Abstract
There are conflicting data about the frequency and role of regulatory T cells (Tregs) during the course of HIV infection. Peripheral blood of a large cohort of HIV-infected patients (n = 131) at different stages of disease, including 15 long-term nonprogressors and 21 elite controllers, was analyzed to determine the frequency and phenotype of Tregs, defined as CD4+, CD25high, CD127low, FoxP3high cells. A significantly increased relative frequency of Tregs within the CD4+ compartment of HIV+ patients compared to that of healthy controls (P < 0.0001) was observed. Additionally, the relative frequency of Tregs directly correlated with HIV viral load and inversely with CD4+ counts. However, the absolute Treg number was reduced in HIV-infected patients versus healthy controls (P < 0.0001), with the exception of elite controllers (P > 0.05). The loss of absolute Treg numbers coincided with rising markers of immune activation (P < 0.0006). The initiation of antiviral therapy significantly increased absolute Treg numbers (P < 0.0031). We find that the expression of CD39, a newly defined ectonucleotidase with immunomodulatory functions on Tregs, correlated with progressive HIV disease, HIV viral load, and immune activation. Of note, when tested in peripheral blood mononuclear cells of healthy volunteers, the in vitro capacity to suppress T-cell proliferation was limited to CD4+, CD25high, CD39+ T cells. Interestingly, Tregs of elite controllers exhibited not only the highest expression of CCR5, CTLA-4, and ICOS but also the lowest level of CD39. The data presented here reconcile the seemingly contradictory results of previous studies looking at Tregs in HIV and highlight the complexity of Treg-mediated immunoregulation during human viral infections.
Increasing evidence suggests a critical role for regulatory T cells (Tregs) for the coordination and maintenance of virus-specific immune responses that are necessary to control chronic viral infections, such as HIV (50, 54). It has been proposed that excessive Treg reactivity suppresses multiple cell types and leads to the faster progression of HIV pathogenesis (27). On the other hand, Tregs might protect individuals from the deleterious effects of immune activation that is typically observed in chronic HIV infection (5, 18). There is increasing evidence that the clinical progression of HIV infection is critically linked to a state of immunological hyperactivation and its negative consequences (15, 23, 31, 59). The results of studies that assessed the role of Tregs in HIV infection have been largely inconclusive due to technical reasons, such as the suboptimal definition of Tregs in many studies that relied only on the coexpression of CD4+ and CD25high (43, 52, 62). However, since activated CD4+ T cells also express CD25, purified CD4+ CD25high Tregs are likely to be contaminated with an unknown proportion of activated conventional CD4+ T cells. The intracellular expression of the transcriptional activator FoxP3 (forkhead box protein 3) is commonly regarded as a more definitive marker after cell fixation (18, 19, 49). In addition, recent studies have suggested additional markers for the characterization of regulatory T cells ex vivo (55). For this study, a multicolor flow panel was developed to determine the frequency and phenotype of Tregs, defined as CD4+ CD25+ CD127low FoxP3high cells. Peripheral blood mononuclear cell (PBMC) samples from a large, well-characterized cohort of 131 HIV-infected patients at different stages of disease were analyzed. We examined the phenotype of Treg populations further using a selection of differentiation markers, including CCR5, CTLA-4, ICOS, as well as CD39, a recently described molecule with immunomodulatory properties (36). Notably, we studied 36 patients with nonprogressive disease, including 21 who met the criteria of elite control with HIV plasma viral loads (VL) below the limit of detection (<50 RNA copies/ml) and stable CD4 counts in the absence of highly active anti-retroactive therapy (HAART), to understand whether the frequency or quality of regulatory T cells is associated with slow disease progression. A subset of patients was also monitored longitudinally before and after the initiation of HAART therapy. Moreover, we compared Tregs isolated from lymph nodes with corresponding samples from peripheral blood to better understand the distribution of these regulatory T cells in lymphoid tissues. We observed that the relative frequency of Tregs in the CD4+ T cell compartment increased with disease progression and showed strong association with viral replication. However, this was mainly due to the loss of conventional CD4+ T cells. Overall, we find that the absolute number of Tregs declined at a slow pace, with progressive general immune activation during the course of untreated HIV infection. The results of this study reconcile the seemingly contradictory conclusions of previous reports looking at Tregs in HIV and further highlight the complexity of Treg-mediated immunoregulation during human viral infections (18, 25, 35).
MATERIALS AND METHODS
Study subjects and samples.
PBMC as well as lymph node mononuclear cells (LNMC) of HIV+ patients were collected at the University Medical Center Hamburg-Eppendorf, University of Frankfurt, University of Cologne, University of Bonn, and the University of Hannover, Germany. The HIV nonprogressor and elite controller group consisted of HIV-infected subjects recruited from the NaViC (natural virus controller) study group, which is composed of patients selected from a detailed clinical and laboratory database of more than 6,000 individuals of a German network of clinical HIV centers. Healthy individuals (n = 20) served as controls for the validation of the immunological tests. Written informed consent was obtained from all patients enrolled into this study, which was approved by the respective Institutional Review Boards. The time and duration of HIV infection and the definition of the different stages of the disease were extracted from the electronic databases of the participating centers and confirmed by the treating physicians according to standard classifications and by criteria commonly used in the literature (13, 46). HIV-1 viral load was determined using COBAS amplicor assays with a limit of detection of <50 RNA copies/ml. HIV CDC status, antiretroviral treatment, and slopes of CD4+ T cell counts were determined via chart review. All patients enrolled in this study were hepatitis C virus (HCV) seronegative and had no serological signs of chronic HBV infection.
Immunophenotypic analysis.
For immunophenotypic staining, cryopreserved PBMC or LNMC were thawed using standardized techniques in the laboratory. After incubation with EDTA for 15 min at 20°C in the dark, at least 5 × 105 PBMC were stained with appropriate fluorochrome-conjugated surface antibodies, including anti-CD3, anti-CD4, anti-CD8, anti-CD25, anti-CD127, anti-HLA-DR, anti-Ki67, anti-CD39, anti-CTLA-4 (CD152), anti-ICOS (CD278), or anti-CCR5 (CD195) (all from BD Biosciences Pharmingen, Heidelberg, Germany), for 30 min at 4°C in the dark to characterize the Treg population. Cells were washed once and fixed with 0.5% paraformaldehyde. After surface staining, the intracellular staining of FoxP3 (Alexa Fluor647; clone 259D/C7) was performed using the FoxP3 staining buffer set (eBiosciences, San Diego, CA) by following the manufacturer's protocol and as published previously (9). All samples were resuspended in 0.5% paraformaldehyde, and data were collected on a 6-channel FACS Canto flow cytometer using FACSDiva version 5 (Becton-Dickinson, Heidelberg, Germany).
Cell purification and suppression assays.
To characterize CD39+ Tregs, intracellular cytokine staining (ICS) assays and suppression assays were performed. For both assays, fresh PBMC from healthy volunteers were stained with anti-human CD3, CD4, CD25, and CD39 antibodies and sorted into four populations (CD39+CD25high/CD39−CD25high and CD39+CD25low/CD39−CD25low) on a BD FACSAria (BD Biosciences). Sorted cells were analyzed for purity by flow cytometry (data not shown). ICS assays were performed as described previously with at least 50,000 sorted cells. Phorbol myristate acetate (PMA) (50 ng/ml final concentration)-ionomycin (0.67 μM final concentration) was used to stimulate the cells for 1 h before the addition of brefeldin A (10 μg/ml). The cells were incubated for an additional 12 h at 37°C and 5% CO2. Then PBMC were washed and stained with surface antibodies; after being washed, the PBMC were fixed and permeabilized, and the anti-gamma interferon (IFN-γ) monoclonal antibody (MAb) and the interleukin-10 (IL-10) MAb (both Becton Dickinson) were added. To analyze the suppressive capacity of CD4+ CD25high CD39+ and CD4+ CD25high CD39− cells, live cells subjected to fluorescence-activated cell sorting (FACS) were mixed at a ratio of 5:1 with full PBMC and plated at 100,000 cells per well in 96-well U-bottom plates (TPP, Switzerland) in 200 μl R10 in quadruplicates. Staphylococcus aureus enterotoxin B (SEB) was used to stimulate the PBMC at a final concentration of 1 μg/ml. After 6 days of incubation at 37°C and 5% CO2, cells were pulsed for 6 h with 1 μCi [3H]thymidine and harvested on filters. For the purpose of data interpretation, a stimulation index (SI) above 5 was considered significant, as previously reported (53). The stimulation index was calculated as SI = (mean of counts per minute [cpm] of stimulated cells/mean of cpm of unstimulated cells).
Statistical analysis.
All flow-cytometric data were analyzed using FlowJo version 8.0 software (Treestar), and statistical analysis was done using Prism 5.0 software (GraphPad Software, San Diego, CA). The Kolmogorov-Smirnov test was applied to investigate the normal distribution of metric data analyzed. Due to the normal distribution of metric data, parametric tests of significance were performed throughout all analyses, using unpaired and paired t tests for intergroup comparisons and the Spearman rank test for bivariate correlation analyses. One-way analysis of variance was used to test for differences between the groups using the F distribution. All data are expressed as means ± standard deviations. P values of 0.05 or less were considered significant. To adjust for pairwise multiple comparisons, thereby controlling the overall type I error rate, Bonferroni correction was applied, with a conservative level of statistical significance set at αBonf = 0.001 (7).
RESULTS
Study cohort and flow-cytometric assessment of Tregs.
The aim of this study was to assess the frequency and phenotype of regulatory T cells (Tregs) in a large, well-characterized cohort of patients with HIV-1 infection, including individuals at different stages of chronic disease and a large number of patients who spontaneously control HIV viremia in the absence of HAART. All patients enrolled in this study were negative for serological markers for concomitant chronic hepatitis B or C infection. Altogether, 131 HIV+ patients and 20 healthy controls were included. The clinical and immunological characteristics of our cohort are shown in Table 1. To correlate immunological results with clinical progression, this cohort was divided into the following separate subgroups: (i) 31 patients were aviremic (VL < 50 copies/ml) for more than a year of HAART treatment (median CD4 cells/μl, 208 [range, 95 to 906 cells/μl]); (ii) 43 patients with viral loads between 50 and 1 × 105 copies/ml (CD4 cells/μl, 523 [12 to 1,023 cells/μl]); (iii) 21 patients with HIV-associated disease (CDC stages B and C) and VL of >1 × 105 copies/ml were designated progressors (CD4 cells/μl, 105 [3 to 544 cells/μl]); and (iv) the group of 36 clinical HIV controllers. This group was further split into two groups: (i) 15 long-term nonprogressors (LTNP) with CDC stage A, a stable VL of <2,000 copies/ml, and CD4+ counts of >500/μl for more than 5 years of being treatment naïve; and (ii) 21 elite controllers (EC) with CDC stage A, a stable CD4+ count of >500/μl, and an undetectable VL (<50 copies/ml) in the absence of therapy. These definitions were chosen according to widely accepted criteria (13).
TABLE 1.
Clinical, immunological, and virological characteristics of the cohort
| Patient classification | Gendera | Ageb (yr) | Viral load (copies/ml)b | CD4 countb (cells/μl) | Treatmentc (no.) |
|---|---|---|---|---|---|
| HAART treatment for >1 yr (n = 31) | 27 M, 4 F | 50 (33-73) | <50 | 208 (95-906) | HAART, 31 |
| VL of 50 to 105 copies/ml (n = 43) | 37 M, 6 F | 42 (19-78) | 1.5 × 104 (84-7.9 × 104) | 523 (12-1,023) | Naïve, 28; HAART, 8; IoT, 7 |
| Progressors (n = 21) | 19 M, 2 F | 39 (23-72) | 2.9 × 105 (1 × 105-3 × 106) | 105 (3-544) | Naïve, 16; HAART, 1; IoT, 5 |
| Nonprogressors (n = 36) | 22 M, 14 F | 44 (23-81) | 1055 (<50-8,600) | 689 (265-1,622) | Naïve, 36; LTNP, 15; EC, 21 |
M, male; F, female.
Values are medians (ranges).
IoT, interruption of treatment; LTNP, long-term nonprogressor (>5 years, stable VL and CD4 counts, untreated); EC, elite controller (VL < 50 copies/ml, CD4 count stable, untreated).
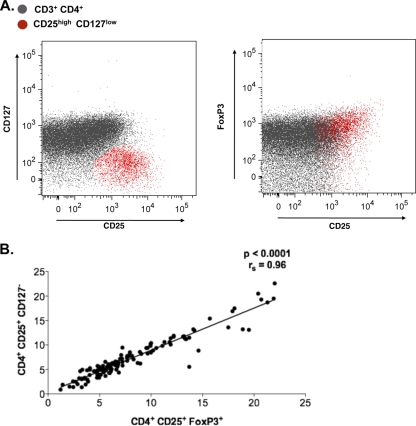
To discriminate Tregs from the remainder of CD4+ T cells in this study, we incorporated a combination of cell surface markers that included CD4, CD25, and the IL-7 receptor, CD127 (55), as well as the intracellular marker FoxP3 into our flow cytometry analyses, thereby defining Tregs as CD4+, CD25high, CD127low, and FoxP3high. Representative plots using this gating strategy for patients at different stages of HIV-1 disease are depicted in Fig. 1A and B and Fig. 2A. CD127 (unlike FoxP3) is an extracellular protein and therefore appears to serve as a good marker to sort live Tregs. Clearly, the overwhelming majority of CD25high CD127low cells coincided with the FoxP3high population (Fig. 1A and B) (34, 55, 58). Notably, this also was true for viremic patients, and our results are in contrast to a recent report that saw a disconnect in this group of patients (14).
FIG. 1.
(A and B) CD25high CD127low surface staining coincides with CD25high FoxP3+ cells. The overlay of the CD25high CD127low population and CD25high FoxP3high shows strong correlation between populations (P < 0.0001; rs = 0.96).
FIG. 2.
Relative increase but absolute decrease of Treg frequency during HIV disease progression. (A) Representative Treg stains (FoxP3high CD25high CD127low) of patients in different stages of the disease. Cells were stained as previously described and gated on CD3+, CD4+, CD127low FoxP3high, and CD25high, showing differences in the relative Treg frequency, while (B) the relative Treg frequency was similar in long-term nonprogressors as well as in elite controllers and the relative Treg frequencies were significantly increased, even in patients that suppressed the virus due to HAART for more than a year. The relative Treg frequency inversely correlated with CD4+ counts for the entire cohort (C), while the frequency directly correlated with the HIV VL (only patients with detectable VL were plotted) (D). (E) When absolute Treg numbers (Tregs/μl) were plotted, we found a decrease of Treg frequency with HIV disease progression. Interestingly, only the elite controllers had Treg frequencies comparable to those of healthy controls. (F and G) Absolute Treg numbers correlated directly with CD4+ count and inversely with HIV VL.
Relative increase and absolute decrease of the Treg frequency with progressive HIV disease.
Overall, we observed a significant increase in the relative frequency of Tregs within the CD4+ compartment of the entire HIV+ cohort (7.4% ± 5.2%) compared to that of healthy controls (3.2% ± 1.7%). Furthermore, significant differences were observed depending upon the status of HIV infection (Fig. 2B). Patients with high HIV-1 viremia and a particularly progressive disease course showed a statistically significant elevation in relative Treg frequency (P < 0.0001; 11.9% ± 8.5%). Interestingly, patients with long-term controller status (3.9% ± 1.5%) as well as elite controllers (4.1% ± 1.5%) had relative Treg frequencies comparable to those of healthy individuals (P > 0.05). Patients who were successfully treated with antiretroviral drugs for more than a year (all VL were <50 copies/ml) displayed a lower frequency of Tregs than that of HIV progressors (7.8% ± 3.5%; P = 0.0001). However, this frequency was still higher than that for healthy controls (P < 0.0001). In addition, we observed a statistically significant positive correlation between relative Treg frequency and HIV VL (rs = 0.54, where rs is regression calculated with the Spearman rank correlation test; P < 0.0001) and an inverse correlation with CD4+ counts (rs = −0.56; P < 0.0001) (Fig. 2C and D). Interestingly, regulatory T cells of patients with HIV infection, regardless of their clinical status, had a higher expression (measured as MFI, for mean fluorescence intensity) of FoxP3, and the MFI also correlated positively with VL and inversely with the CD4 count (data not shown). It remains to be determined whether the higher expression (MFI) of FoxP3 correlates with Treg activation or function (9, 55). As expected, following the in vitro depletion of CD25high FoxP3high Tregs, we observed the enhanced proliferation of HIV-specific CD4+ T cells (data not shown).
Since CD4+ counts of the majority of HIV-infected patients decrease significantly during the course of natural infection (1, 15, 31, 33), we next plotted the proportion of Tregs of all CD3+ T cells against CD4+ counts (in cells/μl) to obtain the absolute number of Tregs in the peripheral blood, as described previously (9). The absolute number of Treg cells in the peripheral blood was significantly decreased in our cohort of HIV-infected patients (8.8 ± 8.3 Tregs/μl; P < 0.0001), including the subgroup of long-term nonprogressors (11.9 ± 11.56 Tregs/μl; P < 0.0001). Only in HIV elite controllers did we observe absolute Treg numbers that were comparable to those of healthy controls (32 ± 14 Tregs/μl; P > 0.05) (Fig. 2 E). The absolute number of Tregs also correlated with the CD4+ count (rs = 0.70; P < 0.0001) and showed an inverse correlation with viral load (rs = −0.52; P < 0.0001) (Fig. 2F and G). Interestingly, we find a significant correlation between T-cell activation as measured by HLA-DR expression of the non-Tregs and the loss of absolute Treg numbers (rs = −0.46; P < 0.0006) (see Fig. S1F in the supplemental material). Finally, the absolute Treg number of patients on HAART was only slightly higher than that of the progressor group and did not reach statistical significance (11.6 ± 7.2 Tregs/μl; P > 0.05), indicating that the absolute number of Tregs does not fully recover after the initiation of therapy.
The distinction between the absolute number of Tregs and the relative Treg frequency is important and might help to explain the contradictory results about the role of Tregs in HIV infection (9-11, 17, 18, 42, 54, 61). Our data indirectly indicate that the Treg population is decreasing over time (maybe due to the deleterious effects of immune activation) at a lower rate than the (memory) CD4+ T cell population.
Phenotypical characterization of Tregs: CD39 expression of Tregs correlates with progressive HIV disease.
To date, several studies have characterized Treg populations in HIV patients; however, few have systematically and comprehensively analyzed the expression of further surface molecules of Tregs in HIV patients (18, 34). For this study, we focused on the expression of three surface molecules that play a critical role in controlling the adaptive arm of immune responses and are deemed critical for Treg function. These include two members of the CD28 family of receptors, the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) as well as the inducible costimulator (ICOS), and CD39, a member of the E-NTPDases (ectonucleoside triphosphate diphosphohydrolases). The ligation of CTLA-4 inhibits T-cell activation by reducing the production of interleukin-2 and arresting cell cycle progression (26), ICOS is suggested to stimulate the induction of interleukin-10 (29), and CD39 is strongly associated with suppressive immunomodulatory functions by removing proinflammatory ATP and producing anti-inflammatory adenosine (8).
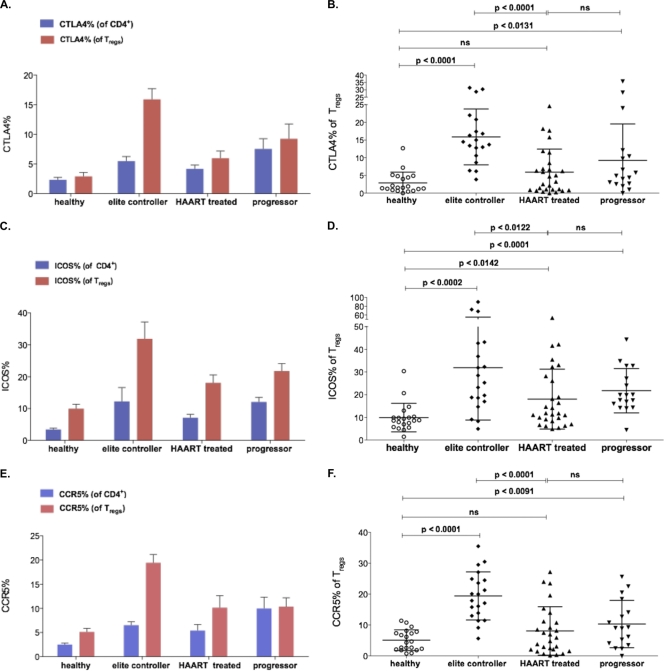
In our study, we observed elevated levels of the surface expression of these molecules on the Treg population compared to those of other CD4+ T cells, regardless of HIV infection status (Fig. 3). We also detected significantly increased levels of CTLA-4 (9.2% ± 8.4%; P < 0.0007), ICOS (21.6% ± 16.3%; P < 0.0002), and CD39 (46.5% ± 20.2%; P < 0.0011) expression on Tregs of HIV patients compared to those of a group of healthy controls (CTLA4, 2.9% ± 2.0%; ICOS, 9.9% ± 6.2%; CD39, 30.5% ± 17.0%, respectively) (data not shown). No association was detected between the Treg expression of CTLA-4 or ICOS and HIV VL or CD4+ counts in HIV-infected individuals. However, the levels of expression differed according to the stage of the disease, and Tregs from the subgroup of elite controllers displayed elevated levels of CTLA-4 and ICOS compared to levels for those who progressed (ICOS, 31.9% ± 23.0%; P < 0.0314; CTLA4, 15.9% ± 7.8%; P < 0.0043) (Fig. 3A to D), although these trends did not reach statistical significance after Bonferroni correction. Due to the limited sample size, we were able to assess these markers in only a minority of HIV nonprogressors. These limited assays showed levels of CTLA-4 and ICOS expression comparable to those of elite controllers (data not shown).
FIG. 3.
Surface expression of CTLA-4, ICOS, and CCR5 on Tregs of HIV-infected patients. CTLA4 (A and B) and ICOS (C and D) were more highly expressed on the surface of Tregs of HIV-infected patients (CTLA-4, 9.2% ± 8.4%, P < 0.0007; ICOS, 21.6% ± 16.3%, P < 0.0002) compared to the healthy control group (CTLA4, 2.9% ± 2.0%; ICOS, 9.9% ± 6.2%). Elite controllers showed elevated levels of CTLA-4 and ICOS compared to those with progressive disease (ICOS, 31.9% ± 23.0, P < 0.0314; CTLA4, 15.9% ± 7.8, P < 0.0043). (E and F) CCR5 expression was higher on CD4+ T cells and Tregs of HIV-infected patients (10.3% ± 7.6%; P < 0.0091) than for the healthy control group (5.1% ± 3.3%). Tregs of elite controllers showed the highest expression of CCR5 (19.4% ± 7.7%).
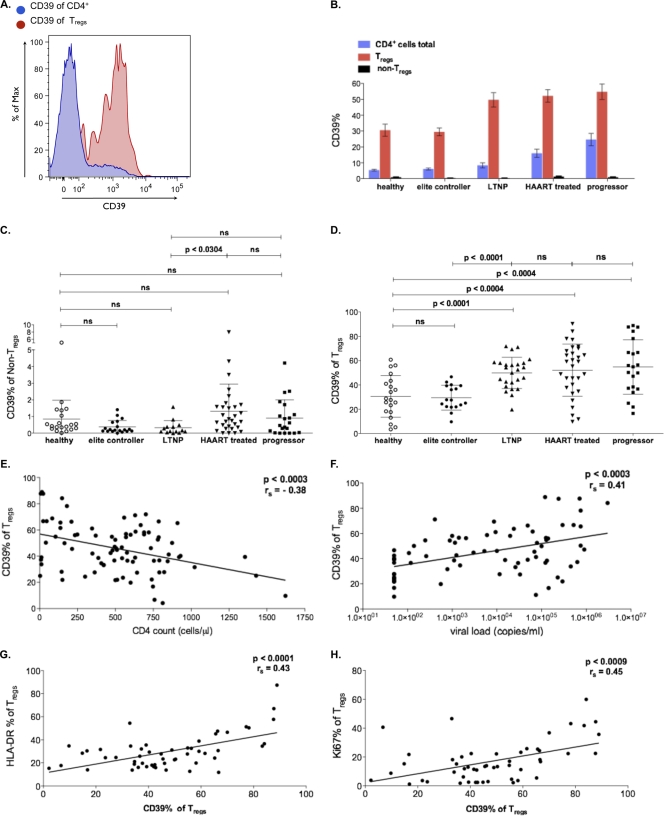
We observed a significant increase of CD39 expression in regulatory T cells of patients with HIV infection (P < 0.0011). Notably, we also found a strong association between CD39 expression on Tregs and disease progression. CD39 expression of Tregs correlated inversely with CD4+ counts (rs = −0.38; P < 0.0003) and directly with HIV VL (P < 0.0003; rs = 0.41). Interestingly, compared to that of the Treg population defined by our markers, we observed only the low surface expression of CD39 on the CD4+ non-Treg population (Fig. 4A to F). These data show that CD39 expression is largely restricted to Tregs. In accordance with the data by Borsellino et al. (8), we find a positive correlation between CD39 expression and the activation status of the Tregs as determined by HLA-DR and Ki67 expression (Fig. 4G and H). So far, there are few published data about the functionality of CD39+ T cells. To investigate the functionality of CD39+ T cells, we live-sorted CD4+ T cells according to the expression of CD25 and/or CD39 from PBMC of healthy volunteers (see Fig. S2 in the supplemental material). Interestingly, the capability to suppress the proliferation of PBMC was confined to the CD4+, CD39+, CD25high cell compartment (see Fig. S2C).
FIG. 4.
CD39 expression correlates with HIV disease progression. (A) Representative fluorescence-activated cell sorter plot showing CD39 expression (red) on CD4+ cells (blue). (B and C) CD39 expression was restricted to Tregs and compared to conventional CD4+ T cells (non-Tregs) regardless of the stage of the disease. (D) CD39 expression on Tregs positively correlated with disease progression and was expressed to a higher extent on Tregs of all HIV-infected patients regardless of the stage of the disease (46.5% ± 20.2%) compared to that of healthy controls (30.5% ± 17.0%, P < 0.0011), with the exception of elite controllers (P > 0.05). (E and F) CD39 expression on Tregs correlated inversely with CD4+ counts (P < 0.0003; rs = −0.38) and positive with HIV viral load (P < 0.0003; rs = 0.41). (G and H) Expression of CD39 shows a strong correlation with the activation status of Tregs, as measured by the Treg expression of HLA-DR (P < 0.0001; rs = 0.43) and Ki67 (P < 0.0009; rs = 0.45).
We next examined the surface expression of the viral coreceptor CCR5 to address the potential susceptibility of Tregs to infection with HIV. First, CCR5 expression was detected on Tregs from healthy controls and appeared slightly higher on Tregs than other CD4+ T-cell subpopulations. Overall, we observed a significant induction of CCR5 expression of Tregs during HIV disease (10.3% ± 7.6%; P < 0.0091) compared to that of the healthy control group (5.1% ± 3.3%), but this did not appear to correlate with HIV VL (Fig. 3E and F). Of note, the CCR5 expression level was highest in the group of elite controllers (19.4% ± 7.7%), which differed significantly from that of progressors (P < 0.0001). While it is conceivable that an enrichment of CCR5+ Tregs in elite controllers is merely a consequence of suppressed infection, it also is important to note that CCR5 is a homing marker influencing Treg trafficking, which may contribute in some way to viral control in these individuals. In particular, recent research has emphasized a beneficial role for Tregs at the site of infection, where they can orchestrate rather than dampen the effector cell response at early stages of infection (35, 51). Thus, we aimed to study the distribution of Tregs in lymphoid tissue, an important site of viral replication.
Higher relative frequency of Tregs in lymph node tissue compared to peripheral blood of HIV-infected patients.
We were able to compare the frequencies of Tregs in peripheral blood with concurrent lymph node samples collected early during HIV infection in 10 patients enrolled previously in a sexually transmitted infection (STI) cohort (Table 2). We found a higher relative Treg frequency (10.8% ± 3.1%) and FoxP3 MFI (1,593 ± 272) in the lymph nodes compared to those of PBMC (Tregs, 7.6% ± 3.0%; P < 0.0028; FoxP3 MFI, 1,095 ± 199; P < 0.0001) (see Fig. S3 in the supplemental material). These preliminary findings are somewhat limited, in that we do not have matching lymph node mononuclear cells (LNMC) from healthy controls; however, the striking difference observed in HIV patients is notable and illustrates the necessity for further studies to examine Tregs in different tissues. More detailed analysis of the kinetics, dynamics, phenotype, and tissue distribution of these cells over the course of untreated HIV infection and following therapy is clearly warranted (42).
TABLE 2.
Clinical characteristics of patient lymph node mononuclear cells
| Characteristic | Value |
|---|---|
| Gender | 10 M, 0 F |
| Age | 53 yr (33-78)a |
| Viral load (copies/ml) | <50 (<50-9,500)a |
| CD4 count (cells/μl) | 418 (140-1691)a |
| Treatment | HAARTb |
Values are medians (ranges).
Data of three patients were unknown.
Longitudinal assessment of Tregs in HIV and influence of HAART treatment.
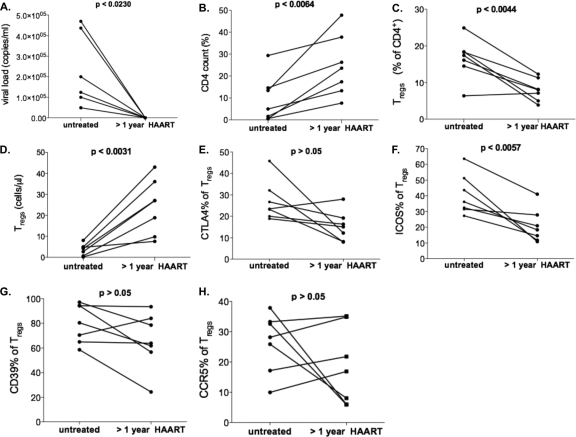
Finally, we examined PBMC samples from seven patients collected before as well as more than 1 year after the initiation of successful HAART treatment, with the full suppression of viremia, to determine the impact of antiretroviral therapy on the frequency and phenotype of Tregs (Fig. 5A to H). As expected, increasing numbers of conventional CD4+ T cells (P < 0.0064) corresponded with a decrease of the relative Treg frequency (baseline, 16.5% ± 5.5%; after >1 year of HAART, 7.9% ± 3.0%; P < 0.0044). Accordingly, we observed an increase in the absolute number of Treg following treatment (baseline, 3.6 ± 2.7 cells/μl; after >1 year of HAART, 24.2 cells/μl ± 13.0; P < 0.0031). The surface expression of CTLA-4, ICOS, CCR5, and CD39 on Tregs did not decrease after the initiation of HAART (data not shown), suggesting that these phenotypic changes are not fully reversible, or they slowly revert in the absence of viremia.
FIG. 5.
Longitudinal analysis of Tregs during HAART. Seven patients were analyzed during HAART (each on therapy for more than a year). (A) HIV VL load (<50 ± 0 copies/ml; P > 0.0230) was below the limit of detection (50 RNA copies/ml) in all patients during HAART. (B) CD4+ count (9.3% ± 8.6%) increased during HAART (24.8% ± 14.4%; P < 0.0064). (C and D) While relative Treg frequencies decreased (baseline, 16.5% ± 5.5%; after >1 year of HAART, 7.9% ± 3.0%; P < 0.0044), we detected an increase in absolute Treg numbers, defined as the proportion of Tregs of total CD3+/CD4+ T cells (baseline, 3.6 ± 2.7 cells/μl; after >1 year of HAART, 24.2 ± 13.0 cells/μl; P < 0.0031). (E to H) Surface expression of Treg markers CTLA-4, ICOS, CCR5, and CD39 showed a trend toward lower expression, but this trend does not reach statistical significance.
DISCUSSION
Generalized chronic immune activation and the progressive loss of the number and function of CD4+ T lymphocytes have been demonstrated as leading events in HIV-1 pathogenesis. There is now compelling evidence that regulatory T cells play an important role in maintaining a balance between the induction and suppression of immune activation (5, 25). However, the precise role of Tregs in HIV pathogenesis still is controversial. On one hand, Tregs may prevent chronic immune activation and therefore are beneficial for the preservation of CD4+ T cells. Conversely, Tregs may dampen the antiviral immune response and therefore play a detrimental role for the containment of HIV (27, 28). Previous data to address this topic were generated using a suboptimal definition of Treg markers and/or were collected from rather small and heterogeneous HIV patient cohorts. We sought to overcome these limitations by examining the frequency and phenotype of a precisely defined Treg population using a large and well-characterized cohort of HIV-infected patients, including elite controllers, longitudinal samples from HAART-treated patients, as well as lymph node samples for comparison to healthy controls. In this respect, we believe that our compilation of data is rather unique.
While we find that an increase of Treg frequency within the CD4+ T cell compartment is associated with HIV disease progression, we assume that this relative increase most likely is due to the loss of absolute CD4+ T-cell counts rather than a true increase of absolute Treg numbers. Our calculations indeed indicate that the absolute number of Tregs actually decreases during disease progression and is correlated with increased immune activation (see Fig. S1 in the supplemental material) and plasma viral load.
These results confirm recent data (9) from a study that examined patients from the MACS cohort but for whom HIV viral load data were not available. Importantly, we extended the observations of previous studies (6, 9) by examining a large group of nonprogressing patients and elite controllers. Notably, our results indicate that the absolute number of Tregs is normal or even elevated in these individuals.
Differences in peripheral Treg frequency or Treg numbers may be explained by a redistribution of these cells from other tissue compartments or by the differential proliferation or conversion of CD4+ T cells into Tregs. Our comparison of lymph node and peripheral blood samples indeed suggests that Tregs preferentially migrate to lymphoid tissues and therefore are underrepresented in PBMC (2).
In the current study, we not only compared the relative frequencies and absolute numbers of Tregs in different groups of patients but also extended our studies to a comprehensive analysis of additional phenotypical markers. Here, elite controllers not only exhibited levels of Tregs (relative frequency and absolute numbers) comparable to those of the healthy control group but also displayed a distinct phenotypic expression pattern. We observed the highest levels of CCR5, Foxp3 MFI, and CTLA4 expression in Tregs of elite controllers. Additional work will be necessary to fully examine the function and homing properties of these cells and to see how they may differ from Tregs present in healthy controls and HIV-infected patients with chronic progressive disease. It is intriguing to hypothesize that this phenotype contributes to the control of viral replication and whether Tregs are more (or less) susceptible to HIV infection than conventional CD4+ T cells (42, 44, 45, 47, 56, 60). It has been reported that HIV replication is slower in Tregs than in conventional CD4+ T cells on a per-cell basis, so it remains unclear whether they can function as productive target cells in vivo. Previous in vitro data demonstrated an increased susceptibility of Tregs to infection (10, 45), but these in vitro assays may alter the Treg phenotype and therefore do not fully represent the in vivo situation (56). In the present study, we examined the expression of CCR5 on Tregs ex vivo and detected higher CCR5 expression of Tregs from HIV-infected patients than from healthy controls. These data are consistent with recent reports by other groups and indicate that viral attachment and entry is possible in Tregs using this pathway (39). However, CCR5+ effector memory CD4+ subpopulations that are resistant to R5-tropic virus recently have been described (44). The low rate of viral replication seen in Tregs may be explained by the potential interaction of FoxP3, NF-κB, and the HIV-1 long terminal repeat (LTR) (56). NF-κB is essential for viral replication (21), and it has been shown that the FoxP3 interfers with NF-κB activation and suppressed HIV-1 LTR-specific transcription (22). Additionally, it has been shown that CTLA-4, which is upregulated in Tregs, reduces NF-κB activation (20), altogether conceivably leading not to a lower rate of infection but to reduced virus replication capacity. In turn, this would be beneficial for the survival and maintenance of the functionality of Tregs in HIV infection. However, all of these hypotheses (and others [39, 42]) have to be confirmed by future studies on the rate of the infection of Tregs in vivo at different stages of the disease (39).
A critical role of CD39 has been described for Tregs in general, but few studies have analyzed the expression of CD39 during HIV disease (32). In particular, to our knowledge this is the first study to demonstrate a significant correlation between the CD39 expression of Tregs and progressive HIV disease.
While the expression of CD39 could be seen as a mere surrogate marker for immune activation in these individuals (11, 47), recent studies suggest a functional role for CD39 in pathogenesis (3, 8, 30, 40). CD39 functions as a nucleoside triphosphate diphsphohydrolase 1 (NTPDase 1), which converts ATP (a proinflammatory stimulus) into AMP, and also boosts the production of adenosine (8), thereby dampening the general immune activation (4, 16). Adenosine suppresses CD4+ and CD8+ T cells directly through binding to the adenosine 2a receptor, and CD39/CD73 expression of Tregs has been implicated in T cell dysfunction via this mechanism (12). Originally CD39 expression had been linked to the activation of lymphoid cells (36). Recently, Borsellino et al. reported that CD39 is constitutively expressed by FoxP3+ regulatory T cells (8). The same group also described an association between the activation status of murine Tregs and CD39 expression. We confirm those findings in our human study and show that the high expression of CD39 is restricted mainly to FoxP3+ Tregs (Fig. 4A and B) and increases with disease progression and immune activation (Fig. 4G).
Interestingly, even long-term nonprogressors with low viral loads show significantly higher levels of CD39 on Tregs than those of elite controllers. This may underline the sensitivity of CD39 in immune regulation and its importance as a functional molecule on Tregs, which may be useful to predict disease progression. According to functional experiments performed by us (see Fig. S2 in the supplemental material) and others (38), the capability of Tregs to suppress proliferation seems to be confined exclusively to the CD4+, CD25high, CD39+ cell compartment. At the moment, it remains speculative whether CD39 acts as a switch for the suppressive function of Tregs. According to this model, Tregs are consecutively switched on in patients with even minimal HIV viremia, while the majority of CD39− Tregs detected in healthy people and elite controllers are in a nonfunctional status. Paradoxically, in a recent publication the subset CD4+, CD25−, CD39+ T cells, a subset that also is increased with rising HIV viral loads in our cohort, was functionally described to have an inducer phenotype, promoting T-cell proliferation, and it is intriguing to speculate whether an increased ratio of T inducer cells/Tregs would be of any importance for HIV pathogenesis. However, further functional studies have to confirm those recent results, and in first experiments performed by us, these CD4+, CD25low, CD39+ cells did not produce gamma interferon after stimulation (see Fig. S2B). Taken together, our data and work by other groups (8, 37, 38, 40) warrant further functional investigations on the role of CD39 in HIV infection, possibly opening new avenues for future immunomodulatory interventions by blocking the CD39 molecule in vivo by specific inhibitors (32, 57).
In summary, our results explain the discrepancies of previously published data on the role of Tregs in HIV infection. We find an increase of the relative Treg frequency within the CD4+ compartment but a decrease of the absolute Treg number in PBMC associated with HIV disease progression, indicating a loss of Tregs at a lower rate than that of conventional CD4+ T cells. The loss of the absolute Treg number is partially reversible by HAART. We found a strong association between the quantity and quality of Tregs and different courses of HIV disease.
Notably, elite controllers, but not long-term nonprogressors, exhibited high frequencies of Tregs with a distinct phenotype. Future studies will be needed to establish whether this phenotypic pattern in elite controllers is associated with specific immune functions, which may be directly involved in the unique capability to control virus replication observed in these patients (10, 11). Although we are waiting for a further confirmation of a causative relation, our data indirectly indicate a beneficial and complex role of Tregs for the orchestration of the immune response in HIV infection. This immunomodulatory capacity exceeds the current view, which implies that the role of Tregs is merely to dampen the adaptive antiviral response. Further functional (40, 47) and longitudinal analyses of Tregs, and especially the role of CD39 expression, the thorough assessment of the distribution of these cells in different tissues (35, 42, 51) (e.g., lymph node, gut, and/or liver) as well as an analysis of CD8+ Tregs (41) are necessary to fully comprehend the role of Tregs in HIV infection. Due to the complex role of Tregs in vivo, more detailed studies have to be completed before clinical immunotherapeutic interventions involving Tregs can be attempted in HIV or other viral infections (48).
Supplementary Material
Acknowledgments
We thank the patients who participated in this study and Silke Kummer and Verena Matzat for technical help. We thank Mark Brockman for helpful discussions.
This work was supported by the German Federal Ministry of Research and Education (J.v.L., J.H., C.L.; BMBF grant 01KI0771), the Forschungsförderungsfond Medizin, UKE (J.S.Z.W.), and the German Research Agency (J.S.Z.W.; SCHU 2482/1-1, SFB841 project A6).
Footnotes
Published ahead of print on 3 November 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Alimonti, J. B., T. B. Ball, and K. R. Fowke. 2003. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J. Gen. Virol. 84:1649-1661. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, J., A. Boasso, J. Nilsson, R. Zhang, N. J. Shire, S. Lindback, G. M. Shearer, and C. A. Chougnet. 2005. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J. Immunol. 174:3143-3147. [DOI] [PubMed] [Google Scholar]
- 3.Barat, C., G. Martin, A. R. Beaudoin, J. Sevigny, and M. J. Tremblay. 2007. The nucleoside triphosphate diphosphohydrolase-1/CD39 is incorporated into human immunodeficiency type 1 particles, where it remains biologically active. J. Mol. Biol. 371:269-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beigi, R. D., S. B. Kertesy, G. Aquilina, and G. R. Dubyak. 2003. Oxidized ATP (oATP) attenuates proinflammatory signaling via P2 receptor-independent mechanisms. Br. J. Pharmacol. 140:507-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belkaid, Y., and B. T. Rouse. 2005. Natural regulatory T cells in infectious disease. Nat. Immunol. 6:353-360. [DOI] [PubMed] [Google Scholar]
- 6.Bi, X., Y. Suzuki, H. Gatanaga, and S. Oka. 2009. High frequency and proliferation of CD4+ FOXP3+ Treg in HIV-1-infected patients with low CD4 counts. Eur. J. Immunol. 39:301-309. [DOI] [PubMed] [Google Scholar]
- 7.Bland, J. M., and D. G. Altman. 1995. Multiple significance tests: the Bonferroni method. BMJ 310:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borsellino, G., M. Kleinewietfeld, D. Di Mitri, A. Sternjak, A. Diamantini, R. Giometto, S. Hopner, D. Centonze, G. Bernardi, M. L. Dell'Acqua, P. M. Rossini, L. Battistini, O. Rotzschke, and K. Falk. 2007. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110:1225-1232. [DOI] [PubMed] [Google Scholar]
- 9.Cao, W., B. D. Jamieson, L. E. Hultin, P. M. Hultin, and R. Detels. 2009. Regulatory T cell expansion and immune activation during untreated HIV type 1 infection are associated with disease progression. AIDS Res. Hum. Retrovir. 25:183-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Card, C. M., P. J. McLaren, C. Wachihi, J. Kimani, F. A. Plummer, and K. R. Fowke. 2009. Decreased immune activation in resistance to HIV-1 infection is associated with an elevated frequency of CD4(+)CD25(+)FOXP3(+) regulatory T cells. J. Infect. Dis. 199:1318-1322. [DOI] [PubMed] [Google Scholar]
- 11.Chase, A. J., H. C. Yang, H. Zhang, J. N. Blankson, and R. F. Siliciano. 2008. Preservation of FoxP3+ regulatory T cells in the peripheral blood of human immunodeficiency virus type 1-infected elite suppressors correlates with low CD4+ T-cell activation. J. Virol. 82:8307-8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deaglio, S., K. M. Dwyer, W. Gao, D. Friedman, A. Usheva, A. Erat, J. F. Chen, K. Enjyoji, J. Linden, M. Oukka, V. K. Kuchroo, T. B. Strom, and S. C. Robson. 2007. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204:1257-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deeks, S. G., and B. D. Walker. 2007. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 27:406-416. [DOI] [PubMed] [Google Scholar]
- 14.Del Pozo-Balado Mdel, M., M. Leal, G. Mendez-Lagares, and Y. M. Pacheco. 2010. CD4(+)CD25(+/hi)CD127(lo) phenotype does not accurately identify regulatory T cells in all populations of HIV-infected persons. J. Infect. Dis. 201:331-335. [DOI] [PubMed] [Google Scholar]
- 15.Douek, D. C. 2003. Disrupting T-cell homeostasis: how HIV-1 infection causes disease. AIDS Rev. 5:172-177. [PubMed] [Google Scholar]
- 16.Elliott, M. R., F. B. Chekeni, P. C. Trampont, E. R. Lazarowski, A. Kadl, S. F. Walk, D. Park, R. I. Woodson, M. Ostankovich, P. Sharma, J. J. Lysiak, T. K. Harden, N. Leitinger, and K. S. Ravichandran. 2009. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461:282-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epple, H. J., C. Loddenkemper, D. Kunkel, H. Troger, J. Maul, V. Moos, E. Berg, R. Ullrich, J. D. Schulzke, H. Stein, R. Duchmann, M. Zeitz, and T. Schneider. 2006. Mucosal but not peripheral FOXP3+ regulatory T cells are highly increased in untreated HIV infection and normalize after suppressive HAART. Blood 108:3072-3078. [DOI] [PubMed] [Google Scholar]
- 18.Fazekasde St. Groth, B., and A. L. Landay. 2008. Regulatory T cells in HIV infection: pathogenic or protective participants in the immune response? AIDS 22:671-683. [DOI] [PubMed] [Google Scholar]
- 19.Fontenot, J. D., M. A. Gavin, and A. Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330-336. [DOI] [PubMed] [Google Scholar]
- 20.Fraser, J. H., M. Rincon, K. D. McCoy, and G. Le Gros. 1999. CTLA4 ligation attenuates AP-1, NFAT and NF-kappaB activity in activated T cells. Eur. J. Immunol. 29:838-844. [DOI] [PubMed] [Google Scholar]
- 21.Ganesh, L., E. Burstein, A. Guha-Niyogi, M. K. Louder, J. R. Mascola, L. W. Klomp, C. Wijmenga, C. S. Duckett, and G. J. Nabel. 2003. The gene product Murr1 restricts HIV-1 replication in resting CD4+ lymphocytes. Nature 426:853-857. [DOI] [PubMed] [Google Scholar]
- 22.Grant, C., U. Oh, K. Fugo, N. Takenouchi, C. Griffith, K. Yao, T. E. Newhook, L. Ratner, and S. Jacobson. 2006. Foxp3 represses retroviral transcription by targeting both NF-kappaB and CREB pathways. PLoS Pathog. 2:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hazenberg, M. D., S. A. Otto, D. Hamann, M. T. Roos, H. Schuitemaker, R. J. de Boer, and F. Miedema. 2003. Depletion of naive CD4 T cells by CXCR4-using HIV-1 variants occurs mainly through increased T-cell death and activation. AIDS 17:1419-1424. [DOI] [PubMed] [Google Scholar]
- 24.Reference deleted.
- 25.Kassiotis, G., and A. O'Garra. 2008. Immunology. Immunity benefits from a little suppression. Science 320:1168-1169. [DOI] [PubMed] [Google Scholar]
- 26.Kaufmann, D. E., and B. D. Walker. 2009. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J. Immunol. 182:5891-5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinter, A., J. McNally, L. Riggin, R. Jackson, G. Roby, and A. S. Fauci. 2007. Suppression of HIV-specific T cell activity by lymph node CD25+ regulatory T cells from HIV-infected individuals. Proc. Natl. Acad. Sci. U. S. A. 104:3390-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinter, A. L., R. Horak, M. Sion, L. Riggin, J. McNally, Y. Lin, R. Jackson, A. O'Shea, G. Roby, C. Kovacs, M. Connors, S. A. Migueles, and A. S. Fauci. 2007. CD25+ regulatory T cells isolated from HIV-infected individuals suppress the cytolytic and nonlytic antiviral activity of HIV-specific CD8+ T cells in vitro. AIDS Res. Hum. Retrovir. 23:438-450. [DOI] [PubMed] [Google Scholar]
- 29.Kohyama, M., D. Sugahara, S. Sugiyama, H. Yagita, K. Okumura, and N. Hozumi. 2004. Inducible costimulator-dependent IL-10 production by regulatory T cells specific for self-antigen. Proc. Natl. Acad. Sci. U. S. A. 101:4192-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar, V., and A. Sharma. 2009. Adenosine: an endogenous modulator of innate immune system with therapeutic potential. Eur. J. Pharmacol. 616:7-15. [DOI] [PubMed] [Google Scholar]
- 31.Lawn, S. D., S. T. Butera, and T. M. Folks. 2001. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin. Microbiol. Rev. 14:753-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leal, D. B., C. A. Streher, M. Bertoncheli Cde, L. F. Carli, C. A. Leal, J. E. da Silva, V. M. Morsch, and M. R. Schetinger. 2005. HIV infection is associated with increased NTPDase activity that correlates with CD39-positive lymphocytes. Biochim. Biophys. Acta 1746:129-134. [DOI] [PubMed] [Google Scholar]
- 33.Letvin, N. L., and B. D. Walker. 2003. Immunopathogenesis and immunotherapy in AIDS virus infections. Nat. Med. 9:861-866. [DOI] [PubMed] [Google Scholar]
- 34.Lim, A., M. A. French, and P. Price. 2009. CD4+ and CD8+ T cells expressing FoxP3 in HIV-infected patients are phenotypically distinct and influenced by disease severity and antiretroviral therapy. J. Acquir. Immune. Defic. Syndr. 51:248-257. [DOI] [PubMed] [Google Scholar]
- 35.Lund, J. M., L. Hsing, T. T. Pham, and A. Y. Rudensky. 2008. Coordination of early protective immunity to viral infection by regulatory T cells. Science 320:1220-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maliszewski, C. R., G. J. Delespesse, M. A. Schoenborn, R. J. Armitage, W. C. Fanslow, T. Nakajima, E. Baker, G. R. Sutherland, K. Poindexter, C. Birks, et al. 1994. The CD39 lymphoid cell activation antigen. Molecular cloning and structural characterization. J. Immunol. 153:3574-3583. [PubMed] [Google Scholar]
- 37.Mandapathil, M., B. Hilldorfer, M. J. Szczepanski, M. Czystowska, M. Szajnik, J. Ren, S. Lang, E. K. Jackson, E. Gorelik, and T. L. Whiteside. 2010. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J. Biol. Chem. 285:7176-7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moncrieffe, H., K. Nistala, Y. Kamhieh, J. Evans, A. Eddaoudi, S. Eaton, and L. R. Wedderburn. 2010. High expression of the ectonucleotidase CD39 on T cells from the inflamed site identifies two distinct populations, one regulatory and one memory T cell population. J. Immunol. 185:134-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreno-Fernandez, M. E., W. Zapata, J. T. Blackard, G. Franchini, and C. A. Chougnet. 2009. Human regulatory T cells are targets for human immunodeficiency virus (HIV) infection, and their susceptibility differs depending on the HIV type 1 strain. J. Virol. 83:12925-12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ndhlovu, L. C., F. E. Leal, I. G. Eccles-James, A. R. Jha, M. Lanteri, P. J. Norris, J. D. Barbour, D. J. Wachter, J. Andersson, K. Tasken, E. A. Torheim, E. M. Aandahl, E. G. Kallas, and D. F. Nixon. 2010. A novel human CD4+ T-cell inducer subset with potent immunostimulatory properties. Eur. J. Immunol. 40:134-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nigam, P., V. Velu, S. Kannanganat, L. Chennareddi, S. Kwa, M. Siddiqui, and R. R. Amara. 2010. Expansion of FOXP3+ CD8 T cells with suppressive potential in colorectal mucosa following a pathogenic simian immunodeficiency virus infection correlates with diminished antiviral T cell response and viral control. J. Immunol. 184:1690-1701. [DOI] [PubMed] [Google Scholar]
- 42.Nilsson, J., A. Boasso, P. A. Velilla, R. Zhang, M. Vaccari, G. Franchini, G. M. Shearer, J. Andersson, and C. Chougnet. 2006. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood 108:3808-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nixon, D. F., E. M. Aandahl, and J. Michaelsson. 2005. CD4+CD25+ regulatory T cells in HIV infection. Microbes Infect. 7:1063-1065. [DOI] [PubMed] [Google Scholar]
- 44.Oswald-Richter, K., S. M. Grill, M. Leelawong, M. Tseng, S. A. Kalams, T. Hulgan, D. W. Haas, and D. Unutmaz. 2007. Identification of a CCR5-expressing T cell subset that is resistant to R5-tropic HIV infection. PLoS Pathog. 3:e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oswald-Richter, K., S. M. Grill, N. Shariat, M. Leelawong, M. S. Sundrud, D. W. Haas, and D. Unutmaz. 2004. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2:E198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pereyra, F., M. M. Addo, D. E. Kaufmann, Y. Liu, T. Miura, A. Rathod, B. Baker, A. Trocha, R. Rosenberg, E. Mackey, P. Ueda, Z. Lu, D. Cohen, T. Wrin, C. J. Petropoulos, E. S. Rosenberg, and B. D. Walker. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 197:563-571. [DOI] [PubMed] [Google Scholar]
- 47.Prendergast, A., J. G. Prado, Y. H. Kang, F. Chen, L. A. Riddell, G. Luzzi, P. Goulder, and P. Klenerman. 2010. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. AIDS 24:491-502. [DOI] [PubMed] [Google Scholar]
- 48.Riley, J. L., C. H. June, and B. R. Blazar. 2009. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity 30:656-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roncarolo, M. G., and S. Gregori. 2008. Is FOXP3 a bona fide marker for human regulatory T cells? Eur. J. Immunol. 38:925-927. [DOI] [PubMed] [Google Scholar]
- 50.Rouse, B. T., P. P. Sarangi, and S. Suvas. 2006. Regulatory T cells in virus infections. Immunol. Rev. 212:272-286. [DOI] [PubMed] [Google Scholar]
- 51.Ruckwardt, T. J., K. L. Bonaparte, M. C. Nason, and B. S. Graham. 2009. Regulatory T cells promote early influx of CD8+ T cells in the lungs of respiratory syncytial virus-infected mice and diminish immunodominance disparities. J. Virol. 83:3019-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151-1164. [PubMed] [Google Scholar]
- 53.Schulze zur Wiesch, J., G. M. Lauer, C. L. Day, A. Y. Kim, K. Ouchi, J. E. Duncan, A. G. Wurcel, J. Timm, A. M. Jones, B. Mothe, T. M. Allen, B. McGovern, L. Lewis-Ximenez, J. Sidney, A. Sette, R. T. Chung, and B. D. Walker. 2005. Broad repertoire of the CD4+ Th cell response in spontaneously controlled hepatitis C virus infection includes dominant and highly promiscuous epitopes. J. Immunol. 175:3603-3613. [DOI] [PubMed] [Google Scholar]
- 54.Seddiki, N., and A. D. Kelleher. 2008. Regulatory T cells in HIV infection: who's suppressing what? Curr. Infect. Dis. Rep. 10:252-258. [DOI] [PubMed] [Google Scholar]
- 55.Seddiki, N., B. Santner-Nanan, J. Martinson, J. Zaunders, S. Sasson, A. Landay, M. Solomon, W. Selby, S. I. Alexander, R. Nanan, A. Kelleher, and B. Fazekas de St. Groth. 2006. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 203:1693-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Selliah, N., M. Zhang, S. White, P. Zoltick, B. E. Sawaya, T. H. Finkel, and R. Q. Cron. 2008. FOXP3 inhibits HIV-1 infection of CD4 T-cells via inhibition of LTR transcriptional activity. Virology 381:161-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sitkovsky, M., D. Lukashev, S. Deaglio, K. Dwyer, S. C. Robson, and A. Ohta. 2008. Adenosine A2A receptor antagonists: blockade of adenosinergic effects and T regulatory cells. Br. J. Pharmacol. 153(Suppl. 1):S457-S464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tenorio, A. R., J. Martinson, D. Pollard, L. Baum, and A. Landay. 2008. The relationship of T-regulatory cell subsets to disease stage, immune activation, and pathogen-specific immunity in HIV infection. J. Acquir. Immune Defic. Syndr. 48:577-580. [DOI] [PubMed] [Google Scholar]
- 59.Terzieva, V. 2008. Regulatory T cells and HIV-1 infection. Viral Immunol. 21:285-291. [DOI] [PubMed] [Google Scholar]
- 60.Tran, T. A., M. G. de Goer de Herve, H. Hendel-Chavez, B. Dembele, E. Le Nevot, K. Abbed, C. Pallier, C. Goujard, J. Gasnault, J. F. Delfraissy, A. M. Balazuc, and Y. Taoufik. 2008. Resting regulatory CD4 T cells: a site of HIV persistence in patients on long-term effective antiretroviral therapy. PLoS One 3:e3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsunemi, S., T. Iwasaki, T. Imado, S. Higasa, E. Kakishita, T. Shirasaka, and H. Sano. 2005. Relationship of CD4+CD25+ regulatory T cells to immune status in HIV-infected patients. AIDS 19:879-886. [DOI] [PubMed] [Google Scholar]
- 62.Weiss, L., V. Donkova-Petrini, L. Caccavelli, M. Balbo, C. Carbonneil, and Y. Levy. 2004. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood 104:3249-3256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.