Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) has been identified as a major cause of community-associated (CA) S. aureus infections in the past decade. The main reservoir in the community for MRSA and the factors contributing to its worldwide spread remain poorly defined. Between July 2005 and June 2008, a total of 6,057 healthy children 2 to 60 months of age were screened for carriage of S. aureus and Streptococcus pneumoniae in Taiwan. The prevalence and epidemiological factors influencing MRSA carriage were determined. MRSA strains were tested for antimicrobial susceptibility and underwent molecular characterization. The overall prevalences of MRSA and S. aureus carriage were 7.8% and 23.2%, respectively. A majority (88%) of MRSA isolates belonged to a common Asian-Pacific CA-MRSA lineage, multilocus sequence type 59, and were resistant to multiple non-beta-lactam antibiotics. The carriage rate of MRSA was higher among subjects 2 to 6 months old (P < 0.0001), residing in northern Taiwan (P = 0.0003), and enrolled later in the study (P < 0.0001). MRSA colonization was associated with the number of children in the family (adjusted odds ratio [aOR], 1.114; 95% confidence interval [CI], 1.002 to 1.240; P = 0.0463) and day care attendance (aOR, 1.530; 95% CI, 1.201 to 1.949; P = 0.0006). Breast feeding (P < 0.0001) and colonization with S. pneumoniae (P = 0.0170) were protective against MRSA colonization. We concluded that epidemic CA-MRSA strains increasingly colonized Taiwanese children between 2005 and 2008. The carriage rate varied significantly across different demographical features. Crowding was an independent environmental risk factor that might accelerate CA-MRSA transmission in the community.
Two of the major changes in the epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) in the past decade were the worldwide emergence of community-associated (CA) MRSA strains and an increasing number of CA-MRSA infections in previously healthy hosts (24, 35). The CA-MRSA isolates were initially identified in pediatric populations and subsequently reported in certain adult populations, such as Native Americans, military recruits, prison inmates, drug users, men who have sex with men, and competitive sports participants (3, 12, 14, 15, 23, 31, 38). Although superficial skin and soft tissue infections remained the most common manifestation of CA-MRSA, severe diseases such as necrotizing pneumonitis, necrotizing fasciitis, osteomyelitis, pyomyositis, septic embolism, and venous thrombosis were not uncommon and previously caused death in healthy children (5, 13).
The causes of the increasing incidence of CA-MRSA infection in previously healthy hosts are not completely understood, and the factors influencing CA-MRSA virulence remain an issue of ongoing debate (2, 9, 30). Colonization with S. aureus has been identified as an important risk factor for the development of S. aureus infections in both community and hospital settings (17, 36). Evidence further suggests that, compared to methicillin-susceptible S. aureus (MSSA), colonization with MRSA imposes a significantly greater risk for development of subsequent infections (10). In the past few years, the prevalence of MRSA colonization increased significantly among healthy hosts during CA-MRSA epidemics (8, 19). The carriage of MRSA with CA genotypes was significantly higher among children than among adults (25). Furthermore, living with young children was associated with increased risk of MRSA colonization in adults (37). When combined, these observations strongly suggested young children were the major reservoir of MRSA in the community and were the main population responsible for the accelerated transmission of CA-MRSA. Other host and environmental factors contributing to the worldwide spread of CA-MRSA remain poorly defined. Identification of these factors should expand our understanding of the epidemiology of CA-MRSA colonization and may help in developing measures for controlling its spread.
In this study, we investigated the epidemiological factors associated with MRSA colonization among young children in the community setting. To our best knowledge, this is the largest-scale study of S. aureus colonization that targets young children and simultaneously explores a variety of influencing factors, including microbial interference from another important pediatric pathogen, Streptococcus pneumoniae.
MATERIALS AND METHODS
Study design.
Between July 2005 and June 2008, all 2- to 60-month-old healthy children who visited general health checkup clinics in three representative teaching hospitals in Taiwan were invited to participate in this study. The hospitals were located in the suburban area of northern Taiwan (Linko Medical Center of Chang Gung Memorial Hospital) and in two metropolitan areas of central (Taichung Veterans General Hospital) and southern (Kaohsiung branch, Chang Gung Memorial Hospital) Taiwan. These hospitals all provided primary to tertiary care to children of all ages, and the medical costs of the visits were covered by the compulsory national health insurance system in Taiwan. Therefore, access to the hospitals was not affected by socioeconomic status. Children were enrolled in the study if they met the inclusion criteria (discussed below), and informed consent was obtained from their parents or legal guardians. A swab (BBL CultureSwab Plus; Becton Dickinson and Company) of both anterior nares and another flexible swab (Venturi Transystem; Copan Innovation) from deep in the nasopharyngeal space were obtained from each participant. The swabs were used for isolation of both S. aureus and S. pneumoniae. For the standardization of specimen collection, the sampling procedure was performed by a well-trained nurse at each study site. The nasal and nasopharyngeal swabs were immediately placed in the transport medium and transported to the clinical microbiology laboratory in each institute for detection of S. aureus and S. pneumoniae by standard methods. Identification of S. pneumoniae was confirmed by optochin sensitivity and bile solubility. S. aureus was identified by coagulase testing, and oxacillin susceptibility was assessed by the disc diffusion method according to Clinical and Laboratory Standards Institute 2006 guidelines (7). This study was approved by the institute review boards from the three sampled hospitals.
Demographics and anthropometric variables of subjects.
Children with chronic renal failure, dialysis, indwelling devices, thalassemia major, chronic cardiovascular diseases, chronic lung disease, nephrotic syndrome, liver cirrhosis, diabetes mellitus, congenital immunodeficiency, human immunodeficiency virus infection, asplenia, or malignancy or receiving immunosuppressant agents were excluded from the study. Prematurity without complication was not seen as an underlying condition, and those subjects were eligible for the study. A standardized questionnaire interview of the parents or primary caregivers was used to collect the information needed in this study. In addition to the demographic data (date of birth, gender, and history of breast feeding), we also collected environmental factors and health conditions of the subjects. These variables were hypothesized to be potential factors associated with MRSA colonization. The environmental factors included house size, number of children in the family, frequency of hand washing, passive smoking, sleeping alone or with parents, kindergarten or day care attendance, and the identity of the main caregiver during the day. The children's health-related factors included vaccination history for seasonal influenza and S. pneumoniae, history of acute otitis media, history of recent upper respiratory tract infection, and recent (<2 weeks) antibiotic use.
The administered questionnaires were collected from the three study sites. The data were digitalized and cleaned in a computer core laboratory before we proceeded with statistical analysis.
Molecular characterization of MRSA isolates.
All S. aureus isolates were sent to the Linko Medical Center of Chang Gung Memorial Hospital for further microbiological characterization. A random sample of MRSA isolates (1 out of 2 consecutive isolates from central and southern Taiwan and 3 out of 4 consecutive isolates from northern Taiwan) were prospectively selected for molecular characterization. Pulsed-field gel electrophoresis (PFGE) with SmaI digestion was used in this study to fingerprint the MRSA isolates and was performed according to procedures described by Huang et al. (22). The genotypes were designated in alphabetical order, as in the previous studies (17-19); any new genotype identified was designated consecutively. PFGE patterns with fewer than four band differences from an existing genotype were defined as subtypes of that genotype.
SCCmec typing of isolates was done using a multiplex PCR strategy described previously (19). The control strains for staphylococcal chromosomal cassette mec (SCCmec) types I, II, III, and IVa, kindly provided by Keiichi Hiramatsu, were as follows: type I, NCTC10442; type II, N315; type III, 85/2082; and type IVa, JCSC4744. SCCmec typing for type VT was determined by using a particular primer described elsewhere (20), and strain TSGH-17, kindly provided by Chi-Chien Wang, was used as a control.
The presence of Panton-Valentine leukocidin (PVL) genes was determined by a PCR technique described by Lina et al. (27). Some isolates with representative PFGE patterns were selected and underwent multilocus sequence typing (MLST) (http://www.mlst.net) (11). The allelic profiles were assigned through a comparison of the sequence at each locus with the sequences of the known alleles in the S. aureus MLST database and were defined as sequence types accordingly.
Statistics.
The comparison of categorical variables between study groups was performed with a chi-square test, or with Fisher's exact test where appropriate, while the differences between study groups in the numerical variables were tested by a two-sample t test. Multiple logistic regression analysis was applied to explore factors associated with MRSA and MSSA colonization. Statistical significance was defined as a P value of <0.05 in the tests. The data were analyzed with SAS software version 9.1 (SAS Institute Inc., Cary, NC).
RESULTS
Characteristics of study subjects.
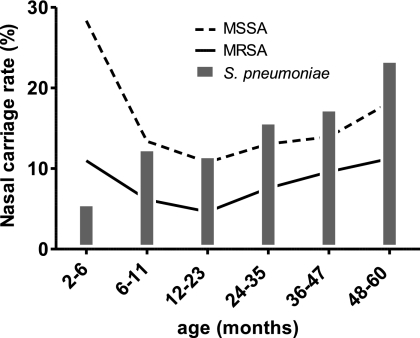
From July 2005 to June 2008, a total of 6,057 children aged from 2 to 60 months (mean age, 25.0 months ± 17.2 months) were enrolled in the study. The majority of the participants were male (54.5%), and 7.3% of the 6,057 children had a birth history of prematurity. The age distribution (P = 0.9799), gender (P = 0.5047), and prematurity (P = 0.0653) did not differ significantly for the subjects across the three regions of Taiwan. Nasal carriage of MRSA and MSSA was detected in 473 (7.8%) and 931 (15.4%) subjects, respectively (Table 1). Infants 2 to 6 months old had the highest incidence of S. aureus carriage but the lowest incidence of S. pneumoniae carriage (Fig. 1). The rate of S. aureus carriage declined to a nadir in the second year of life but gradually increased between 2 and 5 years of age. The carriage of S. pneumoniae generally increased with age.
TABLE 1.
Nasal carriage of MSSA, MRSA, and S. pneumoniae in Taiwanese children aged 2 to 60 months from July 2005 to June 2008 in northern, central, and southern Taiwan
| Area of Taiwan | No. of subjects | No. (%) witha: |
||
|---|---|---|---|---|
| MSSA | MRSA | S. pneumoniae | ||
| Northern | 2,017 | 361 (17.9) | 192 (9.5) | 261 (12.9) |
| Southern | 2,023 | 286 (14.1) | 156 (7.7) | 281 (13.9) |
| Central | 2,017 | 284 (14.1) | 125 (6.2) | 315 (15.6) |
| Total | 6,057 | 931 (15.4) | 473 (7.8) | 857 (14.1) |
Comparison of colonization rates among the three areas (northern, southern, and central Taiwan) by chi-square test: MSSA, P = 0.0006: MRSA, P = 0.0004; S. pneumoniae, P = 0.0469.
FIG. 1.
Age-specific distribution of MRSA, MSSA, and S. pneumoniae carriage in children younger than 6 years old.
The colonization rates of S. aureus were different among the three areas, with the highest incidence in northern Taiwan (27.4%) and the lowest incidence in central Taiwan (20.3%) (Table 1). The geographical variation was observed in both MRSA (P = 0.0004) and MSSA (P = 0.0006) carriage. There were also geographical differences in S. pneumoniae colonization. The highest incidence of S. pneumoniae colonization occurred in central Taiwan, while northern Taiwan had the lowest incidence.
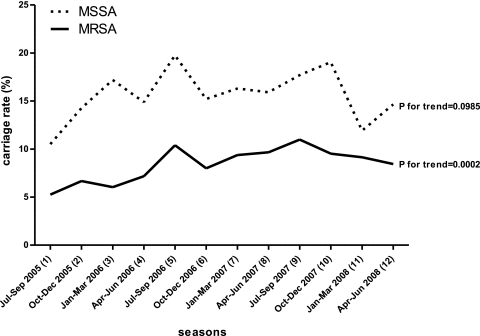
When these rates were graphically compared across the period of study (Fig. 2), an increasing trend of MRSA carriage was identified (P = 0.0002). The increasing temporal trend was highly significant in central and southern Taiwan (P = 0.0129 and P < 0.0001, respectively), but not in northern Taiwan (P = 0.6983). The incidence of MSSA carriage varied greatly, from 10.5% to 19.8%, at different seasons but did not develop a significant trend during the same period for any area (P = 0.1758 for northern Taiwan, P = 0.1870 for central Taiwan, and P = 0.8076 for southern Taiwan).
FIG. 2.
Temporal trend of MRSA and MSSA nasal colonization in Taiwanese children from July 2005 to June 2008.
Association between S. aureus and S. pneumoniae colonization.
S. pneumoniae colonization was identified in 857 (14.1%) subjects of this study. Cocolonization with S. pneumoniae and S. aureus was found in 122 (2%) subjects. A negative correlation was found between S. aureus and S. pneumoniae colonization. Children with S. aureus colonization had a positivity rate of 8.7% for S. pneumoniae colonization, while a positivity rate of 15.8% for S. pneumoniae colonization was identified among children without S. aureus colonization (P < 0.0001). The trend was retained when further stratified by MRSA and MSSA (Table 2). The colonization rates of S. pneumoniae also differed significantly among MRSA and MSSA carriers (12.3% versus 6.9%; P = 0.0008).
TABLE 2.
Association of S. aureus and S. pneumoniae colonization in Taiwanese children
| S. pneumoniae colonization | No. (%) with S. aureus: |
|||
|---|---|---|---|---|
| MRSAa | MSSAa | Noncarrier | Total | |
| Yes | 58 (12.3) | 64 (6.9) | 735 (15.8) | 857 (14.1) |
| No | 415 (87.7) | 867 (93.1) | 3,918 (84.2) | 5,200 (85.9) |
| Total | 473 | 931 | 4,653 | 6,057 |
Comparison of S. pneumoniae colonization rates between S. aureus carriers and noncarriers (Fisher's exact test): MRSA, P = 0.0451; MSSA, P = <0.0001.
Epidemiological factors associated with MRSA and MSSA carriage.
The variables correlated with MRSA and MSSA carriage by means of univariate analysis are displayed in Table 3. MSSA colonization in children appeared to be influenced more readily than MRSA colonization by various health and environmental factors in the univariate analysis. For instance, sleeping with parents, lower frequency of hand washing, influenza vaccination, premature birth, upper respiratory tract infections, and use of antibiotics within the previous 2 weeks were all associated with decreased incidence of MSSA colonization but did not influence colonization by MRSA. Further, a greater number of children in the family and day care attendance both increased the risk of MRSA colonization but were associated with decreased incidence of MSSA colonization.
TABLE 3.
Univariate analysis of epidemiologic factors associated with MRSA and MSSA nasal carriage in Taiwanese children from July 2005 to June 2008
| Factora | Value |
P1b | P2c | ||
|---|---|---|---|---|---|
| MRSA carrier (n = 473) | MSSA carrier (n = 931) | Noncarrier (n = 4,653) | |||
| Demographic | |||||
| Residing in northern Taiwan (%) | 40.6 | 38.8 | 31.5 | <0.0001 | <0.0001 |
| Age 2-6 mo (%) | 18.8 | 24.7 | 10.6 | <0.0001 | <0.0001 |
| Male (%) | 55.8 | 53.1 | 54.7 | 0.6260 | 0.3764 |
| Breast feeding (%) | 61.1 | 66.6 | 70.8 | <0.0001 | 0.0108 |
| Period (mo)d | 4.3 ± 5.3 | 4.8 ± 5.6 | 5.3 ± 5.6 | 0.0032 | 0.0249 |
| Environmental | |||||
| No. of children in the family. | 2.0 ± 0.9 | 1.8 ± 0.9 | 1.89 ± 0.87 | 0.0341 | 0.0478 |
| House size (m2) | 173.8 ± 99.3 | 175.6 ± 115.3 | 178.4 ± 267.9 | 0.4475 | 0.6131 |
| No. of toilets | 2.4 ± 1.1 | 2.4 ± 1.1 | 2.4 ± 1.0 | 0.4297 | 0.8887 |
| Sleeping with parents (%) | 89 | 85.8 | 89.7 | 0.6342 | 0.0006 |
| Hand washing frequency (no./day) | 5.7 ± 4.2 | 5.4 ± 3.6 | 5.9 ± 3.6 | 0.3357 | <0.0001 |
| Passive smoking (%) | 50.5 | 48.3 | 47.8 | 0.2666 | 0.7460 |
| Day-care or kindergarten attendance (%) | 22.4 | 15.9 | 18.7 | 0.0567 | 0.0455 |
| Duration (months)e | 13.0 ± 10.9 | 11.9 ± 10.2 | 10.8 ± 9.3 | 0.0463 | 0.1513 |
| No. of classmatese | 16.9 ± 6.0 | 17.0 ± 6.5 | 17.0 ± 7.0 | 0.8498 | 0.9505 |
| Duration of stay (h/wk)e | 39.7 ± 6.5 | 38.8 ± 7.0 | 39.2 ± 6.5 | 0.5013 | 0.5880 |
| Health conditions | |||||
| Pneumococcal vaccination (%) | 14.2 | 13.1 | 12.0 | 0.1844 | 0.3514 |
| Flu vaccination (%) | 44.6 | 33.5 | 41.1 | 0.1419 | <0.0001 |
| History of AOM (%) | 12.7 | 9.0 | 10.3 | 0.1153 | 0.2574 |
| URI within 2 weeks (%) | 37 | 29.8 | 35.3 | 0.4495 | 0.0013 |
| Premature birth (%) | 7.2 | 5.5 | 7.7 | 0.7851 | 0.0188 |
| Received antibiotics within 2 weeks (%) | 6.8 | 3.7 | 6.9 | 1.0000 | <0.0001 |
AOM, acute otitis media; URI, upper respiratory tract infection.
P1, statistical test between MRSA carriers and noncarriers.
P2, statistical test between MSSA carriers and noncarriers, either the chi-square test or a two-sample t test.
Data derived from those with a history of breast feeding.
Data derived from those attending day care.
Multiple logistic regression analysis has demonstrated factors including residing in northern Taiwan, subject enrollment at a later time in the study, age of 2 to 6 months, a greater number of children in the family, and day care attendance as independent factors for MRSA carriage. Subjects with a history of breast feeding and colonization by S. pneumoniae had lower MRSA carriage rates (Table 4 ). In addition to the significant demographic factors found in the MRSA model, use of antibiotics within the previous 2 weeks and prematurity were two significant variables associated with decreased incidence of MSSA colonization.
TABLE 4.
Risk factors for MRSA and MSSA carriage in Taiwanese children by multiple logistic regression analysis
| Factor | MRSA carrier vs. noncarrier |
MSSA carrier vs. noncarrier |
||
|---|---|---|---|---|
| Adjusted OR (95% confidence interval) | P | Adjusted OR (95% confidence interval) | P | |
| Colonization by S. pneumoniae | 0.697 (0.518-0.938) | 0.0170 | 0.436 (0.331-0.573) | <0.0001 |
| Residing in northern Taiwan | 1.447 (1.187-1.763) | 0.0003 | 1.405 (1.205-1.639) | <0.0001 |
| Timing of subject enrollmenta | 1.059 (1.031-1.087) | <0.0001 | ||
| Age 2-6 mo | 2.236 (1.725-2.898) | <0.0001 | 2.631 (2.133-3.245) | <0.0001 |
| Breast feeding | 0.650 (0.532-0.794) | <0.0001 | 0.767 (0.657-0.897) | 0.0009 |
| No. of children in family | 1.114 (1.002-1.240) | 0.0463 | 0.966 (0.886-1.052) | NSb |
| Day care attendance | 1.530 (1.201-1.949) | 0.0006 | 1.173 (0.955-1.440) | NS |
| Received antibiotics (<2 weeks) | 0.606 (0.417-0.880) | 0.0036 | ||
| Premature birth | 0.696 (0.511-0.946) | 0.0198 | ||
A continuous variable was created by dividing the period of study into 12 study seasons(3 months/season). The seasons were coded from 1 (July to September 2005) to 12 (April to June 2008) as shown in Fig. 2.
NS, not significant.
Molecular analysis and antimicrobial susceptibilities of MRSA isolates.
Of 473 MRSA isolates, 294 (62.2%) were randomly selected for determination of PFGE types, SCCmec types, and the presence of PVL genes. The strains included 74.5% (northern Taiwan), 53.8% (southern Taiwan), and 53.6% (central Taiwan) MRSA isolates. A total of 12 PFGE types with 71 subtypes were identified. The strains were further divided into 3 groups (Table 5). Group 1 contained isolates of four PFGE types belonging to the dominant Asian-Pacific CA-MRSA lineage, ST59, or its single-locus variant, ST338. In this group, two major clones characterized as PFGE type C/SCCmec IV/PVL−, and PFGE type D/SCCmec VT/PVL+ were predominant and identified in 59.2% and 22.8% of the 294 isolates, respectively.
TABLE 5.
Molecular characterizations of 294 MRSA nasal isolates from healthy Taiwanese children from July 2005 to June 2008
| Characteristic | No. from PFGE typea: |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 |
Group 2 |
Group 3 |
||||||||||||
| C (n = 176) | D (n = 77) | AN (n = 3) | A (n = 2) | AF (n = 7) | A (n = 4) | F (n = 3) | AK (n = 7) | AQ (n = 5) | F (n = 1) | AG (n = 1) | AR (n = 1) | BA (n = 1) | U (n = 6) | |
| SCCmec (type) | 174 (IV), 2 (VT) | 10 (IV), 67 (VT) | 3 (IV) | 2 (IV) | 7 (IIv) | 3 (III), 1 (NT) | 2 (II), 1 (IIv) | 7 (IV) | 5 (IV) | 1 (VT) | 1 (IV) | 1 (IV) | 1 (IV) | 6 (NT) |
| PVL+ | 2 | 73 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| MLST (type) | 12/12 (59) | 6/8 (59), 2/2 (338) | 1/1 (59) | 1/1 (59) | 1/1 (89) | 1/1 (239) | 1/1 (5) | 2/2 (508) | 1/1 (508) | 1/1 (9) | 1/1 (30) | 1/1 (15) | 1/1 (5) | 1/1 (573b) |
NT, nontypeable; IIv, SCCmec type II variants.
ST573 is a single-locus variant of ST1.
Group 2 contained 14 isolates carrying SCCmec II or III, which belonged to two pandemic hospital-associated (HA)-MRSA genotypes, ST5 and ST239, and a less commonly reported genotype, ST89. Isolates in group 3 consisted of 7 minor PFGE types and constituted 6 unrelated MLST types. Except for isolates of genotype U, all isolates in this group carried SCCmec IV or VT. Genotype U belonged to ST573, a single-locus variant of ST1, and harbored an untypeable SCCmec. PVL was identified in one isolate of ST30 in this group.
The analysis of the temporal trend for each genotype disclosed no significant change during the period of the study, except for genotype U in group 3. The genotype carrying an untypeable SCCmec was not detected in any isolates in the first year, appeared in the second year (1.7%), and accounted for 10.9% of the isolates tested in the third year of study (P for the trend, <0.0001). The genotype distributions were not associated with other epidemiological factors, including the geographic areas and nasopharyngeal carriage of S. pneumoniae. The MRSA isolates exhibited extremely high rates of resistance to erythromycin (92.4%), clindamycin (85.6%), and multiple (≥2) non-beta-lactam antibiotics (88.8%). Resistance to trimethoprim-sulfamethoxazole (TMP-SMX), fusidic acid, doxycycline, and glycopeptides was rare and was identified in 2.5%, 0.8%, 3.6%, and 0% of isolates, respectively.
DISCUSSION
Pooled data incorporating 8,350 patients from 10 studies of MRSA carriage (mostly in the United States) between 1998 and 2002 showed a MRSA prevalence of 1.3% (34). A much lower rate (≤0.24%) was estimated among persons without health care-associated risks. In contrast to the data from the meta-analysis, results from this study indicated a strikingly high incidence of MRSA carriage (7.8%) among healthy Taiwanese children from 2005 to 2008, with rates ranging from 6.2% to 9.5% in different geographic areas. The carriage rate in children reported here was also significantly higher than in the Taiwanese adult populations surveyed during the same period (3.8%; P < 0.0001) (37). This observation may suggest unique epidemiology for MRSA in Taiwanese children or, more likely, a universal increase in MRSA carriage in the past decade. We previously identified a sharp rise in the MRSA carriage rate in children living in northern Taiwan from 1.9% in 2001 and 2002 to 9.5% in 2005 and 2006 (20, 21). The carriage rate appeared to plateau at 9.5% in this study. The incidence of MRSA carriage in southern Taiwan also increased from 3.3% in 2001 to 6.7% in 2005 and 2006, and further to 7.7% by 2008 (20, 28). A lower rate of MRSA carriage was identified in central Taiwan, but it still increased from 4.8% in 2005 and 2006 to 6.2% in 2005 to 2008 (20).
Two major clones with multidrug resistance belonging to the ST59 lineage accounted for the increasing incidence of MRSA colonization. The two clones also predominated in the clinical CA-MRSA isolates that have been identified in nearly 60% of CA S. aureus infections in Taiwan (18). These observations clearly revealed an accelerated, island-wide transmission of MRSA in the community. Children harboring MRSA played an important role in this changing epidemiology and could be one of the major forces boosting the incidence of CA-MRSA infections in previously healthy individuals.
The factors associated with CA-MRSA carriage in children have never been comprehensively surveyed. In this study, we collected samples from different geographic areas over a 3-year span, which allowed us to simultaneously analyze the demographic, environmental, and host factors influencing MRSA colonization. The interplay between S. aureus and another common pediatric pathogen, S. pneumoniae, was also explored, while the other epidemiological factors were controlled. It was intriguing that, except for the demographic factors, there were no host or environmental factors that increased the risk of MSSA colonization. Furthermore, a variety of factors, including recent exposure to antibiotics and premature birth history, surrogates for exposure to health care facilities and antimicrobial agents, were associated with a decreasing incidence of MSSA carriage. MRSA appeared not to be affected by these factors. The findings indicated a contributing role of the emergence of antibiotic resistances in MRSA transmission within the community. Moreover, crowded environments, such as living with a greater number of children and attending day care, significantly increased the risk of MRSA colonization. These observations may explain the increasing incidence of MRSA colonization in the past decade while the MSSA colonization rate remained stable during the same period.
The innate immunity of the host has been implicated in the mechanisms of S. aureus colonization (26, 32). Our finding that a history of breast feeding and the feeding period after birth affected the colonization rates of MRSA and MSSA may be explained by the involvement of host humoral immunity. The factor was not previously reported to be associated with S. aureus colonization. Further studies are needed to explore the roles of active and passive immunity in CA-MRSA nasal colonization.
The inverse relationship between S. aureus and S. pneumoniae colonization has been previously observed among both healthy children and children with acute illness (2, 29). The mechanisms of microbial interference were not completely understood and may involve the bactericidal effect of S. pneumoniae against S. aureus mediated by the production of hydrogen peroxide (33). The inhibiting effect on S. aureus colonization seemed restricted to hosts without HIV-1 infections and specific to S. pneumoniae serotypes included in the 7-valent pneumococcal conjugate vaccine (1, 29). Our analysis further indicated that colonization by S. pneumoniae had a significantly greater impact on the colonization rate of MSSA than on that of MRSA (odds ratio [OR], 1.61; P = 0.0165), which supported the idea that the epidemic CA-MRSA was less susceptible to environmental factors. However, the finding was in contrast to a report from South Africa investigating a group of children with a high rate (67.3%) of HIV-1 infection and hospitalization for severe pneumonia (29). There was a negative correlation between S. pneumoniae and MRSA, but not MSSA. Multiple factors may be responsible for these observations, including the patients' ethnicities, HIV infection, and the presence of severe pneumonia. The MRSA isolates in the current study were from healthy children and belonged to the epidemic CA-MRSA clone, which may behave differently than the MRSA strains identified in the South African patients with high rates of HIV-1 infection.
Most of the MRSA isolates (82%) in the current study belonged to one of two major clones, characterized as PFGE C/SCCmec IV/PVL− and PFGE D/SCCmec VT/PVL+. These isolates were from a single genetic lineage, ST59, and demonstrated high rates of resistance to several non-beta-lactam antibiotics. The PFGE D/SCCmec VT/PVL+ clone, though accounting for a smaller part (22.8%) of the colonizing isolates in this survey, were identified in a majority (69%) of clinical CA-MRSA isolates in one prospective study in children between 2004 and 2005 (18). In the same study, the PFGE C/SCCmec IV/PVL− clone was responsible for only 8.8% of the clinical isolates. The discrepancy of clonal distributions in clinical and colonizing isolates was also identified in other studies (6, 37). The finding suggested greater virulence of the clone positive for PVL than of its sister clone negative for PVL. Another study is under way to investigate the virulence of CA-MRSA by comparing the two major clones in Taiwan.
Although they accounted for only 7.5% of all MRSA isolates, emerging CA genotypes (carrying type IV, V, or untypeable SCCmec in group 3) appeared to be increasingly identified during the period of the study. Genotype U, belonging to ST573 and carrying an untypeable SCCmec, was of particular interest due to its rapid increase in nasal colonization. The finding indicated that the overall increase of MRSA carriage in Taiwanese children was in part due to the appearance of emerging MRSA clones in the community. The impact of the emerging clones remained unclear and requires further molecular study of clinical CA-MRSA isolates.
The high rates of multidrug resistance of CA-MRSA in the current study were not observed in several prevalent CA-MRSA clones in other areas of the world (2). The characteristics of the CA-MRSA strains complicated the treatment of CA S. aureus infections and limited the choice of antimicrobial agents in putative S. aureus infections in previously healthy children (4). However, most isolates from this study were susceptible to TMP-SMX and fusidic acid, which appeared to be an attractive alternative choice for empirical therapy of putative CA S. aureus infections in Taiwanese children with noninvasive diseases.
There were several limitations in this study. First, the nose and pharynx are not the only sites of S. aureus colonization. The rate of S. aureus colonization may be underestimated without simultaneous swabs of other body sites (e.g., axillae, groin, and anus). Second, some epidemiological factors influencing MRSA and MSSA carriage may not have been collected. For instance, we did not sample the household members whose status of S. aureus carriage may have influenced the study subjects (16). Information related to exposure to health care facilities was not available for the other household members; however, we believe this should not be an important factor in the study. The type II or III staphylococcal chromosomal cassette mec elements, presumed to be characteristic of HA-MRSA isolates, were carried by very few MRSA isolates (4.8%) (Table 5) in this study. This finding suggested that the dominant HA-MRSA isolates have very limited, if any, spread in the community. Third, subjects were recruited from three main hospitals; therefore, the prevalence of MRSA and MSSA carriage may not be generalized to the entire pediatric population in Taiwan. We noted that the MRSA carriage rate varied significantly from site to site. However, the impact of these geographic differences and temporal trends was controlled in the multivariate analysis of epidemiological risks. The significance of the identified factors associated with MRSA and MSSA carriage should be valid in and applicable to the general population of preschool age children in Taiwan.
In summary, we observed a high rate and an increasing incidence of MRSA colonization in preschool age Taiwanese children between 2005 and 2008. The carriage rate varied with age and within different geographic areas. Crowded environments increased the risk of MRSA colonization, whereas a history of breast feeding and colonization by S. pneumoniae were protective from MRSA colonization in healthy children. Compared to MSSA, MRSA appeared to be less susceptible to environmental factors, including microbial interference by S. pneumoniae. This characteristic can contribute to the increasing incidence of CA-MRSA colonization and infections in previously healthy hosts and may partly explain the worldwide spread of this “superbug.”
Acknowledgments
We thank Keiichi Hiramatsu and Chi-Chien Wang for providing control strains of MRSA for SCCmec typing.
The work was supported by grants from Chang Gung Memorial Hospital (CMRPG450121, CMRPG450122). The funding organization had no role in the design and conduct of the study and the collection, analysis, and interpretation of the data.
Footnotes
Published ahead of print on 17 November 2010.
REFERENCES
- 1.Bogaert, D., et al. 2004. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 363:1871-1872. [DOI] [PubMed] [Google Scholar]
- 2.Boyle-Vavra, S., and R. S. Daum. 2007. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine leukocidin. Lab. Invest. 87:3-9. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Diseases Control and Prevention. 2003. Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections—Los Angeles County, California, 2002-2003. MMWR Morb. Mortal. Wkly. Rep. 52:88. [PubMed] [Google Scholar]
- 4.Chen, C. J., et al. 2007. Experience with linezolid therapy in children with osteoarticular infections. Pediatr. Infect. Dis. J. 26:985-988. [DOI] [PubMed] [Google Scholar]
- 5.Chen, C. J., et al. 2007. Clinical features and molecular characteristics of invasive community-acquired methicillin-resistant Staphylococcus aureus infections in Taiwanese children. Diagn. Microbiol. Infect. Dis. 59:287-293. [DOI] [PubMed] [Google Scholar]
- 6.Chen, F. J., et al. 2005. Methicillin-resistant Staphylococcus aureus in Taiwan. Emerg. Infect. Dis. 11:1760-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial disk susceptibility tests; approved standard, 9th ed. CLSI, Wayne, PA.
- 8.Creech, C. B., II, D. S. Kernodle, A. Alsentzer, C. Wilson, and K. M. Edwards. 2005. Increasing rates of nasal carriage of methicillin-resistant Staphylococcus aureus in healthy children. Pediatr. Infect. Dis. J. 24:617-621. [DOI] [PubMed] [Google Scholar]
- 9.Deleo, F. R., M. Otto, B. N. Kreiswirth, and H. F. Chambers. 2010. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375:1557-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis, M. W., D. R. Hospenthal, D. P. Dooley, P. J. Gray, and C. K. Murray. 2004. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin. Infect. Dis. 39:971-979. [DOI] [PubMed] [Google Scholar]
- 11.Enright, M. C., N. P. Day, C. E. Davies, S. J. Peacock, and B. G. Spratt. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert, M., et al. 2006. Outbreak in Alberta of community-acquired (USA300) methicillin-resistant Staphylococcus aureus in people with a history of drug use, homelessness or incarceration. CMAJ 175:149-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez, B. E., et al. 2005. Severe Staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics 115:642-648. [DOI] [PubMed] [Google Scholar]
- 14.Groom, A. V., et al. 2001. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA 286:1201-1205. [DOI] [PubMed] [Google Scholar]
- 15.Herold, B. C., et al. 1998. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 279:593-598. [DOI] [PubMed] [Google Scholar]
- 16.Huang, Y. C., et al. 2009. Association of Staphylococcus aureus colonization in parturient mothers and their babies. Pediatr. Infect. Dis. J. 28:742-744. [DOI] [PubMed] [Google Scholar]
- 17.Huang, Y. C., Y. H. Chou, L. H. Su, R. I. Lien, and T. Y. Lin. 2006. Methicillin-resistant Staphylococcus aureus colonization and its association with infection among infants hospitalized in neonatal intensive care units. Pediatrics 118:469-474. [DOI] [PubMed] [Google Scholar]
- 18.Huang, Y. C., C. F. Ho, C. J. Chen, L. H. Su, and T. Y. Lin. 2008. Comparative molecular analysis of community-associated and healthcare-associated methicillin-resistant Staphylococcus aureus isolates from children in northern Taiwan. Clin. Microbiol. Infect. 14:1167-1172. [DOI] [PubMed] [Google Scholar]
- 19.Huang, Y. C., C. F. Ho, C. J. Chen, L. H. Su, and T. Y. Lin. 2007. Nasal carriage of methicillin-resistant Staphylococcus aureus in household contacts of children with community-acquired diseases in Taiwan. Pediatr. Infect. Dis. J. 26:1066-1068. [DOI] [PubMed] [Google Scholar]
- 20.Huang, Y. C., K. P. Hwang, P. Y. Chen, C. J. Chen, and T. Y. Lin. 2007. Prevalence of methicillin-resistant Staphylococcus aureus nasal colonization among Taiwanese children in 2005 and 2006. J. Clin. Microbiol. 45:3992-3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, Y. C., L. H. Su, C. J. Chen, and T. Y. Lin. 2005. Nasal carriage of methicillin-resistant Staphylococcus aureus in school children without identifiable risk factors in northern Taiwan. Pediatr. Infect. Dis. J. 24:276-278. [DOI] [PubMed] [Google Scholar]
- 22.Huang, Y. C., et al. 2004. Molecular epidemiology of clinical isolates of methicillin-resistant Staphylococcus aureus in Taiwan. J. Clin. Microbiol. 42:307-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kazakova, S. V., et al. 2005. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N. Engl. J. Med. 352:468-475. [DOI] [PubMed] [Google Scholar]
- 24.King, M. D., et al. 2006. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA300 clone as the predominant cause of skin and soft-tissue infections. Ann. Intern. Med. 144:309-317. [DOI] [PubMed] [Google Scholar]
- 25.Kuehnert, M. J., et al. 2006. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001-2002. J. Infect. Dis. 193:172-179. [DOI] [PubMed] [Google Scholar]
- 26.Li, M., et al. 2009. Staphylococcus aureus mutant screen reveals interaction of the human antimicrobial peptide dermcidin with membrane phospholipids. Antimicrob. Agents Chemother. 53:4200-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lina, G., et al. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29:1128-1132. [DOI] [PubMed] [Google Scholar]
- 28.Lu, P. L., et al. 2005. Risk factors and molecular analysis of community methicillin-resistant Staphylococcus aureus carriage. J. Clin. Microbiol. 43:132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNally, L. M., et al. 2006. Lack of association between the nasopharyngeal carriage of Streptococcus pneumoniae and Staphylococcus aureus in HIV-1-infected South African children. J. Infect. Dis. 194:385-390. [DOI] [PubMed] [Google Scholar]
- 30.Otto, M. 2010. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu. Rev. Microbiol. 64:143-162. [DOI] [PubMed] [Google Scholar]
- 31.Pan, E. S., et al. 2003. Increasing prevalence of methicillin-resistant Staphylococcus aureus infection in California jails. Clin. Infect. Dis. 37:1384-1388. [DOI] [PubMed] [Google Scholar]
- 32.Peschel, A. 2002. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 10:179-186. [DOI] [PubMed] [Google Scholar]
- 33.Regev-Yochay, G., K. Trzcinski, C. M. Thompson, R. Malley, and M. Lipsitch. 2006. Interference between Streptococcus pneumoniae and Staphylococcus aureus: in vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae. J. Bacteriol. 188:4996-5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salgado, C. D., B. M. Farr, and D. P. Calfee. 2003. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin. Infect. Dis. 36:131-139. [DOI] [PubMed] [Google Scholar]
- 35.Vandenesch, F., et al. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9:978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N. Engl. J. Med. 344:11-16. [DOI] [PubMed] [Google Scholar]
- 37.Wang, J. T., et al. 2009. Prevalence of and risk factors for colonization by methicillin-resistant Staphylococcus aureus among adults in community settings in Taiwan. J. Clin. Microbiol. 47:2957-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zinderman, C. E., et al. 2004. Community-acquired methicillin-resistant Staphylococcus aureus among military recruits. Emerg. Infect. Dis. 10:941-944. [DOI] [PMC free article] [PubMed] [Google Scholar]