Abstract
Phosphorylation represents one the most abundant and important posttranslational modifications of proteins, including viral proteins. Virus-encoded serine/threonine protein kinases appear to be a feature that is unique to large DNA viruses. Although the importance of these kinases for virus replication in cell culture is variable, they invariably play important roles in virus virulence. The current review provides an overview of the different viral serine/threonine protein kinases of several large DNA viruses and discusses their function, importance, and potential as antiviral drug targets.
Protein kinases are characterized by their potential to catalyze the transfer of a phosphate group from a nucleoside triphosphate (generally ATP) to an amino acid residue of a protein substrate. This phosphorylation usually results in a functional change of the target protein by interfering with its enzymatic activity, cellular location, and/or association with other proteins. Depending on the specificity of the substrate amino acids, protein kinases are subdivided in serine/threonine (S/T) or tyrosine (Tyr) kinases. In addition, rare dual-specificity kinases have also been identified. Protein kinases phosphorylate either a serine, a threonine, or a tyrosine residue when it is present in a specific stretch of other amino acids, known as the consensus sequence. The catalytic core of protein kinases consists of 12 conserved subdomains that fold into a common structure. Several strongly conserved residues were identified as indispensable for kinase activity, such as the conserved lysine in subdomain VIb, which is critical for ATP binding, and the conserved aspartate in the catalytic loop (subdomain VIb) (77). These residues are frequently used as targets for mutagenesis in the construction of kinase-negative mutants.
The human genome contains about 500 protein kinase genes, constituting approximately 2% of all human genes (127). Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction (127).
Since viruses have evolved complex interactions with their host and often mimic cellular proteins in order to usurp the cellular machinery for their own benefit, it might not be surprising that several viruses encode protein kinases. In the late 1970s, the first reports speculating about the existence of viral kinases were published (40, 206); it was only 10 years later that the first viral kinases were identified (115, 117). Over the last few years, several new insights have been gained concerning the often pleiotropic functions of viral protein kinases.
Both serine/threonine and tyrosine viral protein kinases have been identified. The best-characterized viral tyrosine kinases are the oncogenic tyrosine kinases encoded by acute transforming retroviruses. These kinases are captured forms of normal cellular genes (proto-oncogenes), and this incorporation may actually be considered an “accident de parcours” during virus replication, typically rendering the virus replication defective. These viral tyrosine kinases will not be covered in this review but have been covered elsewhere (126). Viral serine/threonine kinases, on the other hand, often display little homology to any of the known cellular kinases. They appear to be encoded exclusively by large DNA viruses and form the subject of the current review.
HERPESVIRUSES
Herpesviruses are large, enveloped viruses with a linear, double-stranded DNA genome that varies in size from 125 to 245 kb. The majority of herpesviruses have been classified in one of three subfamilies: the alpha-, beta-, and gammaherpesviruses. Eight human herpesviruses have been described: the alphaherpesviruses herpes simplex virus 1 (HSV-1) and HSV-2 and varicella-zoster virus (VZV), the betaherpesviruses human cytomegalovirus (HCMV) and human herpesvirus 6 (HHV-6) and HHV-7, and the gammaherpesviruses Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV).
Invariably, primary infections are followed by lifelong persistence. Specific circumstances may trigger reactivation, with or without viral shedding and/or clinical symptoms. Disease symptoms vary greatly, and include cold sores (HSV-1), genital lesions (HSV), chickenpox (VZV), shingles (VZV), congenital disease (HCMV), posttransplant diseases (HCMV), roseola (HHV-6, -7), infectious mononucleosis (EBV, HCMV), lymphoproliferative disorders (EBV), and Kaposi's sarcoma (KSHV).
All herpesviruses encode serine/threonine kinases. Two types of conserved herpesvirus serine/threonine kinases have been described. One type is conserved over the three different subfamilies. The prototypical example of this kinase is the UL13 kinase of HSV. These conserved kinases will be designated conserved herpesvirus protein kinases (CHPK) throughout the text. Another type of herpesvirus protein kinases, exemplified by the US3 kinase of HSV, is conserved in the alphaherpesvirus subfamily. In addition, serine/threonine protein kinase activity has been suggested to be present in the large subunit of the ribonucleotide reductase of HSV-2.
CHPK.
Orthologs of CHPK include UL13 of HSV, ORF47 of VZV, UL97 of HCMV, U69 of HHV-6, BGLF4 of EBV, and ORF36 of KSHV and murine herpesvirus 68 (MHV-68).
In 1989, by using sequencing assays, Chee and colleagues identified a gene encoding a putative protein kinase that appeared to be conserved in the herpesvirus family, since it was present in the alphaherpesviruses HSV-1 and VZV, the betaherpesviruses HCMV and HHV-6, and the gammaherpesvirus EBV (35). The corresponding protein was confirmed a few years later to display serine protein kinase activity for the CHPK of the alphaherpesviruses VZV and porcine pseudorabies virus (PRV) (54, 159). In vitro studies using cell cultures and primary cells showed that the importance of CHPK for virus growth in vitro varies from crucial for viral replication to unimportant, depending on both the virus and the cell (45, 184, 185). In vivo studies, however, invariably point to an important role of the kinase in virus virulence (45, 86, 150, 180). Although the kinase is conserved among herpesviruses, CHPK from different herpesvirus subfamilies show considerable variation in amino acid sequence. Although some sequence preferences for substrate specificity have been described for individual CHPK, there is no consensus phosphorylation sequence for all CHPK (10, 30, 95, 96, 100).
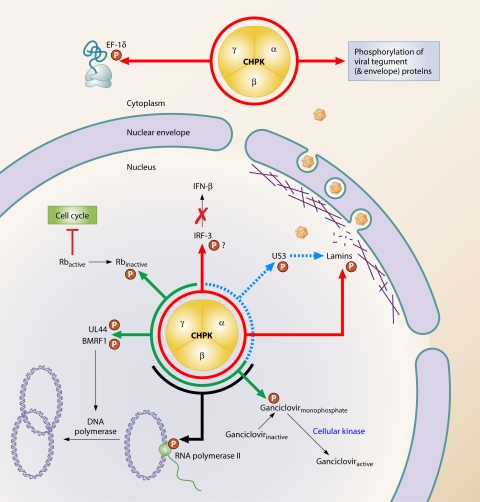
Despite these differences, partial functional conservation has been reported (193). Characteristics and functions of CHPK that appear to be conserved to some extent include its autophosphorylating activity, tegument incorporation, phosphorylation of cellular elongation factor 1 delta (EF-1δ), evasion of the interferon (IFN) response, and phosphorylation of ganciclovir. Specific subfamily- and virus-specific functions have also been described. Several of the CHPK funtions are shown in Fig. 1, and different substrates are shown in Table 1.
FIG. 1.
Some of the major functions associated with the conserved herpesvirus protein kinase (CHPK). Red lines indicate functions that are conserved over the three herpesvirus subfamilies. Green lines indicate functions that are common to beta- and gammaherpesviruses. Blue and black lines indicate functions that have been reported only for alpha- and betaherpesviruses, respectively.
TABLE 1.
Human CHPK and their cellular and viral substrates
| Virus | CHPK | Substrate (reference[s]) |
|
|---|---|---|---|
| Cellular | Viral | ||
| HSV-1 | UL13 | IRF-3 (82) | US3 (91) |
| EF-1δ (98) | gE, gI (161) | ||
| CKIIβ (96) | ICP0 (166) | ||
| RNA polymerase II (125) | ICP22/Us1.5 (184, 185) | ||
| p60 (26) | UL13 (46, 47) | ||
| VP13/14 (192) | |||
| VP22 (45) | |||
| UL41 (167) | |||
| HSV-2 | UL13 | Lamin A/C, limited (31) | US3 (31) |
| EF-1δ (97) | UL13 (30) | ||
| VP22 (70) | |||
| VZV | ORF47 | Akt (186) | ORF32 (187) |
| ORF47 (159) | |||
| IE62 (160) | |||
| IE63 (101) | |||
| gE (99) | |||
| HCMV | UL97 | Lamin A/C (130) | UL44 (109, 129) |
| Rb tumor suppressor (81, 181) | pp65 (88) | ||
| RNA polymerase II (12) | UL97 (79) | ||
| Histone H2B (141, 210) | |||
| IRF-3 (82) | |||
| EF-1δ (97) | |||
| HHV-6/HHV-7 | U69 | Lamin A/C (111) | U69 (4) |
| Rb tumor suppressor (111) | |||
| EBV | BGLF4 | Lamin A/C (118) | BRMF1 (36) |
| Rb tumor suppressor (111) | BGLF4 (92) | ||
| H2AX (209) | EBNA-LP (208) | ||
| IRF-3 (82, 225) | |||
| EF-1δ (92) | |||
| CKIIβ (96) | |||
| KSHV | ORF36 | Lamin A/C (111) | ORF36 (76) |
| Rb tumor suppressor (111) | |||
| IRF-3 (82) | |||
(i) Autophosphorylation and tegument incorporation.
Like many cellular serine/threonine kinases, all herpesviral CHPK orthologs appear to possess both autophosphorylation and transphosphorylation activities (95). Autophosphorylation was first demonstrated for the VZV CHPK by immunoprecipitation experiments with infected cells and by protein kinase assays (159).
The importance of autophosphorylation for the activity of CHPK orthologs is not entirely clear. For example, there have been conflicting reports on whether the autophosphorylating activity of HCMV CHPK is required for phosphorylation of exogenous substrates, such as histone 2B (10, 129, 141). Autophosphorylation has been reported to be important for the ability of the KSHV CHPK to activate JNK signaling (76).
All CHPK orthologs of HSV, VZV, HCMV, and EBV are incorporated into the virus particle (7, 168, 219). More specifically, the viral kinase is located in the tegument, a proteinaceous layer in herpesvirus virions between the capsid and the envelope.
(ii) Phosphorylation of EF-1δ.
The translation elongation factor 1 delta (EF-1δ) consists of two forms, a hypo- and a hyperphosphorylated form. In 1997, Kawaguchi et al. reported that whereas the hyperphosphorylated form of EF-1δ cells was a minor species in mock-infected cells, it became the predominant form in cells infected with HSV-1 (94). One year later, the same group identified the HSV-1 CHPK as the kinase responsible for the observed EF-1δ hyperphosphorylation (98). Hyperphosphorylation of EF-1δ was subsequently observed for members of the three different herpesvirus subfamilies, and the HCMV CHPK ortholog could compensate for the HSV CHPK to induce EF-1δ hyperphosphorylation in HSV-1-infected cells (97). EF-1δ is a subunit of EF-1, a complex of proteins which mediate the elongation of polypeptide chains during translation of mRNA. Although the physiological role of EF-1δ hyperphosphorylation is not known, this function of CHPK is likely to regulate and/or stimulate the translation process in infected cells.
The cellular cyclin-dependent kinase cdc2 also phosphorylates EF-1δ, and research on the interaction of CHPK with EF-1δ provided a first clue that CHPK orthologs to some extent mimic the function and substrate specificity of cdc2. It was demonstrated that different CHPK phosphorylate EF-1δ at the same amino acid residue (Ser-133) as for cdc2 (8, 96). Other features of CHPK, discussed below, also point toward a significant functional similarity between CHPK and cyclin-dependent kinases. However, Cano-Monreal et al. reported that the CHPK of the alphaherpesvirus HSV-2 did not phosphorylate standard cdc2 peptide substrates in vitro, suggesting that this similarity is less obvious in alphaherpesviruses (30). In support of this are the findings that all human beta- and gammaherpesvirus CHPK, except for the KSHV CHPK, are able to rescue a G1-to-S cell cycle defect in Saccharomyces cerevisiae lacking cyclin-dependent kinase function, whereas alphaherpesvirus CHPK are unable to do so (81, 111).
(iii) Nuclear egress.
Thus far, for all CHPK, a fraction has been described to be present in the nucleus of infected cells (47, 49, 71, 72, 84, 143, 169, 226), indicating that the kinase may play important, conserved roles in the nuclear phase of infection.
A function that appears to be largely conserved is a role of CHPK in nuclear egress of progeny capsids. Herpesvirus capsids assemble in the nucleus and, because of their relatively large size, need to (locally) disrupt the dense meshwork of the nuclear lamina to gain access to the nuclear envelope to continue the process of virion assembly and egress. Disassembly and reassembly of the nuclear lamina, a key process in cell division, occurs through phosphorylation and dephosphorylation of lamins, e.g., by cyclin-dependent kinases and protein kinase C. Phosphorylation of lamins during herpesvirus infection appears to involve both cellular kinases, such as protein kinase C (144, 155, 170), but also the viral CHPK kinases. This is best documented for beta- and gammaherpesvirus CHPK. Indeed, both the CHPK of EBV and that of HCMV have been reported to phosphorylate lamins (75, 118, 130), and lack of these kinases is correlated with accumulation of capsids in the nucleus (109, 180, 233). Interestingly, the CHPK of both EBV and HCMV phosphorylate lamins on residues that are also substrates for phosphorylation by cdc2 (75, 118), further highlighting the similarities in substrate specificity between the beta- and gammaherpesvirus CHPK and cdc2. Recently, lamin A/C phosphorylation was confirmed for all human beta- and gammaherpesviruses (111).
A role for CHPK of alphaherpesviruses in nuclear egress is less clear, possibly because these viruses encode a second viral kinase, US3, which appears to fulfill at least part of this function (see below). The CHPK of HSV-1 is still thought to have some function in nuclear egress, albeit indirectly, by phosphorylating the viral US3 kinase and thereby regulating the function of US3 in nuclear egress (91). In support of a combined role of CHPK and US3, deletion of both in the genome of the porcine alphaherpesvirus PRV resulted in strongly reduced production of infectious virus (54). A direct effect of alphaherpesvirus CHPK on lamins and the nuclear lamina is either absent or very limited. A recent study reported a failure to detect phosphorylation of lamin A or disruption of the nuclear lamina by HSV-1 and VZV CHPK (111). For HSV-2 CHPK, a noticeable, but limited, effect on lamin phosphorylation and nuclear lamina integrity was observed, in line with an earlier report (31, 111).
(iv) Virus replication.
Besides their role in viral nuclear egress, CHPK have been reported to play other functions in the nucleus that may affect virus replication efficiency.
In 2008, two groups independently showed that the HCMV CHPK directly phosphorylates and thereby inactivates the retinoblastoma tumor suppressor (Rb), again similar to one of the functions of cdc2 (81, 181). Recently, Rb phosphorylation was demonstrated for all human beta- and gammaherpesvirus CHPK, but not the alphaherpesvirus counterparts (111). Nevertheless, the HSV-1 CHPK has also been reported to affect regulation of the cell cycle via an increase in cellular cdc2 activity (2).
HSV-1 CHPK also promotes the expression of a subset of viral genes, including ICP22 and several late genes (2, 184, 185), although this effect appears to be independent of the kinase activity of the protein (207). Interestingly, the CHPK of the betaherpesvirus HCMV could compensate for the loss of HSV CHPK with regard to its role in viral gene expression (162), demonstrating that there is some functional overlap between alpha- and betaherpesvirus CHPK members.
Deletion of the CHPK in HCMV results in reduced viral gene expression (109, 233). In vitro kinase assays showed that the HCMV CHPK is able to directly phosphorylate RNA polymerase II, which is also phosphorylated in HCMV-infected cells (12). Inhibition of this phosphorylation by the addition of roscovitine resulted in defective immediate-early gene expression, suggesting a causal relationship between RNA polymerase II phosphorylation and increased viral gene expression (205). However, it is unclear whether the effect of roscovitine can be attributed solely to the CHPK. Roscovitine is a purine derivative that also inhibits several cellular cyclin-dependent kinases by competing with ATP binding, and as such is being used in clinical trials as a treatment for various tumors (110, 135, 196).
Reduced viral gene expression observed in the absence of the HCMV CHPK has also been attributed to the modest reduction in viral DNA accumulation observed with a CHPK-deleted virus (109, 233). This defect in DNA accumulation may correlate with the finding that this CHPK phosphorylates the viral DNA polymerase processivity factor UL44, an essential component of the replication complex (109, 129). Like the HCMV CHPK, the CHPK of EBV phosphorylates the DNA processivity factor BMRF1, which is homologous to HCMV UL44 (36, 65). Phosphorylation of BMRF1 may affect its transactivation activity (234). EBV CHPK also phosphorylates the EBV coactivator EBNA-LP, one of the EBV proteins involved in B-cell transformation. Phosphorylation of EBNA-LP is important for its function, but phosphorylation does not critically rely on CHPK, since cellular cdc2 is able to phosphorylate EBNA-LP on the same residue (93).
Effects of CHPK on viral gene expression may also be related to phosphorylation of histones. In vitro, HCMV CHPK phosphorylates histone H2B, which has also been hypothesized to affect gene expression (11). More recently, CHPK of EBV and MHV-68 were found to induce phosphorylation of histone H2AX, in contrast to the CHPK counterparts of the related virus KSHV and the more distantly related virus HSV (209). Phosphorylated H2AX is involved in the cellular DNA damage response (28). Lack of MHV-68 CHPK or H2AX resulted in inefficient MHV-68 replication in primary macrophages, indicating that a virus-activated DNA damage response facilitates MHV-68 lytic replication (209). Recently, the lack of CHPK or H2AX was also correlated with decreased levels of MHV-68 latency in mice (210). Clearly, more research is needed to clarify the role of CHPK in viral gene expression, DNA replication, and viral pathogenesis.
(v) Evasion of the interferon response.
A potential role for CHPK in evasion of the IFN response was first suggested for HSV-1. In 2001, Shibaki et al. reported that a CHPK-null virus was rapidly cleared upon intraperitoneal inoculation of mice. The authors showed that this correlated with increased levels of type I IFN induced by the mutant virus and greater sensitivity of the mutant toward the antiviral effects of type I IFN (200). Later, HSV-1 was found to induce the expression of a negative regulator of IFN, suppressor of cytokine signaling 3 (SOCS-3). Deletion of the CHPK resulted in less efficient induction of SOCS-3 (236).
Recently, the involvement of CHPK orthologs in counteracting the interferon response was further substantiated and was found to be conserved over the three different herpesvirus subfamilies. The CHPK of HSV-1, HCMV, EBV, KSHV, and MHV-68 were all found to subvert the type I IFN response by suppressing the activity of interferon regulatory factor 3 (IRF-3) (82, 225). The viral kinase interacts with activated IRF-3, thereby interfering with IRF-3 recruitment to the IFN promoter (82, 225). Although the interaction of EBV CHPK with IRF-3 leads to phosphorylation of IRF-3 (225), the kinase activity of the CHPK does not appear to be absolutely required for its effect on the IFN response (82).
(vi) Phosphorylation of viral tegument and membrane proteins.
CHPK-mediated phosphorylation of tegument proteins (other than the CHPK itself) and envelope proteins is best documented for alphaherpesviruses.
The CHPK of VZV is essential for infection of differentiated human T cells and skin xenografts in SCID-hu mice (150). The VZV CHPK phosphorylates the tegument proteins IE62 and IE63 (101, 102, 160), and phosphorylation of IE62 has been correlated with CHPK-mediated virulence in SCID-hu mice. IE62 is the major immediate-early transactivating protein, and the interaction between ORF47 and IE62, rather than IE62 phosphorylation per se, appears to be a pivotal element in CHPK-mediated virulence in SCID-hu mice (19).
The VZV CHPK also phosphorylates the viral glycoprotein gE (99). Kenyon et al. reported that ORF47-mediated phosphorylation of gE may be associated with the surprising increase in virus spread observed for a CHPK-null VZV recombinant in MeWo cells. Phosphorylation by the CHPK redirected gE to the plasma membrane instead of the trans-Golgi network, where herpesvirus budding is thought to occur, possibly explaining the suppressive effect on viral spread (99).
The HSV CHPK also phosphorylates gE and its complexing partner gI (159). In addition, the CHPK of HSV phosphorylates the tegument proteins VP13/14 and VP22, which may facilitate their release from the capsid during entry (45, 70, 152). Alphaherpesvirus CHPK-mediated phosphorylation of tegument and/or cellular proteins in combination with phosphorylation by the alphaherpesvirus kinase US3 (see below) may regulate the directionality of microtubule-based virus transport in neuronal axons (39). HSV CHPK also phosphorylates the virion host shutoff protein (UL41), a viral tegument protein involved in shutting down host translation. Inactivation of CHPK resulted in the production of virions that did not possess host shutoff activity, although they did incorporate UL41 (167).
For HCMV, the CHPK interacts with and phosphorylates the tegument protein pp65 in vitro (88). Although the biological consequences of this phosphorylation are unclear, in the absence of CHPK activity, HCMV-infected cells produce large nuclear aggregates that contain substantial quantities of pp65 (179). The association of HCMV CPHK with pp65 most likely explains the protein kinase activity associated with immunoprecipitates of pp65 (25, 88), although pp65 itself has also been suggested to display kinase activity (201, 235).
(vii) Phosphorylation of GCV.
The remarkable success of the nucleoside analogue acyclovir (ACV) in antiviral therapy against HSV and VZV is based on its selectivity: the viral thymidine kinase converts ACV to its monophosphate form and cellular kinases then further process it to its active triphosphate form. Ganciclovir (GCV) was synthesized in 1980 at Syntex Laboratories as an analogue of acyclovir with an improved structural resemblance to natural nucleosides. GCV turned out to display potent antiviral activity against HCMV. This was somewhat surprising, since HCMV does not encode a thymidine kinase-like enzyme. In 1992, the CHPK of HCMV was found to be responsible for the conversion of GCV to its monophosphate form (123, 202). Hence, the HCMV CHPK possesses the unusual ability to act as both a protein kinase and a nucleoside kinase. The lack of homology to any known nucleoside kinase and the inability of the HCMV CHPK to phosphorylate natural nucleosides indicate that it is not a natural nucleoside kinase (143). Besides GCV, this CHPK also phosphorylates ACV and penciclovir (238). The ability of CHPK orthologs to phosphorylate GCV is conserved with various levels of efficiency in some, but not all, betaherpesviruses and gammaherpesviruses, but apparently not in alphaherpesviruses (4, 29, 136, 142, 193, 222).
US3 Protein kinase orthologs.
In the second part of the 1980s, the US3 orthologs of HSV-1 and -2 and VZV were reported to show homology to eukaryotic protein kinases (133) and soon thereafter, by using antisera and a kinase assay, HSV US3 was identified as a bona fide viral protein kinase (67). The US3 kinase is conserved throughout the alphaherpesvirus subfamily but is not present in other herpesvirus genomes. The consensus target sequence for US3 of the porcine alphaherpesvirus PRV was characterized as RnX(S/T)ZZ, where n is greater than or equal to 2; X can be absent or Arg, Ala, Val, Pro, or Ser; and Z can be any amino acid except an acidic residue (116, 183) and was found to be broadly similar for HSV-1 and -2 and VZV (48, 58, 183) and the cellular protein kinase A (17, 89). Recent findings indicate that HSV-1 US3 is more promiscuous than previously thought and suggest the existence of more substrates than originally predicted (153). US3 autophosphorylation at a site that corresponds to serine 147 in HSV-1 has been reported to affect US3 function and localization in both HSV-1 and HSV-2 (90, 151). Several possible US3 phosphorylation substrates have been proposed, but few have been confirmed as biologically relevant thus far.
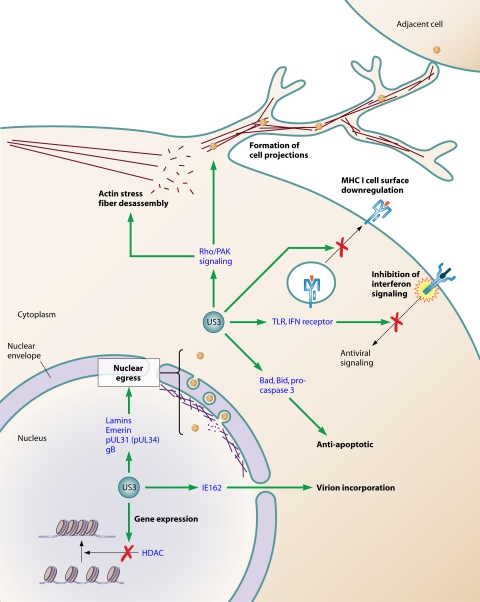
Based on studies showing that recombinant US3 mutant viruses have modestly impaired growth properties in cell cultures but are severely attenuated in animal models, US3 was identified as a positive regulator of viral replication and viral pathogenicity (134, 191, 218). Several lines of evidence suggest the involvement of US3 in a variety of functions, some of which are conserved among the alphaherpesviruses, while others appear to be unique for a specific virus. The kinase activity of US3 was shown to be crucial for most of its functions. Different functions of US3 are shown in Fig. 2.
FIG. 2.
Major functions associated with US3 kinase orthologs of alphaherpesviruses. Proteins in blue represent US3 substrates that may be involved in the different functions of US3.
(i) Nuclear egress.
In 1995, a first report was published pointing at the involvement of US3 in nuclear egress (221). Alphaherpesviruses, and herpesviruses in general, use a unique system to transport progeny nucleocapsids out of the nucleus and into the cytoplasm. Nucleocapsids undergo primary envelopment by budding in the inner nuclear membrane, followed by fusion with the outer nuclear membrane, releasing the virion into the cytoplasm (reviewed in reference 140). In cells infected with US3-null PRV, HSV-1, and Marek's disease virus (MDV), virions aggregate aberrantly within the perinuclear space in large invaginations (105, 191, 198, 221), suggesting a conserved role for the US3 kinase in the de-envelopment step during nuclear egress. This defect in capsid nuclear export is, however, not absolute, since extracellular virus titers are typically only mildly reduced in the absence of US3 (39, 191, 194, 215, 218).
Since these initial reports, US3 has been implicated in different steps of the nuclear egress pathway. First, lamin A/C and emerin, key elements of the nuclear lamina network, can be phosphorylated by HSV-1 US3 (114, 153). This leads to the disruption of the nuclear lamina, which represents a barrier for the virions to reach the inner nuclear membrane. Second, infection with US3-null HSV-1 or PRV results in an altered distribution of the viral UL34 and UL31 proteins, both of which are crucial regulators of primary envelopment of nucleocapsids at the nuclear membrane, from a roughly continuous distribution to a distribution in discrete aggregates in the inner nuclear membrane (105, 190). This relocalization of the envelopment machinery was recently found to be regulated by phosphorylation of the N terminus of UL31 by HSV-1 US3 (154). A third potential involvement of US3 in nuclear egress has also been put forward. HSV-1 US3 phosphorylates the cytoplasmic domain of the envelope glycoprotein gB (83). The gB protein is one of the viral proteins that is essential to mediate fusion between envelope and host membrane during viral entry. For HSV-1, gB, together with gH, has been suggested to be involved in the fusion between the primary enveloped virion in the perinuclear space and the outer nuclear membrane (232). Recent evidence indicates that phosphorylation of gB by US3 drives this process (232). The latter may not be a conserved function of alphaherpesviruses since, for PRV, neither gB nor gH appears to be present at the nuclear membranes and neither protein functions in the nuclear egress of virions (74, 104).
(ii) Antiapoptotic activity.
US3 possesses antiapoptotic properties in HSV, PRV, and MDV, but possibly not in bovine herpesvirus 1 (BoHV-1) (9, 69, 119, 197, 204). The kinase activity of HSV-1, PRV, and MDV US3 is necessary for this US3 function (33, 52), and several phosphorylation targets implicated in the HSV-1 US3-mediated suppression of apoptosis have been identified, including Bad, Bid, and procaspase 3, pointing at the involvement of US3 in different antiapoptotic pathways (18, 32). This may explain how US3 is able to inhibit apoptosis induced by very diverse apoptotic stimuli, including herpesvirus infection itself, cytotoxic T lymphocytes and granzyme B, overexpression of BclX, staurosporine, and sorbitol (9, 32, 33, 78, 87, 119, 157, 165).
(iii) Actin rearrangements.
The US3 kinase induces drastic rearrangements of the cytoskeleton, including disassembly of actin stress fibers and the formation of actin- and microtubule-containing cellular projections, which are implicated in intercellular spread (63). The kinase activity of US3 is essential for these processes in HSV-2, PRV, and BoHV-1, but not in MDV, suggesting different or multiple cytoskeleton-affecting activities of US3 (27, 66, 156, 197, 215). Recent evidence with PRV US3 indicates that the kinase interferes with actin-controlling Rho-GTPase signaling to cause the cytoskeletal alterations. PRV US3 phosphorylates and thereby activates p21-activated kinases (PAKs), key regulators of Rho GTPase signaling (216).
(iv) Gene expression.
Over the last couple of years, it has become evident that US3 also affects gene expression. US3 orthologs of HSV-1, HSV-2, PRV, and VZV phosphorylate HDAC1 and -2, thereby inhibiting the deacetylation of histones, which otherwise silence gene expression (151, 176, 223, 224). US3-mediated phosphorylation of HDAC2 occurs at a C-terminally located conserved serine residue (224). HSV-1 US3 is able to phosphorylate HDAC-1 and -2 directly (177, 178), although there are indications that VZV, PRV, and HSV-1 phosphorylate HDAC indirectly by activating a cellular kinase pathway (223, 224). For PRV, hyperphosphorylation of HDAC2 was still observed in cells infected with a US3-null recombinant (224). This is in contrast to findings for HSV-1 and VZV and indicates virus-specific US3-independent mechanisms of HDAC hyperphosphorylation. Inhibition of HDAC activity increased plaquing efficiency of US3-null virus for PRV and VZV, but not for HSV-1, pointing to virus- and cell-dependent differences in the functional significance of US3-mediated HDAC modification (223, 224).
An additional role in gene expression has been described for the US3 ortholog of VZV, ORF66. Both ORF66 and the CHPK of VZV have a unique viral substrate, IE62, a nuclear transcription regulatory protein. After phosphorylation by ORF66, IE62 accumulates in the cytoplasm, where it is incorporated in newly formed virions. This points at a role for the VZV US3 ortholog in reducing nuclear import of IE62 and thereby regulating gene expression (58, 60).
(v) Immune evasion.
Apart from their ability to protect cells from apoptotic cell death, US3 orthologs have been linked to other aspects of evasion of the host immune response. The VZV ORF66 was reported to be involved in downregulating the surface expression of the major histocompatibility complex class I (MHC-I) in VZV-infected cells and ORF66-transfected cells (1, 59), suggesting a role for ORF66 in immune evasion. The kinase activity of ORF66 protein kinase was beneficial but not absolutely required for MHC-I downregulation (59). Recently, PRV US3 was also shown to be able to downregulate MHC-I surface expression. However, this ability was highly cell-type dependent. The underlying mechanism is unclear but appears different from that for VZV ORF66 since transfection of PRV US3 did not affect MHC-I downregulation (53). Still, the potential effect of US3 orthologs on MHC-I surface expression appears to be cell-type dependent and not always very robust; therefore, further investigation may be needed to determine its importance.
For VZV- and PRV-infected cells, the lack of US3 reduced the capacity of the virus to interfere with induction of the IFN signaling pathway following exposure to IFN (175, 195). Recently, Peri et al. reported that US3-null HSV-1 induces a strong activation of IRF-3 and type I IFN mRNA expression and that HSV-1 US3 downregulates the intracellular expression of TLR3 and the type I interferon-inducible protein MxA, which is known to posses antiviral activity (171). In addition, HSV-1 US3 phosphorylates the gamma interferon (IFN-γ) receptor, which interferes with IFN-γ-dependent gene expression (120).
Ribonucleotide reductase.
Alphaherpesviruses, like other herpesviruses, encode a ribonucleotide reductase that is involved in generating deoxyribonucleotides. Therefore, these viruses do not depend on cellular S-phase enzymes, allowing them to replicate in nondividing cells, including neuronal cells (34). The ribonucleotide reductase consists of two subunits, R1 and R2. The large R1 subunit (ICP10) of the HSV-2 ribonucleotide reductase has been suggested to contain protein kinase activity, which may be responsible for the antiapoptotic activity associated with ICP10 (173). Inhibition of apoptosis by ICP10 involves a c-Raf-1-dependent mechanism and induction of the antiapoptotic protein Bag-1 by the activated ERK survival pathway. ICP10 is also responsible for an increased activation/stability of the transcription factor CREB and stabilization of the antiapoptotic protein Bcl-2 (172). This antiapoptotic function could be exploited in cancer therapy, based on the observation that an HSV-2-based oncolytic virus with a deletion of the putative protein kinase domain of the ICP10 gene is a potent inducer of apoptotic death in tumor cells (41, 68). Although ICP10 indisputably displays antiapoptotic activity, there has been some controversy as to whether the protein contains intrinsic protein kinase activity. Based on biochemical studies, it has been suggested that the observed kinase activity is caused by contaminating cellular kinases, in particular casein kinase II (34, 44, 112).
POXVIRUSES
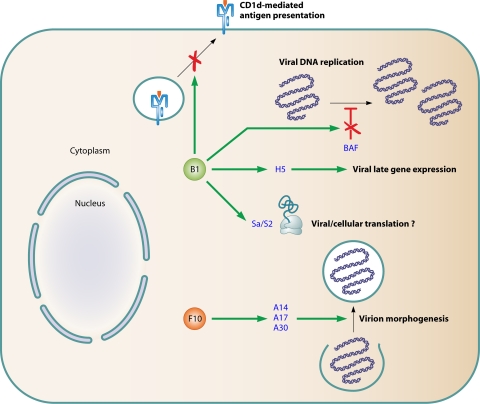
The Poxviridae family can be subdivided into the Entomopoxvirinae, which infect insects, and the Chordopoxvirinae, which infect vertebrates. All poxviruses are double-stranded DNA viruses with an envelope and a very large genome, ranging from 130 to 360 kb. All poxviruses encode serine/threonine kinases. Unlike the vast majority of DNA viruses, poxviruses replicate in the cytoplasm since they encode all necessary replication enzymes and therefore do not rely on cellular nuclear enzymes. Poxviruses share this property with all members of a proposed clade of very large DNA viruses of eukaryotes, the nucleo-cytoplasmic large DNA viruses (NCLDV). Poxviruses and African swine fever virus are the only NCLDV with mammalian hosts (106). Several poxviruses have been reported to infect humans. Most notable is the smallpox virus (variola virus), for which humans are the only known natural host. Smallpox has caused millions of deaths and has had a major impact on human history (132). Although smallpox was eradicated in 1980, the virus is still considered to be a threat as a potential bioterrorist weapon (6). Other poxviruses able to infect humans include vaccinia virus (origin unknown; used for immunization against smallpox), cowpox virus (causes ulcerative lesions on the hands of dairy workers), molluscum contagiosum virus (causes infectious warty papules of the skin), monkeypox virus (causes rare smallpox disease of children in central Africa), pseudocowpox virus (causes nonulcerating nodules on the hands of dairy workers), and Orf virus (causes single lesions on the skin of people handling sheep and goats). Major functions associated with conserved poxviral kinases are shown in Fig. 3.
FIG. 3.
Major functions associated with B1 and F10 kinases of poxviruses. Proteins in orange represent phosphorylation substrates that may be involved in the different functions of B1 and F10.
B1 kinase.
Almost 30 years ago, a wide array of temperature-sensitive mutants of vaccinia virus, the Condit collection, was constructed (42, 43). The ts2 and ts25 mutants were found to be arrested at the stage of DNA replication, and the mutations were located in the B1 gene (42, 189). Sequencing of this region of the genome revealed that the B1 gene displays strong homology to serine/threonine kinases (80, 212). Soon thereafter, B1 was indeed confirmed to encode a catalytically active viral serine/threonine kinase of 34 kDa, which was expressed early in infection, associated with cytoplasmic virus factories, and incorporated as a minor component in the virion (14, 122). Interestingly, based on sequence homology, identification of B1 resulted in the subsequent discovery of a novel class of cellular serine/threonine kinases that share up to 40% amino acid identity with B1, the vaccinia virus B1 kinase-related kinases (VRKs) (158, 163). VRK orthologs are present in mammals, fruit flies, and nematodes, but apparently not in yeast. They reside predominantly in the nucleus, where they fulfill several crucial roles in successful DNA replication, mitosis, and gene expression (reviewed in reference 103).
Nearly all known poxviruses encode B1 orthologs; the exception is molluscum contagiosum virus (199). Although not proven as of yet, it is possible that the molluscum contagiosum virus is able to compensate for the lack of B1 by usurping related host kinases, such as VRKs (108). In line with this idea, expression of the VRK1 genes of humans and, to a lesser extent, mice in the B1-defective ts2 mutant of vaccinia virus rescues the inhibition of DNA replication, virus production, and plaque formation observed with this mutant (24).
The phenotypes associated with temperature-sensitive mutants of B1 show that the kinase is critically involved in at least two key stages of the virus cycle: DNA replication and production of intermediate viral proteins (108, 189).
(i) DNA replication.
In 2007, a paper by Wiebe and Traktman provided fascinating insights into the mechanism of B1-mediated vaccinia virus DNA replication and, at the same time, uncovered a novel aspect of the innate, cellular antiviral response (229). The paper showed that B1 acts on barrier-to-autointegration factor (BAF). BAF is a DNA-binding protein, known to participate in nuclear reorganization and reassembly during mitosis by bridging chromatin to the nuclear membrane (reviewed in reference 128). The B1-related VRKs had already previously been shown to be able to phosphorylate BAF, thereby abrogating the DNA-binding abilities of BAF (164). In cells infected with vaccinia virus, but not with the B1-defective ts2 mutant, BAF phosphorylation was increased and BAF was prevented from association with virus DNA replication sites. Importantly, RNA interference-mediated silencing of BAF rescued the defect in virus growth of the ts2 mutant. Hence, cytoplasmic BAF likely constitutes a novel component of the innate viral response, acting by binding and thereby sequestering foreign, cytoplasmic DNA. B1 allows vaccinia virus to overcome this defense barrier of the cell by phosphorylating BAF, thereby interfering with its DNA-binding abilities.
(ii) Production of intermediate viral proteins.
The mechanism underlying the role of B1 in expression of viral intermediate proteins (108) is less clear. Likely important in this respect is that B1 phosphorylates viral late gene transcription factor 4 (VLTF-4; H5) (15, 16). Although H5 is a late gene transcription factor, B1-mediated phosphorylation of H5 may also affect intermediate protein synthesis, since H5 is the only VLTF that is expressed prior to DNA replication (107). Since H5 is also involved in transcription elongation (via interaction with A18 and G2), DNA replication (via interaction with A20), and virion morphogenesis, phosphorylation by B1 may also affect these aspects of the virus cycle (23, 50, 131). However, arguing against a direct involvement of B1-mediated H5 phosphorylation in DNA replication is the finding that rescue of DNA replication in the ts2 mutant by expression of the B1-like VRK1 was not associated with H5 phosphorylation (24). An alternative role for the B1/H5 interaction may lie in evasion of the innate antiviral response, since both proteins appear to be involved in inhibiting CD1d-mediated antigen presentation to natural killer T (NKT) cells (228).
Besides the effects of B1 on BAF and H5, the viral kinase has been shown to phosphorylate two ribosomal proteins, Sa and S2, which may have consequences on the efficiency of viral versus cellular translation (13). In vitro kinase assays have shown that the A30 protein, which is involved in virion morphogenesis, may also be a B1 substrate (139).
F10 kinase.
In 1994, a second kinase, encoded by the F10 gene, was identified in the vaccinia virus genome. The F10 kinase has a molecular weight of 50 kDa, and as is the case for B1, the kinase is incorporated into virions and is essential for viability of the virus. The majority of kinase activity packaged in the vaccinia virion core is the product of the F10 gene (121). F10 is strongly conserved in poxvirus genomes, with orthologs occurring not only in chordopoxviruses but also in entomopoxviruses (57). Unlike B1, F10 shows very little similarity to cellular protein kinases and has a very atypical structure, which probably explains the relatively late discovery of this viral kinase. Kinase subdomains II (ATP-binding pocket) and VI (catalytic loop) are the only typical kinase domains that are obviously recognizable in F10 (182). F10 has been characterized as a dual-specificity kinase, a relatively rare type of kinase that is able to phosphorylate not only serine/threonine but also tyrosine residues (51).
Experiments using temperature-sensitive mutants from the Condit collection showed that lack of F10 did not prevent genome replication or gene expression, but halted the virus at the earliest points of virion morphogenesis (213, 227). Subsequent studies revealed that F10 phosphorylates, and thereby influences several key viral factors involved in wrapping of electron-dense virosomes by membrane crescents to form the immature virion. F10 phosphorylates two viral membrane proteins that are critical in early morphogenesis, A14 and A17, which is thought to be required to form the membranes associated with immature virions (20, 51, 138). Together with viral proteins A30 and G7, F10 makes part of a viral assembly complex, which stimulates F10-mediated A17 phosphorylation (203). Additional components of this viral assembly complex were subsequently identified and include J1, A15, D2, and D3 (37, 203). A30, G7, and F10 itself are phosphorylated in an F10-mediated manner in this complex (139, 203). In vitro kinase assays confirmed A30 as a potential substrate for F10, whereas G7 was not phosphorylated by F10 in vitro, leaving the possibility that F10-mediated G7 phosphorylation occurs via a cellular kinase or occurs only in vivo (139).
In vitro kinase assays showed that F10 can phosphorylate E8R and thereby abrogates the DNA-binding capacity of this protein (55). Although there are indications that F10-mediated E8R phosphorylation may occur inside virus particles, thereby potentially facilitating DNA exit from the core during virus entry, this remains to be formally demonstrated (55).
BACULOVIRUSES
Members of the family Baculoviridae are large, double-stranded, enveloped DNA viruses that infect mainly insects. In 1983, Miller and coworkers reported the presence of serine/threonine kinase activity associated with both the extracellular and the occluded forms of a baculovirus (145). Until now, only one protein kinase gene has been identified in all baculovirus genomes sequenced: PK1. Sequences of PK1 of baculoviruses Spodoptera litura nucleopolyhedrovirus (SpltNPV) (148), Choristoneura fumiferana granulovirus (ChfuGV) (73), Lymantria dispar nuclear polyhedrosis virus (LdNPV) (22), Helicoverpa armigera single nucleopolyhedrovirus (HaSNVP) (237), and Autographa californica nucleopolyhedrovirus (AcNPV) (188) were analyzed and were found to contain typical serine/threonine kinase domains. Recombinant PK1 of AcNPV, SpltNPV, and LdNPV phosphorylates exogenous substrates, such as histone H1 and myelin basic protein (MBP) (22, 148, 188). Based on genome sequence analyses, the existence of a viral protein kinase in Anagrapha falcifera multinuclear polyhedrosis virus (AfMNPV) and Neodiprion lecontei nucleopolyhedrovirus (NeleNPV) was also predicted (64, 113). Baculovirus kinase activity was first suggested to be essential for release of viral DNA from virions during entry (230, 231). More recently, PK1 has been shown to regulate transcription from very late promoters, such as the polyhedrin (polh) and p10 promoters. This was first reported in a study of the temperature-sensitive AcNPV mutant (62) and was recently confirmed for both AcNPV and SpltNPV (146-149). AcNPV PK1 also plays a role in the phosphorylation of the LEF8 protein, which is a component of the very late gene transcription initiation complex (147).
OTHER VIRUSES
Apart from the above-mentioned viruses, viral kinases have been described or predicted based on homology to kinase domains in several other large DNA viral families, including the asfavirus, nimavirus, phycodnavirus, iridovirus, and mimivirus families. In irido- and phycodnavirus families, multiple copies of protein kinase genes were identified (85, 211). A comparison of nine sequenced iridoviruses revealed the presence of two conserved S/T kinases (56).
Phylogenetic trees have been constructed based on the pox-, herpes-, asfa-, nima-, phycodna-, irido-, and baculovirus protein kinase sequences to obtain new insights in the evolution of the PK genes and/or taxonomic classification of viruses (124, 217), which confirm broadly the evolutionary relationships of the different virus families. Although the existence and apparent conservation of viral protein kinases in the above-mentioned viral families point toward potentially important roles during their replication, further research is needed to address their exact biological roles.
CONCLUDING THOUGHTS
One of the curious aspects of viral serine/threonine kinases is that they appear to be encoded exclusively by large and evolutionarily old DNA viruses, such as herpesviruses, poxviruses, and baculoviruses.
Trying to explain this requires some speculation. One possibility would be that viral kinases merely are an “optional feature” of viruses, encoded only by those viral species that have a large enough genome to be able to afford them. Alternatively, they may be evolutionary relics, present in many of the evolutionarily ancient, complex DNA viruses and selected away in viruses of more recent origin. The fact that all studied viral kinases constitute important virulence factors argues against this possibility in favor of a third possibility: viral serine/threonine kinases may actually be one of the key elements of these old, large DNA viruses and may have aided them in still being around after millions of years of host evolution.
Indeed, evolutionarily very distinct members of the herpesviruses, with natural hosts ranging from koi to humans, are predicted to encode viral kinases (5). Throughout the tremendous evolution of their hosts, herpesviruses have successfully adapted themselves and kept their ability to establish lifelong infections. Apparently, viral kinases were not selected away in the evolutionary process but instead may have aided in the adaptation of these viruses to the increasing complexity of their host.
Intrinsic to the limited size of their genome, in order to be able to replicate and spread in a host, viruses have to do a lot with a little. Hence, it comes as no surprise that many viral proteins are multifunctional. This is especially true for viral kinases, which have the ability to phosphorylate, and thereby alter, several viral and cellular targets, which may result in a plethora of different effects. Some of these effects are intentional and beneficial for viral replication, and others may actually be side effects that are biologically irrelevant and thereby artifactual. One of the main tasks in the field of viral kinase research will be to better discriminate these possibilities. This is especially challenging since the major roles of viral kinases in pathogenesis do not appear to translate easily to obvious phenotypes in vitro. Indeed, as indicated above, many kinases, including the herpesvirus kinases, are often not absolutely required for growth in cell culture, although lack of viral kinase activity results in strongly reduced virulence. One important issue may be that, in contrast to the typical immortalized cell cultures, many of the viral target cells in vivo comprise highly differentiated, nonproliferating cells. These in vivo target cells are therefore less suitable for viral replication, which may increase the importance of viral kinases, especially since many of these kinases have been reported to promote viral replication and gene expression. As an example to support this idea, mutation of the CHPK kinase of VZV does not substantially affect viral growth in cell culture, whereas it is essential for replication in human T cells and human thymus/liver xenografts in SCID-hu mice (150). Also indicative of the caution needed when interpreting results from immortalized cell lines is that SV40 T antigens have been reported to induce lamin A/C phosphorylation in 293T cells, thereby compensating for the defect in viral egress observed for a CHPK-null EBV recombinant in 293 cells (137).
In order to meet the need to better define the biological importance of individual functions of viral kinases, several approaches may be followed. Studies of important primary target cells and/or organ cultures may highlight which kinase-dependent phenotypes manifest themselves in these cells. Since many of the kinase targets are cellular proteins, homologous studies, using primary cells of the natural host of the virus, will be particularly informative. In addition, a search for mutations in the viral kinases that abrogate their interaction with specific, but not all, substrates may be vital in revealing the importance of individual functions of viral kinases. Alternatively, complementing kinase deficiency with cellular or viral factors that restore only selected functions of the kinase should also yield important data in this respect. For example, replacing the US3 gene in alphaherpesviruses with specific antiapoptotic factors will allow us to better define the importance of the antiapoptotic activity of this kinase in virus biology.
There are several indications that viral serine/threonine protein kinases may be promising future targets for the development of antiviral drugs. First, although sometimes not associated with obvious phenotypes in cell culture, as described above, all viral protein kinases identified appear to play important and frequently essential roles in virus virulence. Second, and perhaps equally important, most viral serine/threonine protein kinases are evolutionarily very distinct from cellular protein kinases, which should facilitate the selection of drugs that do not interfere with cellular kinase activity.
A first proof of principle that viral kinases may represent interesting drug targets is maribavir. Maribavir is a drug directed against the UL97 kinase of HCMV and displays potent antiviral activity against HCMV (21). It is important to keep in mind that the drug of preference used against HCMV is GCV and that UL97 plays an important role in activating GCV by phosphorylating it to its monophosphate form (123, 202). Hence, the two treatment strategies may interfere with each other (38, 61). Maribavir successfully passed phase I and phase II clinical trials (214). Although this demonstrates the potential of viral kinases as therapeutic targets, unfortunately, maribavir very recently did not pass phase III clinical trials conducted by Viropharma. Despite this mishap, viral serine/threonine protein kinases should still be considered one of the promising avenues for the design of novel antiviral drugs (3). One example may be the B1 kinase, which is of critical importance in the poxvirus replication cycle. A first indication for the potential of B1 as a target for antiviral therapy was demonstrated, since interfering RNAs that target B1 effectively reduced vaccinia and monkeypox virus yields and plaques in in vitro assays, especially when administered together with the proven antiviral drug cidofovir (220).
An aspect of viral kinases that may warrant further research is their effect on cellular signaling networks and cascades. Phosphorylation and dephosphorylation arguably represent the most important regulatory switches of signaling proteins, affecting virtually every aspect of the biology of a cell and the host organism (174). Up to now, relatively little information has been available on the potential effect of viral serine/threonine kinases on cellular signaling cascades. This is even more relevant when considering that most, if not all, of these viral kinases are incorporated in the virus particle. Although largely unexplored at this time, there is thus the possibility that viral kinases may affect cellular signaling cascades at the very early stages of infection, during viral entry, thereby preparing and perhaps steering the otherwise hostile cellular environment to their own benefit and needs.
Obviously, there is a need for more research on viral protein kinases to fully address their invariably multifunctional and pivotal roles in viral replication, spread, and immune evasion. This information, together with rational drug design strategies, is needed to explore the therapeutic potential of these viral enzymes.
Acknowledgments
Work by the authors has been supported by grants from the Fonds Wetenschappelijk Onderzoek—Vlaanderen (FWO-Vlaanderen) (grants G.0196.06 and G.0835.09) and a concerted research action from the special research fund of Ghent University. C.V.D.B. is supported by a postdoctoral grant by the FWO-Vlaanderen.
Footnotes
Published ahead of print on 17 November 2010.
REFERENCES
- 1.Abendroth, A., I. Lin, B. Slobedman, H. Ploegh, and A. M. Arvin. 2001. Varicella-zoster virus retains major histocompatibility complex class I proteins in the Golgi compartment of infected cells. J. Virol. 75:4878-4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Advani, S. J., R. Brandimarti, R. R. Weichselbaum, and B. Roizman. 2000. The disappearance of cyclins A and B and the increase in activity of the G(2)/M-phase cellular kinase cdc2 in herpes simplex virus 1-infected cells require expression of the alpha22/U(S)1.5 and U(L)13 viral genes. J. Virol. 74:8-15. [PMC free article] [PubMed] [Google Scholar]
- 3.Andrei, G., E. De Clercq, and R. Snoeck. 2009. Drug targets in cytomegalovirus infection. Infect. Disord. Drug Targets 9:201-222. [DOI] [PubMed] [Google Scholar]
- 4.Ansari, A., and V. C. Emery. 1999. The U69 gene of human herpesvirus 6 encodes a protein kinase which can confer ganciclovir sensitivity to baculoviruses. J. Virol. 73:3284-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoki, T., et al. 2007. Genome sequences of three koi herpesvirus isolates representing the expanding distribution of an emerging disease threatening koi and common carp worldwide. J. Virol. 81:5058-5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Artenstein, A. W., and J. D. Grabenstein. 2008. Smallpox vaccines for biodefense: need and feasibility. Expert Rev. Vaccines 7:1225-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asai, R., et al. 2006. Epstein-Barr virus protein kinase BGLF4 is a virion tegument protein that dissociates from virions in a phosphorylation-dependent process and phosphorylates the viral immediate-early protein BZLF1. J. Virol. 80:5125-5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asai, R., T. Ohno, A. Kato, and Y. Kawaguchi. 2007. Identification of proteins directly phosphorylated by UL13 protein kinase from herpes simplex virus 1. Microbes Infect. 9:1434-1438. [DOI] [PubMed] [Google Scholar]
- 9.Asano, S., et al. 1999. US3 protein kinase of herpes simplex virus type 2 plays a role in protecting corneal epithelial cells from apoptosis in infected mice. J. Gen. Virol. 80:51-56. [DOI] [PubMed] [Google Scholar]
- 10.Baek, M. C., P. M. Krosky, and D. M. Coen. 2002. Relationship between autophosphorylation and phosphorylation of exogenous substrates by the human cytomegalovirus UL97 protein kinase. J. Virol. 76:11943-11952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baek, M. C., P. M. Krosky, Z. He, and D. M. Coen. 2002. Specific phosphorylation of exogenous protein and peptide substrates by the human cytomegalovirus UL97 protein kinase: importance of the P+5 position. J. Biol. Chem. 277:29593-29599. [DOI] [PubMed] [Google Scholar]
- 12.Baek, M. C., P. M. Krosky, A. Pearson, and D. M. Coen. 2004. Phosphorylation of the RNA polymerase II carboxyl-terminal domain in human cytomegalovirus-infected cells and in vitro by the viral UL97 protein kinase. Virology 324:184-193. [DOI] [PubMed] [Google Scholar]
- 13.Banham, A. H., D. P. Leader, and G. L. Smith. 1993. Phosphorylation of ribosomal proteins by the vaccinia virus B1R protein kinase. FEBS Lett. 321:27-31. [DOI] [PubMed] [Google Scholar]
- 14.Banham, A. H., and G. L. Smith. 1992. Vaccinia virus gene B1R encodes a 34-kDa serine/threonine protein kinase that localizes in cytoplasmic factories and is packaged into virions. Virology 191:803-812. [DOI] [PubMed] [Google Scholar]
- 15.Beaud, G., R. Beaud, and D. P. Leader. 1995. Vaccinia virus gene H5R encodes a protein that is phosphorylated by the multisubstrate vaccinia virus B1R protein kinase. J. Virol. 69:1819-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaud, G., and R. Beaud. 2000. Temperature-dependent phosphorylation state of the H5R protein synthesised at the early stage of infection in cells infected with vaccinia virus ts mutants of the B1R and F10L protein kinases. Intervirology 43:67-70. [DOI] [PubMed] [Google Scholar]
- 17.Benetti, L., and B. Roizman. 2004. Herpes simplex virus protein kinase US3 activates and functionally overlaps protein kinase A to block apoptosis. Proc. Natl. Acad. Sci. U. S. A. 101:9411-9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benetti, L., and B. Roizman. 2007. In transduced cells, the US3 protein kinase of herpes simplex virus 1 precludes activation and induction of apoptosis by transfected procaspase 3. J. Virol. 81:10242-10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Besser, J., et al. 2003. Differentiation of varicella-zoster virus ORF47 protein kinase and IE62 protein binding domains and their contributions to replication in human skin xenografts in the SCID-hu mouse. J. Virol. 77:5964-5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Betakova, T., E. J. Wolffe, and B. Moss. 1999. Regulation of vaccinia virus morphogenesis: phosphorylation of the A14L and A17L membrane proteins and C-terminal truncation of the A17L protein are dependent on the F10L kinase. J. Virol. 73:3534-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biron, K. K., et al. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole l-riboside with a unique mode of action. Antimicrob. Agents Chemother. 46:2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bischoff, D. S., and J. M. Slavicek. 1994. Identification and characterization of a protein kinase gene in the Lymantria dispar multinucleocapsid nuclear polyhedrosis virus. J. Virol. 68:1728-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black, E. P., N. Moussatche, and R. C. Condit. 1998. Characterization of the interactions among vaccinia virus transcription factors G2R, A18R, and H5R. Virology 245:313-322. [DOI] [PubMed] [Google Scholar]
- 24.Boyle, K. A., and P. Traktman. 2004. Members of a novel family of mammalian protein kinases complement the DNA-negative phenotype of a vaccinia virus ts mutant defective in the B1 kinase. J. Virol. 78:1992-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Britt, W. J., and D. Auger. 1986. Human cytomegalovirus virion-associated protein with kinase activity. J. Virol. 59:185-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruni, R., B. Fineschi, W. O. Ogle, and B. Roizman. 1999. A novel cellular protein, p60, interacting with both herpes simplex virus 1 regulatory proteins ICP22 and ICP0 is modified in a cell-type-specific manner and is recruited to the nucleus after infection. J. Virol. 73:3810-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brzozowska, A., M. Rychlowski, A. D. Lipinska, and K. Bienkowska-Szewczyk. 2010. Point mutations in BHV-1 US3 gene abolish its ability to induce cytoskeletal changes in various cell types. Vet. Microbiol. 143:8-13 [DOI] [PubMed] [Google Scholar]
- 28.Burma, S., B. P. Chen, M. Murphy, A. Kurimasa, and D. J. Chen. 2001. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 276:42462-42467. [DOI] [PubMed] [Google Scholar]
- 29.Cannon, J. S., F. Hamzeh, S. Moore, J. Nicholas, and R. F. Ambinder. 1999. Human herpesvirus 8-encoded thymidine kinase and phosphotransferase homologues confer sensitivity to ganciclovir. J. Virol. 73:4786-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cano-Monreal, G. L., J. E. Tavis, and L. A. Morrison. 2008. Substrate specificity of the herpes simplex virus type 2 UL13 protein kinase. Virology 374:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cano-Monreal, G. L., K. M. Wylie, F. Cao, J. E. Tavis, and L. A. Morrison. 2009. Herpes simplex virus 2 UL13 protein kinase disrupts nuclear lamins. Virology 392:137-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cartier, A., E. Broberg, T. Komai, M. Henriksson, and M. G. Masucci. 2003. The herpes simplex virus-1 Us3 protein kinase blocks CD8T cell lysis by preventing the cleavage of Bid by granzyme B. Cell Death Differ. 10:1320-1328. [DOI] [PubMed] [Google Scholar]
- 33.Cartier, A., T. Komai, and M. G. Masucci. 2003. The Us3 protein kinase of herpes simplex virus 1 blocks apoptosis and induces phosporylation of the Bcl-2 family member Bad. Exp. Cell Res. 291:242-250. [DOI] [PubMed] [Google Scholar]
- 34.Chabaud, S., et al. 2007. The ribonucleotide reductase domain of the R1 subunit of herpes simplex virus type 2 ribonucleotide reductase is essential for R1 antiapoptotic function. J. Gen. Virol. 88:384-394. [DOI] [PubMed] [Google Scholar]
- 35.Chee, M. S., G. L. Lawrence, and B. G. Barrell. 1989. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J. Gen. Virol. 70:1151-1160. [DOI] [PubMed] [Google Scholar]
- 36.Chen, M. R., S. J. Chang, H. Huang, and J. Y. Chen. 2000. A protein kinase activity associated with Epstein-Barr virus BGLF4 phosphorylates the viral early antigen EA-D in vitro. J. Virol. 74:3093-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiu, W. L., P. Szajner, B. Moss, and W. Chang. 2005. Effects of a temperature sensitivity mutation in the J1R protein component of a complex required for vaccinia virus assembly. J. Virol. 79:8046-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chou, S., and G. I. Marousek. 2006. Maribavir antagonizes the antiviral action of ganciclovir on human cytomegalovirus. Antimicrob. Agents Chemother. 50:3470-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coller, K. E., and G. A. Smith. 2008. Two viral kinases are required for sustained long distance axon transport of a neuroinvasive herpesvirus. Traffic 9:1458-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collett, M. S., and R. L. Erikson. 1978. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc. Natl. Acad. Sci. U. S. A. 75:2021-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colunga, A. G., J. M. Laing, and L. Aurelian. 2010. The HSV-2 mutant DeltaPK induces melanoma oncolysis through nonredundant death programs and associated with autophagy and pyroptosis proteins. Gene Ther. 17:315-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Condit, R. C., A. Motyczka, and G. Spizz. 1983. Isolation, characterization, and physical mapping of temperature-sensitive mutants of vaccinia virus. Virology 128:429-443. [DOI] [PubMed] [Google Scholar]
- 43.Condit, R. C., and A. Motyczka. 1981. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology 113:224-241. [DOI] [PubMed] [Google Scholar]
- 44.Conner, J. 1999. The unique N terminus of herpes simplex virus type 1 ribonucleotide reductase large subunit is phosphorylated by casein kinase 2, which may have a homologue in Escherichia coli. J. Gen. Virol. 80:1471-1476. [DOI] [PubMed] [Google Scholar]
- 45.Coulter, L. J., H. W. Moss, J. Lang J. and D. J. McGeoch. 1993. A mutant of herpes simplex virus type 1 in which the UL13 protein kinase gene is disrupted. J. Gen. Virol. 74:387-395. [DOI] [PubMed] [Google Scholar]
- 46.Cunningham, C., et al. 1992. The UL13 virion protein of herpes simplex virus type 1 is phosphorylated by a novel virus-induced protein kinase. J. Gen. Virol. 73:303-311. [DOI] [PubMed] [Google Scholar]
- 47.Daikoku, T., et al. 1997. Purification and characterization of the protein kinase encoded by the UL13 gene of herpes simplex virus type 2. Virology 253:82-93. [DOI] [PubMed] [Google Scholar]
- 48.Daikoku, T., Y. Yamashita, T. Tsurumi, K. Maeno, and Y. Nishiyama. 1993. Purification and biochemical characterization of the protein kinase encoded by the US3 gene of herpes simplex virus type 2. Virology 197:685-694. [DOI] [PubMed] [Google Scholar]
- 49.De Bolle, L., et al. 2002. Role of the human herpesvirus 6 u69-encoded kinase in the phosphorylation of ganciclovir. Mol. Pharmacol. 62:714-721. [DOI] [PubMed] [Google Scholar]
- 50.DeMasi, J., and P. Traktman. 2000. Clustered charge-to-alanine mutagenesis of the vaccinia virus H5 gene: isolation of a dominant, temperature-sensitive mutant with a profound defect in morphogenesis. J. Virol. 74:2393-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Derrien, M., A. Punjabi, M. Khanna, O. Grubisha, and P. Traktman. 1999. Tyrosine phosphorylation of A17 during vaccinia virus infection: involvement of the H1 phosphatase and the F10 kinase. J. Virol. 73:7287-7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deruelle, M., K. Geenen, H. J. Nauwynck, and H. W. Favoreel. 2007. A point mutation in the putative ATP binding site of the pseudorabies virus US3 protein kinase prevents Bad phosphorylation and cell survival following apoptosis induction. Virus Res. 128:65-70. [DOI] [PubMed] [Google Scholar]
- 53.Deruelle, M. J., C. Van den Broeke, H. J. Nauwynck, T. C. Mettenleiter, and H. W. Favoreel. 2009. Pseudorabies virus US3- and UL49.5-dependent and -independent downregulation of MHC I cell surface expression in different cell types. Virology 395:172-181. [DOI] [PubMed] [Google Scholar]
- 54.de Wind, N., J. Domen, and A. Berns. 1992. Herpesviruses encode an unusual protein-serine/threonine kinase which is nonessential for growth in cultured cells. J. Virol. 66:5200-5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doglio, L., A. De Marco, S. Schleich, N. Roos, and J. Krijnse Locker. 2002. The vaccinia virus E8R gene product: a viral membrane protein that is made early in infection and packaged into the virions' core. J. Virol. 76:9773-9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eaton, H. E., J. Metcalf, E. Penny, V. Tcherepanov, C. Upton, and C. R. Brunetti. 2007. Comparative genomic analysis of the family Iridoviridae: re-annotating and defining the core set of iridovirus genes. Virol. J. 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ehlers, A., J. Osborne, S. Slack, R. L. Roper, and C. Upton. 2002. Poxvirus orthologous clusters (POCs). Bioinformatics 18:1544-1545. [DOI] [PubMed] [Google Scholar]
- 58.Eisfeld, A. J., S. E. Turse, S. A. Jackson, E. C. Lerner, and P. R. Kinchington. 2006. Phosphorylation of the varicella-zoster virus (VZV) major transcriptional regulatory protein IE62 by the VZV open reading frame 66 protein kinase. J. Virol. 80:1710-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eisfeld, A. J., M. B. Yee, A. Erazo, A. Abendroth, and P. R. Kinchington. 2007. Downregulation of class I major histocompatibility complex surface expression by varicella-zoster virus involves open reading frame 66 protein kinase-dependent and -independent mechanisms. J. Virol. 81:9034-9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Erazo, A., and P. R. Kinchington. 2010. Varicella-zoster virus open reading frame 66 protein kinase and its relationship to alphaherpesvirus US3 kinases. Curr. Top. Microbiol. Immunol. 342:79-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Evers, D. L., et al. 2002. Interactions among antiviral drugs acting late in the replication cycle of human cytomegalovirus. Antiviral Res. 56:61-72. [DOI] [PubMed] [Google Scholar]
- 62.Fan, X., K. Thirunavukkarasu, and R. F. Weaver. 1996. Temperature-sensitive mutations in the protein kinase-1 (pk-1) gene of the Autographa californica nuclear polyhedrosis virus that block very late gene expression. Virology 224:1-9. [DOI] [PubMed] [Google Scholar]
- 63.Favoreel, H. W., G. Van Minnebruggen, D. Adriaensen, and H. J. Nauwynck. 2005. Cytoskeletal rearrangements and cell extensions induced by the US3 kinase of an alphaherpesvirus are associated with enhanced spread. Proc. Natl. Acad. Sci. U. S. A. 102:8990-8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Federici, B. A., and R. H. Hice. 1997. Organization and molecular characterization of genes in the polyhedrin region of the Anagrapha falcifera multinucleocapsid NPV. Arch. Virol. 142:333-348. [DOI] [PubMed] [Google Scholar]
- 65.Feederle, R., A. M. Mehl-Lautscham, H. Bannert, and H. J. Delecluse. 2009. The Epstein-Barr virus protein kinase BGLF4 and the exonuclease BGLF5 have opposite effects on the regulation of viral protein production. J. Virol. 83:10877-10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Finnen, R. L., B. B. Roy, H. Zhang, and B. W. Banfield. 2010. Analysis of filamentous process induction and nuclear localization properties of the HSV-2 serine/threonine kinase Us3. Virology 397:23-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frame, M. C., F. C. Purves, D. J. McGeoch, H. S. Marsden, and D. P. Leader. 1987. Identification of the herpes simplex virus protein kinase as the product of viral gene US3. J. Gen. Virol. 68:2699-2704. [DOI] [PubMed] [Google Scholar]
- 68.Fu, X., L. Tao, and X. Zhang. 2007. An HSV-2-based oncolytic virus deleted in the PK domain of the ICP10 gene is a potent inducer of apoptotic death in tumor cells. Gene Ther. 14:1218-1225. [DOI] [PubMed] [Google Scholar]
- 69.Geenen, K., H. W. Favoreel, L. Olsen, L. W. Enquist, and H. J. Nauwynck. 2005. The pseudorabies virus US3 protein kinase possesses anti-apoptotic activity that protects cells from apoptosis during infection and after treatment with sorbitol or staurosporine. Virology 331:144-150. [DOI] [PubMed] [Google Scholar]
- 70.Geiss, B. J., J. E. Tavis, L. M. Metzger, D. A. Leib, and L. A. Morrison. 2001. Temporal regulation of herpes simplex virus type 2 VP22 expression and phosphorylation. J. Virol. 75:10721-10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gershburg, E., M. Marschall, K. Hong, and J. S. Pagano. 2004. Expression and localization of the Epstein-Barr virus-encoded protein kinase. J. Virol. 78:12140-12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gershburg, S., L. Murphy, M. Marschall, and E. Gershburg. 2010. Key motifs in EBV (Epstein-Barr virus)-encoded protein kinase for phosphorylation activity and nuclear localization. Biochem. J. 431:227-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giannopoulos, P. N., N. Nassoury, L. Lamontagne, C. Guertin, and K. K. Rashidan. 2005. Choristoneura fumiferana granulovirus pk-1: a baculoviral protein kinase. J. Biochem. Mol. Biol. 38:457-467. [DOI] [PubMed] [Google Scholar]
- 74.Granzow, H., B. G. Klupp, W. Fuchs, J. Veits, N. Osterrieder, and T. C. Mettenleiter. 2001. Egress of alphaherpesviruses: comparative ultrastructural study. J. Virol. 75:3675-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hamirally, S., et al. 2009. Viral mimicry of Cdc2/cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress. PLoS Pathog. 5:e1000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamza, M. S., et al. 2004. ORF36 protein kinase of Kaposi's sarcoma herpesvirus activates the c-Jun N-terminal kinase signaling pathway. J. Biol. Chem. 279:38325-38330. [DOI] [PubMed] [Google Scholar]
- 77.Hanks, S. K., and T. Hunter. 1995. Protein kinases 6: the eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9:576-596. [PubMed] [Google Scholar]
- 78.Hata, S., et al. 1999. Antiapoptotic activity of herpes simplex virus type 2: the role of US3 protein kinase gene. Microbes Infect. 1:601-607. [DOI] [PubMed] [Google Scholar]
- 79.He, Z., et al. 1997. The human cytomegalovirus UL97 protein is a protein kinase that autophosphorylates on serines and threonines. J. Virol. 71:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Howard, S. T., and G. L. Smith. 1989. Two early vaccinia virus genes encode polypeptides related to protein kinases. J. Gen. Virol. 70:3187-3201. [DOI] [PubMed] [Google Scholar]
- 81.Hume, A. J., et al. 2008. Phosphorylation of retinoblastoma protein by viral protein with cyclin-dependent kinase function. Science 320:797-799. [DOI] [PubMed] [Google Scholar]
- 82.Hwang, S., et al. 2009. Conserved herpesviral kinase promotes viral persistence by inhibiting the IRF-3-mediated type I interferon response. Cell Host Microbe 5:166-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Imai, T., K. Sagou, J. Arii, and Y. Kawaguchi. 2010. Effects of phosphorylation of herpes simplex virus 1 envelope glycoprotein B by Us3 kinase in vivo and in vitro. J. Virol. 84:153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Isegawa, Y., et al. 2008. Characterization of the human herpesvirus 6 U69 gene product and identification of its nuclear localization signal. J. Virol. 82:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jakob, N. J., K. Müller, U. Bahr, and G. Darai. 2001. Analysis of the first complete DNA sequence of an invertebrate iridovirus: coding strategy of the genome of Chilo iridescent virus. Virology 286:182-196. [DOI] [PubMed] [Google Scholar]
- 86.Jarosinski, K. W., and N. Osterrieder. 2010. Further analysis of Marek's disease virus horizontal transmission confirms that UL44 (gC) and UL13 protein kinase activity are essential, while US2 is nonessential. J. Virol. 84:7911-7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jerome, K. R., et al. 1999. Herpes simplex virus inhibits apoptosis through the action of two genes, Us5 and Us3. J. Virol. 73:8950-8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kamil, J. P., and D. M. Coen. 2007. Human cytomegalovirus protein kinase UL97 forms a complex with the tegument phosphoprotein pp65. J. Virol. 81:10659-10668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kato, A., J. Arii, I. Shiratori, H. Akashi, H. Arase, and Y. Kawaguchi. 2009. Herpes simplex virus 1 protein kinase Us3 phosphorylates viral envelope glycoprotein B and regulates its expression on the cell surface. J. Virol. 83:250-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kato, A., et al. 2008. Identification of a physiological phosphorylation site of the herpes simplex virus 1-encoded protein kinase Us3 which regulates its optimal catalytic activity in vitro and influences its function in infected cells. J. Virol. 82:6172-6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kato, A., et al. 2006. Herpes simplex virus 1-encoded protein kinase UL13 phosphorylates viral Us3 protein kinase and regulates nuclear localization of viral envelopment factors UL34 and UL31. J. Virol. 80:1476-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kato, K., et al. 2001. Epstein-Barr virus-encoded protein kinase BGLF4 mediates hyperphosphorylation of cellular elongation factor 1delta (EF-1delta): EF-1delta is universally modified by conserved protein kinases of herpesviruses in mammalian cells. J. Gen. Virol. 82:1457-1463. [DOI] [PubMed] [Google Scholar]
- 93.Kato, K., et al. 2003. Identification of protein kinases responsible for phosphorylation of Epstein-Barr virus nuclear antigen leader protein at serine-35, which regulates its coactivator function. J. Gen. Virol. 84:3381-3392. [DOI] [PubMed] [Google Scholar]
- 94.Kawaguchi, Y., R. Bruni, and B. Roizman. 1997. Interaction of herpes simplex virus 1 alpha regulatory protein ICP0 with elongation factor 1delta: ICP0 affects translational machinery. J. Virol. 71:1019-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kawaguchi, Y., and K. Kato. 2003. Protein kinases conserved in herpesviruses potentially share a function mimicking the cellular protein kinase cdc2. Rev. Med. Virol. 13:331-340. [DOI] [PubMed] [Google Scholar]
- 96.Kawaguchi, Y., et al. 2003. Conserved protein kinases encoded by herpesviruses and cellular protein kinase cdc2 target the same phosphorylation site in eukaryotic elongation factor 1delta. J. Virol. 77:2359-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kawaguchi, Y., T. Matsumura, B. Roizman, and K. Hirai. 1999. Cellular elongation factor 1delta is modified in cells infected with representative alpha-, beta-, or gammaherpesviruses. J. Virol. 73:4456-4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1998. Eukaryotic elongation factor 1delta is hyperphosphorylated by the protein kinase encoded by the U(L)13 gene of herpes simplex virus 1. J. Virol. 72:1731-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kenyon, T. K., J. I. Cohen, and C. Grose. 2002. Phosphorylation by the varicella-zoster virus ORF47 protein serine kinase determines whether endocytosed viral gE traffics to the trans-Golgi network or recycles to the cell membrane. J. Virol. 76:10980-10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kenyon, T. K., and C. Grose. 2010. VZV ORF47 serine protein kinase and its viral substrates. Curr. Top. Microbiol. Immunol. 342:99-111. [DOI] [PubMed] [Google Scholar]
- 101.Kenyon, T. K., J. Lynch, J. Hay, W. Ruyechan, and C. Grose. 2001. Varicella-zoster virus ORF47 protein serine kinase: characterization of a cloned, biologically active phosphotransferase and two viral substrates, ORF62 and ORF63. J. Virol. 75:8854-8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kinchington, P. R., J. K. Hougland, A. M. Arvin, W. T. Ruyechan, and J. Hay. 1992. The varicella-zoster virus immediate-early protein IE62 is a major component of virus particles. J. Virol. 66:359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Klerkx, E. P., P. A. Lazo, and P. Askjaer. 2009. Emerging biological functions of the vaccinia-related kinase (VRK) family. Histol. Histopathol. 24:749-759. [DOI] [PubMed] [Google Scholar]
- 104.Klupp, B., J. Altenschmidt, H. Granzow, W. Fuchs, and T. C. Mettenleiter. 2008. Glycoproteins required for entry are not necessary for egress of pseudorabies virus. J. Virol. 82:6299-6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Klupp, B. G., H. Granzow, and T. C. Mettenleiter. 2001. Effect of the pseudorabies virus US3 protein on nuclear membrane localization of the UL34 protein and virus egress from the nucleus. J. Gen. Virol. 82:2363-2371. [DOI] [PubMed] [Google Scholar]
- 106.Koonin, E. V., and N. Yutin. 2010. Origin and evolution of eukaryotic large nucleo-cytoplasmic DNA viruses. Intervirology 53:284-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kovacs, G. R., R. Rosales, J. G. Keck, and B. Moss. 1994. Modification of the cascade model for regulation of vaccinia virus gene expression: purification of a prereplicative, late-stage-specific transcription factor. J. Virol. 68:3443-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kovacs, G. R., N. Vasilakis, and B. Moss. 2001. Regulation of viral intermediate gene expression by the vaccinia virus B1 protein kinase. J. Virol. 75:4048-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Krosky, P. M., M. C. Baek, and D. M. Coen. 2003. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J. Virol. 77:905-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krystof, V., and S. Uldrijan. 2010. Cyclin-dependent kinase inhibitors as anticancer drugs. Curr. Drug Targets 11:291-302. [DOI] [PubMed] [Google Scholar]
- 111.Kuny, C. V., K. Chinchilla, M. R. Culbertson, and R. F. Kalejta. 2010. Cyclin-dependent kinase-like function is shared by the beta- and gamma- subset of the conserved herpesvirus protein kinases. PLoS Pathog. 6:e1001092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Langelier, Y., et al. 1998. The R1 subunit of herpes simplex virus ribonucleotide reductase is a good substrate for host cell protein kinases but is not itself a protein kinase. J. Biol. Chem. 273:1435-1443. [DOI] [PubMed] [Google Scholar]
- 113.Lauzon, H. A., C. J. Lucarotti, P. J. Krell, Q. Feng, A. Retnakaran, and B. M. Arif. 2004. Sequence and organization of the Neodiprion lecontei nucleopolyhedrovirus genome. J. Virol. 78:7023-7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leach, N., et al. 2007. Emerin is hyperphosphorylated and redistributed in herpes simplex virus type 1-infected cells in a manner dependent on both UL34 and US3. J. Virol. 81:10792-10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leader, D. P. 1993. Viral protein kinases and protein phosphatases. Pharmacol. Ther. 59:343-389. [DOI] [PubMed] [Google Scholar]
- 116.Leader, D. P., A. D. Deana, F. Marchiori, F. C. Purves, and L. A. Pinna. 1991. Further definition of the substrate specificity of the alpha-herpesvirus protein kinase and comparison with protein kinases A and C. Biochim. Biophys. Acta 1091:426-431. [DOI] [PubMed] [Google Scholar]
- 117.Leader, D. P., and M. Katan. 1988. Viral aspects of protein phosphorylation. J. Gen. Virol. 69:1441-1464. [DOI] [PubMed] [Google Scholar]
- 118.Lee, C. P., et al. 2008. Epstein-Barr virus BGLF4 kinase induces disassembly of the nuclear lamina to facilitate virion production. J. Virol. 82:11913-11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Leopardi, R., C. Van Sant, and B. Roizman. 1997. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc. Natl. Acad. Sci. U. S. A. 94:7891-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liang, L., and B. Roizman. 2008. Expression of gamma interferon-dependent genes is blocked independently by virion host shutoff RNase and by US3 protein kinase. J. Virol. 82:4688-4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin, S., and S. S. Broyles. 1994. Vaccinia protein kinase 2: a second essential serine/threonine protein kinase encoded by vaccinia virus. Proc. Natl. Acad. Sci. U. S. A. 91:7653-7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lin, S., W. Chen, and S. S. Broyles. 1992. The vaccinia virus B1R gene product is a serine/threonine protein kinase. J. Virol. 66:2717-2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Littler, E., A. D. Stuart, and M. S. Chee. 1992. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature 358:160-162. [DOI] [PubMed] [Google Scholar]
- 124.Liu, W. J., et al. 2001. Cloning, characterization, and phylogenetic analysis of a shrimp white spot syndrome virus gene that encodes a protein kinase. Virology 289:362-377. [DOI] [PubMed] [Google Scholar]
- 125.Long, M. C., V. Leong, P. A. Schaffer, C. A. Spencer, and S. A. Rice. 1999. ICP22 and the UL13 protein kinase are both required for herpes simplex virus-induced modification of the large subunit of RNA polymerase II. J. Virol. 73:5593-5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maeda, N., H. Fan, and Y. Yoshikai. 2008. Oncogenesis by retroviruses: old and new paradigms. Rev. Med. Virol. 18:387-405. [DOI] [PubMed] [Google Scholar]
- 127.Manning, G., D. B. Whyte, R. Martinez, T. Hunter, and S. Sudarsanam. 2002. The protein kinase complement of the human genome. Science 298:1912-1934. [DOI] [PubMed] [Google Scholar]
- 128.Margalit, A., A. Brachner, J. Gotzmann, R. Foisner, and Y. Gruenbaum. 2007. Barrier-to-autointegration factor: a BAFfling little protein. Trends Cell Biol. 17:202-208. [DOI] [PubMed] [Google Scholar]
- 129.Marschall, M., et al. 2003. The protein kinase pUL97 of human cytomegalovirus interacts with and phosphorylates the DNA polymerase processivity factor pUL44. Virology 311:60-71. [DOI] [PubMed] [Google Scholar]
- 130.Marschall, M., et al. 2005. Cellular p32 recruits cytomegalovirus kinase pUL97 to redistribute the nuclear lamina. J. Biol. Chem. 280:33357-33367. [DOI] [PubMed] [Google Scholar]
- 131.McCraith, S., T. Holtzman, B. Moss, and S. Fields. 2000. Genome-wide analysis of vaccinia virus protein-protein interactions. Proc. Natl. Acad. Sci. U. S. A. 97:4879-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McFadden, G. 2010. Killing a killer: what next for smallpox? PLoS Pathog. 6:e1000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McGeoch, D. J., and A. J. Davison. 1986. Alphaherpesviruses possess a gene homologous to the protein kinase gene family of eukaryotes and retroviruses. Nucleic Acids Res. 14:1765-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Meignier, B., R. Longnecker, and B. Roizman. 1988. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020: construction and evaluation in rodents. J. Infect. Dis. 158:602-614. [DOI] [PubMed] [Google Scholar]
- 135.Meijer, L., et al. 1997. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 243:527-536. [DOI] [PubMed] [Google Scholar]
- 136.Meng, Q., et al. 2010. The Epstein-Barr virus (EBV)-encoded protein kinase, EBV-PK, but not the thymidine kinase (EBV-TK), is required for ganciclovir and acyclovir inhibition of lytic viral production. J. Virol. 84:4534-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Meng, Q., S. R. Hagemeier, C. V. Kuny, R. F. Kalejta, and S. C. Kenney. 2010. Simian virus 40 T/t antigens and lamin A/C small interfering RNA rescue the phenotype of an Epstein-Barr virus protein kinase (BGLF4) mutant. J. Virol. 84:4524-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mercer, J., and P. Traktman. 2003. Investigation of structural and functional motifs within the vaccinia virus A14 phosphoprotein, an essential component of the virion membrane. J. Virol. 77:8857-8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mercer, J., and P. Traktman. 2005. Genetic and cell biological characterization of the vaccinia virus A30 and G7 phosphoproteins. J. Virol. 79:7146-7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mettenleiter, T. C., B. G. Klupp, and H. Granzow. 2009. Herpesvirus assembly: an update. Virus Res. 143:222-234. [DOI] [PubMed] [Google Scholar]
- 141.Michel, D., et al. 1999. Amino acids of conserved kinase motifs of cytomegalovirus protein UL97 are essential for autophosphorylation. J. Virol. 73:8898-8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Michel, D., and T. Mertens. 2004. The UL97 protein kinase of human cytomegalovirus and homologues in other herpesviruses: impact on virus and host. Biochim. Biophys. Acta 1697:169-180. [DOI] [PubMed] [Google Scholar]
- 143.Michel, D., et al. 1996. The UL97 gene product of human cytomegalovirus is an early-late protein with a nuclear localization but is not a nucleoside kinase. J. Virol. 70:6340-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Milbradt, J., S. Auerochs, and M. Marschall. 2007. Cytomegaloviral proteins pUL50 and pUL53 are associated with the nuclear lamina and interact with cellular protein kinase C. J. Gen. Virol. 88:2642-2650. [DOI] [PubMed] [Google Scholar]
- 145.Miller, L. K., M. J. Adang, and D. Browne. 1983. Protein kinase activity associated with the extracellular and occluded forms of the baculovirus Autographa californica nuclear polyhedrosis virus. J. Virol. 46:275-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mishra, G., P. Chadha, I. Chaudhury, and R. H. Das. 2008. Inhibition of Autographa californica nucleopolyhedrovirus (AcNPV) polyhedrin gene expression by DNAzyme knockout of its serine/threonine kinase (pk1) gene. Virus Res. 135:197-201. [DOI] [PubMed] [Google Scholar]
- 147.Mishra, G., P. Chadha, and R. H. Das. 2008. Serine/threonine kinase (pk-1) is a component of Autographa californica multiple nucleopolyhedrovirus (AcMNPV) very late gene transcription complex and it phosphorylates a 102 kDa polypeptide of the complex. Virus Res. 137:147-149. [DOI] [PubMed] [Google Scholar]
- 148.Mishra, G., and R. H. Das. 2007. Characterization of a eukaryotic type serine/threonine kinase in Spodoptera litura nucleopolyhedrovirus-I (SpltNPV-I). Virus Res. 128:126-134. [DOI] [PubMed] [Google Scholar]
- 149.Mishra, G., H. K. Gautam, and R. H. Das. 2007. Serine/Threonine kinase dependent transcription from the polyhedrin promoter of SpltNPV-I. Biochem. Biophys. Res. Commun. 358:942-947. [DOI] [PubMed] [Google Scholar]
- 150.Moffat, J. F., et al. 1998. The ORF47 and ORF66 putative protein kinases of varicella-zoster virus determine tropism for human T cells and skin in the SCID-hu mouse. Proc. Natl. Acad. Sci. U. S. A. 95:11969-11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Morimoto, T., et al. 2009. Differences in the regulatory and functional effects of the Us3 protein kinase activities of herpes simplex virus 1 and 2. J. Virol. 83:11624-11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Morrison, E. E., Y. F. Wang, and D. M. Meredith. 1998. Phosphorylation of structural components promotes dissociation of the herpes simplex virus type 1 tegument. J. Virol. 72:7108-7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mou, F., T. Forest, and J. D. Baines. 2007. US3 of herpes simplex virus type 1 encodes a promiscuous protein kinase that phosphorylates and alters localization of lamin A/C in infected cells. J. Virol. 81:6459-6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mou, F., E. Wills, and J. D. Baines. 2009. Phosphorylation of the UL31 protein of herpes simplex virus 1 by the US3 encoded kinase regulates localization of the nuclear envelopment complex and egress of nucleocapsids. J. Virol. 83:5181-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Muranyi, W., J. Haas, M. Wagner, G. Krohne, and U. H. Koszinowski. 2002. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science 297:854-857. [DOI] [PubMed] [Google Scholar]
- 156.Murata, T., F. Goshima, T. Daikoku, H. Takakuwa, and Y. Nishiyama. 2000. Expression of herpes simplex virus type 2 US3 affects the Cdc42/Rac pathway and attenuates c-Jun N-terminal kinase activation. Genes Cells 5:1017-1027. [DOI] [PubMed] [Google Scholar]
- 157.Murata, T., et al. 2002. Phosphorylation of cytokeratin 17 by herpes simplex virus type 2 US3 protein kinase. Microbiol. Immunol. 46:707-719. [DOI] [PubMed] [Google Scholar]
- 158.Nezu, J., A. Oku, M. H. Jones, and M. Shimane. 1997. Identification of two novel human putative serine/threonine kinases, VRK1 and VRK2, with structural similarity to vaccinia virus B1R kinase. Genomics 45:327-331. [DOI] [PubMed] [Google Scholar]
- 159.Ng, T. I., and C. Grose. 1992. Serine protein kinase associated with varicella-zoster virus ORF 47. Virology 191:9-18. [DOI] [PubMed] [Google Scholar]
- 160.Ng, T. I., L. Keenan, P. R. Kinchington, and C. Grose. 1994. Phosphorylation of varicella-zoster virus open reading frame (ORF) 62 regulatory product by viral ORF 47-associated protein kinase. J. Virol. 68:1350-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ng, T. I., W. O. Ogle, and B. Roizman. 1998. UL13 protein kinase of herpes simplex virus 1 complexes with glycoprotein E and mediates the phosphorylation of the viral Fc receptor: glycoproteins E and I. Virology 241:37-48. [DOI] [PubMed] [Google Scholar]
- 162.Ng, T. I., C. Talarico, T. C. Burnette, K. Biron, and B. Roizman. 1996. Partial substitution of the functions of the herpes simplex virus 1 U(L)13 gene by the human cytomegalovirus U(L)97 gene. Virology 225:347-358. [DOI] [PubMed] [Google Scholar]
- 163.Nichols, R. J., and P. Traktman. 2004. Characterization of three paralogous members of the mammalian vaccinia related kinase family. J. Biol. Chem. 279:7934-7946. [DOI] [PubMed] [Google Scholar]
- 164.Nichols, R. J., M. S. Wiebe, and P. Traktman. 2006. The vaccinia-related kinases phosphorylate the N′ terminus of BAF, regulating its interaction with DNA and its retention in the nucleus. Mol. Biol. Cell 17:2451-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ogg, P. D., P. J. McDonell, B. J. Ryckman, C. M. Knudson, and R. J. Roller. 2004. The HSV-1 Us3 protein kinase is sufficient to block apoptosis induced by overexpression of a variety of Bcl-2 family members. Virology 319:212-224. [DOI] [PubMed] [Google Scholar]
- 166.Ogle, W. O., T. I. Ng, K. L. Carter, and B. Roizman. 1997. The UL13 protein kinase and the infected cell type are determinants of posttranslational modification of ICP0. Virology 235:406-413. [DOI] [PubMed] [Google Scholar]
- 167.Overton, H., D. McMillan, L. Hope, and P. Wong-Kai-In. 1994. Production of host shutoff-defective mutants of herpes simplex virus type 1 by inactivation of the UL13 gene. Virology 202:97-106. [DOI] [PubMed] [Google Scholar]
- 168.Overton, H. A., D. J. McMillan, L. S. Klavinskis, L. Hope, A. J. Ritchie, and P. Wong-kai-in. 1992. Herpes simplex virus type 1 gene UL13 encodes a phosphoprotein that is a component of the virion. Virology 190:184-192. [DOI] [PubMed] [Google Scholar]
- 169.Park, J., D. Lee, T. Seo, J. Chung, and J. Choe. 2000. Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) open reading frame 36 protein is a serine protein kinase. J. Gen. Virol. 81:1067-1071. [DOI] [PubMed] [Google Scholar]
- 170.Park, R., and J. D. Baines. 2006. Herpes simplex virus type 1 infection induces activation and recruitment of protein kinase C to the nuclear membrane and increased phosphorylation of lamin B. J. Virol. 80:494-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Peri, P., et al. 2008. Herpes simplex virus type 1 Us3 gene deletion influences toll-like receptor responses in cultured monocytic cells. Virol. J. 5:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Perkins, D., E. F. Pereira, and L. Aurelian. 2003. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) functions as a dominant regulator of apoptosis in hippocampal neurons involving activation of the ERK survival pathway and upregulation of the antiapoptotic protein Bag-1. J. Virol. 77:1292-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Perkins, D., E. F. Pereira, M. Gober, P. J. Yarowsky, and L. Aurelian. 2002. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway. J. Virol. 76:1435-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Petretti, C., and C. Prigent. 2005. The protein kinase resource: everything you always wanted to know about protein kinases but were afraid to ask. Biol. Cell 97:113-118. [DOI] [PubMed] [Google Scholar]
- 175.Piroozmand, A., et al. 2004. Role of Us3 gene of herpes simplex virus type 1 for resistance to interferon. Int. J. Mol. Med. 14:641-645. [PubMed] [Google Scholar]
- 176.Poon, A. P., H. Gu, and B. Roizman. 2006. ICP0 and the US3 protein kinase of herpes simplex virus 1 independently block histone deacetylation to enable gene expression. Proc. Natl. Acad. Sci. U. S. A. 103:9993-9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Poon, A. P., and B. Roizman. 2005. Herpes simplex virus 1 ICP22 regulates the accumulation of a shorter mRNA and of a truncated US3 protein kinase that exhibits altered functions. J. Virol. 79:8470-8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Poon, A. P., and B. Roizman. 2007. Mapping of key functions of the herpes simplex virus 1 U(S)3 protein kinase: the U(S)3 protein can form functional heteromultimeric structures derived from overlapping truncated polypeptides. J. Virol. 81:1980-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Prichard, M. N., W. J. Britt, S. L. Daily, C. B. Hartline, and E. R. Kern. 2005. Human cytomegalovirus UL97 kinase is required for the normal intranuclear distribution of pp65 and virion morphogenesis. J. Virol. 79:15494-15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Prichard, M. N., et al. 1999. A recombinant human cytomegalovirus with a large deletion in UL97 has a severe replication deficiency. J. Virol. 73:5663-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Prichard, M. N., et al. 2008. Human cytomegalovirus UL97 kinase activity is required for the hyperphosphorylation of retinoblastoma protein and inhibits the formation of nuclear aggresomes. J. Virol. 82:5054-5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Punjabi, A., and P. Traktman. 2005. Cell biological and functional characterization of the vaccinia virus F10 kinase: implications for the mechanism of virion morphogenesis. J. Virol. 79:2171-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Purves, F. C., A. D. Deana, F. Marchiori, D. P. Leader, and L. A. Pinna. 1986. The substrate specificity of the protein kinase induced in cells infected with herpesviruses: studies with synthetic substrates [corrected] indicate structural requirements distinct from other protein kinases. Biochim. Biophys. Acta 889:208-215. [DOI] [PubMed] [Google Scholar]
- 184.Purves, F. C., W. O. Ogle, and B. Roizman. 1993. Processing of the herpes simplex virus regulatory protein alpha 22 mediated by the UL13 protein kinase determines the accumulation of a subset of alpha and gamma mRNAs and proteins in infected cells. Proc. Natl. Acad. Sci. U. S. A. 90:6701-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Purves, F. C., and B. Roizman. 1992. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein alpha 22. Proc. Natl. Acad. Sci. U. S. A. 89:7310-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rahaus, M., N. Desloges, and M. H. Wolff. 2007. Varicella-zoster virus requires a functional PI3K/Akt/GSK-3alpha/beta signaling cascade for efficient replication. Cell. Signal. 19:312-320. [DOI] [PubMed] [Google Scholar]
- 187.Reddy, S. M., E. Cox, I. Iofin, W. Soong, and J. I. Cohen. 1998. Varicella-zoster virus (VZV) ORF32 encodes a phosphoprotein that is posttranslationally modified by the VZV ORF47 protein kinase. J. Virol. 72:8083-8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Reilly, L. M., and L. A. Guarino. 1994. The pk-1 gene of Autographa californica multinucleocapsid nuclear polyhedrosis virus encodes a protein kinase. J. Gen. Virol. 75:2999-3006. [DOI] [PubMed] [Google Scholar]
- 189.Rempel, R. E., M. K. Anderson, E. Evans, and P. Traktman. 1990. Temperature-sensitive vaccinia virus mutants identify a gene with an essential role in viral replication. J. Virol. 64:574-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Reynolds, A. E., et al. 2001. U(L)31 and U(L)34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J. Virol. 75:8803-8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Reynolds, A. E., E. G. Wills, R. J. Roller, B. J. Ryckman, and J. D. Baines. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 76:8939-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Roizman, B., and P. E. Pellet. 2001. The family Herpesviridae: a brief introduction, p. 2381-2397. In D. M. Knipe et al. (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, PA.
- 193.Romaker, D., et al. 2006. Analysis of the structure-activity relationship of four herpesviral UL97 subfamily protein kinases reveals partial but not full functional conservation. J. Med. Chem. 49:7044-7053. [DOI] [PubMed] [Google Scholar]
- 194.Ryckman, B. J., and R. J. Roller. 2004. Herpes simplex virus type 1 primary envelopment: UL34 protein modification and the US3-UL34 catalytic relationship. J. Virol. 78:399-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schaap, A., et al. 2005. T-cell tropism and the role of ORF66 protein in pathogenesis of varicella-zoster virus infection. J. Virol. 79:12921-12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schang, L. M., et al. 2002. Pharmacological cyclin-dependent kinase inhibitors inhibit replication of wild-type and drug-resistant strains of herpes simplex virus and human immunodeficiency virus type 1 by targeting cellular, not viral, proteins. J. Virol. 76:7874-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Schumacher, D., C. McKinney, B. B. Kaufer, and N. Osterrieder. 2008. Enzymatically inactive U(S)3 protein kinase of Marek's disease virus (MDV) is capable of depolymerizing F-actin but results in accumulation of virions in perinuclear invaginations and reduced virus growth. Virology 375:37-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Schumacher, D., B. K. Tischer, S. Trapp, and N. Osterrieder. 2005. The protein encoded by the US3 orthologue of Marek's disease virus is required for efficient de-envelopment of perinuclear virions and involved in actin stress fiber breakdown. J. Virol. 79:3987-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Senkevich, T. G., E. V. Koonin, J. J. Bugert, G. Darai, and B. Moss. 1997. The genome of molluscum contagiosum virus: analysis and comparison with other poxviruses. Virology 233:19-42. [DOI] [PubMed] [Google Scholar]
- 200.Shibaki, T., T. Suzutani, I. Yoshida, M. Ogasawara, and M. Azuma. 2001. Participation of type I interferon in the decreased virulence of the UL13 gene-deleted mutant of herpes simplex virus type 1. J. Interferon Cytokine Res. 21:279-285. [DOI] [PubMed] [Google Scholar]
- 201.Somogyi, T., S. Michelson, and M. J. Masse. 1990. Genomic location of a human cytomegalovirus protein with protein kinase activity (PK68). Virology 174:276-285. [DOI] [PubMed] [Google Scholar]
- 202.Sullivan, V., et al. 1992. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature 358:162-164. [DOI] [PubMed] [Google Scholar]
- 203.Szajner, P., A. S. Weisberg, and B. Moss. 2004. Physical and functional interactions between vaccinia virus F10 protein kinase and virion assembly proteins A30 and G7. J. Virol. 78:266-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Takashima, Y., H. Tamura, X. Xuan, and H. Otsuka. 1999. Identification of the US3 gene product of BHV-1 as a protein kinase and characterization of BHV-1 mutants of the US3 gene. Virus Res. 59:23-34. [DOI] [PubMed] [Google Scholar]
- 205.Tamrakar, S., A. J. Kapasi, and D. H. Spector. 2005. Human cytomegalovirus infection induces specific hyperphosphorylation of the carboxyl-terminal domain of the large subunit of RNA polymerase II that is associated with changes in the abundance, activity, and localization of cdk9 and cdk7. J. Virol. 79:15477-15493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tan, K. B. 1975. Comparative study of the protein kinase associated with animal viruses. Virology 64:566-570. [DOI] [PubMed] [Google Scholar]
- 207.Tanaka, M., Y. Nishiyama, T. Sata, and Y. Kawaguchi. 2005. The role of protein kinase activity expressed by the UL13 gene of herpes simplex virus 1: the activity is not essential for optimal expression of UL41 and ICP0. Virology 341:301-312. [DOI] [PubMed] [Google Scholar]
- 208.Tanaka, M., et al. 2002. Conserved region CR2 of Epstein-Barr virus nuclear antigen leader protein is a multifunctional domain that mediates self-association as well as nuclear localization and nuclear matrix association. J. Virol. 76:1025-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tarakanova, V. L., et al. 2007. Gamma-herpesvirus kinase actively initiates a DNA damage response by inducing phosphorylation of H2AX to foster viral replication. Cell Host Microbe 1:275-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tarakanova, V. L., E. Stanitsa, S. M. Leonardo, T. M. Bigley, and S. B. Gauld. 2010. Conserved gammaherpesvirus kinase and histone variant H2AX facilitate gammaherpesvirus latency in vivo. Virology 405:50-61. [DOI] [PubMed] [Google Scholar]
- 211.Tidona, C. A., and G. Darai. 1997. The complete DNA sequence of lymphocystis disease virus. Virology 230:207-216. [DOI] [PubMed] [Google Scholar]
- 212.Traktman, P., M. K. Anderson, and R. E. Rempel. 1989. Vaccinia virus encodes an essential gene with strong homology to protein kinases. J. Biol. Chem. 264:21458-21461. [PubMed] [Google Scholar]
- 213.Traktman, P., A. Caligiuri, S. A. Jesty, K. Liu, and U. Sankar. 1995. Temperature-sensitive mutants with lesions in the vaccinia virus F10 kinase undergo arrest at the earliest stage of virion morphogenesis. J. Virol. 69:6581-6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Trofe, J., L. Pote, E. Wade, E. Blumberg, and R. D. Bloom. 2008. Maribavir: a novel antiviral agent with activity against cytomegalovirus. Ann. Pharmacother. 42:1447-1457. [DOI] [PubMed] [Google Scholar]
- 215.Van den Broeke, C., M. Deruelle, H. J. Nauwynck, K. E. Coller, G. A. Smith, J. Van Doorsselaere, and H. W. Favoreel. 2009. The kinase activity of pseudorabies virus US3 is required for modulation of the actin cytoskeleton. Virology 385:155-160. [DOI] [PubMed] [Google Scholar]
- 216.Van den Broeke, C., M. Radu, M. Deruelle, H. Nauwynck, C. Hofmann, Z. M. Jaffer, J. Chernoff, and H. W. Favoreel. 2009. Alphaherpesvirus US3-mediated reorganization of the actin cytoskeleton is mediated by group A p21-activated kinases. Proc. Natl. Acad. Sci. U. S. A. 106:8707-8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Van Hulten, M. C., and J. M. Vlak. 2001. Identification and phylogeny of a protein kinase gene of white spot syndrome virus. Virus Genes. 22:201-207. [DOI] [PubMed] [Google Scholar]
- 218.Van Minnebruggen, G., H. W. Favoreel, L. Jacobs, and H. J. Nauwynck. 2003. Pseudorabies virus US3 protein kinase mediates actin stress fiber breakdown. J. Virol. 77:9074-9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.van Zeijl, M., J. Fairhurst, E. Z. Baum, L. Sun, and T. R. Jones. 1997. The human cytomegalovirus UL97 protein is phosphorylated and a component of virions. Virology 231:72-80. [DOI] [PubMed] [Google Scholar]
- 220.Vigne, S., et al. 2009. Inhibition of vaccinia virus replication by two small interfering RNAs targeting B1R and G7L genes and their synergistic combination with cidofovir. Antimicrob. Agents Chemother. 53:2579-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wagenaar, F., et al. 1995. The US3-encoded protein kinase from pseudorabies virus affects egress of virions from the nucleus. J. Gen. Virol. 76:1851-1859. [DOI] [PubMed] [Google Scholar]
- 222.Wagner, M., et al. 2000. Comparison between human cytomegalovirus pUL97 and murine cytomegalovirus (MCMV) pM97 expressed by MCMV and vaccinia virus: pM97 does not confer ganciclovir sensitivity. J. Virol. 74:10729-10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Walters, M. S., A. Erazo, P. R. Kinchington, and S. Silverstein. 2009. Histone deacetylases 1 and 2 are phosphorylated at novel sites during varicella-zoster virus infection. J. Virol. 83:11502-11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Walters, M. S., P. R. Kinchington, B. W. Banfield, and S. Silverstein. 2010. Hyperphosphorylation of histone deacetylase 2 by alphaherpesvirus US3 kinases. J. Virol. 84:9666-9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wang, J. T., et al. 2009. Epstein-Barr virus BGLF4 kinase suppresses the interferon regulatory factor 3 signaling pathway. J. Virol. 83:1856-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang, J. T., et al. 2005. Detection of Epstein-Barr virus BGLF4 protein kinase in virus replication compartments and virus particles. J. Gen. Virol. 86:3215-3225. [DOI] [PubMed] [Google Scholar]
- 227.Wang, S., and S. Shuman. 1995. Vaccinia virus morphogenesis is blocked by temperature-sensitive mutations in the F10 gene, which encodes protein kinase 2. J. Virol. 69:6376-6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Webb, T. J., R. A. Litavecz, M. A. Khan, W. Du, J. Gervay-Hague, G. J. Renukaradhya, and R. R. Brutkiewicz. 2006. Inhibition of CD1d1-mediated antigen presentation by the vaccinia virus B1R and H5R molecules. Eur. J. Immunol. 36:2595-2600. [DOI] [PubMed] [Google Scholar]
- 229.Wiebe, M. S., and P. Traktman. 2007. Poxviral B1 kinase overcomes barrier to autointegration factor, a host defense against virus replication. Cell Host Microbe 1:187-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wilson, M. E., and R. A. Consigli. 1985. Characterization of a protein kinase activity associated with purified capsids of the granulosis virus infecting Plodia interpunctella. Virology 143:516-525. [DOI] [PubMed] [Google Scholar]
- 231.Wilson, M. E., and R. A. Consigli. 1985. Functions of a protein kinase activity associated with purified capsids of the granulosis virus infecting Plodia interpunctella. Virology 143:526-535. [DOI] [PubMed] [Google Scholar]
- 232.Wisner, T. W., et al. 2009. Herpesvirus gB-induced fusion between the virion envelope and outer nuclear membrane during virus egress is regulated by the viral US3 kinase. J. Virol. 83:3115-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wolf, D. G., C. T. Courcelle, M. N. Prichard, and E. S. Mocarski. 2001. Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc. Natl. Acad. Sci. U. S. A. 98:1895-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yang, P. W., S. S. Chang, C. H. Tsai, Y. H. Chao, and M. R. Chen. 2008. Effect of phosphorylation on the transactivation activity of Epstein-Barr virus BMRF1, a major target of the viral BGLF4 kinase. J. Gen. Virol. 89:884-895. [DOI] [PubMed] [Google Scholar]
- 235.Yao, Z. Q., et al. 2001. Site-directed mutation in a conserved kinase domain of human cytomegalovirus-pp65 with preservation of cytotoxic T lymphocyte targeting. Vaccine 19:1628-1635. [DOI] [PubMed] [Google Scholar]
- 236.Yokota, S., et al. 2004. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 contributes to inhibition of the interferon signaling pathway. J. Virol. 78:6282-6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhang, C. X., G. Wang, C. Hu, and X. F. Wu. 1997. Nucleotide sequence analysis of HaSNPV protein kinase. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 29:322-326. [PubMed] [Google Scholar]
- 238.Zimmermann, A., D. Michel, I. Pavić, W. Hampl, A. Lüske, J. Neyts, E. De Clercq, and T. Mertens. 1997. Phosphorylation of aciclovir, ganciclovir, penciclovir and S2242 by the cytomegalovirus UL97 protein: a quantitative analysis using recombinant vaccinia viruses. Antiviral Res. 36:35-42. [DOI] [PubMed] [Google Scholar]