Abstract
The risk of the transmission of ruminant transmissible spongiform encephalopathy (TSE) to humans was thought to be low due to the lack of association between sheep scrapie and the incidence of human TSE. However, a single TSE agent strain has been shown to cause both bovine spongiform encephalopathy (BSE) and human vCJD, indicating that some ruminant TSEs are transmissible to humans. While the transmission of cattle BSE to humans in transgenic mouse models has been inefficient, indicating the presence of a significant transmission barrier between cattle and humans, BSE has been transmitted to a number of other species. Here, we aimed to further investigate the human transmission barrier following the passage of BSE in a sheep. Following inoculation with cattle BSE, gene-targeted transgenic mice expressing human PrP showed no clinical or pathological signs of TSE disease. However, following inoculation with an isolate of BSE that had been passaged through a sheep, TSE-associated vacuolation and proteinase K-resistant PrP deposition were observed in mice homozygous for the codon 129-methionine PRNP gene. This observation may be due to higher titers of the BSE agent in sheep or an increased susceptibility of humans to BSE prions following passage through a sheep. However, these data confirm that, contrary to previous predictions, it is possible that a sheep prion is transmissible to humans and that BSE from other species is a public health risk.
The transmissible spongiform encephalopathies (TSEs) are a group of fatal infectious neurodegenerative diseases that include scrapie in sheep, bovine spongiform encephalopathy (BSE) in cattle, and Creutzfeldt-Jakob disease (CJD) in humans. TSEs are characterized by the accumulation in the brain of PrPSc, which is a conformational variant of the normal cellular host prion protein (PrPC). The abnormal form of the protein is protease resistant and detergent insoluble, and it aggregates in diffuse or amyloid deposits in the central nervous system (CNS) and lymphoreticular system of infected animals. TSEs are infectious diseases and can be transmitted between animals of the same and different species by a number of routes, including oral, environmental, or iatrogenic exposure. The host range is a specific characteristic of each strain, but TSE agents usually transmit more readily within rather than between species. Low transmission rates often are observed upon transmission to a new species, but on further passage in the new species increased transmission rates and shorter incubation times usually are observed. This effect is referred to as the species barrier. The ability of individual TSE agents to cross a species barrier can be examined experimentally by the direct inoculation of different species or modeled in transgenic mice expressing PrP sequences from these species. Modeling species barriers in mice is particularly important when assessing the risks of infection in humans. Such experiments can assess risk posed by different TSE agents and also the potential for the mutation and adaptation of the agent to the new species. These experiments can highlight changes that may result in the emergence of a new agent strain with a much wider or unknown host range.
One TSE agent that has shown the ability to transmit to several different species is BSE, where infection has been observed in captive and domestic feline species and exotic ungulates (kudu and nyala), probably due to the ingestion of contaminated feed (13). In 1996 a new variant form of CJD (vCJD) was reported in humans that presented with unusual pathology and at an extremely young age range compared to those of other human TSEs (30). Strain typing experiments demonstrated that vCJD was caused by the same agent strain as BSE (9, 23), indicating that exposure to contaminated foods also resulted in the transmission of BSE to humans. Humans previously had been thought to be at low risk to contract ruminant TSEs, as sheep scrapie has been endemic in many countries for hundreds of years without any related foci of human TSE. However, the link between BSE and vCJD proved that ruminant TSEs are a public health risk, and that new ruminant TSEs may be transmissible to humans.
During the BSE epidemic, sheep undoubtedly were exposed to the BSE agent; however, no cases of BSE in sheep have been documented in the field. Sheep can, however, be infected with BSE via oral, intravenous, and intracerebral routes (17, 18), producing clinical TSE with incubation periods ranging from months to years (depending on the PrP genotype of the sheep), proving that this species is susceptible to infection with the BSE agent. It is possible that low-level BSE infection did exist in sheep during the height of the BSE epidemic; however, it is unknown whether this could have been masked by coinfection with scrapie. BSE has, however, been documented in goats (14, 25), providing evidence that the infection of small ruminants in the field has occurred. Whether the presence of BSE infection in sheep and goats represents a risk to humans currently is unclear. No previous association between sheep scrapie and human TSE has been documented. This may be due to incompatibility between sheep and human PrP or the inability of natural scrapie strains to replicate efficiently in a human host. If the agent strain ultimately is responsible for this lack of transmission rather than a sheep-human barrier, it is possible that BSE in other ruminant species pose a risk to humans.
To address the transmissibility of cattle- and sheep-derived BSE to humans, we performed inoculations of BSE-infected sheep brain into a panel of gene-targeted transgenic mice expressing human PrP under the same spatial and temporal controls as wild-type PrP (4). Three lines of transgenic mice were used, representing the genetic diversity in the human population due to the PrP codon 129-methionine/valine polymorphism: HuMM (40%), HuMV (50%), and HuVV (10%). This polymorphism is known to affect human susceptibility to TSE, and to date all confirmed clinical cases of vCJD have occurred in individuals who are methionine homozygous at PrP codon 129. Although previous experiments showed no disease transmission from cattle BSE in any of these human transgenic mouse lines (4), we show here that experimental sheep BSE produced pathological evidence of disease transmission in ∼70% of HuMM transgenic mice, suggesting that sheep BSE is a greater risk to humans than cattle BSE.
MATERIALS AND METHODS
Transgenic mice.
Inbred gene-targeted human PrP transgenic mice with the codon 129-methionine/valine polymorphism have been described previously (4). Transgenic lines homozygous for the polymorphism were crossed to produce all three genotypes represented in the United Kingdom population (designated HuMM, HuMV, and HuVV, respectively) as previously described (4). Brain tissue from a group of uninoculated HuMM and HuVV mice that had previously been allowed to age to more than 700 days were utilized as controls in chemical analyses. In addition, a gene-targeted bovine transgenic line expressing bovine PrP with the 6-octapeptide repeat region (Bov6) and wild-type 129/Ola mice were used as control lines (4). The Bov6 gene-targeted transgenic line is the same line as that described as BovTg in Bishop et al. (4).
Preparation of inocula.
Brain tissue from the cortex of a female, Cheviot sheep (NPU J2501; ARQ/ARQ) experimentally infected via the oral route with cattle BSE (19) was used to prepare two separate inocula (inoculum 1 and inoculum 2). The sheep were culled with confirmed clinical and pathological BSE at 596 days postinoculation. Natural scrapie isolates (VRQ/VRQ) from the NPU flock were used as controls. Inocula were prepared from cortex tissue in sterile saline at a concentration of 10% (wt/vol). The cattle BSE brainstem pool used in comparative experiments was supplied by the Veterinary Laboratories Agency, Weybridge, United Kingdom (infectivity titer, 103.3 50% infective doses [ID50]U/g tissue, measured in RIII mice; M. Simmons and R. Lockey, personal communication).
Inoculation of transgenic lines.
Experimental sheep BSE inoculum 1 was used to infect groups of gene-targeted transgenic mice expressing human PrP with the codon 129-methionine/valine polymorphism (HuMM, HuVV, and HuMV), control 129/Ola mice, and gene-targeted Bov6 mice (4). In a later experiment, the same transgenic panel was inoculated with experimental sheep BSE inoculum 2 to confirm data obtained with inoculum 1. Data shown (for comparison) from the inoculation of HuMM, HuMV, HuVV, and Bov6 transgenic mice with a cattle BSE brainstem pool (provided by the Veterinary Laboratories Agency, Weybridge, United Kingdom) was generated and published previously (4). The 129/Ola wild-type mice inoculated with cattle BSE described here were inoculated in the same manner with the same BSE brainstem pool as part of this work, but they were not included in the original publication (4).
All mice were intracerebrally (i.c.) inoculated with 0.02 ml of inoculum per mouse into the right midhemisphere. Following the inoculation, mice were monitored daily and scored once a week for signs of clinical disease. Mice were culled at a predefined clinical endpoint (12) or due to welfare reasons, and brain tissue was recovered postmortem. One-half of the brain was fixed in formal saline, further trimmed to expose a number of different regions of the brain (frontal cortex, cortex, hippocampus, thalamus, cerebellum, and brain stem), and then wax embedded to allow 6-μm sections to be cut for use in the pathological analysis of the tissue. The second half of each brain and the spleen (where available) were snap-frozen in liquid nitrogen for biochemical analysis. Each mouse was genotyped postmortem to confirm PrP genotype. All mouse experiments were reviewed and approved by the Local Ethical Review Committee and performed under license from the United Kingdom Home Office in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986.
Analysis of vacuolar pathology.
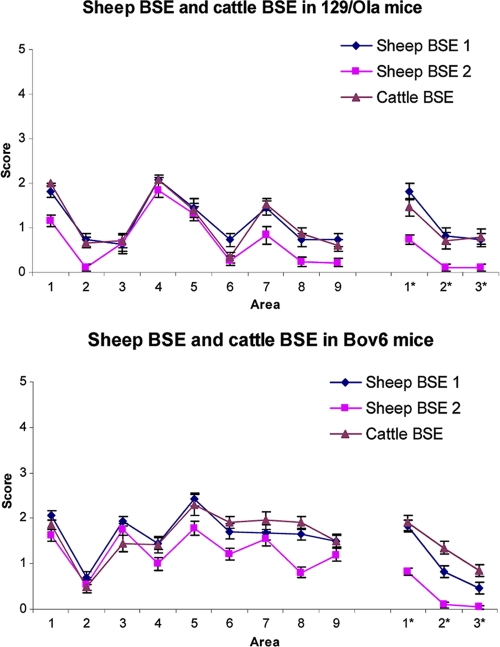
Sections were cut (6 μm) from each mouse brain and stained using hematoxylin and eosin (H&E). Nine regions of the gray matter (dorsal medulla, cerebellar cortex, superior colliculus, hypothalamus, thalamus, hippocampus, septum, cerebral cortex, and forebrain cerebral cortex) and three regions of white matter (cerebellar white matter, midbrain white matter, and cerebral peduncle) were examined and scored on a scale of 0 (no vacuolation) to 5 (severe vacuolation) for the presence and severity of vacuolation. Mean vacuolation scores for each mouse group in each experiment were calculated and plotted with standard errors of means (SEM) against scoring areas to produce a lesion profile (21).
IHC analysis of PrP deposition in brain.
To identify PrP deposits in the brain, two methods of immunohistochemical (IHC) analysis were employed. (i) For the ABC kit (Vectastain), brain sections (6 μm) were pretreated using hydrated autoclaving at 121°C for 15 min and exposure to formic acid (95%) for 5 min prior to incubation with 0.44 μg/ml anti-PrP monoclonal antibody (MAb) 6H4 (Prionics) at room temperature overnight. Biotinylated secondary anti-mouse antibody (Jackson Immuno Research Laboratories, United Kingdom) was added at 2.6 μg/ml and incubated at room temperature for 1 h. PrPSc was visualized by a reaction with hydrogen peroxidase-activated diaminobenzidine (DAB). (ii) When levels of PrPSc detected using the ABC kit were low or zero, the Dako catalyzed signal amplification kit (CSA II [Vectastain]) was used. IHC was performed using the same principals as those for i, but a streptavidin-biotin-peroxidase amplification step was added (see the manufacturer's information). Sections were pretreated as described above prior to incubation with 0.44 μg/ml monoclonal antibody 6H4 at room temperature overnight. Anti-mouse immunoglobulins, supplied with the CSA II kit, were added and incubated for 60 min at room temperature. PrPSc was detected by a reaction with hydrogen peroxidase-activated DAB.
Immunohistochemical detection of glial activation.
To detect astrocyte activation, brain sections (6 μm) were incubated with 1.45 μg/ml anti-glial fibrillary acidic protein (GFAP; Dako UK Ltd.) antibody at room temperature for 1 h. To detect microglia activation, brain sections (6 μm) were pretreated using hydrated microwaving for 10 min prior to incubation with 0.05 μg/ml anti-Iba1 antibody (Wako Chemicals GmbH) at room temperature for 1 h. For both primary antibodies, a biotinylated secondary anti-rabbit antibody (Jackson Immuno Research Laboratories, United Kingdom) was added at 2.6 μg/ml and incubated at room temperature for 1 h. Astrocytes and microglia were visualized by a reaction with hydrogen peroxidase-activated DAB.
Detection of amyloid plaques by thioflavin fluorescence.
Sections (6 μm) were processed and exposed to 1% thioflavin-S (Sigma, United Kingdom) solution as described previously (28). Viewed under a fluorescence microscope, amyloid deposits fluoresce bright green.
Identification of PrPSc in spleen tissue.
Spleen tissues that were available from HuMM transgenic mice inoculated with experimental sheep BSE inoculum 2 were screened by the IDEXX HerdChek assay by following the manufacturer's guidelines. Buffer volumes for homogenization were adjusted to ensure that all homogenates were 30% (wt/vol) for consistency.
Identification of PrPSc by immunoblotting.
Brain tissues from HuMM transgenic mice, Bov6 transgenic mice, and wild-type 129/Ola mice inoculated with experimental sheep BSE inoculum 2 were prepared for analysis by immunoblotting. Samples from the experimental sheep BSE source brain and from isolates of natural scrapie also were prepared as controls. Due to the focus of PrP deposition and the amyloid nature of much of the disease-associated PrP in HuMM transgenic mice, a centrifugal concentration extraction procedure (including proteinase K digestion) was performed as described previously (24) to maximize the possibility of identifying PrP-res. Samples were loaded and run on 16% Tris-glycine acrylamide gels (Novex, Invitrogen) at various concentrations (0.6 to 64 mg brain equivalent) to allow comparison between lanes and immunoblotted onto a polyvinylidene difluoride membrane. To achieve a detectable signal, approximately 64 mg brain equivalent was loaded from HuMM brain tissue, whereas 0.88 mg brain equivalent was loaded from experimental sheep BSE-infected Bov6 controls. Monoclonal antibodies 6H4 (0.1 μg/ml) and 12B2 (0.2 μg/ml) were used to detect PrP, and bands were visualized using horseradish peroxidase (HRP)-labeled anti-mouse secondary antibody (Jackson Immuno Research Laboratories, United Kingdom) and a chemiluminescence substrate (Roche).
RESULTS
BSE strain characteristics are retained following transmission in sheep.
For both experimental sheep BSE inoculum 1 and inoculum 2, 100% transmission rates were observed in 129/Ola and Bov6 control mice. Experimental sheep BSE inoculum 1 produced incubation times in 129/Ola and Bov6 mice of 474 ± 22 days and 564 ± 8 days, respectively (Table 1), which are similar to those observed in a previous experiment following inoculation of these lines with a cattle BSE brain pool (4). Inoculum 2, prepared from the same BSE-infected sheep, gave incubation times of 403 ± 17 days and 487 ± 3 days in 129/Ola and Bov6 mice, respectively (Table 1). In each line of mice, the lesion profiles of cattle and sheep BSE were similar, indicating no change in the targeting properties of BSE following passage through sheep (Fig. 1). Lesion profiles of sheep BSE inoculum 1 and inoculum 2 also were similar, although the degree of vacuolation was slightly reduced for inoculum 2. This may represent the shortened incubation times observed in these mice. Although these shortened incubation times may reflect a higher level of agent replication in the tissue sample used to prepare inoculum 2, overall the incubation time ratio and targeting of cattle BSE and sheep BSE in control mice indicates no major change in agent characteristics following passage in sheep.
TABLE 1.
Transmission of cattle BSE, experimental sheep BSE, and natural scrapie to gene-targeted human and bovine transgenic mice
| TSE isolate | Mouse line |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 129/Ola |
Bov6 |
HuMM |
HuVV |
HuMV |
||||||
| Incubation timea | No. affectedb | Incubation time | No. affected | Incubation time | No. affected | Incubation time | No. affected | Incubation time | No. affected | |
| Cattle BSE (brain pool) | 447 ± 27 | 8/8 | 551 ± 12c | 22/22c | >765 | 0/18c | >793 | 0/22c | >749 | 0/23c |
| Sheep BSE inoculum 1 | 474 ± 22 | 11/11 | 564 ± 8 | 17/17 | >812 | 1/20 | >812 | 0/23 | >812 | 0/23 |
| Sheep BSE inoculum 2 | 403 ± 17 | 23/23 | 487 ± 3 | 24/24 | >750 | 16/23 | >650 | 0/23 | >708 | 0/24 |
| Natural scrapie 1 | 594, 705 | 2/15 | >811 | 0/21 | >685 | 0/24 | >776 | 0/22 | >671 | 0/23 |
| Natural scrapie 2 | 510 ± 17 | 15/23 | >647 | 0/24 | >730 | 0/24 | >710 | 0/24 | >682 | 0/24 |
Measured as days ± standard errors of the means and calculated from mice showing both clinical and pathological signs of TSE. >n represents the survival in days of the oldest mouse in groups where both clinical and pathological signs of disease were not observed in any animals.
Number of mice showing TSE pathology (vacuolation and/or PrP deposition)/number of mice inoculated.
Data are from Bishop et al. (4).
FIG. 1.
Pattern of vacuolation observed in brains of 129/Ola wild-type mice (a) and Bov6 mice (b) following inoculation with the cattle BSE brainstem pool and experimental sheep BSE inoculum 1 and inoculum 2. A profile was produced from nine gray matter areas (1, dorsal medulla; 2, cerebellar cortex; 3, superior colliculus; 4, hypothalamus; 5, thalamus; 6, hippocampus; 7, septum; 8, cerebral cortex; 9, forebrain cerebral cortex) and three white matter areas (1*, cerebellar white matter; 2*, midbrain white matter; 3*, cerebral peduncle). Average scores were taken from a minimum of six mice per group and plotted against brain area ± standard errors of the means.
Susceptibility of human PrP transgenic mice to experimental sheep BSE.
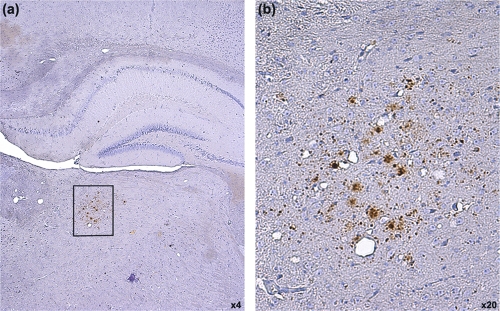
Following inoculation with experimental sheep BSE inoculum 1, three human transgenic mice (one each of HuMM, HuVV, and HuMV) were scored as showing clinical signs of TSE disease at 449, 609, and 707 days postinoculation, respectively, but had no confirmatory vacuolar pathology in the brain. All other human transgenic mice showed no clinical signs of TSE disease and no TSE-associated vacuolar pathology (Table 1). This agreed with previous data generated following the inoculation of these human transgenic mouse lines with cattle BSE (4), indicating the presence of a significant transmission barrier to the BSE agent in humans. However, to rule out the presence of subclinical disease, the oldest mice were screened for abnormal PrP deposition by immunohistochemistry (IHC) using the anti-PrP antibody 6H4. Of all animals screened, one HuMM mouse, which was culled due to old age (706 days postinoculation), showed a small focus of PrP deposition in the thalamus (Fig. 2). No other deposition was observed in this animal or any of the other animals examined.
FIG. 2.
Limited PrPSc accumulation in the thalamus of one HuMM mouse 706 days postinoculation with experimental sheep BSE (inoculum 1). Panel b is a higher-magnification image of the boxed area in panel a. (b) PrPSc deposition appears to be restricted to the thalamus. Images obtained after staining with anti-PrP antibody 6H4 and counterstained with hematoxylin. Magnification is as shown.
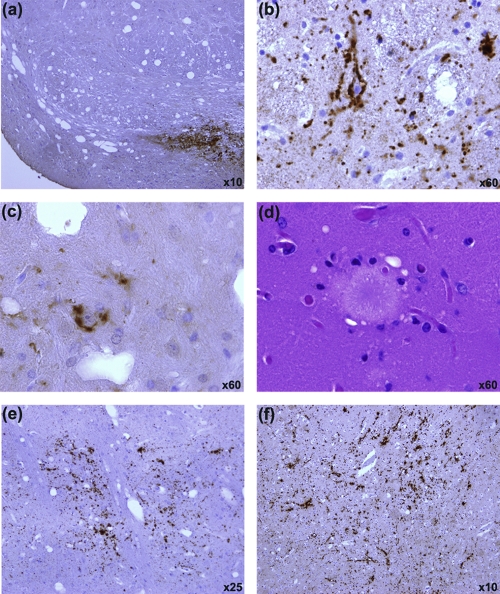
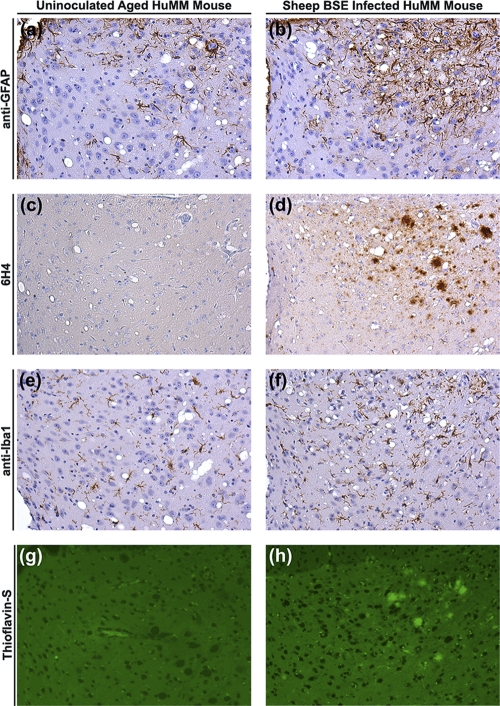
To confirm this observation, a second separate sample was taken from the same BSE-infected sheep brain to prepare inoculum 2, which was used to infect a repeat panel of human transgenic mice. In this experiment, four mice (two each of HuVV and HuMV) were scored as showing clinical signs of TSE disease (between 494 and 539 days postinoculation) but showed no confirmatory TSE-associated pathology in the CNS (vacuolation or PrP deposition). All other human transgenic mice were negative for clinical signs of TSE disease (Table 1). On pathological examination, all HuVV and HuMV mice were negative for vacuolar pathology and PrP deposition. However, 16/23 HuMM mice contained abnormal PrP deposition in the brain, and 3 of the 16 scored positive for vacuolar pathology (Tables 1 and 2). PrP deposition was present in 50% of HuMM mice culled (due to welfare reasons) between 377 and 589 days postinoculation (n = 14) and in all of the remaining HuMM mice culled between 600 and 750 days (n = 9). Variable patterns and levels of PrP deposition were observed in HuMM mice (Table 2 and Fig. 3 and 4). Staining was evident in the cochlear nucleus (when identifiable in the tissue section) as punctate-like deposits in a pattern similar to that of the targeting of BSE in wild-type mice (Fig. 3a) (9). Punctate perineuronal and intraneuronal staining (Fig. 3b and c) also was evident in the midbrain and areas of the thalamus. Most strikingly, thioflavin-S fluorescent PrP amyloid plaques were present in the hippocampus, corpus callosum, dorsal lateral geniculate nucleus (dLGN), and thalamus of 8/16 mice positive for TSE pathology (Fig. 4h; also see Fig. S1 in the supplemental material). The eight mice showing thioflavin-S fluorescence all were more than 589 days postinoculation (Table 2), indicating that plaque formation was occurring later in the disease process, and that PrP deposition in animals culled prior to 589 days was not amyloid. The PrP plaques were shown to be bilateral in a number of tissues where whole brains, or significant portions of the contralateral half, had been analyzed (see Fig. S2 in the supplemental material). Florid plaques (associated with vCJD disease in humans) also were evident in the hippocampus (Fig. 3d). The vacuolar pathology observed in three of the PrP-positive HuMM mice was extremely limited, with the main focus of vacuolation occurring in the hippocampus and thalamus and being closely linked with the presence of PrP amyloid plaques (Fig. 4d). No PrP deposition was observed in aged (750 days old) uninoculated HuMM and HuVV mice that were stained with 6H4 to control for possible age-related PrP deposition due to the transgene (Fig. 4c).
TABLE 2.
Disease profile of HuMM mice inoculated with experimental sheep BSE inoculum 2
| Mouse no. | Survivala (days) | Clinical signs | Vacuolar pathology | Thioflavin | PrPd scoreb | Spleen IDEXXc |
|---|---|---|---|---|---|---|
| 1 | 377 | − | − | − | − | − |
| 2 | 414 | − | − | − | + | + |
| 3 | 422 | − | − | − | − | + |
| 4 | 434 | − | − | − | + | − |
| 5 | 463 | − | − | − | + | − |
| 6 | 514 | − | − | − | − | NAd |
| 7 | 568 | − | − | − | − | + |
| 8 | 568 | − | − | − | − | + |
| 9 | 568 | − | − | − | + | + |
| 10 | 583 | − | − | − | − | NA |
| 11 | 583 | − | − | − | + | + |
| 12 | 583 | − | − | − | + | + |
| 13 | 589 | − | − | − | − | − |
| 14 | 589 | − | − | + | ++ | + |
| 15 | 603 | − | − | − | ++ | + |
| 16 | 622 | − | − | − | ++ | + |
| 17 | 623 | − | − | + | ++ | − |
| 18 | 681 | − | − | + | +++ | NA |
| 19 | 687 | − | + | + | ++ | − |
| 20 | 692 | − | − | + | +++ | NA |
| 21 | 705 | − | − | + | +++ | NA |
| 22 | 723 | − | + | + | +++ | NA |
| 23 | 750 | − | + | + | +++ | − |
Animals died or were culled for welfare reasons.
PrP deposition scored as the following: −, no deposition; +, very low deposition; ++, low deposition; +++, moderate deposition.
An optical density reading above the positive threshold value for the IDEXX assay is represented as a plus.
NA, no spleen tissue available.
FIG. 3.
Variation in pattern and location of PrPSc accumulation in the brain of HuMM mice infected with experimental sheep BSE (inoculum 2). (a) Punctate depositions in the cochlear nucleus are similar to those of BSE targeting in wild-type mice. Perineuronal (b) and intraneuronal (c) PrP depositions are seen in the midbrain and areas of the thalamus. (d) Hematoxylin and eosin stain of a large mature florid plaque located in the hippocampus. Punctate (e) and linear (f) PrP deposition in the thalamic region. Images a to c, e, and f were obtained after samples were stained with anti-PrP antibody 6H4 and counterstained with hematoxylin. Magnification is as shown.
FIG. 4.
Comparative analysis of serial sections through the lateral geniculate nucleus (thalamus) of an uninoculated aged (750 days) HuMM mouse and a HuMM mouse infected with experimental sheep BSE (inoculum 2). A HuMM mouse infected with sheep BSE (inoculum 2) shows astro- and microgliosis (b and f) visible when stained with anti-GFAP and anti-Iba1 (respectively). Several amyloid plaques are clearly visible, fluorescing green with thioflavin-S (h) and being stained with anti-PrP antibody 6H4 (d). (a, c, e, g) Sections from a control aged HuMM mouse show mild astro- and microgliosis and the absence of PrP deposits or amyloid plaques. Sections used for immunohistochemical analysis were counterstained with hematoxylin. Magnification, ×20.
As PrP deposition in HuMM transgenic mice was focused to specific brain areas, PrPSc had to be concentrated by centrifugal purification (SAF prep) to be visualized by immunoblotting. PrPSc was extracted from a HuMM transgenic mouse (with PrP deposition; the same animal as that shown in Fig. 4b, d, f, and h), a Bov6 transgenic mouse, and a wild-type mouse inoculated with experimental sheep BSE inoculum 2 and analyzed by immunoblotting alongside PrPSc that had been extracted from the source BSE-inoculated sheep and four scrapie controls. Blots probed with MAb 6H4 revealed a low level of proteinase K-resistant PrP (PrP-res) in the HuMM mouse compared to that of Bov6 and 129/Ola controls (Fig. 5a). The HuMM sample represented an approximately one-sixth brain equivalent, reflecting the foci of deposition in the original tissue (Fig. 4d) and the amyloid nature of much of the deposited PrP, which may not have been resolved in the polyacrylamide gel. PrP-res levels were too low in both the HuMM mouse and the original source BSE-infected sheep brain to determine the size of the low-molecular-weight PrP-res band. However, when the gel was reprobed with the N-terminal MAb 12B2, reduced levels of staining were observed in lanes containing the source BSE-infected sheep brain, and brain homogenate from the HuMM mouse, 129/Ola mouse, and Bov6 mouse infected with experimental sheep BSE (Fig. 5b). Such reduced staining with MAb 12B2 is characteristic of BSE infection (27, 31).
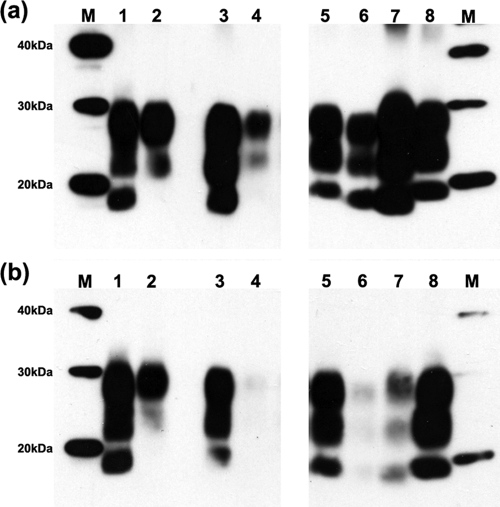
FIG. 5.
Comparative Western blot (WB) analysis of the proteinase K-resistant fragment (PrPSc) of the prion protein. Discrimination between BSE and natural scrapie is achieved using two monoclonal antibodies, 6H4 (a) and 12B2 (b). Lane 1, 4.2 mg equivalent of brain material (mgE) of natural scrapie isolate from the NPU flock. Lane 2, 20 mg equivalent of inocula NPU J2501, Cheviot sheep experimentally infected via the oral route with cattle BSE. Lanes 3 and 5, 1.2 and 1.5 mg equivalent of 129/Ola mice infected with a natural scrapie isolate. Lane 4, 64 mg equivalent of HuMM transgenic mouse infected with experimental sheep BSE (inoculum 2). Lanes 6 and 7, 2.8 and 0.88 mg equivalent of 129/Ola and Bov6 (respectively) infected with experimental sheep BSE (inoculum 2). Lane 8, 0.6 mg equivalent of ME7/SV control. Molecular markers (M) of the standards are indicated on either side of the panels (in kDa).
Glial activation in infected HuMM transgenic mice.
Brian sections from selected HuMM, HuVV, and HuMV transgenic mice inoculated with experimental sheep BSE inoculum 2 were screened for the activation of astrocytes (anti-GFAP) and microglia (anti-Iba1). Aged (∼750 days) uninoculated control HuMM and HuVV mice showed modest GFAP and Iba1 immunoreactivity in isolated cells with slender processes (Fig. 4a and e). PrP amyloid deposits were not seen in these animals (Fig. 4c and g). In contrast, astro- and microgliosis were observed in HuMM mice inoculated with sheep BSE inoculum 2 in brain areas with abundant PrP amyloid deposition, e.g., the lateral geniculate nucleus (LGN) (Fig. 4b, d, f, and h). Gliosis was not detected in experimental sheep BSE-inoculated HuMV and HuVV mice (data not shown).
Peripheral accumulation of PrPSc in spleen.
The IDEXX HerdChek assay was used to analyze spleen tissue available from 17 of the 23 HuMM mice inoculated with experimental sheep BSE inoculum 2. Positive assay readouts were obtained for 10 of the 17 spleens analyzed (Table 2). The detection of abnormal PrP in spleen tissue by the IDEXX HerdChek assay did not correlate with PrP deposition observed in the brain of the same animal. Of the 10 IDEXX-positive samples, three were from mice in which the brain was scored negative by IHC with anti-PrP antibody 6H4 (culled at 422 and 658 days postinoculation). In contrast, the three oldest spleen samples available (623, 687, and 750 days postinoculation) all were negative, despite moderate PrP deposition in the brain (Table 2).
Susceptibility of human transgenic mice to natural sheep scrapie.
Brain homogenate from two VRQ/VRQ sheep with clinically and pathologically confirmed scrapie was used to inoculate the transgenic mouse panel. For both isolates, the presence of clinical and pathological signs of TSE disease were observed only in some control 129/Ola wild-type mice with long incubation times (Table 1), as has been observed previously in C57 and RIII mice (8). Clinical signs were recorded in several other mice (two Bov6 mice, eight HuMV mice, and one HuVV mouse), but none of these animals showed signs of TSE-associated pathology. No clinical signs of disease were observed in any remaining transgenic mice, and animals were culled for intercurrent disease or due to old age. No evidence of TSE-associated vacuolar pathology or PrP deposition was observed in the human or bovine PrP gene-targeted transgenic lines following the pathological examination of brain tissue (data not shown).
DISCUSSION
While the transmission of BSE from cattle to humans via oral exposure has been proposed as the origin of vCJD, the risk posed to humans from BSE infection in other species currently is unknown. Sheep can be experimentally infected with BSE, and it has long been a concern that sheep have become infected during the BSE epidemic. Although no evidence of such infection has been identified in the field, cases of BSE in goats have been reported (14, 25). Here, we have shown that the inoculation of experimental sheep BSE into gene-targeted HuMM transgenic mice resulted in the identification of TSE-related pathology (PrP deposition, vacuolation, and gliosis) in ∼70% of the animals overall and 100% of HuMM mice surviving more than 600 days. PrPSc was detected in brain tissue by immunoblotting and in spleen tissue of several mice using the IDEXX HerdChek assay. Some of the oldest HuMM mice (623 to 750 days postinoculation) that showed PrP deposition in the brain did not have detectable PrPSc in spleen. This variability is not unusual, as extremely old mice that show PrPSc in the brain often have no corresponding deposition in the spleen. This likely is due to the loss of germinal centers in the spleen caused by aging (7). No evidence of disease transmission was observed in HuMV or HuVV mice, mirroring the prevalence of vCJD disease observed to date in the United Kingdom population. Although the presence of disease pathology indicates agent replication and early-phase disease, we cannot predict whether these mice would have developed clinical disease if their life spans had been extended, or if this represents a persistent subclinical state. The subpassage of brain material from HuMM mice will be performed to confirm agent replication and assess the adaptation and host range of the agent.
Our results cast new light on the existing data concerning BSE transmission to humans. Since the identification of the link between BSE and vCJD, many studies have been performed to demonstrate or model the transmission of BSE to humans using in vitro conversion techniques or by the inoculation of transgenic mice expressing human PrP. In these transmission studies, cattle BSE has shown limited transmissibility to human PrP transgenic mice (∼0 to 30%), and considerable variation in susceptibility exists between different transgenic lines with various constructs and protein expression levels (1, 3, 4, 11). The highest levels of susceptibility to BSE in mice expressing human PrP with codon 129-methionine (∼30%) were reported by Asante et al. (1) in the two-times overexpression Tg35 model (Hu-129 M), which included the identification of both limited clinical disease and subclinical disease. However, lower attack rates of approximately 20% have been reported in Tg650 mice, which have a higher expression level of 129-Met human PrP of around 5- to 8-fold (3, 26). The gene-targeted transgenic mice utilized in our studies, which express wild-type levels of human PrP from the endogenous mouse Prnp locus, previously showed no incidence of disease following cattle BSE inoculation (4). These observations have led to the assumption that the overexpression of PrP is essential to model human disease susceptibility in mice, and that rodent models with wild-type physiological levels of PrP expression do not live long enough to display signs of disease, as would be seen in the longer-life-span human species. It therefore is significant that the inoculation of experimental sheep BSE described here has resulted in the identification of TSE-related pathology in the gene-targeted human PrP transgenic mice. Additionally, previously published data have shown that short incubation times can be achieved in HuMM and HuVV gene-targeted mice (5). Our data show clearly that gene-targeted transgenic lines are useful in the study of cross-species susceptibility, and that such susceptibility depends on the agent/host combination rather than the life span of a mouse. The inclusion of data obtained from both overexpressing and gene-targeted transgenic mice therefore may inform more accurately on the assessment of the true zoonotic potential of a particular TSE isolate.
The reasons for the increased susceptibility of HuMM mice to experimental sheep BSE in respect to cattle BSE currently are unknown and are the subject of further investigation in our laboratory. One possible explanation is that our BSE-infected sheep brain contained a significantly higher titer of BSE than that found in the cattle BSE brainstem pool, resulting in the shortened incubation times in control 129/Ola and Bov6 mice with experimental sheep BSE inoculum 2 compared to that of cattle BSE, and the pathological features observed in HuMM mice. Incubation times for control mice also were shorter for experimental sheep BSE inoculum 2 than for inoculum 1, although the ratio between 129/Ola and Bov6 mice was similar for each inoculum. Such variation in incubation time on the primary passage of experimental sheep BSE is, however, common, and it has been observed in previous experiments (see Table S1 in the supplemental material). The observed difference in incubation times between inoculum 1 and inoculum 2 therefore is not unexpected. Previous studies by Gonzalez et al. (22) have shown relatively high infectivity titers of sheep-passaged BSE in RIII mice, which were equivalent to those obtained in Romney sheep. The infectivity titer of the cattle BSE brainstem pool used in our transmissions was 103.3 ID50 U/g in RIII mice (R. Lockey and M. Simmons, personal communication). Those reported by Gonzalez et al. (22) for sheep-passaged BSE were 105 ID50 U/g in RIII mice, suggesting that higher titers may indeed be attained in sheep brain. However, reported infectivity titers for BSE in cattle have been variable (6, 20, 22). We therefore are performing the titration analyses of experimental sheep BSE brainstem in Bov6 mice to provide a direct comparison to titration data already available for the cattle BSE brainstem pool.
An alternative hypothesis is that the passage of BSE through a sheep has altered the strain characteristics of the agent, producing a variant with increased virulence and/or host range. This possibility is supported by recent data describing the enhanced virulence of experimental sheep BSE in bovine PrP transgenic mice (BoPrP-Tg110) and porcine PrP transgenic mice (PoPrP-Tg001) compared to that of cattle BSE (15, 16). BoPrP-Tg110 mice and PoPrP-Tg001 mice (which overexpress PrP 8× and 4×, respectively) produced significantly shorter incubation times following inoculation with an experimental sheep BSE brainstem pool than with cattle BSE isolates. The differences in incubation time observed in BoPrP-Tg110 mice were maintained on subpassage (15), indicating that the original variation probably was not due to infectivity titer discrepancies between the two BSE sources. However, the full titration of these tissue homogenates in mice would be required to confirm that this was indeed the case. In PoPrP-Tg001 mice, incubation times shortened significantly on subpassage and were maintained on further subpassage, indicating adaptation to the new host (16). In the study described here, lesion profiles obtained from control 129/Ola mice and Bov6 transgenic mice were similar for both cattle and experimental sheep BSE. Although we were unable to resolve the size of the PrP-res low-molecular-weight band in both the experimental sheep BSE brain homogenate and the experimental sheep BSE-infected HuMM mouse, both showed reduced staining with MAb 12B2, which is characteristic of BSE infection (27). Therefore, there were no differences in strain characteristics between experimental sheep BSE and cattle BSE, with the exception of the transmissibility to HuMM mice (which could be due to increased infectivity titer).
The altered agent properties of sheep BSE observed by Espinosa and colleagues (15, 16) suggest that passage through a sheep causes BSE to transmit in a manner more similar to that of natural scrapie than that of cattle BSE. To investigate this, we inoculated our transgenic panel with two isolates of natural sheep scrapie. No disease pathology was observed in any transgenic mice following inoculation with either isolate of natural scrapie. Hence, in the experiments described here, the susceptibility of the HuMM mice to experimental sheep BSE does not appear to be due to a general susceptibility to ovine prions but is instead linked specifically to the replication of the BSE agent strain in sheep brain. Although agent strain characteristics of BSE are not altered when assayed in Bov6 or 129/Ola mice following passage in sheep, both samples of experimental sheep BSE did show positive TSE pathology in HuMM transgenic mice, which has not been seen previously with cattle BSE inoculations in these mice. Whether this is due simply to agent infectivity titer or a more subtle change in agent characteristics is the subject of further analysis in our laboratory.
Although sheep can be experimentally infected with BSE by oral, intravenous, or intracerebral exposure (18), no cases of sheep BSE have been reported in the field. The possible increased risk of disease transmission identified in these studies thus is not of major concern to the public at present. Natural BSE infection has, however, been identified in goats (14, 25), indicating that small ruminants have been exposed to sources of contamination. We cannot rule out the possibility that sheep have been infected with BSE during the height of the BSE epidemic, as these animals undoubtedly were exposed to similar feed sources (although with different levels of exposure compared to those of cattle). Such infection may have been limited and/or localized and resolved very quickly. BSE in small ruminants may, however, represent an increased risk to humans due to the wider distribution of BSE infectivity identified in peripheral sheep tissues (2, 17, 19, 29) compared to that of BSE in cattle, mainly which is restricted to the CNS (10). While TSEs remain in the environment and continue to infect animals (even at low prevalence), there remains the potential for cross-species transmission and the emergence of TSE isolates with altered strain properties or host ranges. Our data therefore emphasize the need for continued surveillance to identify, monitor, and characterize any new emerging TSE agents that are identified in ruminants and the assessment of the potential risks posed to other species.
Supplementary Material
Acknowledgments
We thank I. McConnell, V. Thomson, S. Cumming, S. Shillinglaw, R. Greenan, and K. Hogan for experimental setup, care, and scoring of the animals; A. Coghill, A. Boyle, S. Mack, and G. McGregor for histology processing and scoring; M. Jeffrey (Veterinary Laboratories Agency, Lasswade, United Kingdom) for pathology advice; and the Veterinary Laboratories Agency, Weybridge, United Kingdom, for providing the cattle BSE brain pool.
This work was funded by contracts SE1439 and SE1441 from the United Kingdom Department for Environment, Food and Rural Affairs (Defra), and NIH-NIAID agreement Y1-A1-4893-02 and FDA agreement 224-05-1307.
The findings and conclusions in this article have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any agency determination or policy.
Footnotes
Published ahead of print on 17 November 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Asante, E. A., et al. 2002. BSE prions propagate as either variant CJD-like or sporadic CJD-like prion strains in transgenic mice expressing human prion protein. EMBO J. 21:6358-6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellworthy, S. J., et al. 2005. Tissue distribution of bovine spongiform encephalopathy infectivity in Romney sheep up to the onset of clinical disease after oral challenge. Vet. Rec. 156:197-202. [DOI] [PubMed] [Google Scholar]
- 3.Béringue, V., et al. 2008. Transmission of atypical bovine prions to mice transgenic for human prion protein. Emerg. Infect. Dis. 14:1898-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop, M. T., et al. 2006. Predicting susceptibility and incubation time of human-to-human transmission of vCJD. Lancet Neurol. 5:393-398. [DOI] [PubMed] [Google Scholar]
- 5.Bishop, M. T., R. G. Will, and J. C. Manson. 2010. Defining sporadic Creutzfeldt-Jakob disease strains and their transmission properties. Proc. Natl. Acad. Sci. 107:12005-12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley, R. 2001. Bovine spongiform encephalopathy and its relationship to the new variant form of Creutzfeldt-Jakob disease, p. 105-144. In H. F. Rabeneau, J. Cinatl, and H. W. Basel (ed.), Prions. A challenge for science and public health system. Contributions to microbiology, vol. 7. Karger Publishers, Basel, Switzerland. [DOI] [PubMed] [Google Scholar]
- 7.Brown, K. L., G. J. Wathne, J. Sales, M. E. Bruce, and N. A. Mabbott. 2009. The effects of host age on follicular dendritic cell status dramatically impair scrapie agent neuroinvasion in aged mice. J. Immunol. 183:5199-5207. [DOI] [PubMed] [Google Scholar]
- 8.Bruce, M. E., et al. 2002. Strain characterization of natural sheep scrapie and comparison with BSE. J. Gen. Virol. 83:695-704. [DOI] [PubMed] [Google Scholar]
- 9.Bruce, M. E., et al. 1997. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 389:498-501. [DOI] [PubMed] [Google Scholar]
- 10.Buschmann, A., and M. H. Groschup. 2005. Highly bovine spongiform encephalopathy-sensitive transgenic mice confirm the essential restriction of infectivity to the nervous system in clinically diseased cattle. J. Infect. Dis. 192:934-942. [DOI] [PubMed] [Google Scholar]
- 11.Collinge, J., et al. 1995. Unaltered susceptibility to BSE in transgenic mice expressing human prion protein. Nature 378:779-783. [DOI] [PubMed] [Google Scholar]
- 12.Dickinson, A. G., V. M. Meikle, and H. Fraser. 1968. Identification of a gene which controls the incubation period of some strains of scrapie agent in mice. J. Comp. Pathol. 78:293-299. [DOI] [PubMed] [Google Scholar]
- 13.Doherr, M. G. 2003. Bovine spongiform encephalopathy (BSE)-infectious, contagious, zoonotic or production disease? Acta Vet. Scand. Suppl. 98:33-42. [PubMed] [Google Scholar]
- 14.Eloit, M., et al. 2005. BSE agent signatures in a goat. Vet. Rec. 156:523-524. [DOI] [PubMed] [Google Scholar]
- 15.Espinosa, J. C., et al. 2007. Sheep-passaged bovine spongiform encephalopathy agent exhibits altered pathobiological properties in bovine-PrP transgenic mice. J. Virol. 81:835-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espinosa, J. C., et al. 2009. Transgenic mice expressing porcine prion protein resistant to classical scrapie but susceptible to sheep bovine spongiform encephalopathy and atypical scrapie. Emer. Infect. Dis. 15:1214-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster, J. D., M. Bruce, I. McConnell, A. Chree, and H. Fraser. 1996. Detection of BSE infectivity in brain and spleen of experimentally infected sheep. Vet. Rec. 138:546-548. [DOI] [PubMed] [Google Scholar]
- 18.Foster, J. D., J. Hope, and H. Fraser. 1993. Transmission of bovine spongiform encephalopathy to sheep and goats. Vet. Rec. 133:339-341. [DOI] [PubMed] [Google Scholar]
- 19.Foster, J. D., D. W. Parnham, N. Hunter, and M. Bruce. 2001. Distribution of the prion protein in sheep terminally affected with BSE following experimental oral transmission. J. Gen. Virol. 82:2319-2326. [DOI] [PubMed] [Google Scholar]
- 20.Fraser, H., M. E. Bruce, A. Chree, I. McConnell, and G. A. Wells. 1992. Transmission of bovine spongiform encephalopathy and scrapie to mice. J. Gen. Virol. 73:1891-1897. [DOI] [PubMed] [Google Scholar]
- 21.Fraser, H., and A. G. Dickinson. 1967. Distribution of experimentally induced scrapie lesions in the brain. Nature 216:1310-1311. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez, L., et al. 2007. Comparative titration of experimental ovine BSE infectivity in sheep and mice. J. Gen. Virol. 88:714-717. [DOI] [PubMed] [Google Scholar]
- 23.Hill, A. F., et al. 1997. The same prion strain causes vCJD and BSE. Nature 389:448-450. [DOI] [PubMed] [Google Scholar]
- 24.Hope, J., G. Multhaup, L. J. Reekie, R. H. Kimberlin, and K. Beyreuther. 1988. Molecular pathology of scrapie-associated fibril protein (PrP) in mouse brain affected by the ME7 strain of scrapie. Eur. J. Biochem. 172:271-277. [DOI] [PubMed] [Google Scholar]
- 25.Jeffrey, M., et al. 2006. Immunohistochemical features of PrPd accumulation in natural and experimental goat transmissible spongiform encephalopathies. J. Comp. Pathol. 134:171-181. [DOI] [PubMed] [Google Scholar]
- 26.Kong, Q., et al. 2008. Evaluation of the human transmission risk of an atypical bovine spongiform encephalopathy prion strain. J. Virol. 82:3697-3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langeveld, J., et al. 2006. Rapid and discriminatory diagnosis of scrapie and BSE in retro-pharyngeal lymph nodes of sheep. BMC Vet. Res. 2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piccardo, P., et al. 1998. Phenotypic variability of Gerstmann-Straussler-Scheinker disease is associated with prion protein heterogeneity. J. Neuropathol. Exp. Neurol. 57:979-988. [DOI] [PubMed] [Google Scholar]
- 29.van Keulen, L. J. M., M. E. W. Vromans, C. H. Dolstra, A. Bossers, and F. G. van Zijderveld. 2008. Pathogenesis of bovine spongiform encephalopathy in sheep. Arch. Virol. 153:445-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Will, R. G., et al. 1996. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347:921-925. [DOI] [PubMed] [Google Scholar]
- 31.Yull, H. M., et al. 2006. Detection of type 1 prion protein in variant Creutzfeldt-Jakob disease. Am. J. Pathol. 168:151-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.