Abstract
HIV-1 gp41 envelope antibodies, which are frequently induced in HIV-1-infected individuals, are predominantly nonneutralizing. The rare and difficult-to-induce neutralizing antibodies (2F5 and 4E10) that target gp41 membrane-proximal epitopes (MPER) are polyspecific and require lipid binding for HIV-1 neutralization. These results raise the questions of how prevalent polyreactivity is among gp41 antibodies and how the binding properties of gp41-nonneutralizing antibodies differ from those of antibodies that are broadly neutralizing. In this study, we have characterized a panel of human gp41 antibodies with binding specificities within the immunodominant cluster I (gp41 amino acids [aa] 579 to 613) or cluster II (gp41 aa 644 to 667) for reactivity to autoantigens, to the gp140 protein, and with MPER peptide-lipid conjugates. We report that while none of the gp41 cluster I antibodies studied were polyspecific, all three gp41 cluster II antibodies bound either to lipids or autoantigens, thus showing the propensity of cluster II antibodies to manifest polyreactivity. All cluster II gp41 monoclonal antibodies (MAbs), including those that were lipid reactive, failed to bind to gp41 MPER peptide-lipid complexes. Cluster II antibodies bound strongly with nanomolar binding affinity (dissociation constant [Kd]) to oligomeric gp140 proteins, and thus, they recognize conformational epitopes on gp41 that are distinct from those of neutralizing gp41 antibodies. These results demonstrate that lipid-reactive gp41 cluster II antibodies are nonneutralizing due to their inability to bind to the relevant neutralizing epitopes on gp41.
Anti-HIV-1 gp41 envelope (Env) antibodies (Abs) are frequently induced in HIV-1-infected individuals (6, 8, 58). The antigenic determinants of gp41 have been mapped with a large panel of human gp41 antibodies (21, 32, 55, 61). Whereas cluster I monoclonal Abs (MAbs) are directed against the immunodominant region (gp41 amino acids [aa] 579 to 613) of the gp41 envelope, a subset of human HIV-1 MAbs specific for “cluster II” (55) show binding specificities for the gp41 region, which includes HR-2 (heptad repeat 2) (aa 644 to 667) (21, 35, 55). The broadly neutralizing MAbs 2F5 and 4E10 recognize epitopes that are within the gp41 membrane-proximal external region (MPER), with the 2F5 epitope being adjacent to the cluster II region and that of 4E10 mapping outside the cluster II and being more membrane proximal (cluster III). There is some overlap between cluster II epitopes and the epitope recognized by MPER MAb 2F5 (56). Thus, cluster II human MAbs (98-6, 126-6, and 167-D) can partially cross-block 2F5 MAb binding to HIV-1 Env gp140 oligomers (3).
Anti-gp41 antibodies whose epitopes are within the gp41 HR-2 region are largely nonneutralizing, with only rare neutralizing MAbs (9, 36, 45, 61). One each of cluster I (clone 3) and cluster II (98-6) MAbs have been reported to weakly neutralize select HIV-1 strains (19, 25). Both cluster I and cluster II MAbs, however, can bind HIV-1-infected cells and HIV-1 virions and also have been reported to mediate antibody-dependent cellular cytotoxicity (ADCC) (41, 51, 59). However, the determinants on the HIV-1 viral spikes that are recognized by cluster II antibodies are not well defined.
Among the broadly neutralizing MAbs, 2F5 and 4E10 show reactivity toward anionic phospholipids and other autoantigens (5, 22, 38, 39); in addition, MPER MAb Z13 has been shown to react weakly with cardiolipin (33). Although a third broadly neutralizing MAb, 2G12, did not show any reactivity for the tested autoantigens (24), the epitope recognized by this gp120 MAb, 2G12, is composed primarily of a cluster of high-mannose oligosaccharides that are similar to “self” carbohydrates (4, 42, 50). Thus, the rarity of broadly neutralizing MAbs after either natural infection or immunization (7, 30) raises the question of whether expression of these specificities for gp41 or carbohydrates may be immunoregulated and whether cross-reactivity with self antigens, such as phospholipids, is essential for anti-gp41 MAbs to neutralize HIV-1 (1, 2). Recent studies have shown that for 2F5 and 4E10 to neutralize HIV, the hydrophobic heavy chain complementarity determining region 3 (CDR H3) must be intact, since mutations of hydrophobic amino acids disrupt both lipid binding and neutralization capacity (2). In contrast, a mouse MPER MAb that also partially cross blocks 2F5 binding to gp41 MPER peptide (13H11) neither binds to phospholipids nor binds to peptide-lipid complexes (1). It is therefore not clear why certain antibodies that bind to HIV-1 gp41 and recognize epitopes close to the 2F5 nominal epitope show no neutralizing capability. In the case of the murine MAb 13H11, the lack of lipid reactivity could explain its inability to interact with a critical residue (L669) immersed in membrane lipids and thus explain its failure to neutralize (1, 43). Lipid reactivities of 4E10 and 2F5 allow them the capability to extract membrane-immersed critical residues (44, 46) and also to position close to a transiently expressed gp41 neutralizing determinant (2). Thus, a fundamental question is whether all gp41 MAbs show reactivity to lipids or other autoantigens and whether such polyreactivity is associated with neutralization of HIV-1 (1, 22, 24).
In the present study, we have probed the relationship of the binding of gp41 cluster II MAbs to phospholipid and other autoantigens with HIV-1 neutralization; for this, we have compared a panel of neutralizing and nonneutralizing gp41 MAbs for binding to Env gp140s, phospholipids, and gp41 peptide-lipid conjugates. Our data show that polyreactivity is not unique to human neutralizing antibodies, since all three nonneutralizing cluster II MAbs were polyreactive and two of them also bound to lipids. However, unlike 2F5, cluster II MAbs bind strongly to oligomeric forms of Env gp140 but not to gp41 peptide complexes. Thus, polyreactivity is necessary but not sufficient for neutralization.
MATERIALS AND METHODS
Antibodies.
Anti-gp41 cluster I MAbs (240D, 246D, 50-69D, and 181) and cluster II MAbs (98-6, 167-D, and 126-6) were derived from HIV-infected individuals and have been described previously (21, 55). Human MAbs 71-31D (anti-p24) (18), 847D (anti-C2) (35), and 1570 (anti-CD4-binding site) (26) were used as controls. The murine anti-MPER MAb 13H11 was made following immunization with the group M consensus Env ConS gp140 oligomer as described earlier (1). HIV-1 Env gp41 MPER MAbs 2F5 and 4E10 were purchased from Polymun Scientific (Vienna, Austria) (60).
Recombinant proteins.
The recombinant oligomeric gp140 proteins Con S gp140, JRFL gp140, and Con B gp140 were produced and purified as described earlier (28).
Phospholipids.
Chloroform stocks of the phospholipids 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), 1,2-dimyristoyl-sn-glycero-3-phosphate (DMPA), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoserine (POPS), bovine heart cardiolipin, and cholesterol were purchased from Avanti Polar Lipids.
SPR assays.
Surface plasmon resonance (SPR) binding assays were performed on a BIAcore 3000 instrument (BIAcore Inc., Piscataway, NJ) maintained at 20°C, and analyses were done using the BIAEval 4.1 software program (BIAcore Inc., Pisacattaway, NJ). Lipid binding assays were done using a BIAcore L1 chip that had 2,500 to 3,000 resonance units (RU) of bovine serum albumin (BSA) immobilized on each flow cell. POPC-POPS (25:75, molar ratio) and POPC-cardiolipin (25:75, molar ratio) liposomes were injected over the chip at a 5-μl/min flow rate until 500 RU of capture was achieved. Antibodies were injected over the liposomes for 2 min at a 20-μl/min flow rate. Surfaces were regenerated by injecting 100 μl of 40 mM n-octyl-glucopyranoside at a 100-μl/min flow rate followed by a 10-μl injection of 25 mM NaOH at a 50-μl/min flow rate. The responses due to nonspecific binding of MAbs to the blank flow cell were subtracted to obtain the specific binding shown in the figures. The binding of MAbs to MPER peptide liposomes was done as reported earlier (1, 11). The interactions of MAbs with MPER peptides were done using biotinylated versions of the HIV-1 gp41 MPER peptide SP62 (QQEKNEQELLELDKWASLWN) and a control peptide with a scrambled sequence, SP62 Scrambled (NKEQDQAEESLQLWEKLNWL). These peptides were individually anchored on a BIAcore SA sensor chip as described previously (1). Each peptide was injected until 100 to 150 RU of binding to streptavidin was observed. Specific binding responses of MAb binding were obtained following subtraction of nonspecific binding on the scrambled 2F5 peptide surface. Rate constants were measured using the bivalent analyte model (to account for the avidity of bivalent Ig molecules) and global curve fitting to binding curves obtained from MAb titrations, which ranged from 0.01 to 119 nM and 6.0 to 1,400 nM for MAbs 2F5 and 4E10, respectively. MAbs were injected at 30 μl/min for 2 to 6 min, and glycine-HCl (pH 2.0) and the surfactant P20 (0.01%) were used as the regeneration buffer. The interactions of MAbs with gp140 oligomers were studied by immobilizing gp140 oligomers on a BIAcore CM5 chip and flowing MAbs over the immobilized gp140 oligomers as described previously (3).
Autoantigen assay.
The Luminex AtheNA Multi-Lyte ANA test (Wampole Laboratories, Princeton, NJ) was used to test for MAb reactivity to SSA/Ro, SS-B/La, Sm, ribonucleoprotein (RNP), Jo-1, double-stranded DNA (dsDNA), centromere B, and histone and was performed per the manufacturer's specifications and as previously described (22). Cardiolipin reactivity was measured in an enzyme-linked immunosorbent assay (ELISA) as previously described (22).
RESULTS
Reactivities of HIV-1 gp41 MAbs with phospholipids.
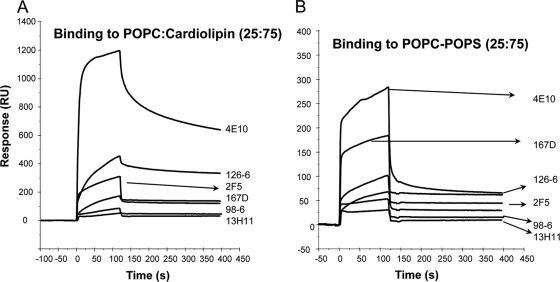
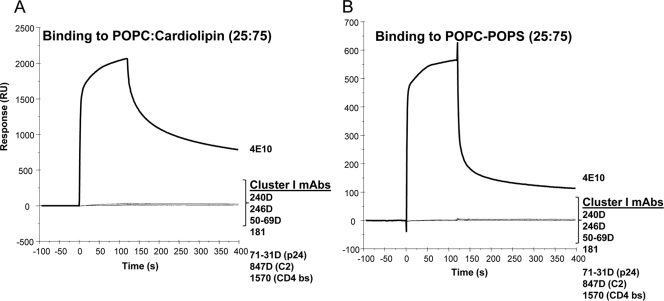
Since two of the previously studied broadly neutralizing MAbs (2F5 and 4E10) that bind to the MPER region of gp41 were polyspecific (1, 22, 38, 39), we first asked how prevalent polyreactivity is among MAbs that bind to both cluster I and cluster II regions of gp41 Env. In SPR binding assays, two of the three human cluster II MAbs (126-6 and 167D) bound to the cardiolipin- and phosphatidylserine (PS)-containing liposomes (Fig. 1A and B) and showed no binding to phosphatidylcholine liposomes (not shown). The 126-6 MAb bound more strongly to cardiolipin-containing liposomes than did the 2F5 and 167-D MAbs, while MAb 98-6 did not bind any phospholipids. As previously reported, the nonneutralizing anti-gp41 MPER MAb 13H11 did not bind to cardiolipin or to PS-containing liposomes (1) (Fig. 1). Compared to MAb 4E10, all cluster II gp41 MAbs showed lower reactivity with phospholipids. In contrast, no binding of any of the cluster I MAbs (240D, 246D, 50-69D, and 181) to cardiolipin- or PS-containing liposomes was observed (Fig. 2). Thus, in this panel of gp41 MAbs, all of gp41 human cluster II bound to phospholipids, with 98-6 being the only exception.
FIG. 1.
Binding of gp41 cluster II MAbs to anionic phospholipids. SPR sensograms are shown for the binding of 100 μg/ml of 126-6, 167D, 98-6, 4E10, 2F5, and 13H11 MAbs to POPC-cardiolipin (25:75) (A) or POPC-POPS (25:75) (B) liposomes captured on a Biacore L1 chip.
FIG. 2.
gp41 cluster I MAbs do not bind to anionic lipids. SPR sensograms are shown for the binding of 100 μg/ml of 240D, 246D, 50-69D, 181, 71-31D, 847D, 1570, and 4E10 MAbs to POPC-cardiolipin (25:75) (A) or POPC-POPS (25:75) (B) liposomes captured on a Biacore L1 chip.
Reactivity of human anti-gp41 monoclonal antibodies with protein autoantigens.
In Table 1, we show that while MAb 98-6 had no reactivity with phospholipids, it was positive for reactivity with several other autoantigens, which include Ro, RNP, Jo1, dsDNA, centromere B, and histones. The other two cluster II MAbs, 126-6 and 167-D, did not react with any of the protein autoantigens tested. However, as shown, they were reactive in ELISA with cardiolipin, and as described above, they were reactive by SPR with phospholipids. Overall, all of the three human anti-cluster II MAbs studied were polyreactive, showing binding to either lipids or protein autoantigens.
TABLE 1.
Reactivities of anti-HIV-1 human MAbs with autoantigens
| MAb | Reactivity with autoantigena |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CL | Ro (SSA) | SSB | Sm | RNP | Scl 70 | Jo1 | dsDNA | CentrB | Histones | |
| 4E10 | ++ | 365 | − | − | − | − | − | − | − | − |
| 98-6 | − | 725 | − | − | 233 | − | 217 | 265 | 198 | 375 |
| 126-6 | + | − | − | − | − | − | − | − | − | − |
| 167-D | + | − | − | − | − | − | − | − | − | − |
| 17B | − | − | − | − | − | − | − | − | − | − |
| Positive control serum | 1,095 | 703 | 822 | 788 | 502 | 921 | 729 | 577 | 824 | |
Autoantigen reactivity was measured in a Luminex AtheNA Multi-Lyte ANA assay (Wampole Laboratories, Princeton, NJ). −, <120 relative units. MAbs were used at 300 μg/ml. The relative units shown in the table are for each MAb at 300 μg/ml. The cardiolipin binding ELISA was done using MAbs at concentrations ranging from 50 to 0.02 μg MAb/ml. CL, cardiolipin; Ro (SSA), Sjogren's syndrome antigen A; SSB, Sjogren syndrome antigen B; Sm, Smith antigen; RNP, ribonucleoprotein; Scl 70, scleroderma 70; Jo1, antigen; CentrB, centromere B.
Binding of anti-gp41 MAbs to Env gp140 oligomers.
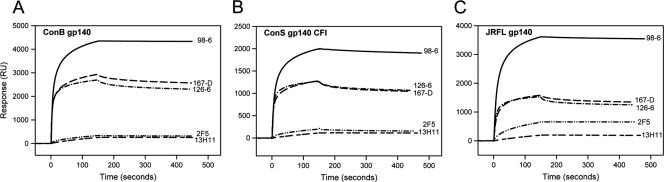
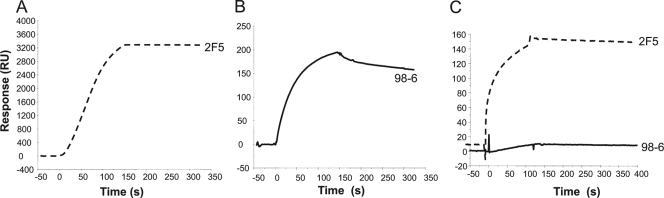
It has been suggested that broadly neutralizing MPER gp41 MAbs target epitopes that are transiently exposed on gp41 during HIV-1 fusion (15, 43) and thus are less likely to bind strongly to conformations that are presented on prefusion or postfusion states of gp41. This is consistent with previous observations that gp41 MPER MAb 2F5 bound only to select Env gp140 oligomers (JRFL gp140) and showed weak or no binding to most recombinant Env gp140 oligomers (1, 15). In contrast, all three human cluster II MAbs bound to Env gp140 oligomers with comparable affinities, with dissociation constant (Kd) values in the nanomolar range (Fig. 3 and Table 2). While MAb 98-6 bound to Env gp140 with relatively faster on-rates, the binding of both 126-6 and 167-D showed relatively slower association and dissociation rates (Table 2). Like MAb 2F5, the murine gp41 MPER MAb 13H11 bound weakly to all three gp140 oligomers, with much faster dissociation kinetics (Fig. 3) (1).
FIG. 3.
Nonneutralizing human gp41 cluster II MAbs bind strongly to oligomeric gp140 Env proteins. SPR sensograms are displayed for the binding of 98-6, 167D, 126-6, 2F5, and 13H11 MAbs to the ConB gp140 (A), ConS gp140 CFI (B), and JRFL gp140 (C) Env proteins immobilized on a Biacore CM5 chip.
TABLE 2.
Binding kinetics of cluster II MAbs for Env gp140 oligomersa
| Oligomer or MAb | ka (×103 M−1 s−1) | kd (×10−3 s−1) | KD (nM) |
|---|---|---|---|
| JRFL gp140 | |||
| 98-6 | 111.0 | 1.6 | 14.8 |
| 126-6 | 10.8 | 0.3 | 30.7 |
| 167-D | 8.3 | 0.3 | 35.1 |
| ConS gp140 | |||
| 98-6 | 132.0 | 3.1 | 23.3 |
| 126-6 | 7.3 | 0.3 | 30.5 |
| 167-D | 8.4 | 0.2 | 26.3 |
13H11 MAb bound too weakly to Env gp140 for Kd measurements. Binding of 2F5 to ConS gp140 was also too weak, and the Kd of 2F5 binding to JRFL gp140 is 164 nM (1). ka, association rate constant; kD, dissociation rate constant.
The above results are in contrast to those observed with linear gp41 HR-2 peptides, which include the 2F5 nominal epitope. While the 2F5 MAb bound with higher avidity to the 2F5 peptide (Kd = 10 nM), the binding of the nonneutralizing gp41 MAb 13H11 was weaker (Kd = 435 and 431 nM, respectively), with about 15- to 20-fold-faster dissociation rates (3). Like the nonneutralizing 13H11 MAb, the human cluster II MAbs (98-6, 126-6, and 167-D) also bound with faster dissociation rates. However, binding of MAb 98-6 to the 2F5-MPER peptide was relatively stronger and demonstrated slower off-rates than did that of the other cluster II MAbs. We have also previously shown that MAb 98-6 also cross-blocked 2F5 binding to HR-2 peptides while the other cluster II MAbs could only partially block 2F5 binding (3). Taken together, these results suggest that the human cluster II gp41 MAbs recognize conformational epitopes on gp41 that are distinct from those recognized by the broadly neutralizing 2F5 MAb, which bound more strongly to linear peptides. Thus, while the nonneutralizing cluster II MAbs bound strongly to gp140 oligomers (Kd values in the nanomolar range), their binding to HR-2 or the 2F5 nominal epitope peptides was much weaker (>1 μM).
Reactivities of cluster II MAbs with gp41 MPER peptide-lipid conjugates.
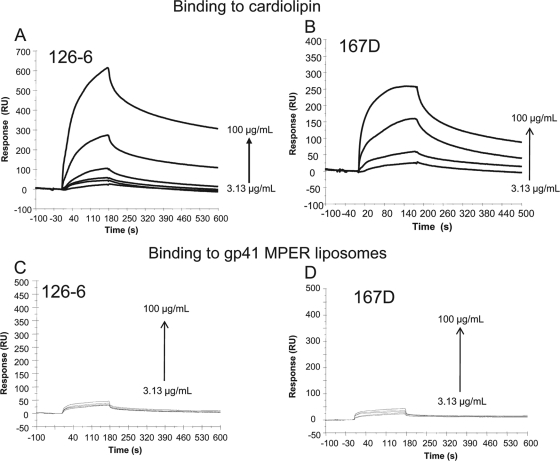
We have previously reported that the binding of MPER MAbs 2F5 and 4E10 to peptide-lipid conjugates follows a 2-step encounter-docking model (1, 11). Binding of 2F5 and 4E10 to gp41 peptide-lipid complexes was selective for the broadly neutralizing MAbs, since the nonneutralizing gp41 MPER MAb 13H11, which did not react with lipids, also failed to bind to gp41 epitopes when presented in the context of the membrane. Thus, we next tested whether the polyreactive cluster II MAbs used in this study, particularly those that were lipid reactive, would interact with gp41 epitopes complexed to lipid membranes. When we tested binding of human cluster II MAbs to peptide-lipid conjugates, we found that all three of the nonneutralizing human cluster II MAbs failed to bind to 2F5 peptide liposomes (Fig. 4C and D and 5B). Among the two MAbs, 126-6 and 167-D, that showed stronger reactivity with phospholipids, no binding was observed with 2F5 peptide-lipid complexes, even at a concentration of 100 μg/ml (Fig. 4C and D). Similarly, 98-6, which shows relatively stronger reactivity with HR-2 peptides (3), also failed to bind to peptide-lipid complexes (Fig. 5). These data suggest that the lipid reactivity of three human cluster II MAbs, in the absence of reactivity with gp41 neutralizing determinants, is not sufficient for HIV-1 neutralization.
FIG. 4.
gp41 cluster II MAbs that show lipid reactivity do not bind to the gp41 peptide-lipid complexes. The binding of 126-6 and 167D MAbs at different concentrations as indicated to POPC-cardiolipin (25:75) liposomes (A and B) or to gp41 MPER peptide liposomes (C and D), respectively, is shown.
FIG. 5.
gp41 cluster II MAb 98-6 binds to 2F5 nominal epitope peptide but not to 2F5 peptide-lipid conjugates. SPR sensograms are shown for the interaction of 2F5 and 98-6 MAbs with the 2F5 epitope peptide (A and B) or 2F5 peptide-liposome conjugates (C).
DISCUSSION
In this study, we have shown that polyreactivity is common among human gp41 cluster II but not cluster I antibodies. Although lipid reactivity of gp41 antibodies per se was not associated with HIV-1 neutralization, neutralizing and nonneutralizing gp41 MAbs are similar in being polyreactive for lipids and/or other autoantigens. Thus, polyreactivity is associated with HIV-1 Env antibodies that target epitopes within the cluster II region and within the gp41 MPER region, which induces neutralizing antibodies. Since the nonneutralizing cluster II MAbs bind strongly to epitopes presented on oligomeric gp140 but not to gp41 peptides or peptide-lipid complexes, they recognize antigenic determinants distinct from those recognized by broad neutralizing gp41 antibodies.
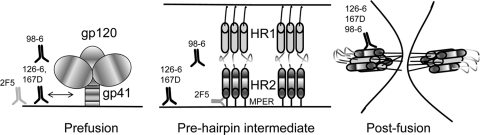
During the course of HIV-1 fusion, gp41 is believed to present at least three distinct conformations—a native prefusion configuration, a “prehairpin” intermediate, and a postfusion state (37, 54) (Fig. 6). In this model, the relevant MPER neutralizing epitopes are transient in nature and are exposed only in a “prehairpin intermediate” state (13, 23) that is induced upon CD4 and/or coreceptor binding (40). 2F5 and 4E10 have been proposed to target the prehairpin intermediate and not bind to the prefusion Env protein (15). 2F5 and 4E10, however, can bind reversibly to lipids (1, 2) and bind strongly with almost irreversible off-rates to the gp41-inter protein, a mimic of the prehairpin state of gp41 (15). Thus, while the prehairpin intermediate is the target for neutralizing gp41 antibodies, binding of 2F5 and 4E10 to lipids allows them to preconcentrate on the virion surface and to subsequently engage the transient MPER neutralizing epitopes in the intermediate conformation (2). Based on this model, the cluster II MAbs (126-6 and 167-D) that bind to lipids would also be able to preconcentrate on the virion surface, while the nonreactive 98-6 would not (Fig. 6). However, lipid binding is unlikely to be a requirement for binding of cluster II MAbs to HIV-1 Env due to the following reasons. First, their epitopes are more distal from the membrane, and second, a recent study has shown that cluster II MAbs do not bind to the prehairpin intermediate conformation of gp41 (14). On the viral surface, where the density of Env spike is low (29, 57), polyreactivity might be an advantage in terms of enhancing avidity by promoting bivalent interactions (27, 31, 53) and promoting heteroligation (31). Simultaneous interactions of 126-6 and 167-D with membrane lipid and gp41, in either the prefusion or the extended intermediate state, would be hindered due to the membrane-distal nature of their epitopes. Although the 98-6 MAb did not show any lipid reactivity, it bound to several autoantigens and potentially could benefit from bivalent interactions with two distinct ligands that would include cross-reactivity with a host antigen, as has been described for the 21c antibody (12). For this heteroligation to occur, however, the proximity of the two ligands would have to accommodate the 15-nm span of the two Fab arms of the IgG molecule. Thus, unlike the broad neutralizing antibodies 2F5 and 4E10, antigen recognition of cluster II antibodies is likely not to benefit from the ability of the antibodies to bind to lipids.
FIG. 6.
Differential recognition of gp41 conformational states by neutralizing and nonneutralizing gp41 antibodies during the course of membrane fusion. The three distinct conformational states of gp41 widely believed to be present during the Env-mediated membrane fusion are shown schematically. In the prefusion state, cluster II MAbs 126-6 and 167D will bind to viral lipid, which may facilitate their interaction with HIV-1 Env. 98-6, with no lipid reactivity, can only potentially interact with Env or host antigens. The membrane-distal nature of the epitopes of the cluster II MAbs will not favor simultaneous binding of the MAbs to gp41 and the lipids. The cluster II MAbs will not bind to the transient prehairpin intermediate but will bind strongly to the postfusion conformation of gp41. Due to their inability to engage the prehairpin intermediate conformation, the cluster II MAbs will fail to disrupt progression of the fusion process. By binding first to membrane lipids, 2F5 can efficiently target the transient prehairpin intermediate and block membrane fusion.
Several observations indicate that it is more likely that the cluster II MAbs bind only to the postfusion state of gp41. Both 126-6 and 167-D react with complexed HR-1 and HR-2 peptides (the gp41 N51-C43 peptide complex) but not with either alone (21, 56). This is consistent with our results that both MAbs bound strongly to gp140 oligomers and showed no reactivity with MPER peptide-lipid conjugates. The epitope for MAb 126-6 has been mapped to a region N-terminal to gp41 aa 648, and its requirement for a gp140 trimer-specific conformation suggests that the MAb likely targets the postfusion state of gp41 (56). Of the three cluster II MAbs, 98-6 is distinct in that it bound to linear MPER peptides and gp140 oligomers. These results are consistent with previous reports that 98-6 is reactive with both the free HR-2 peptide and the complexed HR-1/HR-2 peptides (21) and is reactive with dimeric and trimeric forms of gp140 (56). The lack of reactivity of MAb 98-6 with MPER peptide complexes suggest that it may fail to recognize the constrained conformation on the membrane surface since the epitope of this MAb is located N-terminal to the 2F5 core (56) and such residues are more likely to be solvent exposed (11, 46). Exposure of the 98-6 epitope on infected cells was enhanced following soluble CD4 treatment, which suggests that the conformational changes induced upon CD4 binding result in the exposure of gp41 epitopes that include the 98-6 binding site (40, 47). It is also likely that 98-6 binds to a more advanced fusion intermediate than 2F5, and as a result, 98-6 will have a shorter window of opportunity for binding and blocking membrane fusion. Thus, the exposure of the 98-6 epitope might occur too late in the fusion process for the MAb to potently neutralize HIV-1. This is consistent with the finding that 98-6 MAb can be more effective in blocking fusion and viral entry under certain restricted conditions, e.g., incubation at a suboptimal temperature, which slows down the steps leading to fusion (16, 17). Thus, the lack of lipid reactivity of 98-6 would preclude it from engaging efficiently with a transient conformation of gp41, while it would be able to interact stably with the postfusion conformation. That the nonneutralizing cluster II MAbs indeed bind to gp41 in its postfusion conformation has now been reported in two recent structural studies (14, 34). Thus, nonneutralizing cluster II MAbs bind strongly to postfusion gp41; while some may bind to lipids, they fail to engage the prehairpin intermediate and block fusion (Fig. 6).
Finally, the finding that a number of gp41-nonneutralizing MAbs are polyreactive and bind to autoantigens is of interest regarding their potential mode of immunoregulation. Many of the cluster II MAbs are derived from the VH1-69 heavy chain family (4E10, 126-6, and 167-D), which is frequently used by antibodies which display autoreactivity (20). Thus, HIV-1 Env epitopes within gp41 may target immunoglobulin VH family-specific germ line sequences. We have suggested that the lipid reactivities of 2F5 and 4E10 may subject these types of antibodies to control by tolerance mechanisms (52), as with antiphospholipid and other autoantibodies (10). While neutralizing antibodies that bind to the MPER region are uncommon (found in only 0.3 to 1% of infected patients [48, 49]), nonneutralizing cluster II antibodies are more common (∼30% of subjects) and when present arise ∼5 to 10 weeks after HIV-1 transmission (49). Immunization strategies designed to break tolerance and induce anti-dsDNA antibodies also induce nonneutralizing cluster II antibodies in mouse strains that otherwise do not make these antibodies (3). Thus, there may be immunoregulatory controls on both neutralizing MPER and nonneutralizing cluster II antibodies depending on the affinity of binding to autoantigens and perhaps the nature of the interactions with virion lipid-Env complexes. Alternatively, these data can also suggest that it is the MPER neutralizing determinant that is critical for induction of tolerance control. Data in support of this notion comes from the observation that a majority of residual peripheral B cells that have escaped central clonal deletion in 2F5 VH knock-in mice have lost their MPER reactivity but retain their lipid reactivity (L. Verkoczy and B. F. Haynes, unpublished observations). Thus, reactivity to the MPER neutralizing determinant for 2F5-like antibodies is a more important factor in limiting antibody induction by tolerance mechanisms than is antibody lipid reactivity. That both neutralizing MPER and nonneutralizing cluster II antibodies cross-react with self antigens demonstrates that both specificities of antibodies may be subjected to regulation by immune control; however, interaction of such antibodies with their respective Env conformations appears to be the critical determinant of both the antibodies' ability to be induced and ability to neutralize HIV-1.
Acknowledgments
This research was conducted as part of the Collaboration for AIDS Vaccine Discovery (CAVD) with support from the Bill & Melinda Gates Foundation to B.F.H. (38643) and S.Z-P. (38631_01), the Center for HIV/AIDS Vaccine Immunology (CHAVI), grant AI067854 from the NIAID, NIH grants HL59725 (to S.Z.P.) and AI27742 (NYU CFAR), and research funds from the Department of Veterans Affairs.
We thank Laurent Verkoczy for comments and suggestions and Millie McAdams for technical assistance in performing SPR assays.
Footnotes
Published ahead of print on 24 November 2010.
REFERENCES
- 1.Alam, S. M., et al. 2007. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J. Immunol. 178:4424-4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam, S. M., et al. 2009. Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc. Natl. Acad. Sci. U. S. A. 106:20234-20239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alam, S. M., et al. 2008. Human immunodeficiency virus type 1 gp41 antibodies that mask membrane proximal region epitopes: antibody binding kinetics, induction, and potential for regulation in acute infection. J. Virol. 82:115-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Astronomo, R. D., et al. 2008. A glycoconjugate antigen based on the recognition motif of a broadly neutralizing human immunodeficiency virus antibody, 2G12, is immunogenic but elicits antibodies unable to bind to the self glycans of gp120. J. Virol. 82:6359-6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, B. K., et al. 2007. Monoclonal antibodies to phosphatidylinositol phosphate neutralize human immunodeficiency virus type 1: role of phosphate-binding subsites. J. Virol. 81:2087-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchacher, A., et al. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retroviruses 10:359-369. [DOI] [PubMed] [Google Scholar]
- 7.Burton, D. R., R. L. Stanfield, and I. A. Wilson. 2005. Antibody vs. HIV in a clash of evolutionary titans. Proc. Natl. Acad. Sci. U. S. A. 102:14943-14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calarota, S., et al. 1996. Immunodominant glycoprotein 41 epitope identified by seroreactivity in HIV type 1-infected individuals. AIDS Res. Hum. Retroviruses 12:705-713. [DOI] [PubMed] [Google Scholar]
- 9.Cardoso, R. M., et al. 2005. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity 22:163-173. [DOI] [PubMed] [Google Scholar]
- 10.Chen, C., Z. Nagy, E. L. Prak, and M. Weigert. 1995. Immunoglobulin heavy chain gene replacement: a mechanism of receptor editing. Immunity 3:747-755. [DOI] [PubMed] [Google Scholar]
- 11.Dennison, S. M., et al. 2009. Stable docking of neutralizing human immunodeficiency virus type 1 gp41 membrane-proximal external region monoclonal antibodies 2F5 and 4E10 is dependent on the membrane immersion depth of their epitope regions. J. Virol. 83:10211-10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diskin, R., P. M. Marcovecchio, and P. J. Bjorkman. 2010. Structure of a clade C HIV-1 gp120 bound to CD4 and CD4-induced antibody reveals anti-CD4 polyreactivity. Nat. Struct. Mol. Biol. 17:608-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 14.Frey, G., et al. 14 November 2010. Distinct conformational states of HIV-1 gp41 are recognized by neutralizing and non-neutralizing antibodies. Nat. Struct. Mol. Biol. [Epub ahead of print.] doi: 10.1038/nsmb.1950. [DOI] [PMC free article] [PubMed]
- 15.Frey, G., et al. 2008. A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc. Natl. Acad. Sci. U. S. A. 105:3739-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golding, H., et al. 2002. Dissection of human immunodeficiency virus type 1 entry with neutralizing antibodies to gp41 fusion intermediates. J. Virol. 76:6780-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golding, H., et al. 2002. Dissection of human immunodeficiency virus type 1 entry with neutralizing antibodies to gp41 fusion intermediates. J. Virol. 76:6780-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorny, M. K., V. Gianakakos, S. Sharpe, and S. Zolla-Pazner. 1989. Generation of human monoclonal antibodies to human immunodeficiency virus. Proc. Natl. Acad. Sci. U. S. A. 86:1624-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorny, M. K., et al. 2004. The v3 loop is accessible on the surface of most human immunodeficiency virus type 1 primary isolates and serves as a neutralization epitope. J. Virol. 78:2394-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorny, M. K., et al. 2009. Preferential use of the VH5-51 gene segment by the human immune response to code for antibodies against the V3 domain of HIV-1. Mol. Immunol. 46:917-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorny, M. K., and S. Zolla-Pazner. 2000. Recognition by human monoclonal antibodies of free and complexed peptides representing the prefusogenic and fusogenic forms of human immunodeficiency virus type 1 gp41. J. Virol. 74:6186-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haynes, B. F., et al. 2005. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 308:1906-1908. [DOI] [PubMed] [Google Scholar]
- 23.Haynes, B. F., and D. C. Montefiori. 2006. Aiming to induce broadly reactive neutralizing antibody responses with HIV-1 vaccine candidates. Expert Rev. Vaccines 5:347-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haynes, B. F., M. A. Moody, L. Verkoczy, G. Kelsoe, and S. M. Alam. 2005. Antibody polyspecificity and neutralization of HIV-1: a hypothesis. Hum. Antibodies 14:59-67. [PMC free article] [PubMed] [Google Scholar]
- 25.Hioe, C. E., et al. 1997. Neutralization of HIV-1 primary isolates by polyclonal and monoclonal human antibodies. Int. Immunol. 9:1281-1290. [DOI] [PubMed] [Google Scholar]
- 26.Jeffs, S. A., et al. 2001. Characterization of human monoclonal antibodies selected with a hypervariable loop-deleted recombinant HIV-1(IIIB) gp120. Immunol. Lett. 79:209-213. [DOI] [PubMed] [Google Scholar]
- 27.Klein, J. S., and P. J. Bjorkman. 2010. Few and far between: how HIV may be evading antibody avidity. PLoS Pathog. 6:e1000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao, H. X., et al. 2006. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology 353:268-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, J., A. Bartesaghi, M. J. Borgnia, G. Sapiro, and S. Subramaniam. 2008. Molecular architecture of native HIV-1 gp120 trimers. Nature 455:109-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGaughey, G. B., et al. 2003. HIV-1 vaccine development: constrained peptide immunogens show improved binding to the anti-HIV-1 gp41 MAb. Biochemistry 42:3214-3223. [DOI] [PubMed] [Google Scholar]
- 31.Mouquet, H., et al. 2010. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature 467:591-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muster, T., et al. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson, J. D., et al. 2007. An affinity-enhanced neutralizing antibody against the membrane-proximal external region of human immunodeficiency virus type 1 gp41 recognizes an epitope between those of 2F5 and 4E10. J. Virol. 81:4033-4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicely, N. I., et al. 14 November 2010. Crystal structure of a non-neutralizing antibody to the HIV-1 gp41 membrane-proximal external region. Nat. Struct. Mol. Biol. [Epub ahead of print.] doi: 10.1038/nsmb.1944. [DOI] [PMC free article] [PubMed]
- 35.Nyambi, P. N., et al. 2000. Conserved and exposed epitopes on intact, native, primary human immunodeficiency virus type 1 virions of group M. J. Virol. 74:7096-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ofek, G., et al. 2004. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J. Virol. 78:10724-10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roux, K. H., and K. A. Taylor. 2007. AIDS virus envelope spike structure. Curr. Opin. Struct. Biol. 17:244-252. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Martinez, S., et al. 2006. Specific phospholipid recognition by human immunodeficiency virus type-1 neutralizing anti-gp41 2F5 antibody. FEBS Lett. 580:2395-2399. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Martinez, S., M. Lorizate, H. Katinger, R. Kunert, and J. L. Nieva. 2006. Membrane association and epitope recognition by HIV-1 neutralizing anti-gp41 2F5 and 4E10 antibodies. AIDS Res. Hum. Retroviruses 22:998-1006. [DOI] [PubMed] [Google Scholar]
- 40.Sattentau, Q. J., and J. P. Moore. 1991. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J. Exp. Med. 174:407-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sattentau, Q. J., S. Zolla-Pazner, and P. Poignard. 1995. Epitope exposure on functional, oligomeric HIV-1 gp41 molecules. Virology 206:713-717. [DOI] [PubMed] [Google Scholar]
- 42.Scanlan, C. N., et al. 2002. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1→2 mannose residues on the outer face of gp120. J. Virol. 76:7306-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen, X., et al. 2010. Prolonged exposure of the HIV-1 gp41 membrane proximal region with L669S substitution. Proc. Natl. Acad. Sci. U. S. A. 107:5972-5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song, L., et al. 2009. Broadly neutralizing anti-HIV-1 antibodies disrupt a hinge-related function of gp41 at the membrane interface. Proc. Natl. Acad. Sci. U. S. A. 106:9057-9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stiegler, G., et al. 2001. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses 17:1757-1765. [DOI] [PubMed] [Google Scholar]
- 46.Sun, Z. Y., et al. 2008. HIV-1 broadly neutralizing antibody extracts its epitope from a kinked gp41 ectodomain region on the viral membrane. Immunity 28:52-63. [DOI] [PubMed] [Google Scholar]
- 47.Taniguchi, Y., et al. 2000. Human monoclonal antibody 98-6 reacts with the fusogenic form of gp41. Virology 273:333-340. [DOI] [PubMed] [Google Scholar]
- 48.Tomaras, G. D., and B. F. Haynes. 2009. HIV-1-specific antibody responses during acute and chronic HIV-1 infection. Curr. Opin. HIV AIDS 4:373-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomaras, G. D., et al. 2008. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J. Virol. 82:12449-12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trkola, A., et al. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tyler, D. S., et al. 1990. Identification of sites within gp41 that serve as targets for antibody-dependent cellular cytotoxicity by using human monoclonal antibodies. J. Immunol. 145:3276-3282. [PubMed] [Google Scholar]
- 52.Verkoczy, L., et al. 2010. Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance. Proc. Natl. Acad. Sci. U. S. A. 107:181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, P., and X. Yang. 2010. Neutralization efficiency is greatly enhanced by bivalent binding of an antibody to epitopes in the V4 region and the membrane-proximal external region within one trimer of human immunodeficiency virus type 1 glycoproteins. J. Virol. 84:7114-7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884-1888. [DOI] [PubMed] [Google Scholar]
- 55.Xu, J. Y., M. K. Gorny, T. Palker, S. Karwowska, and S. Zolla-Pazner. 1991. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using ten human monoclonal antibodies. J. Virol. 65:4832-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan, W., et al. 2009. Oligomer-specific conformations of the human immunodeficiency virus (HIV-1) gp41 envelope glycoprotein ectodomain recognized by human monoclonal antibodies. AIDS Res. Hum. Retroviruses 25:319-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu, P., et al. 2006. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature 441:847-852. [DOI] [PubMed] [Google Scholar]
- 58.Zolla-Pazner, S., M. K. Gorny, P. N. Nyambi, T. C. VanCott, and A. Nadas. 1999. Immunotyping of human immunodeficiency virus type 1 (HIV): an approach to immunologic classification of HIV. J. Virol. 73:4042-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zolla-Pazner, S., et al. 1995. Serotyping of primary human immunodeficiency virus type 1 isolates from diverse geographic locations by flow cytometry. J. Virol. 69:3807-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zwick, M. B., and D. R. Burton. 2007. HIV-1 neutralization: mechanisms and relevance to vaccine design. Curr. HIV Res. 5:608-624. [DOI] [PubMed] [Google Scholar]
- 61.Zwick, M. B., et al. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75:10892-10905. [DOI] [PMC free article] [PubMed] [Google Scholar]