Abstract
Dermatophilus congolensis, which affects animal species, is an uncommon human infection. Few cases, mainly in tropical areas, have been reported. We describe the first human infection in Spain in a traveler returning from Central America. Diagnosis of human infection may be underestimated in people in contact with animals.
CASE REPORT
In September 2009, a 26-year-old woman came to the Tropical Diseases Service, Hospital Carlos III, Madrid, Spain, with skin lesions on her right wrist and no other symptoms. Two months prior to her presentation, the patient spent 15 days in Costa Rica working as a volunteer on a dairy farm. She reported close contact with animals, including feeding and milking cows, as well as drinking raw milk. She did not report any other contact with livestock in Spain, either professionally or in leisure activities. The patient had been vaccinated against diphtheria and tetanus in 2003. In addition, she was vaccinated against hepatitis B (third dose), hepatitis A (first dose), typhoid fever, and rabies 2 weeks before her trip. She followed a correct malaria prophylaxis with chloroquine. No other previous medical history was of interest. Five days after her arrival in Costa Rica, she noticed a vesicular eruption over a scratched area on her right wrist that evolved to pustules and crust 4 days later. The eruption relapsed on several occasions and was painful and itchy. Neither fever nor lymph node swelling was present. She had begun self-treatment with topical gentamicin and corticosteroid ointment 1 month before medical consultation, with a mild improvement. The patient's physical examination revealed five erythematous, desquamative lesions of less than 0.5 cm in diameter and with elevated edges. Topical treatment was discontinued, and a sample for microbiological analysis was taken. One month later, the lesions had disappeared.
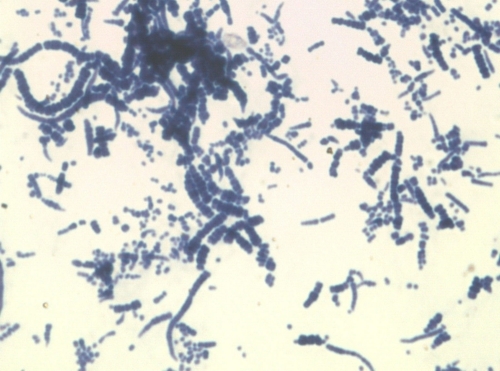
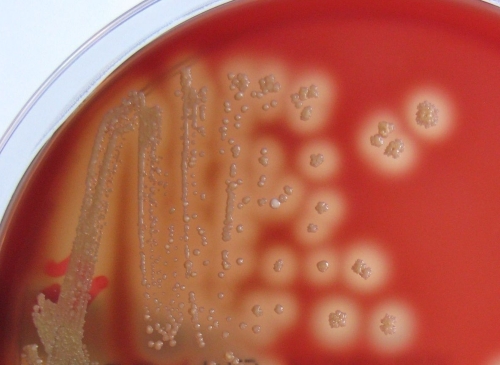
Swab samples were taken from the lesions and sent to our laboratory for bacterial and fungal culture analyses. They were directly inoculated in blood and chocolate agar, thioglycolate broth, Sabouraud chloramphenicol, and Sabouraud cycloheximide (Actidione)-chloramphenicol agar. Blood and chocolate agar and thioglycolate broth were incubated at 35°C in an aerobic atmosphere, the chocolate agar was incubated in air supplemented with 5% CO2, and the blood agar was also incubated in an anaerobic atmosphere. Sabouraud chloramphenicol and Sabouraud cycloheximide-chloramphenicol agar plates were incubated at 32°C. After 24 h, a pure culture of tiny, point-like, smooth, creamy white-colored, beta-hemolytic colonies adherent to the media grew in aerobic blood agar and chocolate agar. Gram staining showed hypha-like, branching filaments with “train track” form and clusters of sporangia as well as coccoid Gram-positive forms, mostly in chains (Fig. 1). After 48 h, crowded colonies became yellowish and mucoid, with a great variation in colonial morphology, e.g., pulvinate, umbonate, or cake crumb-like (Fig. 2). At that time, released sporangia were the main finding in the Gram stain. Colonies were reinoculated and incubated at 25°C, 35°C, and 42°C, but only growth at 35°C was successful. Fungal culture was negative after 28 days.
FIG. 1.
Gram stain with characteristic branching filaments with “train track” form or hypha-like chains that released sporangium Gram-positive cells (magnification, ×1,000).
FIG. 2.
Beta-hemolytic colonies after 2 days of incubation at 37°C on blood agar medium, with pleomorphic appearance in pulvinate, umbonate, or cake crumb-like form.
Biochemical tests were carried out for phenotypic identification (Table 1). The organism was presumptively identified as Dermatophilus congolensis. Definitive genotypic identification of the aerobic culture was made by sequential analysis of two overlapped fragments of a partial 16S rRNA gene of 660 and 817 bp. Fragments were amplified by PCR using specific primers for the Dermatophilus congolensis partial 16S rRNA gene after a primer BLAST search. Primers used were TGCCGTAAACGTTGGGCGCT and CGTGCAGTGGGTACGGGCAG as the forward primers for the 660-bp and 817-bp fragments, respectively, and TGTTACTTGATCCCCAATCGCCAGT as the reverse primer. The PCR was performed in a 50-μl volume with 5 μl of DNA, 1 μl 25 μM primers, 25 μl of PCR Master Mix 2× (PCR Master Mix, Promega, Madison, WI), and 18 μl of nuclease-free water, using the following cycling program: 10 min at 94°C, 40 cycles of 30 s at 94°C, 30 s at 60°C, and 1 min and 15 s at 72°C, and a final extension for 1 min at 72°C. Amplicons were sequenced using the dye Terminator FS rhodamine kit in a 3100 genetic analyzer (ABI). Sequence analysis was performed with the DNASTAR Lasergene 7.1 software suite for sequence analysis. The obtained sequences were 99.9% identical to the DMS 44180 type strain (GenBank accession no. AJ243918). The antimicrobial susceptibility test was performed by the disk diffusion method. The isolate was susceptible to penicillins (amoxicillin, amoxicillin-clavulanic acid, ampicillin, methicillin, oxacillin, and penicillin), cephalosporins (cefazolin, cefepime, cefotaxime, ceftriaxone, and cefuroxime), aminoglycosides (amikacin, gentamicin, and tobramycin), clindamycin, tetracycline, chloramphenicol, rifampin, cotrimoxazole, glycopeptides (vancomycin and teicoplanin), linezolid, imipenem, and macrolides (erythromycin). Antibiotic resistance was found only against quinolones (ciprofloxacin and levofloxacin).
TABLE 1.
Biochemical reactions
| Test | Test resulta |
|
|---|---|---|
| D. congolensis | Patient isolate | |
| Hemolysis | Beta in 3-7 days | Beta in 1-2 days |
| Growth at: | ||
| 25°C | − | − |
| 35°C | + | + |
| 42°C | − | − |
| Catalase | + | + |
| Urea, Christensen's | + | + |
| Oxidase | + | + |
| Nitrate reduction | − | − |
| Indole | − | − |
| Hydrolysis of: | ||
| Tyrosine | − | − |
| Xanthine | − | − |
| Esculin | − | − |
| Gelatin | + | + |
| Starch | + | + |
| Casein | + | + |
| Voges-Proskauer | − | − |
| Acid from: | ||
| Glucose | + | + |
| Fructose | + | + |
| Ribose | + | + |
| Galactose | + | + |
| Xylose | − | − |
| Mannitol | − | − |
| Lactose | − | − |
| Sucrose | − | − |
| Maltose | − | − |
Dermatophilus congolensis is an aerobic actinomycete (facultatively anaerobic) that usually affects animals, causing dermatophilosis. It is distributed worldwide, prevailing in tropical areas, and related to humid environments and other factors, such as poor veterinary services, coinfection with other bacteria, like Pseudomonas aeruginosa, immunosuppression status, and hygiene conditions, which favor its occurrence and spread (11). The first case was reported in Congo in 1915. Since then it has been isolated in animal infection mainly in Africa (Kenya, Ethiopia, Tanzania, Nigeria, South Africa), Asia (Turkey, India, China), and Central and South America (Argentina, Uruguay, Brazil) but also in Australia, the United States (New York, Kentucky, Florida, Texas), Canada, and Europe (France, Spain, Germany) as a chronic endemic disease and, more rarely, as an acute and epidemic infection (5, 10, 11). It is most commonly associated with goats, sheep, cows, and horses but also affects a worldwide variety of domestic and wild animals, such as cats, antelope, buffalo, and deer. Animals are infected by motile zoospores. The main routes of transmission seem to be tick bites and close contact with contaminated fomites or debris. The infection occurs most frequently in keratinized tissues, and it remains localized in the upper layers. The hyphae grow in the epidermis and penetrate cells; after a maturation process, they release coccoid forms, sporangia, which transform into zoospores in the environment, closing the life cycle of the microorganism (8). The main presentation of infection is the skin disease, an exudative dermatitis, but it has also been increasingly related to systemic infections, like placentitis and funiculitis, and abortion. There are also many asymptomatic carriers (3).
Very few cases have been reported in humans. Although the transmission mechanism is not clearly known, mechanical transfer from infected animals is commonly accepted. Previous skin lesions seem to facilitate the infection. However, in several cases, the origin of infection remains unknown. There is a large clinical spectrum that involves only the living epidermis: pustular, exudative, and scaling lesions, recalcitrant verruca, folliculitis, hairy leukoplakia of the tongue, pitted keratolysis, chronic nodular disease, and asymptomatic infection (2, 4, 5, 7, 9). There is no specific treatment for dermatophilosis. Animals have been treated with a variety of topical and parenteral antibiotics and other preparations, but they have been largely ineffective. Human infections may be self-limiting and regress without treatment, although they can recur, especially in wet environments (3).
To our knowledge, this is the first human-imported case of infection by D. congolensis reported in Spain. Although conditions of growth, colonial characteristics, and microscopic morphology as well as biochemical reactions were illustrative for a presumptive diagnosis in various stages of its life cycle, epidemiological and clinical data together with nucleic acid analyses were essential for a final identification.
Infection by D. congolensis can affect many animal species, but D. congolensis rarely produces human infection. However, it should be considered in lesions developed on a previous minor skin trauma along with animal contact, mainly in tropical regions, in order to improve the measures of control and development of animal production. A good identification and antimicrobial susceptibility should be determined for every isolate, as well as a description of geographical location and a study of possible environmental sources related to microorganism pathogenicity. We suggest correct management of incubation times in suspected lesions in patients with animal contact; Gram stains on consecutive days during prolonged incubation are helpful for a correct identification. Nucleic acid-based techniques are highly specific. In the framework of an increase in the number of pathogenic species of aerobic actinomycetes (6), it is feasible that the diagnosis of human infection is underestimated.
Nucleotide sequence accession number.
The nucleotide sequence of the largest fragment of the partial 16S rRNA gene was submitted to GenBank and provided with the accession no. HQ113103.
Acknowledgments
The authors report no conflicts of interest.
Footnotes
Published ahead of print on 17 November 2010.
REFERENCES
- 1.Abu-Samra, M. T. 1978. Morphological, cultural and biochemical characteristics of Dermatophilus congolensis. Zentralbl. Veterinarmed. B 25:668-688. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht, R., et al. 1974. Dermatophilus congolensis chronic nodular disease in man. Pediatrics 53:907-913. [PubMed] [Google Scholar]
- 3.Ambrose, N. C. 1996. The pathogenesis of dermatophilosis. Trop. Anim. Health Prod. 28:29S-37S. [DOI] [PubMed] [Google Scholar]
- 4.Bunker, M. L., L. Chewning, S. E. Wang, and M. A. Gordon. 1988. Dermatophilus congolensis and “hairy” leukoplakia. Am. J. Clin. Pathol. 89:683-687. [DOI] [PubMed] [Google Scholar]
- 5.Burd, E. M., L. A. Juzych, J. T. Rudrik, and F. Habib. 2007. Pustular dermatitis caused by Dermatophilus congolensis. J. Clin. Microbiol. 45:1655-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conville, P. S., and F. G. Witebsky. 2007. Nocardia, Rhodococcus, Gordonia, Actinomadura, Streptomyces, and other aerobic actinomycetes, p. 515-542. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 7.Gillum, R. L., S. M. H. Qadri, M. N. Al-Ahdal, D. H. Connor, and A. J. Strano. 1988. Pitted keratolysis: a manifestation of human dermatophilosis. Dermatologica 177:305-308. [DOI] [PubMed] [Google Scholar]
- 8.Gordon, M. A. 1964. The genus Dermatophilus. J. Bacteriol. 88:509-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaminski, G. W., and I. I. Suter. 1976. Human infection with Dermatophilus congolensis. Med. J. Aust. 1:443-447. [PubMed] [Google Scholar]
- 10.Towersey, L., et al. 1993. Dermatophilus congolensis human infection. J. Am. Acad. Dermatol. 29:351-354. [DOI] [PubMed] [Google Scholar]
- 11.Zaria, L. T. 1993. Dermatophilus congolensis infection (dermatophilosis) in animals and man! An update. Comp. Immun. Microbiol. Infect. Dis. 16:179-222. [DOI] [PubMed] [Google Scholar]