Abstract
While chronic wasting disease (CWD) prion transmission, entry, and trafficking remain incompletely elucidated, natural exposure of the oral and/or nasal mucous membranes seems certain. Cervids commonly sustain minor lesions on oral mucous membranes that could have an impact on susceptibility to prion infection. To explore this potential cofactor, we studied cohorts of cervid PrP transgenic mice with or without superficial abrasions on the lingual mucosa to determine whether minor oral mucosa lesions may enhance susceptibility to CWD infections. Results demonstrated that minor lingual abrasions substantially facilitate CWD transmission, revealing a cofactor that may be significant in cervids and perhaps other species.
Chronic wasting disease (CWD) is a fatal neurodegenerative prion disease affecting deer, elk, and moose. The efficiency by which CWD spreads suggests that transmission occurs primarily by horizontal means (22-25). Body fluids and excreta, including blood, saliva, urine, and feces from infected cervids, have been shown to contain infectious CWD prions (13, 20, 35). While the exact mechanisms of CWD prion transmission, entry, and trafficking remain incompletely elucidated, transmission by contact with the oral and/or nasal mucous membranes seems certain. Whether prion infection occurs after mucous membrane exposure may be influenced by cofactors beyond dose, such as particle association with soil and the status of the mucous membrane barrier (12, 16, 17, 30).
Oral inoculation studies with sheep scrapie and CWD have indicated that early amplification of the resistant prion protein (PrPSc/CWD) occurs within the tonsils, retropharyngeal lymph nodes, and/or Peyer's patches (6, 10, 32). Prion dissemination can also occur through lymphoreticular system (LRS)-independent pathways (4, 7, 28). The oral cavity is highly innervated (38), and contact between prions and free nerve endings of the cranial-facial nerves may provide a direct site for prion uptake.
Cervids acquire lesions in the oral mucous membranes through foraging and tooth eruption (31, 33) that could impact susceptibility to prion entry by facilitating direct contact with exposed nerves or blood vessels. The present work was prompted by a study by Bartz et al. (5), who demonstrated in the transmissible mink encephalopathy (TME) system that lesions on or injection into the tongue enhanced susceptibility to prion infections. We therefore explored the hypothesis that CWD transmission may be facilitated by small lesions on the oral epithelial surface.
Transgenic mice that express the elk prion protein [Tg(CerPrP-E226)5037+/− mice; Telling laboratory, University of Kentucky] and are susceptible to CWD infection (1) were used in these studies. Clinical criteria for assessing CWD infection included ataxia, lethargy, tail rigidity, poor coat quality, and weight loss. Mice were euthanized when distinct signs of neurologic disease were evident. CWD agent- and sham-inoculated mice were housed in separate rooms to minimize potential for cross contamination.
The CWD inoculum consisted of brain homogenate from a CWD prion-infected white-tailed deer (no. 104). The negative control was brain homogenate from a CWD prion-naïve white-tailed deer (no. 123). Brain homogenates were prepared in 1× phosphate-buffered saline (PBS) to a concentration of 10% (wt/vol). Two cohorts of Tg(CerPrP-E226)5037+/− mice (12 CWD mice and 12 control mice) were inoculated per os (p.o.) by pipette instillation of 10 μl of the 10% (wt/vol) brain homogenate onto the lingual surface. Two additional cohorts of Tg(CerPrP-E226)5037+/− mice (12 CWD mice and 12 sham-inoculated mice) were anesthetized using a ketamine-xylazine mix, and 3 minor abrasions (∼3 mm) were created by lightly scratching the lingual surface with a 27-gauge needle. The epithelium was disrupted and small amounts of blood were evident at the scarification sites. Mice were inoculated as described above. Three mice from each cohort were sacrificed at 90 days postinoculation (p.i.) and analyzed for early PrPCWD detection. The remaining mice (n = 9/cohort) were monitored until clinical symptoms became apparent or until study termination at 738 days p.i.
Tissues from necropsied mice were prepared for Western blotting (WB) as described previously (9); samples were electrophoresed for 1.5 h at 125 V and then transferred onto a PVDF membrane (0.22 μm; Millipore) for 1.0 h and processed using the SNAP i.d. system (Millipore). Membranes were blocked in a mixture of 0.5% casein-Tris-buffered saline (TBS; Thermo Scientific) and 0.1% Tween 20 (Sigma) for 3 min, incubated with monoclonal antibody BAR-224 (Spi-Bio) conjugated with horseradish peroxidase (HRP) at a 1:20,000 dilution in a mixture of 0.5% casein-TBS and 0.1% Tween 20 for 7 min, and washed 3 times with 1× TBS-0.02% Tween 20.
Tissues were prepared for immunohistochemistry (IHC) analyses as described previously (9). Staining was performed by hand using a TSA (tyramide signal amplification) Plus dinitrophenyl (DNP)-HRP kit (PerkinElmer). Slides were blocked with 3% H2O2 in methanol for 45 min; blocked with either TNB buffer (0.1 M Tris HCl [pH 7.5], 0.15 M Nacl, 0.5% blocking reagent) or a mouse-on-mouse (M.O.M.) IgG blocking reagent (Vector Laboratories) for 45 min, with a secondary M.O.M. protein concentrate block for 5 min; and incubated with a 1:500 dilution of HRP-conjugated BAR-224 in TNB or the M.O.M. protein concentrate for 90 min. Samples were then subjected to amplification with a 1:50 dilution of 1× DNP amplification reagent in diluent for 15 min, enhanced with a 1:100 dilution of anti-DNP-HRP in TNB for 30 min, developed with chromogen AEC (Dako) for 30 min, and counterstained with hematoxylin for 5 min and bluing reagent for 1 min. Brain sections stained by hematoxylin and eosin (H&E) and IHC staining were assessed for transmissible spongiform encephalopathy (TSE) lesions which included primary neuronal loss, vacuolation, and gliosis.
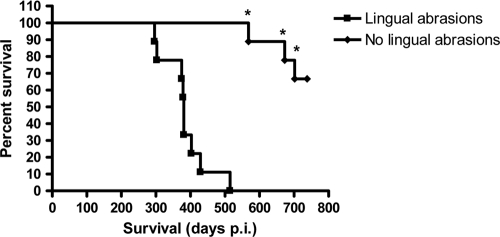
Of the CWD brain homogenate-inoculated Tg(CerPrP-E226)5037+/− mice (n = 9) that did not receive lingual lesions, 2 were euthanized due to neoplasias unrelated to CWD; the remaining 7 mice survived until study termination at >700 days p.i. (Fig. 1) without exhibiting clinical signs of CWD infection. None of the negative control-inoculated mice developed signs of TSE, although 3 were euthanized due to issues unrelated to CWD. WB analysis of brains from CWD agent- and sham-inoculated mice were negative for PrPCWD (data not shown). Despite application of the sensitive TSA IHC methodology, the presence of PrPCWD could not be demonstrated in the brain, tongue, salivary glands, trachea, esophagus, spleen, stomach, or gastrointestinal tract in either the mice sacrificed early or the mice surviving to study termination (data not shown). A summary of these results can be found in Table 1.
FIG. 1.
Kaplan-Meier survival plot for Tg(CerPrP-E226) mice with and without lingual abrasions inoculated orally with CWD brain homogenate. * denotes mice that died of causes unrelated to CWD.
TABLE 1.
Summary of CWD inoculation results for Tg(CerPrP-E226)5037+/− mice
| Lingual abrasion status | Inoculum | Attack ratea | Survival (days p.i.) | % PrPCWD positiveb |
|---|---|---|---|---|
| Present | CWD-positive brain homogenate | 9/9 (100) | 385 ± 62 | 100 |
| CWD-negative brain homogenate | 0/9 (0) | 388 ± 61c | 0 | |
| Absent | CWD-positive brain homogenate | 0/9 (0) | 708 ± 57 | 0 |
| CWD-negative brain homogenate | 0/9 (0) | 607 ± 212c | 0 |
No. affected/total(%).
Positive by Western blot and immunohistochemistry analyses.
One control mouse was sacrificed for each symptomatic mouse euthanized.
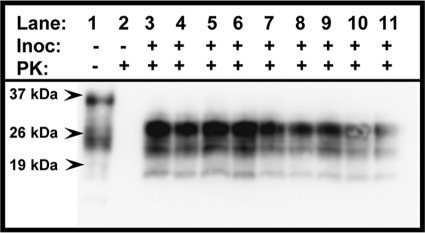
Nine (100%) of 9 CWD brain homogenate-inoculated mice with lingual abrasions developed clinical symptoms consistent with TSE between 296 and 515 days p.i. and were euthanized (Fig. 1). None of the negative control-inoculated mice (0 of 9) displayed evidence of TSE throughout the observation period of 515 days. PrPCWD was detected in the brains of all 9 CWD brain homogenate-inoculated mice by WB (Fig. 2, lanes 3 to 11) and IHC (Fig. 3A) analyses but was absent in negative control-inoculated mice (Fig. 2, lane 2, and 3B). Both H&E-stained and IHC sections revealed evidence of neuronal loss and gliosis consistent with CWD infection (data not shown). TSA IHC analysis of peripheral tissues was again unable to detect PrPCWD. A summary of these results is in Table 1.
FIG. 2.
Western blot detection of PrPCWD (lanes 3 to 11) in brains of Tg(CerPrPE226)5037+/− mice with lingual lesions exposed to CWD prions. Samples from mice with lesions exposed to the negative-control inoculum (lanes 1 and 2) showed no evidence of PrPCWD. PK, proteinase K.
FIG. 3.
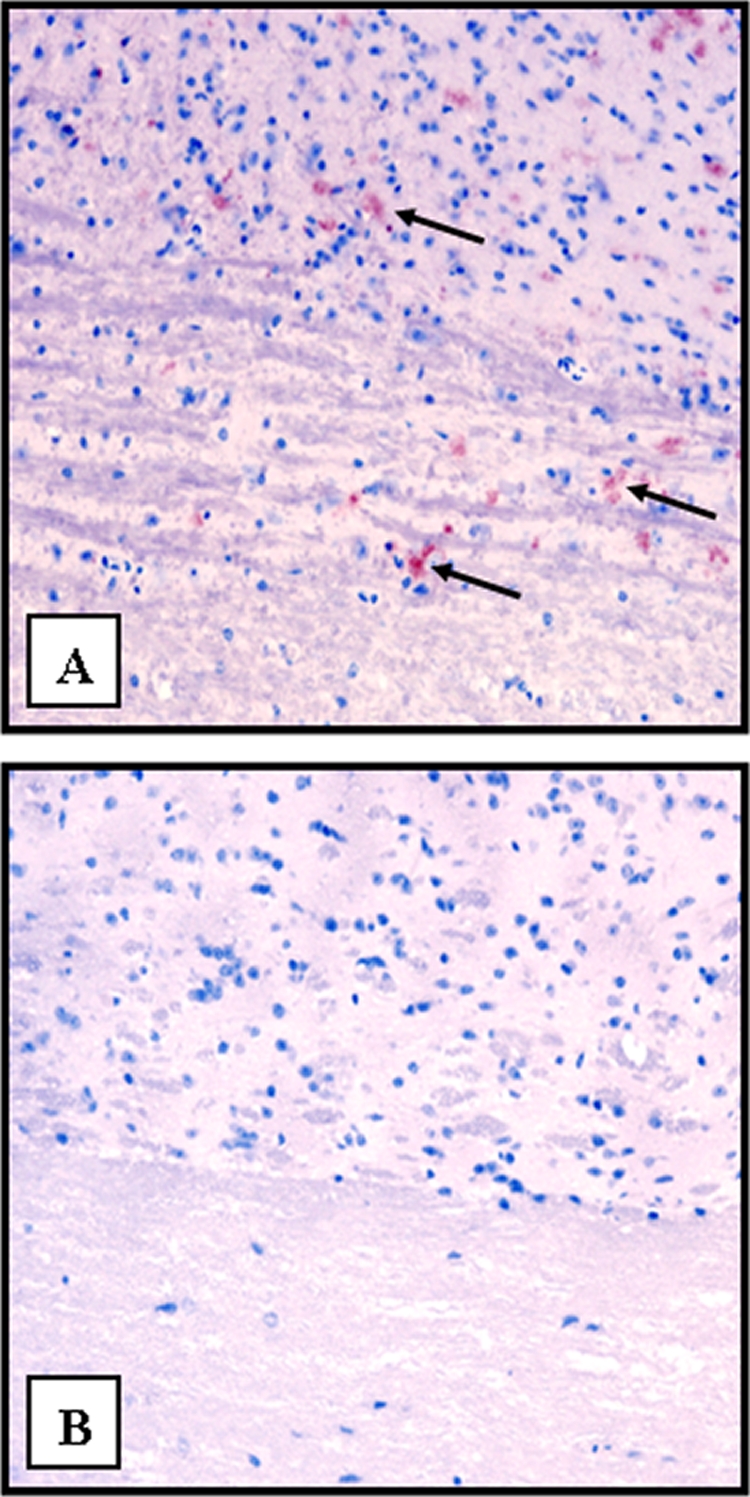
IHC analysis of the obex region of the medulla from a Tg(CerPrP-E226)5037+/− mouse with lingual lesions demonstrating aggregates of PrPCWD (arrows) (A) and a mouse with lesions exposed to the negative-control inoculum (B). Original magnification, ×20.
Transmission of CWD prions to deer by oral inoculation has been demonstrated experimentally, and the oral route is considered to be the most plausible route of infection in nature (10, 14, 19, 20, 24, 32). Cervids naturally experience minor oral lesions as part of foraging (2, 33), although the frequency at which this occurs remains undocumented. Such lesions may provide a cofactor for prion entry that could help explain the facile transmission of CWD. The present study models this natural event in cervid PrP transgenic mice.
Available evidence indicates that cervid PrP transgenic mice are much less susceptible to oral CWD infection than cervids. Establishment of infection requires a potent inoculum, and inoculated mice have relatively low attack rates (29, 37). Here, we show that this substantial resistance is negated by breeches in the lingual epithelium, enhancing CWD infection rates from 0 to 100%. While Tg(CerPrP-E226)5037+/− mice support PrPCWD replication in the spleen and lymph nodes after peripheral inoculation (D. M. Seelig, G. L. Mason, G. C. Telling, and E. A. Hoover, unpublished results), the lack of demonstrable PrPCWD in nonneural tissues of the affected mice in the present study suggests that transit to the brain occurred by an LRS-independent pathway.
Gajdusek (11) suggested that lesions on the conjunctiva or skin or mucosal injury might facilitate the transmission of kuru. This premise was supported by studies indicating that scarification of either skin or gingival tissues enhanced the transmission of scrapie (8, 12, 15, 36). The work of Bartz and Bessen et al. (5, 7) with hamsters orally exposed to TME most clearly demonstrated that lingual scarification produces significantly increased attack rates, shortened survival, and infection with substantially smaller prion doses. The present study suggests that the enhancement of CWD infection by oral inoculation is even greater.
We surmise that mucosal lesions may have made possible direct neural contact, as the tongue is heavily innervated (5, 38). However, we were unable to detect PrPCWD in tongue or lymphoid tissues by immunostaining at 90 days p.i. or in terminally affected mice. In that CWD is lymphotropic (10, 32, 34), the lack of discernible PrPCWD in LRS tissues again suggests that prion ascension to the brain occurred independently of the LRS. Caveats in this conclusion include that small PrPCWD aggregates in peripheral tissues may be below the sensitivity limits of TSA IHC protocols and that such aggregates may exist in a formalin- or protease-sensitive form. Nevertheless, while entry via a lymphatic or hematogenous route cannot be excluded, the most plausible pathway would seem to be trafficking along lingual and facial nerves—a prion phenomenon demonstrated previously by the work of several investigators (5, 15, 26).
Dose dependency in prion infections has been demonstrated extensively (3, 18, 21, 27, 29). While environmental contamination almost surely plays a role in CWD transmission, prion concentrations in excretions, soil, and the environment are very low. The results of the present study may help explain how low concentrations of environmental prions (13, 20, 35) may be able to transit the mucosal barrier to initiate infection in foraging cervids and perhaps in other species.
In summary, superficial lesions on the lingual surface in cervid PrP transgenic mice substantially enhanced susceptibility to oral CWD infection. The absence of PrPCWD detection in the tongue, lymphoid tissues, or any peripheral tissue site suggested a direct neural route of invasion. These results implicate one cofactor that could facilitate CWD infection in cervids after oral exposure to very low concentrations of prions in nature.
Acknowledgments
This work was supported by funding from NIH/NIAID contract NOI-AI-25491 (to E.A.H. and G.C.T.), the Stephen G. and Susan E. Denkers Family Foundation (to N.D.D.), USDA CSREES training grant 2005-38420-15813 (to N.D.D.), and NIH grant RO1-NS-040334 (to G.C.T.).
Special thanks to Jeanette Hayes-Klug and Kelly Anderson for the care of the transgenic mice and to Davis Seelig for his expertise and help with PrPC and PrPCWD immunohistochemistry.
Footnotes
Published ahead of print on 17 November 2010.
REFERENCES
- 1.Angers, R. C., et al. 2009. Chronic wasting disease prions in elk antler velvet. Emerg. Infect. Dis. 15:696-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayers, E., et al. 2001. Oral lesions in sheep and cattle in Dumfries and Galloway. Vet. Rec. 148:720-723. [PubMed] [Google Scholar]
- 3.Baier, M., et al. 2003. Prion diseases: infectious and lethal doses following oral challenge. J. Gen. Virol. 84:1927-1929. [DOI] [PubMed] [Google Scholar]
- 4.Bartz, J. C., C. Dejoia, T. Tucker, A. E. Kincaid, and R. A. Bessen. 2005. Extraneural prion neuroinvasion without lymphoreticular system infection. J. Virol. 79:11858-11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartz, J. C., A. E. Kincaid, and R. A. Bessen. 2003. Rapid prion neuroinvasion following tongue infection. J. Virol. 77:583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beekes, M., and P. A. McBride. 2000. Early accumulation of pathological PrP in the enteric nervous system and gut-associated lymphoid tissue of hamsters orally infected with scrapie. Neurosci. Lett. 278:181-184. [DOI] [PubMed] [Google Scholar]
- 7.Bessen, R. A., S. Martinka, J. Kelly, and D. Gonzalez. 2009. Role of the lymphoreticular system in prion neuroinvasion from the oral and nasal mucosa. J. Virol. 83:6435-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carp, R. I. 1982. Transmission of scrapie by oral route: effect of gingival scarification. Lancet i:170-171. [DOI] [PubMed] [Google Scholar]
- 9.Denkers, N. D., D. M. Seelig, G. C. Telling, and E. A. Hoover. 2010. Aerosol and nasal transmission of chronic wasting disease in cervidized mice. J. Gen. Virol. 91:1651-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox, K. A., J. E. Jewell, E. S. Williams, and M. W. Miller. 2006. Patterns of PrPCWD accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus). J. Gen. Virol. 87:3451-3461. [DOI] [PubMed] [Google Scholar]
- 11.Gajdusek, D. C. 1985. Subacute spongiform virus encephalopathies cuased by unconventional viruses, p. 483-544. In K. M. J. McKelvey (ed.), Subviral pathogens of plants and animals: viroids and prions. Academic Press, New York, NY.
- 12.Glaysher, B. R., and N. A. Mabbott. 2007. Role of the draining lymph node in scrapie agent transmission from the skin. Immunol. Lett. 109:64-71. [DOI] [PubMed] [Google Scholar]
- 13.Haley, N. J., D. M. Seelig, M. D. Zabel, G. C. Telling, and E. A. Hoover. 2009. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS One 4:e4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamir, A. N., et al. 2006. Preliminary observations of genetic susceptibility of elk (Cervus elaphus nelsoni) to chronic wasting disease by experimental oral inoculation. J. Vet. Diagn. Invest. 18:110-114. [DOI] [PubMed] [Google Scholar]
- 15.Ingrosso, L., F. Pisani, and M. Pocchiari. 1999. Transmission of the 263K scrapie strain by the dental route. J. Gen. Virol. 80(Pt. 11):3043-3047. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, C. J., J. A. Pedersen, R. J. Chappell, D. McKenzie, and J. M. Aiken. 2007. Oral transmissibility of prion disease is enhanced by binding to soil particles. PLoS Pathog. 3:e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, C. J., et al. 2006. Prions adhere to soil minerals and remain infectious. PLoS Pathog. 2:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimberlin, R. H., and C. A. Walker. 1989. Pathogenesis of scrapie in mice after intragastric infection. Virus Res. 12:213-220. [DOI] [PubMed] [Google Scholar]
- 19.Kreeger, T. J., D. L. Montgomery, J. E. Jewell, W. Schultz, and E. S. Williams. 2006. Oral transmission of chronic wasting disease in captive Shira's moose. J. Wildl. Dis. 42:640-645. [DOI] [PubMed] [Google Scholar]
- 20.Mathiason, C. K., et al. 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314:133-136. [DOI] [PubMed] [Google Scholar]
- 21.McLean, A. R., and C. J. Bostock. 2000. Scrapie infections initiated at varying doses: an analysis of 117 titration experiments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:1043-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, M. W., and M. A. Wild. 2004. Epidemiology of chronic wasting disease in captive white-tailed and mule deer. J. Wildl. Dis. 40:320-327. [DOI] [PubMed] [Google Scholar]
- 23.Miller, M. W., M. A. Wild, and E. S. Williams. 1998. Epidemiology of chronic wasting disease in captive Rocky Mountain elk. J. Wildl. Dis. 34:532-538. [DOI] [PubMed] [Google Scholar]
- 24.Miller, M. W., and E. S. Williams. 2003. Prion disease: horizontal prion transmission in mule deer. Nature 425:35-36. [DOI] [PubMed] [Google Scholar]
- 25.Miller, M. W., E. S. Williams, N. T. Hobbs, and L. L. Wolfe. 2004. Environmental sources of prion transmission in mule deer. Emerg. Infect. Dis. 10:1003-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulcahy, E. R., J. C. Bartz, A. E. Kincaid, and R. A. Bessen. 2004. Prion infection of skeletal muscle cells and papillae in the tongue. J. Virol. 78:6792-6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prusiner, S. B., S. P. Cochran, and M. P. Alpers. 1985. Transmission of scrapie in hamsters. J. Infect. Dis. 152:971-978. [DOI] [PubMed] [Google Scholar]
- 28.Race, R., M. Oldstone, and B. Chesebro. 2000. Entry versus blockade of brain infection following oral or intraperitoneal scrapie administration: role of prion protein expression in peripheral nerves and spleen. J. Virol. 74:828-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seelig, D. M., G. L. Mason, G. C. Telling, and E. A. Hoover. 2010. Pathogenesis of chronic wasting disease in cervidized transgenic mice. Am. J. Pathol. 176:2785-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seidel, B., et al. 2007. Scrapie agent (strain 263K) can transmit disease via the oral route after persistence in soil over years. PLoS One 2:e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Severinghaus, C. W. 1949. Tooth development and wear as criteria of age in white-tailed deer. J. Wildl. Manage. 13:195-216. [Google Scholar]
- 32.Sigurdson, C. J., et al. 1999. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). J. Gen. Virol. 80(Pt. 10):2757-2764. [DOI] [PubMed] [Google Scholar]
- 33.Spraker, T. R., et al. 1997. Spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus) and Rocky Mountain elk (Cervus elaphus nelsoni) in northcentral Colorado. J. Wildl. Dis. 33:1-6. [DOI] [PubMed] [Google Scholar]
- 34.Spraker, T. R., et al. 2002. Comparison of histological lesions and immunohistochemical staining of proteinase-resistant prion protein in a naturally occurring spongiform encephalopathy of free-ranging mule deer (Odocoileus hemionus) with those of chronic wasting disease of captive mule deer. Vet. Pathol. 39:110-119. [DOI] [PubMed] [Google Scholar]
- 35.Tamguney, G., et al. 2009. Asymptomatic deer excrete infectious prions in faeces. Nature 461:529-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor, D. M., I. McConnell, and H. Fraser. 1996. Scrapie infection can be established readily through skin scarification in immunocompetent but not immunodeficient mice. J. Gen. Virol. 77(Pt. 7):1595-1599. [DOI] [PubMed] [Google Scholar]
- 37.Trifilo, M. J., G. Ying, C. Teng, and M. B. Oldstone. 2007. Chronic wasting disease of deer and elk in transgenic mice: oral transmission and pathobiology. Virology 365:136-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weddell, G., J. A. Harpman, and D. G. Lambley. 1940. The innervation of the musculature of the tongue. J. Anat. 74:255-267. [PMC free article] [PubMed] [Google Scholar]