Abstract
We have established that human cytomegalovirus (HCMV) infection modulates the biology of target primary peripheral blood monocytes, allowing HCMV to use monocytes as “vehicles” for its systemic spread. HCMV infection of monocytes results in rapid induction of phosphatidylinositol-3-kinase [PI(3)K] and NF-κB activities. Integrins, which are upstream of the PI(3)K and NF-κB pathways, were shown to be involved in HCMV binding to and entry into fibroblasts, suggesting that receptor ligand-mediated signaling following viral binding to integrins on monocytes could trigger the functional changes seen in infected monocytes. We now show that integrin engagement and the activation of the integrin/Src signaling pathway are essential for the induction of HCMV-infected monocyte motility. To investigate how integrin engagement by HCMV triggers monocyte motility, we examined the infected-monocyte transcriptome and found that the integrin/Src signaling pathway regulates the expression of paxillin, which is an important signal transducer in the regulation of actin rearrangement during cell adhesion and movement. Functionally, we observed that paxillin is activated via the integrin/Src signaling pathway and is required for monocyte motility. Because motility is intimately connected to cellular cytoskeletal organization, a process that is also important in viral entry, we investigated the role paxillin regulation plays in the process of viral entry into monocytes. New results confirmed that HCMV entry into target monocytes was significantly reduced in cells deficient in paxillin expression or the integrin/Src/paxillin signaling pathway. From our data, HCMV-cell interactions emerge as an essential trigger for the cellular changes that allow for HCMV entry and hematogenous dissemination.
Human cytomegalovirus (HCMV), a betaherpesvirus, is a prevalent infectious agent, with seropositivity reaching 50 to 80% among adults in the United States (15). In immunocompromised individuals, viral infection can lead to significant morbidity and mortality (19, 35). HCMV is the leading cause of congenital central nervous system damage and a leading opportunistic pathogen in AIDS and transplant patients (19, 35). In immunocompetent individuals, HCMV infection is usually mild or asymptomatic, although results now show that HCMV is a strong risk factor in the development of cardiovascular diseases (CVDs) (25, 42-44, 47).
After primary infection, HCMV establishes a lifelong persistent infection, with frequent reactivations and active infection of new target cells. The virus can be shed in nearly all body fluids, illustrating HCMV's broad cellular tropism and capacity to spread to and infect most organ systems (5, 27, 54). It is postulated that effective multiorgan viral spread is critical for HCMV survival and persistence within infected hosts (35). HCMV has been shown to infect circulating cells in the blood (30), such as monocytes, and to use these cells as viral carriers, allowing dissemination to target tissues (37). In support, HCMV infection is characterized by a cell-associated viremia, in particular a monocyte-associated viremia, prior to the onset of viral pathogenesis (34, 35, 37, 39, 55).
The emerging critical role for circulating monocytes in dissemination and persistence during HCMV infection necessitates a better understanding of the influence of HCMV infection on the biology of these cells. Because monocytes are short-lived cells, infection by HCMV seems counterintuitive for establishing lifelong viral persistence. However, when HCMV-infected monocytes enter organ tissues, they appear to differentiate into long-lived macrophages (8, 37). The available data suggest that HCMV infection of monocytes results in a change from their traditional sentinel role in the immune system to a viral carrier, which allows for the effective spread of the virus throughout the host (3, 37, 39, 55). In support, we have shown that HCMV infection of monocytes leads to the overexpression and secretion of inflammatory cytokines, to an induction of cellular motility, along with increased expression of adhesion molecules, allowing for tight adhesion of infected monocytes to endothelial cells, to transendothelial migration, and to the promotion of cellular differentiation (37, 39, 55). We have also documented that HCMV upregulates the expression of inflammatory genes in monocytes by triggering the phosphatidylinositol-3-kinase [PI(3)K] and nuclear factor κB (NF-κB) signaling pathways and promotes the polarization of monocyte differentiation toward a unique proinflammatory M1-like macrophage (8-10).
Monocytes are not permissive for HCMV viral gene expression and replication upon initial infection; this fact, when coupled with the time frame of observed biological changes in monocytes following infection, suggests that the virus begins to trigger biological changes in monocytes during the process of binding to the cell. It was previously shown that the HCMV major glycoproteins, gB and gH, can initiate rapid activation of transcription factors in infected fibroblasts and monocytes via receptor-ligand engagement (55, 56). UV-irradiated virus shows an influence on monocyte biology similar to that of live virus, further suggesting that HCMV is a bona fide ligand responsible for triggering specific biological changes during binding to target cells (37-39).
Even though viral glycoproteins have been found to bind to and activate infected monocytes (55), the cellular binding partner in these interactions remains elusive. Although several cellular receptors, including the epidermal growth factor receptor (EGFR) (12, 50), the platelet-derived growth factor α receptor (PDGFRα) (41), and integrins (14, 49), have been reported to play a role in HCMV attachment and entry, along with a documented immunological activation of toll-like receptor 2 (TLR2) during viral binding (6, 13), how important they are for HCMV infection remains unresolved and seems to be cell type specific. Because EGFR, PDGFRα, and integrin engagement is upstream of PI(3)K activation (20, 41, 46) and is important in HCMV entry (12, 14, 41, 49, 50), we initiated an investigation of the role these receptors play in HCMV-infected monocyte signaling. Because PDGFRα is not expressed on the surface of monocytes (21), which we confirmed (12), we did not pursue the influence of PDGFRα on HCMV infection of monocytes. Recently, we demonstrated that HCMV interacts with EGFR expressed on the surface of monocytes, leading to the activation of cellular signaling (12). This virus-EGFR engagement in turn promoted enhanced monocyte motility and efficient viral entry into these cells (12). Although our data identify the important role that EGFR plays in modulating monocyte biology (12), our results also show that EGFR engagement is not responsible for the entirety of the virus-mediated signaling, suggesting that engagement of another receptor, such as cellular integrins, might also be necessary for translating extracellular stimuli into the biological changes seen in HCMV-infected monocytes (8, 11, 37-39, 54-56).
Integrins are heterodimeric receptors composed of a single alpha chain and a single beta chain. There are 19 known alpha chains and 8 known beta chains, which bind to form 25 different integrin receptors. It is known that integrin engagement of their cognate ligands leads to the induction of specific signaling pathways initiated by phosphorylation of Src tyrosine kinases, which in turn is translated downstream into a cascade of cellular signaling (20, 51). Integrins have been implicated in HCMV binding to and entry into human fibroblasts (49), an established in vitro model for HCMV infection. Because we have documented that HCMV has a unique influence on monocytes, resulting in cellular differentiation and long-term cellular survival (8, 37-39), changes not observed in fibroblasts, we suggest that, although the virus may signal through integrins in multiple cell types, the resulting signal is distinct to each cell type. Hence, we hypothesized that viral binding to cellular integrins on target monocytes serves as a key trigger of the specific cellular signaling leading to the functional changes in monocytes that we observed (37-39, 55).
To test our hypothesis, we investigated the role of integrin-mediated signaling, as well as the role of viral binding to these receptors, in the induction of pathogenic monocyte motility triggered by HCMV infection. Moreover, we explored the impact of integrin engagement by the virus on the global regulation of gene expression in infected monocytes. Using this transcriptome-based analysis, we discovered a molecular regulator activated by integrin-mediated signaling upon HCMV infection that plays a central role as a convergent point linking the specific cellular signaling triggered during viral binding to the induction of enhanced cellular motility and to efficient viral entry into monocytes. We propose that the net result of these functional changes mediated by the virus during viral binding in vivo is the exit of infected monocytes from the blood into peripheral tissue, where the seeding of host organs occurs, allowing for HCMV spread to other hosts in multiple body fluids and the establishment of lifelong persistence.
MATERIALS AND METHODS
HCMV culture and infection.
The HCMV Towne/E (passages 39 to 41) (55) and green fluorescent protein (GFP)-labeled TB40/E-UL32 (TB40/E) (3, 36) strains were cultured in human embryonic lung (HEL) fibroblasts with 4% heat-inactivated fetal bovine serum (FBS). HCMV was purified on a sucrose gradient and resuspended in RPMI 1640 (Cellgro; Mediatech, Herndon, VA). Monocytes were infected with purified virus at the multiplicity of infection (MOI) of 4 to 5 for each experiment unless otherwise stated. Mock infection was carried out by adding an equivalent volume of RPMI 1640 (Cellgro) to monocytes. In some experiments, HCMV was pretreated for 1 h with blocking antibodies to gB (20 μg/ml; ViroStat, Portland, ME) or gH (20 μg/ml; ViroStat).
Human peripheral blood monocyte isolation.
Double-density gradient centrifugation was used to purify human peripheral blood monocytes (57). Whole blood was collected from donors by venipuncture. Mononuclear cells were then collected by centrifugation through a Ficoll Histopaque 1077 (Sigma-Aldrich, Inc., St. Louis, MO) gradient. Next, the collected cells were washed in 1× phosphate-buffered saline (PBS; Mediatech, Inc., Herndon, VA) to remove platelets. Monocytes were then isolated by centrifugation through a Percoll (Pharmacia Biotech, Inc., Piscataway, NJ) gradient and suspended in RPMI 1640 (Cellgro) supplemented with 1% human serum (Sigma-Aldrich Inc.). All Health Insurance Portability and Accountability Act (HIPAA) and university Institutional Review Board (IRB) guidelines were followed for the use of human subjects in our study.
Monocyte treatment and culture.
After isolation, monocytes were cultured under nonadherent conditions (if not specified otherwise) in RPMI 1640 (Cellgro) supplemented with 1% human serum (Sigma-Aldrich Inc.) at 37°C with 5% CO2 overnight, prior to any treatment. The following standard treatments were employed in our study: dimethyl sulfoxide (DMSO; Sigma-Aldrich Inc.) as a solvent control, 1 μM Src tyrosine kinase inhibitor PP2 (EMB Biosciences, Inc., La Jolla, CA), 1 μM EGFR tyrosine kinase inhibitor AG1478 (EMB Biosciences, Inc.), blocking antibodies specific for EGFR (1 μg/ml; Millipore, Billerica, MA) or integrins (β1 or β3; 5 μg/ml; Millipore), or an isotype-matched IgG (5 μg/ml; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Treatments were given 1 h prior to HCMV infection at 37°C with 5% CO2. Phorbol 12-myristate 13-acetate (PMA; Acros Organics, Morris Plains, NJ) at the concentration of 10 ng/ml was used in some experiments upon treatment with blocking antibodies.
Phagokinetic track motility assay.
Colloidal-gold-coated coverslips were prepared as previously described (1, 37). Monocytes (2.5 × 104) were pretreated with pharmacological agents or blocking antibodies and incubated in nonadherent conditions prior to HCMV infection or PMA treatment for 1 h. Monocytes were transferred to colloidal-gold-coated coverslips for 24 h at 37°C with 5% CO2. Individual images were captured. The average area (in arbitrary units) of colloidal gold cleared by 10 cells was determined for each experimental arm from the captured images using ImageJ software (28). Results are plotted as means ± the standard errors of the means (SEM). Student′s t tests were performed, and a P value of <0.05 was used as the measure of statistical significance between samples.
Western blot analysis.
Monocytes (2 × 106) were pretreated with pharmacological agents or blocking antibodies, infected, and incubated under nonadherent conditions. To examine kinetics of receptor-mediated signaling pathways, cells were harvested over time in a lysis buffer (50 mM Tris-HCl at pH 7.5 [Fisher Scientific, Fair Lawn, NJ], 5 mM EDTA [Bio-Rad Laboratories, Hercules, CA], 100 mM sodium chloride [Fisher Scientific], 1% Triton X-100 [Fisher Scientific], 0.1% sodium dodecyl sulfate [SDS; MP Biomedicals, Inc., Solon, OH], and 10% glycerol [MP Biomedicals, Inc.]). Samples were mixed with Laemmli′s SDS sample buffer (Boston BioProducts, Boston, MA) containing 2-mercaptoethanol (Fisher Scientific). Equal protein amounts of the different samples were separated by continuous polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to ImmunoBlot polyvinylidene difluoride membranes (Bio-Rad Laboratories). Western blot analyses were performed using primary antibodies recognizing the phosphorylated and nonphosphorylated forms of Src (phospho-Src [Tyr416] antibody [Cell Signaling Technology] and pan-Src [Santa Cruz Biotechnology, Inc.]), of paxillin (phospho-paxillin [Tyr18] antibody and pan-paxillin antibody [Cell Signaling Technology], of Akt (phospho-Akt [Ser473] antibody and pan-Akt antibody [Cell Signaling Technology]), and of Erk (phospho-Erk [204] antibody and pan-Erk1 antibody [Santa Cruz Biotechnology, Inc.]). Probing for actin (Santa Cruz Biotechnology, Inc.) was a loading control. Donkey anti-rabbit (GE Healthcare Life Sciences, Piscataway, NJ), donkey anti-mouse (GE Healthcare Biosciences), and donkey anti-goat (Santa Cruz Biotechnology, Inc.) antibodies conjugated with horseradish peroxidase (HRP) were used as secondary antibodies. Western blots were developed using ECL Plus Western blotting detection reagents (GE Healthcare Life Sciences).
Quantitative and semiquantitative reverse transcriptase PCR.
Monocytes were pretreated, infected, and incubated under nonadherent conditions (if not stated otherwise). Total cellular RNA or DNA was harvested with E.Z.N.A. total RNA kit I (Omega Bio-Tek, Inc., Norcross, GA) or E.Z.N.A. tissue DNA kit (Omega Bio-Tek, Inc.), respectively. RNA samples were DNase treated according to the manufacturer's protocol (Promega, Madison, WI). Then, samples were reverse transcribed with 400 U of Moloney murine leukemia virus reverse transcriptase (Invitrogen Corp., Carlsbad, CA) in 1× reverse transcriptase buffer along with 80 U of RNasin (Promega), random hexamers (0.1 μg/μl; Invitrogen Corp.), and 1 mM deoxynucleotides (Promega). Reaction mixtures were incubated at 25°C for 10 min, 48°C for 30 min, and 95°C for 5 min. Amplification and detection were performed with the iCycler iQ real-time PCR detection system in 25-μl reaction mixtures containing 50 ng of the transcription reaction mixture (cDNA), 12.5 μl of iQ SYBR green Supermix, and 0.4 μM primers (Bio-Rad Laboratories). The incubation conditions consisted of 3 min at 95°C for polymerase activation and 40 cycles of 15 s at 95°C and 1 min at 60°C for IE1-72 and 18S rRNA or 1 min at 65°C for paxillin, integrin β1, and integrin β3. Target mRNA and DNA levels were calculated using the absolute standard curve method as described in user bulletin no. 2 for the ABI Prism 7700 sequence detection system. After the amount of target cDNA or DNA was calculated from a standard curve, it was normalized to the amount of human 18S rRNA in each sample. Primers specific for paxillin (sense, 5′-AAACCCGACTGAAACTGGAACCCT-3′; antisense, 5′-GGCTGCACTGCTGAAATATGAGGAAG-3′), integrin β1 (sense, 5′-TGCGAGTGTGGTGTCTGTAAGTGT-3′; antisense, 5′-CCCGTGTCCCATTTGGCATTGGCATTCATT-3′), integrin β3 (sense, 5′-ACACTGGCAAGGATGCAGTGAATTGTA-3′; antisense, 5′-CGTGATATTGGTGAAGGTAGACGTGGC-3′), HCMV IE1-72 (sense, 5′-AGTGACCGAGGATTGCAACG-3′; antisense, 5′-CCTTGATTCTATGCCGCACC-3′), and 18S rRNA (sense, 5′-CGAGCCGCCTGGATACC-3′; antisense, 5′-CAGTTCCGAAAACCAACAAAATAG-3′) were used to amplify target sequences.
Semiquantitative PCR was also used to assess the expression of paxillin mRNA. Reverse transcription products were amplified using an MyCycler thermocycler (Bio-Rad Laboratories). Reverse transcription was performed in 1× iTaq buffer (Bio-Rad Laboratories) containing 1.25 U of iTaq DNA polymerase (Invitrogen Corp.) and a 50 μM concentration of each deoxynucleotide (Invitrogen Corp.). Amplification of actin (sense, 5′-TTCCTTCCTGGGCATGGAGT-3′; antisense, 5′-CTTGATCTTCATTGTGCTGGGTGC-3′) served as a control. All primers were obtained from Integrated DNA Technologies, Inc. (Coralville, IA).
Affymetrix gene array and analysis.
Isolated monocytes (1 × 107) were pretreated with 1 μM PP2, 1 μM AG1478, or DMSO for 1 h and incubated nonadherently. Monocytes were then mock infected or HCMV infected (Towne/E; MOI of 4 to 5) for 24 h. Total RNA was then harvested with the RNA STAT-60 isolation kit (Tel-Test Inc., Friendswood, TX), according to the manufacturer's protocol. Affymetrix, Inc. (Santa Clara, CA), human genome U95Av2 arrays were used to examine the cellular transcriptome changes in primary human monocytes from six different human donors. RNA sample preparation and the microarray procedure were performed as previously described (8). Affymetrix Microarray Suite version 5.0 was used to determine changes in gene expression. Data Mining Tool version 3.0 (Affymetrix, Inc.) was used to compile data from each of the replicates, and one-way analysis of variance (ANOVA) tests were performed to calculate P values. Signal-to-log ratios were collected for all data sets, and the data from individual experimental arms were averaged. Student's t tests were then performed, and a P value of <0.05 was used as the measure of significant changes in gene expression across the replicants. For our analysis, genes were considered upregulated or downregulated if the average signal-to-log ratio was increased or decreased 1.5-fold, respectively. Those genes that were differentially and statistically up- or downregulated by infection were first analyzed and grouped to generate the HCMV-infected-monocyte transcriptome, which was compared to the mock-infected-monocyte transcriptome. Second, Genesifter software (Geospiza, Inc., Seattle, WA) was used to compare the genes differentially and statistically regulated during infection to those modulated by EGFR and integrin signaling to generate gene grouping (ontologies) of gene sets that were statistically regulated by EGFR- and integrin-mediated signaling following infection.
siRNA transfection.
Isolated monocytes (3 × 106) were resuspended in supplemented human monocyte Nucleofector solution (Lonza Group Ltd., Basel, Switzerland) containing 300 nM paxillin small interfering RNA (siRNA) (Dharmacon, Inc., Lafayette, CO; sequence: 5′-GUGUGGAGCCUUCUUUGGUUU-3′), 300 nM control siRNA (Santa Cruz Biotechnology, Inc.), or RNase-free water (Invitrogen Corp.) and then transfected using an Amaxa Nucleofector (Lonza Group Ltd.). siRNA-transfected monocytes were mixed with preequilibrated human monocyte Nucleofector medium (Lonza Group Ltd.) supplemented with 10% human serum (Sigma-Aldrich Inc.) and incubated for 48 h at 37°C in 5% CO2.
HCMV entry assay.
Briefly (12), isolated monocytes (1 × 106) were treated and then plated onto 6-well plates for 1 h at 37°C in 5% CO2. Unbound monocytes were removed by washing with 1× PBS (Mediatech, Inc., Herndon, VA). Monocytes were HCMV infected (Towne/E or TB40/E; MOI of 0.5) for 1 h at 4°C, washed with 1× PBS (Mediatech, Inc.), and temperature shifted to 37°C for 1 h. Monocytes were washed and treated with proteinase K (Promega) for 1 h at 4°C. Monocytes were then harvested, and quantitative real-time PCR was performed using primers complementary to genomic HCMV DNA (the UL123/IE1-72 exon 1 region) and cellular 18S rRNA. Results are plotted as means ± SEM. Student's t tests were performed, and a P value of <0.05 was used as the measure of statistical significance between samples. To monitor the accuracy of the assay, representative samples of infected monocytes were kept at 4°C without a temperature shift and processed as described above.
Flow cytometry.
Monocytes (1 × 106) were incubated nonadherently. In some experiments, monocytes were transfected with paxillin siRNA or control siRNA. Some infections were also carried out with the TB40/E strain of HCMV. Monocytes were fixed in 2% paraformaldehyde, were blocked with 10% human serum (Sigma-Aldrich Inc.), 10% normal mouse serum (Santa Cruz Biotechnology Inc.), 10% normal goat serum (Calbiochem, La Jolla, CA), and 5% bovine serum albumin (BSA; Sigma-Aldrich Inc.) in fluorescence-activated cell sorting (FACS) buffer, and were stained with CD14-allophycocyanin (APC)-Cy7 monoclonal antibody (MAb) (BD Biosciences, San Jose, CA), CD29-APC MAb (BD Biosciences), and CD61-fluorescein isothiocyanate (FITC) MAb (BD Biosciences) for 60 min. FITC anti-GFP secondary antibody (BD Biosciences, San Jose, CA) was used to increase the fluorescent signal from GFP-labeled HCMV. Following antibody staining, cells were washed two times in FACS buffer and analyzed by flow cytometry using a FACSCalibur system (BD Biosciences). Only CD14+ cells are represented in histograms discussed below.
Microarray data accession number.
The GEO accession number for the gene grouping data is GSE24238.
RESULTS
EGFR and integrin engagement along with EGFR and Src kinase activities is required for monocyte motility following HCMV infection.
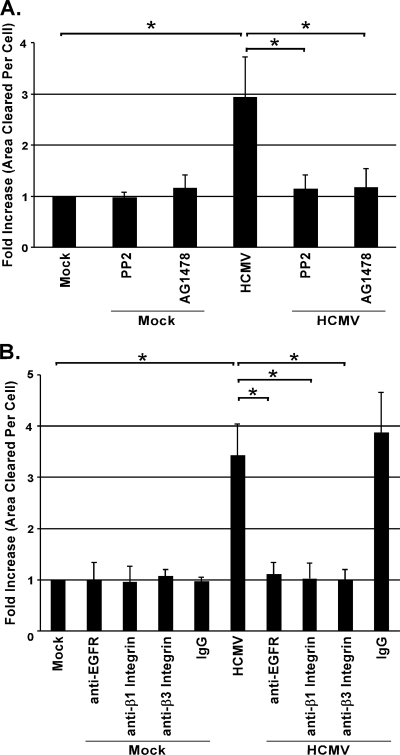
We previously reported that HCMV infection of monocytes leads to an induction of cellular motility and transendothelial migration through NF-κB- and PI(3)K-dependent signaling pathways (37-39). Because integrins and EGFR are upstream of the induction of PI(3)K and NF-κB (4, 32) and have been implicated in HCMV binding to and entry into human fibroblasts (14, 49, 50), we investigated if these receptors were involved in virus-induced monocyte motility. Using phagokinetic track motility assays (37-39), we observed that infected monocytes were 3.5 times more motile than mock-infected cells, consistent with previous studies (37-39). However, when monocytes were pretreated with PP2 (a specific inhibitor of Src tyrosine kinase activity [17]) or AG1478 (an inhibitor of EGFR tyrosine kinase activity [23]), their motility returned to that observed in mock-infected cells (Fig. 1A).
FIG. 1.
EGFR and integrin engagement and EGFR and Src kinase activity are required for monocyte motility following HCMV infection. Monocytes were treated with 1 μM PP2 (a Src kinase activity inhibitor), 1 μM AG1478 (an EGFR kinase activity inhibitor), or the solvent control DMSO (A) or with blocking antibodies specific for EGFR (1 μg/ml) or integrins (β1 or β3; 5 μg/ml) or an isotype-matched IgG (5 μg/ml) (B) for 1 h prior to HCMV infection or underwent mock treatment (A and B) for 1 h. Cells were transferred to colloidal-gold-coated coverslips and incubated for 24 h. Results are plotted as means ± SEM. Student's t tests were performed, and a P value of <0.05 (indicated by asterisks) was used as the measure of statistical significance between samples. The experiments were repeated at least three times.
To examine the direct role of receptor engagement in the enhanced motility following infection, we pretreated cells with blocking antibodies to select β-integrins and EGFR. Pretreatment of monocytes with blocking antibodies to β1 and β3 integrins or EGFR prior to HCMV infection resulted in significant inhibition (3.5-fold decrease) of motility of infected cells compared to the motility of untreated or isotype control IgG-pretreated infected monocytes (Fig. 1B). The role of EGFR in enhanced motility of infected monocytes is consistent with previous data (12). Together the results of Fig. 1A and B demonstrate that engagement of both integrin and EGFR is required for monocyte motility following HCMV infection. Pretreatment of mock-infected monocytes with neutralizing antibodies to β1 and β3 integrins or EGFR did not affect basal levels of monocyte motility (Fig. 1B). Furthermore, we demonstrated that pretreatment with neutralizing antibodies to β1 and β3 integrins did not influence the enhanced motility of PMA-treated monocytes (see Fig. S1 in the supplemental material), providing additional evidence that HCMV receptor-mediated activation is directly responsible for enhanced monocyte motility upon infection. From our data, we conclude that EGFR and integrin engagement, as well as EGFR and Src kinase activities, is required for increased monocyte motility following HCMV infection.
HCMV activates integrin-mediated cellular signaling pathways in monocytes.
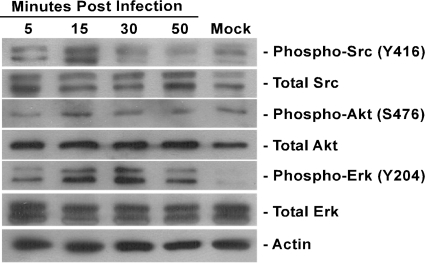
We have shown that HCMV interacts with EGFR, leading to an activation of cellular signaling (12). However, our data from the monocyte motility assay also strongly suggest that integrin engagement is essential for translating extracellular stimuli into biological changes seen in HCMV-infected monocytes. The activation of Src tyrosine kinase is directly initiated upon an engagement of integrins (26). To investigate the signaling cascade(s) triggered by HCMV binding to cellular integrins at the surfaces of monocytes, we performed Western blot analysis probing for phosphorylated/activated forms of Src at Tyr416, as well as Akt at Ser473 and Erk at Tyr204, which are reported to be downstream of the Src signal transduction pathway (2, 29). We examined monocyte activation over a time course from 5 min to 50 min postinfection (p.i.). Upon HCMV infection, we observed a gradual increase in the phosphorylated form of Src, with peak Src activation at 15 min p.i., followed by its decrease (Fig. 2). We also examined the activation of the downstream Akt and Erk signaling cascades. A similar profile of activation was seen (Fig. 2). The levels of total Src, Akt, and Erk did not change throughout the time course of the experiment (Fig. 2). Our data demonstrate that HCMV infection activates integrin-mediated cellular signaling pathways in monocytes through the activation of Src.
FIG. 2.
HCMV activates integrin-mediated cellular signaling pathways in monocytes. Monocytes were cultured in low serum for 24 h after isolation and then mock infected or HCMV infected. Cells were harvested at the time points indicated. Western blot analyses were performed using antibodies recognizing the phosphorylated and nonphosphorylated forms of Src, Akt, and Erk. Actin was used as a loading control. The experiment was repeated at least three times and representative results are shown.
Paxillin mRNA and protein expression are upregulated following HCMV infection.
It is known that signal transduction pathways initiated by surface receptor engagement can regulate multiple cellular processes. To provide clues to how viral receptor-ligand engagement activates monocyte motility, we examined the infected-monocyte transcriptome and focused on those integrin- and EGFR-regulated genes associated with motility and extravasation. Using Genesifter software, we found that the paxillin transcript was significantly expressed in HCMV-infected monocytes and that its expression was regulated by both the integrin- and EGFR-mediated signaling pathways (change in paxillin transcript expression in HCMV-induced monocytes compared to expression in mock-infected cells, 3.3-fold; inhibition of paxillin transcript expression in HCMV-infected monocytes by pretreatment with PP2 or AG1478 prior to infection, −2.2-fold and −3.0-fold, respectively; values are from six experiments). We became interested in paxillin, as it is a scaffolding and/or signal transduction protein important in regulating the interaction between multiple proteins involved in cell motility and adhesion (7). Our data suggest that paxillin regulation during infection might be an important aspect of HCMV-induced changes in monocyte motility.
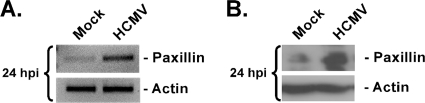
To confirm our transcriptome results showing HCMV-induced paxillin expression, we analyzed paxillin mRNA and protein expression in HCMV-infected monocytes. Semiquantitative PCR was performed using primers complementary to paxillin (Fig. 3A, upper band) or actin (Fig. 3A, lower band). The PCR results confirmed that paxillin mRNA expression increases during HCMV infection (Fig. 3A). Next, our analysis showed that the higher level of expression of paxillin mRNA observed in infected cells (Fig. 3A) correlates with higher levels of paxillin protein expression in infected cells, compared to levels in mock-infected cells, as shown by Western blot analysis (Fig. 3B).
FIG. 3.
Paxillin mRNA and protein expression is upregulated following HCMV infection. Monocytes were mock infected or HCMV infected and then harvested at 24 h p.i. (A) Semiquantitative PCR was performed using primers complementary to the paxillin or actin transcripts. Actin was used as a control. (B) Western blot analysis was performed using a specific antibody to paxillin or actin. Actin was used as a loading control. The experiments were repeated at least three times, and representative results are shown.
Paxillin activation (phosphorylation) occurs through integrin-mediated signaling following HCMV infection of monocytes.
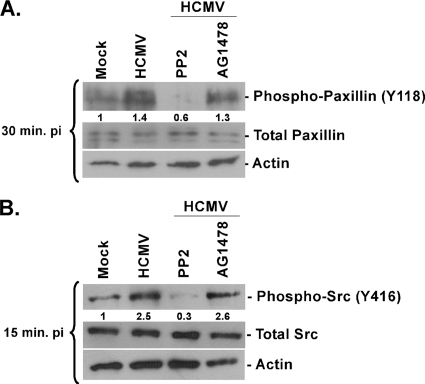
Paxillin is a known signal transducer that plays an important role in modulating interactions between proteins involved in signaling/cytoskeleton rearrangement, cellular motility, and adhesion (7). For paxillin to function as a signal integrator, it must be activated through appropriate phosphorylation (7). We performed time course analyses and examined paxillin activation; we observed that peak paxillin activation occurred between 15 min and 30 min p.i. (data not shown). Shown in Fig. 4 are the data from 15 and 30 min p.i., depicting the dependence of paxillin activation on Src activation. Using PP2 to pretreat cells, we observed a decrease in paxillin activation to below that seen in mock-infected cells (Fig. 4A). This effect was not seen when cells were pretreated with AG1478 (Fig. 4A). As a control, we show a corresponding decrease in Src phosphorylation in PP2- but not AG1478-pretreated monocytes (Fig. 4B). The obtained data suggest that paxillin is activated in an integrin-dependent manner and that Src and paxillin activation is not regulated via the EGFR-mediated signaling pathway. Moreover, our results indicate that, even though paxillin mRNA expression is regulated by integrin and EGFR signaling together, the functional activation of paxillin is mediated exclusively through integrin/Src-mediated signaling, at least soon after infection.
FIG. 4.
Paxillin activation (phosphorylation) occurs through integrin-mediated signaling following HCMV infection of monocytes. (A and B) Monocytes were isolated and cultured in low serum for 24 h. Monocytes were then pretreated with PP2, AG1478, or DMSO as a solvent control. Cells were mock infected or HCMV infected and harvested at 15 (B) or 30 (A) min p.i. Western blot analyses were performed using antibodies specific for the phosphorylated and nonphosphorylated forms of paxillin (A) and Src (B). Actin was used as a loading control. The experiments were repeated at least three times, and representative results are shown. The results were also measured by densitometry with relative numbers shown in the figures.
Paxillin phosphorylation is induced through receptor-ligand interaction between HCMV and cellular integrins on the surfaces of monocytes.
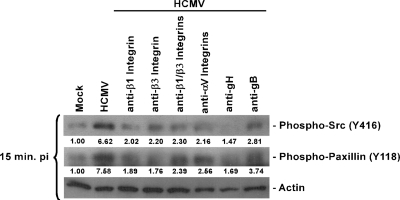
The above-mentioned results suggest that HCMV is able to trigger Src and paxillin phosphorylation in monocytes through the engagement of integrins present on the cell surface during viral binding. To investigate the possible direct interaction of HCMV with cellular integrins, we used blocking antibodies to various integrins, as well as blocking antibodies to gB and gH. Monocytes were harvested 15 min p.i. Monocytes infected with HCMV were characterized by higher levels of phosphorylated paxillin and Src (Fig. 5). Pretreatment of monocytes with blocking β1, β3, or αV integrin antibodies caused a decrease in the phosphorylated forms of Src (70%, 67%, or 67% decrease, respectively) and of paxillin (75%, 77%, or 66% decrease, respectively). Preincubation of monocytes with blocking antibodies to both β1 and β3 integrins prior to infection had similar effects on Src (65% decrease) and paxillin (69% decrease) activation (Fig. 5). By pretreating purified virus with blocking antibody to gH, we showed a strong inhibition of both Src (78% decrease) and paxillin (78% decrease) phosphorylation in HCMV-infected monocytes (Fig. 5). This incubation effect was not as profound when virus was pretreated prior to infection with blocking antibody to gB; we saw a 58% decrease in Src and a 51% decrease in paxillin phosphorylation (Fig. 5). The obtained results suggest that HCMV interacts with cellular β1, β3, and αV integrins, possibly via the gH glycoprotein and, to a lesser extent, via the gB glycoprotein.
FIG. 5.
Paxillin phosphorylation is induced through receptor-ligand interaction between HCMV and cellular integrins on the surfaces of monocytes. Monocytes were isolated and cultured in low serum for 24 h. Monocytes were then pretreated with blocking antibodies specific for β1, β3, or αV integrins, or virus was pretreated with blocking antibodies to gB or gH for 1 h. Cells were mock infected or HCMV infected and harvested at 15 min p.i. Western blot analyses were performed using antibodies specific for the phosphorylated forms of paxillin and Src. Actin was used as a loading control. The experiment was repeated at least three times, and representative results are shown. The results were also measured by densitometry with relative numbers shown in the figures.
Paxillin is required for the increased motility observed in HCMV-infected monocytes.
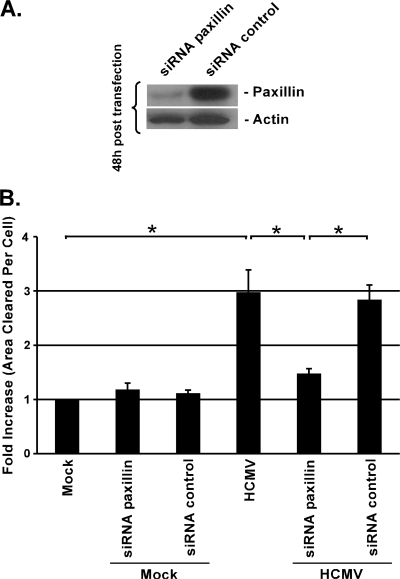
Because paxillin is known to regulate cellular motility, we next asked if paxillin activity is required for the enhanced monocyte motility observed following infection. We used siRNA to knock down paxillin expression. Isolated blood monocytes were transfected using the Amaxa Nucleofector (12) with specific siRNA complementary to paxillin or a scrambled control siRNA. Based on Western blot analysis, we were able to knock down paxillin expression by 80 to 90% (Fig. 6A). We also examined paxillin knockdown at the level of mRNA: an 80 to 90% knockdown of paxillin mRNA was observed (data not shown).
FIG. 6.
Paxillin is required for the increase in motility observed in HCMV-infected monocytes. (A) Monocytes were transfected with siRNA complementary to paxillin or a control siRNA. After 48 h, monocytes were harvested and Western blot analyses were performed using antibodies recognizing paxillin or actin. Actin was used as a loading control. (B) Forty-eight hours posttransfection, monocytes were mock infected or HCMV infected for 1 h, then transferred to colloidal-gold-coated glass coverslips and incubated for an additional 24 h. Results are plotted as means ± SEM. Student's t tests were performed, and a P value of <0.05 (indicated by asterisks) was used as the measure of statistical significance between samples. The experiments were repeated at least three times.
Next, we determined if the enhanced monocyte motility seen following HCMV infection was paxillin dependent. To address this question, we used a phagokinetic track motility assay (1, 38). Our assay revealed that paxillin-deficient monocytes had a significantly reduced capacity for movement compared to untransfected or scrambled siRNA control-treated cells (Fig. 6B). Mock-infected monocytes were characterized as having a low basal level of motility, and knockdown of paxillin in mock-infected cells did not influence this basal motility. Our data suggest that, during infection, HCMV upregulates paxillin activity to exploit its cellular regulatory role in order to promote enhanced monocyte motility.
Paxillin is required for efficient HCMV entry into monocytes.
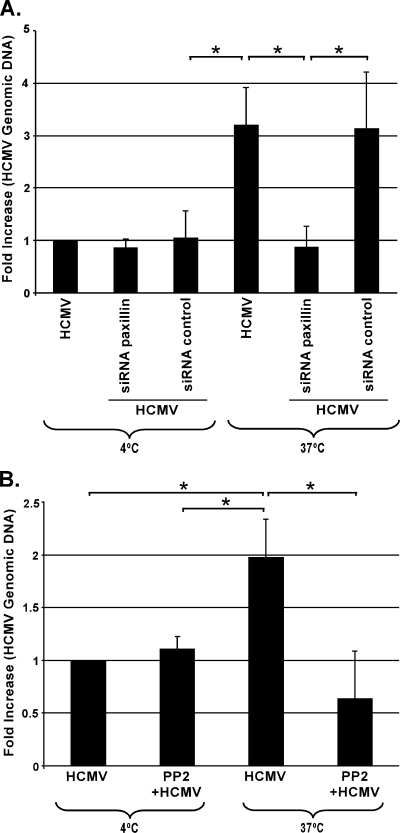
Because cytoskeletal rearrangements have been documented to be important for HCMV entry into fibroblasts (14, 49), we hypothesized that the viral regulation of paxillin that we observed in infected monocytes might also be a central player in viral entry into these cells. Monocytes transfected with paxillin siRNA or a scrambled control siRNA were investigated for levels of HCMV entry using our HCMV entry assay (12). Cells were infected at an MOI of 0.5 at 4°C to allow the virus to bind to cells. The temperature was then shifted to 37°C to permit HCMV entry into monocytes. Unbound and noninternalized virus was removed from monocytes by thorough washing and treatment with proteinase K. Total DNA was isolated from infected monocytes, and quantitative real-time PCR was performed. We observed strong inhibition (<3-fold decrease) of viral entry into monocytes transfected with paxillin siRNA compared to entry into untreated, infected monocytes or monocytes transfected with a scrambled siRNA control prior to infection (Fig. 7A). To monitor the accuracy of the assay, we also kept representative samples of infected monocytes at 4°C without a temperature shift and proceeded as described above. These cells had a <3-fold-smaller amount of internalized HCMV genomic DNA then infected cells shifted to 37°C (with or without transfected siRNA) (Fig. 7A).
FIG. 7.
Paxillin and integrin-mediated signaling are required for efficient HCMV entry into monocytes. (A) Monocytes were transfected with siRNA complementary to paxillin or a control siRNA. (B) Monocytes were pretreated with PP2. Cells were then mock infected or HCMV infected, and the ability of HCMV to enter the cells was investigated based on the relative amount of viral genomic DNA inside the cells as measured by quantitative real-time PCR. Results are plotted as means ± SEM. Student's t tests were performed, and a P value of <0.05 (indicated by asterisks) was used as the measure of statistical significance between samples. The experiments were repeated at least three times.
Because the introduction of siRNA into monocytes could influence viral binding to cells and thus affect viral entry, we examined viral binding to paxillin siRNA- and control siRNA-treated monocytes. For this purpose, we utilized flow cytometric analysis: siRNA-transfected monocytes were infected with GFP-tagged HCMV (TB40/E) and subsequently stained for CD14 and GFP. Based on CD14 expression, monocytes were gated and then analyzed for the presence of a GFP signal on those cells. We did not observe a difference in levels of HCMV binding to untreated, paxillin siRNA-treated, or control siRNA-treated cells (see Fig. S2 in the supplemental material).
We also investigated if paxillin siRNA-treated cells had changes in levels of β1 and β3 integrin expression on the surfaces of monocytes. Isolated monocytes were stained for the expression of the cellular marker CD14, as well as for the β1 (CD29) and β3 (CD61) integrins. The results demonstrated that treatment of monocytes with paxillin siRNA did not change the pattern of β1 and β3 integrin expression on monocytes (see Fig. S3 in the supplemental material). Moreover, we examined mRNA levels of β1 and β3 integrin expression using quantitative real time-PCR. Treatment of monocytes with paxillin siRNA or control siRNA did not change the expression of β1 and β3 integrin message (data not shown). Together, these data revealed that not only is paxillin an important player in the enhanced monocyte motility following HCMV infection but also that it acts as a crucial regulator for efficient HCMV entry into these cells.
Integrin-mediated signaling is important for efficient HCMV entry into monocytes.
Because paxillin activation appears to be strictly regulated through an integrin/Src-mediated signaling pathway, we next investigated if Src-mediated signaling triggered through integrin engagement is required for efficient HCMV entry into monocytes. HCMV was not able to efficiently enter monocytes pretreated with PP2 prior to infection (∼3-fold decrease), compared to entry into untreated, HCMV-infected monocytes (Fig. 7B). We also kept representative samples of infected monocytes at 4°C without a temperature shift and proceeded as described above. In these cells, only a basal level of HCMV genomic DNA was observed (Fig. 7B).
DISCUSSION
Peripheral blood monocytes are primary targets of HCMV infection in vivo and are thought to be a central cell type that allows for viral dissemination to multiple host organ systems (34, 35, 40, 45). Previously, we demonstrated that HCMV modifies the biology of infected monocytes, resulting in the overexpression and secretion of inflammatory cytokines, an induction of “hyper” cellular motility, adhesion to endothelial cells, transendothelial migration, and the promotion of cellular differentiation (8, 9, 37, 39, 55). Here we demonstrate that HCMV engagement of cellular receptors is responsible for early modulation of monocyte biology. Integrins and EGFR are known to initiate signaling pathways through activation/phosphorylation of the nonreceptor Src family of kinases and the intrinsic EGFR tyrosine kinase, respectively (20, 24, 51), resulting in PI(3)K and NF-κB activation (4, 32). These two receptors have been found to be important in HCMV binding to and entry into human fibroblasts (14, 49, 50). Although, fibroblasts are an established model for examining HCMV infection (49), we have previously documented that there are key differences in the effects of HCMV infection on these two cell types. For example, HCMV infection of monocytes results in the induction of cellular differentiation, long-term cellular survival, and PI(3)K-independent HCMV entry into the cells (8, 12, 37-39), which are results not observed in fibroblasts. The specific changes seen following HCMV infection and the lack of viral replication in monocytes suggest that cellular signaling may play a larger role in the biology of HCMV-infected monocytes than in that of other cell types.
To attempt to better define how signaling can impact the biology of monocytes during viral infection, we focused on the role that integrins and EGFR play in the early events associated with viral binding, entry, and cellular activation. Based on available data (14, 48-50) and our previous results (4, 12, 38, 39), we hypothesized that HCMV binding triggered both receptors simultaneously to mediate a unique activation of cellular signaling pathways, which in turn resulted in the functional changes in infected monocytes required for viral spread and persistence. Because of the complexity of these HCMV receptor-mediated changes in infected monocytes, we decided to initially focus separately on integrin- and EGFR-mediated regulation (12). In this paper, we concentrated on the role integrins play in the early events following HCMV infection of monocytes.
To test our hypothesis, we first used a phagokinetic motility track assay to quantitatively measure the motility of infected monocytes. We demonstrated that β1 and β3 integrin engagement through activation of the Src kinase was essential for the induction of infected-monocyte motility. HCMV uses multiple integrin receptors to modulate monocyte biology, in contrast to its infection of fibroblasts, where only a single β-integrin appears to be involved (14, 49). Additionally, we showed that the initial activation of the Src/integrin nexus translates downstream to the activation of other signaling molecules. Our data showing that both integrin and EGFR signaling pathways are required for motility support our hypothesis of the need for the engagement of multiple receptors during viral binding to monocytes.
To further investigate how integrin engagement during HCMV infection modulates monocyte biology, we examined the infected-monocyte transcriptome and found a disproportionate induction of genes associated with cell motility and adhesion, regulated by viral ligand-receptor engagement. Our analysis showed that integrin and EGFR signaling pathways are in part independently utilized by HCMV to regulate the expression of several genes in this group. However, we also discovered that a significant number of motility-associated genes are controlled simultaneously by both receptors, underlining the importance of the synchronized regulation of these two receptor-mediated signaling pathways by the virus. In this group of identified genes, we discovered paxillin to be significantly controlled. Paxillin is a scaffolding and signal transducer protein important for cell motility and adhesion that is known to regulate multiple components of these important cellular processes (7). Paxillin needs to be phosphorylated in order to function. This process of paxillin activation can be regulated through a number of cellular receptors, such as integrins, TLRs, G protein-coupled receptors, and EGFR (18, 31, 33).
Here we show that paxillin is indeed phosphorylated upon HCMV infection, with the kinetics of its activation being similar to that observed for Src phosphorylation. We also demonstrated that pretreatment of cells with PP2 prior to HCMV infection blocks paxillin phosphorylation, suggesting that, in infected monocytes, paxillin activation is triggered solely by cellular integrin-mediated signaling. Reports have documented that paxillin regulation can be directly initiated by pathogen-host cell interactions. For example, bacterial lipopolysaccharide (LPS) and fimbriae of the Gram-negative anaerobe Porphyromonas gingivalis can trigger paxillin phosphorylation (18, 52, 53). Additionally, the HIV transactivator protein Tat has been demonstrated to activate paxillin; however, this process was not a result of a direct interaction of the virus with the cell, as the Tat protein must be expressed to have a stimulating effect on surrounding cells (16). The targeting of paxillin by multiple pathogens hints at the central role the protein plays in cellular regulation and suggests that by controlling this process the pathogen gains a competitive advantage during infection. Nevertheless, our studies appear to be the initial evidence that viral binding to target cells regulates paxillin function.
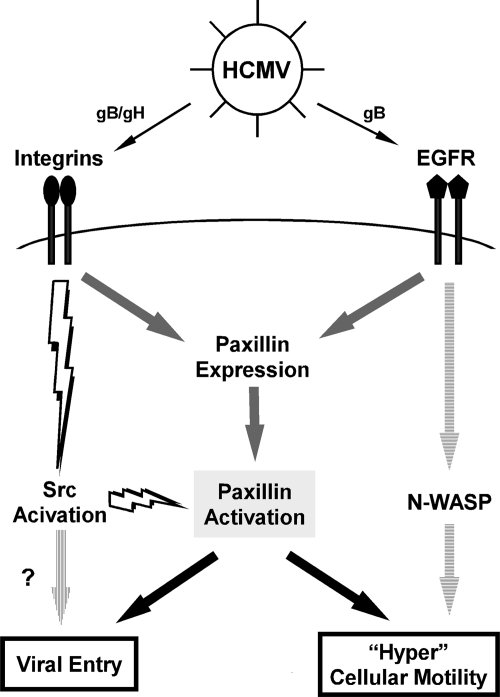
Because of the role paxillin plays in cellular motility, adhesion, and signaling and its binary regulatory mechanism at the level of message expression and activation (phosphorylation) seen following HCMV infection, we postulated that paxillin is a central player enabling the virus to effectively disseminate and persist in the host (Fig. 8). We specifically proposed that the rapid activation of paxillin through its phosphorylation allows for efficient viral entry into monocytes and that the initial activation of cellular signaling along with prolonged upregulation of paxillin expression upon HCMV infection governs the cytoskeletal changes responsible for pathogenic motility of infected monocytes. In support, we demonstrated that paxillin-deficient monocytes are characterized by an inhibited motility upon infection compared to the motility of control cells. Moreover, we determined that the importance of paxillin during HCMV infection is not limited to cellular movement; this protein also participates in another critical viral process: viral entry. Together, these results indicate that HCMV likely specifically targets paxillin function in order to exploit the intrinsic ability of this protein to control cytoskeletal function. Monocyte motility is inseparably connected to the regulation of the cytoskeleton, because of the role cytoskeletal reorganization plays in lamelipodium formation (22). In addition, because the cytoskeleton has also been proposed to play a key role in viral entry of fibroblasts (14, 49), we believe our data identify paxillin as a key link between the cytoskeletal changes required for motility and that required for entry. Hence, our results suggest a potentially novel role for paxillin in HCMV entry into monocytes; this is, to our knowledge, the initial discovery of a mechanism for how HCMV engagement of integrins may modulate viral entry into monocytes and how integrin signaling through the direct regulation of paxillin is involved in pathogenic motility of HCMV-infected monocytes.
FIG. 8.
Our model: paxillin activation is a molecular convergence point where the signaling required for the regulation of HCMV-induced pathogenic motility in monocytes meets the signaling required for efficient HCMV entry into monocytes. Integrin- and EGFR-mediated signaling upregulates paxillin mRNA expression and thus the total levels of paxillin expression (gray arrows). Yet only the cellular signaling initiated from integrin engagement during the viral binding process through downstream Src activation is responsible for the functional activation/phosphorylation of paxillin (lightning bolts). Paxillin activation is a convergence point essential for HCMV-induced pathogenic monocyte motility, as well as for efficient viral entry into target monocytes (black arrows). Note that our laboratory also documented that EGFR separately regulates expression of the neural Wiskott-Aldrich syndrome protein (N-WASP), but, unlike paxillin, N-WASP plays a role only in the induction of monocyte motility (horizontally striped arrows). There is also the possibility that Src activation may regulate other mechanisms of HCMV entry into monocytes (vertically striped arrow).
Our work begins to elucidate molecular mechanisms used by HCMV to trigger changes in monocyte biology that are likely responsible for enhanced viral survival and pathogenesis. This work provides insight into specific cellular molecules regulated by HCMV that dictate the efficiency of infection and control virus-mediated biological changes in target cells. Specifically, we found that HCMV regulates paxillin, underlying the crucial role of this molecular regulator, as well as the cytoskeletal changes governed by this protein that affect the pathogenic motility of and the efficient entry of HCMV into primary cells. Because other pathogens also target paxillin function (52, 53), our results suggest that this may be a key mechanism utilized by pathogens in order to allow for persistence in the infected host. Because little is known about viral entry in monocytes, this work will likely have global implications for the understanding of the viral entry process and the early steps in the infection of monocytes and other clinically relevant cell types.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (AI050677, HD-051998, and P20-RR018724), a Malcolm Feist cardiovascular research fellowship, and an American Heart Association predoctoral fellowship (10PRE4200007).
We have no financial conflict of interest.
Footnotes
Published ahead of print on 17 November 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Albrecht-Buehler, G. 1977. The phagokinetic tracks of 3T3 cells. Cell 11:395-404. [DOI] [PubMed] [Google Scholar]
- 2.Arcaro, A., et al. 2007. Critical role for lipid raft-associated Src kinases in activation of PI3K-Akt signalling. Cell. Signal. 19:1081-1092. [DOI] [PubMed] [Google Scholar]
- 3.Bentz, G. L., et al. 2006. Human cytomegalovirus (HCMV) infection of endothelial cells promotes naive monocyte extravasation and transfer of productive virus to enhance hematogenous dissemination of HCMV. J. Virol. 80:11539-11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bentz, G. L., and A. D. Yurochko. 2008. Human CMV infection of endothelial cells induces an angiogenic response through viral binding to EGF receptor and beta1 and beta3 integrins. Proc. Natl. Acad. Sci. U. S. A. 105:5531-5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bissinger, A. L., C. Sinzger, E. Kaiserling, and G. Jahn. 2002. Human cytomegalovirus as a direct pathogen: correlation of multiorgan involvement and cell distribution with clinical and pathological findings in a case of congenital inclusion disease. J. Med. Virol. 67:200-206. [DOI] [PubMed] [Google Scholar]
- 6.Boehme, K. W., M. Guerrero, and T. Compton. 2006. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J. Immunol. 177:7094-7102. [DOI] [PubMed] [Google Scholar]
- 7.Brown, M. C., and C. E. Turner. 2004. Paxillin: adapting to change. Physiol. Rev. 84:1315-1339. [DOI] [PubMed] [Google Scholar]
- 8.Chan, G., E. R. Bivins-Smith, M. S. Smith, P. M. Smith, and A. D. Yurochko. 2008. Transcriptome analysis reveals human cytomegalovirus reprograms monocyte differentiation toward an M1 macrophage. J. Immunol. 181:698-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan, G., E. R. Bivins-Smith, M. S. Smith, and A. D. Yurochko. 2009. NF-κB and phosphatidylinositol 3-kinase activity mediates the HCMV-induced atypical M1/M2 polarization of monocytes. Virus Res. 144:329-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan, G., E. R. Bivins-Smith, M. S. Smith, and A. D. Yurochko. 2008. Transcriptome analysis of NF-κB- and phosphatidylinositol 3-kinase-regulated genes in human cytomegalovirus-infected monocytes. J. Virol. 82:1040-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan, G., M. T. Nogalski, G. L. Bentz, and A. D. Yurochko. 2010. PI3K-dependent upregulation of Mcl-1 by human cytomegalovirus is mediated by epidermal growth factor receptor and inhibits apoptosis in short-lived monocytes. J. Immunol. 184:3213-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan, G., M. T. Nogalski, and A. D. Yurochko. 2009. Activation of EGFR on monocytes is required for human cytomegalovirus entry and mediates cellular motility. Proc. Natl. Acad. Sci. U. S. A. 106:22369-22374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Compton, T., et al. 2003. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J. Virol. 77:4588-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feire, A. L., H. Koss, and T. Compton. 2004. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc. Natl. Acad. Sci. U. S. A. 101:15470-15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowler, K. B. 1991. Sexually transmitted diseases in mothers of neonates with congenital cytomegalovirus infection. J. Infect. Dis. 164:259-264. [DOI] [PubMed] [Google Scholar]
- 16.Ganju, R. K., et al. 1998. Human immunodeficiency virus tat modulates the Flk-1/KDR receptor, mitogen-activated protein kinases, and components of focal adhesion in Kaposi's sarcoma cells. J. Virol. 72:6131-6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanke, J. H., et al. 1996. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 271:695-701. [DOI] [PubMed] [Google Scholar]
- 18.Hazeki, K., et al. 2003. Toll-like receptor-mediated tyrosine phosphorylation of paxillin via MyD88-dependent and -independent pathways. Eur. J. Immunol. 33:740-747. [DOI] [PubMed] [Google Scholar]
- 19.Huang, E. S., and T. F. Kowalik. 1993. The pathogenicity of human cytomegalovirus: an overview, p. 1-45. In Y. Becker, G. Darai, and E. S. Huang (ed.), Molecular aspects of human cytomegalovirus diseases. Springer-Verlag, New York, NY.
- 20.Hynes, R. O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110:673-687. [DOI] [PubMed] [Google Scholar]
- 21.Inaba, T., et al. 1993. Expression of platelet-derived growth factor beta receptor on human monocyte-derived macrophages and effects of platelet-derived growth factor BB dimer on the cellular function. J. Biol. Chem. 268:24353-24360. [PubMed] [Google Scholar]
- 22.Le Clainche, C., and M. F. Carlier. 2008. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol. Rev. 88:489-513. [DOI] [PubMed] [Google Scholar]
- 23.Levitzki, A., and A. Gazit. 1995. Tyrosine kinase inhibition: an approach to drug development. Science 267:1782-1788. [DOI] [PubMed] [Google Scholar]
- 24.Lo, H. W., S. C. Hsu, and M. C. Hung. 2006. EGFR signaling pathway in breast cancers: from traditional signal transduction to direct nuclear translocalization. Breast Cancer Res. Treat. 95:211-218. [DOI] [PubMed] [Google Scholar]
- 25.Melnick, J. L., E. Adam, and M. E. DeBakey. 1995. Cytomegalovirus and atherosclerosis. Bioessays 17:899-903. [DOI] [PubMed] [Google Scholar]
- 26.Miyamoto, S., et al. 1995. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J. Cell Biol. 131:791-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mocarski, E. S., T. Shenk, and R. F. Pass. 2007. Cytomegalovirus, p. 2701-2772. In D. M. Knipe et al. (ed.), Fields virology. Lippincott, Williams & Wilkins, Philadelphia, PA.
- 28.Rasband, W. S. 2010. ImageJ. National Institutes of Health, Bethesda, MD.
- 29.Reyes-Reyes, M., N. Mora, G. Gonzalez, and C. Rosales. 2002. β1 and β2 integrins activate different signalling pathways in monocytes. Biochem. J. 363:273-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rice, G. P., R. D. Schrier, and M. B. Oldstone. 1984. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc. Natl. Acad. Sci. U. S. A. 81:6134-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rozengurt, E. 2007. Mitogenic signaling pathways induced by G protein-coupled receptors. J. Cell. Physiol. 213:589-602. [DOI] [PubMed] [Google Scholar]
- 32.Scatena, M., et al. 1998. NF-κB mediates αvβ3 integrin-induced endothelial cell survival. J. Cell Biol. 141:1083-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider, I. C., C. K. Hays, and C. M. Waterman. 2009. Epidermal growth factor-induced contraction regulates paxillin phosphorylation to temporally separate traction generation from de-adhesion. Mol. Biol. Cell 20:3155-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinclair, J., and P. Sissons. 1996. Latent and persistent infections of monocytes and macrophages. Intervirology 39:293-301. [DOI] [PubMed] [Google Scholar]
- 35.Sinzger, C., and G. Jahn. 1996. Human cytomegalovirus cell tropism and pathogenesis. Intervirology 39:302-319. [DOI] [PubMed] [Google Scholar]
- 36.Sinzger, C., et al. 1999. Modification of human cytomegalovirus tropism through propagation in vitro is associated with changes in the viral genome. J. Gen. Virol. 80:2867-2877. [DOI] [PubMed] [Google Scholar]
- 37.Smith, M. S., G. L. Bentz, J. S. Alexander, and A. D. Yurochko. 2004. Human cytomegalovirus induces monocyte differentiation and migration as a strategy for dissemination and persistence. J. Virol. 78:4444-4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith, M. S., G. L. Bentz, P. M. Smith, E. R. Bivins, and A. D. Yurochko. 2004. HCMV activates PI(3)K in monocytes and promotes monocyte motility and transendothelial migration in a PI(3)K-dependent manner. J. Leukoc. Biol. 76:65-76. [DOI] [PubMed] [Google Scholar]
- 39.Smith, M. S., et al. 2007. Roles of phosphatidylinositol 3-kinase and NF-κB in human cytomegalovirus-mediated monocyte diapedesis and adhesion: strategy for viral persistence. J. Virol. 81:7683-7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soderberg, C., S. Larsson, S. Bergstedt-Lindqvist, and E. Moller. 1993. Definition of a subset of human peripheral blood mononuclear cells that are permissive to human cytomegalovirus infection. J. Virol. 67:3166-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soroceanu, L., A. Akhavan, and C. S. Cobbs. 2008. Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection. Nature 455:391-395. [DOI] [PubMed] [Google Scholar]
- 42.Speir, E., et al. 1994. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science 265:391-394. [DOI] [PubMed] [Google Scholar]
- 43.Streblow, D. N., S. L. Orloff, and J. A. Nelson. 2001. Do pathogens accelerate atherosclerosis? J. Nutr. 131:2798S-2804S. [DOI] [PubMed] [Google Scholar]
- 44.Streblow, D. N., et al. 2003. Human cytomegalovirus chemokine receptor US28-induced smooth muscle cell migration is mediated by focal adhesion kinase and Src. J. Biol. Chem. 278:50456-50465. [DOI] [PubMed] [Google Scholar]
- 45.Taylor-Wiedeman, J., J. G. Sissons, L. K. Borysiewicz, and J. H. Sinclair. 1991. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J. Gen. Virol. 72:2059-2064. [DOI] [PubMed] [Google Scholar]
- 46.Vivanco, I., and C. L. Sawyers. 2002. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer 2:489-501. [DOI] [PubMed] [Google Scholar]
- 47.Waldman, W. J., P. W. Adams, D. A. Knight, and D. D. Sedmak. 1997. CMV as an exacerbating agent in transplant vascular sclerosis: potential immune-mediated mechanisms modelled in vitro. Transplant. Proc. 29:1545-1546. [DOI] [PubMed] [Google Scholar]
- 48.Wang, H. Q., et al. 2003. Epidermal growth factor receptor-dependent, NF-κB-independent activation of the phosphatidylinositol 3-kinase/Akt pathway inhibits ultraviolet irradiation-induced caspases-3, -8, and -9 in human keratinocytes. J. Biol. Chem. 278:45737-45745. [DOI] [PubMed] [Google Scholar]
- 49.Wang, X., D. Y. Huang, S. M. Huong, and E. S. Huang. 2005. Integrin αvβ3 is a coreceptor for human cytomegalovirus. Nat. Med. 11:515-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, X., S. M. Huong, M. L. Chiu, N. Raab-Traub, and E. S. Huang. 2003. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424:456-461. [DOI] [PubMed] [Google Scholar]
- 51.Yamada, K. M., and S. Miyamoto. 1995. Integrin transmembrane signaling and cytoskeletal control. Curr. Opin. Cell Biol. 7:681-689. [DOI] [PubMed] [Google Scholar]
- 52.Yilmaz, O., K. Watanabe, and R. J. Lamont. 2002. Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cell. Microbiol. 4:305-314. [DOI] [PubMed] [Google Scholar]
- 53.Yilmaz, O., P. A. Young, R. J. Lamont, and G. E. Kenny. 2003. Gingival epithelial cell signalling and cytoskeletal responses to Porphyromonas gingivalis invasion. Microbiology 149:2417-2426. [DOI] [PubMed] [Google Scholar]
- 54.Yurochko, A. D. 2008. Human cytomegalovirus modulation of signal transduction. Curr. Top. Microbiol. Immunol. 325:205-220. [DOI] [PubMed] [Google Scholar]
- 55.Yurochko, A. D., and E. S. Huang. 1999. Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J. Immunol. 162:4806-4816. [PubMed] [Google Scholar]
- 56.Yurochko, A. D., et al. 1997. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-κB during infection. J. Virol. 71:5051-5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yurochko, A. D., D. Y. Liu, D. Eierman, and S. Haskill. 1992. Integrins as a primary signal transduction molecule regulating monocyte immediate-early gene induction. Proc. Natl. Acad. Sci. U. S. A. 89:9034-9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.