Abstract
The external resistance (Rext) of microbial fuel cells (MFCs) regulates both the anode availability as an electron acceptor and the electron flux through the circuit. We evaluated the effects of Rext on MFCs using acetate or glucose. The average current densities (I) ranged from 40.5 mA/m2 (9,800 Ω) to 284.5 mA/m2 (150 Ω) for acetate-fed MFCs (acetate-fed reactors [ARs]), with a corresponding anode potential (Ean) range of −188 to −4 mV (versus a standard hydrogen electrode [SHE]). For glucose-fed MFCs (glucose-fed reactors [GRs]), I ranged from 40.0 mA/m2 (9,800 Ω) to 273.0 mA/m2 (150 Ω), with a corresponding Ean range of −189 to −7 mV. ARs produced higher Coulombic efficiencies and energy efficiencies than GRs over all tested Rext levels because of electron and potential losses from glucose fermentation. Biogas production accounted for 14 to 18% of electron flux in GRs but only 0 to 6% of that in ARs. GRs produced similar levels of methane, regardless of the Rext. However, total methane production in ARs increased as Rext increased, suggesting that Ean might influence the competition for substrates between exoelectrogens and methanogens in ARs. An increase of Rext to 9,800 Ω significantly changed the anode bacterial communities for both ARs and GRs, while operating at 970 Ω and 150 Ω had little effect. Deltaproteobacteria and Bacteroidetes were the major groups found in anode communities in ARs and GRs. Betaproteobacteria and Gammaproteobacteria were found only in ARs. Bacilli were abundant only in GRs. The anode-methanogenic communities were dominated by Methanosaetaceae, with significantly lower numbers of Methanomicrobiales. These results show that Rext affects not only the Ean and current generation but also the anode biofilm community and methanogenesis.
The bioanode, a crucial component in bioelectrochemical systems (BESs), is composed of an anode biofilm and a conductive electrode. The main catalytic components of interest in anode biofilms are exoelectrogens, microorganisms that are capable of exocellular electron transfer (31). In mixed-culture systems, exoelectrogens compete for electron donors with other functional groups such as fermenters, acetogens, and methanogens. The complexity of anode biofilms makes it hard to elucidate electrochemical mechanisms at the bioanode, but a precise understanding of exoelectrogenesis and competition in anode biofilms will aid in improving the performance of BESs. Several reviews provide insightful summaries and perspectives regarding bioanodes (31, 34, 40, 43, 51).
The anode potential (Ean) is defined as the potential difference between the anode and the surrounding electrolyte (14). Ean also refers to the electromotive force that drives electrons to flow into an anode, and it is regarded as a measure of electron affinity (14). Ean is affected by intrinsic factors such as electrode material, electrolyte composition, electrochemical reactions, and catalysts (anode biofilm in BESs) (14). Ean also can be controlled extrinsically using a potentiostat or external resistances (Rext). Potentiostats polarize the anode by varying the electrical energy input, while external resistances simply regulate Ean and the current without energy input. For example, the application of a low external resistance allows a relatively high Ean and current when the anode is connected to a highly oxidizing cathode.
There are conflicting results about the influence of Ean on start-up and sustained performance in BESs. Application of a high Ean (+200 mV versus Ag/AgCl) shortened the start-up of electrogenesis compared with the Ean of −360 mV (56). In another study, Ean did not affect the start-up time due to the use of an exoelectrogenic inoculum from another BES (1). At the start of this study, an anode poised at −200 mV versus Ag/AgCl had the highest maximum power density among systems poised at 0, −200, and −400 mV, but the performance of these systems converged by the end of the 1-month study. Ean was demonstrated to influence anode biofilm communities in microbial electrolysis cells (MECs) (52). A high Ean (370 mV versus standard hydrogen electrode [SHE]) created highly diverse and thin anode biofilms, with low current density and a low proportion of Geobacteraceae-like cells, while lower Ean values (−150, −90, and 20 mV) preferentially selected Geobacteraceae-like cells and produced high current density and thick biofilms (53). It was recently suggested that Rext cannot be a valid tool for the improvement of anode biofilm performance because similar levels of maximum power were produced from potentially different anode biofilm communities enriched at different Rext (32). However, this study did not provide electrochemical characterization to demonstrate whether the system was anode limited, nor did it address whether changes in Rext influenced electron losses to competing metabolisms. Another recent study of cellulose-fed microbial fuel cells (MFCs) showed that Rext affected power densities, with the highest maximum power density observed for reactors with the lowest Rext (46). While this study showed screening-level differences in 16S rRNA genes from anode biofilm and planktonic communities, the effect of Rext on specific microbial populations was not determined.
Methanogenesis in BESs has also been investigated in terms of Ean. Methane production in a glucose-fed MFC incorporating a ferricyanide catholyte was not affected by Ean values between −220 to 200 mV versus SHE (13). An acetate-fed batch test with these glucose-acclimated communities showed negligible production of methane at an Rext of 20 Ω (Ean of >100 mV versus SHE), suggesting that acetoclastic methanogenesis was not significant in these MFCs. That may have been influenced by the high current not allowing acetate utilization by the slow-growing acetoclastic methanogens. Another study showed that a flowthrough system fed with acetate (5.4 kg chemical oxygen demand [COD]/m3/day) had increasing methane production as the anode potential decreased in the anode potential range of −300 mV < Ean < −100 mV versus SHE (55). In an ethanol-fed MFC, hydrogenotrophic Methanomicrobiales was the only detected methanogen (38). In MEC systems, hydrogenotrophic methanogenesis has been proposed as the main methanogenic pathway due to hydrogen flux to the anode (7, 26).
Control of Rext is a simple method for studying bioelectrochemistry and exoelectrogenic ecology in MFCs. Furthermore, it can be a promising operational tool for MFC-incorporating wastewater treatment processes, as it is a system element that can be variable after design. The purpose of this study was to evaluate how Rext affects the anode microbial community, electricity production, methane production, and electron flow without energy input from potentiostats. To achieve this goal, triplicate anode biofilms were developed at the same Rext in H-shaped MFCs using glucose or acetate. Variations among anode microbial communities in triplicate MFCs were small, and their compositions were stable after four batch cycles (18). Then, they were operated and monitored at different Rext. H-shaped MFCs provided comparatively stable Ean values during most of the batch cycles, which maintained nearly constant pressure for each anode microbial community. Our results showed that Rext affected anode microbial community evolution when Ean substantially decreased. We also showed for the first time that acetoclastic Methanosaetaceae made up the main methanogenic group in acetate-fed MFCs, and their methanogenesis rate was affected by Ean. Rext was demonstrated as a potential tool for controlling electrogenesis, methanogenesis, and anode microbial community.
MATERIALS AND METHODS
MFC construction and operation.
H-shaped MFCs (two chambers separated by a Nafion membrane) were constructed as previously described with 25.2 cm2 of anode (carbon paper; E-TEK) and 13.0 cm2 of cathode (carbon paper coated with 0.35 mg Pt/cm2; E-TEK) (18). Anode chambers were inoculated with anaerobic sludge from a secondary digester at the Pennsylvania State University Wastewater Treatment Plant. Two sets of triplicate reactors, acetate-fed reactors (ARs) fed sodium acetate (Sigma-Aldrich, MO) and glucose-fed reactors (GRs) fed d-glucose (EM Science, NJ), were operated with an Rext of 970 Ω for four batches (stages 0 to 3, ca. 65 days). Afterwards, an Rext of 9,800, 970, or 150 Ω was correspondingly applied to AR1/GR1, AR2/GR2, and AR3/GR3 for an additional three batch cycles (stages 4 to 6). Acetate-fed control reactors (ACs) and glucose-fed control reactors (GCs) were operated in an open circuit for stages 0 to 3 and then in a closed circuit at 970 Ω for stages 4 to 6 (Table 1). Anode chambers were filled with 210 ml medium (50 mM phosphate-buffered glucose minimal [GM] medium with 200 mg COD/liter or 25 meq electrons [e−]/liter) in an anaerobic glove box. At the start of stages 1 to 3, the anode bottles were cleaned during the medium change, but this was discontinued at the end of stage 3. Cathode chambers were refilled with phosphate buffer (50 mM, pH 7.0) in each cycle and continuously aerated. Reactors were operated at 30°C.
TABLE 1.
MFC operation scheme depicting applied Rext
| MFC |
Rext (Ω) at indicated stages |
|
|---|---|---|
| 1 to 3 | 4 to 6 | |
| AC | Open circuit | 970 |
| AR1 | 970 | 9,800 |
| AR2 | 970 | 970 |
| AR3 | 970 | 150 |
| GC | Open circuit | 970 |
| GR1 | 970 | 9,800 |
| GR2 | 970 | 970 |
| GR3 | 970 | 150 |
Electrochemical measurements.
Cell voltage was monitored using a multiple data acquisition system (Pico, United Kingdom). Cathode potential was intermittently measured with a Ag/AgCl reference electrode (MF-2079; BASi) located about 5 mm from the cathode electrode, and anode potential was calculated by subtracting cathode potential from cell voltage. Electrode potentials were converted into SHE values by adding 195 mV and are reported throughout versus SHE. The Coulombic efficiency (CE) and the energy efficiency (EE) were calculated as previously described (29). Ohmic resistances of abiotic MFCs filled with the anolytes and catholyte mentioned above were measured using a potentiostat (PC 4/750; Gamry Instrument Inc., PA), and internal resistances (Rint) were calculated from the slopes of polarization curves.
Chemical analyses.
Liquid samples were filtered with a syringe filter (0.2-μm Supor membrane; Pall Life Science, NY), and the soluble COD was measured using the colorimetric method (catalog no. 21258-15; Hach Co.). Duplicate headspace gas samples (100 μl) were collected using a gas-tight syringe for gas composition analyses at the end of each batch (24). Methane, carbon dioxide, hydrogen, and nitrogen concentrations in the headspace were analyzed by a GC (model 8610; SRI Instruments, CA) equipped with a thermal conductivity detector and a stainless steel column (1.8 m by 1/8 in.) packed with Porapak Q (Alltech, Deerfield, IL).
DNA extraction and PCR.
Anode electrode samples (2 cm2) were sliced with sterilized scissors at the end of each batch in an anaerobic chamber for isolation of genomic DNA. DNA was extracted with the PowerSoil DNA isolation kit (Mo Bio, CA), according to the manufacturer's instructions. The V3 regions of 16S rRNA genes were amplified with 534r (5′-ATTACCGCGGCTGCTGG-3′) and 341f (5′-CCTACGGGAGGCAGCAG-3′), with a GC clamp (5′-CGCCCGGGGCGCGCCCCGGGCGGGGCGGGGGCACGGGGGG-3′) attached to the 5′ termini (36). PCR amplifications were performed in a 10-μl volume containing 1.75 μl of DNA, 0.25 μM each primer (IDT, Inc., IA), 0.4 mg/ml of bovine serum albumin (Promega, WI), and Taq PCR master mix (Qiagen, CA), giving final concentrations as follows: 0.5 U of Taq polymerase, 200 μM of each deoxynucleoside triphosphate, and 3.0 mM MgCl2. An iCycler IQ thermocycler (Bio-Rad, CA) was used for the PCR with the following program: initial denaturation at 95°C for 10 min; 10 touchdown cycles of denaturation at 94°C for 1 min, annealing at 65 to 55°C for 1 min (decreasing 1°C each cycle), and extension at 72°C for 2 min; 25 standard cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 2 min; and a final extension at 72°C for 30 min. Archaeal 16S rRNA genes were amplified with primers Arch958r (5′-YCCGGCGTTGAMTCCAATT-3′) and A20f (5′-TTCCGGTTGATCCYGCCRG-3′) in the above-described PCR composition using a PCR program described elsewhere (10).
Community analysis.
Denaturing gradient gel electrophoresis (DGGE) was performed using the DCode universal mutation detection system (Bio-Rad, CA) for bacterial community analysis. PCR amplicons (10 μl) mixed with a loading dye were loaded onto 8% (wt/vol) polyacrylamide gels containing a gradient of denaturant ranging from 30 to 60% (100% denaturant was 7 M urea and 40% formamide). DGGE was run in 0.5× TAE (Tris-acetate-EDTA) buffer at 25 V for 15 min and subsequently at 200 V for 6 h (60°C). DGGE gels were silver stained (44). DGGE bands were excised and transferred to 30 μl elution buffer (Qiagen, CA) and incubated overnight at 4°C. Eluted DNAs were purified by PCR-DGGE until a single band was obtained in the target region to remove comigrated DNA with other melting domains. Operational taxonomic units (OTUs) were defined based on the melting domain. Single bands were eluted as described above and purified using DNA Clean & Concentrator-5 (Zymo, CA), followed by sequencing. Archaeal clone libraries were constructed as previously described (18, 37). DNAs were sequenced at the Penn State University Nucleic Acid Facility. The DGGE profiles were converted into a matrix based on the locations and intensities of DGGE bands, and Minitab15 (Minitab Inc., PA) was used for principal component analysis (PCA) to convert the matrix into a two-dimensional PCA plot.
qPCR.
Quantification of Geobacteraceae was performed with the primer set Geobacteraceae-494f (5′-AGGAAGCACCGGCTAACTCC-3′) and Geo825r (5′-TACCCGCRACACCTAGT-3′) (16). Methanosaetaceae and Methanomicrobiales were quantified using primer set Mst702f (5′-TAATCCTYGARGGACCACCA-3′) and Mst862r (5′-CCTACGGCACCRACMAC-3′) and primer set MMB282f (5′-ATCGRTACGGGTTGTGGG-3′) and MMB832r (5′-CACCTAACGCRCATHGTTTAC-3′) (58), respectively. Quantitative PCR (qPCR) was done in 20-μl volumes containing 1 μl DNA, 0.25 μM each primer (IDT, Inc., IA), 0.4 mg/ml bovine serum albumin (Promega, WI), and Taq PCR master mix (Qiagen, CA). qPCR was done in an iCycler IQ thermocycler (Bio-Rad, CA) with the program used by Yu et al. (58). Standard curves were made using plasmids containing an insert sequence of each targeted group. Plasmid concentrations were determined with a NanoDrop 2000 (Thermo Scientific). Total cell numbers were estimated using 16S rRNA genomic operon numbers of 2 for Geobacteraceae (35), 2 for Methanosaetaceae, and 2.67 for Methanomicrobiales (27).
Nucleotide sequence accession numbers.
Nucleotide sequences were deposited in the GenBank database (accession numbers HM193296 to HM193368).
RESULTS
Electrochemistry.
Exoelectrogenic community evolution, system performance, and replicate reactor reproducibility at 970 Ω for batches 0 to 3 were reported elsewhere (18). Following the stable and reproducible performance that was observed after 2 months at identical Rext values, the Rext was increased in one of the triplicate reactors and decreased in another for acetate- and glucose-fed systems. Low Rext allowed high current flow and elevated the Ean (Table 2 ). Average currents in the voltage plateau ranged from 40.5 mA/m2 (9,800 Ω) to 284.5 mA/m2 (150 Ω) for ARs, with a corresponding Ean range of −188 to −4 mV. For the GRs, currents ranged from 40.0 mA/m2 (9,800 Ω) to 273.0 mA/m2 (150 Ω), with a corresponding Ean range of −189 to −7 mV. Cathode potential was also affected by Rext, ranging from ∼50 to ∼330 mV. Lower average internal resistances of ARs (1,322 ± 107 Ω) than those of GRs (1,520 ± 126 Ω) contributed to higher power from ARs.
TABLE 2.
Electrochemical properties of MFCs and performances under different Rext conditions
| MFC | Rext(Ω) | Rohm(Ω)a | Rint(Ω)b | Ean (mV)c | Ecat (mV)c | I (mA/m2)d | P (mW/m2)d |
|---|---|---|---|---|---|---|---|
| ACe | ∞ | −274 ± 10 | NA | NA | |||
| AR1 | 9,800 | 1,003 | 1,200 ± 28 | −188 ± 5 | 328 ± 5 | 40.5 ± 0.6 | 20.9 ± 0.6 |
| AR2 | 970 | 1,003 | 1,368 ± 16 | −76 ± 3 | 160 ± 4 | 187.5 ± 0.9 | 44.3 ± 0.4 |
| AR3 | 150 | 1,115 | 1,398 ± 43 | −4 ± 3 | 52 ± 3 | 284.5 ± 5.6 | 15.8 ± 0.6 |
| GCe | ∞ | −269 ± 9 | NA | NA | |||
| GR1 | 9,800 | 1,179 | 1,449 ± 18 | −189 ± 4 | 320 ± 4 | 40.0 ± 0.4 | 20.4 ± 0.4 |
| GR2 | 970 | 1,195 | 1,666 ± 41 | −70 ± 3 | 142 ± 4 | 168.1 ± 1.7 | 35.6 ± 0.7 |
| GR3 | 150 | 1,122 | 1,445 ± 33 | −7 ± 3 | 46 ± 3 | 273.0 ± 3.5 | 14.5 ± 0.4 |
Ohmic resistance of abiotic MFCs, measured by a potentiostat at an open circuit.
Internal resistance of MFCs, measured from polarization curves.
Anode potential (Ean) and cathode potential (Ecat) in the voltage plateau. E (versus SHE) = 195 mV + E (versus Ag/AgCl); n = 8.
Current/power density based on the cathode (13 cm2) because the anode area was variable due to anode sampling after each batch. NA, not applicable.
Rext is infinity under an open circuit during stages 2 and 3.
Electron flow and energy recovery.
In lower Rext (higher Ean) systems, the total substrate consumption rate was higher due to increased rates of electrogenesis (Table 3). COD loss not accounted for in measured current, hydrogen, and methane production and estimated aerobic respiration increased with Rext, reaching 74% (ACs) and 64% (GCs) in an open circuit (Rext = ∞). Acetate consistently resulted in higher CEs and EEs than glucose because of potential losses from glucose fermentation (13, 18). Biogas production accounted for 14 to 18% of electron loss in GRs but only 0 to 6% of that in ARs. The high availability of the anode at low Rext resulted in high CEs for both substrates. EEs were highest at 970 Ω (14% for AR2 and 9% for GR2), which was closest to the internal resistances of tested MFCs. Higher power density is earned when Rext equals Rint, according to the following equation: P = OCV2 {Rext/[A(Rext + Rint)2]} (6), where P is the power density, OCV is the open-circuit voltage, and A is the electrode area.
TABLE 3.
Average headspace gas compositions and electron flux distributions before and after changing external resistances
| MFC | Headspace gas composition (μmol) in stages: |
Electron flux (μeq/day) in stages 5 and 6g |
Electron distribution and energy efficiency (%) in stages 5 and 6g |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 and 3 |
5 and 6 |
|||||||||||
| CH4 | CO2 | CH4 | CO2 | Total | Electricity | Methane | CEa | Biogasb | Aerobicc | Undefinedd | EEe | |
| ACf | 1 ± 2 | 0 ± 0 | 0 ± 1 | 58 ± 21 | 143 ± 17 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 26 ± 3 | 74 ± 3 | 0 ± 0 |
| AR1 | 28 ± 6 | 59 ± 9 | 30 ± 17 | 25 ± 9 | 193 ± 58 | 43 ± 2 | 10 ± 1 | 25 ± 6 | 6 ± 1 | 21 ± 9 | 47 ± 5 | 11 ± 2 |
| AR2 | 30 ± 8 | 63 ± 13 | 24 ± 0 | 62 ± 17 | 347 ± 20 | 207 ± 3 | 14 ± 0 | 67 ± 6 | 5 ± 1 | 12 ± 2 | 17 ± 4 | 14 ± 1 |
| AR3 | 13 ± 3 | 57 ± 11 | 0 ± 0 | 88 ± 4 | 481 ± 4 | 327 ± 6 | 0 ± 0 | 76 ± 10 | 0 ± 0 | 9 ± 1 | 16 ± 4 | 4 ± 1 |
| GCf | 51 ± 6 | 0 ± 0 | 90 ± 13 | 49 ± 8 | 170 ± 5 | 0 ± 0 | 25 ± 2 | 0 ± 0 | 15 ± 1 | 21 ± 1 | 64 ± 1 | 0 ± 0 |
| GR1 | 46 ± 28 | 73 ± 6 | 91 ± 22 | 25 ± 16 | 224 ± 105 | 45 ± 2 | 29 ± 1 | 23 ± 9 | 18 ± 8 | 19 ± 12 | 41 ± 6 | 8 ± 3 |
| GR2 | 43 ± 67 | 67 ± 7 | 92 ± 9 | 50 ± 16 | 343 ± 31 | 191 ± 2 | 57 ± 1 | 56 ± 4 | 17 ± 0 | 10 ± 1 | 17 ± 3 | 9 ± 1 |
| GR3 | 54 ± 42 | 42 ± 37 | 84 ± 7 | 64 ± 14 | 543 ± 23 | 297 ± 0 | 67 ± 1 | 62 ± 5 | 14 ± 1 | 7 ± 1 | 16 ± 4 | 2 ± 0 |
CE, Coulombic efficiency.
Biogas = methane and hydrogen.
Aerobic oxidation calculated based on the oxygen transfer coefficient (1.45 × 10−4 cm/s) through the proton exchange membrane (Nafion) (22).
Undefined electron sink = 1 − (CE + biogas + aerobic).
EE, energy efficiency.
Control reactors were changed from open to closed circuit after stage 3.
For AC and GC, values were taken from stages 2 and 3 when they were operated in an open circuit (Rext = ∞).
Biogas production.
Headspace biogas consisted mainly of methane and carbon dioxide, with negligible hydrogen detected. For acetate-fed reactors, total methane production increased as Rext increased and the current decreased (Table 3). The nonwashing of anode chambers between batches did not affect methane production in ARs. Regardless of the current generation, ACs produced very little methane. GRs produced 43 to 54 μmol methane during stages 1 to 3 and 84 to 92 μmol in stages 5 and 6, during which the anode bottles were not washed with each medium change as they had been during stages 1 to 3 (Table 3). Regardless of the Rext, GRs produced similar levels of methane, but cycle length-averaged methanogenesis rates varied significantly due to longer operation in higher Rext. The nonelectrogenic GC produced an amount of methane similar to that of the closed-circuit GRs. The amount of produced carbon dioxide increased with decreasing Rext and increasing current generation, and no carbon dioxide was detected in nonelectrogenic ACs and GCs.
Rext effect on anode prokaryotic communities.
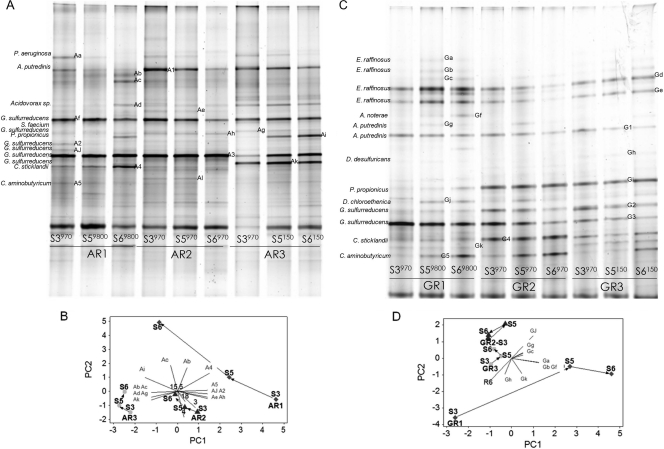
Bacterial 16S rRNA genes were screened by DGGE and identified by band sequence analysis to determine the Rext effect on the anode community composition and evolution (Fig. 1 and Table 4). Anode bacterial communities enriched at 970 Ω for four batches were compared with those that developed after the Rext changes. Densitometry-based PCA showed that Rext increasing to 9,800 Ω for both acetate- and glucose-fed MFCs changed the anode bacterial communities significantly, while continued operation at 970 Ω and a reduction to 150 Ω had little effect on them. The principal components for the community evolution observed in the 9,800-Ω systems were OTUs Ac, Ad, and Ai for AR1 and OTUs Ga, Gg, and GJ for GR1. OTUs G1, G2, G3, G4, and G5 of glucose-fed anode communities had nearly identical sequences and shared the same melting domains in DGGE correspondingly with OTUs A1, A2, A3, A4, and A5 of acetate-fed communities. Deltaproteobacteria and Bacteroidetes were the major taxonomic groups commonly found in the anode communities of ARs and GRs. Clostridia was also a common group found in ARs and GRs. Betaproteobacteria (Acidovorax species) and Gammaproteobacteria (Pseudomonas species) were found only in ARs, and Bacilli (Enterococcus species) were abundantly detected only in GRs. In order to identify targets for quantifying methanogenic communities, clone libraries of archaeal 16S rRNA genes were constructed. The methanogenic communities in our anode biofilms were dominated by Methanosaetaceae. Most of the retrieved clones (40 out of 42) had the closest match to Methanosaeta concilii Opfikon (GenBank accession number NR_028242), with >99% similarity. The other two clones were affiliated with uncultured hydrogenotrophic Methanomicrobiales (19).
FIG. 1.
Bacterial 16S rRNA gene-derived DGGE profiles (A and C) and their corresponding PCA plots (B and D), showing community evolution of anode biofilms in ARs (A and B) and GRs (C and D). S3 to S6, stages 3 to 6. Different external resistances were applied after stage 3. (Anode communities at stage 4 were not characterized.) A. putredinis, Alistipes putredinis; S. faecium, Sphingobacterium faecium; E. raffinosus, Enterococcus raffinosus; A. noterae, Acetoanaerobium noterae; D. desulfuricans, Desulfovibrio desulfuricans; D. chloroethenica, Desulfuromonas chloroethenica.
TABLE 4.
Phylogenetic identification of prominent DGGE bands
| Band | GenBank closest isolate or enrichment-derived sequence (accession no.) | Classa | Identity (%) |
|---|---|---|---|
| A1/G1 | Alistipes putredinis (AJ518876) | Bacteroidetes | 92 |
| A2/G2 | G. sulfurreducens (AE017180) | Deltaproteobacteria | 98 |
| A3/G3 | G. sulfurreducens (AE017180) | Deltaproteobacteria | 99 |
| A4/G4 | C. sticklandii (M26494) | Clostridia | 100 |
| A5/G5 | C. aminobutyricum (X76161) | Clostridia | 99 |
| Aa | P. aeruginosa(EF650089) | Gammaproteobacteria | 100 |
| Ac | Bacterial enrichment (FJ802369) | NA | 93 |
| Ad | Acidovorax sp. strain Ic3 (DQ421392) | Betaproteobacteria | 100 |
| Ae | Bacterial enrichment (EU082059) | NA | 93 |
| Af | G. sulfurreducens (AE017180) | Deltaproteobacteria | 96 |
| Ag | Sphingobacterium faecium (NR_025537) | Sphingobacteria | 88 |
| Ah | G. sulfurreducens (AE017180) | Deltaproteobacteria | 96 |
| Ai | P. propionicus (CP000482) | Deltaproteobacteria | 96 |
| AJ | G. sulfurreducens (AE017180) | Deltaproteobacteria | 98 |
| Ak | G. sulfurreducens (AE017180) | Deltaproteobacteria | 95 |
| Ga | Enterococcus raffinosus (FN600541) | Bacilli | 99 |
| Gb | Enterococcus raffinosus (FN600541) | Bacilli | 100 |
| Gd | Enterococcus raffinosus (FN600541) | Bacilli | 99 |
| Ge | Enterococcus raffinosus (FN600541) | Bacilli | 99 |
| Gg | Alistipes putredinis (AJ518876) | Bacteroidetes | 93 |
| Gh | Desulfovibrio desulfuricans (FJ655841) | Deltaproteobacteria | 97 |
| Gi | P. propionicus (CP000482) | Deltaproteobacteria | 96 |
| GJ | Desulfuromonas chloroethenica (NR_026012) | Deltaproteobacteria | 91 |
NA, not applicable.
Quantification of three functional groups in anode biofilms.
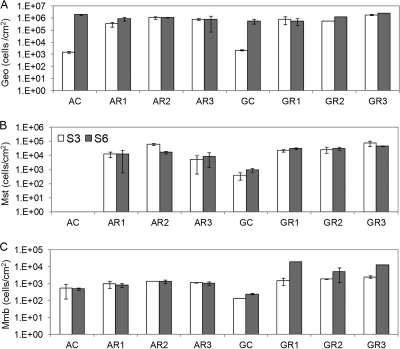
qPCR was used to quantify the cell number changes of three major functional groups (Geobacteraceae, Methanosaetaceae, and Methanomicrobiales) in the anode biofilms. In general, the Rext change did not consistently affect the abundances of these functional groups (Fig. 2), though these data do not reveal their relative abundances in the total anode communities. The average cell numbers of Geobacteraceae were 1.7 × 106 ± 5.8 × 105 cells/cm2 in ARs and 2.5 × 106 ± 1.6 × 106 cells/cm2 in GRs. When ACs and GCs produced electricity, Geobacteraceae cell numbers increased drastically. The average Methanosaetaceae density was 2.0 × 104 ± 2.2 × 104 cells/cm2 in ARs and 3.9 × 104 ± 1.9 × 104 cells/cm2 in GRs. The average Methanomicrobiales density was 1.1 × 103 ± 2.1 × 102 cells/cm2 in ARs. However, the average Methanomicrobiales density in GRs increased ca. 5 times from 2.0 × 103 cells/cm2 in stage 3 to 1.2 × 104 cells/cm2 in stage 6, with a doubling of methane production (Table 3). This coincided with the discontinuation of anode chamber washing between feedings, but nonwashing did not affect methane production or methanogen cell numbers in ARs. Regardless of the current generation, ACs produced very little methane and had only Methanomicrobiales cells. The nonelectrogenic GCs had less methanogenic cells in the anode biofilm but produced amounts of methane similar to those of the closed-circuit GRs.
FIG. 2.
Densities of Geobacteraceae (Geo), Methanosaetaceae (Mst), and Methanomicrobiales (Mmb) in anode biofilms at stage 3 (white) and stage 6 (gray), measured by quantitative PCR (n = 2).
Electricity production of open-circuit-acclimated anode biofilms.
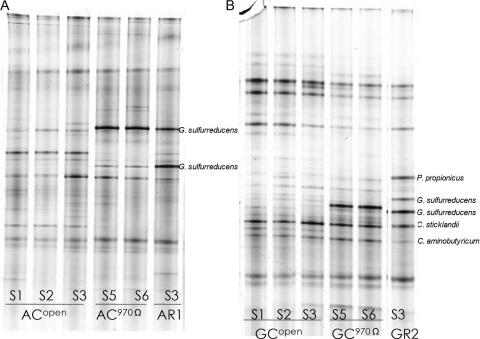
The open-circuit Ean values of ACs and GCs were initially ca. 50 mV, gradually decreasing to ca. −300 mV at the end of stage 1. In stage 1, acetate was barely consumed in ACs, while nearly all of the glucose added to GCs was converted to and accumulated as acetate. In successive batches, Ean remained ca. −300 mV, and metabolite concentrations fluctuated in both reactors. When circuits were connected with 970 Ω at stage 4, the current was generated immediately in both systems, and their Ean values rapidly increased to levels similar to those of AR2 and GR2. DGGE profiles of anode communities during stages 1 to 3 were similar and showed that Geobacteraceae sequences existed in the anode biofilms during open-circuit operation. Despite the unavailability of the anode for exoelectrogenic activity under the open-circuit condition, substantial substrate was consumed in these reactors during stages 2 and 3 (Table 3). Bands representing Geobacteraceae increased their intensities after circuit connection, which is consistent with the qPCR quantification, showing that the Geobacteraceae cell number increased ca. 103 times (Fig. 3). Anode biofilm profiles of electrogenic ACs and GCs had high similarities to those of AR1 and GR2, respectively.
FIG. 3.
Evolution of bacterial 16S rRNA gene-derived DGGE profiles from control reactor anode biofilms before and after electrogenesis. (A) Acetate-fed MFCs; (B) glucose-fed MFCs. S1 to S6, stages 1 to 6. Control reactors were changed from open to closed circuit after stage 3.
DISCUSSION
External resistance effects on anode community evolution.
Our results show that high Rext affected the exoelectrogenic communities that had been established at 970 Ω. Rext regulates Ean, which is equivalent to the anode availability as an electron acceptor. Ean influenced the competition between exoelectrogenic and nonexoelectrogenic community members, as supported by changes in the MFC performance with respect to electron distribution and biogas production (Table 3). Ean might also influence competition among exoelectrogens, either indirectly through microenvironmental conditions or directly through anode utilization, though we do not have direct evidence of this. For example, Rext influences the substrate consumption rate (Table 3) and the associated proton production rate in the anode biofilm, so it can affect pH in the anode biofilm (12, 50). The anode biofilm community has been shown previously to be affected by pH in biohydrogenesis processes (44).
Regarding the direct effects of Ean on competition among exoelectrogenic populations, the lowest Ean (where the community changes were most pronounced) would select for exoelectrogens that can still meet their metabolic energy needs with such a small potential gradient between the redox potential of their electron donor and the anode. Most known Geobacter strains become significantly limited below potentials of about −0.15 V (45). Bacteria might adapt themselves to a specific potential by regulating intracellular reducing equivalents, such as the NADH/NAD+ ratio, depending on the potential of the electron acceptor (30). For Escherichia coli, the NADH/NAD+ ratio was 0.094 in aerobic growth and 0.22 in anaerobic growth (28) and increased progressively with decreasing midpoint potential of the electron acceptor (9). More fundamental bioelectrochemical studies are needed to address this redox issue.
Anode bacterial ecology.
Geobacter strains appear to need a small amount of bicarbonate in the medium for crucial reactions, such as carbon fixation mediated by pyruvate-ferredoxin oxidoreductase (33). Accordingly, a low density of Geobacter cells in MFCs was thought to be associated with their poor growth in phosphate-buffered medium because high numbers of Geobacter 16S rRNA gene sequences were detected in carbonate-buffered anode chambers with scarce oxygen (4, 17). However, our result showing Geobacter-like cell dominance in phosphate-buffered MFCs with the headspace filled with nitrogen is coincident with previous results under the same condition (18, 25, 57). It shows that the initial buffer species might not be an important factor in terms of Geobacter dominance in mixed-culture BESs, in which bicarbonate can be produced by other community members.
Desulfuromonas-like sequences were found in our GRs and were also detected in the anode community of an ethanol-fed MFC (23). Desulfuromonas acetoxidans is an acetate-utilizing electricity-producing species (2) and also oxidizes ethanol coupled with iron reduction (48). Pelobacter-like sequences detected in our systems were observed in sediment MFCs (15) and were predominant in ethanol-fed MECs (39). Pelobacter propionicus-like sequences were also abundant in an acetate-fed MEC system (5). Acidovorax sp. strain Ic3 is a hydrogenotrophic denitrifying bacterium (54), and similar sequences in acetate-fed anode biofilms (3, 57) and a cathode biofilm (42) were detected. These molecular evidences imply more research opportunities on potential electrogenesis of these genera. Pseudomonas aeruginosa produces electricity using electron shuttles (pyocyanin) (41), and related sequences in the anode biofilm of an acetate-fed MFC (57) were found, consistent with our results. Enterococcus is a genus of fermentative lactic acid bacteria, and an isolate of Enterococcus gallinarum exhibited oxidation and reduction peaks in cyclic voltammetry (21).
Bacteroidetes and Clostridia were common taxonomic groups in the anode biofilms of ARs and GRs. Bacteroidetes have been found in anode microbial communities of BESs fed starch-processing wastewater (20), cellulose (47), and acetate or glucose (57), but whether they contribute an exoelectrogenic phenotype in these communities is not known due to the absence of isolates from BESs. Clostridium sticklandii performs Stickland reactions, oxidizing an amino acid by reducing another amino acid (49). Clostridium aminobutyricum (GenBank accession number X76161; 99%) ferments 4-aminobutyrate to ammonia, acetate, and butyrate. Both species were also found in acetate- or glucose-fed single-chamber MFCs (57).
Methanogenic archaeal ecology.
Methanogenesis was not affected by Ean in GRs. The nonwashing of anode chambers might have resulted in higher levels of methanogenesis during stages 5 and 6 in GRs, due to adhered microbial communities on the reactor walls that were not reflected in the qPCR data from anode electrodes. In ARs, the nonwashing of the anode chambers did not increase methanogenesis. Methanogenesis occurred only in MFCs harboring Methanosaetaceae-like sequences in their anode biofilms, suggesting that acetoclastic Methanosaetaceae can be the main methanogenic source in acetate-fed MFCs. As the Ean became higher, the current generation increased and methanogenesis diminished (Table 3). Ean might affect competition between exoelectrogens and acetoclastic methanogens in acetate-fed MFCs, with Methanosaetaceae being outcompeted in higher-current MFCs due to their lower affinity for and specific substrate utilization rate with acetate. For instance, pure culture Geobacter sulfurreducens has a half-saturation concentration (Ks) for acetate of 6.4 mg COD/liter (0.10 mM) and a specific substrate utilization rate (qmax) of 22.7 mg COD/mg volatile suspended solids (VSS) day−1 under ferric citrate-respiring conditions, while Methanosaeta spp. in methanogenic reactors have a Ks of 49 mg COD/liter and a qmax of 10.1 mg COD/mg VSS day−1 (8, 11). Thus, if anode availability is not limited, exoelectrogens could have a kinetic advantage over methanogens for acetate.
Not only acetoclastic Methanosaetaceae but also hydrogenotrophic Methanomicrobiales were detected in our systems. Hydrogenotrophic methanogenesis was suggested to be a main methanogenic pathway in MECs (7, 26). In an ethanol-fed MFC, Methanomicrobiales were the only methanogen (38); Methanosaetaceae might have been suppressed by the high concentration of substrate (initial COD of 2,500 mg/liter of ethanol) in this system due to their inhibition by high organic carbon concentrations (19). No Methanosaetaceae in the anode biofilms of ACs could be due to the initial oxic condition. During anode biofilm acclimation, the higher biomasses of the exoelectrogenic communities in closed-circuit ARs could create an ecological niche for Methanosaetaceae by consuming oxygen.
The differences between the internal resistances and ohmic resistances were 282 ± 84 Ω for ARs and 355 ± 104 Ω for GRs. Assuming that the overpotentials of abiotic cathodes were similar due to the high reproducibility of commercial cathode electrodes used in this experiment, the larger difference for GRs could be due to current-dependent anode overpotential, such as microbial activation, or metabolic loss, such as indirect glucose oxidation by mixed cultures (13, 18, 57) and higher diversity of anode biofilms in GRs. However, the high ohmic resistance of H-shaped MFCs precludes further electrochemical analysis on anode biofilm kinetics because power densities and current densities are limited by ohmic resistances in H-shaped MFCs, as shown previously (18). This ohmic limitation should be resolved by using BESs with low ohmic resistances.
Acknowledgments
This research was supported by National Science Foundation grant CBET-0834033.
We thank Jungrae Kim (Sustainable Environment Research Center, United Kingdom) and Kion Kim (Department of Statistics, Penn State University) for their useful suggestions.
Footnotes
Published ahead of print on 12 November 2010.
REFERENCES
- 1.Aelterman, P., S. Freguia, J. Keller, W. Verstraete, and K. Rabaey. 2008. The anode potential regulates bacterial activity in microbial fuel cells. Appl. Microbiol. Biotechnol. 78:409-418. [DOI] [PubMed] [Google Scholar]
- 2.Bond, D. R., D. E. Holmes, L. M. Tender, and D. R. Lovley. 2002. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295:483-485. [DOI] [PubMed] [Google Scholar]
- 3.Borole, A. P., C. Y. Hamilton, T. Vishnivetskaya, D. Leak, and C. Andras. 2009. Improving power production in acetate-fed microbial fuel cells via enrichment of exoelectrogenic organisms in flow-through systems. Biochem. Eng. J. 48:71-80. [Google Scholar]
- 4.Call, D. F., R. C. Wagner, and B. E. Logan. 2009. Hydrogen production by Geobacter species and a mixed consortium in a microbial electrolysis cell. Appl. Environ. Microbiol. 75:7579-7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chae, K.-J., M.-J. Choi, J. Lee, F. F. Ajayi, and I. S. Kim. 2008. Biohydrogen production via biocatalyzed electrolysis in acetate-fed bioelectrochemical cells and microbial community analysis. Int. J. Hydrogen Energy 33:5184-5192. [Google Scholar]
- 6.Cheng, S., H. Liu, and B. E. Logan. 2006. Increased power generation in a continuous flow MFC with advective flow through the porous anode and reduced electrode spacing. Environ. Sci. Technol. 40:2426-2432. [DOI] [PubMed] [Google Scholar]
- 7.Clauwaert, P., and W. Verstraete. 2009. Methanogenesis in membraneless microbial electrolysis cells. Appl. Microbiol. Biotechnol. 82:829-836. [DOI] [PubMed] [Google Scholar]
- 8.Conklin, A., H. D. Stensel, and J. Ferguson. 2006. Growth kinetics and competition between Methanosarcina and Methanosaeta in mesophilic anaerobic digestion. Water Environ. Res. 78:486-496. [DOI] [PubMed] [Google Scholar]
- 9.de Graef, M. R., S. Alexeeva, J. L. Snoep, and M. J. Teixeira de Mattos. 1999. The steady-state internal redox state (NADH/NAD) reflects the external redox state and is correlated with catabolic adaptation in Escherichia coli. J. Bacteriol. 181:2351-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLong, E. F., L. T. Taylor, T. L. Marsh, and C. M. Preston. 1999. Visualization and enumeration of marine planktonic archaea and bacteria by using polyribonucleotide probes and fluorescent in situ hybridization. Appl. Environ. Microbiol. 65:5554-5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esteve-Nunez, A., M. Rothermich, M. Sharma, and D. Lovley. 2005. Growth of Geobacter sulfurreducens under nutrient-limiting conditions in continuous culture. Environ. Microbiol. 7:641-648. [DOI] [PubMed] [Google Scholar]
- 12.Franks, A. E., K. P. Nevin, H. Jia, M. Izallalen, T. L. Woodard, and D. R. Lovley. 2009. Novel strategy for three-dimensional real-time imaging of microbial fuel cell communities: monitoring the inhibitory effects of proton accumulation within the anode biofilm. Energy Environ. Sci. 2:113-119. [Google Scholar]
- 13.Freguia, S., K. Rabaey, Z. G. Yuan, and J. Keller. 2008. Syntrophic processes drive the conversion of glucose in microbial fuel cell anodes. Environ. Sci. Technol. 42:7937-7943. [DOI] [PubMed] [Google Scholar]
- 14.Hamann, C., A. Hamnett, and W. Vielstich. 2007. Electrochemistry, 2nd ed., p. 77. Wiley-VCH, Weinheim, NY.
- 15.Holmes, D. E., D. R. Bond, R. A. O'Neil, C. E. Reimers, L. R. Tender, and D. R. Lovley. 2004. Microbial communities associated with electrodes harvesting electricity from a variety of aquatic sediments. Microb. Ecol. 48:178-190. [DOI] [PubMed] [Google Scholar]
- 16.Holmes, D. E., K. T. Finneran, R. A. O'Neil, and D. R. Lovley. 2002. Enrichment of members of the family Geobacteraceae associated with stimulation of dissimilatory metal reduction in uranium-contaminated aquifer sediments. Appl. Environ. Microbiol. 68:2300-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishii, S. I., K. Watanabe, S. Yabuki, B. E. Logan, and Y. Sekiguchi. 2008. Comparison of electrode reduction activities of Geobacter sulfurreducens and an enriched consortium in an air-cathode microbial fuel cell. Appl. Environ. Microbiol. 74:7348-7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung, S., and J. M. Regan. 2007. Comparison of anode bacterial communities and performance in microbial fuel cells with different electron donors. Appl. Microbiol. Biotechnol. 77:393-402. [DOI] [PubMed] [Google Scholar]
- 19.Karakashev, D., D. J. Batstone, and I. Angelidaki. 2005. Influence of environmental conditions on methanogenic compositions in anaerobic biogas reactors. Appl. Environ. Microbiol. 71:331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, B. H., H. S. Park, H. J. Kim, G. T. Kim, I. S. Chang, J. Lee, and N. T. Phung. 2004. Enrichment of microbial community generating electricity using a fuel-cell-type electrochemical cell. Appl. Microbiol. Biotechnol. 63:672-681. [DOI] [PubMed] [Google Scholar]
- 21.Kim, G. T., M. S. Hyun, I. S. Chang, H. J. Kim, H. S. Park, B. H. Kim, S. D. Kim, J. W. Wimpenny, and A. J. Weightman. 2005. Dissimilatory Fe(III) reduction by an electrochemically active lactic acid bacterium phylogenetically related to Enterococcus gallinarum isolated from submerged soil. J. Appl. Microbiol. 99:978-987. [DOI] [PubMed] [Google Scholar]
- 22.Kim, J. R., S. Cheng, S. E. Oh, and B. E. Logan. 2007. Power generation using different cation, anion, and ultrafiltration membranes in microbial fuel cells. Environ. Sci. Technol. 41:1004-1009. [DOI] [PubMed] [Google Scholar]
- 23.Kim, J. R., S. H. Jung, J. M. Regan, and B. E. Logan. 2007. Electricity generation and microbial community analysis of alcohol powered microbial fuel cells. Bioresour. Technol. 98:2568-2577. [DOI] [PubMed] [Google Scholar]
- 24.Kim, J. R., B. Min, and B. E. Logan. 2005. Evaluation of procedures to acclimate a microbial fuel cell for electricity production. Appl. Microbiol. Biotechnol. 68:23-30. [DOI] [PubMed] [Google Scholar]
- 25.Lee, H.-S., P. Parameswaran, A. Kato-Marcus, C. I. Torres, and B. E. Rittmann. 2008. Evaluation of energy-conversion efficiencies in microbial fuel cells (MFCs) utilizing fermentable and non-fermentable substrates. Water Res. 42:1501-1510. [DOI] [PubMed] [Google Scholar]
- 26.Lee, H.-S., C. S. I. Torres, P. Parameswaran, and B. E. Rittmann. 2009. Fate of H2 in an upflow single-chamber microbial electrolysis cell using a metal-catalyst-free cathode. Environ. Sci. Technol. 43:7971-7976. [DOI] [PubMed] [Google Scholar]
- 27.Lee, Z. M., C. Bussema III, and T. M. Schmidt. 2009. rrnDB: documenting the number of rRNA and tRNA genes in bacteria and archaea. Nucleic Acids Res. 37:D489-D493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leonardo, M. R., Y. Dailly, and D. P. Clark. 1996. Role of NAD in regulating the adhE gene of Escherichia coli. J. Bacteriol. 178:6013-6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, H., S. Cheng, and B. E. Logan. 2005. Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ. Sci. Technol. 39:658-662. [DOI] [PubMed] [Google Scholar]
- 30.Logan, B. E. 2008. Microbial fuel cells. Wiley-Interscience, Hoboken, NJ.
- 31.Logan, B. E., and J. M. Regan. 2006. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 14:512-518. [DOI] [PubMed] [Google Scholar]
- 32.Lyon, D. Y., F. Buret, T. M. Vogel, and J.-M. Monier. 2010. Is resistance futile? Changing external resistance does not improve microbial fuel cell performance. Bioelectrochemistry 78:2-7. [DOI] [PubMed] [Google Scholar]
- 33.Mahadevan, R., D. R. Bond, J. E. Butler, A. Esteve-Nunez, M. V. Coppi, B. O. Palsson, C. H. Schilling, and D. R. Lovley. 2006. Characterization of metabolism in the Fe(III)-reducing organism Geobacter sulfurreducens by constraint-based modeling. Appl. Environ. Microbiol. 72:1558-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcus, A. K., C. I. Torres, and B. E. Rittmann. 2007. Conduction-based modeling of the biofilm anode of a microbial fuel cell. Biotechnol. Bioeng. 98:1171-1182. [DOI] [PubMed] [Google Scholar]
- 35.Methe, B. A., K. E. Nelson, J. A. Eisen, I. T. Paulsen, W. Nelson, J. F. Heidelberg, D. Wu, M. Wu, N. Ward, M. J. Beanan, R. J. Dodson, R. Madupu, L. M. Brinkac, S. C. Daugherty, R. T. DeBoy, A. S. Durkin, M. Gwinn, J. F. Kolonay, S. A. Sullivan, D. H. Haft, J. Selengut, T. M. Davidsen, N. Zafar, O. White, B. Tran, C. Romero, H. A. Forberger, J. Weidman, H. Khouri, T. V. Feldblyum, T. R. Utterback, S. E. Van Aken, D. R. Lovley, and C. M. Fraser. 2003. Genome of Geobacter sulfurreducens: metal reduction in subsurface environments. Science 302:1967-1969. [DOI] [PubMed] [Google Scholar]
- 36.Muyzer, G., T. Brinkhoff, U. Nubel, C. Santegoeds, H. Schafer, and C. Wawer. 2004. Molecular microbial ecology manual, 2nd ed., p. 743-770. Kluwer Academic Publishers, Dordrecht, Netherlands.
- 37.Nicol, G. W., D. Tscherko, T. M. Embley, and J. I. Prosser. 2005. Primary succession of soil Crenarchaeota across a receding glacier foreland. Environ. Microbiol. 7:337-347. [DOI] [PubMed] [Google Scholar]
- 38.Parameswaran, P., C. I. Torres, H.-S. Lee, R. Krajmalnik-Brown, and B. E. Rittmann. 2009. Syntrophic interactions among anode respiring bacteria (ARB) and non-ARB in a biofilm anode: electron balances. Biotechnol. Bioeng. 103:513-523. [DOI] [PubMed] [Google Scholar]
- 39.Parameswaran, P., H. Zhang, C. I. Torres, B. E. Rittmann, and R. Krajmalnik-Brown. 2009. Microbial community structure in a biofilm anode fed with a fermentable substrate: the significance of hydrogen scavengers. Biotechnol. Bioeng. 105:69-78. [DOI] [PubMed] [Google Scholar]
- 40.Pham, T. H., P. Aelterman, and W. Verstraete. 2009. Bioanode performance in bioelectrochemical systems: recent improvements and prospects. Trends Biotechnol. 27:168-178. [DOI] [PubMed] [Google Scholar]
- 41.Rabaey, K., N. Boon, M. Hofte, and W. Verstraete. 2005. Microbial phenazine production enhances electron transfer in biofuel cells. Environ. Sci. Technol. 39:3401-3408. [DOI] [PubMed] [Google Scholar]
- 42.Rabaey, K., S. T. Read, P. Clauwaert, S. Freguia, P. L. Bond, L. L. Blackall, and J. Keller. 2008. Cathodic oxygen reduction catalyzed by bacteria in microbial fuel cells. ISME J. 2:519-527. [DOI] [PubMed] [Google Scholar]
- 43.Rabaey, K., J. Rodriguez, L. L. Blackall, J. Keller, P. Gross, D. Batstone, W. Verstraete, and K. H. Nealson. 2007. Microbial ecology meets electrochemistry: electricity-driven and driving communities. ISME J. 1:9-18. [DOI] [PubMed] [Google Scholar]
- 44.Ren, N., D. Xing, B. E. Rittmann, L. Zhao, T. Xie, and X. Zhao. 2007. Microbial community structure of ethanol type fermentation in bio-hydrogen production. Environ. Microbiol. 9:1112-1125. [DOI] [PubMed] [Google Scholar]
- 45.Richter, H., K. P. Nevin, H. Jia, D. A. Lowy, D. R. Lovley, and L. M. Tender. 2009. Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type IV pili, and protons in extracellular electron transfer. Energy Environ. Sci. 2:506-516. [Google Scholar]
- 46.Rismani-Yazdi, H., A. D. Christy, S. M. Carver, Z. Yu, B. A. Dehority, and O. H. Tuovinen. 2011. Effect of external resistance on bacterial diversity and metabolism in cellulose-fed microbial fuel cells. Bioresour. Technol. 102:278-283. [DOI] [PubMed] [Google Scholar]
- 47.Rismani-Yazdi, H., A. D. Christy, B. A. Dehority, M. Morrison, Z. Yu, and O. H. Tuovinen. 2007. Electricity generation from cellulose by rumen microorganisms in microbial fuel cells. Biotechnol. Bioeng. 97:1398-1407. [DOI] [PubMed] [Google Scholar]
- 48.Roden, E. E., and D. R. Lovley. 1993. Dissimilatory Fe(III) reduction by the marine microorganism Desulfuromonas acetoxidans. Appl. Environ. Microbiol. 59:734-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stadtman, T. C., and L. S. McClung. 1957. Clostridium sticklandii nov. spec. J. Bacteriol. 73:218-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torres, C. I., A. Kato Marcus, and B. E. Rittmann. 2008. Proton transport inside the biofilm limits electrical current generation by anode-respiring bacteria. Biotechnol. Bioeng. 100:872-881. [DOI] [PubMed] [Google Scholar]
- 51.Torres, C. I., A. K. Marcus, H. S. Lee, P. Parameswaran, R. Krajmalnik-Brown, and B. E. Rittmann. 2010. A kinetic perspective on extracellular electron transfer by anode-respiring bacteria. FEMS Microbiol. Rev. 34:3-17. [DOI] [PubMed] [Google Scholar]
- 52.Torres, C. I., A. K. Marcus, P. Parameswaran, and B. E. Rittmann. 2008. Kinetic experiments for evaluating the Nernst-Monod model for anode-respiring bacteria (ARB) in a biofilm anode. Environ. Sci. Technol. 42:6593-6597. [DOI] [PubMed] [Google Scholar]
- 53.Torres, C. S. I., R. Krajmalnik-Brown, P. Parameswaran, A. K. Marcus, G. Wanger, Y. A. Gorby, and B. E. Rittmann. 2009. Selecting anode-respiring bacteria based on anode potential: phylogenetic, electrochemical, and microscopic characterization. Environ. Sci. Technol. 43:9519-9524. [DOI] [PubMed] [Google Scholar]
- 54.Vasiliadou, I. A., S. Siozios, I. T. Papadas, K. Bourtzis, S. Pavlou, and D. V. Vayenas. 2006. Kinetics of pure cultures of hydrogen-oxidizing denitrifying bacteria and modeling of the interactions among them in mixed cultures. Biotechnol. Bioeng. 95:513-525. [DOI] [PubMed] [Google Scholar]
- 55.Virdis, B., K. Rabaey, Z. Yuan, R. A. Rozendal, and J. R. Keller. 2009. Electron fluxes in a microbial fuel cell performing carbon and nitrogen removal. Environ. Sci. Technol. 43:5144. [DOI] [PubMed] [Google Scholar]
- 56.Wang, X., Y. Feng, N. Ren, H. Wang, H. Lee, N. Li, and Q. Zhao. 2009. Accelerated start-up of two-chambered microbial fuel cells: effect of anodic positive poised potential. Electrochim. Acta 54:1109-1114. [Google Scholar]
- 57.Xing, D., S. Cheng, J. M. Regan, and B. E. Logan. 2009. Change in microbial communities in acetate- and glucose-fed microbial fuel cells in the presence of light. Biosens. Bioelectron. 25:105-111. [DOI] [PubMed] [Google Scholar]
- 58.Yu, Y., C. Lee, J. Kim, and S. Hwang. 2005. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol. Bioeng. 89:670-679. [DOI] [PubMed] [Google Scholar]