Abstract
Escherichia coli strains (KJ060 and KJ073) that were previously developed for succinate production have now been modified for malate production. Many unexpected changes were observed during this investigation. The initial strategy of deleting fumarase isoenzymes was ineffective, and succinate continued to accumulate. Surprisingly, a mutation in fumarate reductase alone was sufficient to redirect carbon flow into malate even in the presence of fumarase. Further deletions were needed to inactivate malic enzymes (typically gluconeogenic) and prevent conversion to pyruvate. However, deletion of these genes (sfcA and maeB) resulted in the unexpected accumulation of d-lactate despite the prior deletion of mgsA and ldhA and the absence of apparent lactate dehydrogenase activity. Although the metabolic source of this d-lactate was not identified, lactate accumulation was increased by supplementation with pyruvate and decreased by the deletion of either pyruvate kinase gene (pykA or pykF) to reduce the supply of pyruvate. Many of the gene deletions adversely affected growth and cell yield in minimal medium under anaerobic conditions, and volumetric rates of malate production remained low. The final strain (XZ658) produced 163 mM malate, with a yield of 1.0 mol (mol glucose−1), half of the theoretical maximum. Using a two-stage process (aerobic cell growth and anaerobic malate production), this engineered strain produced 253 mM malate (34 g liter−1) within 72 h, with a higher yield (1.42 mol mol−1) and productivity (0.47 g liter−1 h−1). This malate yield and productivity are equal to or better than those of other known biocatalysts.
The U.S. Department of Energy has identified malic acid and other 1,4-dicarboxylic acids (fumaric and succinic) as building block chemicals that could be made in large quantities from renewable carbohydrates and converted to high-volume products (41). Presently, malic acid usage is limited to pharmaceuticals, cosmetics, and acidulants in the food industry (3, 33). It is produced as a racemic mixture by chemical synthesis (hydration of maleic or fumaric acid) or as enantiomerically pure l-malate by the enzymatic hydration of fumarate (immobilized cells or fumarase) (3, 9, 30). Substrates for the synthesis of malic acid (maleic acid, fumaric acids, maleic anhydride) are derived from petroleum (32). Increases in oil and gas prices coupled with concerns about climate change and global warming have renewed interest in the production of malic acid by microbial fermentation (10).
Malate can be made by a wide range of microorganisms using aerobic or microaerophilic processes (Table 1) (2, 21, 27, 28, 37). Aspergillus flavus is the best-known producer (2). This organism can ferment glucose to malate with a relatively high yield (1.28 mol malate/mol glucose), titer (113 g liter−1), and productivity (0.59 g liter−1 h−1). However, this biocatalyst is not useful in industrial processes because of the potential for aflatoxin production (2, 8). A sugar-tolerant yeast, Zygosaccharomyces rouxii, was recently found to produce 75 g liter−1 malic acid when cultured aerobically in complex medium containing 300 g liter−1 glucose (37). Malate has also been produced by engineered strains of Saccharomyces cerevisiae (29, 44). Overexpression of plasmid-born genes encoding pyruvate carboxylase, cytosolic malate dehydrogenase, and a heterologous malate transporter resulted in the production of 59 g liter−1 malate (44).
TABLE 1.
Comparison of malate production by natural and metabolically engineered microorganisms
| Microorganism | Medium/conditions | Titer (g liter−1) | Yield (mol mol−1) | Productivity (g liter−1 h−1) | Reference |
|---|---|---|---|---|---|
| Natural malate producers | |||||
| A. flavus | Glucose (120 g liter−1) in mineral salts medium, 90 g liter−1 CaCO3, microaerobic, 25°C, pH 7-5 | 113 | 1.26 | 0.59 | 2 |
| Z. rouxii | Glucose (300 g liter−1) with 5 g liter−1 yeast extract, 10 g liter−1 peptone, 5 g liter−1 glutamate; microaerobic, 25°C, pH 5 | 75 | 0.52 | 0.54 | 37 |
| Engineered strains | |||||
| E. coli WGS-10(p104ManPck) | Glucose (20 g liter−1) in mineral salts medium, aerobic batch, 37°C, pH 6.7 | 9.25 | 0.56 | 0.74 | 25 |
| S. cerevisiae | Glucose (188 g liter−1) in mineral salts medium with 150 g liter−1 CaCO3, aerobic flask, 30°C, pH 6 | 59 | 0.42 | 0.19 | 44 |
| E. coli XZ658 | Glucose (50 g liter−1) in mineral salts medium with 100 mM KHCO3, anaerobic batch, 37°C, pH 7 | 22 | 1.0 | 0.15 | This study |
| E. coli XZ658 | Glucose (50 g liter−1) in mineral salts medium with 100 mM KHCO3, two-stage process, 37°C, pH 7 | 34 | 1.42 | 0.47 | This study |
Escherichia coli has proven to be an excellent biocatalyst platform for metabolic engineering. Derivatives of E. coli have been constructed for the production of succinate (6, 16, 22, 24) and other monomers for plastics and rubber (5, 38, 42, 50), renewable fuels (1, 14, 46), antimalarial drug precursors (31), amino acids (26, 45, 48), and other compounds (4, 7, 18). E. coli was previously engineered in our lab for succinate production by increasing the expression of pyruvate carboxykinase, an energy-conserving reaction (16, 17, 47, 49). Malate is an intermediate in this process (Fig. 1) but requires only a single reducing equivalent for synthesis from phosphoenolpyruvate (PEP). A homomalate fermentation could produce up to 2 mol of malate/mol of glucose at redox balance, preserve all glucose carbon, and incorporate two additional molecules of CO2 (greenhouse gas) with a product yield of 149% of that of glucose (weight basis).
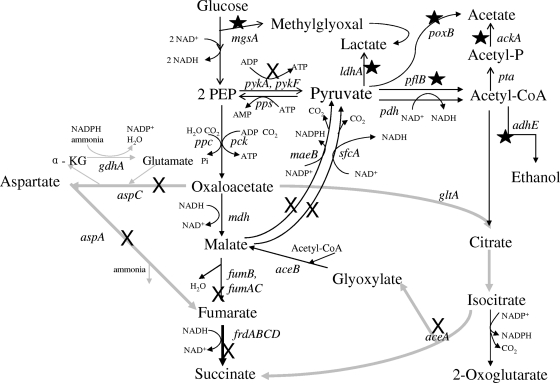
FIG. 1.
Engineering of a pathway for malate production. The central pathway for succinate in K060 and K073 is portrayed, showing the inactivation of fumarase genes predicted to accumulate malate. The stoichiometry of succinate production is shown assuming excess reductant. G6P, glucose-6-phosphate.
In this study, E. coli succinate-producing strains KJ060 (ΔldhA ΔackA ΔadhE ΔpflB) (16) and KJ073 (ΔldhA ΔackA ΔadhE ΔpflB ΔmgsA ΔpoxB) (16) were further engineered for malate production by selective gene deletions. Initial modifications based on current literature (35) and observations of the central pathway (rational design) were surprisingly ineffective. The core mutation required to promote malate accumulation in a succinate-producing strain was the inactivation of fumarate reductase (ΔfrdBC).
MATERIALS AND METHODS
Strains, media, and growth conditions.
The strains used in this study are listed in Table 2. KJ060 and KJ073 were previously engineered for succinate production (16). During strain construction, cultures were grown aerobically at 30°C, 37°C, or 39°C in Luria broth (per liter, 10 g Difco tryptone, 5 g Difco yeast extract, and 5 g NaCl) containing 2% (wt/vol) glucose or 5% (wt/vol) arabinose. Ampicillin (50 mg liter−1), kanamycin (50 mg liter−1), or chloramphenicol (40 mg liter−1) was added as needed.
TABLE 2.
Strains used in this study
| Strain | Relevant characteristics | Reference |
|---|---|---|
| KJ060a | E. coli ATCC 8739 (ΔldhA ΔackA ΔadhE ΔpflB) | 16 |
| XZ273 | KJ060 ΔfumB | This study |
| XZ276 | XZ273 ΔfumAC | This study |
| XZ277 | XZ276, sequential subculture to improve growth | This study |
| XZ278 | XZ277 ΔaspA | This study |
| XZ280 | XZ277 ΔaspC | This study |
| XZ282 | XZ277 ΔaceA | This study |
| XZ372 | KJ060 ΔfrdBC | This study |
| KJ073 | KJ060 ΔmgsA ΔpoxB | 16 |
| XZ316 | KJ073 ΔfrdBC | This study |
| XZ347 | XZ316 ΔsfcA | This study |
| XZ654 | XZ347 ΔmaeB | This study |
| XZ656 | XZ654 ΔfumB | This study |
| XZ658 | XZ656 ΔfumAC | This study |
| XW009 | XZ658 ΔpykF | This study |
| XW036 | XZ658 ΔpykA | This study |
| XW051 | XZ658 Δpck | This study |
Genetic methods.
Chromosomal genes were deleted seamlessly without leaving segments of foreign DNA as described previously (17, 48). Red recombinase technology (Gene Bridges GmbH, Dresden, Germany) was used to facilitate chromosomal integration. For the plasmids and primers used during construction, see Table S1 in the supplemental material.
Enzyme assays.
Cells were grown in pH-controlled fermentors and harvested (70% of the maximal cell density) by centrifugation (7,000 × g for 5 min, 4°C) for the determination of fumarase activity. Cells were washed twice in 50 mM sodium phosphate buffer (pH 7.0) and disrupted using a Fastprep-24 (MP Biomedicals, Solon, OH) in the presence of 1 mM dithiothreitol (DTT). After clarification at 13,000 × g (10 min, 4°C), the protein concentration was determined by the bicinchoninic acid method (Pierce Research Products, Rockford, IL) using bovine serum albumin as the standard. Fumarase activity was determined by measuring the conversion of malate to fumarate (the extinction coefficient of fumarate at 240 nm is 2,530 M−1 cm−1) (38). The reaction mixture contained 100 mM sodium phosphate buffer (pH 7.0), 50 mM malate, and 1 mM DTT. One unit of activity is the amount of protein required to produce 1 μmol of fumarate/min. Note that DTT was essential to preserve activity with the Fastprep-24 bead disruptor.
Fumarate reductase activity was measured by coupling with benzyl viologen as described by Lemire and Weiner (23). Both fumarate-dependent and malate-dependent activities were investigated. Argon was used to minimize exposure to oxygen.
l-Specific and d-specific lactate dehydrogenase enzymes (Sigma Scientific, St. Louis, MO) were used to determine the chirality of the lactate produced during fermentation. Reaction mixtures contained 200 mM Tricine buffer (pH 9.0), 5.5 mM NAD+, and 1 U of commercial enzyme. Activity was measured by monitoring the formation of NADH at 340 nm (13). The lactate dehydrogenase activities from different strains were determined by measuring the conversion of pyruvate to lactate by oxidizing NADH (extinction coefficient of 6,220 M−1 cm−1) (13). Reaction mixtures contained 200 mM phosphate buffer (pH 7.0), 0.2 mM NADH, and 1 mM pyruvate (13). One unit of activity is the amount of protein required to convert 1 μmol of pyruvate to lactate/min.
Fermentation.
Strains were grown without antibiotics at 37°C in NBS (New Brunswick Scientific) mineral salts medium (4) supplemented with 2% or 5% (wt/vol) glucose and 100 mM potassium bicarbonate unless stated otherwise. Supplementation of a small amount of acetate at the beginning of fermentation was helpful for the growth of early succinate strains even though additional acetate was produced during fermentation (16). Acetate (10 mM) was also included to improve growth for malate strains. Preinocula for fermentations were grown by transferring fresh colonies into a 250-ml flask (100 ml NBS medium, 2% glucose). After 16 h (37°C, 120 rpm), this culture was diluted into a small 500-ml fermentation vessel containing 300 ml NBS medium (5% glucose, 100 mM potassium bicarbonate) to provide an inoculum of 0.033 g (cell dry weight [CDW]) liter−1. For a microaerobic process, fermentation was carried out with a 3-liter bioreactor (BioFlo 110; New Brunswick Scientific, Edison, NJ) containing 1.5 liters NBS medium (5% glucose, 100 mM potassium bicarbonate). The inoculum was 0.017 g (CDW) liter−1 with low aeration (0.1 vol/vol/min [vvm]) to provide microaerobic conditions.
A two-stage process was also investigated using a 3-liter bioreactor (BioFlo 110) containing 1.2 liters NBS medium (5% glucose, 100 mM potassium bicarbonate; inoculum of 0.017 g [CDW] liter−1). An airflow of 1.0 vvm was used for the initial aerobic growth. After 16 h (cell mass of 2.5 g liter−1), the airflow was stopped and incubation was continued for anaerobic malate production. All fermentations were maintained at pH 7.0 by the automatic addition of base containing additional CO2 (base for neutralization: 2.4 M potassium carbonate and 1.2 M potassium hydroxide).
Analysis.
CDW was estimated by measuring optical density at 550 nm. Organic acids and glucose were measured by high-performance liquid chromatography (HPLC) (48).
RESULTS
Inactivation of fumarase for malate production.
E. coli KJ060 and derivatives were previously engineered in our lab for the production of succinate (16). In these engineered strains, the phosphotransferase system is inactive and phosphoenolpyruvate (PEP) is carboxylated to oxaloacetate (OAA) by PEP carboxykinase (pck), conserving energy as ATP. OAA is reduced by malate dehydrogenase (mdh), converted to fumarate by fumarase (fumB and fumAC), and reduced to succinate by fumarate reductase (frdABCD) (Fig. 1). Based on this central pathway, elimination of fumarase activity would be expected to cause the accumulation of malate.
E. coli contains three fumarase isoenzymes encoded by fumB, fumA, and fumC (20, 39). Fumarase C is the dominant enzyme during aerobic growth and oxidative metabolism but has low activity during anaerobic growth. Fumarase A is the dominant isoenzyme under microaerobic conditions (1 to 2% oxygen) and is also synthesized under anaerobic conditions (39). Fumarase B is induced under anaerobic conditions, where it serves as the dominant isoenzyme during fermentation. Based on inspection of this pathway (Fig. 1) and published literature (35), inactivation of the fumB-encoded isoenzyme would be expected to block the fermentative production of fumarate and cause accumulation of malate as the primary reduced product. However, accumulation of malate did not occur after the deletion of fumB in KJ060 (strain XZ273) and succinate remained the dominant product (Table 3). Succinate yield and cell yield were both reduced in XZ273 by over 60%, consistent with fumarase B serving as the dominant isoenzyme during fermentation (39). Deletion of the genes encoding all three isoenzymes (strain XZ276) reduced succinate production by 97% and cell yield by 75% compared to those of KJ060, with the accumulation of a small amount of malate (27 mM) after 6 days. With further anaerobic incubation, this malate was converted to succinate despite the inactivation of the three known fumarase genes.
TABLE 3.
Effects of gene deletions on succinate production
| Strain | Genetic modification(s) | Time (days) | Cell mass (g liter−1) | Fermentation product concn (mM)b |
||||
|---|---|---|---|---|---|---|---|---|
| Mal | Suc | Pyr | Ace | For | ||||
| KJ060a | ATCC 8739 ΔldhA ΔackA ΔadhE ΔpflB | 2 | 1.9 | 127 | 31 | 2 | ||
| XZ273 | KJ060 ΔfumB | 4 | 0.6 | 47 | 10 | |||
| XZ276 | KJ060 ΔfumB ΔfumAC | 6 | 0.47 | 27 | 4 | 4 | 15 | |
| XZ276 | KJ060 ΔfumB ΔfumAC | 9 | 1.1 | 56 | 45 | |||
| XZ277c | KJ060 ΔfumB ΔfumAC | 2 | 1.07 | 1 | 71 | 35 | ||
| XZ278 | XZ277 ΔaspA | 2 | 1.37 | 2 | 82 | 43 | ||
| XZ280 | XZ277 ΔaspC | 2 | 1.17 | 1 | 78 | 31 | ||
| XZ282 | XZ277 ΔaceA | 2 | 1.43 | 1 | 97 | 46 | ||
Strain KJ060 and derivatives also contain spontaneous mutations in pck and ptsI and affecting galP that were acquired during selection for improvements in growth (16, 17, 47, 49).
Fermentations were carried out in NBS mineral salts medium with 2% glucose and 100 mM potassium bicarbonate (37°C, pH 7.0, 150 rpm). Anaerobiosis was achieved during growth with added bicarbonate to ensure an atmosphere of CO2. The acetate concentration in the medium was measured at the end of fermentation. Abbreviations: Mal, malate; Suc, succinate; Pyr, pyruvate; Ace, acetate; For, formate.
Sequential subculture (improvement in growth).
Strain XZ276 was subcultured at 24-h intervals for 2 weeks, during which time growth improved substantially. A clone was isolated and designated XZ277. Strain XZ277 lacking all three fumarase isoenzymes produced 71 mM succinate after 2 days and only 1 mM malate (Table 3).
Succinate accumulation in strain XZ277 (ΔfumAC ΔfumB).
Five mechanisms were envisioned that could be responsible for succinate accumulation in fumarase-deficient strains (Fig. 2). Aspartate metabolism could be activated. A combination of aspartate transaminase (aspC) and aspartate ammonia-lyase (aspA) could serve as a bypass route to malate (and succinate) without producing fumarate. The glyoxylate bypass could be activated by the deletion of fumarase genes and convert OAA and acetyl coenzyme A into malate and succinate by using citrate synthase (gltA), aconitate hydratase (acnA and acnB), isocitrate lyase (aceA), and malate synthase (aceB). E. coli could have a cryptic gene encoding fumarase activity. E. coli could use a more complex (unexplored) pathway to produce succinate. Alternatively, small amounts of malate could be spontaneously dehydrated to fumarate (ΔG = +1.3 kcal mol−1) (12) and reduced to succinate to provide an energetically favorable process.
FIG. 2.
Pathways concerned with malate metabolism and succinate production. The native fermentation pathway produces malate as an intermediate between OAA and fumarate. Fumarate is subsequently reduced to succinate. Gray arrows represent alternative routes to succinate which do not involve malate. Succinate can be produced from OAA through either an aspartate bypass (aspartate aminotransferase and aspartase) or by using the glyoxylate bypass (citrate synthase, aconitate hydratase, and isocitrate lyase). Although two genes that encode enzymes for PEP carboxylation to OAA are shown, pck was found to be responsible for 97% of this reaction. Intermediate steps in glycolysis have been omitted for clarity. Original gene deletions in the succinate-producing parent (KJ073) are marked by stars. Sites of new deletions described in this paper are also marked (×). Abbreviations: Acetyl-CoA, acetyl-coenzyme A; Acetyl-P, acetylphosphate; α-KG, α-ketoglutarate.
Potential conversion of malate to fumarate by aspartic acid metabolism (aspartate bypass) or by the glyoxylate bypass was investigated by deleting key genes. Strains XZ278, XZ280, and XZ282 were constructed by deleting aspA, aspC, and aceA in XZ277, respectively. After 2 days of fermentation, all of these strains produced primarily succinate and very little malate (Table 3), reducing the likelihood that activation of either pathway contributes to succinate accumulation.
Fumarase activities were measured in cell lysates of strains KJ060, XZ273 (KJ060 with a fumB deletion), and XZ277 (KJ060 containing deletions in fumAC and fumB). Under substrate-saturating conditions, the fumarase activity of XZ273 (0.13 ± 0.03 U mg−1) was about 20% of the fumarase activity of KJ060 (0.60 ± 0.03 U mg−1). Little activity was detected in XZ277 (0.00015 ± 0.00002 U mg−1), in which all three fumarase genes were deleted. Similar low levels were also observed for boiled lysates of KJ273 (<0.0001 U mg−1) and bovine serum albumin, consistent with the absence of activity from a cryptic fumarase gene.
Although the net rate of spontaneous dehydration was essentially below the limit of detection, the possible production of succinate in a fumarase-negative strain by coupling with the energetically favorable reduction of fumarate was investigated. No malate-dependent fumarate reductase activity could be detected in cell lysates of strain XZ277 (ΔfumAC ΔfumB), however, making such coupling unlikely.
Deletion of fumarate reductase promoted malate and pyruvate accumulation.
An improved-succinate strain (KJ073) became available during the course of this study (16). This strain is a derivative of KJ060 that contains additional mutations in methylglyoxal synthase (ΔmgsA) and pyruvate oxidase (ΔpoxB). Deletion of fumarate reductase in KJ060 and KJ073 (XZ372 and XZ316, respectively) did not cause accumulation of fumarate. However, this mutation eliminated over 90% of the strain's succinate production and promoted the accumulation of malate (Table 4), an earlier intermediate in the pathway. Deletion of fumarate reductase was also accompanied by an increase in pyruvate and a decline in acetate. There are many routes that could lead to an increase in pyruvate. The decrease in acetate production by XZ372 and XZ316 can be attributed, in part, to the decrease in glucose metabolism and lower cell mass.
TABLE 4.
Effects of gene deletions on malate production
| Strain | Genetic modification(s) | Time (days) | Cell mass (g/liter) | Glucose concn used (mM) | Mal yieldb | Fermentation product concn (mM)f |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mal | Fum | Suc | Pyr | Lac | Ace | ||||||
| KJ060a,c | ATCC 8739 ΔldhA ΔackA ΔadhE ΔpflB | 2 | 1.9 | 277 | 0 | 291 | 2 | 125 | |||
| XZ372 | KJ060 ΔfrdBC | 6 | 0.5 | 72 | 0.53 ± 0.08 | 38 ± 6 | 1 | 9 | 16 | 40 | |
| KJ073c | KJ060 ΔmgsA ΔpoxB | 2 | 2.2 | 277 | 0 | 339 | 6 | 115 | |||
| XZ316c | KJ073 ΔfrdBC | 6 | 0.44 | 55 | 0.44 ± 0.06 | 24 ± 3 | 1 | 5 | 74 | 5 | |
| XZ347c | KJ073 ΔfrdBC ΔsfcA | 6 | 0.53 | 99 | 0.71 ± 0.10 | 70 ± 11 | 1 | 9 | 51 | 15 | |
| XZ654c | KJ073 ΔfrdBC ΔsfcA ΔmaeB | 6 | 0.33 | 45 | 0.89 ± 0.11 | 40 ± 5 | 1 | 4 | 3 | 2 | 10 |
| XZ658c | KJ073 ΔfrdBC ΔsfcA ΔmaeB ΔfumB ΔfumAC | 6 | 0.75 | 163 | 1.0 ± 0.13 | 163 ± 22 | 1 | 4 | 13 | 78 | 6 |
| XZ658d | KJ073 ΔfrdBC ΔsfcA ΔmaeB ΔfumB ΔfumAC | 6 | 0.85 | 216 | 0.91 ± 0.06 | 197 ± 13 | 14 | 50 | 156 | 15 | |
| XW009c | KJ073 ΔfrdBC ΔsfcA ΔmaeB ΔfumB ΔfumAC ΔpykF | 6 | 0.46 | 85 | 1.3 ± 0.07 | 111 ± 15 | 2 | 21 | 5 | 11 | |
| XW036c | KJ073 ΔfrdBC ΔsfcA ΔmaeB ΔfumB ΔfumAC ΔpykA | 6 | 0.42 | 74 | 1.1 ± 0.04 | 84 ± 7 | 3 | 33 | 5 | 11 | |
| XW051 | KJ073 ΔfrdBC ΔsfcA ΔmaeB ΔfumB ΔfumAC Δpck | 6 | 0.26 | 25 | 0.3 ± 0.1 | 6.4 ± 1 | 0.5 | 4 | 14 | ||
| XZ658e | KJ073 ΔfrdBC ΔsfcA ΔmaeB ΔfumB ΔfumAC | 3 | 2.5 | 182 | 1.42 | 253 | 10 | 12 | 8 | ||
Strain KJ060 and derivatives also contain spontaneous mutations in pck and ptsI and affecting galP that were acquired during selection for improvements in growth (16, 17, 47, 49).
Yield was calculated as moles of malate produced per mole of glucose consumed.
Fermentations were carried out in a 500-ml flask with 300 ml NBS mineral salts medium with 5% glucose, 10 mM acetate, and 100 mM potassium bicarbonate (37°C, pH 7.0, 150 rpm). Anaerobiosis was achieved during growth with added bicarbonate to ensure an atmosphere of CO2. The acetate concentration in the medium was measured at the end of fermentation.
The fermentation medium was supplemented with 57 mM pyruvate.
XZ658 was tested with a two-stage process (aerobic cell growth and anaerobic malate production).
Abbreviations: Mal, malate; Fum, fumarate; Suc, succinate; Pyr, pyruvate; Lac, d-lactate; Ace, acetate.
Our results demonstrate that deletion of fumarate reductase genes results in an accumulation of malate, consistent with the idea that fumarate is an intermediate in succinate production even in the absence of fumarase genes (Tables 3 and 4). The metabolic source of this malate remains unknown. Sugar metabolism, cell yield, and succinate production were higher in KJ073 than in KJ060 (Table 4), and this strain was used in further studies to engineer improvements in malate production.
Pyruvate accumulation in XZ316 attributed to malic enzymes (encoded by scfA and maeB).
Conversion of malate to pyruvate is a thermodynamically favorable reaction (12). Although genes encoding malic enzymes (gluconeogenic) have been shown to be repressed by glucose during oxidative metabolism (19, 36), these enzymes represent potential routes to pyruvate (Fig. 2). There are two malic enzymes in E. coli, NAD+-dependent SfcA and NADP+-dependent MaeB. The genes for both were sequentially deleted. Deletion of sfcA to produce strain XZ347 increased the cell yield by 20% and increased malate production to 70 mM, 3-fold that of the parent XZ316. Subsequent deletion of the NADPH-linked malic enzyme (maeB) to produce XZ654 further increased the malate yield but decreased the malate titer, glucose metabolism, and cell yield. With the deletion of both genes (XZ654), pyruvate production was substantially eliminated (3 mM), establishing SfcA and MaeB as the primary sources of pyruvate. Thus, both malic enzymes are able to participate in glucose fermentation, in addition to their role in gluconeogenesis during oxidative metabolism. During fermentation, a futile cycle composed of Pck, Mdh, MaeB, and PEP synthetase (pps) could effectively convert NADH to NADPH for biosynthesis at the cost of a single ATP equivalent (Fig. 2).
Deletion of fumarase isoenzymes in a fumarate reductase mutant.
Malate accumulated as a primary fermentation product only after the deletion of fumarate reductase (Tables 3 and 4). Since small amounts of malate were produced by fumarase-deficient strain XZ276 after fermentation for 6 days, deletion of fumarase genes (fumB and fumAC) in XZ654 to produce XZ658 was expected to be of minor benefit. However, deletion of these fumarase genes (strain XZ658) more than doubled the cell yield (127% increase) and increased the malate titer by 4-fold (Table 4). The higher cell yield and malate titer were accompanied by an increase in lactate production during fermentation. However, lactate levels were reduced by 90% when aerobically grown cells were used in a two-step fermentation process (Table 4).
Lactate accumulation reduced by pyruvate kinase deletions.
Deletion of genes encoding the three fumarase isoenzymes (XZ658) caused a large and unexpected increase in lactate (78 mM; Table 4) despite the absence of lactate dehydrogenase (ΔldhA) and methylglyoxal synthase (ΔmgsA). Testing with chiral-specific lactate dehydrogenases indicated that only the d-lactate enantiomer was present. No lactate dehydrogenase activity could be detected in disrupted cells of XZ658, and the pathway leading to this d-lactate in E. coli remains unknown. However, we observed that the addition of pyruvate (57 mM) to the fermentation medium increased the accumulation of d-lactate (and malate), cell yield, and the lactate/malate ratio (Table 4). The effects of added pyruvate are complex and may be quite indirect due to its central role in metabolism. However, this suggested an approach to lactate reduction.
Deletion of pykA or pykF was tested as a means of reducing the supply of pyruvate from PEP (Table 4). Deletion of either isoenzyme of pyruvate kinase reduced lactate production by over 90%. Cell yield and succinate production were also decreased by the deletion of either pykA or pykF. Together, these results indicate that both isoenzymes of pyruvate kinase function during glucose fermentation and that lactate production is related in part to an excess supply of pyruvate.
Bacteria such as Lactobacillus delbrueckii contain enzymes capable of converting malate to l(+)-lactate (43). Genes encoding these enzymes were used to search the sequence of E. coli C for homologues. Two related genes were found, sfcA and maeB, and both encode malic enzymes. Deletion of these genes individually or in combination did not eliminate lactate production (Table 4). Direct testing for the production of lactate from malate by a cell lysate of E. coli XZ658 was also unsuccessful (detection by HPLC). The pathway for lactate production in XZ658 remains unknown.
Improving the production of malate.
Although malate was the dominant fermentation product of XZ658, cell growth (0.75 g/liter) and malate productivity (0.15 g liter−1 h−1) were low in comparison to those achieved with our previous biocatalysts for succinate (16, 17, 49) and lactate (11). A spontaneous mutation leading to increased production of pyruvate carboxykinase was a key event for development of the succinate production strains (47, 49). The importance of pck for malate production was also confirmed. Deletion of pyruvate carboxykinase in XZ658 reduced the titer of malate from 163 mM to 6 mM, a 96% reduction (Table 4).
Microaerobic (4, 5) and two-stage processes (aerobic cell growth followed by anaerobic fermentation) have been used previously to improve succinate production (34, 40). Both approaches were investigated to improve malate production. After 6 days under microaerobic conditions (0.1 vvm air), malate production (120 mM) and malate yield (0.49 mol/mol glucose) were even poorer than during anaerobic fermentations (Table 4). A two-stage process proved more effective for malate production. Cells were grown aerobically (1.0 vvm air) for 16 h (2.5 g [CDW] liter−1) and then shifted to anaerobic conditions for malate production (72 h). With this approach, 253 mM malate was produced with a yield of 1.42 mol/mol glucose. Under these conditions, very little lactate was produced. Productivity of malate during the anaerobic phase averaged 0.47 g liter−1 h−1.
DISCUSSION
None of the natural malate-producing microorganisms appears suitable for large-scale commercial production due to either toxin production (aflatoxin [A. flavus]) or dependence on complex medium and low yields (Z. rouxii) (2, 37) (Table 1). S. cerevisiae and E. coli are excellent platforms for biologically based chemicals, and both have been investigated as biocatalysts for malate production (25, 44). S. cerevisiae was engineered for aerobic malate production by overexpressing pyruvate carboxylase, cytosolic malate dehydrogenase, and a malate transporter from Schizosaccharomyces pombe. This strain produced 59 g liter−1 malate in flask cultures after 192 h (44). However, the malate yield (0.42 mol mol−1) and productivity (0.29 g liter−1 h−1) were low. E. coli was previously engineered for aerobic malate production by overexpressing “Mannheimia succiniciproducens” PEP carboxykinase and inactivating acetate production (25). Although the productivity was high (0.74 g liter−1h−1), the final titer and yield were low (9.25 g liter−1 and 0.56 mol mol−1, respectively). Our engineering strategy provides some new insights into the improvement of microbial malate production.
Several metabolically engineered E. coli strains were previously constructed for the efficient production of succinate from glucose under anaerobic conditions (16, 17). Two key genetic changes were subsequently identified (47, 49). The native gluconeogenic PEP carboxykinase was recruited as the primary carboxylation reaction by mutational activation, conserving energy as additional ATP. The native glucose PEP-dependent phosphotransferase system was inactivated and replaced with the native GalP permease and glucokinase, increasing the availability of PEP for carboxylation to OAA. These changes are presumed to be important for the efficient production of malate, an upstream intermediate in the fermentative succinate pathway. We have confirmed that PEP carboxykinase is needed for malate production. Deletion of pck in XZ658 led to dramatic decreases in cell growth and malate production (Table 4). Our best strain, XZ658, produced malate as the major fermentation product with a yield of 1.0 mol/mol glucose during batch fermentation under anaerobic conditions. This yield was increased to 1.42 mol mol−1 using a two-stage process (aerobic growth followed by anaerobic fermentation), a higher yield than previously reported (Table 1). Malate productivity with XZ658 was 0.47 g liter−1 h−1, too low for most commercial uses but comparable to that of the best natural malate-producing microorganisms (Table 1). The titer of malate with the succinate-producing parental strain (KJ073) was less than 0.5 mM. Through metabolic engineering and the use of a two-stage process, this was increased by over 500-fold (XZ658). Since homomalate production is a redox-balanced pathway, there is still room for further titer and yield improvement.
The fermentation products from malate-producing strains were generally more oxidized than expected and not in redox balance. This was particularly true for cultures with very limited glucose metabolism, consistent with the scavenging of small amounts of oxygen in the fermentor. Small unidentified peaks (refractive index detector) were present in HPLC profiles that eluted between lactate and ethanol. These could be reduced products that contribute to redox balance. The redox balance was much closer for strain XZ658, a strain that metabolized most of the available sugar.
Initial rational designs of pathway modifications were surprisingly ineffective for a malate biocatalyst. Gene deletions often produced unexpected results. Deletion of the three fumarase genes did not cause the accumulation substrate (malate) but instead led to the accumulation of succinate as the primary product. Similarly, deletion of the fumarate reductase genes did not cause accumulation of the substrate (fumarate) but instead caused the accumulation of malate. The primary basis of both results is not understood. The thermodynamic equilibrium favors the hydration of fumarate to malate (ΔG = −1.3 kcal mol−1) (12), although this reaction is reversible under physiological conditions when fumarase is present. Fumarate appears to be the immediate precursor for succinate production in a fumarase-negative background. The metabolic source of this fumarate is unknown. Malate accumulated only in mutants lacking fumarate reductase, with or without a functional fumarase.
Strains that accumulated malate or succinate in fermentation broth at relatively high concentrations were readily developed. Both of these dicarboxylic acids can be actively transported by E. coli, and each can be used efficiently as a sole carbon source under oxidative conditions (15). The ability to utilize these diacids as sole carbon sources when alternative electron acceptors become available may have been an evolutionary advantage.
Supplementary Material
Acknowledgments
This research was supported by grants from the U.S. Department of Energy (DE-FG36-08-GO88142) and Myriant Technologies, LLC.
Footnotes
Published ahead of print on 19 November 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Atsumi, S., T. Hanai, and J. C. Liao. 2008. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451:86-89. [DOI] [PubMed] [Google Scholar]
- 2.Battat, E., Y. Peleg, A. Bercovitz, J. S. Rokem, and I. Goldberg. 1991. Optimization of l-malic acid production by Aspergillus flavus in a stirred fermentor. Biotechnol. Bioeng. 37:1108-1116. [DOI] [PubMed] [Google Scholar]
- 3.Bressler, E., O. Pines, I. Goldberg, and S. Braun. 2002. Conversion of fumaric acid to l-malic by sol-gel immobilized Saccharomyces cerevisiae in a supported liquid membrane bioreactor. Biotechnol. Prog. 18:445-450. [DOI] [PubMed] [Google Scholar]
- 4.Causey, T. B., K. T. Shanmugam, L. P. Yomano, and L. O. Ingram. 2004. Engineering Escherichia coli for efficient conversion of glucose to pyruvate. Proc. Natl. Acad. Sci. U. S. A. 101:2235-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Causey, T. B., S. Zhou, K. T. Shanmugam, and L. O. Ingram. 2003. Engineering the metabolism of Escherichia coli W3110 for the conversion of sugar to redox-neutral and oxidized products: homoacetate production. Proc. Natl. Acad. Sci. U. S. A. 100:825-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee, R., C. S. Millard, K. Chamion, D. P. Clark, and M. I. Donnelly. 2001. Mutation of the ptsG gene results in increased succinate in fermentation of glucose by Escherichia coli. Appl. Environ. Microbiol. 67:148-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cirino, P. C., J. W. Chin, and L. O. Ingram. 2006. Engineering Escherichia coli for xylitol production from glucose-xylose mixtures. Biotechnol. Bioeng. 95:1167-1176. [DOI] [PubMed] [Google Scholar]
- 8.Geiser, D. M., J. I. Pitt, and J. W. Taylor. 1998. Cryptic speciation and recombination in the aflatoxin-producing fungus Aspergillus flavus. Proc. Natl. Acad. Sci. U. S. A. 95:388-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giorno, L., E. Drioli, G. Carvoli, A. Cassano, and L. Donato. 2001. Study of an enzyme membrane reactor with immobilized fumarase for production of l-malic acid. Biotechnol. Bioeng. 72:77-84. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg, I., J. S. Rokem, and O. Pines. 2006. Organic acids: old metabolites, new themes. J. Chem. Technol. Biotechnol. 81:1601-1611. [Google Scholar]
- 11.Grabar, T. B., S. Zhou, K. T. Shanmugam, L. P. Yomano, and L. O. Ingram. 2006. Methylglyoxal bypass identified as source of chiral contamination in l(+) and d(−)-lactate fermentations by recombinant Escherichia coli. Biotechnol. Lett. 28:1527-1535. [DOI] [PubMed] [Google Scholar]
- 12.Henry, C. S., M. D. Jankowski, L. J. Broadbelt, and V. Hatzimanikatis. 2006. Genome-scale thermodynamic analysis of Escherichia coli metabolism. Biophys. J. 90:1453-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howell, B. F., S. McCune, and R. Schaffer. 1979. Lactate-to-pyruvate or pyruvate-to-lactate assay for lactate dehydrogenase: a re-examination. Clin. Chem. 25:269-272. [PubMed] [Google Scholar]
- 14.Ingram, L. O., T. Conway, D. P. Clark, G. P. Sewell, and J. F. Preston. 1987. Genetic engineering of ethanol production in Escherichia coli. Appl. Environ. Microbiol. 53:2420-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janausch, I. G., E. Zientz, Q. H. Tran, A. Kroger, and G. Unden. 2002. C4-dicarboxylate carriers and sensors in bacteria. Biochim. Biophys. Acta 1553:39-56. [DOI] [PubMed] [Google Scholar]
- 16.Jantama, K., et al. 2008. Combining metabolic engineering and metabolic evolution to develop nonrecombinant strains of Escherichia coli C that produce succinate and malate. Biotechnol. Bioeng. 99:1140-1153. [DOI] [PubMed] [Google Scholar]
- 17.Jantama, K., et al. 2008. Eliminating side products and increasing succinate yields in engineered strains of Escherichia coli C. Biotechnol. Bioeng. 101:881-893. [DOI] [PubMed] [Google Scholar]
- 18.Jin, Y. S., and G. Stephanopoulos. 2007. Multi-dimensional gene target search for improving lycopene biosynthesis in Escherichia coli. Metab. Eng. 9:337-347. [DOI] [PubMed] [Google Scholar]
- 19.Kao, K. C., L. M. Tran, and J. C. Liao. 2005. A global regulatory role of gluconeogenic genes in Escherichia coli revealed by transcriptome network analysis. J. Biol. Chem. 280:36079-36087. [DOI] [PubMed] [Google Scholar]
- 20.Karp, P. D., et al. 2007. Multidimensional annotation of the Escherichia coli K-12 genome. Nucleic Acids Res. 35:7577-7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawagoe, M., K. Hyakumura, S. I. Suye, K. Miki, and K. Naoe. 1997. Application of bubble column fermenters to submerged culture of Schizophyllum commune for production of l-malic acid. J. Ferment. Bioeng. 84:333-336. [Google Scholar]
- 22.Kim, P., M. Laivenieks, C. Vieille, and J. G. Zeikus. 2004. Effect of overexpression of Actinobacillus succinogenes phosphoenolpyruvate carboxykinase on succinate production in Escherichia coli. Appl. Environ. Microbiol. 70:1238-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemire, B. D., and J. H. Weiner. 1986. Fumarate reductase of Escherichia coli. Methods Enzymol. 126:377-386. [DOI] [PubMed] [Google Scholar]
- 24.Lin, H., G. N. Bennett, and K.-Y. San. 2005. Metabolic engineering of aerobic succinate production systems in Escherichia coli to improve process productivity and achieve the maximum theoretical succinate yield. Metab. Eng. 7:116-127. [DOI] [PubMed] [Google Scholar]
- 25.Moon, S. Y., S. H. Hong, T. Y. Kim, and S. Y. Lee. 2008. Metabolic engineering of Escherichia coli for the production of malic acid. Biochem. Eng. J. 40:312-320. [Google Scholar]
- 26.Park, J. H., K. H. Lee, T. Y. Kim, and S. Y. Lee. 2007. Metabolic engineering of Escherichia coli for the production of l-valine based on transcriptome analysis and in silico gene knockout simulation. Proc. Natl. Acad. Sci. U. S. A. 104:7797-7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peleg, Y., J. S. Rokem, and I. Goldberg. 1990. A simple plate-assay for the screening of l-malic acid producing microorganisms. FEMS Microbiol. Lett. 55:233-236. [DOI] [PubMed] [Google Scholar]
- 28.Pines, O., et al. 1996. The cytosolic pathway of l-malic acid synthesis in Saccharomyces cerevisiae: the role of fumarase. Appl. Microbiol. Biotechnol. 46:393-399. [DOI] [PubMed] [Google Scholar]
- 29.Pines, O., S. Shemesh, E. Battat, and I. Goldberg. 1997. Overexpression of cytosolic malate dehydrogenase (MDH2) causes overproduction of specific organic acids in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 48:248-255. [DOI] [PubMed] [Google Scholar]
- 30.Presecki, A. V., and D. Vasic-Racki. 2005. Production of l-malic acid by permeabilized cells of commercial Saccharomyces sp. strains. Biotechnol. Lett. 27:1835-1839. [DOI] [PubMed] [Google Scholar]
- 31.Ro, D. K., et al. 2006. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440:940-943. [DOI] [PubMed] [Google Scholar]
- 32.Roa Engel, C. A., A. J. Straathof, T. W. Zijlmans, W. M. van Gulik, and L. A. van der Wielen. 2008. Fumaric acid production by fermentation. Appl. Microbiol. Biotechnol. 78:379-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg, M., H. Mikova, and L. Kristofikova. 1999. Formation of l-malic acid by yeasts of the genus Dipodascus. Lett. Appl. Microbiol. 29:221-223. [DOI] [PubMed] [Google Scholar]
- 34.Sánchez, A. M., G. N. Bennett, and K. Y. San. 2005. Efficient succinic acid production from glucose through overexpression of pyruvate carboxylase in an Escherichia coli alcohol dehydrogenase and lactate dehydrogenase mutant. Biotechnol. Prog. 21:358-365. [DOI] [PubMed] [Google Scholar]
- 35.Sawers, R. G., and D. P. Clark. July 2004, posting date. Chapter 3.5.3. Fermentative pyruvate and acetyl-coenzyme A metabolism. In R. Curtiss III et al. (ed.), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC. http://www.ecosal.org/. [DOI] [PubMed]
- 36.Stols, L., and M. I. Donnelly. 1997. Production of succinic acid through overexpression of NAD(+)-dependent malic enzyme in an Escherichia coli mutant. Appl. Environ. Microbiol. 63:2695-26701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taing, O., and K. Taing. 2007. Production of malic and succinic acids by sugar-tolerant yeast Zygosaccharomyces rouxii. Eur. Food Res. Technol. 224:343-347. [Google Scholar]
- 38.Tong, I. T., H. H. Liao, and D. C. Cameron. 1991. 1,3-Propanediol production by Escherichia coli expressing genes from the Klebsiella pneumoniae dha regulon. Appl. Environ. Microbiol. 57:3541-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tseng, C. P., C. C. Yu, H. H. Lin, C. Y. Chang, and J. T. Kuo. 2001. Oxygen- and growth rate-dependent regulation of Escherichia coli fumarase (FumA, FumB, and FumC) activity. J. Bacteriol. 183:461-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vemuri, G. N., M. A. Eiteman, and E. Altman. 2002. Succinate production in dual-phase Escherichia coli fermentations depends on the time of transition from aerobic to anaerobic conditions. J. Ind. Microbiol. Biotechnol. 28:325-332. [DOI] [PubMed] [Google Scholar]
- 41.Werpy, T., and G. Petersen. 2004. Top value added chemicals from biomass. U.S. Department of Energy, Washington, DC. http://www1.eere.energy.gov/biomass/pdfs/35523.pdf.
- 42.Whited, G. W., et al. 2010. Development of a gas-phase bioprocess for isoprene-monomer production using metabolic pathway engineering. Ind. Biotechnol. 6:152-163. [Google Scholar]
- 43.Williams, S. A., R. A. Hodges, T. L. Strike, R. Snow, and R. E. Kunkee. 1984. Cloning the gene for the malolactic fermentation of wine from Lactobacillus delbrueckii in Escherichia coli and yeasts. Appl. Environ. Microbiol. 47:288-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zelle, R. M., et al. 2008. Malic acid production by Saccharomyces cerevisiae: engineering of pyruvate carboxylation, oxaloacetate reduction, and malate export. Appl. Environ. Microbiol. 74:2766-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, K., L. Han, K. M. Cho, and J. C. Liao. 2010. Expanding metabolism for total biosynthesis of the nonnatural amino acid l-homoalanine. Proc. Natl. Acad. Sci. U. S. A. 107:6234-6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, K., M. R. Sawaya, D. S. Eisenberg, and J. C. Liao. 2008. Expanding metabolism for biosynthesis of nonnatural alcohols. Proc. Natl. Acad. Sci. U. S. A. 105:20653-20658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang, X., et al. 2009. Metabolic evolution of energy-conserving pathways for succinate production in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 106:20180-20185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, X., K. Jantama, J. C. Moore, K. T. Shanmugam, and L. O. Ingram. 2007. Production of L-alanine by metabolically engineered Escherichia coli. Appl. Microbiol. Biotechnol. 77:355-366. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, X., K. Jantama, K. T. Shanmugam, and L. O. Ingram. 2009. Reengineering Escherichia coli for succinate production in mineral salts medium. Appl. Environ. Microbiol. 75:7807-7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou, S., T. B. Causey, A. Hasona, K. T. Shanmugam, and L. O. Ingram. 2003. Production of optically pure d-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl. Environ. Microbiol. 69:399-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.