Abstract
Helicobacter pullorum represents a potential food-borne pathogen, and avian species appear to be a relevant reservoir of this organism. In this study, the prevalence of H. pullorum was investigated at 30 conventional farms where 169 ceca from 34 flocks were tested, at eight organic farms where 39 ceca from eight flocks were tested, and at seven free-range farms where 40 ceca from eight flocks were tested. All of the ceca were obtained from healthy broiler chickens. Moreover, amplified fragment length polymorphism, pulsed-field gel electrophoresis, and automated ribotyping were employed to estimate the levels of genetic variability of H. pullorum broiler isolates within and between flocks. Overall, Gram-negative, slender, curved rods, identified as H. pullorum by PCR, were isolated at 93.3% of the farms tested. The percentage of positive free-range farms (54.2%) was significantly lower than that of conventional (100%) or organic (100%) farms (P < 0.001). The level of within-flock genetic variability, calculated as the number of flocks colonized by isolates genetically different by all of the typing methods, was 34.9%. Isolates showing identical profiles by each typing method were observed in 11.6% of the flocks, but they were never detected between flocks. However, groups of isolates clustered together with an overall similarity level of ≥85%. Our results suggest that even though a high level of genetic variability is attributable to H. pullorum broiler isolates, their hierarchical genotyping produces data useful for epidemiological investigations.
Helicobacter species are Gram-negative, curved to spiral-shaped or fusiform bacteria which naturally colonize the gastrointestinal tract surface. Based on their preferential niche, Helicobacter species isolated from humans are divided into gastric helicobacters such as Helicobacter pylori and enterohepatic helicobacters which predominantly colonize the intestine and the hepatobiliary system (11). Helicobacter pullorum is a member of this last group, along with Helicobacter canadensis, Helicobacter canis, Helicobacter cinaedi, Helicobacter fennelliae, Helicobacter sp. strain flexispira taxon 8, and Helicobacter winghamensis.
H. pullorum may infect the intestinal tracts of avian species and humans. It has been detected in the intestinal contents of broiler chickens, laying hens (2, 9, 20, 28, 32), and guinea fowls (20) and in a psittacine bird (10). Moreover, H. pullorum has been isolated in fecal samples from humans with gastroenteritis (5, 8, 18, 19, 29) and its DNA was isolated from the biliary trees and gallbladders of women suffering from chronic cholecystitis and from livers of patients suffering from cirrhosis and/or hepatocellular carcinoma (12, 22, 24, 26). In vitro studies focused on the role of H. pullorum as a putative trigger of intestinal inflammation have demonstrated its proinflammatory properties involving cytolethal distending toxin and lipopolysaccharide via activation of the NF-κB pathway (30, 31). However, H. pullorum signaling in epithelial cells is still not fully understood.
Few studies of the occurrence of H. pullorum in poultry have been conducted, and these are difficult to compare because they have been performed using different culture methods or PCR protocols, as well as different kinds of samples, such as frozen versus fresh samples. Burnens and colleagues (6) found H. pullorum occurring in 4% of the cecal contents sampled from 150 healthy broiler chickens and isolated H. pullorum from 9 out of 18 ceca of laying hens affected by vibrionic hepatitis. Atabay and Corry (1) isolated Campylobacter-like organisms, which were successfully identified as H. pullorum (2), in 9 out of 15 chicken carcasses taken from the line in a poultry factory immediately after evisceration and from nine cecal contents from the positive carcasses. Ceelen et al. (9) carried out a study on the gastrointestinal tracts and liver tissues of 110 broiler chickens originating from 11 different flocks. Using a PCR assay amplifying a 447-bp fragment of the 16S rRNA gene of H. pullorum, they found samples belonging to 7 of the 11 flocks tested to be positive. In particular, using cecal tissue, they found that 37 of 110 animals were positive. Moreover, they obtained positive results with 31.8% of the colon, 10.9% of the jejunum, and 4.6% of the liver tissue samples tested. Using the filter technique of Steel and McDermott (28), the same authors found only 16 animals positive for H. pullorum from two out of seven PCR-positive flocks. Pilon et al. (23) detected 100% positive samples taken from the cecal contents of 10 chickens tested using real-time PCR targeting the gene coding for subunit A of the DNA gyrase. In Italy, Zanoni et al. (32) tested the cecal contents of 60 animals from nine commercial laying hen and six broiler chicken farms and found that 51 broiler chickens were H. pullorum positive by using the modified filter technique of Steel and McDermott (28) and incubating the plates before removing the filters under a hydrogen atmosphere. Nebbia and colleagues (20) found 80% of 130 DNA poultry samples (i.e., chickens, laying hens, and Guinea fowl) were H. pullorum positive by 16S rRNA gene sequence analysis.
There are fewer data on H. pullorum genotyping than on the occurrence of this pathogen. Whole-cell protein profiles of H. pullorum isolates provided some indication of phenotypic diversity within the species, although overall, they are conserved and have been used as a means of species identification rather than for distinguishing between isolates (2, 27). Gibson and colleagues (15) analyzed human and poultry H. pullorum isolates collected in four different countries by amplified fragment length polymorphism (AFLP) and pulsed-field gel electrophoresis (PFGE), establishing that they had distinct genotypes and a high degree of genetic diversity. Ceelen et al. (9) fingerprinted 16 isolates from two broiler flocks by AFLP and showed that isolates from each of the individual flocks examined clustered according to their flock of origin. Ribotyping with HaeIII successfully differentiated the intraspecies variation of both H. pylori and H. felis strains (7, 17). However, no data on typeability and the discriminatory power of ribotyping for H. pullorum isolates have been reported yet.
In the present study, to contribute to a better understanding of the prevalence and level of genetic diversity of H. pullorum in poultry, 45 farms applying conventional, organic, and free-range farming techniques were tested. At these farms, 50 flocks and 248 ceca from healthy broiler chickens were analyzed. The H. pullorum isolates were then characterized using three different genotyping methods (i.e., AFLP, PFGE, and automated ribotyping) to compare the suitability of these typing methods for H. pullorum characterization and also to evaluate the level of genetic variability between and within flocks.
MATERIALS AND METHODS
Samples tested.
Between September 2005 and November 2006, a total of 45 broiler farms, 50 flocks, and 248 ceca were tested. The farms reared healthy broiler chickens, and the ceca were collected in seven slaughterhouses. On average, five ceca were obtained from each flock tested. Specifically, 169 ceca were from 34 conventionally reared flocks across 30 farms, 39 ceca were from 8 organic flocks reared across 8 farms, and 40 ceca were from 8 free-range flocks reared across 7 farms. The samples were collected and processed as follows, avoiding cross-contamination. The complete intestinal tract was obtained from each bird directly after evisceration at the slaughterhouse, packed into a separate sterile plastic bag using fresh disposable gloves, and kept cool until microbiological processing.
The farms tested belonged to seven different Italian poultry companies, designated A to G, located in seven different regions of Italy. All of the birds belonging to the flocks tested were fed diets without antibiotic growth promoters. In accordance with Council Directive 2007/43/EC, conventionally reared flocks were raised within environmentally controlled poultry houses, with daily free ad libitum access to water and feed. The maximum stocking density did not exceed 42 kg (live weight)/m2. Female and male birds were slaughtered at minimum ages of 38 and 49 days, respectively. According to regulation EEC 1538/91, free-range birds were reared in poultry houses with free access to open-air runs. The maximum stocking density did not exceed 27.5 kg (live weight)/m2. The open-air runs comprised an area covered mainly by vegetation of not less than 1 m2 per chicken. Moreover, the feed formulation used in the growing phase contained at least 70% cereals. The birds were slaughtered at a minimum age of 56 days. In accordance with European Communities Council Regulation 1804/99, birds belonging to the organic flocks were reared under organic growing conditions. Poultry houses had at least one-third of their solid floors covered with bedding litter. The open-air runs were covered mainly with vegetation and permitted animals to have easy access to adequate numbers of drinking and feeding troughs. Birds were fed ad libitum organic raw materials without any supplementation of synthetic additives such as amino acids, vitamins, antioxidants, or pigments. The stocking density did not exceed 21 kg (live weight)/m2. The birds were slaughtered at a minimum age of 81 days.
Isolation procedure.
Within 5 h of sample collection, approximately 5 g of cecal contents was suspended into 5 ml of sterile saline and shaken using a Vortex mixer to obtain a homogeneous suspension. Samples were inoculated onto BBL Brucella Agar (Becton Dickinson & Co., Sparks, MD) supplemented with 5% sheep blood using a modified Steele and McDermott (28) filter technique as described by Zanoni et al. (32). Plates were incubated at 37 ± 1°C in a microaerobic atmosphere with hydrogen in a jar. The microaerobic atmosphere with hydrogen was obtained by the gas replacement method (3) with an anaerobic gas mixture (10% H2, 10% CO2, and 80% N2). The plates were examined daily for 1 week. Up to five grayish white colonies of Gram-negative, gently curved, slender rod bacteria presumed to be H. pullorum were subcultured and cloned from each plate. All five colonies were identified as H. pullorum by the PCR assay described by Stanley et al. (27) using the Redextract-N-Amp Tissue PCR kit (Sigma Chemical Co., St. Louis, MO) and primers pullF (5′ ATGAATGCTAGTTGTTGTCAG 3′) and pullR (5′ GATTGGCTCCACTTCACA 3′), targeting the16S rRNA gene and producing an amplicon of 467 bp. Although specific for H. pullorum, these primers are also able to amplify the 16S rRNA gene of the closely related species H. canadensis. In order to distinguish H. pullorum from H. canadensis, a 1,200-bp PCR product of the 16S rRNA genes gene was amplified using primers C97 (5′ GCTATGACGGGTATCC 3′) and C05 (5′ ACTTCACCCCAGTCGCTG 3′) and subsequently subjected to the ApaLI (Fermentas, International Inc., Burlington, Ontario, Canada) digestion assay as described by Fox et al. (13). H. pullorum CIP 104787T and Campylobacter jejuni ATCC 33560 were used as positive and negative controls, respectively, in the first PCRs. H. canadensis CCUG 47163T was used as a positive-control strain in the second PCRs.
A randomly selected average of two H. pullorum isolates per positive flock were from different ceca and stored at −70°C for further genotyping analysis by AFLP, PFGE, and automated ribotyping.
AFLP.
AFLP analysis was performed using HindIII and HhaI as previously described (21). The AFLP fragments detected in the 75- to 500-bp size range were considered for numerical analysis. Genemapper-processed data files containing bacterial AFLP profiles were imported into Bionumerics 5.1 software (Applied Maths, Saint-Martens-Latem, Belgium). Normalized AFLP profiles were compared using the Dice correlation coefficient and clustered by the unweighted-pair group method using average linkages (UPGMA). Isolates showing a similarity level of >90% were grouped in the same AFLP type.
PFGE.
A PFGE protocol suitable for H. pullorum genotyping was set up based on the PulseNet protocol for C. jejuni (25) using 40 U of SacII (Fermentas, St. Leon-Rot, Germany) (14). The restriction fragments were separated by electrophoresis in 1.5% Certified Megabase Agarose (Bio-Rad Laboratories, Hercules, CA) for 22.5 h at 5 V/cm at 14°C. The pulse time, which changed linearly, was 0.5 to 40 s. A Lambda DNA ladder (Bio-Rad Laboratories) was used as the size standard. H. pullorum CIP 104787 was used as a positive-control strain in each PFGE gel, and C. jejuni LMG 8842 was used as a positive run control. The PFGE profiles were subjected to comparative numerical analysis using the Diversity Database (Bio-Rad Laboratories). Similarity among profiles was calculated by the Dice correlation coefficient and clustered by UPGMA. Isolates showing a similarity level of >80% were grouped in the same PFGE type.
Automated ribotyping.
Automated ribotyping was performed using the RiboPrinter according to the manufacturer's instructions (4), with 53 U of HaeIII (New England BioLabs Inc., Ipswich, MA) per run. The ribotype patterns of the H. pullorum isolates typed in this study were stored in the RiboPrinter database and compared to each other. The characterization consisted of combining profiles within a similarity range, as calculated using the RiboPrinter's proprietary algorithm, of greater than 0.93 to form a dynamic ribotype or ribogroup that reflected the genetic relatedness of the isolates (4).
Statistical analysis.
The data collected were analyzed with the Statgraphics package (ver. 5.1; StatSoft, Inc.). In particular, the data on H. pullorum prevalence obtained by testing ceca were subjected to analysis of variance using the different kinds of farms (i.e., conventional, free-range, and organic farms) as the independent variable. The percentages of H. pullorum-positive samples at the farm, flock, and cecum levels were compared by using the Tukey honestly significant difference test. A P value of ≤0.05 was considered statistically significant.
Discriminatory index.
Simpson's discriminatory index (D), as described by Hunter and Gaston (16), was calculated for AFLP, PFGE, and automated ribotyping. This index represents the probability that two randomly chosen isolates would be distinguished by one typing method. As the numerical index approaches the maximum value of 1 (representing 100% discriminatory ability), the greater the probability that the typing method under study would be able to discriminate between two unrelated isolates.
Cluster analysis.
Data from each typing method were imported into Bionumerics 5.1 software (Applied Maths, Saint-Martens-Latem, Belgium) and included in a composite data set to assess the global genetic relatedness of the isolates. Profiles were clustered by UPGMA.
RESULTS
Prevalence of H. pullorum at poultry farms.
All of the farms belonging to companies A, D, E, F, and G were H. pullorum positive, whereas 22.2 and 11.1% of the farms belonging to companies B and C, respectively, were H. pullorum negative (Table 1). Overall, the percentage of positive free-range farms (i.e., 57.1%) was significantly lower than that of conventional (i.e., 100%) and organic (i.e., 100%) farms (Table 2). Even if the statistical comparisons of conventional, free-range, and organic farms, as well as flocks and ceca, were made on a different number of samples, the number of samples tested takes into account the frequency of diverse poultry husbandry practices in Italy and Europe, where 91.7% of the broiler flocks are conventional, 6% are free range, and 1% are organic (http://www.efsa.europa.eu/en/scdocs/scdoc/1522.htm). Within each flock, the proportion of positive ceca ranged between 20% (i.e., one in five ceca) and 100%. It is important to stress that after 72 to 96 h of incubation, all of the first presumptively positive isolation plates showed a large number of pinpoint grayish white colonies (>50 CFU) of Gram-negative, slender, curved rods referable to H. pullorum.
TABLE 1.
Prevalence of H. pullorum in the farms, flocks, and collected ceca within each poultry company
| Poultry company | No. of positive farms/total (%)a | No. of positive flocks/total (%)b | No. of positive ceca/total (%) | Type of rearing |
|---|---|---|---|---|
| A | 5/5 (100) | 5/5 (100) | 23/25 (92) | Conventional |
| B | 5/5 (100) | 6/6(100) | 18/30 (60) | Conventional |
| 2/4 (50) | 2/4 (50) | 10/20 (50) | Free range | |
| C | 6/6 (100) | 7/7 (100) | 30/35 (85.7) | Conventional |
| 2/3 (66.7) | 3/4 (75) | 13/20 (65) | Free range | |
| D | 4/4 (100) | 4/4 (100) | 20/20 (100) | Conventional |
| 6/6 (100) | 6/6 (100) | 29/30 (96.7) | Organic | |
| E | 5/5 (100) | 7/7 (100) | 32/35 (91.4) | Conventional |
| F | 5/5 (100) | 5/5 (100) | 19/24 (79.2) | Conventional |
| G | 2/2 (100) | 2/2 (100) | 9/9 (100) | Organic |
| Total | 42/45 (93.3) | 47/50 (94) | 203/248 (81.8) |
Positive farms were considered farms at which at least one H. pullorum-positive flock was detected.
Positive flocks were considered flocks at which at least one H. pullorum-positive cecum was collected.
TABLE 2.
Comparison of prevalences of H. pullorum in conventional, free-range, and organic farms, flocks, and ceca
| Category | No. positive/total (%)a |
P value | ||
|---|---|---|---|---|
| Conventionalb | Free rangec | Organicd | ||
| Farms | 30/30 (100)† | 4/7 (57.1)‡ | 8/8 (100)† | <0.001 |
| Flocks | 34/34 (100)† | 5/8 (62.5)‡ | 8/8 (100)† | <0.001 |
| Ceca | 142/169 (84)† | 23/40 (57.5)‡ | 38/39 (97.4)† | <0.001 |
Percentages with different symbols in the same row are significantly different (P ≤ 0.005).
Slaughtering age, 38 days for females and 49 for males.
Slaughtering age, 56 days.
Slaughtering age, 81 days.
Molecular characterization.
A total of 89 isolates were subjected to AFLP, PFGE, and automated ribotyping. Specifically, 84 isolates were from 30 conventional, 5 free-range, and 7 organic flocks (i.e., 2 isolates per flock); 2 isolates were from 2 different conventional flocks (i.e., 1 isolate per flock); and 3 isolates were from the same organic flock. Unfortunately, no isolate from two positive conventional flocks was tested because no cultivable isolate was recovered after sample storage at −70°C.
All of the isolates were successfully characterized by all of the techniques employed (see Table S1 in the supplemental material). AFLP grouped the 89 isolates within 67 different AFLP types. Sixteen types contained two to four isolates, whereas 51 types were unique. Thirteen common AFLP types (i.e., A-18, A-20, A-21, A-23, A-25, A-30, A-31, A-35, A-40, A-56, A-58, A-59, and A-64) were associated with 31 isolates from 20 conventional flocks, AFLP type A-36 was associated with 2 isolates from the same free-range flock, AFLP type A-49 was associated with 2 isolates from the same organic flock, and AFLP type A-47 was associated with 2 isolates from the same free-range flock and 1 isolate from an organic flock. The D value achieved for the 89 isolates typed by AFLP was 0.992.
PFGE grouped the isolates into 59 different pulsotypes, with the number of bands ranging between 11 and 21 and the sizes ranging from approximately 48 to 437 kbp. Twenty-three pulsotypes were shared by two to four isolates, whereas 36 were unique. Sixteen common pulsotypes (i.e., P-2, P-15, P-16, P-17, P-18, P-19, P-20, P-31, P-35, P-36, P-39, P-41, P-46, P-49, P-52, and P-59) were associated with 36 isolates from 19 conventional flocks, three pulsotypes (i.e., P-9, P-11, and P-43) were associated with 6 isolates from 3 free-range flocks, three pulsotypes (i.e., P-5, P-38, and P-57) were associated with 6 isolates from 3 organic flocks, and pulsotype P-55 was associated with 3 isolates from 2 different conventional flocks and 1 isolate from 1 organic flock. The D value achieved by the PFGE typing method for the 89 isolates was 0.990.
Automated ribotyping discriminated the 89 isolates into 48 different ribotypes corresponding to ribotyping profiles characterized by two to five bands with molecular sizes ranging from 1.1 to 48 kbp. Fourteen ribotypes were shared by two to seven isolates, whereas 34 were unique. Six common ribotypes (i.e., 153-313-S-3, 153-313-S6, 153-393-S-7, 153-394-S-3, 153-394-S-7, and 153-406-S-1) were associated with 15 isolates from nine conventional flocks, four ribotypes (i.e., 153-313-S-1, 153-390-S-1, 153-392-S-6, 163-393-S-3) were associated with 9 isolates from seven conventional flocks and 9 isolates from seven organic flocks; two ribotypes (i.e., 153-313-S-5 and 153-395-S1) were associated with 8 isolates from six conventional flocks, 2 isolates from two different free-range flocks, and 2 isolates from two organic flocks, ribotype 153-390-S-6 was associated with 5 isolates from five different conventional flocks and 2 isolates from two free-range flocks, and ribotype 153-396-S-3 was associated with 1 isolate from one organic flock and 1 isolate from one free-range flock. The D value achieved using automated ribotyping was 0.971.
In 43 of 47 positive flocks, it was possible to compare the AFLP, PFGE, and ribotyping profiles of two, and in one case (i.e., flock 99) three, isolates within the same flock. In two additional positive flocks (i.e., flocks 234 and 236), only one isolate was available for typing because a single cecum was positive. Finally, no cultivable isolates were obtained from the remaining two positive flocks. Corresponding results in terms of similarity or difference among the AFLP, PFGE, and ribotyping results of isolates belonging to the same flock were observed in 20 (46.5%) of the 43 flocks. In fact, in four conventional flocks and one organic flock, all of the isolates were identified as clonal by each typing method. Moreover, in nine conventional flocks, five organic flocks, and one free-range flock, isolates from the same flock showed different genotypes by all of the typing methods. Complete agreement between the AFLP and ribotyping results was observed for isolates belonging to an additional 13 flocks. In fact, in 10 conventional flocks, 2 free-range flocks, and 1 organic flock, isolates from the same flock showed only identical pulsotypes. Agreement between ribotyping and PFGE results was obtained across five additional flocks. In fact, in three conventional flocks and one free-range flock, the isolates were genetically similar only by AFLP. In one additional conventional flock, the isolates were genetically similar by PFGE and ribotyping but showed different AFLP profiles. Finally, isolates from three conventional flocks, one free-range flock, and one organic flock appeared identical by PFGE and AFLP but showed different ribotyping profiles.
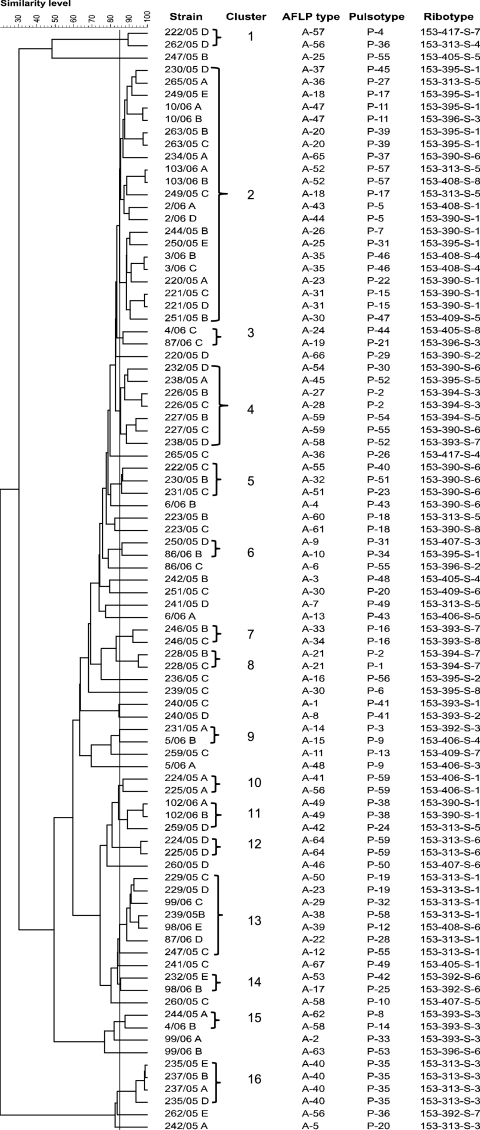
On the basis of all of the typing methods, the isolates clustered at similarity levels ranging from 30 to 99% (Fig. 1). A similarity of ≥85% was observed between isolates from the same farm (i.e., clusters 7 and 8), between isolates from different farms belonging to the same company (i.e., clusters 10, 12 and 16), between isolates from different companies applying the same rearing system (i.e., clusters 1 and 4), and between isolates from companies applying different rearing systems (i.e., clusters 2, 3, 5, 6, 9, 11, and 13 to 15) (Fig. 1). Therefore, based on the results of this study, no correlation between cluster and management or company of ownership was observed.
FIG. 1.
Clusters of isolates based on their fingerprinting by AFLP, PFGE, and automated HaeIII ribotyping.
DISCUSSION
H. pullorum represents an enterohepatic Helicobacter species to which humans have a high risk of exposure. The data collected in this study address not only birds conventionally reared (i.e., 169) but also organic (i.e., 39) and free-range (i.e., 40) birds, which have never been investigated before. Overall, the prevalence of H. pullorum among the farms (i.e., 42/45) and flocks (i.e., 47/50) tested in this study was higher than 93% but the individual bird prevalence (i.e., 203/248) was 82% (Table 2). This finding agrees with the level of occurrence detected in individual birds by Zanoni et al. (32). Moreover, according to those authors, the detection of an elevated number of H. pullorum colonies in the medium of the first isolation of all of the samples suggests that this microorganism, when present, colonizes the cecum at a high concentration. Birds reared using free-range husbandry (i.e., 57%) were significantly less contaminated than conventionally (i.e., 84%) or organically (i.e., 97.4%) reared birds. However, this finding will be investigated further in order to clarify if this result is due to environmental reasons (i.e., density, open air), the age of the birds, or their diet. The level of genetic diversity of H. pullorum isolates collected in this study was investigated using AFLP, PFGE, and automated HaeIII ribotyping. AFLP and PFGE sample variations through the whole genome, whereas automated ribotyping measures variations within the three rRNA operons present on the H. pullorum chromosome. All of the typing methods were able to discriminate the isolates, with D values approaching the maximum value of 1. AFLP and ribotyping present several advantages in terms of reproducibility and throughput over PFGE, but the latter typing method is less costly and can be easily applicable in all laboratories. On the other hand, the use of capillary electrophoresis with an automatic sequencer for the accurate sizing of fragments and specific software for data management and rapid analysis of fingerprinting makes AFLP particularly effective for H. pullorum typing. It is important to stress that the three genotyping methods gave corresponding results for 46.5% of the flocks where it was possible to compare two, and in one case three, isolates. In fact, 11% of the flocks for which more than one isolate was available for genotyping were colonized by clonal isolates showing identical AFLP, PFGE, and ribotyping profiles. Moreover, 34.8% of the flocks were composed of genetically different isolates when typed by the three typing methods. Multiple clonal isolates were never detected in the ceca of birds reared in different flocks. However, 16 clusters of isolates showing an overall similarity level of ≥85% were identified and 69% of them included isolates from different companies applying the same husbandry protocol or different husbandry protocols. This finding seemed to suggest that even if a high level of genetic variability between and within flocks is associated with H. pullorum broiler isolates, their hierarchical genotyping would support epidemiological investigations tracing sources and transmission routes of specific type strains back to humans. Genetic variability within flocks was tested by typing two isolates per flock, which is quite a low number. However, the results obtained allow prediction of the possible rate of flock colonization by genetically different isolates by all of the typing methods and the rate of flocks colonized by clonal isolates. A high level of genomic diversity was also observed by Gibson et al. (15), who tested 13 human and 7 poultry H. pullorum strains collected in Switzerland, the United Kingdom, Germany, and Canada. However, no additional results are currently available for comparison.
In conclusion, the results of this study showed an overall farm prevalence of 93.3% and that the free-range farms tested were significantly less contaminated than conventional and organic farms (i.e., 57 versus 100%). The hierarchical genotyping of H. pullorum, characterized by a high level of genetic variability both within and between flocks, evidenced genetic similarities which appear relevant for epidemiological investigations designed to clarify the possible impact of this organism on human health.
Supplementary Material
Acknowledgments
This study was financed within European Union project EU-FOOD-CT-200X-007076, named POULTRYFLORGUT.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 19 November 2010.
REFERENCES
- 1.Atabay, H. I., and J. E. L. Corry. 1997. The prevalence of campylobacters and arcobacters in broiler chickens. J. Appl. Microbiol. 83:619-626. [DOI] [PubMed] [Google Scholar]
- 2.Atabay, H. I., J. E. L. Corry, and S. L. W. On. 1998. Identification of unusual Campylobacter like isolates from poultry products as Helicobacter pullorum. J. Appl. Microbiol. 84:1017-1024. [DOI] [PubMed] [Google Scholar]
- 3.Bolton, F. J., D. R. A. Wareing, M. B. Skirrow, and D. N. Hutchinson. 1992. Identification and biotyping of campylobacters, p. 151-161. In R. G. Board, D. Jones, and F. A. Skinner (ed.), Identification methods in applied environmental microbiology. Academic Press, London, United Kingdom.
- 4.Bruce, J. 1996. Automated system rapidly identifies and characterizes micro-organisms in foods. Food Technol. 50:77-78. [Google Scholar]
- 5.Burnens, A. P., J. Stanley, R. Morgenstern, and J. Nicolet. 1994. Gastroenteritis associated with Helicobacter pullorum. Lancet 344:1569-1570. [DOI] [PubMed] [Google Scholar]
- 6.Burnens, A. P., J. Stanley, and J. Nicolet. 1996. Possible association of Helicobacter pullorum with lesions of vibrionic hepatitis in poultry, p. 291-293. In D. G. Newell, J. M. Ketley, and R. A. Feldman. (ed.), Campylobacter, Helicobacter and related organisms. Plenum Press, New York, NY.
- 7.Burucoa, C., V. Lhomme, and J. L. Fauchere. 1999. Performance criteria of DNA fingerprinting methods for typing of Helicobacter pylori isolates: experimental results and meta-analysis. J. Clin. Microbiol. 37:4071-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceelen, L., A. Decostere, G. Verschraegen, R. Ducatelle, and F. Haesebrouck. 2005. Prevalence of Helicobacter pullorum among patients with gastrointestinal disease and clinically healthy persons. J. Clin. Microbiol. 43:2984-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceelen, L. M., A. Decostere, K. Van den Bulck, S. L. On, M. Baele, R. Ducatelle, and F. Haesebrouck. 2006. Helicobacter pullorum in chickens, Belgium. Emerg. Infect. Dis. 12:263-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceelen, L., A. Decostere, A. Martel, F. Pasmans, and F. Haesebrouck. 2006. First report of H. pullorum in the faeces of diarrhoeic psittacine bird (Psephotus haematogaster). Vet. Rec. 159:389-390. [DOI] [PubMed] [Google Scholar]
- 11.Fox, J. G. 1997. The expanding genus of Helicobacter: pathogenic and zoonotic potential. Semin. Gastrointest. Dis. 8:124-141. [PubMed] [Google Scholar]
- 12.Fox, J. G., et al. 1998. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology 114:755-763. [DOI] [PubMed] [Google Scholar]
- 13.Fox, J. G., et al. 2000. Helicobacter canadensis sp. nov. isolated from humans with diarrhea as an example of an emerging pathogen. J. Clin. Microbiol. 38:2546-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson, J. R., K. Sutherland, and R. J. Owen. 1994. Inhibition of DNase activity in PFGE analysis of DNA from Campylobacter jejuni. Lett. Appl. Microbiol. 19:357-358. [DOI] [PubMed] [Google Scholar]
- 15.Gibson, J. R., M. A. Ferrus, D. Woodward, J. Xerry, and R. J. Owen. 1999. Genetic diversity in Helicobacter pullorum from human and poultry sources identified by an amplified fragment length electrophoresis. J. Appl. Microbiol. 87:602-610. [DOI] [PubMed] [Google Scholar]
- 16.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jalava, K., et al. 1999. Characterization of Helicobacter felis by pulsed-field gel electrophoresis, plasmid profiling and ribotyping. Helicobacter 4:17-27. [DOI] [PubMed] [Google Scholar]
- 18.Kuijper, E. J., S. Stevens, T. Imamura, B. De Wever, and E. C. Claas. 2003. Genotypic identification of erythromycin-resistant Campylobacter isolates as Helicobacter species and analysis of resistance mechanism. J. Clin. Microbiol. 41:3732-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melito, P. L., et al. 2000. Differentiation of clinical Helicobacter pullorum isolates from related Helicobacter and Campylobacter species. Helicobacter 5:142-147. [DOI] [PubMed] [Google Scholar]
- 20.Nebbia, P., et al. 2007. Identification of enteric Helicobacter in avian species. Schweiz. Arch. Tierheilkd. 149:403-407. [DOI] [PubMed] [Google Scholar]
- 21.Parisi, A., et al. 2010. Amplified fragment length polymorphism and multi-locus sequence typing for high-resolution genotyping of Listeria monocytogenes from foods and the environment. Food Microbiol. 27:101-108. [DOI] [PubMed] [Google Scholar]
- 22.Pellicano, R., et al. 2004. Helicobacter species sequences in liver samples from patients with and without hepatocellular carcinoma. World J. Gastroenterol. 10:598-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pilon, C., V. Prouzel-Mauléon, A. Ménard, and F. Mégraud. 2005. Development of a real-time quantitative PCR specific to Helicobacter pullorum, p. 62. In V. Korolik, A. Lee, H. Mitchell, G. Mendez, B. Fry, and P. Coloe (ed.), Abstracts of scientific presentations: 13th International Workshop on Campylobacter, Helicobacter and Related Organisms (CHRO), Gold Coast, Queensland, Australia.
- 24.Ponzetto, A., et al. 2000. Helicobacter infection and cirrhosis in hepatitis C virus carriage: is it an innocent bystander or a troublemaker? Med. Hypotheses 54:275-277. [DOI] [PubMed] [Google Scholar]
- 25.Ribot, E. M., C. Fitzgerald, K. Kubota, B. Swaminathan, and T. J. Barrett. 2001. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. J. Clin. Microbiol. 39:1889-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocha, M., et al. 2005. Association of Helicobacter species with hepatitis C cirrhosis with or without hepatocellular carcinoma. Gut 54:396-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanley, J., et al. 1994. Helicobacter pullorum sp. nov.: genotype and phenotype of new species isolated from poultry and from human patients with gastroenteritis. Microbiology 140:3441-3449. [DOI] [PubMed] [Google Scholar]
- 28.Steele, T. W., and S. N. McDermott. 1984. The use of membrane filters applied directly to the surface of agar plates for the isolation of Campylobacter jejuni from faeces. Pathology 16:263-265. [DOI] [PubMed] [Google Scholar]
- 29.Steinbrueckner, B., et al. 1997. Isolation of Helicobacter pullorum from patients with enteritis. Scand. J. Infect. Dis. 29:315-318. [DOI] [PubMed] [Google Scholar]
- 30.Varon, C., et al. 2009. Study of Helicobacter pullorum proinflammatory properties on human epithelial cells in vitro. Gut 58:629-635. [DOI] [PubMed] [Google Scholar]
- 31.Young, V. B., et al. 2000. Cytolethal distending toxin in avian and human isolates of Helicobacter pullorum. J. Infect. Dis. 182:620-623. [DOI] [PubMed] [Google Scholar]
- 32.Zanoni, R. G., M. Rossi, D. Giacomucci, V. Sanguinetti, and G. Manfreda. 2007. Occurrence and antibiotic susceptibility of Helicobacter pullorum from boiler chickens and commercial laying hens in Italy. J. Food Microbiol. 116:168-173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.