Abstract
Partial sleep deprivation is increasingly common in modern society. This study examined for the first time if partial sleep deprivation alters circadian phase shifts to bright light in humans. Thirteen young healthy subjects participated in a repeated-measures counterbalanced design with 2 conditions. Each condition had baseline sleep, a dim-light circadian phase assessment, a 3-day phase-advancing protocol with morning bright light, then another phase assessment. In one condition (no sleep deprivation), subjects had an 8-h sleep opportunity per night during the advancing protocol. In the other condition (partial sleep deprivation), subjects were kept awake for 4 h in near darkness (<0.25 lux), immediately followed by a 4-h sleep opportunity per night during the advancing protocol. The morning bright light stimulus was four 30-min pulses of bright light (~5000 lux), separated by 30-min intervals of room light. The light always began at the same circadian phase, 8 h after the baseline dim-light melatonin onset (DLMO). The average phase advance without sleep deprivation was 1.8 ± 0.6 (SD) h, which reduced to 1.4 ± 0.6 h with partial sleep deprivation (p < 0.05). Ten of the 13 subjects showed reductions in phase advances with partial sleep deprivation, ranging from 0.2 to 1.2 h. These results indicate that short-term partial sleep deprivation can moderately reduce circadian phase shifts to bright light in humans. This may have significant implications for the sleep-deprived general population and for the bright light treatment of circadian rhythm sleep disorders such as delayed sleep phase disorder.
Keywords: circadian, human, light, melatonin, phase, sleep deprivation
There is increasing evidence that much of modern society is partially sleep deprived. The results of the 2010 National Sleep Foundation poll suggest that up to 27% of Americans regularly sleep 6 h or less each weeknight and up to 15% of Americans do the same on weekends (National Sleep Foundation, 2010). This trend appears to be increasing over time, as the number of full-time workers who reported sleeping less than 6 h per day in time diaries significantly increased between 1975 and 2006 (Knutson et al., 2010). Potential causes of this increasing trend of curtailing sleep include increased time spent working and commuting and a reluctance to curtail social and leisure time for more sleep (Basner et al., 2007; Knutson et al., 2010).
Previous research has examined the interaction of circadian phase with varying durations of wakefulness on measures of mood and performance (Boivin et al., 1997). However, one basic unresolved question is whether sleep deprivation can alter circadian phase shifts to light in humans. As bright light is used to treat a variety of circadian rhythm sleep disorders (Reid and Burgess, 2005), it is important to learn if partial sleep deprivation alters phase shifts to light. Studies in hamsters and mice suggest that sleep deprivation can reduce phase shifts to light. One study in Syrian hamsters indicated that after as little as 6 h of sleep deprivation, phase delays in response to 30 min of 50-lux light decreased to less than 15% of the phase delays seen in non–sleep-deprived hamsters (Mistlberger et al., 1997). A second study in mice found that after 8 h of sleep deprivation, phase delays in response to a 10-min pulse of 100 lux were reduced by 30% when compared to non–sleep-deprived mice (Challet et al., 2001).
Recently, our group reported that in humans, 2 weeks of sleeping 6.6 h per day significantly attenuated both phase advances and phase delays to light when compared to 2 weeks of sleeping 8.0 to 8.3 h per day (Burgess and Eastman, 2005, 2006). While these findings suggested that curtailing one’s sleep would reduce phase shifts to light, these results could have been due to several factors, including not only partial sleep deprivation but also associated changes in the light/dark cycle. Whether sleep deprivation per se can alter phase shifts to light in humans has not been previously investigated. Therefore, this study examined the effect of partial sleep deprivation on phase shifts to light in humans, while controlling for changes in the light-dark cycle.
MATERIALS AND METHODS
Subjects
Fourteen healthy subjects participated (9 men, 5 women; mean age ± SD, 28.0 ± 5.2 years; body mass index, 25.2 ± 2.7 kg/m2). All subjects were nonsmokers, consumed moderate caffeine (<300 mg/d) and alcohol doses (<2 drinks/day), and reported no medical, psychiatric, or sleep disorders as assessed from in-person interviews and several screening questionnaires (Minnesota Multiphasic Personality Inventory-2 [Butcher et al., 1989], Beck Depression Inventory [Beck et al., 1961], Pittsburgh Sleep Quality Index [Buysse et al., 1989], and part of a general health questionnaire [Tasto et al., 1978]). All subjects were medication free except for one woman who used hormonal birth control. A urine drug screen confirmed that all subjects were free of common drugs of abuse. No subject was color blind as determined by the Ishihara test. No subject had worked night shifts or traveled across more than 1 time zone in the month preceding the study. As part of the screening for the study, subjects were required to record their bed and wake times for a week. Subjects with an average sleep length of <6.5 h or >8.4 h were excluded. The self-reported mean (±SD) sleep schedule in the week before the study was 0019 ± 1.1 h to 0812 ± 1.2 h. Morningness-eveningness was assessed (Horne and Ostberg, 1976) prior to the start of the study, and there was 1 moderate morning, 11 neither, and 2 moderate evening types. The protocol was approved by the Rush University Medical Center Institutional Review Board. Subjects were recruited via fliers and Internet postings. All subjects gave written informed consent prior to participation and were compensated for their participation.
Protocol
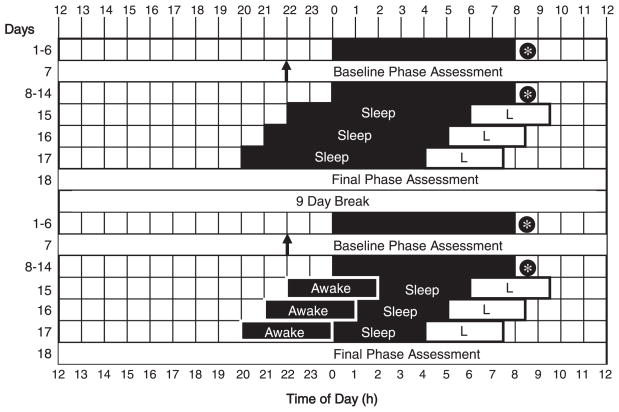
The study was a within-subjects repeated-measures design consisting of 2 parts (Fig. 1). Each part had 6 baseline nights at home, a baseline phase assessment in the laboratory, 7 more baseline nights at home, followed by a 3-day phase-advancing protocol and final phase assessment in the laboratory. The 2 parts differed in the length of sleep opportunity subjects had during the 3-day phase-advancing protocol. In one condition (“no sleep deprivation”), subjects had an 8-h sleep opportunity per night in the phase-advancing protocol. In the other condition (“partial sleep deprivation”), subjects were kept awake for 4 h in near darkness, immediately followed by a 4-h sleep opportunity per night during the phase-advancing protocol. In this way, the light-dark cycle was very similar in both conditions, but the duration of the sleep opportunity varied. There was a 9-day break between the 2 parts of the study during which subjects returned to their baseline sleep times. The 2 parts of the study were counterbalanced across subjects: 6 subjects completed the no sleep deprivation condition first, and 8 subjects completed the partial sleep deprivation condition first.
Figure 1.
A sample protocol for a subject who typically slept from midnight to 0800 h. There were 6 days of baseline sleep at home (days 1–6), a baseline phase assessment in the laboratory (day 7), a further week of baseline sleep at home (days 8–14), followed by a 3-day phase-advancing protocol in the laboratory (days 15–17) and then a final phase assessment (day 18). After a 9-day break, this sequence was repeated. In the no sleep deprivation condition (top), there was an 8-h sleep opportunity per night in the phase-advancing protocol. In the partial sleep deprivation condition (bottom), there was 4 h of enforced wakefulness in near darkness, followed by a 4-h sleep opportunity per night in the phase-advancing protocol. Thus, the light-dark cycle was similar in both conditions, but the duration of the sleep opportunity varied. The 2 conditions were counterbalanced across subjects. Black shading = sleep/dark episodes at night. * = at least 10 min of morning outdoor light. ↑= time of dim light melatonin onset. L = advancing bright light stimulus: four 30-min bright light pulses alternating with room light, starting 8 h after the baseline dim light melatonin onset, and advancing by 1 h on the second and third mornings. For clarity, the phase assessments are shown as starting and ending at 1200 h.
Sleep at Home
All subjects slept at home except during the 3 days of the phase-advancing protocol when they slept in the laboratory. Subjects were assigned a home sleep schedule that was within 1 h of their self-reported habitual sleep times. During their scheduled sleep/dark episodes, subjects were instructed to lie in bed and try to sleep. Subjects were not permitted to read, watch television, listen to music, or talk on the telephone at this time. To ensure compliance to the study requirements, all subjects were required to call the laboratory voice mail (time and date of call was recorded) before turning out their lights at night and at their wake time each morning. Subjects completed daily sleep logs, noting bed time, estimated sleep-onset time, any awakenings during the night, and time of final awakening. Subjects were also required to go outside to receive a minimum of 10 min of morning light to mimic the morning light that many people receive every day. The light exposure had to occur within a 1-h window, starting 30 min after their scheduled wake time. Each subject also wore a waterproof actigraphy monitor with photosensor (Actiwatch-L, Respironics, Bend, OR) on their nondominant wrist and a second monitor attached to a cord around their neck during the entire study. This equipment recorded activity and light exposure every 30 sec. Subjects came to the laboratory every 1 to 3 days to meet with research staff, and the sleep logs and activity and light data from both monitors were inspected in their presence to ensure compliance to the study requirements.
Phase Assessments
Each subject experienced 4 dim-light phase assessments to determine their endogenous melatonin profiles (Fig. 1). Melatonin is a hormone synthesized and released from the pineal gland (Moore, 1978) and in dim light is a reliable marker of the circadian clock (Klerman et al., 2002; Lewy et al., 1999). The phase assessments were 21 to 24 h long and began between 1200 and 1900 h. During the phase assessments, subjects remained awake and seated in dim light (<5 lux, at the level of the subjects’ eyes, in the direction of gaze) (Minolta TL-1 light meter, Ramsey, NJ). Subjects gave a saliva sample every 30 min using Salivettes (Sarstedt, Newton, NC). Subjects were not permitted to consume any alcohol or caffeine after the third baseline day of the study and were breathalyzed at the start of each laboratory stay. Nonsteroidal anti-inflammatory drugs were not permitted during the entire study as these drugs can suppress melatonin (Murphy et al., 1996). Toothpaste or mouthwash was not allowed during the phase assessments. Small snacks and fluids were permitted, except in the 10 min before each sample, and subjects were required to rinse and brush their teeth with water while remaining seated 10 min before each sample if they had consumed food or drink. The samples were centrifuged immediately upon collection and frozen. These samples were shipped on dry ice to Pharmasan Laboratories (Osceola, WI) and radioimmunoassayed for melatonin. The sensitivity of the assay was 0.7 pg/mL, and intra-assay and interassay coefficients of variability were 12.1% and 13.2%, respectively. After each phase assessment, subjects traveled home either via a ride with friends/family or a reimbursed taxi ride. Subjects were only permitted to nap on the day a phase assessment ended, during a 3-h window centered 12 h from the center of their baseline sleep schedule.
Phase-Advancing Protocol
The timing of each phase-advancing protocol was dependent on the timing of each subject’s dim light melatonin onset (DLMO), as determined from the previous baseline phase assessment (Fig. 1). Subjects arrived at the laboratory 3 h before their baseline DLMO on day 15 and remained in the laboratory until the end of the final phase assessment. In the no sleep deprivation condition, subjects were put to bed in an individual temperature-controlled dark bedroom at the time of their DLMO. In the partial sleep deprivation condition, starting at the time of the baseline DLMO, subjects reclined in dim light (<5 lux, at the level of the subjects’ eyes, in the direction of gaze) (Minolta TL-1 light meter) and put on welder’s goggles (Flex Seal with Infra Dura 5.0 lens, Uvex, Fuerth, Germany). These goggles have a flexible silicone inner shield that provides a continuous seal to the face. The goggles were adjusted to fit each individual subject prior to the laboratory stay to ensure the goggles were “light tight.” As the lens in the goggles transmits only 0% to 5% of visible light, subjects received less than 0.25 lux at the level of the eye. Subjects wore the goggles continuously for the next 4 h and were kept awake by staff through conversation and word games. Subjects then had a 4-h sleep opportunity. Subjects only removed the goggles after the dim ambient light was extinguished and the room was dark.
Subjects were awakened at the end of each 8-h dark episode in both conditions and exposed to bright intermittent light spanning 3.5 h (mean intensity ± SD, 5068 ± 866 lux), 30 min alternating with 30 min of ordinary, dim room light of <120 lux (measured periodically at angle of gaze with Minolta TL-1 light meter). The bright light was produced by a single light box (61 × 61 × 10 cm, Enviro-Med, Vancouver, WA) placed on a desk about 40 cm in front of the subject’s eyes. Each light box had a diffuser screen and contained four 54-cm-long 40-W fluorescent horizontal tubes (Philips PL-L40W/41/RS/IS, 4100K, Amsterdam, the Netherlands). At this distance, subjects received 5.1 × 1015 photons/cm2/sec, and specifically 1.1 × 1015 photons/cm2/sec in the blue range (400–490 nm), with an irradiance of 1741 μW/cm2 (400–750 nm). The room light was produced by a ceiling fixture containing 3 fluorescent tubes. The timing of the sleep/dark episode and bright light exposure was advanced by 1 h per day over the next 2 days of the phase-advancing protocol. Following each 3-day phase-advancing protocol, subjects had a final phase assessment.
Measures of Mood and Performance
Subjects completed a “How Are You Feeling Right Now?” (HAYFRN) questionnaire 4 times per day throughout the study on a portable handheld device: within 15 min after their assigned wake time, in the 15 min before bed time, and at 2 additional times evenly spaced across the day. This questionnaire contains the Stanford Sleepiness Scale (SSS) (Hoddes et al., 1973) and 6 additional items relating to physical fatigue, mental fatigue, sadness, anxiety, irritability, and gastrointestinal distress, each rated on a 10-point scale (1 = “very little” to 10 = “very much”). After the questionnaire, subjects also completed a 5-min Psychomotor Vigilance Test (PVT) on the same device (Lamond et al., 2008). The PVT is a simple reaction time test designed to evaluate the ability to sustain attention and respond in a timely manner to salient signals (Dinges and Powell, 1985). In the PVT task, a bull’s-eye target appears on the screen at various intervals ranging between 2000 to 10,000 msec. When the response button is pressed (adjusted for left or right handedness), the reaction time appears in the center of the bull’s eye, providing feedback to the subject.
Data Analysis
Sleep, performance, and mood parameters
Total sleep time was derived from the wrist actigraphy recordings with the Actiware 5 program (medium sensitivity, Respironics). Total sleep time was also calculated from the sleep logs as the time between estimated sleep onset to final awakening minus any awakenings during the night. The results of each PVT test were analyzed to yield mean reaction time and mean number of lapses (reaction time >500 msec) (Lamond et al., 2008). The PVT variables and HAYFRN questionnaires completed 4 times per day were averaged to produce one value per day and then averaged again to produce one value per condition (days 15–17). Due to the high interindividual variability in response to sleep deprivation, total sleep time and all PVT and HAYFRN variables were expressed as relative to the preceding baseline (days 1–6 and 8–14).
Circadian phase and area under the curve
Two phase markers were derived from each melatonin profile, the dim light melatonin onset (DLMO) and dim light melatonin offset (DLMOff). For each subject’s melatonin profile, a threshold was calculated as the mean of 5 low consecutive day-time values plus twice the standard deviation of these points (Voultsios et al., 1997). This method yields low thresholds that are typically close to the physiological onset of melatonin secretion. Each subject’s DLMO was the point in time (as determined with linear interpolation) when the melatonin concentration exceeded the threshold. The DLMOff was the point in time when melatonin levels fell below the threshold. The phase shift during each condition was calculated as the baseline DLMO (or DLMOff) minus the final DLMO (or DLMOff). The area under the curve (AUC) was also calculated using the trapezoidal method (Salas and Hille, 1982). To ensure AUC calculations were not confounded by differences in the number of saliva samples, all melatonin profiles were truncated to 37 samples by removing extra low day-time points as needed.
Statistical analysis
To confirm that subjects were significantly sleep deprived in the partial sleep deprivation condition, total sleep time from the sleep logs and actigraphy analysis were analyzed in a 1-way MANOVA with within-subject factor “condition” (no sleep deprivation, partial sleep deprivation). Similarly, the HAYFRN and PVT variables were each analyzed in 1-way MANOVAs with within-subject factor “condition” (no sleep deprivation, partial sleep deprivation). As the MANOVAs were significant, each variable was separately analyzed with a paired t test.
The phase shifts in the DLMO and DLMOff were analyzed with a 3-way repeated-measures MANOVA with a within-subjects factor “condition” (no sleep deprivation v. partial sleep deprivation), within-subjects factor “time” (circadian phase before and after phase-advancing protocol), and a between-subjects factor “order” (no sleep deprivation first v. no sleep deprivation second). The MANOVA was significant, but the factor “order” was not, so the DLMO and DLMOff were then separately analyzed with a 2-way repeated-measures ANOVA with within-subjects factors “condition” and “time.” The condition × time interaction was of most interest as it would indicate if the magnitude of phase advances differed between conditions. The AUC was similarly analyzed. Statistical significance for all analyses was determined with 2-tailed tests at p < 0.05.
RESULTS
Sleep, Mood, and Performance
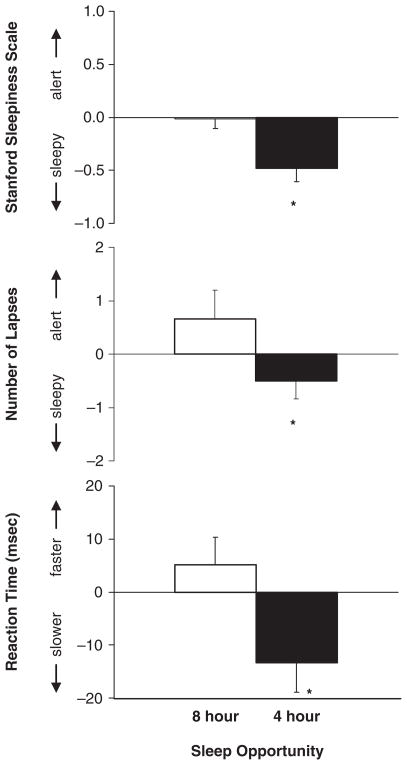
The analysis of the actigraphy, sleep logs, and HAYFRN and PVT variables confirmed that subjects were significantly more sleep deprived in the partial sleep deprivation condition than in the no sleep deprivation condition (Fig. 2 and Table 1). Subjects had a significantly shorter total sleep time in the partial sleep condition (actigraphy t13 = 28.63, p < 0.001; sleep logs t13 = 14.56, p < 0.001). Subjects reported significantly higher levels of sleepiness on the Stanford Sleepiness Scale (t13 = 4.62, p < 0.001) and significantly higher levels of physical fatigue (t13 = 2.33, p < 0.04) and mental fatigue (t13 = 2.60, p < 0.03) in the partial sleep deprivation condition. Subjects’ ratings of sadness, anxiety, irritability, and gastrointestinal distress did not significantly differ between conditions (p > 0.05). Subjects also had significantly longer mean reaction times (t13 = 2.95, p < 0.02) and more lapses (t13 = 2.37, p < 0.04) on the PVT in the partial sleep deprivation condition than in the no sleep deprivation condition.
Figure 2.
The average difference from baseline in ratings of sleepiness on the Stanford Sleepiness Scale and number of lapses and mean reaction time on the Psychomotor Vigilance Test when subjects had an 8-h sleep opportunity (no sleep deprivation condition) and a 4-h sleep opportunity per night (partial sleep deprivation condition). Subjects reported higher levels of sleepiness and had more lapses and slower reaction times in the partial sleep deprivation condition (*p < 0.02 on all paired t tests). Error bars represent SEMs.
Table 1.
Total sleep time derived from wrist actigraphy and sleep logs at baseline and in each condition.
| Baseline |
No Sleep Deprivation |
Partial Sleep Deprivation |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Sleep logs | ||||||
| Total sleep time (min) | 462 | 14 | 432 | 52 | 231 | 4.3 |
| Actigraphy | ||||||
| Total sleep time (min) | 417 | 16 | 417 | 28 | 218 | 7.0 |
Circadian Phase Shifts
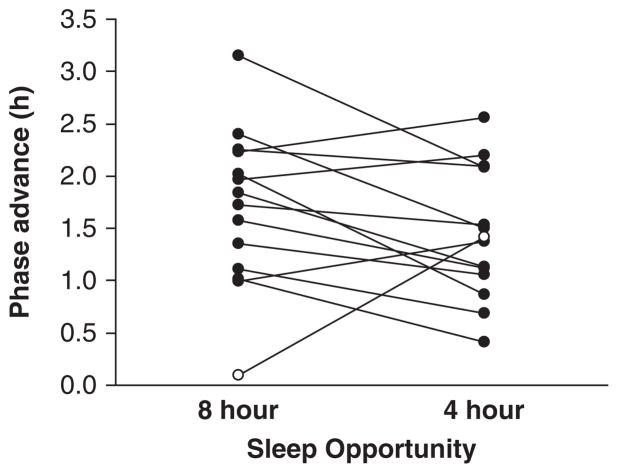
Figure 3 shows the phase shifts in the 2 conditions. One male subject showed a negligible phase advance (0.1 h) during the no sleep deprivation (8-h sleep opportunity) condition. This lack of response to the morning white bright light is unusual and is the smallest phase advance observed in subjects run in 3-day phase-advancing protocols with 8-h sleep opportunities (Burgess et al., 2003; Revell et al., 2006). This subject was also identified as a statistical outlier according to the Tukey test (Tukey, 1977) and was therefore removed from further statistical analysis (Santhi et al., 2008).
Figure 3.
The phase advances observed in each individual subject who underwent a 3-day phase-advancing protocol with either an 8-h (no sleep deprivation condition) or 4-h (partial sleep deprivation condition) sleep opportunity per night. The phase shifts for an individual subject are connected by a line. The subject identified as an outlier is represented with the open circles. Ten of the remaining 13 subjects showed reduced phase advances when sleep deprived.
The DLMO phase advanced significantly less in the partial sleep deprivation condition than in the no sleep deprivation condition (1.4 ± 0.6 h v. 1.8 ± 0.6 h; condition × time interaction: F1,12 = 7.68, p < 0.02). Ten of the remaining 13 subjects showed reduced phase advances in the DLMO in the partial sleep deprivation condition as compared to the no sleep deprivation condition (Fig. 3). In these 10 subjects, the average reduction in phase advance was 0.6 h, ranging from 0.2 to 1.2 h. The DLMOff also phase advanced, but there was no difference between conditions (condition × time interaction: F1,12 = 0.73, p > 0.40). There was no difference in the DLMO thresholds between the no sleep deprivation and partial sleep deprivation conditions (t27 = 0.09, p = 0.93). The average baseline DLMOs in the no sleep deprivation and partial sleep deprivation conditions were at 2114 h and 2058 h, respectively. The average advances in sleep/dark in the no sleep deprivation and partial sleep deprivation conditions (day 15) were 2.8 h and 2.9 h, respectively, and they did not correlate with the phase shifts observed (both p > 0.30). There was also nothing unusually different about the phase shifts observed in the 2 moderate evening type subjects. The AUC was significantly reduced after the phase-advancing protocol in both conditions (time: F1,12 = 25.17, p < 0.001; condition × time interaction: F1,12 = 0.87, p = 0.37).
DISCUSSION
This study is the first to show that partial sleep deprivation can lead to moderate reductions in phase shifts to light in humans. Thus, partial sleep deprivation per se (and not just the associated changes in the light-dark cycle) can reduce circadian responsiveness to light in humans. Importantly, these results cannot be due to differences in the timing of the bright light stimulus between the partial sleep deprivation and no sleep deprivation conditions because the subjects were exposed to the bright light stimulus at the same circadian phase, starting 8 h after the baseline DLMO, at a time that produces large phase advances (Burgess and Eastman, 2005). The lack of change in the phase advance in the DLMOff between conditions is probably because the DLMOff is influenced by individual differences in the metabolism and clearance of melatonin from the circulation and thus can be a less reliable marker of the circadian clock.
While sleep was not directly measured with poly-somnography, several measures confirmed that subjects were significantly sleep deprived in the partial sleep deprivation condition. As expected, the actigraphy recordings and sleep logs indicated significantly less sleep was obtained. While the magnitude of the changes was moderate, subjects did report significantly higher levels of sleepiness and fatigue and performed significantly worse when they phase advanced with a 4-h sleep opportunity versus 8-h sleep opportunity per night. While the actigraphy results suggest that no sleep deprivation occurred when the subjects’ 8-h sleep opportunity was advanced during the phase-advancing protocol, the sleep logs reveal an average sleep loss of 30 min per night relative to baseline (Table 1). This may reflect actigraphy failing to distinguish between quiet wakefulness and sleep during the required time in bed. However, subjects’ subjective sleepiness, fatigue, mood, and PVT performance did not worsen during the 3 days of phase advancing in the no sleep deprivation condition relative to baseline.
When subjects were sleep deprived in this experiment, they were kept awake in near darkness (<0.25 lux) that resulted from a combination of dim ambient light (<5 lux) and the use of very dark tight-fitting goggles that transmitted 5% or less of visible light. The dim ambient light was needed so that research staff could navigate the laboratory space and ensure that the subjects remained awake. Recent work demonstrates that rod photoreceptors may transmit low irradiance light signals to the circadian pacemaker (Lall et al., 2010). However, other available data suggest that the central circadian clock in humans is not significantly influenced by extremely dim light (<0.25 lux) and is unlikely to have influenced the phase advances observed in the sleep deprivation condition. For example, logistic model fits to experimental data suggest that such extremely dim light will not significantly phase shift the human circadian clock (Zeitzer et al., 2005). Furthermore, when subjects lived for 9 days in <0.2 lux with fixed sleep times, their melatonin rhythms appeared to free run (Danilenko et al., 2003), again suggesting that the human circadian system is not significantly influenced by such extremely dim light. Finally, while it is possible that the social interaction (and associated increased arousal) during the sleep deprivation condition may have contributed toward the observed results, social interactions per se in humans are currently thought to have little if any effect on the human circadian pacemaker (Mistlberger and Skene, 2004).
This finding that sleep deprivation can reduce phase shifts to light is consistent with previous reports of sleep deprivation reducing phase shifts to light in non-human animals. As described above, 2 reports have examined the effects of sleep deprivation on phase shifts to light in hamsters (Mistlberger et al., 1997) and mice (Challet et al., 2001), and both found phase delays to light were reduced in size when the animals were sleep deprived. The mechanism of action may be that sleep deprivation increases serotonergic activity in the suprachiasmatic nuclei (SCN), the site of the central circadian pacemaker in mammals, and that this reduces the photic sensitivity of the circadian system (Challet et al., 2001; Mistlberger et al., 1997). More recently, it has been shown that sleep deprivation reduces electrical activity in the rat SCN and that this effect continues into recovery sleep (Deboer et al., 2007). Consistent with this, it has very recently been reported that slow wave activity (a marker of sleep homeostatic pressure) in humans is inversely proportional to SCN activity (as indexed by functional magnetic resonance imaging) (Schmidt et al., 2009). Thus, our finding adds to a growing body of research that suggests that sleep deprivation may directly alter circadian functioning in mammals and that the interactions between the sleep and circadian systems may be more complex than originally thought (Deboer et al., 2007; Dijk and Archer, 2010). Future work should examine if partial sleep deprivation reduces phase delays to bright light and also phase shifts to exogenous melatonin, independent of the light-dark cycle.
It is reasonable to question whether the small reduction in phase advance due to partial sleep deprivation observed here is clinically relevant. There is some evidence to suggest that it is. An earlier study reported results from a within-subjects, double-blind, placebo-controlled design with 2 conditions (Yang et al., 2001). In both conditions, subjects delayed their habitual sleep schedule by 2 h to simulate a delayed weekend sleep pattern on Friday and Saturday nights. When they took a placebo pill on the Sunday afternoon, the average DLMO delayed by 31.6 min. When they took 6 mg of exogenous melatonin on the Sunday afternoon, the DLMO did not shift, presumably because the exogenous melatonin phase advanced the circadian clock, which counteracted the phase delay due to the later sleep schedule. Importantly, the young healthy subjects slept the same amount on the Sunday night, regardless of whether they had taken a placebo or melatonin pill. Of interest here is that the 30-min delay in circadian phase was enough to cause the subjects to rate themselves as significantly more “sleepy” and “overall feeling worse” on the Monday morning. Thus, while more research is required to investigate human sensitivity to such small degrees of circadian misalignment, it may well be that a phase difference as small as 0.5 h can significantly affect subjective mood and overall feelings of well-being.
Our group previously reported that a series of short (6-h) nights produces dramatic reductions in phase shifts to bright light as compared to a series of long (9-h) nights (Burgess and Eastman, 2005, 2006). These studies consisted of 2 weeks of short or long nights prior to and also during phase-shifting protocols. As detailed in these papers, potential causes of the significant attenuation in phase shifts to light with short nights include 1) the previous 2-week history of short nights (“photoperiodic history”), 2) the increased exposure to ambient evening light during the short nights, and 3) partial sleep deprivation. The results of the current study add 2 additional insights into this data set. First, the magnitude of the phase advances observed here in the no sleep deprivation condition (1.8 h, with 8-h nights) lies between the phase advances observed previously with 6- and 9-h nights (1.4 h and 2.9 h, respectively). Therefore, the results suggest that phase advances to bright light progressively decrease when the sleep/dark episode decreases from 9- to 8- to 6-h nights. Second, partial sleep deprivation likely contributed to the reduced phase advances observed with 6-h nights but cannot fully account for all of the reduction in phase advance. Thus, future studies should also explore the importance of avoiding ambient evening light exposure to secure larger phase advances to bright morning light.
Finally, these findings help reveal how the human circadian system is influenced by and responds to relatively recent trends in voluntary human behavior. These results suggest serious implications for the significant and growing proportion of society that report truncating their sleep (Knutson et al., 2010; National Sleep Foundation, 2010). For sleep-deprived individuals, one would expect that jet lag after eastward jet travel will be worse (Eastman and Burgess, 2009), and adjusting to early morning shift work will be more difficult. The efficacy of morning bright light treatment for patients with delayed sleep phase disorder may also be reduced, especially as many of these patients, often adolescents, are regularly sleep deprived due to socially mandated early work or school start times (Wyatt, 2004).
Acknowledgments
I thank Elisabeth Beam, Jillian Canton, Corrine Eckstein, Heather Gunn, Heather Holly, Thomas Molina, Jacqueline Munoz, Meredith Rathert, Dr. Mark Smith, Jessica Stroup, Christina Suh, and Nicole Woodrick for their assistance with data collection; Dr. Louis Fogg for his statistical advice; and our medical director, Margaret Park, MD. I also thank Dr. Stephanie Crowley and especially Dr. Charmane Eastman for their comments on the paper. Enviro-Med donated the light boxes.
This project was supported by an R01 grant (HL083971) and administrative supplement (HL083971S1) from the National Heart, Lung and Blood Institute to H.J.B. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the National Institutes of Health.
References
- Basner M, Fomberstein KM, Razavi FM, Banks S, William JH, Rosa RR, Dinges DF. American time use survey: sleep time and its relationship to waking activities. Sleep. 2007;30:1085–1095. doi: 10.1093/sleep/30.9.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Boivin DB, Czeisler CA, Dijk DJ, Duffy JF, Folkard S, Minors DS, Totterdell P, Waterhouse JM. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch Gen Psychiatry. 1997;54:145–152. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Crowley SJ, Gazda CJ, Fogg LF, Eastman CI. Preflight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright light. J Biol Rhythms. 2003;18:318–328. doi: 10.1177/0748730403253585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. Short nights attenuate light-induced circadian phase advances in humans. J Clin Endocrinol Metab. 2005;90:4437–4440. doi: 10.1210/jc.2005-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. Short nights reduce light-induced circadian phase delays in humans. Sleep. 2006;29:25–30. doi: 10.1093/sleep/29.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher JN, Dahlstrom WG, Graham JR, Tellegen A, Kaemmer B. MMPI-2 (Minnesota Multiphasic Personality Inventory-2): Manual for Administration and Scoring. Minneapolis: University of Minnesota Press; 1989. [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Challet E, Turek FW, Laute M, Van Reeth O. Sleep deprivation decreases phase-shift responses of circadian rhythms to light in the mouse: role of serotonergic and metabolic signals. Brain Res. 2001;909:81–91. doi: 10.1016/s0006-8993(01)02625-7. [DOI] [PubMed] [Google Scholar]
- Danilenko KV, Cajochen C, Wirz-Justice A. Is sleep per se a zeitgeber in humans? J Biol Rhythms. 2003;18:170–178. doi: 10.1177/0748730403251732. [DOI] [PubMed] [Google Scholar]
- Deboer T, Detari L, Meijer JH. Long term effects of sleep deprivation on the mammalian circadian pacemaker. Sleep. 2007;30:257–262. doi: 10.1093/sleep/30.3.257. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Archer SN. PERIOD3, circadian phenotypes, and sleep homeostasis. Sleep Med Rev. 2010;14:151–160. doi: 10.1016/j.smrv.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput. 1985;17:652–655. [Google Scholar]
- Eastman CI, Burgess HJ. How to travel the world without jet lag. Sleep Med Clin. 2009;4:241–255. doi: 10.1016/j.jsmc.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Horne J, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Van Cauter E, Rathouz PJ, DeLeire T, Lauderdale DS. Trends in the prevalence of short sleepers in the USA: 1975–2006. Sleep. 2010;33:37–45. doi: 10.1093/sleep/33.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lall GS, Revell VL, Momiji H, Enazi JE, Altimus CM, Guler AD, Aguilar C, Cameron MA, Allender S, Hankins MW, Lucas RJ. Distinct contributions of rod, cone and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–428. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamond N, Jay SM, Dorrian J, Ferguson SA, Roach GD, Dawson D. The sensitivity of a palm-based psychomotor vigilance task to severe sleep loss. Behav Res Methods. 2008;40:347–352. doi: 10.3758/brm.40.1.347. [DOI] [PubMed] [Google Scholar]
- Lewy A, Cutler NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms. 1999;14:227–236. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Landry GL, Marchant EG. Sleep deprivation can attenuate light-induced phase shifts of circadian rhythms in hamsters. Neurosci Lett. 1997;238:5–8. doi: 10.1016/s0304-3940(97)00815-x. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Skene DJ. Social influences on mammalian circadian rhythms: animal and human studies. Biol Rev Camb Philos Soc. 2004;79:533–556. doi: 10.1017/s1464793103006353. [DOI] [PubMed] [Google Scholar]
- Moore RY. The innervation of the mammalian pineal gland. Prog Reprod Biol. 1978;4:1–29. [Google Scholar]
- Murphy PJ, Myers BL, Badia P. Nonsteroidal anti-inflammatory drugs alter body temperature and suppress melatonin in humans. Physiol Behav. 1996;59:133–139. doi: 10.1016/0031-9384(95)02036-5. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation. Sleep in America Poll summary of findings. 2010 Available at: www.sleepfoundation.org.
- Reid KJ, Burgess HJ. Circadian rhythm sleep disorders. Prim Care. 2005;32:449–473. doi: 10.1016/j.pop.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Revell VL, Burgess HJ, Gazda CJ, Smith MR, Fogg LF, Eastman CI. Advancing human circadian rhythms with afternoon melatonin and morning intermittent bright light. J Clin Endocrinol Metab. 2006;91:54–59. doi: 10.1210/jc.2005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas SL, Hille E. Calculus: One and Several Variables with Analytic Geometry. New York: John Wiley and Sons; 1982. [Google Scholar]
- Santhi N, Aeschbach D, Horowitz TS, Czeisler CA. The impact of sleep timing and bright light exposure on attentional impairment during night work. J Biol Rhythms. 2008;23:341–352. doi: 10.1177/0748730408319863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Collette F, Leclercq Y, Sterpenich V, Vandewalle G, Berthomier P, Berthomier C, Phillips C, Tinguely G, Darsaud A, et al. Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area. Science. 2009;324:516–519. doi: 10.1126/science.1167337. [DOI] [PubMed] [Google Scholar]
- Tasto DL, Colligan MJ, Skjei EW, Polly SJ. NIOSH Publication #78–154. Cincinnati, OH: National Institute for Occupational Safety and Health; 1978. Health Consequences of Shift Work. [Google Scholar]
- Tukey JW. Exploratory Data Analysis. Reading, MA: Addison-Wesley; 1977. [Google Scholar]
- Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- Wyatt JK. Delayed sleep phase syndrome: pathophysiology and treatment options. Sleep. 2004;27:1195–1203. doi: 10.1093/sleep/27.6.1195. [DOI] [PubMed] [Google Scholar]
- Yang CM, Spielman AJ, D’Ambrosio P, Serizawa S, Nunes J, Birnbaum J. A single dose of melatonin prevents the phase delay associated with a delayed weekend sleep pattern. Sleep. 2001;24:272–281. doi: 10.1093/sleep/24.3.272. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Khalsa SB, Boivin DB, Duffy JF, Shanahan TL, Kronauer RE, Czeisler CA. Temporal dynamics of late-night photic stimulation of the human circadian timing system. Am J Physiol. 2005;289:R839–R844. doi: 10.1152/ajpregu.00232.2005. [DOI] [PubMed] [Google Scholar]