilian surgical outbreak that occurred between 2004 and 2008 were analyzed. Identification results obtained with PRA-hsp65 and rpoB sequencing were congruent for most (90.4%) isolates. Discordant results were observed with five isolates from the same patient, and additional sequencing analysis allowed their classification as M. abscessus subsp. bolletii, in agreement with the PRA-hsp65 pattern of M. abscessus type 2. A similar finding was already reported in a previous study with a sputum isolate from PA (19). Divergent identification obtained with rpoB and hsp65 analysis has been observed by other authors. Kim et al. (15) identified two isolates as M. massiliense by rpoB sequence analysis and M. abscessus by hsp65 sequence analysis. These authors used additional sodA and 16S-23S ITS sequencing analysis to confirm that both strains were M. massiliense. Zelazny et al. (29) sequenced the hsp65, secA, and 16S-23S ITS regions and identified 5 out of 42 clinical isolates as M. massiliense, while rpoB identification resulted in identification as M. abscessus. Macheras et al. (21) obtained presumptive identifications for 13 of 15 isolates with discordant identifications by analysis of eight different housekeeping genes. These authors questioned the definition of species on the basis of a few gene sequences and disputed the suitability of classifying M. abscessus, M. bolletii, and M. massiliense as three distinct species. Recently, Leao et al. (18) demonstrated that M. bolletii and M. massiliense cannot be separated from M. abscessus on the basis of DNA-DNA hybridization and an extensive analysis of phenotypic and genotypic characteristics, which is consistent with all these findings.
Another aspect that was examined in the present study was the maintenance of the rpoB sequences and PFGE patterns in isolates from the same patient collected at different times. The stability of these markers during the period of this study was confirmed, and only minor polymorphisms in PFGE patterns were observed after analysis of independent isolates from the same patient (Fig. 3). PFGE has been important in confirming the clonality of the epidemic strain in earlier studies, but the discriminatory power of PFGE for mycobacteria showing the M. abscessus type 2 PRA-hsp65 pattern has not been evaluated before (5, 12, 19, 28). This study allowed the calculation of the discriminatory power of PFGE using two different enzymes, DraI and AseI. The calculated D value for DraI PFGE was 0.978, and for AseI PFGE it was 0.986. In a previous study, a discriminatory index of 0.972 was obtained for PFGE using DraI-digested DNA of isolates showing the M. abscessus type 1 PRA-hsp65 pattern (23). According to Hunter and Gaston (14), an index higher than 0.90, associated with high typeability and reproducibility, would be desirable for a typing method. Using these criteria, we have confirmed that PFGE, using DraI or AseI, is a suitable typing method for the discrimination of isolates belonging to both proposed subspecies of M. abscessus.
PFGE helped to uncover the epidemic strain in isolates from other clinical sources in three different states: Ceara (CE), RJ, and SP. Three isolates (CRM 552, CRM 622, IAL 034), two from RJ and one from SP, were obtained from bronchoalveolar lavage fluid specimens. No specific investigation to isolate mycobacteria from bronchoscopes was carried out, especially because the procedure consisted of a routine evaluation for diagnosis which did not include surveillance of medical devices. No additional cases were reported in the same settings. One isolate (IAL 020) was obtained from a single sputum specimen in a city from SP in 2006, where just one case of surgical-site infection related to the outbreak was reported 2 years later. One isolate was obtained from urine during diagnostic investigation of renal tuberculosis in a state in northeast Brazil (CE) with only one reported case of surgical-site infection. The epidemic strain was also detected in a sputum specimen from PA in previous studies from our group (7, 19).
The DraI and AseI PFGE patterns of two sputum isolates (CRM 270 and CRM 273) were highly similar (91.45%) to those of the epidemic strain, and the rpoB sequences were 100% similar. Both patients had confirmed pulmonary disease and lived in RJ, where more than 1,000 possible surgical-site infection cases were reported to ANVISA (12), but these isolates harbor distinct biological properties, such as clarithromycin resistance and susceptibility to glutaraldehyde at <1.5%, while the epidemic strain is susceptible to clarithromycin and tolerant of glutaraldehyde. Therefore, they may be considered a different strain or a biological variant of the epidemic strain (12, 20).
The finding of two additional clusters (Fig. 2, boxes B and C) of isolates from different geographic regions and sources, grouped by PFGE and rpoB sequencing, demonstrates that some strains predominate and are widely distributed. This could point to the existence of virulence factors or other properties that render these particular strains more pathogenic for humans and more adapted to diverse environmental conditions. These strains, as well as the epidemic strain, should be monitored to map their spread and quickly detect new outbreaks.
Mycobacteria can be quickly identified in the clinical laboratory by the PRA-hsp65 method (6), which is also useful for separation of the two proposed subspecies of M. abscessus, M. abscessus subsp. abscessus, which shows the M. abscessus type 1 pattern, and M. abscessus subsp. bolletii, which shows the M. abscessus type 2 pattern (17a).
In this study, M. abscessus type 2 isolates were considered clinically significant in 30 out of 38 patients, 21 of whom had confirmed NTM lung disease and 9 had probable disease. Previous pulmonary tuberculosis treatment was a frequent finding, but additional studies are necessary to confirm if tuberculosis can be considered a predisposing factor for pulmonary disease caused by M. abscessus type 2 or if these patients had undetected NTM lung disease from the beginning. The identification of the epidemic strain that caused surgical infections in isolates obtained with the use of bronchoscopes points to the possible dissemination of this particular strain by endoscopy equipment, which has never been reported.
Acknowledgments
This study received financial support from the Foundation for Research Support of the State of São Paulo (FAPESP; process no. 06/01533-9) and the National Council for Scientific and Technological Development (CNPq; Universal 475238/2008-7). C.K.M. is the recipient of a fellowship from FAPESP (process no. 08/01451-8).
We acknowledge Creusa Lima Campelo (CE); Celso R. Amaral and Eduardo Alexandrino, Sérvulo de Medeiros (SP); Ana Lucia Viana Atta Sarmento, and Francisco Duarte (Distrito Federal); Eunice Atsuko Totumi Cunha (Mato Grosso do Sul); and Franko de Arruda e Silva (MT) for providing clinical and epidemiological information. Finally, we thank A. Leyva for his help with English editing of the manuscript.
Footnotes
Published ahead of print on 17 November 2010.
REFERENCES
- 1.Adekambi, T., P. Berger, D. Raoult, and M. Drancourt. 2006. rpoB gene sequence-based characterization of emerging non-tuberculous mycobacteria with descriptions of Mycobacterium bolletii sp. nov., Mycobacterium phocaicum sp. nov. and Mycobacterium aubagnense sp. nov. Int. J. Syst. Evol. Microbiol. 56:133-143. [DOI] [PubMed] [Google Scholar]
- 2.Adékambi, T., P. Colson, and M. Drancourt. 2003. rpoB-based identification of nonpigmented and late-pigmenting rapidly growing mycobacteria. J. Clin. Microbiol. 41:5699-5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adekambi, T., M. Reynaud-Gaubert, G. Greub, M. J. Gevaudan, B. La Scola, D. Raoult, and M. Drancourt. 2004. Amoebal coculture of “Mycobacterium massiliense” sp. nov. from the sputum of a patient with hemoptoic pneumonia. J. Clin. Microbiol. 42:5493-5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ANVISA. 2009, posting date. Casos de infeccao por micobacterias nao tuberculosas notificados (Notified cases of nontuberculous mycobacterial infection). ANVISA, Brasilia, DF, Brazil. (In Portuguese.) http://www.anvisa.gov.br/hotsite/hotsite_micobacteria/notificados.pdf.
- 5.Cardoso, A. M., E. Martins de Sousa, C. Viana-Niero, F. Bonfim de Bortoli, Z. C. Pereira das Neves, S. C. Leão, A. P. Junqueira-Kipnis, and A. Kipnis. 2008. Emergence of nosocomial Mycobacterium massiliense infection in Goias, Brazil. Microbes Infect. 10:1552-1557. [DOI] [PubMed] [Google Scholar]
- 6.Chimara, E., L. Ferrazoli, S. Y. Ueky, M. C. Martins, A. M. Durham, R. D. Arbeit, and S. C. Leão. 2008. Reliable identification of mycobacterial species by PCR-restriction enzyme analysis (PRA)-hsp65 in a reference laboratory and elaboration of a sequence-based extended algorithm of PRA-hsp65 patterns. BMC Microbiol. 8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.da Costa, A. R., M. L. Lopes, S. C. Leão, M. P. Schneider, M. S. de Sousa, P. N. Suffys, T. C. Corvelo, and K. V. Lima. 2009. Molecular identification of rapidly growing mycobacteria isolates from pulmonary specimens of patients in the state of Para, Amazon region, Brazil. Diagn. Microbiol. Infect. Dis. 65:358-364. [DOI] [PubMed] [Google Scholar]
- 8.da Silva Rocha, A., C. da Costa Leite, H. M. Torres, A. B. de Miranda, M. Q. Pires Lopes, W. M. Degrave, and P. N. Suffys. 1999. Use of PCR-restriction fragment length polymorphism analysis of the hsp65 gene for rapid identification of mycobacteria in Brazil. J. Microbiol. Methods 37:223-229. [DOI] [PubMed] [Google Scholar]
- 9.da Silva Rocha, A., A. M. Werneck Barreto, C. E. Dias Campos, M. Villas-Boas da Silva, L. Fonseca, M. H. Saad, W. M. Degrave, and P. N. Suffys. 2002. Novel allelic variants of mycobacteria isolated in Brazil as determined by PCR-restriction enzyme analysis of hsp65. J. Clin. Microbiol. 40:4191-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devallois, A., K. S. Goh, and N. Rastogi. 1997. Rapid identification of mycobacteria to species level by PCR-restriction fragment length polymorphism analysis of the hsp65 gene and proposition of an algorithm to differentiate 34 mycobacterial species. J. Clin. Microbiol. 35:2969-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Vos, P., and H. G. Trüper. 2000. Judicial Commission of the International Committee on Systematic Bacteriology. IXth International (IUMS) Congress of Bacteriology and Applied Microbiology. Minutes of the meetings, 14, 15 and 18 August 1999, Sydney, Australia. Int. J. Syst. Evol. Microbiol. 50:2239-2244. [Google Scholar]
- 12.Duarte, R. S., M. C. Lourenço, L. S. Fonseca, S. C. Leão, E. L. Amorim, I. L. Rocha, F. S. Coelho, C. Viana-Niero, K. M. Gomes, M. G. da Silva, N. S. Lorena, M. B. Pitombo, R. M. Ferreira, M. H. Garcia, G. P. de Oliveira, O. Lupi, B. R. Vilaça, L. R. Serradas, A. Chebabo, E. A. Marques, L. M. Teixeira, M. Dalcolmo, S. G. Senna, and J. L. Sampaio. 2009. Epidemic of postsurgical infections caused by Mycobacterium massiliense. J. Clin. Microbiol. 47:2149-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffith, D. E., T. Aksamit, B. A. Brown-Elliott, A. Catanzaro, C. Daley, F. Gordin, S. M. Holland, R. Horsburgh, G. Huitt, M. F. Iademarco, M. Iseman, K. Olivier, S. Ruoss, C. F. von Reyn, R. J. Wallace, Jr., and K. Winthrop. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175:367-416. [DOI] [PubMed] [Google Scholar]
- 14.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, H. Y., Y. Kook, Y. J. Yun, C. G. Park, N. Y. Lee, T. S. Shim, B. J. Kim, and Y. H. Kook. 2008. Proportions of Mycobacterium massiliense and Mycobacterium bolletii strains among Korean Mycobacterium chelonae-Mycobacterium abscessus group isolates. J. Clin. Microbiol. 46:3384-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lapage, S. P., P. H. A. Sneath, E. F. Lessel, V. B. D. Skerman, H. P. R. Seeliger, and W. A. Clark. 1992. International code of nomenclature of bacteria (1990 revision). American Society for Microbiology, Washington, DC. [PubMed]
- 17.Larkin, M. A., G. Blackshields, N. P. Brown, R. Chenna, P. A. McGettigan, H. McWilliam, F. Valentin, I. M. Wallace, A. Wilm, R. Lopez, J. D. Thompson, T. J. Gibson, and D. G. Higgins. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947-2948. [DOI] [PubMed] [Google Scholar]
- 17a.Leão, S. C., E. Tortoli, J. P. Euzéby, and M. J. Garcia. 19 November 2010, posting date. Proposal that the two species Mycobacterium massiliense and Mycobacterium bolletii be reclassified as Mycobacterium abscessus subsp. bolletii comb. nov., designation of Mycobacterium abscessus subsp. abscessus subsp. nov., and emendation of Mycobacterium abscessus. Int. J. Syst. Evol. Microbiol. [Epub ahead of print.] [DOI] [PubMed]
- 18.Leão, S. C., E. Tortoli, C. Viana-Niero, S. Y. Ueki, K. V. Lima, M. L. Lopes, J. Yubero, M. C. Menendez, and M. J. Garcia. 2009. Characterization of mycobacteria from a major Brazilian outbreak suggests that revision of the taxonomic status of members of the Mycobacterium chelonae-M. abscessus group is needed. J. Clin. Microbiol. 47:2691-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leão, S. C., C. Viana-Niero, C. K. Matsumoto, K. V. Lima, M. L. Lopes, M. Palaci, D. J. Hadad, S. Vinhas, R. S. Duarte, M. C. Lourenco, A. Kipnis, Z. C. das Neves, B. M. Gabardo, M. O. Ribeiro, L. Baethgen, D. B. de Assis, G. Madalosso, E. Chimara, and M. P. Dalcolmo. 2010. Epidemic of surgical-site infections by a single clone of rapidly growing mycobacteria in Brazil. Future Microbiol. 5:971-980. [DOI] [PubMed] [Google Scholar]
- 20.Lorena, N. S., M. B. Pitombo, P. B. Cortes, M. C. Maya, M. G. da Silva, A. C. Carvalho, F. S. Coelho, N. H. Miyazaki, E. A. Marques, A. Chebabo, A. D. Freitas, O. Lupi, and R. S. Duarte. 2010. Mycobacterium massiliense BRA100 strain recovered from postsurgical infections: resistance to high concentrations of glutaraldehyde and alternative solutions for high level disinfection. Acta Cir. Bras. 25:455-459. [DOI] [PubMed] [Google Scholar]
- 21.Macheras, E., A. L. Roux, F. Ripoll, V. Sivadon-Tardy, C. Gutierrez, J. L. Gaillard, and B. Heym. 2009. Inaccuracy of single-target sequencing for discriminating species of the Mycobacterium abscessus group. J. Clin. Microbiol. 47:2596-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padoveze, M. C., C. M. Fortaleza, M. P. Freire, D. Brandão de Assis, G. Madalosso, A. C. Pellini, M. L. Cesar, V. Pisani Neto, M. M. Beltramelli, E. Chimara, L. Ferrazoli, M. A. da Silva Telles, J. L. Sampaio, and S. C. Leão. 2007. Outbreak of surgical infection caused by non-tuberculous mycobacteria in breast implants in Brazil. J. Hosp. Infect. 67:161-167. [DOI] [PubMed] [Google Scholar]
- 23.Sampaio, J. L., C. Viana-Niero, D. de Freitas, A. L. Hofling-Lima, and S. C. Leão. 2006. Enterobacterial repetitive intergenic consensus PCR is a useful tool for typing Mycobacterium chelonae and Mycobacterium abscessus isolates. Diagn. Microbiol. Infect. Dis. 55:107-118. [DOI] [PubMed] [Google Scholar]
- 24.Sampaio, J. L. M., E. Chimara, L. Ferrazoli, M. A. da Silva Telles, V. M. Del Guercio, Z. V. Jerico, K. Miyashiro, C. M. Fortaleza, M. C. Padoveze, and S. C. Leão. 2006. Application of four molecular typing methods for analysis of Mycobacterium fortuitum group strains causing post-mammaplasty infections. Clin. Microbiol. Infect. 12:142-149. [DOI] [PubMed] [Google Scholar]
- 25.Suffys, P. N., A. da Silva Rocha, M. de Oliveira, C. E. Campos, A. M. Barreto, F. Portaels, L. Rigouts, G. Wouters, G. Jannes, G. van Reybroeck, W. Mijs, and B. Vanderborght. 2001. Rapid identification of mycobacteria to the species level using INNO-LiPA Mycobacteria, a reverse hybridization assay. J. Clin. Microbiol. 39:4477-4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 27.Ueki, S. Y. M., M. C. Martins, M. A. S. Telles, M. C. Virgilio, C. M. S. Giampaglia, E. Chimara, and L. Ferrazoli. 2005. Nontuberculous mycobacteria: species diversity in Sao Paulo state, Brazil. J. Bras. Patol. Med. Lab. 41:1-8. [Google Scholar]
- 28.Viana-Niero, C., K. V. Lima, M. L. Lopes, M. C. da Silva Rabello, L. R. Marsola, V. C. Brilhante, A. M. Durham, and S. C. Leão. 2008. Molecular characterization of Mycobacterium massiliense and Mycobacterium bolletii in isolates collected from outbreaks of infections after laparoscopic surgeries and cosmetic procedures. J. Clin. Microbiol. 46:850-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zelazny, A. M., J. M. Root, Y. R. Shea, R. E. Colombo, I. C. Shamputa, F. Stock, S. Conlan, S. McNulty, B. A. Brown-Elliott, R. J. Wallace, Jr., K. N. Olivier, S. M. Holland, and E. P. Sampaio. 2009. Cohort study of molecular identification and typing of Mycobacterium abscessus, Mycobacterium massiliense, and Mycobacterium bolletii. J. Clin. Microbiol. 47:1985-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]