Abstract
Phage-coded lysin is an enzyme that destroys the cell walls of bacteria. Phage lysin could be an alternative to conventional antibiotic therapy against pathogens that are resistant to multiple antibiotics. In this study, a novel staphylococcal phage, GH15, was isolated, and the endogenous lytic enzyme (LysGH15) was expressed and purified. The lysin LysGH15 displayed a broad lytic spectrum; in vitro treatment killed a number of Staphylococcus aureus strains rapidly and completely, including methicillin-resistant S. aureus (MRSA). In animal experiments, a single intraperitoneal injection of LysGH15 (50 μg) administered 1 h after MRSA injections at double the minimum lethal dose was sufficient to protect mice (P < 0.01). Bacteremia in unprotected mice reached colony counts of about 107 CFU/ml within 3.5 h after challenge, whereas the mean colony count in lysin-protected mice was less than 104 CFU/ml (and ultimately became undetectable). These results indicate that LysGH15 can kill S. aureus in vitro and can protect mice efficiently from bacteremia in vivo. The phage lysin LysGH15 might be an alternative treatment strategy for infections caused by MRSA.
Staphylococcus aureus is a common and dangerous pathogen that causes various infectious diseases, including skin abscesses, wound infections, endocarditis, osteomyelitis, pneumonia, and toxic shock syndrome (2, 23). Treatment of these infections has become ever more difficult due to the emergence of multidrug-resistant strains, especially methicillin-resistant S. aureus (MRSA) (15, 25, 26, 36, 37). Vancomycin was effective against MRSA, but certain MRSA strains have already acquired resistance to vancomycin as well (vancomycin-resistant S. aureus [VRSA]), raising serious concerns within the medical community (17, 18, 37). Therefore, there is an urgent need for novel therapeutic agents directed against this formidable pathogen (2, 9).
The phage lysin is encoded by the bacteriophage genome and is synthesized at the end of the phage lytic life cycle to lyse the host cell (30). Lysins belong to the family of mureolytic enzymes that directly destroy peptidoglycans in the bacterial cell wall. Previous studies have suggested that lysins from certain phages were highly efficient in lysing bacteria, especially when applied exogenously (11, 14, 21, 22, 29, 35). As a potential antibacterial agent, lysins possess several promising features, namely, a distinct mode of action, species or type specificity, and bactericidal activity independent of the antibiotic susceptibility pattern (1). Indeed, there is a low probability that bacteria will develop resistance against lysin (12, 21).
Some Staphylococcus phage lysins have been isolated and studied, including LysK, ClyS, MV-L, LysWMY, and ΦH5; however, only MV-L and ClyS have been studied in in vivo assays (6, 33). In this study, a novel myovirus phage infecting S. aureus was isolated. The lysin derived from this phage, LysGH15, was expressed and refined. The lysin LysGH15 demonstrated a very broad host range and strong lytic activity. We evaluated the potential of LysGH15 to rescue bacteremia and reduce lethality in a murine model of MRSA infection. Our positive results suggest that lysin treatment might provide an effective approach to control MRSA infections in humans and domestic animals.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Details of bacterial strains used in the study are listed in Table 1. All MRSA strains and most methicillin-susceptible S. aureus (MSSA) strains were isolated from clinical specimens obtained from patients at the First Hospital of Jilin University. All the isolated strains were confirmed as S. aureus by PCR of the 16S rRNA and by the coagulase test. Identification of MRSA strains was achieved by colony formation on selective salt agar plates containing 6 mg/ml oxacillin. Strains that yielded colonies on the plates were tested further for the mecA gene by PCR according to the method of Zhao et al. (38). All bacterial strains were stored at −80°C and routinely grown at 37°C. S. aureus strains and Streptococcus were cultured in brain heart infusion (BHI) broth, while others were cultured in Luria-Bertani (LB) medium.
TABLE 1.
Test organisms for specificity determinations
| Organism | Strain | Sourcea |
|---|---|---|
| S. aureus | N38 | 1 |
| 4B | 1 | |
| J01 | 1 | |
| R6166 | 1 | |
| N4 | 1 | |
| W2 | 2 | |
| N6 | 1 | |
| N3 | 1 | |
| N23 | 1 | |
| N5 | 1 | |
| N25 | 1 | |
| W4727 | 1 | |
| W1 | 2 | |
| W4661 | 1 | |
| J02 | 1 | |
| W3 | 2 | |
| W4 | 2 | |
| W5 | 2 | |
| N1 | 1 | |
| N13 | 1 | |
| N19 | 1 | |
| N30 | 1 | |
| N28 | 1 | |
| N7 | 1 | |
| N26 | 1 | |
| J2 | 1 | |
| CVCC2261 | 3 | |
| ATCC 26003 | 4 | |
| ATCC 25923 | 4 | |
| MRSA | R89 | 1 |
| W3275 | 1 | |
| R81 | 1 | |
| R6012 | 1 | |
| W11 | 1 | |
| R23 | 1 | |
| R5886 | 1 | |
| N10 | 1 | |
| YB57 | 1 | |
| W4552 | 1 | |
| R6491 | 1 | |
| R3784 | 1 | |
| R6246 | 1 | |
| R6994 | 1 | |
| R3659 | 1 | |
| W25 | 1 | |
| R196 | 1 | |
| R75 | 1 | |
| 208 | 1 | |
| R238 | 1 | |
| R3790 | 1 | |
| R6186 | 1 | |
| R6040 | 1 | |
| R6199 | 1 | |
| Streptococcus | CVCC606 | 3 |
| B. subtilis | EA751 | 2 |
| S. enteritidis | CVCC541 | 3 |
| K. pneumoniae | 43816 | 2 |
| E. coli | ATCC 25922 | 4 |
1, isolated from the First Hospital of Jilin University; 2, laboratory collection; 3, purchased from China Institute of Veterinary Drug Control; 4, purchased from the American Type Culture Collection.
Phage isolation, purification, and host range.
Samples of sewage were collected from the Changchun sewerage system and filtered with cotton wool. The MRSA strain W3275 was cultured overnight, together with sewage samples, in BHI broth at 37°C. The culture was centrifuged at 4°C (10,000 × g; 15 min), and the supernatant was filtered through Millipore filters (0.22-μm pore size). To detect the presence of phage, spot tests were carried out as described previously (4). Phage were purified using the double-layer agar plate method (8). After the cycle was repeated until the plaques were homogeneous, the phage were amplified and stored at 4°C or at −80°C in glycerol (3:1 [vol/vol]). The host range was also determined by the spot test method.
Characteristics of phage.
The multiplicity of infection (MOI) was defined as the ratio of virus particles to host cells. W3275 was grown to early log phase and then adjusted to a cell count of approximately 1 × 107 CFU/ml. Bacterial cells were infected with phage at different ratios (phage/bacterium ratio, 0.0001, 0.001, 0.01, 0.1, 1, 10, or 100). After incubation with shaking for 8 h at 37°C, the titers of these lysates were quantified as PFU per milliliter. As a control, W3275 was cultured alone, and the phage titer of the culture was determined.
A culture of W3275 grown to mid-exponential phase was harvested and resuspended in fresh BHI broth. Phage were added at an MOI of 0.1 and allowed to adsorb for 15 min at 4°C. The mixture was then centrifuged, and the pellets were resuspended in 10 ml of BHI. This suspension was incubated at 37°C with shaking at 200 rpm. Samples were taken at 5-min intervals, immediately diluted, and then plated for phage titration. As a control, W3275 was cultured alone, and the phage titer of the culture was determined.
Concentrated GH15 phage preparations were obtained by CsCl density gradient centrifugation following polyethylene glycol 8000 precipitation of BHI lysates. The protocols used were similar to those described previously (24). A sample was applied to copper grids, negatively stained with phosphotungstic acid (PTA) (2% [wt/vol]), and examined by transmission electron microscopy (JEOL JEM-1200EXII; Japan Electronics and Optics Laboratory, Tokyo, Japan) at an accelerating voltage of 80 kV.
The phage genome was purified with a virus genome extraction kit (Takara) and digested with DNase I (20 U/μg), RNase A (5 U/μg), and mung bean nuclease (20 U/μg) (Takara) at 37°C according to the manufacturer's instructions (27). The products of the digested phage nucleic acid were separated by 0.7% agarose gel electrophoresis. The undigested nucleic acid of the phage was quantified as a control.
Cloning, expression, and purification of phage lysin.
The primers lysin-S (5′-GTTAAAAGTAGTGCCATGTCA-3′; nucleotides [nt] 26943 to 26963) and lysin-A (5′-TGAAATTGAAGGTGGTTCAGC-3′; nt 29502 to 29522) were designed according to the phage K gene sequence published in GenBank (accession number AY176327). The template contained the full-length endolysin gene (nt 27072 to 29435). The amplified product was sequenced, and a BLAST search was performed. Primers lysF (5′-CCGCTCGAGATGGCTAAGACT CAAGCAGAA-3′) and lysR (5′-CGGGATCCCTATTTGAATACTCCCCAGGCAA-3′) were designed based on the putative full-length lysGH15 gene. The PCR product (containing 5′ XhoI and 3′ BamHI restriction sites) was digested with XhoI and BamHI and ligated into the pET15b vector. The full expression vector, pET15b-LysGH15, was transformed into competent Escherichia coli BL21 cells (Codon Plus). Exponentially growing cultures were induced with IPTG (isopropyl-β-d-thiogalactopyranoside). Bacterial cells were washed with 20 mM phosphate-buffered saline (PBS), disrupted with an ultrasonic disintegrator, and centrifuged at 4°C (10,000 × g; 15 min). The supernatant and pellet were analyzed by SDS-PAGE using 12% gels. For protein purification, the supernatant was dialyzed against PBS, added to Ni-nitrilotriacetic acid (NTA) (nickel matrix) His-Bind slurry, and eluted according to the manufacturer's instructions (Merck-Novagen).
Characteristics of the lysin.
The mid-exponential-phase W3275 cells were harvested (10,000 × g for 15 min at 4°C) and washed with PBS that contained various concentrations of CaCl2 (0-100 mM). The cell concentration was adjusted to an optical density at 600 nm (OD600) of 1.2-1.5. The lytic activity of the putative lysin toward W3275 was tested at various pH values in 50 mM sodium acetate (pH 4.0-6.0) or in 20 mM PBS (pH 7.0-9.0) at temperatures ranging from 25°C to 45°C. Experiments under each condition (media, pH, and temperature combination) were performed in triplicate.
The enzyme unit was defined as the highest dilution that caused a 50% decrease in absorbance at OD600 after 15 min at 35°C relative to control wells. One unit of LysGH15 was defined as described previously with modifications (21).
Antimicrobial activity of the lysin.
The phage lysin was tested for its ability to form a clearing on live staphylococcal lawns. Fifty-three different S. aureus substrates and other species were tested to determine the host spectrum of the lysin. Bacterial cells were washed and resuspended in sterile PBS, and the colony count was determined. The phage lysin was added to the bacterial suspension (final concentration, 40 μg/ml), and the mixture was incubated for 30 min at 35°C. The colony count was determined again. Enzyme activity in vitro was expressed as the CFU reduction. As a negative control, the bacterial strains were treated with elution buffer under the same conditions.
Mouse experiments.
Female BALB/c mice weighing 20 to 22 g (8 to 12 weeks of age) were purchased from the Experimental Animal Center of Jilin University. Groups of five mice per experiment were injected intraperitoneally (i.p.) with different inocula of MRSA strain YB57 (7 × 106, 7 × 107, 7 × 108, 7 × 109, and 7 × 1010 CFU/mouse) to determine the minimal dose that produced 100% mortality over a 7-day follow-up period (the minimal lethal dose [MLD]). The number of dead mice was recorded daily. Once the MLD had been determined, 2× MLD was used as the infective inoculum (challenge dose). The procedure for this experiment was carried out as described previously, with modifications (3, 20).
To determine the dose dependence of lysin treatment in mice, a single dose of LysGH15 (0 μg, 5 μg, 10 μg, 50 μg, or 100 μg) was administered i.p. at 1 h after bacterial challenge. Each dose group contained five mice. As controls, infected mice were treated with buffer and heat-inactivated LysGH15, and mock-infected mice were treated with 200 μg of LysGH15. An additional experiment was performed to determine whether LysGH15 was capable of protecting mice when injected several hours after challenge. These mice received a single 50-μg injection of LysGH15 at 1, 2, 3, and 4 h after challenge. The ability of LysGH15 to protect the bacteremic mouse was further investigated by colony counting. At intervals, bacterial counts were determined from 10 μl peripheral blood samples taken from the caudal veins of mice treated with either LysGH15 or buffer.
Statistical analysis.
Statistically significant differences in the survival of mice treated with the lytic enzymes versus vehicle were determined using the Pearson chi-square test and Fisher's exact test. A P value of less than 0.05 was accepted as statistically significant. The SPSS statistical software package version 13.0 (SPSS, Inc., Chicago, IL) was used for all statistical analyses.
RESULTS
Phage purification and host range.
Clear plaques were observed wherever phage lysate was spotted onto BHI agar plates covered with a bacterial lawn of MRSA W3275. This indicated that there were indeed phage in the lysate. The host range of all phages was determined, and the phage with the broadest spectrum was chosen and named GH15. The transparent plaque of phage GH15 was 2 mm in diameter. Of the 53 remaining S. aureus samples tested, GH15 was lytic against 25 strains (including 15 MRSA strains, ATCC 25923, ATCC 26003, and 8 MSSA strains). However, the phage GH15 could not lyse other species.
Characteristics of phage.
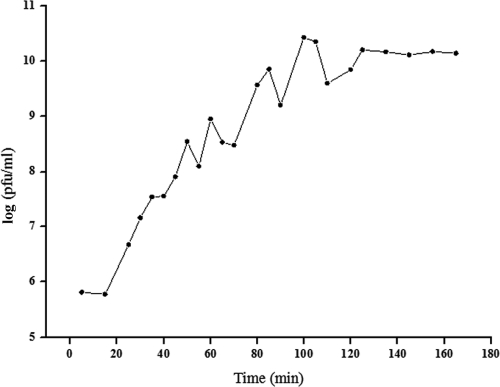
When the MOI was 0.001, the phage titer was highest, reaching 2.7 × 1010 PFU/ml. On the other hand, the titer of the control W3275 culture was 0, so the optimal MOI of phage GH15 was determined to be 0.001. The quantity of GH15 increased quickly in the log phase, and the time interval between two wave crests was about 15 to 20 min (Fig. 1). Moreover, the curve showed a short latency period of about 25 min and a rise period of 80 min.
FIG. 1.
One-step growth curve of phage GH15 in MRSA strain W3275. GH15 was added at an MOI of 0.1 and allowed to adsorb to W3275 cells for 15 min at 4°C. The GH15-exposed bacterial cells were thoroughly washed, transferred into 10 ml fresh BHI medium, and cultured at 37°C with shaking at 200 rpm. The culture samples were harvested at regular intervals, and the quantity of phage particles was measured during the incubation.
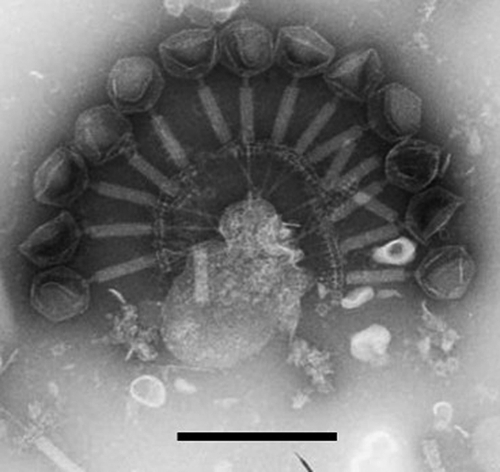
As shown in Fig. 2, phage GH15 had a contractile tail of approximately 125 ± 2 nm, an isometrically hexagonal head 65 ± 3 nm in diameter, and a double-layer baseplate about 60 nm in diameter. The head was separated from the tail sheath by a collar. The phage nucleic acid could be digested only with DNase I, which indicated that it was double-stranded DNA.
FIG. 2.
The morphology of phage GH15 as revealed by transmission electron micrographs of GH15 negatively stained with 2% phosphotungstic acid. The phage has an isometrically hexagonal head 65 ± 3 nm in diameter, a contractile tail of approximately 125 ± 2 nm, and a double-layer baseplate about 60 nm in diameter. The head is always separated from the tail sheath by a collar. These morphological characteristics confirmed that the phage is a member of the family Myoviridae. The bar represents 200 nm.
The sequencing and identification of phage lysin.
A fragment of about 1,704 bp was amplified using the primers lysin-S and lysin-A. The results of BLAST searches in the GenBank database revealed that the amplified product contained an entire open reading frame (ORF) (1,488 bp; GenBank accession no. HMO15284). The sequence of this ORF had 94% homology with those of the lysin genes of the S. aureus bacteriophages K, 812, A5W, and G1. However, the lysin of GH15 did not contain an intron like the lysins of these four phages.
The whole putative GH15 lysin gene (named lysGH15) was amplified using primers lysF and lysR. Exponentially growing cultures of E. coli BL21 containing pET15b-LysGH15 were induced with 1 mM IPTG, followed by incubation for 6 h at 25°C with shaking at 180 rpm. As seen in Fig. 3, SDS-PAGE revealed that there was a band of approximately 55 kDa, corresponding to the predicted size of the phage LysGH15 protein. This band was absent from uninduced cells. In addition, LysGH15 was found in the supernatant as a fusion protein. A homogeneous band also emerged in a sample of purified His-tagged phage lysin.
FIG. 3.

Protein profiles of N-terminal His-6-tagged LysGH15 on a 12% Coomassie brilliant blue (CBB)-stained gel. The lanes were loaded as follows: lane 1, uninduced E. coli BL21 (Coden Plus) cells containing a pET15b-LysGH15 construct; lane 2, pET15b-LysGH15-containing E. coli BL21 (Coden Plus) cells induced with 1 mM IPTG at 25°C; lane 3, supernatant of the induced E. coli BL21 Coden Plus cells containing a pET15b-LysGH15 construct after being crushed; lane 4, the purified LysGH15 fraction eluted from Ni-NTA His-Bind slurry; lane M, molecular mass marker. The arrow indicates the purified LysGH15 protein of about 55 kDa.
Characteristics of the lysin.
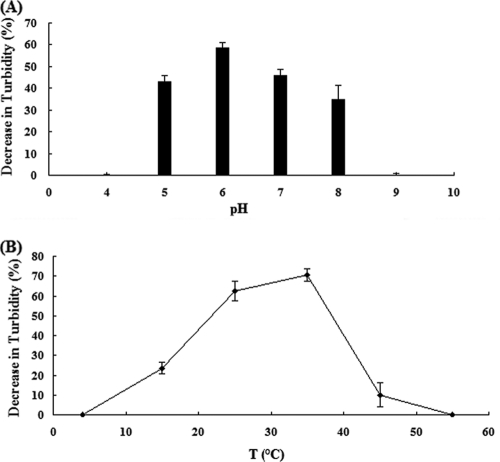
As shown in Fig. 4 A, the greatest enzymatic activity of LysGH15 was detected at pH 6.0, while activity was significantly lower at pH 5.0 or pH 7.0. The activity of lysin was also temperature dependent (Fig. 4B); bacterial cells were most susceptible to the lysin at 35°C, although considerable lysis was seen at temperatures between 25°C and 40°C. This lysin was very sensitive to high temperatures, however, as incubation at 45°C for 30 min could cause inactivation. The activity of LysGH15 was not dependent on the extracellular calcium concentration.
FIG. 4.
Characteristics of LysGH15. Shown is the percent reduction in the turbidity of a MRSA W3275 suspension after treatment with 40 μg/ml lysin for 30 min. (A) pH profile of LysGH15 activity. (B) Temperature profile of lysin activity. The values represent means and standard deviations (SD) (n = 3).
Rapid and specific killing of S. aureus by LysGH15.
The activity unit of LysGH15 was determined using the W3275 strain as the indicator bacterium. Approximately 0.8 μg of purified LysGH15 corresponded to 1 U of lytic activity against W3275.
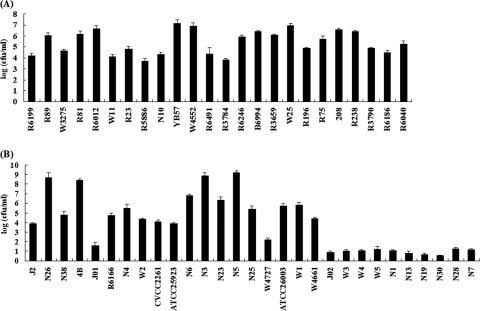
The bactericidal effect of LysGH15 was further investigated against a number of strains. In addition to killing the host bacterial strain, LysGH5 was able to lyse many other S. aureus strains, whether MRSA or MSSA. However, higher susceptibility to the endolysin was observed in MRSA strains, with an average specific activity of 5.4 ± 1.1 compared to 3.9 ± 2.8 for MSSA strains (Fig. 5). No lytic activity against Streptococcus, Bacillus subtilis, Salmonella enteritidis, Klebsiella pneumoniae, or E. coli was detected (data not shown).
FIG. 5.
Lytic activities of the lysin LysGH15 on various strains of S. aureus. LysGH15 caused specific killing of S. aureus. Log-phase cultures of different bacteria were exposed to LysGH15 (final concentration, 40 μg/ml) for 30 min. The results are displayed as the number of viable bacteria after buffer treatment divided by the number of viable bacteria after exposure to LysGH15. (A) MRSA strains. (B) MSSA strains. Averages of triplicate samples for each assay were used for the calculations. The error bars indicate SD.
Elimination of MRSA by LysGH15 in mouse models.
Injection of 7 × 109 and 7 × 1010 CFU/mouse MRSA YB57 (i.p.) was sufficient to produce bacteremia and a 100% mortality rate within 3 days. The average MLD of YB57 was determined to be about 7 × 109 cells.
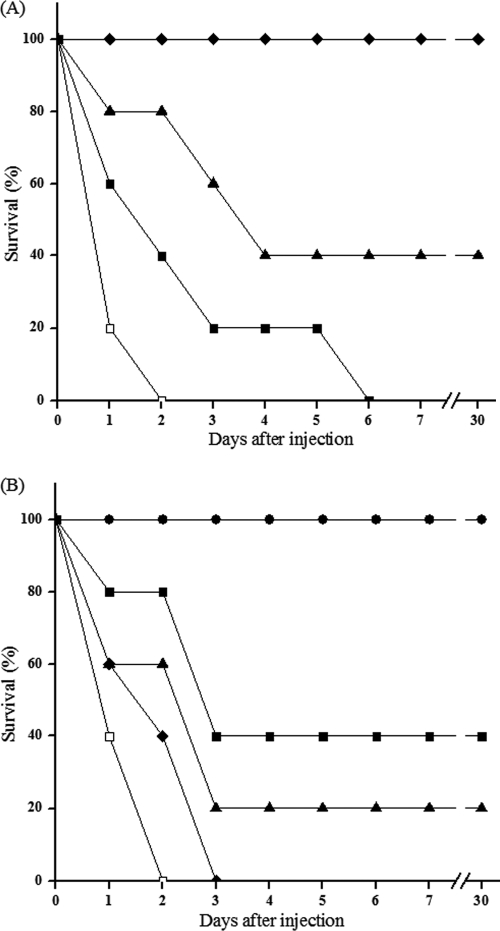
Experimental controls (not shown) demonstrated that high bacteremic rates (>5.4 × 105 CFU/ml) were normally attained in the blood and that the infection was systemic. In fact, CFU values greater than 5 × 103 were detected in heart, liver, spleen, and kidney within 50 min after the 2× MLD challenge. Consequently, the different doses of LysGH15 were administered (i.p.) 1 h after the inoculation. A dose-dependent effect on the state of the animals was visible after 24 h, as shown in Fig. 6 A. All mice recovered and bacteremia was greatly reduced when 50 μg of LysGH15 was injected 1 h after bacterial challenge, as revealed by Fisher's exact test (P < 0.01). The lowest dose of lysin, 10 μg per mouse, was sufficient to protect two out of five mice tested, while doses below 10 μg did not improve the survival rate. Similarly, heat-inactivated LysGH15 was ineffective and did not show any protective effect. Moreover, the administration of a single excess dose of LysGH15 (200 μg) did not produce any adverse effects. A life-saving effect was observed even when 50 μg LysGH15 was administered 2 h after infection, with a protective rate of 40%, as seen in Fig. 6B. The ability of LysGH15 to reduce bacterial counts in blood was investigated (Fig. 7). The group treated with lysin 1 h after inoculation demonstrated a drop of 2 log units by 2.5 h after lysin treatment. This contrasted with bacterial counts in untreated mice, which could increase to 107 CFU/ml and cause death within 2 days.
FIG. 6.
Rescue of mice from lethal MRSA YB57 infection by LysGH15. The mice were inoculated i.p. with 2× MLD YB57. (A) One hour later, 50 μg (black diamonds), 10 μg (black triangles), or 5 μg (black squares) of lysin was injected into the mouse peritoneal cavity. Control mice (white squares) were treated with elution buffer under the same conditions. (B) LysGH15 (50 μg) or buffer was administered into the peritoneal cavities of mice at the indicated time intervals after challenge with 2× MLD YB57. Lysin was given at 1 h (black circles), 2 h (black squares), 3 h (black triangles), or 4 h (black diamond) after infection. As controls, infected mice were treated with buffer (white squares) under the same conditions. Each symbol represents the average of three experiments.
FIG. 7.
Colony counts of blood samples at regular intervals. At the indicated times, bacterial counts (CFU/ml) in five mice treated with either 50 μg of LysGH15 (black symbols) or buffer (white symbols) were determined from peripheral blood samples (10 μl) taken from the caudal vein. Every line with black or white symbols represents the bacterial counts of a single mouse treated with lysin or buffer. The arrow indicates the moment at which the lysin or elution buffer was injected (1 h after challenge).
DISCUSSION
Phage lysin therapy is a possible alternative to antibiotics for the treatment of bacterial infections. Indeed, it has proven to be medically superior to antibiotic therapy in many ways (7). Our results support this notion, as phage lysin was shown to be highly efficacious against infections caused by inoculation with antibiotic-resistant S. aureus.
In this study, a novel phage from the family Myoviridae was isolated and characterized. Most phages that have been isolated belong to the family Siphoviridae or Podoviridae. To our knowledge, only a few Myoviridae members have been studied, including phages K and Twort. The phage GH15 demonstrated high lytic activity and a broad host range, underscoring its great potential to treat S. aureus infection.
The product amplified using lysin-S and lysin-A was shorter than we expected, because the previously cloned lysK has an intron of about 876 bp (31). No intron was found in lysGH15, but otherwise, lysGH15 and lysK showed significant homology (94%). This suggests that the intron of lysGH15 has been lost or shifted or that lysK has obtained an intron (16).
Analysis of the amino acid sequence revealed that LysGH15 is a modular enzyme with three distinct domains similar to those of LysK, including an N-terminal CHAP domain with hydrolytic function, a central amidase domain, and a C-terminal SH3b domain that might be involved in cell wall recognition (22, 28). However, a truncated derivative of the phage endolysin LysK, C165, which contained only the CHAP domain, was shown to have greater lytic power than the native protein (19, 32). This suggests that there may be intramolecular interactions or spatial steric hindrances among domains in LysK that modulate the lytic activity of the lysin (5, 34). While LysGH15 fragments were not tested, the complete enzyme exhibited powerful bactericidal activity against most S. aureus strains tested.
The activity of a lytic enzyme is closely related to its structure (34). There were only four amino acid differences between LysGH15 and LysK (Ile26, Gln113, Asp469, and His485) (31). However, analysis of the amino acid sequence using DNAStar demonstrated that LysGH15 had a different tertiary structure. We found one more β-strand in LysGH15 within the N-terminal CHAP domain due to the change at amino acid 113. In addition, there were three more turn regions and two more coil regions in LysGH15 than in LysK due to amino acid differences at positions 469 and 485. These structural differences may result in the difference in lytic activities.
The lysin LysGH15 displayed a broad lytic spectrum. However, LysGH15 had a significantly different bactericidal effect, depending on resistance to methicillin. A higher susceptibility to the endolysin was observed against MRSA strains. A larger variability was also observed against different MSSA strains. Normally, the C-terminal domain of lysin binds to a specific substrate (usually a carbohydrate) found in the cell wall of the host bacterium (10, 13), and the degree of binding is closely related to the lytic activity (34). It is likely, therefore, that the binding between LysGH15 and MRSA is stronger and that there are different binding moieties in the cell walls of MRSA and MSSA bacteria. Additionally, LysGH15-mediated killing is highly specific to S. aureus, but not to other species, which is consistent with recent investigations by others (29, 33). Thus, LysGH15 has the potential to control infections caused by S. aureus strains, including MRSA and MSSA, without perturbing the commensal microflora.
A single injection of LysGH15 was sufficient to protect mice from fatal MRSA infection. When administered 1 and 3 h after MRSA challenge, 50 μg LysGH15 had protective rates of 100% and 20%, respectively. MV-L and ClyS have also been studied in in vivo assays (6, 33). In comparison, when 500 U MV-L (about 50 μg) was used to treat the murine model 1 h after MRSA challenge, the survival rate reached 60%. The protective rate for MRSA-infected mice was about 30% when 166 μg ClyS (a chimeric lysin) was administrated 3 h after inoculation. Therefore, the protective efficacy of LysGH15 may be higher, at least based on these limited studies. Otherwise, our data indicated that the survival rates of the treated mice would be increased further by changing to a higher dose of LysGH15, even if the administration of lysin was delayed much longer.
The results of these assays demonstrated that the phage lysin LysGH15 had a wide spectrum of lytic activity and was strongly protective against lethal S. aureus infection in mice. Most importantly, we found significant therapeutic efficacy against antibiotic-resistant strains. This alternative therapeutic option will provide a viable tool to control methicillin-resistant S. aureus infections.
Acknowledgments
This research study was supported by the National High Technology Research and Development Program of China (no. 2006AA10A206).
We thank Xiaoming Wang for providing the clinical staphylococcal isolates and Yongjun Yang for his outstanding project management. We are deeply indebted to Xianghong Xu for help with statistical analysis and to Gongpo Long and Xiangpeng Sheng for their generous assistance in collecting sewage samples.
Footnotes
Published ahead of print on 3 November 2010.
REFERENCES
- 1.Borysowski, J., B. Weber-Dabrowska, and A. Gorski. 2006. Bacteriophage endolysins as a novel class of antibacterial agents. Exp. Biol. Med. (Maywood) 231:366-377. [DOI] [PubMed] [Google Scholar]
- 2.Brumfitt, W., and J. Hamilton-Miller. 1989. Methicillin-resistant Staphylococcus aureus. N. Engl. J. Med. 320:1188-1196. [DOI] [PubMed] [Google Scholar]
- 3.Casal, J., L. Aguilar, I. Jado, J. Yuste, M. J. Gimenez, J. Prieto, and A. Fenoll. 2002. Effects of specific antibodies against Streptococcus pneumoniae on pharmacodynamic parameters of beta-lactams in a mouse sepsis model. Antimicrob. Agents Chemother. 46:1340-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, H. C., C. R. Chen, J. W. Lin, G. H. Shen, K. M. Chang, Y. H. Tseng, and S. F. Weng. 2005. Isolation and characterization of novel giant Stenotrophomonas maltophilia phage phiSMA5. Appl. Environ. Microbiol. 71:1387-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng, Q., and V. A. Fischetti. 2007. Mutagenesis of a bacteriophage lytic enzyme PlyGBS significantly increases its antibacterial activity against group B streptococci. Appl. Microbiol. Biotechnol. 74:1284-1291. [DOI] [PubMed] [Google Scholar]
- 6.Daniel, A., C. Euler, M. Collin, P. Chahales, K. J. Gorelick, and V. A. Fischetti. 2010. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 54:1603-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donovan, D. M. 2007. Bacteriophage and peptidoglycan degrading enzymes with antimicrobial applications. Recent Pat. Biotechnol. 1:113-122. [DOI] [PubMed] [Google Scholar]
- 8.Ellis, E. L., and M. Delbruck. 1939. The growth of bacteriophage. J. Gen. Physiol. 22:365-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enright, M. C. 2003. The evolution of a resistant pathogen—the case of MRSA. Curr. Opin. Pharmacol. 3:474-479. [DOI] [PubMed] [Google Scholar]
- 10.Fischetti, V. A. 2005. Bacteriophage lytic enzymes: novel anti-infectives. Trends Microbiol. 13:491-496. [DOI] [PubMed] [Google Scholar]
- 11.Fischetti, V. A. 2003. Novel method to control pathogenic bacteria on human mucous membranes. Ann. N. Y. Acad. Sci. 987:207-214. [DOI] [PubMed] [Google Scholar]
- 12.Fischetti, V. A. 2006. Using phage lytic enzymes to control pathogenic bacteria. BMC Oral Health 6(Suppl. 1):S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.García, E., J. L. Garcia, P. Garcia, A. Arraras, J. M. Sanchez-Puelles, and R. Lopez. 1988. Molecular evolution of lytic enzymes of Streptococcus pneumoniae and its bacteriophages. Proc. Natl. Acad. Sci. U. S. A. 85:914-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García, J. L., E. Garcia, A. Arraras, P. Garcia, C. Ronda, and R. Lopez. 1987. Cloning, purification, and biochemical characterization of the pneumococcal bacteriophage Cp-1 lysin. J. Virol. 61:2573-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto, H. 1994. Drug resistance of methicillin-resistant Staphylococcus aureus (MRSA) in Japan until 1993. Jpn. J. Antibiot. 47:575-584. [PubMed] [Google Scholar]
- 16.Haugen, P., D. M. Simon, and D. Bhattacharya. 2005. The natural history of group I introns. Trends Genet. 21:111-119. [DOI] [PubMed] [Google Scholar]
- 17.Hiramatsu, K., N. Aritaka, H. Hanaki, S. Kawasaki, Y. Hosoda, S. Hori, Y. Fukuchi, and I. Kobayashi. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350:1670-1673. [DOI] [PubMed] [Google Scholar]
- 18.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 19.Horgan, M., G. O'Flynn, J. Garry, J. Cooney, A. Coffey, G. F. Fitzgerald, R. P. Ross, and O. McAuliffe. 2009. Phage lysin LysK can be truncated to its CHAP domain and retain lytic activity against live antibiotic-resistant staphylococci. Appl. Environ. Microbiol. 75:872-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jado, I., R. Lopez, E. Garcia, A. Fenoll, J. Casal, and P. Garcia. 2003. Phage lytic enzymes as therapy for antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J. Antimicrob. Chemother. 52:967-973. [DOI] [PubMed] [Google Scholar]
- 21.Loeffler, J. M., D. Nelson, and V. A. Fischetti. 2001. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294:2170-2172. [DOI] [PubMed] [Google Scholar]
- 22.Loessner, M. J. 2005. Bacteriophage endolysins—current state of research and applications. Curr. Opin. Microbiol. 8:480-487. [DOI] [PubMed] [Google Scholar]
- 23.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 24.Matsuzaki, S., M. Yasuda, H. Nishikawa, M. Kuroda, T. Ujihara, T. Shuin, Y. Shen, Z. Jin, S. Fujimoto, M. D. Nasimuzzaman, H. Wakiguchi, S. Sugihara, T. Sugiura, S. Koda, A. Muraoka, and S. Imai. 2003. Experimental protection of mice against lethal Staphylococcus aureus infection by novel bacteriophage phi MR11. J. Infect. Dis. 187:613-624. [DOI] [PubMed] [Google Scholar]
- 25.Miller, L. G., F. Perdreau-Remington, G. Rieg, S. Mehdi, J. Perlroth, A. S. Bayer, A. W. Tang, T. O. Phung, and B. Spellberg. 2005. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N. Engl. J. Med. 352:1445-1453. [DOI] [PubMed] [Google Scholar]
- 26.Mylotte, J. M., C. McDermott, and J. A. Spooner. 1987. Prospective study of 114 consecutive episodes of Staphylococcus aureus bacteremia. Rev. Infect. Dis. 9:891-907. [DOI] [PubMed] [Google Scholar]
- 27.Nasu, H., T. Iida, T. Sugahara, Y. Yamaichi, K. S. Park, K. Yokoyama, K. Makino, H. Shinagawa, and T. Honda. 2000. A filamentous phage associated with recent pandemic Vibrio parahaemolyticus O3:K6 strains. J. Clin. Microbiol. 38:2156-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navarre, W. W., H. Ton-That, K. F. Faull, and O. Schneewind. 1999. Multiple enzymatic activities of the murein hydrolase from staphylococcal phage phi11. Identification of a D-alanyl-glycine endopeptidase activity. J. Biol. Chem. 274:15847-15856. [DOI] [PubMed] [Google Scholar]
- 29.Nelson, D., L. Loomis, and V. A. Fischetti. 2001. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. U. S. A. 98:4107-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obeso, J. M., B. Martinez, A. Rodriguez, and P. Garcia. 2008. Lytic activity of the recombinant staphylococcal bacteriophage PhiH5 endolysin active against Staphylococcus aureus in milk. Int. J. Food Microbiol. 128:212-218. [DOI] [PubMed] [Google Scholar]
- 31.O'Flaherty, S., A. Coffey, R. Edwards, W. Meaney, G. F. Fitzgerald, and R. P. Ross. 2004. Genome of staphylococcal phage K: a new lineage of Myoviridae infecting gram-positive bacteria with a low G+C content. J. Bacteriol. 186:2862-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Flaherty, S., A. Coffey, W. Meaney, G. F. Fitzgerald, and R. P. Ross. 2005. The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J. Bacteriol. 187:7161-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rashel, M., J. Uchiyama, T. Ujihara, Y. Uehara, S. Kuramoto, S. Sugihara, K. Yagyu, A. Muraoka, M. Sugai, K. Hiramatsu, K. Honke, and S. Matsuzaki. 2007. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage phi MR11. J. Infect. Dis. 196:1237-1247. [DOI] [PubMed] [Google Scholar]
- 34.Sass, P., and G. Bierbaum. 2007. Lytic activity of recombinant bacteriophage phi11 and phi12 endolysins on whole cells and biofilms of Staphylococcus aureus. Appl. Environ. Microbiol. 73:347-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuch, R., D. Nelson, and V. A. Fischetti. 2002. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418:884-889. [DOI] [PubMed] [Google Scholar]
- 36.Tenover, F. C., L. M. Weigel, P. C. Appelbaum, L. K. McDougal, J. Chaitram, S. McAllister, N. Clark, G. Killgore, C. M. O'Hara, L. Jevitt, J. B. Patel, and B. Bozdogan. 2004. Vancomycin-resistant Staphylococcus aureus isolate from a patient in Pennsylvania. Antimicrob. Agents Chemother. 48:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weigel, L. M., D. B. Clewell, S. R. Gill, N. C. Clark, L. K. McDougal, S. E. Flannagan, J. F. Kolonay, J. Shetty, G. E. Killgore, and F. C. Tenover. 2003. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 302:1569-1571. [DOI] [PubMed] [Google Scholar]
- 38.Zhao, W. H., Z. Q. Hu, S. Okubo, Y. Hara, and T. Shimamura. 2001. Mechanism of synergy between epigallocatechin gallate and beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1737-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]