Abstract
Photosynthetic, nitrogen-fixing Anabaena strains are native to tropical paddy fields and contribute to the carbon and nitrogen economy of such soils. Genetic engineering was employed to improve the nitrogen biofertilizer potential of Anabaena sp. strain PCC7120. Constitutive enhanced expression of an additional integrated copy of the hetR gene from a light-inducible promoter elevated HetR protein expression and enhanced functional heterocyst frequency in the recombinant strain. The recombinant strain displayed consistently higher nitrogenase activity than the wild-type strain and appeared to be in homeostasis with compatible modulation of photosynthesis and respiration. The enhanced combined nitrogen availability from the recombinant strain positively catered to the nitrogen demand of rice seedlings in short-term hydroponic experiments and supported better growth. The engineered strain is stable, eco-friendly, and useful for environmental application as nitrogen biofertilizer in paddy fields.
Filamentous, heterocystous, nitrogen-fixing, photosynthetic cyanobacteria naturally abound in tropical paddy fields and significantly contribute to the carbon and nitrogen economy of such soils (23, 26). Under combined nitrogen deprivation, such strains differentiate specialized nitrogen-fixing cells called heterocysts (14, 25). Cyanobacteria, such as Nostoc and Anabaena strains, have great potential as nitrogenous biofertilizer derived from solar energy due to their possession and elegant coordination of photoautotrophy (CO2 fixation through the Calvin cycle by vegetative cells) and diazotrophy (atmospheric dinitrogen fixation by the nitrogenase enzyme complex in heterocysts) (28). Photoautodiazotrophy is the dominant mode of growth of heterocystous cyanobacteria and requires only water, mineral nutrients, carbon dioxide, and light. The heterocyst frequency of wild-type Anabaena strains varies from 5 to 8% under combined-nitrogen-deficient (diazotrophic) conditions (14) and restricts their nitrogen-fixing efficiency. The biofertilizer potential of such strains in tropical rice fields is estimated to be from ∼20 to 30 kg N/ha/season (26), whereas that in legume-Rhizobium symbiosis is ∼150 to 300 kg N/ha/season (29). A relatively higher efficiency of cyanobacterial nitrogen fixation has been recorded in symbiotic association with lichens, bryophytes, and Azolla, where the occurrence of 20 to 30% heterocysts has been reported (15, 16, 18).
Attempts to increase the heterocyst frequency have been made earlier by subjecting cultures to molybdenum deficiency (12) or by exposure to UV rays (13). While such efforts increased heterocyst differentiation, there was no corresponding increase in nitrogen fixation. Identification of the hetR gene (5), encoding a serine-type protease (22), as a master regulator of heterocyst differentiation in recent years has focused the approach around manipulation of this particular gene. The HetR protein has been shown to bind upstream of the hepA, hetR, and patS genes and regulate their expression, including its own, as a homodimer (17). The hetR mutants fail to differentiate heterocysts (5, 6), while the copper-induced overexpression of hetR from a multicopy replicative plasmid in Anabaena resulted in supernumerary heterocysts (7). The nitrogen-fixing potential of such hetR-overexpressing strains was, however, not enhanced. Unfortunately, Cu2+ is not an eco-friendly stimulus that can be employed in environmental applications, and strains overexpressing desired genes from multicopy replicative plasmids are not stable and may aid lateral gene/plasmid transfer to other nontarget organisms in the environment. Thus, there is need for development of a technology for strain improvement involving integrative gene expression from the genome and construction of environmentally stable recombinant strains capable of desired gene expression in response to an eco-friendly stimulus.
Recently, we developed an integrative expression vector, pFPN (9), and demonstrated its utility for the aforesaid objectives (10, 21). The vector pFPN integrates a strong light-inducible Anabaena promoter, PpsbA1, and a selectable gene, nptII, in the Anabaena genome at an innocuous intergenic region (Anabaena sp. PCC7120 chromosome coordinates 4654700 to 4655631) upon homologous double recombination (9). Integrative expression of a desirable gene cloned downstream of the PpsbA1 promoter (i) eliminates the need for antibiotic selection pressure for replicative plasmid maintenance and (ii) avoids the risk of possible horizontal gene transfer through plasmid mobilization (9). Here, we report on the improvement of Anabaena sp. strain PCC7120 (hereafter referred to as An7120) aimed at meeting a continuous and consistently elevated supply of fixed nitrogen to the rice seedlings. To achieve this, the hetR gene was cloned in pFPN, integrated and expressed from PpsbA1 promoter in Anabaena PCC7120, using light as a stimulus. The recombinant strain AnFPNhetR showed elevated continuous heterocyst formation and nitrogen fixation and sustained higher nitrogen availability to rice seedlings.
MATERIALS AND METHODS
Strains and culture conditions.
Anabaena PCC7120 was grown in BG11 medium (8), pH 7.2, with (BG11+) or without (BG11−) combined nitrogen (17 mM NaNO3) at 27°C under continuous illumination (30 μE m−2 s−1) and under either an aeration (3 liters min−1), shaking (150 rpm), or static culture condition, as described earlier (1). Escherichia coli strains were grown in Luria-Bertani (LB) medium supplemented with either 100 μg ml−1 carbenicillin (Cb), 50 μg ml−1 kanamycin (Km), and/or 33 μg ml−1 chloramphenicol (Cm). Recombinant Anabaena strains were grown with 25 μg ml−1 neomycin (Nm) in BG11 agar medium or with 12.5 μg ml−1 in liquid BG11 medium. Growth of An7120 was estimated from the content of chlorophyll a, as described earlier (20), or by measuring the optical density (turbidity) at 750 nm (OD750). All the physiological experiments with AnFPNhetR were performed without antibiotic pressure, unless mentioned otherwise. Growth of E. coli was recorded as turbidity (OD600).
Overexpression and purification of recombinant HetR protein for production of anti-HetR antibody.
The hetR gene was PCR amplified by using hetR forward (5′-GGA ATT CCA TAT GAG TAA CGA CAT CGA TC-3′) and reverse (5′-CGC GGA TCC TTA ATC TTC TTT TCT ACC-3′) primers carrying NdeI and BamHI restriction sites (underlined), described earlier (9). The PCR-amplified hetR gene was sequenced and the sequence was submitted to GenBank. The plasmid vector pET29a (Novagen) was restriction digested with NdeI and BamHI and was ligated with the PCR-amplified hetR gene fragment that had been restriction digested with the same enzymes. The resulting plasmid, designated pET29ahetR, was transformed into E. coli BL21(DE3)pLysS and selected on LB agar plates containing 50 μg ml−1 neomycin. The E. coli BL21 strain carrying plasmid pET29ahetR was grown to an optical density at 600 nm of ∼0.6 and induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 4 h at 30οC. The cells were sonicated on ice in lysis buffer (100 mM Tris Cl, 300 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, pH 8.0) and centrifuged at 13,400 rpm for 15 min. Proteins from the supernatant were extracted in Laemmli's buffer, electrophoretically resolved by 12% SDS-PAGE, and stained with Coomassie brilliant blue G-250 (CBB). The recombinant HetR-6His protein was purified by nickel-nitrilotriacetic acid (Ni2+-NTA) affinity chromatography. Binding of cell extract in lysis buffer to the Ni2+-NTA-agarose resin slurry at 4°C for 2 h, washing, and subsequent elution steps were performed using a protein purification kit (Qiagen, Germany), according to the manufacturer's protocol. To raise antisera against Anabaena HetR, Anabaena HetR-6His protein purified from E. coli was employed for immunization in rabbits. The primary and booster immunizations and collection of the antisera (anti-AnHetR) were performed at Bangalore Genei, Bangalore, India.
Western blotting and immunodetection.
Proteins extracts were electrophoretically resolved by 12% SDS-PAGE and electroblotted onto nitrocellulose membranes. Immunodetection of recombinant HetR-6His protein in cell extracts from E. coli was carried out with a monoclonal anti-His antibody (GE Healthcare). The overexpressed HetR protein in cell extracts from Anabaena was detected with the Anabaena HetR-specific (anti-AnHetR) polyclonal antibody. Both the native and recombinant HetR proteins were visualized by use of a secondary antibody (anti-rabbit IgG-alkaline phosphatase conjugate; Roche Applied Sciences, Germany). The immuno-cross-reacting protein was visualized using nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolylphosphate, toluidine salt (NBT/BCIP), solution (Roche Applied Sciences) in the dark.
Microscopy and phenotypes of different Anabaena strains.
Bright-field and fluorescence microscopy of strains An7120 and AnFPNhetR were performed on a Carl Zeiss Axioscop 40 microscope to visualize the vegetative cells, proheterocysts, and mature heterocysts, as described earlier (9). Fluorescence microscopy was performed using green light excitation (λexcitation, 580 ± 8 nm). Heterocysts were detected by the nearly complete loss of red fluorescence. Alcian blue was used to stain heterocyst-specific envelopes (19) as follows: a 0.5% alcian blue solution in 50% ethanol was added to an equal volume of culture for 15 min, followed by three washes with BG11− medium to remove the free or nonspecifically bound dye from Anabaena cells. At least 2,000 cells were counted per sample to determine the percent frequency of functional heterocysts attached to the filament. The microscope images of the An7120 and AnFPNhetR strains were captured with a charge-coupled-device (CCD) Axiocam MRc (Zeiss) camera attached to the microscope.
Respiration and photosynthesis.
The rates of respiration and photosynthesis of An7120 and hetR-overexpressing AnFPNhetR strains were measured as oxygen consumption in the dark and oxygen evolution in the light, respectively, by 1 ml cells equivalent to an OD750 of 1, using an aqueous-phase Clark-type electrode (Hansatech DW2/2 oxygraph; Hansatech, United Kingdom), as described earlier (10, 21).
Nitrogenase activity.
The An7120 and AnFPNhetR strains, maintained in BG11+ medium, were washed three times with BG11− medium and inoculated in BG11− medium for induction of heterocyst differentiation and nitrogen fixation. Two-milliliter aliquots (cells with 3 μg ml−1 chlorophyll a equivalent) were spun down (15,000 × g, 5 min) and spread on 2 ml of BG11− agar slants in 5-ml glass tubes and allowed to grow for 2 days. Acetylene reduction was assayed as described earlier (2, 3, 11).
Ammonium release.
Ammonium ion release by An7120 and AnFPNhetR strains was measured after the glutamine synthetase (GS) activity was inhibited with 1 μM methionine d,l-sulfoximine (MSX) (24). The release of ammonia in the extracellular medium was spectrophotometrically assessed by the phenol-hypochlorite method, as described earlier (27).
Hydroponic study with rice seedlings.
Rice (Oryza sativa L. cultivar Bura Rata) seeds were soaked in water overnight. For each culture, 15 seeds were laid equidistantly in three 9-in.-diameter petri dishes and allowed to germinate on a wet Whatman filter paper (no. 3) for 2 days in the dark. On the third day, the germinated seedlings were shifted to a growth room and allowed to grow in a 16-h light and 8-h dark regime for 2 days with a light intensity of 30 μE m−2 s−1 at 25 ± 2°C. The mid-log-phase cyanobacterial cultures grown in BG11+ medium were washed three times with BG11− medium, inoculated (cells with the equivalent of 3 μg ml−1 chlorophyll a per plate), and incubated for 15 days in the growth room. Both the uninoculated control plates and petri dishes inoculated with cyanobacteria were supplemented with 2 ml of 0.5× BG11− medium every day to compensate for evaporation. After 15 days, seedlings were analyzed for root and shoot length, fresh weight, and chlorophyll content (4).
Accession numbers.
The nucleotide sequence of the PCR-amplified hetR gene was submitted to GenBank and can be found under accession no. FJ608813. The HetR protein was submitted to the SWISS-PROT database and can be found under accession no. UniProtKB P27709.
RESULTS
Cloning, overexpression, and purification of HetR.
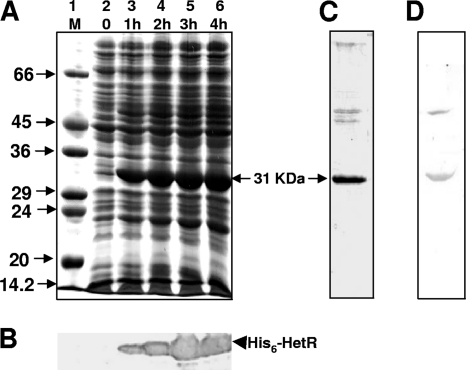
Upon induction with IPTG, the E. coli BL21(DE3)/pLysS/pET29ahetR cells synthesized a 31-kDa protein, visualized by 12% SDS-PAGE and CBB staining (Fig. 1A, lanes 3 to 6). The induction was found to be optimum at 3 h (Fig. 1A, lane 5), and the protein remained in the cytosol, i.e., did not go into inclusion bodies. The identity of the induced HetR protein was confirmed by Western blotting and immunodetection using anti-His antibody (Fig. 1B, lanes 3 to 6). The HetR protein was purified to homogeneity by Ni2+-NTA affinity chromatography (Fig. 1C). The identity and purity of purified HetR protein were further ascertained by peptide mass fingerprinting. The purified protein was used to generate corresponding antiserum. Anti-AnHetR antibody detected the HetR protein in cell extracts of E. coli BL21(pET29ahetR) cells induced with IPTG for 30 min (Fig. 1D).
FIG. 1.
Cloning, overexpression, and purification of HetR from E. coli. (A) Overexpression of HetR protein in E. coli BL21(DE3)/pLysS/pET29ahetR. Various lanes of a 12% SDS-PAGE electrophoretogram stained with CBB depict an SDS-7 protein molecular mass marker (Sigma Chemical Co., United Kingdom) (lane M) and time-dependent induction of the 31-kDa recombinant HetR-His6 protein by 1 mM IPTG up to 4 h (lanes 2 to 6). (B) Western blotting and immunodetection of IPTG-induced recombinant HetR-His6 protein by anti-His antibody at the time points shown in panel A. (C) CBB-stained SDS-polyacrylamide gel showing the purified recombinant HetR protein. (D) Western blot and immunodetection of HetR protein after 30 min IPTG induction in cell extracts of E. coli BL21(pET29ahetR), using the anti-AnHetR antibody.
Overexpression of HetR protein in Anabaena PCC7120.
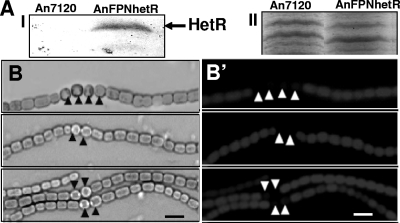
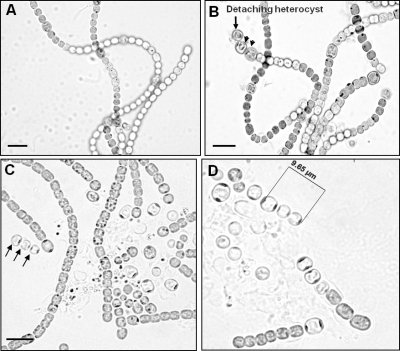
The hetR gene was cloned downstream of the PpsbA1 promoter in the pFPN vector. The resulting construct was transferred to Anabaena by electroporation and was integrated in an innocuous noncoding region of the genome, as reported earlier (9). The HetR protein was overexpressed from the PpsbA1 promoter in the recombinant AnFPNhetR strain. The wild-type Anabaena cells did not show detectable HetR protein (Fig. 2, lane 1). In contrast, the ∼31-kDa HetR protein was detected in the AnFPNhetR strain, maintained without antibiotic, even after 3 days (Fig. 2, lane 2). The elevated levels of HetR protein resulted in multiple intercalary heterocysts (often in a chain) and, frequently, double heterocysts in filaments of the AnFPNhetR strain (Fig. 2B). Loss of red fluorescence confirmed the identity of supernumerary heterocysts in the AnFPNhetR strain (Fig. 2B'). The alcian blue-stained light microphotograph of An7120 and AnFPNhetR filaments confirmed the presence of a laminated polypeptide layer in all the heterocysts (Fig. 3A and B). The multiple heterocysts in the AnFPNhetR strain showed a tendency to detach from the filaments and release their cellular contents (Fig. 3B to D). The frequencies of multiple heterocysts and their detachment were found to be higher in the case of agitated or aerated cultures (Fig. 3D) than in those grown under static culture conditions (Fig. 3C).
FIG. 2.
Effect of constitutive enhanced expression of HetR on heterocyst differentiation in strain AnFPNhetR. (A) Overepression of HetR protein. (I) Western blotting and immunodetection of HetR protein in cell-free extracts of wild-type strain An7120 (lane 1) and recombinant Anabaena strain AnFPNhetR (lane 2) after 72 h of growth in BG11 medium without added neomycin. Proteins (20 μg) were resolved by 12% SDS-PAGE and electroblotted, and HetR was immunodetected with anti-AnHetR antibody. (II) CBB staining of the upper part of the SDS-polyacrylamide gel shown in panel I to ensure equal protein loading. (B and B') Light and fluorescence microscopy, respectively, of an exponentially growing (5-day-old) culture of strain AnFPNhetR to visualize heterocyst differentiation. The identity of visibly mature heterocysts (marked by black arrowheads) in panel B was further confirmed by loss of fluorescence (marked by white arrowheads) in panel B'.
FIG. 3.
Differentiation of heterocysts in Anabaena strains. Alcian blue-stained light photomicrographs of vegetative cells and heterocysts of strains An7120 (A) and AnFPNhetR (B) are shown. Photomicrographs of a 15-day-old culture of strain AnFPNhetR showing chains of heterocysts and their detachment under static culture conditions (C) or under agitation (D) are also shown. Bar, 10 μm; magnification, ×600.
Enhancement of nitrogenase activity upon HetR overexpression.
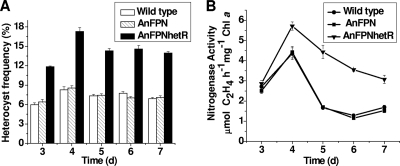
Only the heterocysts attached to Anabaena filaments are functional in N2 fixation. In the An7120 and AnFPN (vector control) strains, the functional heterocyst frequency ranged from 6 to 8%, under the experimental conditions used (Fig. 4A). In comparison, the functional heterocyst frequency in AnFPNhetR was between 12 and 18%. The nitrogenase activity of strain AnFPNhetR was found to be 1.5- to 2-fold higher than the activities of strains An7120 and AnFPN and was sustained at a higher level throughout growth under static culture conditions (Fig. 4B).
FIG. 4.
Functional heterocyst frequency and nitrogen fixation in different Anabaena strains. The strains were compared for functional heterocyst frequency (A) and nitrogenase activity (B), measured by acetylene reduction assays on solid agar slants. Chl a, chlorophyll a.
Growth and general metabolism in strain AnFPNhetR.
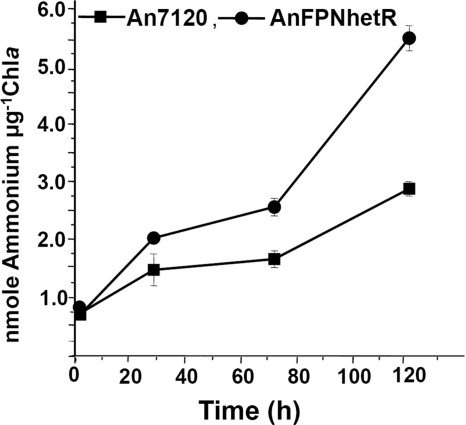
The growth of strain AnFPNhetR was found to be similar to that of wild-type strain An7120 both in combined nitrogen-free (BG11−) and in nitrate-supplemented (BG11+) media for 8 days under aeration, agitation, or static culture conditions (data not shown). Addition of the glutamine synthetase inhibitor MSX resulted in NH4+ excretion from the Anabaena strains. The amount of ammonium released by the AnFPNhetR strain was found to be significantly higher than that released by strain An7120, under diazotrophic culture conditions (Fig. 5). The rates of respiration and photosynthesis were maintained at a slightly higher level in the AnFPNhetR strain than the An7120 and AnFPN strains (Table 1).
FIG. 5.
Ammonium release by diazotrophic cultures of Anabaena strains. Cultures were diazotrophically grown in the presence of 1 μM MSX under static culture conditions. Ammonium release from An7120 and AnFPNhetR cells was monitored for 5 days.
TABLE 1.
Rates of respiration and photosynthesis in An7120, AnFPN, and AnFPNhetR strains
| Strain | Rate (μmol oxygen mg−1 chlorophyll a min−1) |
|
|---|---|---|
| Respiration | Photosynthesis | |
| An7120 | 2.82 ± 0.24 | 10.42 ± 0.27 |
| AnFPN | 2.76 ± 0.13 | 10.84 ± 0.18 |
| AnFPNhetR | 3.29 ± 0.28 | 12.05 ± 0.20 |
Assessment of biofertilizer potential of AnFPNhetR strain for rice seedlings in short-term hydroponic experiment.
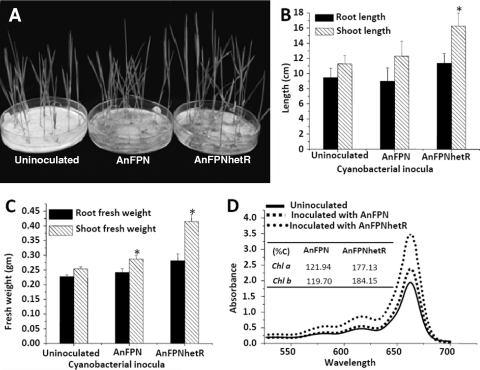
The growth of rice seedlings grown hydroponically with AnFPNhetR cultures was distinctly better than that of rice seedlings grown with strain AnFPN or grown without cyanobacterial inocula (Fig. 6A). There were significant increases in the length (Fig. 6B) and fresh weight of shoots of rice seedlings supplemented with the AnFPNhetR inoculum (Fig. 6C). The chlorophyll a and b contents of rice seedlings inoculated with strain AnFPNhetR were also found to be distinctly higher than the contents of those grown with AnFPN (Fig. 6D).
FIG. 6.
Effect of Anabaena strains on hydroponically grown rice seedling. (A) Photograph of 15-day-old hydroponically raised rice seedlings (Oryza sativa L. cultivar Bura Rata) grown without cyanobacterial inoculum or supplemented with the AnFPN or AnFPNhetR strain. Rice seedlings raised hydroponically with different Anabaena strains were compared for root and shoot lengths (B), fresh weights of roots and shoots (C), and chlorophyll a and chlorophyll b contents (D) after 15 days of growth.
DISCUSSION
The ability of Anabaena/Nostoc strains to grow and be sustained photoautodiazotrophically under tropical light, moisture, and temperature conditions provides an excellent opportunity for the use of these strains as a nitrogen biofertilizer in flooded rice fields. The practice of algalization has therefore been advocated and accepted (23, 26). Under aerobic conditions, N2 fixation depends on heterocyst differentiation, which in turn is positively regulated by the HetR protein in Anabaena/Nostoc strains (5, 6). In Anabaena cells subjected to combined nitrogen deficiency, the HetR protein level is elevated rapidly, reaching maximum expression at 3 h, and thereafter is degraded rapidly to undetectable levels in 6 h (30). Such transient expression of the hetR gene restricts the number of heterocysts produced and eventually limits the nitrogen-fixing potential of the strains. The present work demonstrates that constitutive and continuous hetR overexpression has markedly beneficial consequences for improvement of the diazotrophic potential of Anabaena strains.
To achieve this, an additional copy of the hetR gene was cloned, tagged to a light-stimulated strong PpsbA1 promoter, and integrated at an innocuous site in the An7120 genome (9). The resulting recombinant strain (AnFPNhetR) continuously expressed a high level of the HetR protein, even after 3 days of growth in BG11− medium (Fig. 2A, panel I), resulting in differentiation of a large number of heterocysts (Fig. 2). Such supernumerary heterocysts displayed a normal cell wall structure (Fig. 3B) and were functionally very active (Fig. 2B, lane 4). Overproduction of HetR also resulted in chains of multiple heterocysts (Fig. 2B and B'), which were often detached (Fig. 3C and D) and were rendered nonfunctional. In strain AnFPNhetR, this seemed to be compensated by continuous formation of new heterocysts, which maintained a consistently elevated frequency of functional heterocysts (Fig. 4A) and distinctly superior and sustained nitrogenase activity (Fig. 4B) compared to the activity of wild-type parent strain An7120. To sustain continuous heterocyst development and higher rates of nitrogen fixation, photosynthesis and respiration appeared to be realigned in the recombinant AnFPNhetR strain and maintained homeostasis. As a consequence, strain AnFPNhetR exhibited superior nitrogen fertilizer potential (Fig. 5) and clearly benefited the growth of hydroponically grown rice seedlings in a short-term experiment (Fig. 6).
The genetic engineering approach used in the present study has yielded an improved Anabaena strain with a superior nitrogen fixation ability without compromising any of its inherent capabilities. The recombinant AnFPNhetR strain has been stably maintained in our laboratory without any antibiotic pressure for the last 3 years and continues to overproduce heterocysts. Due to genomic integration of the added hetR gene, the possible risk of lateral transfer of the transgene is minimized in this strain. The AnFPNhetR strain uses a natural stimulus (light) for continuous overproduction of the HetR protein and is an eco-friendly option for environmental application as nitrogen biofertilizer. Its further evaluation in contained greenhouse trials awaits clearances from genetic engineering regulatory agencies.
Acknowledgments
This work was partially supported by a research grant from the Department of Biotechnology, India. A.K.C. acknowledges the award of fellowships from the Department of Biotechnology and Council of Scientific and Industrial Research, New Delhi, India.
We thank Saradha Ramani for help in the hydroponic studies.
Footnotes
Published ahead of print on 5 November 2010.
REFERENCES
- 1.Apte, S. K., and A. A. Bhagwat. 1989. Salinity-stress-induced proteins in two nitrogen-fixing Anabaena strains differentially tolerant to salt. J. Bacteriol. 171:909-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apte, S. K., K. A. David, and J. Thomas. 1978. Conformational changes in the nitrogenase complex in vivo by preincubation under acetylene. Biochem. Biophys. Res. Commun. 83:1157-1163. [DOI] [PubMed] [Google Scholar]
- 3.Apte, S. K., B. R. Reddy, and J. Thomas. 1987. Relationship between sodium influx and salt tolerance of nitrogen-fixing cyanobacteria. Appl. Environ. Microbiol. 53:1934-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruinsma, J. 1963. The quantitative analysis of chlorophylls a and b in higher plant extracts. Photochem. Photobiol. 2:241-249. [Google Scholar]
- 5.Buikema, W. J., and R. Haselkorn. 1991. Isolation and complementation of nitrogen fixation mutants of the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 173:1879-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buikema, W. J., and R. Haselkorn. 1991. Characterization of a gene controlling heterocyst differentiation in the cyanobacterium Anabaena 7120. Genes Dev. 5:321-330. [DOI] [PubMed] [Google Scholar]
- 7.Buikema, W. J., and R. Haselkorn. 2001. Expression of the Anabaena hetR gene from a copper-regulated promoter leads to heterocyst differentiation under repressing conditions. Proc. Natl. Acad. Sci. U. S. A. 98:2729-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castenholz, R. W. 1988. Culturing of cyanobacteria. Methods Enzymol. 167:68-93. [Google Scholar]
- 9.Chaurasia, A. K., A. Parasnis, and S. K. Apte. 2008. An integrative expression vector for strain improvement and environmental applications of the nitrogen fixing cyanobacterium, Anabaena sp. strain PCC7120. J. Microbiol. Methods 73:133-141. [DOI] [PubMed] [Google Scholar]
- 10.Chaurasia, A. K., and S. K. Apte. 2009. Overexpression of the groESL operon enhances the heat and salinity stress tolerance of the nitrogen-fixing cyanobacterium Anabaena sp. strain PCC7120. Appl. Environ. Microbiol. 75:6008-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.David, K. A., S. K. Apte, and J. Thomas. 1978. Stimulation of nitrogenase by acetylene: fresh synthesis or conformational chance? Biochem. Biophys. Res. Commun. 82:39-45. [DOI] [PubMed] [Google Scholar]
- 12.Fay, P., and L. de Vasconcelos. 1974. Nitrogen metabolism and ultrastructure in Anabaena cylindrica. II. The effect of molybdenum and vanadium. Arch. Microbiol. 99:221-230. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes, T. A., and J. Thomas. 1978. Effects of high and low ultraviolet fluences on the filamentous blue-green alga Anabaena L-31. Environ. Exp. Botany 18:229-239. [Google Scholar]
- 14.Haselkorn, R. 1978. Heterocyst. Annu. Rev. Plant Physiol. 29:319-344. [Google Scholar]
- 15.Hill, D. J. 1975. The pattern of development of Anabaena in the Azolla-Anabaena symbiosis. Planta 122:179-184. [DOI] [PubMed] [Google Scholar]
- 16.Hitch, C. J. B., and J. W. Millbank. 1975. Nitrogen metabolism in Lichens. New Phytol. 74:473-476. [Google Scholar]
- 17.Huang, X., Y. Dong, and J. Zhao. 2004. HetR homodimer is a DNA-binding protein required for heterocyst differentiation, and the DNA-binding activity is inhibited by PatS. Proc. Natl. Acad. Sci. U. S. A. 101:4848-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joseph, C. M., and J. C. Meeks. 1987. Regulation of expression of glutamine synthetase in a symbiotic Nostoc strain associated with Anthoceros punctatus. J. Bacteriol. 169:2471-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, D., and J. W. Golden. 2002. hetL overexpression stimulates heterocyst formation in Anabaena sp. strain PCC 7120. J. Bacteriol. 184:6873-6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackinney, G. 1941. Absorption of light by chlorophyll solutions. J. Biol. Chem. 140:315-322. [Google Scholar]
- 21.Rajaram, H., and S. K. Apte. 2008. Nitrogen status and heat-stress-dependent differential expression of the cpn60 chaperonin gene influences thermotolerance in the cyanobacterium Anabaena. Microbiology 154:317-325. [DOI] [PubMed] [Google Scholar]
- 22.Schiefer, W., K. Schutz, W. Hachtel, and T. Happe. 2002. Molecular cloning and characterization of hetR genes from filamentous cyanobacteria. Biochim. Biophys. Acta 1577:139-143. [DOI] [PubMed] [Google Scholar]
- 23.Singh, R. N. 1950. Reclamation of “usar” lands in India through blue-green algae. Nature 165:325-326. [Google Scholar]
- 24.Stewart, W. D. P., and P. Rowell. 1975. Effects of l-methionine-dl-sulphoximine on the assimilation of newly fixed NH3, acetylene reduction and heterocyst production in Anabaena cylindrica. Biochem. Biophys. Res. Commun. 65:846-856. [DOI] [PubMed] [Google Scholar]
- 25.Stewart, W. D. P. 1980. Some aspects of structure and function in nitrogen-fixing cyanobacteria. Annu. Rev. Microbiol. 34:497-536. [DOI] [PubMed] [Google Scholar]
- 26.Venkataraman, G. S. 1979. Algal inoculation in rice field. In N. C. Brady (ed.), Nitrogen and rice. International Rice Research Institute, Los Banos, Philippines.
- 27.Weatherburn, M. W. 1967. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 28:971-974. [Google Scholar]
- 28.Wolk, C. P. 1996. Heterocyst formation. Annu. Rev. Genet. 30:59-78. [DOI] [PubMed] [Google Scholar]
- 29.Zahran, H. H. 1999. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 63:968-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou, R., Z. Cao, and J. Zhao. 1998. Characterization of HetR protein turnover in Anabena sp. PCC7120. Arch. Microbiol. 169:417-423. [DOI] [PubMed] [Google Scholar]