Abstract
While the exact cause of the increase in ethanol consumption during adolescence is not known, age differences in sensitivity to some of ethanol’s effects may play a contributory role. Prior research has shown little difference in the expression of ethanol-induced tachycardia between adolescents and adults following ethanol inhalation. In contrast, there is mounting evidence of ontogenetic differences in ethanol-induced hypothermia, although the nature of the ontogenetic effect observed has been found to vary across studies and even within labs. Relative ontogenetic differences in body temperature after ethanol administration appear to be driven in part by the amount of experimental perturbation associated with the test protocol (Ristuccia et al., 2007), although differing ethanol exposure levels across studies may also have contributed to the variations in ontogenetic patterns that have been observed. To explore the latter possibility, the present study assessed ethanol-induced hypothermia and tachycardia in adolescent and adult male Sprague-Dawley rats examined in their home cages in the presence of their housing partner following intraperitoneal administration of 0.5, 1.5, or 3.0 g/kg ethanol. The results showed that, while adolescents did not show an adult-typical tachycardic effect at any dose, they proved more sensitive than adults to ethanol’s hypothermic effects at the two highest doses. These findings suggest that not only the degree of experimental perturbation, but also the amount of ethanol exposure may differentially effect expression of age differences in ethanol-induced hypothermia, with adolescents showing greater hypothermia than adults at higher doses. Together with previous findings, these data contribute to the emerging picture that age differences in autonomic effects of ethanol appear to be particularly sensitive to dosing parameters and experimental protocols, unlike the generally more consistent ontogenetic findings observed across studies when using behavioral measures of ethanol sensitivity.
Keywords: ethanol, adolescent, autonomic nervous system, heart rate, body temperature
In order to make a successful transition from dependency on the mother to adulthood, young organisms must navigate the transitional developmental period of adolescence. During this time, the nervous system undergoes a number of structural and functional changes that may have evolved in part to facilitate this transition (see Spear, 2000 for review). However, some of the behavioral and physiological changes that occur during this developmental period may leave adolescents vulnerable to drug use and abuse. As has been observed by the Monitoring the Future study, ethanol use increases throughout the adolescent period, and is often associated with relatively high consumption levels, with 30% of high school seniors reporting consumption of five or more drinks in a row within the past two weeks (Johnston et al., 2005). The propensity for adolescents to consume large amounts of ethanol relative to adults is also evident in laboratory animals (e.g. Brunell & Spear, 2005; Doremus et al., 2005), and, hence, may be in part biologically based.
The neurological substrates of this behavior, however, are unknown. Age differences in initial sensitivity to effects of ethanol that normally function to moderate drinking could serve as permissive factors that enable adolescents to drink more ethanol than adults. Indeed, adolescent and adult rats have been shown to differ in their sensitivity to a variety of ethanol effects during a single exposure to ethanol. Adolescents have been reported to be more sensitive to ethanol-induced impairment of spatial memory (Markwiese et al., 1998) as well as ethanol-induced social facilitation (Varlinskaya & Spear, 2002), but less sensitive than adults to the motor-impairing (Silveri & Spear, 2001; White et al., 2002), dysphoric (Shram et al., 2005), anxiolytic (Varlinskaya & Spear, 2002) and hypnotic (Silveri & Spear, 1998) effects of ethanol. Such ontogenetic insensitivity to cues that serve to modulate ethanol intake in adults could permit elevated levels of ethanol intake during adolescence relative to those seen in adults.
When examining ethanol’s hypothermic effect, adolescents have been observed to exhibit less of a decrease in body temperature (BT) than adults when ethanol was administered intragastrically (i.g.) (Ristuccia & Spear, 2004; Silveri & Spear, 2001, but see also Brasser & Spear, 2002) or intraperitoneally (i.p.) (Swartzwelder et al., 1998). However, adolescents were found to be more sensitive to ethanol’s hypothermic effect than adults when ethanol was administered via vapor inhalation (Ristuccia & Spear, 2005). Subsequent work has shown that these differences across studies in ontogenetic patterns of sensitivity to ethanol-induced hypothermia appear to be at least partially dependent on the amount of experimental perturbation associated with the ethanol administration process. That is, when the amount of disruption required to inject the animals with ethanol was attenuated by habituating the animals to the i.p. injection procedure, adolescents exhibited greater sensitivity to ethanol-induced hypothermia than adults (Ristuccia et al., 2007).
However, it is possible that these differences across in age-related sensitivity to ethanol’s hypothermic effects may have been influenced by variations in ethanol exposure levels across studies as well. For example, in comparing across experiments conducted in the same lab, adolescents showed less of a hypothermic response after an i.g. administration of ethanol that produced relatively low blood ethanol concentrations (BECs) in animals of both ages (Ristuccia & Spear, 2004), but a greater hypothermic response than adults when vapor inhalation was used to achieve BECs approximately 60-100 mg/dl higher than those produced by the i.g. administration in the previous study (Ristuccia & Spear, 2005). Brasser & Spear (2002) likewise found younger animals to be more hypothermic than adults after a high dose of ethanol (4.0 g/kg) administered intragastrically. Consequently, adolescent animals may be more sensitive than adults to the hypothermic effects of ethanol at relatively high doses but could potentially be less sensitive than adults at doses that produce lower BECs. Because age differences in these acute autonomic effects of ethanol have not been extensively investigated at relatively low doses, one goal of the work described here was to assess adolescent and adult autonomic responses across a range of ethanol doses.
Ontogenetic patterns of sensitivity to ethanol-induced increases in heart rate (HR) are less well characterized. In previous work using vapor inhalation to expose animals to ethanol, animals of both ages showed equivalent ethanol-induced tachycardia, despite greater sensitivity of the adolescents to the hypothermic effect of ethanol (Ristuccia & Spear, 2005). However, it was unclear whether the similar profile of HR responses across age after ethanol was due to equivalent sensitivity to this effect in adolescents and adults or simply because the ethanol exposure level in that study was sufficiently high to produce a ceiling effect in the animals’ tachycardic responses while perhaps still permitting age differences to appear in the analysis of the BT data. To address these issues, the current experiment examined tachycardic and hypothermic responses to ethanol at three different doses in adolescent and adult rats.
The doses used in this experiment (0.5, 1.5 and 3.0 g/kg) were chosen because doses in this range have been shown in previous research to induce varying degrees of ethanol-induced hypothermia in adult (Crowell et al., 1981; Lê et al., 1984; Lê et al, 1979; Pohorecky et al., 1986; Swartzwelder et al., 1998; York & Chan, 1994) as well as adolescent (Brasser & Spear, 2002; Ristuccia & Spear, 2004, 2007; Silveri & Spear, 2000) rats. In addition, ethanol loads in this range produced via i.p. injection (Peris & Cunningham, 1985, 1986; Pohorecky et al., 1986) or voluntary consumption (Bell et al., 2002) have been shown to produce reliable ethanol-induced tachycardia in adult rats. To the extent that the greater ethanol-induced hypothermia seen in adolescents than adults following ethanol inhalation was at least partly related to relatively high ethanol exposure levels, it was anticipated that adolescents would be more sensitive to ethanol’s hypothermic effect than adults at the highest dose. It was also speculated that a similar pattern of age differences in autonomic sensitivity might emerge with ethanol-induced tachycardia. Evidence for the former but not the latter suggestion was obtained.
Methods
Subjects
The experiment described here used adolescent and adult male Sprague-Dawley rats bred in an AAALAC-accredited vivarium at Binghamton University. Litters were culled on the first day after birth (postnatal day [P] 1) to six males and four females whenever possible. Male offspring were weaned on P21 and housed in pairs with same-sex littermates under a 14/10 hour light/dark cycle until the onset of the experimental procedures as described below; female offspring were used in other projects. For the duration of the experiment, animals were given ad lib access to food and water. All procedures used in the following experiments were approved by the Binghamton University Institutional Animal Care and Use Committee (IACUC).
Procedure
The design of this experiment was a 2 Age X 4 Dose factorial with one repeated measure (dose) (N=24). Adolescent (n=12) and adult (n=12) rats were implanted on P25 or P65-70 respectively with telemetry transmitters (Data Sciences International model #TA10ETA-F20) that monitored HR and BT. Animals were anesthetized with isoflurane and the body of the probe inserted through a small incision in the abdomen and sutured to the peritoneum. Next, the two wire leads for measuring HR were fed through the peritoneum with a 17-gauge needle and tunneled subcutaneously to two locations on opposite sides of the heart where the tip of each lead was sutured into place within the muscle. After closing the peritoneum and skin, animals were allowed to recover for three days. Although shorter than that commonly used in studies involving telemetry implants in adults (e.g., Bell et al, 2002), this recovery period has been shown to be sufficient for recovery from the surgical procedure used to implant this model of telemetry probe in both adolescents and adults (Ristuccia & Spear, 2005), with the implant not affecting weight gain of either adolescents or adults during the post-recovery test period (see results). Post-surgically and throughout the remainder of the experiment, each experimental animal was housed with a non-surgically manipulated housing partner in a solid bottom cage placed on top of an RLA1020 receiving plate (Data Sciences International; St Paul, MN) within the telemetry recording room. The 24 animals used as housing partners were same-sex littermates of the implanted animals. These non-implanted cagemates of the implanted animals were used to assess BEC after ethanol injection so that ethanol load could be estimated without exposing experimental animals to the stress of blood sampling during telemetry data collection. Housing the animals in pairs also prevented isolation of the implanted animals, a condition that has been found to differentially impact some behavioral measures in adolescent and adult rats (Ristuccia & Spear, 2004; Varlinskaya, Spear & Spear, 1999). Three days following surgery, probes were magnetically activated and baseline measurements taken for 24 hours. Beginning during this baseline period and throughout the experiment, telemetry data were sampled for thirty seconds once every ten minutes.
The day following baseline assessments, all animals were given i.p. saline equivalent in volume to the 3.0 g/kg dose of ethanol to be given later. On subsequent days animals were given three i.p. doses of 18.9% ethanol (v/v) (0.5, 1.5, and 3.0 g/kg) administered in semi-random order, using a counterbalanced design whereby all possible permutations of the ordering of doses were represented two times per age group. While this concentration of ethanol was slightly higher than the 12.6% commonly used when injecting animals with low to moderate doses of ethanol (e.g., 0.5-1.5 g/kg — see Varlinskaya & Spear, 2006), it is within the 17.8-20.0% v/v range used to minimize injection volumes when administering ethanol doses in the 3.0-5.0 g/kg range (e.g., Silveri & Spear, 1998; Doremus et al, 2003; Varlinskaya & Spear, 2004). All solutions were warmed shortly prior to injection to approximately 38°C in a closed container to prevent differential evaporation. A one day wash-out period was permitted between ethanol injections. An initial analysis of the telemetry data did not indicate any reliable effects of injection order on HR or BT. Consequently, this variable was not included in the analyses described below.
To minimize any potential influence of circadian variations in BT or HR from influencing the data, all injections were given between 0830 and 1000 hrs. The non-implanted cagemate of each implanted animal was treated identically to the implanted animal, except that tail blood samples were taken from these non-implanted animals at one, two and four hours following each ethanol administration, sampling intervals selected in an attempt to sample from one hour to clearance at the intermediate dose (four hours.), and to allow dose and age-related comparisons at one and two hours. Immediately after collection, samples were frozen at -80°C until later analysis of BECs. Briefly, for analysis frozen samples were thawed and ethanol content determined using an HP5890 Series II Gas Chromatograph and HP7694E autosampler (Hewlett Packard; Wilmington, DE). Peak areas under the curve were compared to known standards using HP3365 Chemstation software (see Varlinskaya & Spear, 2006, for further details).
Data Analysis
Telemetry data were analyzed using a 4 Dose X 2 Age X 11 Hour repeated measures analysis of variance (ANOVA), with dose and time treated as repeated measures. Data were collapsed into hour-long bins by averaging the six samples taken every hour and analyzed over eleven hours to capture the entire duration of the adult post-injection hypothermic period. For analysis of the BEC data, a 2 Age X 3 Dose X 3 Time ANOVA was used, with time and dose treated as repeated measures. In addition, overall weight gain was calculated by subtracting weight prior to the surgery day from the weight on the final injection day.
Results
Body Weight and BEC
As would be expected, adolescents gained significantly more weight than adults throughout the course of this experiment. On the day of the final ethanol injection, adolescents weighed approximately 77.75 ± 1.96 grams (g) more than their weights prior to surgery, while adults only gained an average of 23.38 ± 2.21 g [Age effect: F(1, 44)=330.13, p<.001]. Importantly, regardless of age, there were no differences in weight gain between the implanted animals and the non-implanted cagemates (p>.05), indicating that the surgical procedure did not impair growth at either age. Analysis of tail blood samples from the non-implanted cagemates indicated that adolescents had higher BECs than adults one and two hours after the 3.0 g/kg injection, but not four hours after the injection of this dose [Dose X Time X Age interaction [F(4, 84)=3.62, p<.01]. Post hoc tests did not, however, reveal any reliable age differences in BEC after the 0.5 or 1.5 g/kg doses (Figure 1).
Figure 1.
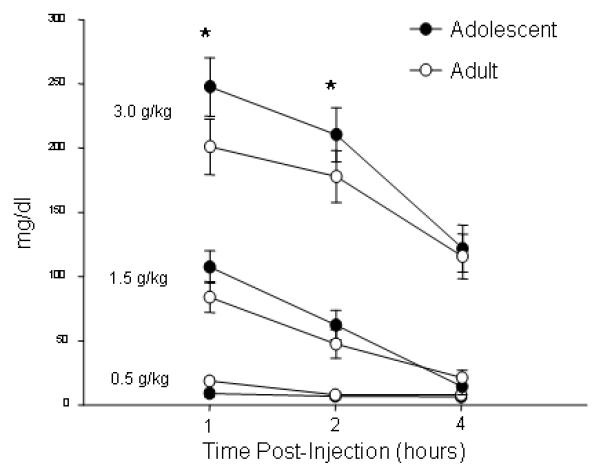
BECs (mg/dl) from adolescent and adult rats after the three ethanol injections in Exp. 1 (mean ± SEM). * indicates significantly higher BECs in adolescents than adults
Heart Rate (HR)
Overall, average HRs were approximately 100 beats/min greater in adolescents than adults, so HR data were analyzed separately at each age. As described below, analysis of the adult data revealed a well-defined relationship between ethanol dose and HR increases, whereas little consistent evidence for ethanol-specific tachycardia emerged in the analysis of the adolescent data (Figure 2).
Figure 2.
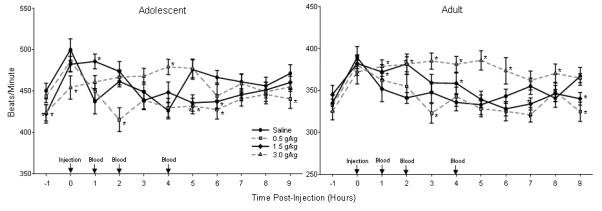
Adolescent (top) and adult (bottom) HR in beats/min after injection of saline and three doses of ethanol in Exp. 1. Data are shown as mean ± SEM for nine hours after as well the hour prior to injection. Injections occurred at time 0 and then blood samples were collected one, two and four hours post-injection. * indicates significant differences in HR between ethanol and saline injection within a single time bin. No comparisons across age were made in this analysis (note the difference in scaling of the y-axis).
Adolescents HRs were rarely higher after ethanol injection than after saline injection (only reaching significance one hour post-injection after 1.5 g/kg; four hours post-injection after 3.0 g/kg) [Dose X Time interaction: F(30, 330)=3.44, p<.001]. Instead, significant bradycardia emerged at a number of time points, an effect most prevalent (but nevertheless sporadic) after the 0.5 and 1.5 g/kg doses. This bradycardia reached significance five and six hours after injection of either of these doses, as well as two and nine hours post-injection after 0.5 g/kg and within the initial hour of injection after the 3.0 g/kg dose. These significant findings may have been driven in part by the considerable fluctuations in HR seen with time following saline injection. Interpretation of these data were further complicated by HR differences evident during the pre-injection period, with HRs lower during the hour before injection of the 1.5 and 3.0 g/kg doses than prior to saline injection, as well as by evidence that some of the significant differences reported at longer post-administration intervals after the 0.5 and 1.5 g/kg doses likely reflected data obtained following ethanol clearance (see Figure 1). Taken together, these data do not provide clear evidence for tachycardic effects specific to ethanol in adolescents under these test circumstances.
In contrast, adult HRs showed a reasonably well-defined dose-response curve after ethanol injection at the three doses used [Dose X Time interaction: F(30, 330)=3.80, p<.001]. Adult HRs were consistently elevated over those seen after saline injection after 3.0 g/kg (reaching significance from one to six hours post-injection and again at eight hours after administration). At the medium dose (1.5 g/kg), elevations in HR over those seen after saline injection were seen episodically (one, two and four hours post-administration). Late-appearing significant HR suppressions also emerged at 0.5 and 1.5 g/kg (with HRs following both doses lower than in adults exposed to saline at seven and nine hours after injection, and with HRs following 0.5 g/kg also lower than after saline at three hours after injection); interpretation of these later appearing HR suppressions are complicated by BEC data suggesting that the ethanol challenge would have been roughly eliminated by two hours at the 0.5 g/kg dose and by four hours at 1.5 g/kg.
Body Temperature (BT)
The pattern of age differences in the hypothermic effect of ethanol varied with the dose administered to the animals, with adolescents generally being more sensitive to ethanol’s hypothermic effect than adults at higher doses. As seen in Fig. 3 and detailed below, adolescents showed a greater magnitude of ethanol-induced hypothermia than adults at both the 1.5 and 3.0 g/kg doses of ethanol. Although it took adolescents longer to recover from the hypothermia induced by the 1.5 g/kg ethanol dose, the duration of the hypothermia was shorter in adolescents than adults following challenge with the 3.0 g/kg dose.
Figure 3.
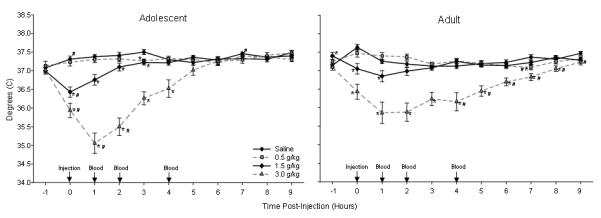
Adolescent (top) and adult (bottom) BT in degrees C after injection of saline and three doses of ethanol in Exp. 1. Data are shown as mean ± SEM for nine hours after as well the hour prior to injection. Injections occurred at time 0 and then blood samples were collected one, two and four hours post-injection. * indicates significant differences in BT between ethanol and saline injection days within a single time bin, while # indicates a significantly lower temperature across age within a time bin.
Adolescents and adults showed significantly different patterns of ethanol-induced hypothermia that varied with dose [Dose X Hour X Age interaction: F(30, 630)=3.75, p<.001]. Neither age showed significant hypothermia after the low dose (0.5 g/kg). However, following the 1.5 g/kg injection, post hoc tests showed that adolescent temperatures were lower than those of adults during the first post-injection hour. In addition, adolescents showed a longer duration of ethanol-induced hypothermia than adults, with adolescent temperatures being lower than after saline injection for four hours while adult temperatures were lower than after saline injection for only the first two hours. Following 3.0 g/kg, adolescent temperatures were lower than adults during the first post-injection hour, as well as during assessments conducted during the time bins beginning one and two hours post-injection, but higher than adults from four to nine hours after administration of 3.0 g/kg. The latter reflected the more rapid recovery of adolescents from the hypothermic response at this dose, with adults showing temperatures lower than those seen after saline injection for ten hours following administration vs. only six hours among the adolescents
Discussion
Relative ethanol sensitivity between adolescents and adults varied with the measure used to assess autonomic functioning in this experiment. On the one hand, adolescents showed little evidence of the ethanol-specific tachycardia that was seen in adults after injection of doses similar to those previously reported to increase HR in adult animals (Peris & Cunningham, 1985; Pohorecky et al., 1986). On the other hand, adolescents appeared more sensitive to the larger doses of ethanol when examining ethanol’s hypothermic effect. Thus, the differences between adolescents and adults in autonomic sensitivity to ethanol that have been observed across studies appeared to depend on both the doses of ethanol used and the variables used to assess autonomic functioning.
Ethanol-induced changes in HR proved difficult to detect in adolescents, perhaps in part because fluctuations in HR seen after saline injection may have obscured detection of changes related to ethanol. As a result, adolescents appeared to be less sensitive to ethanol-induced tachycardia than adults, with HR responses to ethanol similar to that seen in response to saline injection. These results contrast with those seen when ethanol was administered via vapor inhalation, where relatively little difference was observed between adolescents and adults in the tachycardic response to ethanol (Ristuccia & Spear, 2005). The source of these differing findings may be related to the variation across these studies in route of administration and the perturbing effects therein.
At higher doses, adolescent and adult HR changes could be related in part to ethanol’s interference with the baroreflex arc (see Bell et al., 2002 for discussion). This system maintains blood pressure within an acceptable range by decreasing sympathetic input to the heart, thereby decreasing HR when blood pressure rises beyond a certain point. Although the contribution of the baroreflex arc to ethanol-related changes in HR was not directly examined in the present study, prior research has shown that the baroreflex arc in adults can be attenuated by relatively high doses of ethanol through a hypothalamically-mediated mechanism (Zhang et al., 1989), leading to an increase in HR (Abdel-Rahman et al., 1985). No evidence is currently available regarding the influence of ethanol on the baroreflex arc of adolescents. To the extent that the ethanol-induced tachycardia seen in adults at higher doses in this study may have been mediated through the baroreflex arc, adolescents would appear to be relatively insensitive to this effect.
When examining BT, adolescents showed a greater peak hypothermic response than the adults, particularly to the highest dose of ethanol but also after 1.5 g/kg. This finding is similar to that seen following administration of ethanol vapor in an exposure regimen that produced BECs only slightly lower than those seen after the 3.0 g/kg injection used in this study (e.g. 160-200 mg/dl vs. 200-250 mg/dl) (Ristuccia & Spear, 2005). Similar findings have been observed after administration of 4.0 g/kg (i.g.) (Brasser & Spear, 2002), with adolescents again showing slightly but significantly greater hypothermic responses. All of these findings contrast with reports from other studies that showed a greater hypothermic response in adults than adolescents following lower ethanol challenges (Ristuccia & Spear, 2004; Silveri & Spear, 2000; Swartzwelder et al., 1998). The findings of the present experiment indicate that the difference between studies reporting greater or lesser sensitivity to ethanol’s hypothermic effect in adolescents than adults may have been related in part to ethanol dose, with adolescents prone to showing a greater hypothermic response than adults at relatively high ethanol loads.
The greater hypothermic response of the adolescents in the present study may also have been driven partly by pharmacokinetic differences between adolescents and adults, particularly after injection of the highest dose, where adolescent BECs were found to be higher at one and two hrs post-administration than those of the adults. In this case, more rapid absorption of ethanol could have driven a greater hypothermic response in the adolescents. Age differences in ethanol hypothermia are not entirely attributable to pharmacokinetic factors, however, in that adolescents also had a greater hypothermic response than adults after 1.5 g/kg, despite showing no differences in BEC levels from adults.
Together with prior studies (e.g., Brasser & Spear, 2002; Ristuccia et al., 2007; Ristuccia & Spear, 2004, 2005; Silveri & Spear, 2000), the picture that is emerging is that autonomic consequences of ethanol during ontogeny are unusually sensitive to ethanol dosing parameters and other characteristics of the experimental protocol, with differing ontogenetic patterns of sensitivity to ethanol’s autonomic effects emerging across studies (e.g. Brasser & Spear, 2002; Ristuccia & Spear, 2004, 2005; Ristuccia et al., 2007; Silveri & Spear, 2000; Swartzwelder et al., 1998). This apparent lability of autonomic measures contrasts with the generally more consistent ontogenetic findings observed across studies and laboratories when using behavioral measures of ethanol sensitivity (see Spear & Varlinskaya, 2005, for review).
The results of the present study, when considered in light of the findings of Kurtz & Campbell (1994) showing differential stress-related autonomic regulation of HR between adolescents and adults, suggests the presence of notable differences in autonomic system regulation between adolescents and adults. Further investigation of the circumstances driving ontogenetic differences in the autonomic consequences of ethanol seems warranted, particularly given evidence from studies in humans that autonomic reactivity may serve as a somatic marker of the reinforcing properties of alcohol and other drugs of abuse (e.g. Assad et al., 2003). To the extent these autonomic/affect relationships are confirmed and expressed in laboratory animals as well, such HR responses could be used to assess the rewarding value of ethanol in adolescents and adults, potentially shedding more light on the underlying factors that drive the dramatic increase in ethanol and other drug use during the adolescent period.
Acknowledgments
This research was supported by NIAAA grants F31 AA16048 to R.C. Ristuccia and R37 AA12525 to L.P. Spear. In addition, it could not have been completed without input from Dr Elena I. Varlinskaya, Dr Norman E. Spear and Dr Richard L. Bell. Also, the authors would like to thank Judith Sharp for her help in analyzing BECs.
The authors would like to thank Judith Sharp for her help in conducting the BEC assays. This research was supported by NIAAA grants R37-AA12525 to L.P. Spear and F31 AA16048 to R.C. Ristuccia.
References
- Abdel-Rahman AA, Dar MS, Wooles WR. Effect of chronic ethanol administration on arterial baroreceptor function and pressor and depressor responsiveness in rats. J. Pharmacol. Exp. Ther. 1985;232:194–201. [PubMed] [Google Scholar]
- Alberts JR. Huddling by rat pups: Group behavioral mechanisms of temperature regulation and energy conservation. J. Comp. Physiol. Psychol. 1978;92:231–245. doi: 10.1037/h0077459. [DOI] [PubMed] [Google Scholar]
- Araujo NP, Camarini R, Souza-Formigoni MO, Carvalho RC, Abilio VC, Silva RH, Ricardo VP, Ribeiro RA, Frussa-Filho R. The importance of housing conditions on behavioral sensitization and tolerance to ethanol. Pharmacol. Biochem. Behav. 2005;82:40–45. doi: 10.1016/j.pbb.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Assaad JM, Pihl RO, Seguin JR, Nagin D, Vitaro F, Carbonneau R, Tremblay RE. Aggressiveness, family history of alcoholism, and the heart rate response to alcohol intoxication. Exp. Clin. Psychopharmacol. 2003;11:158–166. doi: 10.1037/1064-1297.11.2.158. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd-Henricks Z,A, Webster AA, Lumeng L, Li T, Mcbride WJ, Murphy JM. Heart rate and motor-activating effect of orally self-administered ethanol in alcohol-preferring (P) rats. Alcohol Clin. Exp. Res. 2002;26:1162–1170. doi: 10.1097/01.ALC.0000024126.59174.2C. [DOI] [PubMed] [Google Scholar]
- Boileau I, Assaad J-M, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Brasser SM, Spear N. Physiological and behavioral effects of acute ethanol hangover in juvenile, adolescent, and adult rats. Behav. Neurosci. 2002;116:305–320. doi: 10.1037//0735-7044.116.2.305. [DOI] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. The effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin. Exp. Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Crowell CR, Hinson RE, Siegel S. The role of conditioned drug responses in tolerance to the hypothermic effects of ethanol. Psychopharmacol. 1981;73:51–54. doi: 10.1007/BF00431101. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin. Exp. Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975-2003. Volume I, Secondary school students. National Institute on Drug Abuse; Bethesda, MD: (NIH Publication No. 04-5507) [Google Scholar]
- Kurtz MM, Campbell BA. Paradoxical autonomic responses to aversive stimuli in the developing rat. Behav. Neurosci. 1994;108:962–971. doi: 10.1037//0735-7044.108.5.962. [DOI] [PubMed] [Google Scholar]
- Lê AD, Poulos CX, Cappell H. Conditioned tolerance to the hypothermic effects of ethyl alcohol. Science. 1979;206:1109–1110. doi: 10.1126/science.493999. [DOI] [PubMed] [Google Scholar]
- Lê AD, Khanna JM, Kalant H. Effect of treatment dose and test system on development of ethanol tolerance and physical dependence. Alcohol. 1984;1:447–451. doi: 10.1016/0741-8329(84)90020-x. [DOI] [PubMed] [Google Scholar]
- Peris J, Cunningham CL. Dissociation of tolerance to the hypothermic and tachycardic effects of ethanol. Pharmacol. Biochem. Behav. 1985;22:971–978. doi: 10.1016/0091-3057(85)90305-3. [DOI] [PubMed] [Google Scholar]
- Peris J, Cunningham CL. Handling-induced enhancement of alcohol’s acute physiological effects. Life Sci. 1986;38:273–279. doi: 10.1016/0024-3205(86)90313-9. [DOI] [PubMed] [Google Scholar]
- Peterson JB, Conrod P, Vassileva J, Gianoulakis C, Pihl RO. Differential effects of naltrexone on cardiac, subjective and behavioural reactions to acute ethanol intoxication. J. Psychiatry Neurosci. 2006;31:386–393. [PMC free article] [PubMed] [Google Scholar]
- Philpot RM, Badanich KA, Kirstein CL. Place conditioning: Age-related changes in the rewarding and aversive effects of alcohol. Alcohol Clin. Exp. Res. 2003;27:593–599. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA, Brick J, Carpenter JA. Assessment of the development of tolerance to ethanol using multiple measures. Alcohol Clin. Exp. Res. 1986;10:616–622. doi: 10.1111/j.1530-0277.1986.tb05155.x. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA, Peterson JT, Carpenter JA. Development of tolerance to ethanol-induced tachycardia in rats. Alcohol Drug Res. 1986;6:431–439. [PubMed] [Google Scholar]
- Markwiesse BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin. Exp. Res. 1998;22:416–421. [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Adolescent ethanol sensitivity: Hypothermia and acute tolerance. Ann. N.Y. Acad. Sci. 2004;1021:445–447. doi: 10.1196/annals.1308.061. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Age differences in sensitivity and tolerance to the autonomic effects of ethanol. Alcohol Clin. Exp. Res. 2005;29:1809–1820. doi: 10.1097/01.alc.0000183010.72764.cd. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Hernandez M, Wilmouth CE, Spear LP. Differential expression of ethanol-induced hypothermia in adolescent and adult rats induced by pretest familiarization to the handling/injection procedure. Alcohol Clin. Exp. Res. 2007;31:575–581. doi: 10.1111/j.1530-0277.2007.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Nielsen K. Energy metabolism, body size, and problems of scaling. Proc. of the Am. Soc. Exp. Bio. 1970;29:1524–1531. [PubMed] [Google Scholar]
- Shram MJ, Funk D, Chau V, Li Z, Lê AD. Age differences in the aversive properties of alcohol. Poster presented at the annual meeting of the Res. Soc. Alcohol; Santa Barbara, CA. Jun, 2005. [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin. Exp. Res. 1998;20:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Acute, rapid, and chronic tolerance during ontogeny: Observations when equating ethanol perturbation across age. Alcohol Clin. Exp. Res. 2001;25:1301–1308. [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescent alcohol sensitivity, tolerance, and intake. Recent Dev. Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ. Developmental differences in the acquisition of tolerance to ethanol. Alcohol. 1998;15:311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, Spear NE. Acute effects of ethanol on behavior of adolescent rats: Role of social context. Alcohol Clin. Exp. Res. 2001;25:377–385. [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: Role of familiarity of the test situation. Alcohol Clin. Exp. Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats” social facilitation, social inhibition, and anxiolysis. Developm. Psychobiol. 2006;48:146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol. Biochem. Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- York JL, Chan AW. Age effects on chronic tolerance to ethanol hypnosis and hypothermia. Pharmacol. Biochem. Behav. 1994;46:371–376. doi: 10.1016/0091-3057(94)90436-7. [DOI] [PubMed] [Google Scholar]
- Zhang X, Abdel-Rahman AA, Wooles WR. Impairment of baroreceptor reflex control of heart rate but not sympathetic efferent discharge by central neuroadministration of ethanol. Hypertension. 1989;14:282–292. doi: 10.1161/01.hyp.14.3.282. [DOI] [PubMed] [Google Scholar]


